Abstract
Objectives
Inbreeding increases the probability of homozygosity of deleterious alleles. Inbreeding and Runs of homozygosity (ROH) are associated with increased risk for disease phentoypes, including schizophrenia and other psychiatric disorders. Effects of inbreeding, ROH, homozygous deletions, and other copy number variations (CNVs) on risk for depression and suicide attempt were quantified in an Arab Bedouin Kindred.
Methods
We performed genetic analyses of 439 subjects from an Arab kindred with high rates of depression and suicidal behavior. We obtained complete ascertainment of suicide attempters and first-degree relatives of subjects who have attempted or died by suicide.
Results
We found extensive regions of ROH. On average, 5% of the genome is covered by ROH for these subjects, twofold higher than ROH rates for subjects from populations of European ancestry. Inbreeding and total length of ROH were not associated with risk for depression or attempt. For CNVs, an increased number of duplications >500 kb was associated with increased risk for attempt (OR=2.9, p=0.01; 95% CI 1.3, 6.6). While not significant after correction for multiple testing, risk for suicide attempt appears to increase with copy number for a CNV on chromosome 9p24.1. This possibility is intriguing because the CNV covers GLDC, which encodes glycine dehydrogenase that binds to glycine, a co-agonist at N-methyl-D-aspartate (NMDA) glutamate receptors and is involved in glutamatergic neurotransmission.
Conclusions
Our findings add to the growing evidence of genetic risk factors that act pleiotropically to increase risk for several neuropsychiatric disorders, including depression and suicide attempt, regardless of ancestry.
Keywords: Inbreeding, Runs of Homozygosity, Pedigree, Copy Number Variation, Suicidal behavior, Depression, Arab population
INTRODUCTION
Risk for depression and suicidal behavior, like most psychiatric disorders, has a complex genetic and environmental basis. Myriad genetic factors are involved, most have only a small effect on risk, and they potentially have different modes of inheritance. For all these reasons it has proven difficult to identify individual risk variants and, arguably, different study designs will prove effective at identifying certain risk variants. One successful approach arises from the growing evidence that genetic risk factors are shared across neuropsychiatric disorders. Genome-wide association studies or GWAS from the Psychiatric Genomics Consortium (2013,2014) and others (Malhotra and Sebat, 2012) have identified common variants that are associated with increased risk of a range of neuropsychiatric disorders including major depression, attention deficit hyperactivity disorder, bipolar disorder, schizophrenia, autism, and intellectual function. Parallel results arise from copy number variant (CNV) studies. The largest study examining the role of CNVs in recurrent depression included 3,106 cases and 6,078 controls; it found evidence for association with CNVs such as 1q21.1, 15q13.3, and 22q11.2, when both duplications and deletions were considered together (Rucker et al., 2013). While the burden of rare CNVs >100 kb was not associated with recurrent depression; cases showed significantly higher number of genes falling within deletion CNVs compared to controls (Rucker et al., 2016). The combination of microdeletions and microduplications in 16p11.2 was also associated with major depressive disorder (Degenhardt et al., 2012). To varying degrees these CNVs have been previously associated with autism, schizophrenia, and intellectual disability (Malhotra and Sebat, 2012). In addition, a novel duplication in SLIT3 on 5q35.1 was reported in depression (Glessner et al., 2010).
Pedigree studies have proven tremendously useful for identifying risk variants underlying Mendelian disorders, but so far they are less effective for psychiatric and other complex disorders. Nonetheless there are exceptions, such as the classic study of a DISC1/Boymaw variant segregating with schizophrenia, bipolar disorder and depression in a large family (St Clair et al., 1990,Zhou et al., 2008). CNVs falling in neuronal genes have been found to be enriched in cases in an Amish pedigree segregating bipolar and major depressive disorders (Yang et al., 2009). A study using a 5-generation Palauan family demonstrated segregation of a deletion of SLC1A1 glutamate transporter with schizophrenia and bipolar disorder (Myles-Worsley et al., 2013).
Homozygosity mapping has also been successful in identifying recessive risk variants for disorders showing Mendelian inheritance (Ekstein et al., 2004,Fares et al., 2008); and examples of successful mapping are also emerging for some complex psychiatric disorders, such as autism (Gamsiz et al., 2013,Lim et al., 2013,Yu et al., 2013). There is an increased interest in runs of homozygosity (ROH) given evidence of their association with increased risk for schizophrenia and other psychiatric disorders (Wang et al., 2010,Keller et al., 2012,Gamsiz et al., 2013). Consistent with population genetic theory, human inbreeding results in longer ROH and potentially reveals a greater number of deleterious recessive alleles (Kirin et al., 2010,Szpiech et al., 2013). Direct inbreeding, through consanguineous marriages, has been associated with increased risk for schizophrenia and bipolar disorder in Egypt, Iran, and Arab Bedouin populations (Dobrusin et al., 2009,Mansour et al., 2009,Mansour et al., 2010,Nafissi et al., 2011,Saadat, 2012); and increased risk for suicide by self-inflicted burning in Iran (Saadat and Zendeh-Boodi, 2006). Homozygosity mapping of recurrent major depression in a large extended family in Pakistan with high rate of consanguineous marriages revealed significant linkage on chromosomes 6 and 9 (Ayub et al., 2013).
Inbreeding is common in the Arab world in general and among Bedouins in particular (Jaber et al., 1994,Teebi and Farag, 1997). The Arab world is a mixture of ethnically heterogeneous populations and are the result of admixture with other populations such as Persians, Turks, Southeast Asians, Europeans, and Africans (Teebi and Farag, 1997). In addition to the ethnically heterogeneous populations, homogeneous populations, or isolates, also exist. These include Bedouins, originally from Saudi Arabia or Iraq, who usually belong to tribes and can trace their ancestry to few founders.
Here, we report on a large Arab Bedouin kindred to examine whether inbreeding and resultant longer ROH are related to the high levels of depression and suicidal behavior observed in this kindred. The pedigree consists of 1183 individuals across five generations who can trace their ancestry to a single pair of founders, as well as individuals who are married-in. The suicide attempt rate in this pedigree is 9.1%, much higher than that reported in Lebanon (2%), the United States (5%), and the cross-national prevalence rate across 17 countries (2.7%) (Nock et al., 2008,Hamdan et al., 2012). Similarly, this kindred has a high rate of lifetime depression (25.7%) compared to Lebanon (10.8%), the United States (17.9%), and the cross-national prevalence rate across 17 countries (11.6%) (Kovess-Masfety et al., 2013). Complete ascertainment of suicide attempters and all first-degree relatives of subjects who have completed or attempted suicide was sought. In addition to ROH, the impact of CNVs on risk was also evaluated.
METHODS
Sample
We constructed the pedigree in collaboration with a liaison of the village and sought complete ascertainment of suicide attempters and all first-degree relatives of subjects who have died by or attempted suicide. A trained Arab-speaking Clinical Psychologist (S.H.) directly interviewed study participants and verified the pedigree connections after obtaining informed consent from the subject and their legal guardian for subjects 14 to 17 years of age. Research protocols and procedures were approved by institutional review boards (IRBs) at the University of Pittsburgh and Schneider Children’s Hospital. Subjects were interviewed using the Arabic version of the World Mental Health- Composite International Diagnostic Interview (WMH-CIDI) (Demyttenaere et al., 2004,Kessler et al., 2004,Kessler and Ustun, 2004,Karam et al., 2006), for which the lead author (N.M.) obtained the training of the trainers at the Institute for Development, Research, Advocacy and Applied Care (IDRAAC) in Lebanon, and in turn trained S.H. on the administration of the Arabic WMH-CIDI.
A total sample of 439 subjects participated in the study and provided a blood sample. The acceptance rate for study participation was 94%. The sample was almost equally distributed with regard to sex, 54.7% were female (240 females and 199 males), and the mean age of subjects was 27.1 years (Standard Deviation, SD=11.5, range 14–59). There were 159 affected individuals (36.2%): 113 (25.7%) had a lifetime diagnosis of depression, 40 (9.1%) had made at least one suicide attempt, 117 (26.7%) had anxiety disorder, 6 (1.4%) had schizophrenia, and 3 (0.7%) had bipolar disorder (Table 1). There were 240 individuals unaffected with any of these disorders and 40 individuals with an unknown diagnosis. Of the 40 subjects with a history of suicide attempt, 28 had a lifetime diagnosis of depression, 6 schizophrenia, 5 anxiety disorder, and 1 PTSD. Of the 113 subjects with depression, 67% also had an anxiety disorder. In this study, we focused on the narrow phenotype of suicide attempt (SA) and the broad phenotype of suicide attempt and/or depression (SA-DEP) (n=125, 28.5%).
Table 1.
Characteristics of Study Sample
| Total Sample N=439 |
Genotyped with Affy 6.0 & used for ROH1 N=170 |
Genotyped with Affy 6.0 & used for CNV1 N=159 |
Genotyped with nanostring N=290 |
|
|---|---|---|---|---|
| Affected2 | 159 (36.2) | 84 (49.4) | 78 (49.1) | 110 (37.9) |
| Depression | 113 (25.7) | 58 (34.1) | 56 (35.2) | 79 (27.2) |
| Suicide Attempt | 40 (9.1) | 32 (18.8) | 31 (19.5) | 26 (9.0) |
| Depression and/or Suicide Attempt | 125 (28.5) | 66 (38.8) | 65 (40.9) | 85 (29.3) |
| Anxiety Disorder | 117 (26.7) | 61 (35.9) | 59 (37.1) | 81 (27.9) |
| Bipolar Disorder | 3 (0.7) | 3 (1.8) | 3 (1.9) | 2 (0.7) |
| Schizophrenia | 6 (1.4) | 4 (2.4) | 4 (2.5) | 5 (1.7) |
| Unaffected | 240 (54.7) | 83 (48.8) | 75 (47.1) | 150 (51.7) |
| Unknown Diagnosis | 40 (9.1) | 3 (1.8) | 6 (3.8) | 30 (10.3) |
Quality control checks for ROH and CNV are different;
Affected with any psychiatric diagnosis; Numbers in bold add up to the total N in each column.
Estimating inbreeding coefficient
Inbreeding coefficient was computed based on genotyping data of 401 Short Tandem Repeats (STRs) from Prevention Genetics for all 439 subjects after performing quality control (QC) at the individual and marker level. We also checked for Mendelian errors using PedCheck (O’Connell and Weeks, 1998). We excluded 19 (4.3%) samples with <90% call rate and 29 (7.2%) STR markers based on the distribution of missingness and Mendelian errors. There were 2 (0.4%) discrepancies between the genotype-based gender and the pedigree information based on chromosome Y markers, which were errors on the pedigree and were corrected. Genetic relationships were estimated using all markers and pairwise identity-by-descent (IBD) relationships for all pairs of samples were estimated using maximum likelihood. IBD estimates for different pedigree relationships (parent-offspring, full and half siblings, unrelated) were examined and outliers were identified and investigated. We examined the relationship between genetic-based estimates of inbreeding and risk for the narrow and broad phenotypes using linear regression analyses and computing standard errors for parameter estimates using the cluster option in Stata, which allows for correlations within nuclear families.
Runs of Homozygosity
We genotyped 177 out of the 439 samples using Affymetrix Genomewide Human SNP Array 6.0, which resulted in 909,622 SNPs. We prioritized multiplex nuclear families, with more than one family member affected with any psychiatric disorder, and genotyped all affected and unaffected members. We excluded monomorphic SNPs (15,859 SNPs) and SNPs based on the distribution of missingness (4,778 SNPs with <97 % call rate) and Mendelian errors (57,527 SNPs with > 1 error). We also excluded 7 samples (3.9%) based on the distribution of missingness by sample with <99.7% call rate. Analyses were conducted with 170 subjects (84 affected, 83 unaffected, and 3 with unknown diagnosis) and 832,308 SNPs. We used Plink (Purcell et al., 2007) and Affy 6.0 genotype data to evaluate ROH on autosomes, based on the following settings: a sliding window of 2Mb; an ROH consisted of at least 100 consecutive homozygous SNPs, with no heterozygotes allowed, but up to 2 missing genotypes were allowed; the density specified is a minimum of 1 SNP per 50 kb; and the maximum allowed gap between SNPs in an ROH region was 2 Mb. The last criterion was chosen to prevent ROH spanning the centromere. We compared cases, based on the narrow (SA, n=32) and broad (SA-DEP, n=66) phenotypes, and controls unaffected with any psychiatric disorder (n=83) on the total length of ROH across the genome using linear regression taking into account the clustering within nuclear families (Table 1).
Copy Number Variation analyses
CNVs were called using genotype image intensities and two different calling algorithms: Birdseye of the Birdsuite package (Korn et al., 2008) and PennCNV (Wang et al., 2007). Genotype-based gender was supplied for calling CNVs on X. CNVs were called only when covered by 20 or more SNPs or monomorphic markers (henceforth called ‘probes’). Within this sample and according to Birdsuite, the average count of CNVs per sample was 51; the average size of a CNV was 74.3 kb; and an average CNV was called using 51 probes. According to PennCNVs, the average count of CNVs per sample was 38; the average size of a CNV was 109.5 kb; and an average CNV was called using 58 probes. We analyzed CNVs that were called by both algorithms. Based on the distribution of the number of CNVs per subject, we excluded 18 out of the 177 genotyped samples because of excessive number of CNVs. Of the 159 remaining samples for CNV analyses [74 males (M), 85 females (F)], 78 were affected with a psychiatric disorder, 75 were unaffected with any psychiatric disorder, and 6 had an unknown diagnosis (Table 1). We compared SA (n=31) and controls (n=75) as well as SA-DEP (65) and controls (n=75) on total CNV burden, duplications, deletions, and homozygous deletions called by Birdsuite and PennCNV using logistic regression and taking into account the clustering within nuclear families. Similar analyses from the International Schizophrenia Consortium (2008) and others (Kirov et al., 2009,Malhotra and Sebat, 2012) were conducted for CNVs larger than 500 kb, which are implicated in increased risk for schizophrenia and other neuropsychiatric disorders.
Copy Number Variation molecular validation
We identified CNVs nominally associated with increased risk for SA-DEP in this sample, as measured by the odds ratio, and occurring in genes of relevance to psychiatric disorders, typically identified in studies of samples of European ancestry. The association of these CNVs with affection status was assessed using logistic regression and taking into account clustering within families. Odds ratio (OR) and 95% confidence interval (CI) are reported. When there is a zero cell (undefined or infinity OR), we used the Firth penalized likelihood method (Firth, 1993). Given the relatively small sample size of this study, we do not expect the association of these CNVs with diagnosis to be statistically significant.
We considered risk CNVs for molecular validation if they were called by both Birdsuite and PennCNV algorithms and if they segregated in nuclear families with disorder. We used Nanostring’s nCounter Custom CNV technology to validate risk CNVs in carriers identified based on the two algorithms. We ran Nanostring on 376 out of the 439 samples, which included all samples genotyped with Affymetrix 6.0, all remaining affected subjects, and all remaining controls for whom there remained sufficient DNA. The Nanostring’s nCounter Custom CNV CodeSets included 6 positive control probes, 8 negative control probes, 10 invariant probes designed to autosomal genomic regions that do not cover common CNVs, restriction site controls, and endogenous probes designed to risk CNV regions identified (Table S1). Of the 376 samples, 290 remained after conducting QC checks according to manufacturer’s recommendations [137 males (M), 153 females (F)], 110 (37.9%, 43 M: 67 F) were affected, 150 (51.7%, 81 M: 69 F) were unaffected, and 30 (10.3%, 13 M: 17 F) had an unknown diagnosis (Table 1). We normalized the data to the invariant probes and used a CEPH sample as a reference sample to calculate copy number estimates for each probe. For the selected risk CNV regions, we used finite normal mixture modeling for model-based clustering and classification (mclust in R), to identify samples with duplications and deletions. Only probes with normal distribution of copy number estimates, as evidence of good quality probes, were included in the analyses. Finally, we extracted expression Quantitative Trait Loci (eQTLs) in the cerebellum and parietal regions of the brain from the SNP and CNV Annotation Database (SCAN) for genes falling in CNV regions with increased risk for SA-DEP. We examined the association of the resulting eQTLs with depression in the Psychiatric Genetics Consortium database (Ripke et al., 2013).
RESULTS
Inbreeding
By analysis of 372 STR genotypes per subject, the estimated average inbreeding coefficient ƒ for subjects in this kindred was 0.021 (Standard Deviation, SD=0.03, range 0–0.157). This estimate falls between that expected from the marriage between 2nd cousins (ƒ=0.0156) and 1st cousins (ƒ=0.0625). Only 11% of subjects have ƒ ≥ 0.0625 (Figure S1). We found no significant relationship between inbreeding and SA (β=−0.001, p=0.78; 95% CI −0.01, 0.008) or between inbreeding and SA-DEP (β=0.003, p=0.54; 95% CI −0.006, 0.011).
Runs of Homozygosity
On average, subjects carried 212.4 (SD=69.7, range 8–445) ROH regions with an average length per region of 712.3 kb (SD=257.3, range 406.3–1577.4). The average total ROH length is 160.7 Mb (SD=92.9, range 3.4–400.6) (Figure S2). The inbreeding coefficient was significantly correlated with the number of ROH regions (r=0.573, p<0.001), average length of an ROH (r=0.555, p<0.001), and total length of ROH (r=0.664, p<0.001). As expected, the total length of ROH across the genome was highly correlated with the number of ROH regions (r=0.819, p<0.001) and average length of an ROH (r=0.905, p<0.001). The total length of ROH was not significantly different between cases and controls for either the broad (167.5 ± 11.1 vs. 159.2 ± 11.3; β=9.4, t=0.60, p=0.55; 95% CI −21.6, 40.4) or narrow phenotype (156.9 ± 16.4 vs. 159.2 ± 11.3; β=−0.36, t=−0.02, p=0.99; 95% CI −40.6, 39.9) (Figure S2).
CNV burden
The average number of CNVs, duplications, and deletions per subject is 25.4 (SD=5.8), 6.4 (SD=2.9), and 13.8 (SD=4.1), respectively (Figure S3). Subjects had on average 5.3 (SD=2) homozygous deletions (Figure S4). Looking at CNVs larger than 500 kb, subjects had an average 1.8 CNVs (SD=1), 1.5 (SD=0.7) duplications, and 1.3 (SD=0.8) deletions (Figure S5). None of the subjects genotyped on Affy 6.0 carried homozygous deletions larger than 500 kb. Large duplications were significantly associated with increased risk for SA (OR=2.9, p=0.01; 95% CI 1.3, 6.6) (Figure 1) and SA-DEP (OR=2.1, p=0.02; 95% CI 1.1, 3.9).
Figure 1.
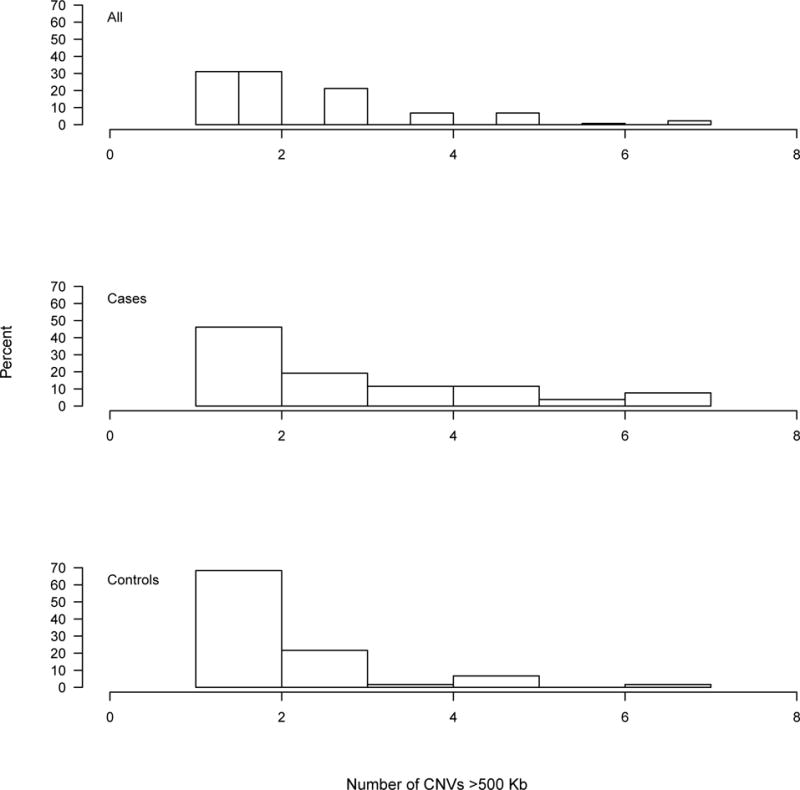
Distribution of large duplications (>500 kb) in all subjects, cases, and controls based on suicide attempt affection status
CNV molecular validation
We detected several CNVs in this Bedouin kindred that could confer risk to depression, SA, and related disorders, as measured by the odds ratio (Table S2). The largest CNV is a1.4 Mb duplication on chromosome 17p12-p13.1 observed in one male subject who had attempted suicide and was diagnosed with depression and anxiety. This duplication spanning 14–15.4 Mb covered several genes including PMP22; duplication of, or mutation in PMP22 causes Charcot-Marie Tooth disease, Type 1A (CMT1A) (OMIM#118220), a neuropathy characterized by progressive loss of muscle tissue and touch sensation. The duplication was called by Birdsuite and PennCNV algorithms and molecularly validated in this subject. None of the samples analyzed with Nanostring showed this duplication.
We also identified duplications and deletions in 9p24.1 covering two genes, UHFR2 and GLDC (Figure S6). The duplication is associated with increased risk based on the odds ratio (OR=5.4, 95% CI, 0.6–49.4) but not the deletion (OR=0.5, 95% CI, 0.12–1.72). However, when we examined the number of copies of this CNV, we find a significantly increased risk for SA with increased copy numbers (OR=13.1, p=0.021, 95% CI, 1.5–115.5), which remained significant after controlling for sex. The duplication, ~193 kb (chr9: 6,484,504–6,676,585) occurred in two affected full sibling females, two affected half-siblings resulting from consanguineous marriages (one male and one female), 1 affected female from a consanguineous marriage, one unaffected female, and one female with an unknown diagnosis (Figure 2). All CNV carriers were related amongst each other. The duplication in all affected subjects was called by both CNV algorithms and was molecularly validated, except in one subject where we were not able to validate it due to poor sample quality on Nanostring (Table S3). The unaffected subject and the one with unknown diagnosis were not genotyped on Affymetrix 6.0 but were detected on Nanostring. The duplication, if it confers risk, is incompletely penetrant; however, the unaffected subject and the one with unknown diagnosis were both 21 years of age at the time of assessment and thus not yet through the age of risk for depression and SA. We have previously shown that incident suicidal behavior continues to occur up to age 30 (Melhem et al., 2007). We have also shown that the risk and protective factors for suicidal behavior in this kindred are similar to those in European populations (Hamdan et al., 2012). For the most proximal and distal boundaries of the 9p24.1 CNV in our study (chr9: 6,484,504–6,676,585), a search of the Database of Genomic Variants (DGV) (Iafrate et al., 2004) with 18,294 healthy subjects found 96 CNVs, of which 26 (27% or 0.14% of the total number of healthy subjects) actually fall within the GLDC gene compared to 8 out of the 78 (10.3%) affected subjects in our sample. Of the 26 DGV CNVs falling in GLDC, there were 13 that covered intronic regions of the gene, 11 covered 1–3 exons, and 2 covered 14–15 exons. All CNV carriers in our sample had duplication or deletion of the full GLDC gene with all of its 25 exons.
Figure 2.
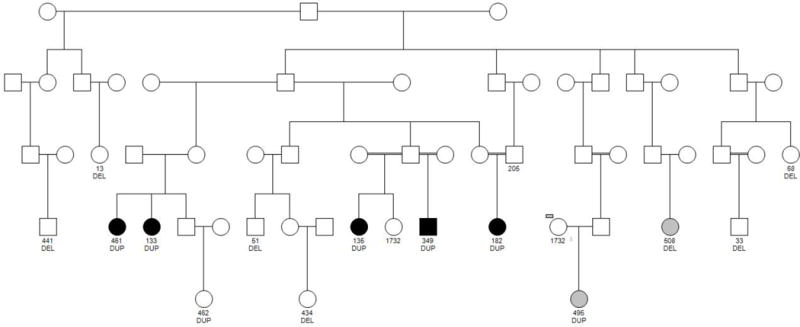
Pedigree relationships between subjects carrying the 9p24.1 duplication. Some of the deletion carriers belong to this pedigree and are indicated. Subjects in black are affected; Subjects in grey have an unknown diagnosis; DUP, duplication; DEL, Deletion
Looking at eQTLs from the SCAN SNP and CNV annotation database, we found two out of 31 eQTL (21 cis and 10 trans) SNPs for GLDC in the parietal region of the brain, rs4363822 (trans eQTL p=1.87×10−6) and rs997295 (trans eQTL p=1.91×10−6), which were associated with depression in the PGC dataset with p-values of 6.10−3 and 6.3.10−3, respectively. These were on chromosome 15 at positions (hg18) 65,779,637 and 65,803,397 bp, respectively, and they fell in MAP2K5. Five other GLDC eQTLs in the parietal region showed p-values between 0.01<p<0.05 for associations with depression in the PGC dataset. There were 87 cis eQTLs for GLDC in the cerebellum, of which 10 SNPs showed p-values between 0.01<p<0.05 for associations with depression in the PGC dataset.
Other CNVs were found that could increase risk for our phenotypes, according to their odds ratio (Tables S4–S8; Figures S7–S16). These included an exonic deletion in 3p11.2-p11.1 covering EPHA3, exonic duplication in 6q21 covering HACE1, intronic duplication in 8p12 covering NRG1, exonic duplication in 10q26.3 covering CYP2E1, and an exonic duplication and deletion in 16p13.11.
DISCUSSION
In this Arab Bedouin kindred, we find extensive regions of homozygosity, consistent with other Arab populations with similar rates of inbreeding (Hunter-Zinck et al., 2010). On average, 5% of the genome is covered by runs of homozygosity (ROH) for subjects from this kindred, twofold higher than ROH rates for subjects from populations of European ancestry (McQuillan et al., 2008). However, this is consistent with ROH in isolated endogamous Berber groups from North African populations consisting of an admixture of sub-Saharan, North African, European, and Middle Eastern ancestral components (Arauna et al., 2017).
Previous studies show inbreeding to be associated with increased risk of schizophrenia, bipolar disorder, and suicidal self-inflicted burns in Egypt, Iran, and Bedouin populations (Saadat and Zendeh-Boodi, 2006,Dobrusin et al., 2009,Mansour et al., 2009,Mansour et al., 2010,Nafissi et al., 2011,Saadat, 2012). However, we did not find estimated level of inbreeding to be associated with increased risk for depression and suicide attempt in this kindred. While our study is limited with respect to characterizing the genotyped sample on other diseases, the effects of inbreeding for other diseases are evident in this kindred where several diseases with a recessive mode of inheritance occur including Limb Girdle Muscular Dystrophy, Thalassemia, Von Willebrand Disease, Hereditary Sensory and Autonomic Neuropathy, and Congenital Motor Nystagmus (Farbstein E, personal communication). Thus, this kindred is similar to Arab populations and specifically, Palestinians, in their increased rates of recessive diseases (Zlotogora, 2002,2010). It is possible that the lack of association between inbreeding and risk for depression and suicide attempt could be an attribute of the genetic structure of this kindred and founder effects rather than being generalizable to the entire Palestinian population.
When we examined CNVs and their impact on risk, we found no significant relationship between burden of homozygous deletions and risk, consistent with our results for inbreeding more generally. However, large duplications were associated with increased risk for suicide attempt and for the broader phenotype. Large CNVs have been previously found to increase risk for schizophrenia (Kirov et al., 2009), recurrent depression (Rucker et al., 2013), and treatment-resistant depression (O’Dushlaine et al., 2014). However, other studies show no evidence for the effect of rare large CNV burden in recurrent depression and lower rates of large CNVs in cases with bipolar disorder compared to controls (Grozeva et al., 2010,Grozeva et al., 2013,Rucker et al., 2016).
Of particular interest, we find a CNV on chromosome 9p24.1 that appears to increase risk for suicide attempt with increased number of copies. This CNV covers GLDC, which encodes glycine dehydrogenase that binds to glycine and results in its degradation. Glycine is a co-agonist at N-methyl-D-aspartate (NMDA) glutamate receptors and thus increases glutamatergic neurotransmission. Plasma levels of glycine are found to be negatively associated with treatment response to the antidepressant Citalopram and a SNP, rs10975641, in GLDC has been associated with treatment outcome (Ji et al., 2011). In addition, plasma levels of the amino acid glycine are significantly increased in depressed patients (Mitani et al., 2006), although other studies report no difference (Maes et al., 1998,Mauri et al., 1998) or lower glycine levels (Altamura et al., 1995,Sumiyoshi et al., 2004). In our sample, only 6 subjects (1.5%) reported the use of psychotropic medications and 4 of these were genotyped and did not carry a duplication or deletion on chromosome 9p24.1. However, we did not collect information about history of treatment response and we did not measure glycine levels. The remaining sample reported that they did not use psychotropic medications (31.5%), did not know if they used them (46%); or refused to answer this question (21%), which may reflect a cultural bias towards underreporting the use of psychotropic medications. According to our results, and assuming the GLDC gene is fully duplicated, the duplication should result in increased glycine degradation and consequently lead to decreased glycine levels. If the duplication were to interfere with gene function, however, it would reduce glycine degradation. A silent exonic GLDC splice mutation was described in a large consanguineous Bedouin kindred with an atypical milder form of glycine encephalopathy characterized with developmental delays, limited expressive speech, hypotonia, aggression, and irritability (Flusser et al., 2005). Intriguingly, impulsive aggression is one of the important predictors of suicidal behavior (Melhem et al., 2007,Mann et al., 2009). Glycine encephalopathy is also characterized by elevated levels of glycine concentrations in blood and cerebrospinal fluid (CSF) and often treated with antagonists at the NMDA receptor site such as ketamine with well-established antidepressant properties (Van Hove et al., 2013,Zunszain et al., 2013). A recent study reported SNPs in glutamatergic genes to be associated with suicide attempt (Sokolowski et al., 2013). Given our results it could be important to further examine the role of the GLDC gene in risk for suicidal behavior.
Our findings add to the growing evidence of genetic risk factors that act pleiotropically to increase risk for several neuropsychiatric disorders, including depression and suicide attempt, regardless of ancestry. While our study is limited in sample size and its ability to examine the association of common and rare genetic variants to depression and suicide attempt, the results underscore the importance of CNVs not only for neurodevelopmental disorders but also for adult-onset disorders. This study reveals several CNVs that could confer risk to depression and suicide attempt, and a CNV in GLDC that specifically confers risk for suicide attempt. It could be important to elucidate the functional role of GLDC expression in risk suicidal behavior.
Supplementary Material
Acknowledgments
Sources of funding. This study work was supported by a K01 grant (MH077930, Melhem NM) from the National Institute of Mental Health, NARSAD Young Investigator Award (Melhem NM), Klingenstein Third Generation Foundation Postdoctoral Fellowship (Melhem NM); and grants from the Pittsburgh Foundation (Melhem NM), United States-Israel Binational Science Foundation (Apter A, Brent D), American Foundation for Suicide Prevention (Apter A), and Knut and Alice Wallenberg Foundation, Sweden (Wasserman D).
Footnotes
Conflict of interests: All authors have no conflicts of interest to disclose except for Dr. David Brent. Dr. Brent receives royalties from Guilford Press, have or will receive royalties from the electronic self-rated version of the C-SSRS from ERT, Inc., is on the Editorial board of UpToDate, is a reviewer for Healthwise, is on the board of Klingenstein Foundation, and receives honoraria for presenting at Continuing Medical Education events.
Supplementary Materials.docx includes Tables S1–S8 and Figures S1–S16
References
- Altamura C, Maes M, Dai J, Meltzer HY. Plasma concentrations of excitatory amino acids, serine, glycine, taurine and histidine in major depression. Eur Neuropsychopharmacol. 1995;5(Suppl):71–75. doi: 10.1016/0924-977x(95)00033-l. [DOI] [PubMed] [Google Scholar]
- Arauna LR, Mendoza-Revilla J, Mas-Sandoval A, Izaabel H, Bekada A, Benhamamouch S, et al. Recent Historical Migrations Have Shaped the Gene Pool of Arabs and Berbers in North Africa. Mol Biol Evol. 2017;34(2):318–329. doi: 10.1093/molbev/msw218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayub M, Irfan M, Maclean A, Naeem F, Blackwood D. Homozygosity mapping of depressive disorder in a large family from Pakistan: significant linkage on chromosome 6 and 9. Am J Med Genet B Neuropsychiatr Genet. 2013;162B(2):157–162. doi: 10.1002/ajmg.b.32126. [DOI] [PubMed] [Google Scholar]
- Cross-Disorder Group of the Psychiatric Genomics Consortium. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381(9875):1371–1379. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt F, Priebe L, Herms S, Mattheisen M, Muhleisen TW, Meier S, et al. Association between copy number variants in 16p11.2 and major depressive disorder in a German case-control sample. Am J Med Genet B Neuropsychiatr Genet. 2012;159B(3):263–273. doi: 10.1002/ajmg.b.32034. [DOI] [PubMed] [Google Scholar]
- Demyttenaere K, Bruffaerts R, Posada-Villa J, Gasquet I, Kovess V, Lepine JP, et al. Prevalence, severity, and unmet need for treatment of mental disorders in the World Health Organization World Mental Health Surveys. JAMA. 2004;291(21):2581–2590. doi: 10.1001/jama.291.21.2581. [DOI] [PubMed] [Google Scholar]
- Dobrusin M, Weitzman D, Levine J, Kremer I, Rietschel M, Maier W, et al. The rate of consanguineous marriages among parents of schizophrenic patients in the Arab Bedouin population in Southern Israel. World J Biol Psychiatry. 2009;10(4):334–336. doi: 10.3109/15622970701849960. [DOI] [PubMed] [Google Scholar]
- Ekstein J, Rubin BY, Anderson SL, Weinstein DA, Bach G, Abeliovich D, et al. Mutation frequencies for glycogen storage disease Ia in the Ashkenazi Jewish population. Am J Med Genet A. 2004;129A(2):162–164. doi: 10.1002/ajmg.a.30232. [DOI] [PubMed] [Google Scholar]
- Fares F, Badarneh K, Abosaleh M, Harari-Shaham A, Diukman R, David M. Carrier frequency of autosomal-recessive disorders in the Ashkenazi Jewish population: should the rationale for mutation choice for screening be reevaluated? Prenat Diagn. 2008;28(3):236–241. doi: 10.1002/pd.1943. [DOI] [PubMed] [Google Scholar]
- Firth D. Bias reduction of maximum likelihood estimates. Biometrika. 1993;80:27–38. [Google Scholar]
- Flusser H, Korman SH, Sato K, Matsubara Y, Galil A, Kure S. Mild glycine encephalopathy (NKH) in a large kindred due to a silent exonic GLDC splice mutation. Neurology. 2005;64(8):1426–1430. doi: 10.1212/01.WNL.0000158475.12907.D6. [DOI] [PubMed] [Google Scholar]
- Gamsiz ED, Viscidi EW, Frederick AM, Nagpal S, Sanders SJ, Murtha MT, et al. Intellectual disability is associated with increased runs of homozygosity in simplex autism. Am J Hum Genet. 2013;93(1):103–109. doi: 10.1016/j.ajhg.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glessner JT, Wang K, Sleiman PM, Zhang H, Kim CE, Flory JH, et al. Duplication of the SLIT3 locus on 5q35.1 predisposes to major depressive disorder. PLoS One. 2010;5(12):e15463. doi: 10.1371/journal.pone.0015463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozeva D, Kirov G, Conrad DF, Barnes CP, Hurles M, Owen MJ, et al. Reduced burden of very large and rare CNVs in bipolar affective disorder. Bipolar Disord. 2013;15(8):893–898. doi: 10.1111/bdi.12125. [DOI] [PubMed] [Google Scholar]
- Grozeva D, Kirov G, Ivanov D, Jones IR, Jones L, Green EK, et al. Rare copy number variants: a point of rarity in genetic risk for bipolar disorder and schizophrenia. Arch Gen Psychiatry. 2010;67(4):318–327. doi: 10.1001/archgenpsychiatry.2010.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdan S, Melhem N, Orbach I, Farbstein I, El-Haib M, Apter A, et al. Protective factors and suicidality in members of Arab kindred. Crisis. 2012;33(2):80–86. doi: 10.1027/0227-5910/a000116. [DOI] [PubMed] [Google Scholar]
- Hunter-Zinck H, Musharoff S, Salit J, Al-Ali KA, Chouchane L, Gohar A, et al. Population genetic structure of the people of Qatar. Am J Hum Genet. 2010;87(1):17–25. doi: 10.1016/j.ajhg.2010.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iafrate AJ, Feuk L, Rivera MN, Listewnik ML, Donahoe PK, Qi Y, et al. Detection of large-scale variation in the human genome. Nat Genet. 2004;36(9):949–951. doi: 10.1038/ng1416. [DOI] [PubMed] [Google Scholar]
- International Schizophrenia Consortium. Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature. 2008;455(7210):237–241. doi: 10.1038/nature07239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaber L, Bailey-Wilson JE, Haj-Yehia M, Hernandez J, Shohat M. Consanguineous matings in an Israeli-Arab community. Arch Pediatr Adolesc Med. 1994;148(4):412–415. doi: 10.1001/archpedi.1994.02170040078013. [DOI] [PubMed] [Google Scholar]
- Ji Y, Hebbring S, Zhu H, Jenkins GD, Biernacka J, Snyder K, et al. Glycine and a glycine dehydrogenase (GLDC) SNP as citalopram/escitalopram response biomarkers in depression: pharmacometabolomics-informed pharmacogenomics. Clin Pharmacol Ther. 2011;89(1):97–104. doi: 10.1038/clpt.2010.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karam EG, Mneimneh ZN, Karam AN, Fayyad JA, Nasser SC, Chatterji S, et al. Prevalence and treatment of mental disorders in Lebanon: a national epidemiological survey. Lancet. 2006;367(9515):1000–1006. doi: 10.1016/S0140-6736(06)68427-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller MC, Simonson MA, Ripke S, Neale BM, Gejman PV, Howrigan DP, et al. Runs of homozygosity implicate autozygosity as a schizophrenia risk factor. PLoS Genet. 2012;8(4):e1002656. doi: 10.1371/journal.pgen.1002656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Ustun TB. The World Mental Health (WMH) Survey Initiative Version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI) Int J Methods Psychiatr Res. 2004;13(2):93–121. doi: 10.1002/mpr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Abelson J, Demler O, Escobar JI, Gibbon M, Guyer ME, et al. Clinical calibration of DSM-IV diagnoses in the World Mental Health (WMH) version of the World Health Organization (WHO) Composite International Diagnostic Interview (WMHCIDI) Int J Methods Psychiatr Res. 2004;13(2):122–139. doi: 10.1002/mpr.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirin M, McQuillan R, Franklin CS, Campbell H, McKeigue PM, Wilson JF. Genomic runs of homozygosity record population history and consanguinity. PLoS One. 2010;5(11):e13996. doi: 10.1371/journal.pone.0013996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirov G, Grozeva D, Norton N, Ivanov D, Mantripragada KK, Holmans P, et al. Support for the involvement of large copy number variants in the pathogenesis of schizophrenia. Hum Mol Genet. 2009;18(8):1497–1503. doi: 10.1093/hmg/ddp043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn JM, Kuruvilla FG, McCarroll SA, Wysoker A, Nemesh J, Cawley S, et al. Integrated genotype calling and association analysis of SNPs, common copy number polymorphisms and rare CNVs. Nat Genet. 2008;40(10):1253–1260. doi: 10.1038/ng.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovess-Masfety V, Alonso J, Angermeyer M, Bromet E, de Girolamo G, de Jonge P, et al. Irritable mood in adult major depressive disorder: results from the world mental health surveys. Depress Anxiety. 2013;30(4):395–406. doi: 10.1002/da.22033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim ET, Raychaudhuri S, Sanders SJ, Stevens C, Sabo A, MacArthur DG, et al. Rare complete knockouts in humans: population distribution and significant role in autism spectrum disorders. Neuron. 2013;77(2):235–242. doi: 10.1016/j.neuron.2012.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Verkerk R, Vandoolaeghe E, Lin A, Scharpe S. Serum levels of excitatory amino acids, serine, glycine, histidine, threonine, taurine, alanine and arginine in treatment-resistant depression: modulation by treatment with antidepressants and prediction of clinical responsivity. Acta Psychiatr Scand. 1998;97(4):302–308. doi: 10.1111/j.1600-0447.1998.tb10004.x. [DOI] [PubMed] [Google Scholar]
- Malhotra D, Sebat J. CNVs: harbingers of a rare variant revolution in psychiatric genetics. Cell. 2012;148(6):1223–1241. doi: 10.1016/j.cell.2012.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann JJ, Arango VA, Avenevoli S, Brent DA, Champagne FA, Clayton P, et al. Candidate endophenotypes for genetic studies of suicidal behavior. Biol Psychiatry. 2009;65(7):556–563. doi: 10.1016/j.biopsych.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour H, Klei L, Wood J, Talkowski M, Chowdari K, Fathi W, et al. Consanguinity associated with increased risk for bipolar I disorder in Egypt. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(6):879–885. doi: 10.1002/ajmg.b.30913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour H, Fathi W, Klei L, Wood J, Chowdari K, Watson A, et al. Consanguinity and increased risk for schizophrenia in Egypt. Schizophr Res. 2010;120(1–3):108–112. doi: 10.1016/j.schres.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauri MC, Ferrara A, Boscati L, Bravin S, Zamberlan F, Alecci M, et al. Plasma and platelet amino acid concentrations in patients affected by major depression and under fluvoxamine treatment. Neuropsychobiology. 1998;37(3):124–129. doi: 10.1159/000026491. [DOI] [PubMed] [Google Scholar]
- McQuillan R, Leutenegger AL, Abdel-Rahman R, Franklin CS, Pericic M, Barac-Lauc L, et al. Runs of homozygosity in European populations. Am J Hum Genet. 2008;83(3):359–372. doi: 10.1016/j.ajhg.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melhem NM, Brent DA, Ziegler M, Iyengar S, Kolko D, Oquendo M, et al. Familial pathways to early-onset suicidal behavior: familial and individual antecedents of suicidal behavior. Am J Psychiatry. 2007;164(9):1364–1370. doi: 10.1176/appi.ajp.2007.06091522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani H, Shirayama Y, Yamada T, Maeda K, Ashby CR, Jr, Kawahara R. Correlation between plasma levels of glutamate, alanine and serine with severity of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(6):1155–1158. doi: 10.1016/j.pnpbp.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Myles-Worsley M, Tiobech J, Browning SR, Korn J, Goodman S, Gentile K, et al. Deletion at the SLC1A1 glutamate transporter gene co-segregates with schizophrenia and bipolar schizoaffective disorder in a 5-generation family. Am J Med Genet B Neuropsychiatr Genet. 2013;162B(2):87–95. doi: 10.1002/ajmg.b.32125. [DOI] [PubMed] [Google Scholar]
- Nafissi S, Ansari-Lari M, Saadat M. Parental consanguineous marriages and age at onset of schizophrenia. Schizophr Res. 2011;126(1–3):298–299. doi: 10.1016/j.schres.2010.11.029. [DOI] [PubMed] [Google Scholar]
- Nock MK, Borges G, Bromet EJ, Alonso J, Angermeyer M, Beautrais A, et al. Cross-national prevalence and risk factors for suicidal ideation, plans and attempts. Br J Psychiatry. 2008;192(2):98–105. doi: 10.1192/bjp.bp.107.040113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell JR, Weeks DE. PedCheck: a program for identification of genotype incompatibilities in linkage analysis. Am J Hum Genet. 1998;63(1):259–266. doi: 10.1086/301904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dushlaine C, Ripke S, Ruderfer DM, Hamilton SP, Fava M, Iosifescu DV, et al. Rare Copy Number Variation in Treatment-Resistant Major Depressive Disorder. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2013.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripke S, Wray NR, Lewis CM, Hamilton SP, Weissman MM, Breen G, et al. A mega-analysis of genome-wide association studies for major depressive disorder. Mol Psychiatry. 2013;18(4):497–511. doi: 10.1038/mp.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker JJ, Tansey KE, Rivera M, Pinto D, Cohen-Woods S, Uher R, et al. Phenotypic Association Analyses With Copy Number Variation in Recurrent Depressive Disorder. Biol Psychiatry. 2016;79(4):329–336. doi: 10.1016/j.biopsych.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker JJ, Breen G, Pinto D, Pedroso I, Lewis CM, Cohen-Woods S, et al. Genome-wide association analysis of copy number variation in recurrent depressive disorder. Mol Psychiatry. 2013;18(2):183–189. doi: 10.1038/mp.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadat M. Influence of parental consanguineous marriages on age at onset of bipolar disorder. Psychiatry Res. 2012;198(2):327–328. doi: 10.1016/j.psychres.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Saadat M, Zendeh-Boodi Z. Correlation between incidences of self-inflicted burns and means of inbreeding coefficients, an ecologic study. Ann Epidemiol. 2006;16(9):708–711. doi: 10.1016/j.annepidem.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowski M, Ben-Efraim YJ, Wasserman J, Wasserman D. Glutamatergic GRIN2B and polyaminergic ODC1 genes in suicide attempts: associations and gene-environment interactions with childhood/adolescent physical assault. Mol Psychiatry. 2013;18(9):985–992. doi: 10.1038/mp.2012.112. [DOI] [PubMed] [Google Scholar]
- St Clair D, Blackwood D, Muir W, Carothers A, Walker M, Spowart G, et al. Association within a family of a balanced autosomal translocation with major mental illness. Lancet. 1990;336(8706):13–16. doi: 10.1016/0140-6736(90)91520-k. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T, Anil AE, Jin D, Jayathilake K, Lee M, Meltzer HY. Plasma glycine and serine levels in schizophrenia compared to normal controls and major depression: relation to negative symptoms. Int J Neuropsychopharmacol. 2004;7(1):1–8. doi: 10.1017/S1461145703003900. [DOI] [PubMed] [Google Scholar]
- Szpiech ZA, Xu J, Pemberton TJ, Peng W, Zollner S, Rosenberg NA, et al. Long runs of homozygosity are enriched for deleterious variation. Am J Hum Genet. 2013;93(1):90–102. doi: 10.1016/j.ajhg.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teebi AS, Farag TI. Genetic disorders among Arab populations. New York: Oxford University Press; 1997. [Google Scholar]
- Van Hove J, Coughlin C, Scharer G. Glycine Encephalopathy. 2013 Available at: http://www.ncbi.nlm.nih.gov/books/NBK1357/.
- Wang K, Li M, Hadley D, Liu R, Glessner J, Grant SF, et al. PennCNV: an integrated hidden Markov model designed for high-resolution copy number variation detection in whole-genome SNP genotyping data. Genome Res. 2007;17(11):1665–1674. doi: 10.1101/gr.6861907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LS, Hranilovic D, Wang K, Lindquist IE, Yurcaba L, Petkovic ZB, et al. Population-based study of genetic variation in individuals with autism spectrum disorders from Croatia. BMC Med Genet. 2010;11:134. doi: 10.1186/1471-2350-11-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Wang K, Gregory B, Berrettini W, Wang LS, Hakonarson H, et al. Genomic landscape of a three-generation pedigree segregating affective disorder. PLoS One. 2009;4(2):e4474. doi: 10.1371/journal.pone.0004474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu TW, Chahrour MH, Coulter ME, Jiralerspong S, Okamura-Ikeda K, Ataman B, et al. Using whole-exome sequencing to identify inherited causes of autism. Neuron. 2013;77(2):259–273. doi: 10.1016/j.neuron.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Geyer MA, Kelsoe JR. Does disrupted-in-schizophrenia (DISC1) generate fusion transcripts? Mol Psychiatry. 2008;13(4):361–363. doi: 10.1038/sj.mp.4002125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotogora J. Molecular basis of autosomal recessive diseases among the Palestinian Arabs. Am J Med Genet. 2002;109(3):176–182. doi: 10.1002/ajmg.10328. [DOI] [PubMed] [Google Scholar]
- Zlotogora J. The molecular basis of autosomal recessive diseases among the Arabs and Druze in Israel. Hum Genet. 2010;128(5):473–479. doi: 10.1007/s00439-010-0890-8. [DOI] [PubMed] [Google Scholar]
- Zunszain PA, Horowitz MA, Cattaneo A, Lupi MM, Pariante CM. Ketamine: synaptogenesis, immunomodulation and glycogen synthase kinase-3 as underlying mechanisms of its antidepressant properties. Mol Psychiatry. 2013;18(12):1236–1241. doi: 10.1038/mp.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


