Abstract
OBJECTIVE
Thyroid dysfunction and obesity during pregnancy have been associated with negative neonatal and obstetric outcomes. Thyroid hormone reference ranges have not been established for the pregnant Hispanic population. This study defines thyroid hormone reference ranges during early pregnancy in Chilean women and evaluates associations of BMI with thyroid function.
DESIGN, PATIENTS, MEASUREMENTS
This is a prospective observational study of 720 healthy Chilean women attending their first prenatal consultation at an outpatient clinic. Thyroid function (TSH, Free T4, Total T4, and TPOAb) and BMI were assessed at 8.8 ± 2.4 weeks of gestational age.
RESULTS
Median, 2.5th percentile (p2.5), and 97.5th percentile (p97.5) TSH values were higher, while median, p2.5, and p97.5 free T4 values were lower in obese patients compared with normal weight patients. Obesity was associated with a median TSH 16% higher (p=0.035) and a median free T4 6.5% lower (p<0.01) than values from patients with normal weight. BMI had a small, but statistically significant effect on TSH (p=0.04) and free T4 (p<0.01) when adjusted by maternal age, TPO antibodies, parity, sex of the newborn, gestational age, and smoking. In all TPOAb (-) patients, median (p2.5–p.97.5) TSH was: 1.96 mIU/L (0.11 – 5.96 mIU/L) and median (p2.5–p.97.5) free T4 was: 14.54 pmol/L (11.1 – 19.02 pmol/L). Applying these reference limits, we found a prevalence of overt and subclinical hypothyroidism of 0.9% and 3.05%, respectively.
CONCLUSIONS
TSH distributes at higher values and free T4 at lower values in obese pregnant women compared to normal weight pregnant women. Thyroid hormone reference ranges derived from Chilean patients with negative TPOAb are different from the fixed internationally proposed reference ranges and may be used in the Hispanic population.
Keywords: pregnancy, hypothyroidism, thyroid hormones reference range, body mass index and thyroid function
INTRODUCTION
Hypothyroidism during pregnancy has been associated with gestational complications for the mother and child including miscarriage, intrauterine growth retardation, preterm delivery and cognitive impairment in the offspring (1–3). Thus, it is critically important to establish precise thyroid function reference ranges during pregnancy that lead to accurate diagnosis and treatment of hypothyroidism in order to avoid these negative outcomes.
International guidelines for the diagnosis and management of thyroid disease during pregnancy recommend that trimester-specific reference intervals for TSH be calculated locally for each centre in a population with optimal iodine intake. If these calculated intervals are not available in the laboratory, fixed TSH reference ranges are recommended for the first trimester of 0.1–2.5 mIU/L and 0.2–3.0 mIU/L for the second trimester (4–6).
Although several studies from different global regions define reference ranges for thyroid hormones during pregnancy (7–17), there are scarce data on this topic in the Hispanic or Latin American population. In 2012, we conducted a pilot study of thyroid disease prevalence during early pregnancy in Chilean women (18) and found it to be much higher than the global prevalence reported until then (8–11, 13–16). This led us to hypothesize that thyroid hormone reference ranges in pregnant Chilean women were different from those reported in international guidelines, and additional factors such as ethnicity, interassay variability, and body mass index may have contributed to these distinct reference ranges.
Interassay differences for TSH are relatively small (r= 0.91–0.98), but free T4 measurements seem to be much more prone to interference and have larger interassay variability (r=0.68–0.89) (19, 20). We postulated that differences in free T4 concentrations between our cohort and other cohorts around the world may be at least in part attributed to assay-related factors and that differences in TSH may be ascribed to other variables including race, ethnicity, regional factors, or weight.
The prevalence of obesity has increased dramatically worldwide to the point of becoming a global epidemic (21). It is known that TSH and body mass index (BMI) are positively correlated in non-pregnant subjects (22). Free T4 concentrations have also been shown to change with increasing BMI (11, 23–26). In 2011, the American Thyroid Association (ATA) guidelines suggested that “serum TSH testing should be carried out in pregnant women with morbid obesity” (5), but there were insufficient data on the relationship between BMI and maternal thyroid function during early pregnancy to routinely consider BMI as a variable when establishing normal TSH values.
Chile has a population with a high prevalence of obesity that affects nearly 25% of adults (21). Given the limited data on thyroid function reference ranges during pregnancy in the Hispanic population, the high prevalence of obesity seen in Chile, and the lack of data on free T4 assays during pregnancy in this population, we conducted the present study to establish local thyroid hormone reference ranges that would hopefully be applicable to the Hispanic population. We also explored the effect of BMI on thyroid function during early pregnancy. Finally, we calculated multiple of medians (MoM) for TSH and free T4 to interpret and compare our upper and lower limits with those of other populations independent of assay differences.
MATERIALS AND METHODS
Subjects
Pregnant women with singleton pregnancies attending their first prenatal care visit in three primary health care centres in Santiago, Chile, were invited to participate in the study during the first half of 2014. The socioeconomic status of these women was uniformly low. We excluded women with multiple gestations, women who presented to their first prenatal visit after week 14, women with prior history of thyroid dysfunction, diabetes, use of medications that can affect thyroid function such as antidepressants, anticonvulsants and antipsychotic drugs, a history of thyroid surgery or radioactive iodine treatment.
Protocol
This study was approved by the Research Ethics Committee of the School of Medicine at the Pontificia Universidad Catolica de Chile and by the South Metropolitan Health Services Ethics Committee. All participants signed an informed consent.
Gestational age was determined by the last menstrual period and confirmed by ultrasound examination. When a major discrepancy between these two dates was found, the date of gestation was ultimately defined by ultrasound examination. Each participant completed a standardized clinical questionnaire of demographic and obstetric details with special emphasis on thyroid disease risk factors described by the Endocrine Society and the ATA guidelines (3,4). The questionnaire included menstrual periodicity, history of previous intrauterine growth restriction (IUGR), history of spontaneous miscarriages, premature labour, preeclampsia, previous offspring with developmental delay, and personal or family history of thyroid diseases such as goitre, neck radiotherapy, or other autoimmune diseases.
A morning fasting venous blood sample was obtained and kept at 4° Celsius. Serum concentrations of TSH, total T4, free T4 (FT4) and anti-thyroid peroxidase antibodies (TPOAb) were measured at the Central Laboratories of the Pontificia Universidad Catolica de Chile, which have international accreditation, ISO 15189.
Every patient was weighed and measured in the morning of the same visit that the blood sample was obtained. Their body mass index (BMI) was calculated by dividing their weight in kilograms by the square of their height in metres (kg/m2). Patients were assigned to the underweight (UW) group when BMI was <20 kg/m2, the normal weight (NW) group when BMI was 20–24.9 kg/m2, the overweight (OW) group when BMI was 25–29.9 kg/m2 and the obese (OB) group when BMI was ≥ 30 kg/m2. Only 10 patients were classified as morbidly obese (BMI≥40 kg/m2). Due to the small number of women in this category, we included them in the obese (OB) group for our analyses. We used universal BMI categories because the hormonal changes from pregnancy and the actual weight of the foetus do not substantially change the mother’s weight in early pregnancy.
Laboratory determinations
Ultrasensitive TSH, total T4, and FT4 were measured by competitive electrochemiluminescent immunoassay (Modular Analytics E 170, Roche, Indianapolis, IN, USA). For TSH, the threshold of detection was 0.005 mIU/L, and the intra- and interassay coefficients of variation were <3.3% at a range of 0.035 to 3.66 mIU/L. For total T4, the analytical sensitivity was 5.4 nmol/L with intra- and interassay coefficients of variation <4.2% at a range of 65.64 to 231.66 nmol/L. For FT4, the analytical sensitivity was 0.296 pmol/L with intra- and interassay coefficients of variation <3.6% at a range of 14.93 to 25.74 pmol/L. The quantitative determination of TPOAb was performed by a microparticle enzyme immunoassay (AXSYM-ABBOT) with an analytical sensitivity of 1 kIU/L. During the study period, the manufacturer’s reference values for non-pregnant women were as follows: TSH: 0.3–4.2 mIU/L; T4: 59.2–154.44 nmol/L; FT4:10.3–23.2 pmol/L; and TPOAb <12 kIU/L.
Statistical analyses
All variables were subjected to a normality test (Kolmogorov-Smirnov test) and were analysed with parametric (Student’s t test) and nonparametric tests (Kruskal-Wallis), as appropriate. The comparison between the frequencies of risk factors was performed with the Chi-square (χ2) test with Yates correction. Risk analysis was performed with logistic regression and linear regression. A multiple linear regression was used to examine the effect of BMI as a continuous variable on TSH and free T4. Changes in BMI were quantified as 1Kg/m2. TSH values were negatively skewed, so a square-root transformation of TSH was used. Free T4 was normally distributed, so no transformations were done. Adjusting covariates included age (in years), TPO antibodies (yes vs. no), parity (one, and two and more children compared to nulliparous women), sex of the newborn (M vs. F), gestational age (in weeks), and smoking (yes vs. no). To test for normality, linearity and heteroscedasticity we predicted raw residuals, standardized residuals, and studentized residuals. We created histograms of these residuals that confirmed the normality assumption and ran rvfplots and component-plus-residual plots (cprplots) to confirm the linearity of our data. We also ran hettest and whitetst to quantitatively test for homoscedasticity and confirmed that indeed all our assumptions were met. Multiple of medians (MoM) were calculated by dividing each individual’s TSH and free T4 values by the trimester-specific population median. The p value was established a priori for statistical significance at 0.05. All statistical analyses were conducted using STATA, version 12.0 (College Station, Texas, USA).
RESULTS
From December 2013 to June 2014, 865 pregnant women were invited to participate in the study. We excluded 93 women who had a history of thyroid disease (10.7%), 34 women who used medications that could affect thyroid function, 8 women who refused to participate, 10 women who did not complete the study. A total of 720 women with singleton pregnancies were included in the final study population.
The demographic, clinical, and obstetric characteristics of the 720 patients are described in Table 1. The mean ± SD maternal age of our patients was 25.4 ± 6.6 years. More than half of the patients were pregnant for the first time and attended a first prenatal visit by week 9; all patients were enrolled before 14 weeks of gestational age. The mean ± SD gestational age at which laboratory and BMI determinations were performed was 8.8 ± 2.4 weeks. Only 81 of 720 women were enrolled between 12 weeks and 14 weeks. The mean ± SD BMI of our pregnant women was 26.3 ± 5.1 kg/m2, but half of our study population was overweight or obese (BMI ≥ 25 kg/m2). The socio-economic status of our pregnant patients was uniformly low.
Table 1.
Clinical and obstetrical characteristics of the study population (n=720)
| Mean maternal age ± SD, years | 25.4 ± 6.6 |
|
| |
| Maternal age, n (%) | |
| Younger than 18 | 59 (8.2) |
| 18–24 | 309 (42.9) |
| 25–34 | 268 (37.2) |
| 35 and over | 84 (11.7) |
|
| |
| Mean gestational age at entry ±SD, weeks | 8.8 ± 2.4 |
|
| |
| Gestational age at first prenatal visit, n (%) | |
| < 9 weeks | 396 (55.0) |
| 9 to <11 weeks | 166 (23.1) |
| 11 to ≤14 weeks | 158 (21.9) |
|
| |
| History of previous pregnancies, n (%) | |
| Nulliparous | 381 (52.9) |
| Parity 1 | 204 (28.3) |
| Parity ≥ 2 | 135 (18.8) |
|
| |
| Smokers, n (%) | 89 (12.4) |
|
| |
| Mean BMI ± SD kg/m2 | 26.3 ± 5.1 |
| BMI range | 16.3 – 45.8 |
|
| |
| Initial weight, n (%) | |
| Underweight (BMI<20) | 56 (7.8) |
| Normal weight (BMI:20–24.9) | 299 (41.5) |
| Overweight (BMI:25–29.9) | 220 (30.6) |
| Obesity (BMI≥30) | 145 (20.1) |
Thyroid hormones, thyroid peroxidase antibodies, and BMI
Obese pregnant women had a median TSH that was 16% higher (2.34 vs. 1.99 mIU/L, p=0.036) and a median free T4 that was 6.5% lower (13.9 vs. 14.8 pmol/L, p<0.01) than pregnant women with a normal weight (Figure 1).
Figure 1.
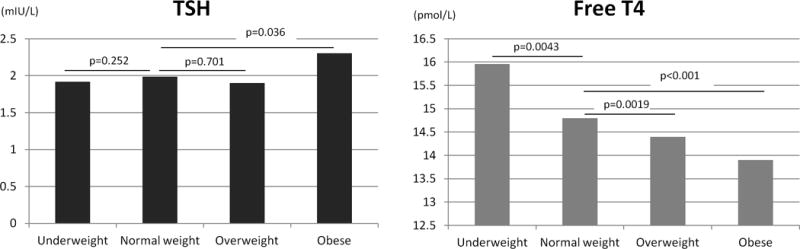
Median TSH, and Free T4 according to BMI categories in all patients (n=720).
Ordinates of panel A are TSH values in mIU/L and ordinates in panel B are Free T4 values in pmol/L.
TPOAb were positive (>12 kIU/L) in 10.1% of the patients (73/720). There was a non-statistically significant increase in TPOAb with increasing BMI: 4/47 (8.5%) in UW, 25/295 (8.5%) in NW, 25/226 (11%) in OW, and 19/152 (12.5%) in OB (p for trend=0.148). The association of decreased thyroid function with increasing BMI was also present in the TPOAb-negative group (Table 2).
Table 2.
Median, p2.5, and p97.5 TSH, total T4, and FreeT4 according to BMI categories in pregnant women without TPOAb (n=647).
| TSH (mIU/L) | Total T4 (nmol/L) | Free T4 (pmol/L) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 2.5 | Median | 97.5 | 2.5 | Median | 97.5 | 2.5 | Median | 97.5 | |
| UW | 0.01 | 1.82 | 6.33 | 92.9 | 128.7 | 254.1 | 11.8 | 16.1 | 42.6 |
| NW | 0.05 | 1.9 | 6.08 | 87.5 | 123.6 | 184.3 | 11.7 | 14.8 | 20.4 |
| OW | 0.33 | 1.87 | 5.94 | 85 | 121 | 171.1 | 10.3 | 14.4 | 18.8 |
| OB | 0.46 | 2.27 | 6.61 | 82.8 | 118.4 | 166.9 | 10.6 | 14 | 18.1 |
| p value* | 0.02 | 0.002 | <0.001 | ||||||
UW: Underweight (n=43); NW: Normal weight (n=270); OW: Overweight (n=201); OB: Obese (n=133)
comparison of medians across four BMI categories using Kruskal-Wallis test.
Using linear regression, we performed multivariate analyses to examine the effects of BMI on TSH and free T4. Higher BMI was significantly associated with higher TSH (p=0.04, Table 3A) and lower free T4 (p<0.001, Table 3B) when adjusted by maternal age, TPO antibodies, parity, sex of the newborn, gestational age, and smoking. The total explained variance (R2) of our model for TSH was 7.97%. BMI explained 1.41% of this variance. TPO antibodies and sex of the newborn were the remaining significant determinants in our model. The R2 change of these determinants were 5% and 1%, respectively. The total explained variance of our model for Free T4 was 16.3%. BMI explained 5.3% of this variance. The remaining significant determinants in our Free T4 model were maternal age, TPO antibodies, and gestational age. The R2 change of these determinants were 2.5%, 1.1%, and 5.1%, respectively.
Table 3.
Multivariate linear regression models examining the effect of body mass index (BMI) on A. TSH and B. Free T4 when adjusted by maternal age, TPO antibodies, parity, sex of the newborn, weeks of gestation, and smoking.
| A. | |||
|---|---|---|---|
| TSH* (n=557) | Regression coefficient | Standard Error | p-value |
| BMI | 0.009057 | 0.0043117 | 0.036 |
| Age | −0.0039257 | 0.0038971 | 0.314 |
| TPO Antibodies | 0.3768184 | 0.0699704 | 0.000 |
| Parity 1 | −0.0935275 | 0.0520008 | 0.073 |
| Parity ≥ 2 | 0.0026789 | 0.0650321 | 0.967 |
| Sex of newborn | 0.0911571 | 0.0413148 | 0.028 |
| Gestation (weeks) | 0.0040729 | 0.0086249 | 0.637 |
| Smoking | −0.0974039 | 0.0639362 | 0.128 |
| Intercept | 1.394582 | 0.1936655 | 0.000 |
| B. | |||
|---|---|---|---|
| Free T4 (n=560) | Regression coefficient | Standard Error | p-value |
| BMI | − 0.0899735 | 0.0167457 | 0.000 |
| Age | − 0.0396639 | 0.0150878 | 0.009 |
| TPO Antibodies | − 0.7663849 | 0.2622203 | 0.004 |
| Parity 1 | 0.1713447 | 0.2008147 | 0.394 |
| Parity 2 | − 0.284663 | 0.251601 | 0.258 |
| Sex of newborn | 0.098168 | 0.1597093 | 0.539 |
| Gestation (weeks) | − 0.1946608 | 0.0332834 | 0.000 |
| Smoking | 0.2690603 | 0.247179 | 0.277 |
| Intercept | 19.18689 | 0.7483954 | 0.000 |
TSH is square-root transformed
¥ R2 = 7.97%
¥ R2 = 16.3%
In the 647 patients without TPOAb (TPOAb-), the median TSH was 1.96 mIU/L, with a p2.5 and p97.5 of 0.11 and 5.96 mIU/L, respectively; the median total T4 was 122.3 nmol/L, with a p2.5 and p97.5 of 86.2 and 181.2 nmol/L, respectively; and the median free T4 was 14.54 pmol/L, with a p2.5 and p97.5 of 11.1 and 19.02 pmol/L, respectively.
Defining thyroid hormone reference ranges and thyroid disease in early pregnancy
With reference values derived from all patients with negative TPOAb (-) (p2.5–p97.5: 0.11–5.96 mIU/L, n=647), the prevalence of overt hypothyroidism was 0.9% and that of subclinical hypothyroidism was 3.05%. Using fixed TSH cutoff concentrations of 2.5 and 3.0 mIU/L for the first and second trimesters, respectively, as described in international guidelines, we would have found a prevalence of overt hypothyroidism of 2.5% and a prevalence of subclinical hypothyroidism of 34.2%. Figure 2 shows a distribution of serum TSH values within the normal reference range (2.5th–97.5th percentiles) of pregnant women in the first trimester (<12 weeks, n=502) without anti-TPOAb. Applying a fixed TSH cutoff concentration of 2.5 mIU/L, 24.4% of the patients would have been misclassified as having an elevated TSH.
Figure 2.
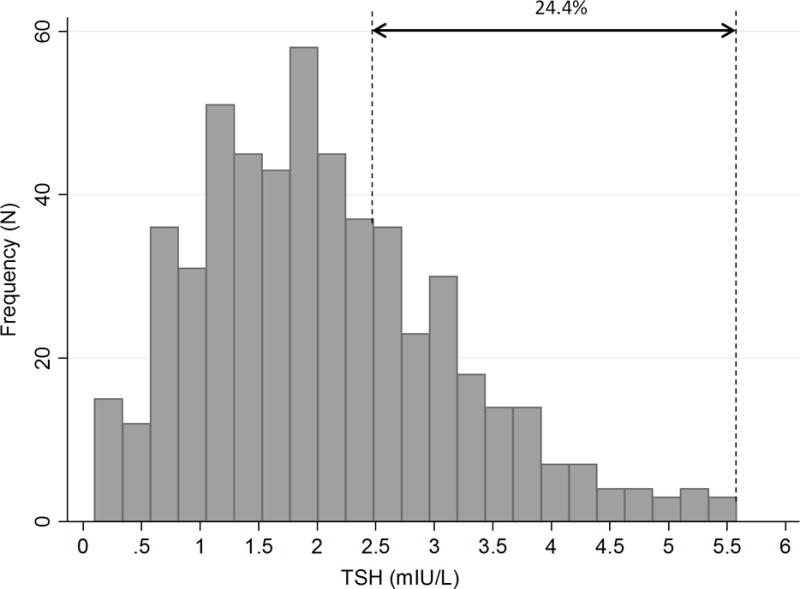
Distribution of serum TSH values within the normal reference interval (2.5th– 97.5th percentiles) in the first trimester in 502 Chilean women with negative TPO antibodies.
To determine if the early pregnancy reference ranges of our population are comparable to those of other populations independent of interassay differences, we calculated the multiple of medians (MoM) for TSH and free T4 values and derived lower (2.5th percentile) and upper (97.5th percentile) limits for both hormones. MoM for TSH: 2.5th–97.5th percentiles were 0.057–2.88, respectively, and MoM for free T4: 2.5th–97.5th percentiles were 0.77–1.28, respectively.
DISCUSSION
To our knowledge, this is the first Latin American population study to define TSH, total T4, and free T4 reference ranges in pregnant patients that explores the relationship between maternal BMI and thyroid function during early pregnancy. We found that TSH reference ranges in pregnant Chilean women were different from the fixed limits proposed in international endocrine guidelines (4–6). As BMI increased, we observed that the TSH distribution curves shifted to higher values, while total T4 and free T4 distributions shifted to lower values. In multivariate models, BMI had a small but statistically significant effect on TSH and free T4 when adjusted by maternal age, TPO antibodies, parity, sex of the newborn, gestational age, and smoking. The multiple of medians (MoM) for the TSH and free T4 lower and upper reference limits were comparable to those of other populations (27).
Our findings suggest that higher BMI in the first trimester of pregnancy is associated with decreased thyroid function. This effect size was small and the overall variance of our models suggests that other parameters in addition to the ones collected likely explain the variability of TSH and Free T4. This association has also been reported by Mannisto et al. in Finland, where BMI was positively correlated with TSH and negatively correlated with free T4 concentrations in early pregnancy (11). Han et al. also found in Chinese pregnant women that TSH in the first trimester shifted to higher values and free T4 shifted to lower values with increasing BMI (23). The association between lower free T4 values with higher BMI in the first trimester appears to be a uniform finding around the world. However, higher TSH values are not consistently associated with higher BMI. For example, Pop et al. found an association between lower free T4 levels and higher BMI, but they could not find a correlation between BMI and TSH levels at 12 weeks of gestation (24). Similarly, Gowachirapant et al. did not see an association between TSH and BMI in iodine-deficient pregnant Thai women (25). Haddow et al. did not find TSH to be associated with weight during early pregnancy (26). All of these studies, including ours, reveal an association between higher BMI and lower free T4 in early pregnancy. Possible explanations for the discrepant findings on the association between TSH and BMI may stem from differences in the number of subjects, differences in iodine sufficiency, and differences in ethnic or regional factors that may make the association between TSH and BMI more evident in some areas rather than others.
High TSH has been associated with elevated BMI in non-pregnant subjects (22, 28, 29), and there are several suggested mechanisms that may lead to increased TSH levels in obesity. Adipose tissue is considered to be an active endocrine organ that produces leptin, cytokines, and other inflammatory factors. Leptin has been shown to dose-dependently stimulate pro-TRH biosynthesis both directly through actions on TRH neurons and indirectly through α-melanocyte-stimulating hormone (30–32). Despite the higher plasma TSH levels, TSH receptors are less expressed on adipocytes of obese vs. lean individuals (33). This reduced TSH receptor expression might induce down-regulation of thyroid hormone receptors and thyroid hormone action, thereby further increasing plasma TSH concentrations and constituting a condition of peripheral thyroid hormone resistance. Another theory is that obesity produces a decrease in peripheral deiodinase activity, thus reducing T3 production from T4 with subsequent feedback stimulation of TSH (34). In keeping with our finding that TPOAb positivity increases as BMI goes up, leptin has also been independently implicated in thyroid autoimmunity (35). Altogether, these observations provide biological hypotheses for the association of decreased thyroid function in patients with elevated BMI.
We defined thyroid function reference ranges during early pregnancy in Chilean women and compared these limits to trimester-specific fixed ranges proposed in international endocrine guidelines (4–6) We found that our thyroid hormone reference ranges were different from static ranges. This difference may be attributed to low iodine intake, although a recent study from China measured first-trimester serum thyroid function and urinary iodine in an iodine-sufficient population and found that low urinary iodine had no significant effect on mean TSH and free T4 concentrations (36). The possibility of a higher prevalence of autoimmune thyroid disease also seemed unlikely because we excluded all women with thyroid disease and TPOAb (+) to define our reference range. Therefore, it is possible that ethnic or regional factors may explain our distinct reference ranges. In this regard, it is worth noting that Indian and Chinese populations showed similar reference ranges as those described by our study (37–39). Differences in TSH levels among pregnant women from different ethnic groups have been shown before (40, 41). More recently, Korevaar et al. (42) reported a significant change in the prevalence of thyroid dysfunction when ethnic-specific reference ranges were applied to four populations of iodine-sufficient pregnant women, suggesting that ethnicity plays a major role in the definition of thyroid hormone reference ranges during pregnancy. Finally, sample processing and assay methodology variations may have contributed to our discrepant findings. During pregnancy, the serum levels of the thyroid hormone binding globulins and albumin are increased. These changes vary by individual, and “free” hormone measurements by immunoassays are influenced by these alterations. Consequently, the results of free hormone measurements may vary depending on the assay. This is particularly true for free T4 (19). When we calculated our upper and lower reference limits expressed as the multiple of medians (MoM), our limits were comparable to those of many published cohorts around the globe (27). This suggests that differences in our early pregnancy thyroid hormone reference ranges with other cohorts or with the fixed values proposed by international guidelines can be at least in part explained by interassay variability.
The strengths of our study include the prospective collection of data in the largest Hispanic cohort of pregnant women reported to date. This study allowed us to define reliable early pregnancy thyroid hormone reference ranges that may be extended to other Hispanic populations. In addition, we were able to examine the effects of BMI on TSH and free T4 distribution and its real impact in multivariate models. Finally, all of our samples were obtained fasting and before 11AM. Circadian variations in TSH with higher values early in the morning that decline thereafter have been previously reported. The standardized sampling in our study provides a clear advantage that minimizes the influence of circadian variations and food intake on TSH results. Despite its strengths, our study has several limitations. First, we only collected thyroid hormone levels in early pregnancy, so the proposed reference ranges only apply to women ≤ 14 weeks of gestational age. Further studies should be conducted to define other trimester-specific thyroid hormone reference ranges. Second, BMI was only calculated at the initial visit, so we could not evaluate longitudinal associations of weight and thyroid function and we are not able to establish causal relationships between weight and thyroid function. Third, the socioeconomic status of these women was uniformly low, so the results of this study may not be generalizable to a population with higher socioeconomic status. Fourth, we did not collect median urine iodine levels to objectively document iodine sufficiency. Lastly, hCG was not evaluated, and it might be hypothesized that the differences in the relationship between BMI and TSH during pregnancy compared to the general (female) population might be hCG related.
In conclusion, the present results provide information on the association between BMI and thyroid hormone concentrations and indicate that obese pregnant patients are more susceptible to thyroid dysfunction in the first trimester. The results of our study have provided additional information on the reference intervals of thyroid hormones during early pregnancy from a thyroid disease-free, antibody-negative Chilean population. Our results may be generalizable to other Hispanic populations.
Acknowledgments
The grant number is P30 CA008748.
Funding: Project FONIS SA10I10078
Footnotes
Financial Disclosure: Nothing to declare.
Disclosure Statement: No competing financial interests exist.
References
- 1.Negro R, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Stagnaro-Green A. Increased pregnancy loss rate in thyroid antibody negative women with TSH levels between 2.5 and 5.0 in the first trimester of pregnancy. The Journal of clinical endocrinology and metabolism. 2010;95(9):E44–8. doi: 10.1210/jc.2010-0340. [DOI] [PubMed] [Google Scholar]
- 2.Medici M, Korevaar TI, Schalekamp-Timmermans S, Gaillard R, de Rijke YB, Visser WE, et al. Maternal early-pregnancy thyroid function is associated with subsequent hypertensive disorders of pregnancy: the generation R study. The Journal of clinical endocrinology and metabolism. 2014;99(12):E2591–8. doi: 10.1210/jc.2014-1505. [DOI] [PubMed] [Google Scholar]
- 3.Chan S, Boelaert K. Optimal management of hypothyroidism, hypothyroxinaemia and euthyroid TPO antibody positivity preconception and in pregnancy. Clinical endocrinology. 2015;82(3):313–26. doi: 10.1111/cen.12605. [DOI] [PubMed] [Google Scholar]
- 4.De Groot L, Abalovich M, Alexander EK, Amino N, Barbour L, Cobin RH, et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. The Journal of clinical endocrinology and metabolism. 2012;97(8):2543–65. doi: 10.1210/jc.2011-2803. [DOI] [PubMed] [Google Scholar]
- 5.Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid: official journal of the American Thyroid Association. 2011;21(10):1081–125. doi: 10.1089/thy.2011.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lazarus J, Brown RS, Daumerie C, Hubalewska-Dydejczyk A, Negro R, Vaidya B. 2014 European thyroid association guidelines for the management of subclinical hypothyroidism in pregnancy and in children. European thyroid journal. 2014;3(2):76–94. doi: 10.1159/000362597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bestwick JP, John R, Maina A, Guaraldo V, Joomun M, Wald NJ, et al. Thyroid stimulating hormone and free thyroxine in pregnancy: expressing concentrations as multiples of the median (MoMs) Clinica chimica acta; international journal of clinical chemistry. 2014;430:33–7. doi: 10.1016/j.cca.2013.12.030. [DOI] [PubMed] [Google Scholar]
- 8.Bocos-Terraz JP, Izquierdo-Alvarez S, Bancalero-Flores JL, Alvarez-Lahuerta R, Aznar-Sauca A, Real-Lopez E, et al. Thyroid hormones according to gestational age in pregnant Spanish women. BMC research notes. 2009;2:237. doi: 10.1186/1756-0500-2-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbert RM, Hadlow NC, Walsh JP, Fletcher SJ, Brown SJ, Stuckey BG, et al. Assessment of thyroid function during pregnancy: first-trimester (weeks 9-13) reference intervals derived from Western Australian women. The Medical journal of Australia. 2008;189(5):250–3. doi: 10.5694/j.1326-5377.2008.tb02015.x. [DOI] [PubMed] [Google Scholar]
- 10.Lambert-Messerlian G, McClain M, Haddow JE, Palomaki GE, Canick JA, Cleary-Goldman J, et al. First- and second-trimester thyroid hormone reference data in pregnant women: a FaSTER (First- and Second-Trimester Evaluation of Risk for aneuploidy) Research Consortium study. American journal of obstetrics and gynecology. 2008;199(1):62, e1–6. doi: 10.1016/j.ajog.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mannisto T, Surcel HM, Ruokonen A, Vaarasmaki M, Pouta A, Bloigu A, et al. Early pregnancy reference intervals of thyroid hormone concentrations in a thyroid antibody-negative pregnant population. Thyroid: official journal of the American Thyroid Association. 2011;21(3):291–8. doi: 10.1089/thy.2010.0337. [DOI] [PubMed] [Google Scholar]
- 12.Medici M, de Rijke YB, Peeters RP, Visser W, de Muinck Keizer-Schrama SM, Jaddoe VV, et al. Maternal early pregnancy and newborn thyroid hormone parameters: the Generation R study. The Journal of clinical endocrinology and metabolism. 2012;97(2):646–52. doi: 10.1210/jc.2011-2398. [DOI] [PubMed] [Google Scholar]
- 13.Pearce EN, Oken E, Gillman MW, Lee SL, Magnani B, Platek D, et al. Association of first-trimester thyroid function test values with thyroperoxidase antibody status, smoking, and multivitamin use. Endocrine practice: official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2008;14(1):33–9. doi: 10.4158/EP.14.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quinn FA, Gridasov GN, Vdovenko SA, Krasnova NA, Vodopianova NV, Epiphanova MA, et al. Prevalence of abnormal thyroid stimulating hormone and thyroid peroxidase antibody-positive results in a population of pregnant women in the Samara region of the Russian Federation. Clinical chemistry and laboratory medicine: CCLM/FESCC. 2005;43(11):1223–6. doi: 10.1515/CCLM.2005.212. [DOI] [PubMed] [Google Scholar]
- 15.Springer D, Zima T, Limanova Z. Reference intervals in evaluation of maternal thyroid function during the first trimester of pregnancy. European journal of endocrinology/European Federation of Endocrine Societies. 2009;160(5):791–7. doi: 10.1530/EJE-08-0890. [DOI] [PubMed] [Google Scholar]
- 16.Stricker R, Echenard M, Eberhart R, Chevailler MC, Perez V, Quinn FA, et al. Evaluation of maternal thyroid function during pregnancy: the importance of using gestational age-specific reference intervals. European journal of endocrinology/European Federation of Endocrine Societies. 2007;157(4):509–14. doi: 10.1530/EJE-07-0249. [DOI] [PubMed] [Google Scholar]
- 17.Vaidya B, Anthony S, Bilous M, Shields B, Drury J, Hutchison S, et al. Detection of thyroid dysfunction in early pregnancy: Universal screening or targeted high-risk case finding? The Journal of clinical endocrinology and metabolism. 2007;92(1):203–7. doi: 10.1210/jc.2006-1748. [DOI] [PubMed] [Google Scholar]
- 18.Mosso L, Martinez A, Rojas MP, Margozzini P, Solari S, Lyng T, et al. Frequency of subclinical thyroid problems among women during the first trimester of pregnancy. Revista medica de Chile. 2012;140(11):1401–8. doi: 10.4067/S0034-98872012001100004. [DOI] [PubMed] [Google Scholar]
- 19.Berta E, Samson L, Lenkey A, Erdei A, Cseke B, Jenei K, et al. Evaluation of the thyroid function of healthy pregnant women by five different hormone assays. Die Pharmazie. 2010;65(6):436–9. [PubMed] [Google Scholar]
- 20.d’Herbomez M, Forzy G, Gasser F, Massart C, Beaudonnet A, Sapin R. Clinical evaluation of nine free thyroxine assays: persistent problems in particular populations. Clinical chemistry and laboratory medicine: CCLM/FESCC. 2003;41(7):942–7. doi: 10.1515/CCLM.2003.143. [DOI] [PubMed] [Google Scholar]
- 21.Haslam DW, James WP. Obesity. Lancet. 2005;366(9492):1197–209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 22.Rimm AA, Werner LH, Yserloo BV, Bernstein RA. Relationship of ovesity and disease in 73,532 weight-conscious women. Public health reports. 1975;90(1):44–51. [PMC free article] [PubMed] [Google Scholar]
- 23.Han C, Li C, Mao J, Wang W, Xie X, Zhou W, et al. High Body Mass Index Is an Indicator of Maternal Hypothyroidism, Hypothyroxinemia, and Thyroid-Peroxidase Antibody Positivity during Early Pregnancy. BioMed research international. 2015;2015:351831. doi: 10.1155/2015/351831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pop VJ, Biondi B, Wijnen HA, Kuppens SM, Lvader H. Maternal thyroid parameters, body mass index and subsequent weight gain during pregnancy in healthy euthyroid women. Clinical endocrinology. 2013;79(4):577–83. doi: 10.1111/cen.12177. [DOI] [PubMed] [Google Scholar]
- 25.Gowachirapant S, Melse-Boonstra A, Winichagoon P, Zimmermann MB. Overweight increases risk of first trimester hypothyroxinaemia in iodine-deficient pregnant women. Maternal & child nutrition. 2014;10(1):61–71. doi: 10.1111/mcn.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haddow JE, Craig WY, Palomaki GE, Neveux LM, Lambert-Messerlian G, Canick JA, et al. Impact of adjusting for the reciprocal relationship between maternal weight and free thyroxine during early pregnancy. Thyroid: official journal of the American Thyroid Association. 2013;23(2):225–30. doi: 10.1089/thy.2012.0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medici M, Korevaar TI, Visser WE, Visser TJ, Peeters RP. Thyroid function in pregnancy: what is normal? Clinical chemistry. 2015;61(5):704–13. doi: 10.1373/clinchem.2014.236646. [DOI] [PubMed] [Google Scholar]
- 28.Knudsen N, Laurberg P, Rasmussen LB, Bulow I, Perrild H, Ovesen L, et al. Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. The Journal of clinical endocrinology and metabolism. 2005;90(7):4019–24. doi: 10.1210/jc.2004-2225. [DOI] [PubMed] [Google Scholar]
- 29.de Moura Souza A, Sichieri R. Association between serum TSH concentration within the normal range and adiposity. European journal of endocrinology/European Federation of Endocrine Societies. 2011;165(1):11–5. doi: 10.1530/EJE-11-0261. [DOI] [PubMed] [Google Scholar]
- 30.Nillni EA, Vaslet C, Harris M, Hollenberg A, Bjorbak C, Flier JS. Leptin regulates prothyrotropin-releasing hormone biosynthesis. Evidence for direct and indirect pathways. The Journal of biological chemistry. 2000;275(46):36124–33. doi: 10.1074/jbc.M003549200. [DOI] [PubMed] [Google Scholar]
- 31.Mantzoros CS, Ozata M, Negrao AB, Suchard MA, Ziotopoulou M, Caglayan S, et al. Synchronicity of frequently sampled thyrotropin (TSH) and leptin concentrations in healthy adults and leptin-deficient subjects: evidence for possible partial TSH regulation by leptin in humans. The Journal of clinical endocrinology and metabolism. 2001;86(7):3284–91. doi: 10.1210/jcem.86.7.7644. [DOI] [PubMed] [Google Scholar]
- 32.Guo F, Bakal K, Minokoshi Y, Hollenberg AN. Leptin signaling targets the thyrotropin-releasing hormone gene promoter in vivo. Endocrinology. 2004;145(5):2221–7. doi: 10.1210/en.2003-1312. [DOI] [PubMed] [Google Scholar]
- 33.Nannipieri M, Cecchetti F, Anselmino M, Camastra S, Niccolini P, Lamacchia M, et al. Expression of thyrotropin and thyroid hormone receptors in adipose tissue of patients with morbid obesity and/or type 2 diabetes: effects of weight loss. International journal of obesity. 2009;33(9):1001–6. doi: 10.1038/ijo.2009.140. [DOI] [PubMed] [Google Scholar]
- 34.Matzen LE, Kvetny J, Pedersen KK. TSH, thyroid hormones and nuclear-binding of T3 in mononuclear blood cells from obese and non-obese women. Scandinavian journal of clinical and laboratory investigation. 1989;49(3):249–53. [PubMed] [Google Scholar]
- 35.Marzullo P, Minocci A, Tagliaferri MA, Guzzaloni G, Di Blasio A, De Medici C, et al. Investigations of thyroid hormones and antibodies in obesity: leptin levels are associated with thyroid autoimmunity independent of bioanthropometric, hormonal, and weight-related determinants. The Journal of clinical endocrinology and metabolism. 2010;95(8):3965–72. doi: 10.1210/jc.2009-2798. [DOI] [PubMed] [Google Scholar]
- 36.Shi X, Han C, Li C, Mao J, Wang W, Xie X, et al. Optimal and safe upper limits of iodine intake for early pregnancy in iodine-sufficient regions: a cross-sectional study of 7190 pregnant women in China. The Journal of clinical endocrinology and metabolism. 2015;100(4):1630–8. doi: 10.1210/jc.2014-3704. [DOI] [PubMed] [Google Scholar]
- 37.Yan YQ, Dong ZL, Dong L, Wang FR, Yang XM, Jin XY, et al. Trimester- and method-specific reference intervals for thyroid tests in pregnant Chinese women: methodology, euthyroid definition and iodine status can influence the setting of reference intervals. Clinical endocrinology. 2011;74(2):262–9. doi: 10.1111/j.1365-2265.2010.03910.x. [DOI] [PubMed] [Google Scholar]
- 38.Li C, Shan Z, Mao J, Wang W, Xie X, Zhou W, et al. Assessment of thyroid function during first-trimester pregnancy: what is the rational upper limit of serum TSH during the first trimester in Chinese pregnant women? The Journal of clinical endocrinology and metabolism. 2014;99(1):73–9. doi: 10.1210/jc.2013-1674. [DOI] [PubMed] [Google Scholar]
- 39.Marwaha RK, Chopra S, Gopalakrishnan S, Sharma B, Kanwar RS, Sastry A, et al. Establishment of reference range for thyroid hormones in normal pregnant Indian women. BJOG: an international journal of obstetrics and gynaecology. 2008;115(5):602–6. doi: 10.1111/j.1471-0528.2008.01673.x. [DOI] [PubMed] [Google Scholar]
- 40.Walker JA, Illions EH, Huddleston JF, Smallridge RC. Racial comparisons of thyroid function and autoimmunity during pregnancy and the postpartum period. Obstetrics and gynecology. 2005;106(6):1365–71. doi: 10.1097/01.AOG.0000185475.61612.ea. [DOI] [PubMed] [Google Scholar]
- 41.La’ulu SL, Roberts WL. Ethnic differences in first-trimester thyroid reference intervals. Clinical chemistry. 2011;57(6):913–5. doi: 10.1373/clinchem.2010.161240. [DOI] [PubMed] [Google Scholar]
- 42.Korevaar TI, Medici M, de Rijke YB, Visser W, de Muinck Keizer-Schrama SM, Jaddoe VW, et al. Ethnic differences in maternal thyroid parameters during pregnancy: the Generation R study. The Journal of clinical endocrinology and metabolism. 2013;98(9):3678–86. doi: 10.1210/jc.2013-2005. [DOI] [PubMed] [Google Scholar]


