Abstract
Objectives
Combination packages for HIV prevention can leverage the effectiveness of biomedical and behavioural elements to lower disease incidence with realistic targets for individual and population risk reduction. We investigated how sexual network structures can maximise the effectiveness of a package targeting sexually active adults in sub-Saharan Africa (SSA) with intervention components for medical male circumcision (MMC) and sexual partnership concurrency (having >1 ongoing partner).
Methods
Network-based mathematical models of HIV type 1 (HIV-1) transmission dynamics among heterosexual couples were used to explore how changes to MMC alone and in combination with changes to concurrency impacted endemic HIV-1 prevalence and incidence. Starting from a base model parameterised from empirical data from West Africa, we simulated the prevalence of circumcision from 10% to 90% and concurrency was modelled at four discrete levels corresponding to values observed across SSA.
Results
MMC and concurrency could contribute to the empirical variation in HIV-1 disease prevalence across SSA. Small reductions in concurrency resulted in large declines in HIV-1 prevalence. Scaling up circumcision in low-concurrency settings yields a greater relative benefit, but the absolute number of infections averted depends on both the circumcision coverage and baseline incidence. Epidemic extinction with this package will require substantial scale-up of MMC in low-concurrency settings.
Conclusions
Dynamic sexual network structure should be considered in the design and targeting of MMC within combination HIV-1 prevention packages. Realistic levels of coverage for these packages within southern Africa could lead to a reduction of incidence to the low levels observed in western Africa, and possibly, epidemic extinction.
INTRODUCTION
The predominant mode of transmission for HIV type 1 (HIV-1) is sexual, with most infections occurring within heterosexual partnerships in low-income and middle-income regions, including sub-Saharan Africa (SSA). Biomedical tools like early antiretroviral therapy (ART) and oral pre-exposure prophylaxis are highly effective for HIV prevention in these settings.1 Medical male circumcision (MMC) is another, surgical approach: the prevalence of circumcision across SSA negatively correlates with HIV-1 prevalence, and randomised controlled trials (RCTs) have observed prevention benefits of over 50% for men.2 Yet MMC interventions alone will likely not eradicate HIV-1 within SSA, due to less than perfect efficacy, high levels of coverage required and the potential for risk compensation.3
Combination packages for HIV-1 prevention leverage individual tools bundled together to achieve synergistic reductions in disease incidence.4 Packages may include interventions like MMC along with behavioural risk reduction components. Although no RCT of a purely behavioural intervention has reduced HIV-1 incidence,5 their combination with biomedical technologies could generate an impact. Even in the absence of behaviour change, targeting packages based on behavioural risk profiles of the population may maximise package efficiency. Ongoing challenges in packaging include estimating the contributions of combination package elements alone and together for package design, and also predicting the best settings for cost-efficient package targeting.
Mathematical modelling has been used to investigate MMC, alone and in combination packages, to estimate impact on HIV incidence.6 One limitation of prior models has been their representation of baseline sexual behaviour (ie, before an intervention) and the type of behavioural change needed for meaningful epidemic control.3 The mathematical framework of these models (deterministic and compartmental) is limited in realistically representing dynamic sexual networks. Network structures have strong influences on transmission potential: small changes in network connectivity can generate large, non-linear reductions in disease incidence.7 Models of HIV interventions with behavioural components should therefore represent behaviour within sexual networks realistically.
In this study, we use mathematical models that explicitly simulate dynamic heterosexual partnership networks to understand an HIV prevention package that combines MMC with changes to network connectivity as a function of sexual partnership concurrency (having more than one ongoing partner).8,9 Using a data-driven approach to simulating sexual behaviour, we address three related questions. First, to validate the behavioural model specifications, do the differences in male circumcision and network structure across SSA help to explain the variation in HIV-1 prevalence across SSA? Second, how do concurrency and MMC combine to reduce HIV incidence in SSA, including to extinction levels? Third, even in the absence of behaviour change, how does MMC intervention performance depend on network structure for the purposes of MMC targeting?
METHODS
This study used stochastic network-based mathematical models of HIV-1 transmission dynamics among heterosexual couples in SSA. More detailed methods, including model parameterisation, simulation and data analysis methods are provided in the online supplementary appendix.
Behavioural parameters governing the base model network structure were estimated from the Migration & HIV in Ghana (MHG) study, a 2012 cross-sectional study of adults in a resource-poor area of Accra, Ghana, designed specifically to parameterise mathematical models.10 A probability sample of the population was obtained; study procedures included a diagnostic HIV-1/2 test and a standardised survey on sexual behaviours, including network data on partnerships.
Dynamic sexual networks
To simulate networks, we used temporal exponential random graph models, a flexible statistical framework for estimating the parameters of partnership formation and dissolution across networks.11 Our models included parameters for heterosexual-only mixing, mean momentary degree (average number of partnerships per person in any cross-section), and the prevalence of concurrent partnerships (ie, degree >1). Degree terms were stratified by sex, as men exhibited both higher mean degree and prevalence of concurrency.12 A parametrical term was used to model sex-asymmetry in age homophily typical of heterosexual partners in SSA: persons tended to select partners similar in age, but asymmetrically, as men were older than their female partners (an average 5.4 years in MHG).
Partnership dissolution was modelled as a constant hazard. We used Kaplan-Meier methods to estimate the dissolution coefficient as a transform of the mean partnership duration. This coefficient was adjusted to minimise the distance between the HIV prevalence observed in MHG (4.7%) and the simulated prevalence at equilibrium. This translated to a reduction in the mean duration by 0.9 years; the expected number of lifetime partners between the MHG estimates (9.6) and simulations after this adjustment (10.1) were similar.12
HIV progression and transmission
We assigned a base CD4 count at infection conditional on sex, with a downward non-linear slope (in the absence of ART) conditional on infection age.13 HIV viral load followed a trajectory of peak viraemia during acute-stage infection, followed by a set point viral load during chronic infection, with a subsequent rise during late-stage infection leading to AIDS and disease-induced mortality.14 Also upon infection, persons were randomly assigned a CD4 count to initiate ART, based on clinical data.15 An upper limit for starting values (CD4 = 350) matched Ghanaian health policy at the study time. We imposed an overall cap on ART coverage given estimates that 30% of those indicated for ART had initiated treatment.16 Upon ART initialisation, persons were partitioned into full and partial adherence groups so that the average levels of HIV viral suppression matched empirical estimates of suppression across SSA.17 ART adherence caused an increase in CD4 to preinfection levels and a reduction in viral load to undetectable levels.18
HIV transmission was simulated over active partnership dyads given the network model structure at each time step. The per-partnership transmission rate was based on the statistical model from Hughes,19 which predicted the per-act transmission probability as a continuous, non-linear function of the infected’s viral load, as well as condom use and the sex, age and circumcision status of the susceptible. The final transmission rate per partnership per unit time was an exponential function of the per-act transmission probability and the number of acts per partnership per unit time.
Base and counterfactual models
Our base model replicated the epidemic observed in the MHG study. We then varied the two key inputs (levels of circumcision and concurrency) over a range of values that included those commonly reported across SSA. For circumcision, the base value was 90%, while counterfactual models covered 10–90%. Point prevalence of concurrency differed strongly by sex in Ghana—17.8% of men and 2.8% of women reported >1 ongoing partner—a differential consistent with most SSA heterosexual populations. Levels of concurrency are known to vary considerably across SSA,8 reflecting both true differences and measurement error. We modelled four concurrency values that captured this observed variation across SSA. The High scenario was the observed level; Moderate was 75%, Medium was 50% and Low was 25% relative to observed. Prevalence of circumcision and concurrency within each scenario was assumed to be fixed at onset of sexual activity, with targeted levels stable for the duration of each simulation. We considered modelling an individual-level correlation between concurrency and circumcision (for men) within each scenario, as circumcised men could have differential rates of concurrency, but found no evidence for this within-person association within either our MHG data set or secondary literature from elsewhere in SSA. Across all scenarios, the mean momentary degree and partnership duration were held constant, such that the per capita number of partnerships at any time, the partnership acquisition rate and the cumulative number of expected lifetime partners (all three functions of mean degree and duration) were all equivalent. Variance in the degree distribution increased minimally with concurrency because we included a maximum degree constraint of three in our models to be consistent with our data collection instrument.
A total of 36 scenarios (4 concurrency levels by 9 circumcision levels) were modelled. Each scenario was simulated 250 times over 100 years in a network size of 10 000 men and women to establish the endemic prevalence and incidence. The summary prevalence and incidence statistics, with associated variance components, represented the final 100 time steps in each simulation set. Given the model stochasticity, 50% credible intervals (CrIs) provided the range of most likely outcomes.
RESULTS
The base scenario model yielded an endemic HIV-1 prevalence of 4.7% (CrI = 3.7%, 5.6%), matching observed prevalence data by design (see online supplementary figure S1). Endemic incidence was 0.43 per 100 person-years (CrI = <0.01, 0.51). Stochastic variability in predicted prevalence and incidence was significant for the base model and other low-prevalence scenarios discussed below.
Table 1 provides a numerical summary of the endemic HIV-1 prevalence across all 36 parameter sets. Prevalence was lower as concurrency decreased and circumcision increased, both independently and jointly, with means ranging from 0% to 34% HIV prevalence at extreme values. Holding concurrency fixed at the observed base levels, endemic prevalence was predicted to be 21.2% if 50% of men were circumcised versus 33.6% if 10% of men were circumcised. In the Low concurrency scenario, where an average of 4.5% of men and 0.7% of women exhibited concurrency, the maximum predicted disease prevalence would be 16.2% in the scenario with 10% circumcision.
Table 1.
Equilibrium HIV type 1 (HIV-1) prevalence with 50% credible intervals (CrIs) and prevalence ratios (PrRs) comparing HIV-1 prevalence by concurrency level within circumcision level
| Concurrency Level* | ||||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| High | Moderate | Medium | Low | |||||
|
|
|
|
|
|||||
| Circumcision level (%) | Prevalence (50% CrI) | PrR | Prevalence (50% CrI) | PrR | Prevalence (50% CrI) | PrR | Prevalence (50% CrI) | PrR |
| 90 | 4.7 (3.5, 5.6) | 1.00 | 0.3 (<0.1, <0.1) | 0.06 | 0.1 (0.0, <0.1) | 0.02 | 0.0 (0.0, 0.0) | – |
| 80 | 9.5 (8.2, 10.8) | 1.00 | 3.4 (2.5, 4.2) | 0.36 | 0.7 (<0.1, 0.1) | 0.07 | 0.3 (<0.1, 0.1) | 0.03 |
| 70 | 14.4 (13.1, 15.7) | 1.00 | 7.1 (6.0, 8.0) | 0.49 | 1.4 (0.1, 1.9) | 0.10 | 0.9 (0.1, 1.2) | 0.06 |
| 60 | 18.5 (17.2, 19.8) | 1.00 | 11.0 (9.8, 12.1) | 0.59 | 4.9 (4.0, 5.8) | 0.26 | 1.8 (1.3, 2.3) | 0.10 |
| 50 | 21.2 (20.0, 22.4) | 1.00 | 14.9 (13.5, 16.1) | 0.70 | 7.4 (6.3, 8.4) | 0.35 | 3.9 (3.1, 4.5) | 0.18 |
| 40 | 24.8 (23.4, 26.0) | 1.00 | 18.2 (17.0, 19.4) | 0.73 | 11.5 (10.2, 12.7) | 0.46 | 6.2 (5.2, 7.1) | 0.25 |
| 30 | 28.3 (27.0, 29.4) | 1.00 | 21.6 (20.2, 22.9) | 0.76 | 15.2 (14.0, 16.4) | 0.54 | 9.6 (8.5, 10.6) | 0.34 |
| 20 | 30.7 (29.6, 31.8) | 1.00 | 24.9 (23.7, 26.1) | 0.81 | 18.7 (17.6, 19.9) | 0.61 | 12.0 (11.1, 13.0) | 0.39 |
| 10 | 33.6 (32.5, 34.7) | 1.00 | 27.6 (26.3, 28.7) | 0.82 | 21.3 (20.1, 22.7) | 0.63 | 16.2 (15.3, 17.3) | 0.48 |
High (observed)=17.8% for men and 2.8% for women; Moderate (75% of observed)=13.4% for men and 2.1% for women; Medium (50% of observed)=8.9% for men and 1.4% for women; and Low (25% of observed)=3.3% for men and 0.5% for women.
The prevalence ratios in table 1 contrast simulated prevalence across concurrency scenarios but within circumcision levels. Compared with the base scenario, prevalence would decline by 94% by changing concurrency to moderate levels. In the 40% circumcision setting (typical of many regions in southern SSA), reducing levels of concurrency by less than 10% for men and less than 2% among women (contrasting the High to Medium scenarios) would be expected to reduce endemic prevalence by over half, from 24.8% to 11.5%. To achieve that level of prevalence reduction by circumcision would require nearly 40% more men become circumcised (contrasting 40% to 80% in the High scenario).
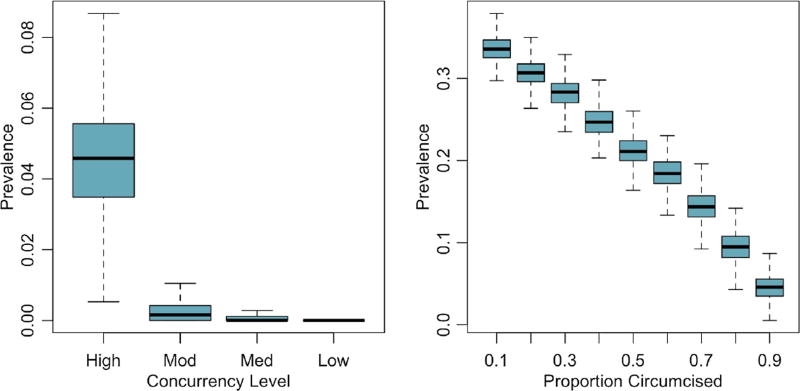
Figure 1 plots the differences in endemic HIV-1 prevalence across two sets of counterfactuals, visualising the stochastic variability in outcomes and the non-linearity in prevalence reductions. Whereas changing concurrency from the base High values results in a strong reduction of prevalence down to extinction levels, differences in prevalence across circumcision scenarios reflects a linear trend between intervention coverage and disease outcomes.
Figure 1.
Distribution of equilibrium HIV prevalence across concurrency levels and circumcision prevalence levels. Concurrency categories: High (observed)=17.8% for men and 2.8% for women; Moderate (75% of observed)=13.4% for men and 2.1% for women; Medium (50% of observed)=8.9% for men and 1.4% for women; and Low (25% of observed)=3.3% for men and 0.5% for women. Left panel compares prevalence given base circumcision value (90%) and varying concurrency scenario. Right panel compares prevalence given base concurrency scenario (High) and varying circumcision value.
In the 90% circumcision scenario, both figure 1 and table 1 show the skewed distributions of outcomes under the Moderate, Medium and Low concurrency scenarios. The means for the prevalence values fell outside their 50% CrI because of epidemic extinction, a phenomenon that occurs as the system nears the threshold for sustainable transmission. Online supplementary figure S2 plots the probability of extinction on a continuous scale. Extinction was only observed in four scenarios, three of which were at this highest circumcision level; extinction probability in the four scenarios at 90% circumcision was 0%, 28%, 61% and 92%.
Table 2 shows incidence rates across the 36 scenarios compared with base model incidence of 0.43 per 100 person-years. Incidence ranges from 3.57 (CrI = 2.14, 4.66) in the highest-risk scenario to effectively 0 in the lowest-risk scenario. The table also shows the incidence rate ratios, comparing the predicted incidence within each of the four concurrency scenarios but varying the prevalence of circumcision. Increasing circumcision coverage from 10% to 50% in the High scenario would reduce the incidence by over 40%, but the relative change in the lower concurrency scenarios would be even greater, with a relative decline of over 75% in the Low scenario with the same change in circumcision coverage.
Table 2.
Equilibrium HIV type 1 incidence rates per 100 person-years with 50% credible intervals (CrIs) and incidence rate ratios (IRRs) comparing incidence by circumcision level within concurrency level
| Concurrency level* | ||||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| High | Moderate | Medium | Low | |||||
|
|
|
|
|
|||||
| Circumcision level (%) | Incidence (50% CrI) | IRR | Incidence (50% CrI) | IRR | Incidence (50% CrI) | IRR | Incidence (50% CrI) | IRR |
| 90 | 0.43 (0.00, 0.52) | 0.119 | 0.03 (0.00, 0.00) | 0.009 | 0.01 (0.00, 0.00) | 0.004 | 0.00 (0.00, 0.00) | 0.000 |
| 80 | 0.90 (0.52, 1.17) | 0.252 | 0.31 (0.00, 0.49) | 0.110 | 0.06 (0.00, 0.00) | 0.027 | 0.02 (0.00, 0.00) | 0.017 |
| 70 | 1.36 (0.61, 1.87) | 0.382 | 0.64 (0.00, 1.04) | 0.230 | 0.12 (0.00, 0.00) | 0.061 | 0.07 (0.00, 0.00) | 0.049 |
| 60 | 1.78 (0.72, 2.59) | 0.499 | 1.02 (0.55, 1.62) | 0.365 | 0.44 (0.00, 0.53) | 0.214 | 0.15 (0.00, 0.45) | 0.101 |
| 50 | 2.09 (1.35, 2.86) | 0.587 | 1.40 (0.62, 1.91) | 0.501 | 0.65 (0.00, 1.06) | 0.319 | 0.33 (0.00, 0.51) | 0.224 |
| 40 | 2.49 (1.54, 3.30) | 0.698 | 1.75 (0.72, 2.53) | 0.627 | 1.05 (0.57, 1.67) | 0.513 | 0.53 (0.00, 0.99) | 0.359 |
| 30 | 2.88 (1.75, 3.79) | 0.808 | 2.11 (1.38, 2.92) | 0.756 | 1.40 (0.64, 1.95) | 0.686 | 0.83 (0.52, 1.17) | 0.564 |
| 20 | 3.18 (1.89, 4.27) | 0.892 | 2.49 (1.56, 3.34) | 0.891 | 1.77 (0.74, 2.30) | 0.864 | 1.06 (0.59, 1.65) | 0.722 |
| 10 | 3.57 (2.14, 4.66) | 1.000 | 2.79 (1.72, 3.67) | 1.000 | 2.05 (0.89, 2.91) | 1.000 | 1.47 (0.68, 2.07) | 1.000 |
High (observed)=17.8% for men and 2.8% for women; Moderate (75% of observed)=13.4% for men and 2.1% for women; Medium (50% of observed)=8.9% for men and 1.4% for women; and Low (25% of observed)=3.3% for men and 0.5% for women.
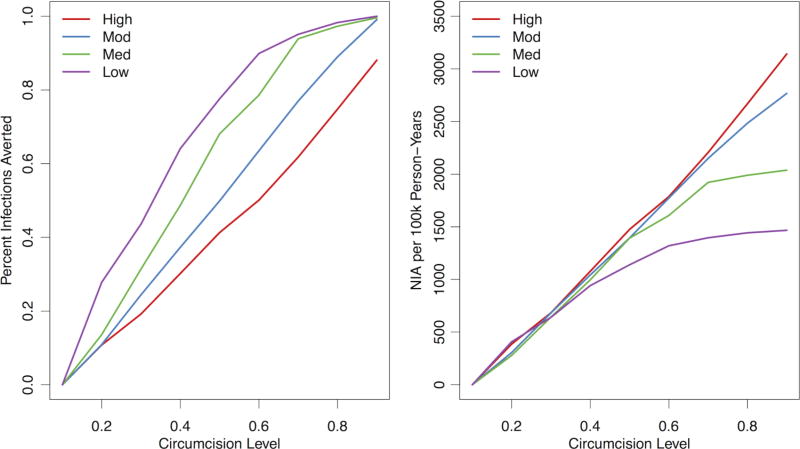
Figure 2 plots the relative (left panel) and absolute (right panel) changes in incidence by circumcision level in the four network scenarios. The per cent of infections averted standardised the differences in incidence rate to the rate in the 10% circumcision scenario. On this metric, MMC scale-up yielded better outcomes in lower-concurrency scenarios: incidence was already lower due to less behavioural risk in the population, and MMC scale-up contributed a larger reduction of that residual incidence. In contrast, with the number of infections averted, which did not standardise to the reference scenario, increasing MMC coverage did not confer differential results up to modest intervention levels (<40% circumcised). But above this, MMC has the greatest impact in high concurrency, and therefore, high-incidence scenarios. Increasing circumcision coverage from 10% to 90% in the High scenario would avert 3142 infections per 100 000 person-years compared with 1467 infections in the Low scenario.
Figure 2.
Estimated per cent of infections averted (left panel) and number of infections averted (NIA) (right panel) comparing levels of circumcision prevalence to lowest simulated value (10%), across the four concurrency levels. Concurrency categories: High (observed)=17.8% for men and 2.8% for women; Moderate (75% of observed)=13.4% for men and 2.1% for women; Medium (50% of observed)=8.9% for men and 1.4% for women; and Low (25% of observed)=3.3% for men and 0.5% for women. The per cent of infections averted is 1—the incidence rate ratio comparing the HIV-1 incidence in each circumcision level of circumcision relative to the 10% scenario. The number of infections averted is expressed in terms of 100 000 person-years at risk of observation time, compared with the starting value in the 10% circumcision scenario.
DISCUSSION
In this mathematical model for HIV-1 transmission, we investigated how changes to the network structure and circumcision rates could impact HIV-1 incidence among heterosexuals in SSA. Using empirically grounded behavioural scenarios reflecting the natural variation in concurrency and circumcision across SSA, differences in only these two features helped to explain much of the variability in HIV prevalence across the region. A large scale-up of a combination package with MMC and behavioural risk reduction related to concurrency could drive the HIV epidemic to extinction in this setting, but only with substantial uptake of both elements. Directing MMC interventions based on network structure could provide a robust, data-driven method for package targeting.
To validate the baseline biobehavioural framework of our model, we explored the endemic HIV-1 prevalence level associated with different levels of MMC and concurrency. Due to technical challenges in modelling dynamic sexual networks, few modelling studies have addressed this question. Previous models have investigated the effects of network structure alone, without circumcision, on HIV transmission. These models also required assumptions about no demographic change,9 or did not incorporate ART.8 Our model implemented the current science related to the HIV care cascade, including disease progression,13 entry into medical care,15 adherence to ART,17,20 and the relationship between disease progression on transmission in long-term and outside sexual partnerships.19
Our main empirical finding was that differences in circumcision alone did not explain the variation in HIV-1 burden across SSA; they were an important and necessary component along with network connectivity.21 Circumcision and concurrency combined to help explain the disparities in HIV-1 prevalence and incidence observed today,17 ranging from 1–2% in western Africa to 7–10% in eastern Africa to 15–25% in southern Africa. Within West Africa, our study sample in Ghana represented a high-risk population: HIV-1 prevalence was over twice that estimated in Ghana nationally (4.7% vs 1.9%). This likely reflects the higher levels of concurrency in this urban population, that is, a hub of circular migration.10 More broadly, South Africa and the neighbouring countries typically have exhibited moderate to high levels of concurrency with lower rates of circumcision (<50%).22 Our model predicted endemic HIV-1 prevalence in this setting of 15–25%, consistent with seroprevalence surveys in those regions.17 Rural Uganda, where circumcision rates are similar to South Africa but concurrency is less common, exhibits prevalence of 6–9% matched by our model predictions.23
These model outcomes have implications for the design and targeting of combination HIV prevention packages with behavioural elements.24 Previous mathematical models have often required extreme behavioural assumptions to both replicate current HIV prevalence levels and also predict the amount of behavioural change needed to meaningfully reduce incidence: one model assumed a baseline 120 lifetime sexual partners on average with the simulated intervention reducing this to 80 lifetime partners.3 These baseline levels have never been observed in population-based data, and no behavioural interventions have attempted at such targets.25 Our models, in contrast, reproduce HIV epidemics with data-driven parameters for sexual networks. This is largely possible by allowing for concurrency: unlike most compartmental models, we can model HIV transmission potential within a heterosexual couple that starts as concordant-negative, then becomes discordant because of an outside partnership, then becomes concordant-positive.26 Our stochastic methods also improve on the predominant deterministic modelling methods by providing a range of plausible outcomes associated with each scenario, including the predicted extinction probability.
For a combination package design, small changes in concurrency had strong, non-linear effects on predicted incidence in our models, consistent with prior research.27 Non-linear thresholds were present when scaling up MMC, but were not as strong. For comparison, the predicted prevalence in a high-concurrency scenario in which there was already 50% circumcision was 21.2% in our model. Reducing concurrency just 4.5% among men and 0.7% among women (the difference between the High and Moderate scenarios) would decrease prevalence to 14.9%, similar to the 14.4% expected prevalence achieved by circumcising another 20% of the population (to 70%).
A persistent challenge to HIV prevention interventions has been targeting and scale-up. The success of packages depends on potentially small changes to individual behavioural or biomedical elements. Given current levels of ART and other demographic features within SSA, we observed that epidemic extinction was only realised in a combination prevention approach that intervened on both the sexual network structure and circumcision. Is this sort of behavioural change possible? Variations in concurrency within West Africa and across SSA suggest that alternative behavioural norms can become established,28 but even small changes to sexual behaviour over a sustained time may be challenging, especially compared with a one-time circumcision surgery.29
Assuming no behavioural change, our study suggested that targeting MMC should depend on epidemiological context. Where to target depended on the baseline conditions and the magnitude of MMC scale-up. Our model predicted MMC yields the greatest relative benefits in low-concurrency settings where baseline incidence is lower. The non-linearity in incidence at epidemic thresholds in low-concurrency scenarios is strong. In comparison, the number of infections averted metric does not standardise to the baseline incidence rate. Epidemic thresholds on this metric also suggested few differences at modest levels of circumcision scale-up (to 40%), but then wide divergence in outcomes at higher levels. At the extreme, a circumcision scale-up of 80% (going from 10% to 90% circumcised) would prevent 1675 more infections per 100 000 person-years in the High versus Low scenario. Circumcising such a large fraction of the population in low-concurrency settings yields fewer infections averted because the baseline incidence is much lower. While the implication of targeting prevention packages at high-incidence settings is intuitive, our study provides clear model-based forecasts of potential impact based on a realistic representation of the behavioural structure of sexual networks.
Limitations
These models required minor calibration for one behavioural parameter, the mean partnership duration, to match observed HIV-1 prevalence in our target population. As described in more detail in the online supplement, this arises from the fact that a single dissolution parameter inadequately captured the heterogeneity in the statistical distribution of duration. However, the calibration results in a difference of 0.5 higher lifetime partners compared to the mean observed in the study. Second, we did not simulate changes to concurrency or circumcision as explicit interventions with a defined time horizon. Instead, the changes in risk were modelled as fixed at sexual debut since we were interested in both the empirical questions of natural epidemic dynamics and intervention scale-up. Counterfactual comparisons should therefore be considered the maximum effect of an intervention. Finally, network-based mathematical models, like all models, are subject to the internal and external validity of their algorithms. For network models explicitly, and other methods implicitly, this raises a question about network boundaries.30 Our base models were based on data collected from a high-risk core in an otherwise low-level epidemic in Ghana. Persons in our sample reported sexual partnerships with those outside our sampling frame, including those outside our age eligibility criteria or the target geography. Secondary analyses suggest few demographic differences in the reported partner population ineligible for the study, but behavioural differences are unknown. It is also unknown whether our sensitivity analyses varying circumcision and concurrency would substantially change if the model were calibrated to another population network.
CONCLUSIONS
The primary contribution of the study was to estimate the synergistic effects of network structure and circumcision, both as empirical patterns explaining the historical HIV-1 burden across SSA, and as intervention components in a combined HIV prevention package. A substantial reduction in HIV-1 incidence will require combined behavioural change and scale-up of biomedical technologies. Where to target prevention packages that implement those depends on the epidemiological context driven by the dynamic network structure.
Supplementary Material
Key messages.
-
▸
Reduction of HIV type 1 incidence among heterosexuals in sub-Saharan Africa critically depends on the development and implementation of highly effective, acceptable prevention strategies.
-
▸
Prevention packages that combine biomedical technologies like male circumcision with sociobehavioural elements related to the structure of heterosexual networks could maximise prevention benefits in these settings.
-
▸
This mathematical modelling study demonstrates how leveraging networks for prevention package design and targeting can contribute to their performance among HIV-discordant heterosexual couples.
Acknowledgments
Funding This work was supported in part by NIH Research Grants NICHD R00 HD057533, NICHD R01 HD68395; an NIH Training Grant NICHD T32 HD007543; and a UW Center for AIDS Research grant (P30 AI027757). Computing support was provided by a NICHD research infrastructure grant (R24 HD042828) to the UW Center for Studies in Demography & Ecology.
Footnotes
Contributors SMJ conceived the idea for the study, and led the analysis and writing of the manuscript. SMG, MM and SC contributed to the concept development and writing of the manuscript. All authors have seen and approved the final version of the manuscript for publication.
Competing interests None declared.
Ethics approval The institutional review boards of the University of Washington and University of Ghana approved all procedures (#41401).
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement Upon publication, the full software code base to run these models will be provided online at github.com.
References
- 1.Hallett TB, Baeten JM, Heffron R, et al. Optimal uses of antiretrovirals for prevention in HIV-1 serodiscordant heterosexual couples in South Africa: a modelling study. PLoS Med. 2011;8:e1001123. doi: 10.1371/journal.pmed.1001123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gray RH, Kigozi G, Serwadda D, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369:657–66. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 3.Hallett TB, Singh K, Smith JA, et al. Understanding the impact of Male circumcision interventions on the spread of HIV in Southern Africa. PLoS ONE. 2008;3:e2212. doi: 10.1371/journal.pone.0002212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boily MC, Mâsse B, Alsallaq R, et al. HIV treatment as prevention: considerations in the design, conduct, and analysis of cluster randomized controlled trials of combination HIV prevention. PLoS Med. 2012;9:e1001250. doi: 10.1371/journal.pmed.1001250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Padian NS, Buve A, Balkus J, et al. Biomedical interventions to prevent HIV infection: evidence, challenges, and way forward. Lancet. 2008;372:585–99. doi: 10.1016/S0140-6736(08)60885-5. [DOI] [PubMed] [Google Scholar]
- 6.Smith RJ, Li J, Gordon R, et al. Can we spend our way out of the AIDS epidemic? A world halting AIDS model. BMC Public Health. 2009;9(Suppl 1):S15. doi: 10.1186/1471-2458-9-S1-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carnegie NB, Morris M. Size matters: concurrency and the epidemic potential of HIV in small networks. PLoS ONE. 2012;7:e43048. doi: 10.1371/journal.pone.0043048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eaton JW, Hallett TB, Garnett GP. Concurrent sexual partnerships and primary HIV infection: a critical interaction. AIDS Behav. 2011;15:687–92. doi: 10.1007/s10461-010-9787-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodreau SM, Cassels S, Kasprzyk D, et al. Concurrent partnerships, acute infection and HIV epidemic dynamics among young adults in Zimbabwe. AIDS Behav. 2012;16:312–22. doi: 10.1007/s10461-010-9858-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cassels S, Jenness SM, Biney AAE, et al. Migration, sexual networks, and HIV in Agbogbloshie, Ghana. Demogr Res. 2014;31:861–88. doi: 10.4054/DemRes.2014.31.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krivitsky PN, Handcock MS. A Separable model for dynamic networks. J R Stat Soc Ser B Stat Methodol. 2014;76:29–46. doi: 10.1111/rssb.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenness SM, Biney AAE, Ampofo WK, et al. Minimal coital dilution in Accra, Ghana. J Acquir Immune Defic Syndr. 2015;69:85–91. doi: 10.1097/QAI.0000000000000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pantazis N, Morrison C, Amornkul PN, et al. Differences in HIV natural history among African and non-African seroconverters in Europe and seroconverters in sub-Saharan Africa. PLoS ONE. 2012;7:e32369. doi: 10.1371/journal.pone.0032369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pilcher CD, Price MA, Hoffman IF, et al. Frequent detection of acute primary HIV infection in men in Malawi. AIDS. 2004;18:517–24. doi: 10.1097/00002030-200402200-00019. [DOI] [PubMed] [Google Scholar]
- 15.Collini P, Schwab U, Sarfo S, et al. Sustained immunological responses to highly active antiretroviral therapy at 36 months in a Ghanaian HIV cohort. Clin Infect Dis. 2009;48:988–91. doi: 10.1086/597353. [DOI] [PubMed] [Google Scholar]
- 16.Ampofo WK. Current Status of HIV/AIDS Treatment, Care and Support Services in Ghana. Ghana Med J. 2009;43:142–3. [PMC free article] [PubMed] [Google Scholar]
- 17.HIV/AIDS JUNP on. The Gap Report. Geneva: UNAIDS; 2014. [Google Scholar]
- 18.Chu H, Gange SJ, Li X, et al. The effect of HAART on HIV RNA trajectory among treatment-naïve men and women: a segmental Bernoulli/lognormal random effects model with left censoring. Epidemiology. 2010;21(Suppl 4):S25–34. doi: 10.1097/EDE.0b013e3181ce9950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hughes JP, Baeten JM, Lingappa JR, et al. Determinants of per-coital-act HIV-1 infectivity among African HIV-1-serodiscordant couples. J Inf Dis. 2012;205:358–65. doi: 10.1093/infdis/jir747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mills EJ, Nachega JB, Buchan I, et al. Adherence to antiretroviral therapy in sub-Saharan Africa and North America: a meta-analysis. JAMA. 2006;296:679–90. doi: 10.1001/jama.296.6.679. [DOI] [PubMed] [Google Scholar]
- 21.Halperin DT, Epstein H. Why is HIV prevalence so severe in Southern Africa?: the role of multiple concurrent partnerships and lack of Male circumcision-implications for HIV prevention: opinion. South Afr J HIV Med. 2007;8:19–25. [Google Scholar]
- 22.Williams BG, Lloyd-Smith JO, Gouws E, et al. The potential impact of Male circumcision on HIV in Sub-Saharan Africa. PLoS Med. 2006;3:e262. doi: 10.1371/journal.pmed.0030262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCreesh N, O’Brien K, Nsubuga RN, et al. Exploring the potential impact of a reduction in partnership concurrency on HIV incidence in rural Uganda: a modeling study. Sex Transm Dis. 2012;39:407–13. doi: 10.1097/OLQ.0b013e318254c84a. [DOI] [PubMed] [Google Scholar]
- 24.Kurth AE, Celum C, Baeten JM, et al. Combination HIV prevention: significance, challenges, and opportunities. Curr HIV/AIDS Rep. 2011;8:62–72. doi: 10.1007/s11904-010-0063-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodreau SM. Is 2 a “high number of partners”? Modeling, data, and the power of concurrency. Sex Transm Dis. 2013;40:61. doi: 10.1097/OLQ.0b013e31827b9d8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bellan SE, Fiorella KJ, Melesse DY, et al. Extra-couple HIV transmission in sub-Saharan Africa: a mathematical modelling study of survey data. Lancet. 2013;381:1561–9. doi: 10.1016/S0140-6736(12)61960-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris M, Kretzschmar M. Concurrent partnerships and the spread of HIV. AIDS. 1997;11:641–8. doi: 10.1097/00002030-199705000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Cori A, Ayles H, Beyers N, et al. HPTN 071 (PopART): a Cluster-Randomized Trial of the Population Impact of an HIV Combination Prevention Intervention Including Universal Testing and Treatment: Mathematical Model. PLoS ONE. 2014;9:e84511. doi: 10.1371/journal.pone.0084511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Padian NS, McCoy SI, Manian S, et al. Evaluation of large-scale combination HIV prevention programs: essential issues. J Acquir Immune Defic Syndr. 2011;58:e23–8. doi: 10.1097/QAI.0b013e318227af37. [DOI] [PubMed] [Google Scholar]
- 30.Helleringer S, Kohler HP, Chimbiri A, et al. The Likoma Network Study: Context, data collection, and initial results. Demogr Res. 2009;21:427–68. doi: 10.4054/DemRes.2009.21.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.