Abstract
Background: Low dietary calcium intake may be a risk factor for hypertension, but studies conflict.
Objective: We evaluated the ability to predict hypertension within 10 y after delivery based on calcium intake during midpregnancy.
Methods: The Norwegian Mother and Child Cohort Study of women delivering in 2004–2009 was linked to the Norwegian Prescription Database (2004–2013) to ascertain antihypertensive medication usage >90 d after delivery. Women with hypertension before pregnancy were excluded, leaving 60,027 mothers for analyses. Age and energy-adjusted cubic splines evaluated dose-response curves, and Cox proportional hazard analyses evaluated HR and 95% CIs by calcium quartiles adjusting for 7 covariates. Analyses were stratified by gestational hypertension and by sodium-to-potassium intake ratio (<0.76 compared with ≥0.76).
Results: Participants had a mean ± SD age of 30.5 ± 4.6 y, a body mass index (in kg/m2) of 24.0 ± 4.3 before pregnancy, and a mean follow-up duration of 7.1 ± 1.6 y. Cubic spline graphs identified a threshold effect of low calcium intake only within the range of dietary inadequacy related to increased risk. The lowest calcium quartile (≤738 mg/d; median: 588 mg/d), relative to the highest quartile (≥1254 mg/d), had an HR for hypertension of 1.34 (95% CI: 1.05, 1.70) among women who were normotensive during pregnancy, and an HR of 1.62 (95% CI: 1.14, 2.35) among women who had gestational hypertension, after adjusting for covariates. Women with gestational hypertension, who were in the lowest quartile of calcium intake, and who had a high sodium-to-potassium intake ratio had a risk of hypertension more than double that of their counterparts with a calcium intake in the highest quartile. Results were attenuated by adjusting for covariates (HR: 1.92; 95% CI: 1.09, 3.39).
Conclusions: The results suggest that low dietary calcium intake may be a risk factor or risk marker for the development of hypertension, particularly for women with a history of gestational hypertension.
Keywords: nutrition, diet, cardiovascular disease, pregnancy, gestational hypertension, preeclampsia, hypertension
Introduction
Hypertension is a major and modifiable risk factor for early mortality and ranks as the third leading factor in increasing the number of disability-adjusted life-years (1). The global burden of this condition is notable: an estimated 26.4% of the worldwide adult population had hypertension in 2000 (2). In women, pregnancy-related de novo hypertension unmasks predispositions for subsequent hypertension, and preeclampsia in particular associates with long-term risks of cardiovascular disease (3–5) and renal disease (6).
Dietary habits and specific nutrient intakes have been associated with blood pressure (BP) and hypertension in adults (7). The importance of calcium intake in hypertension, however, remains controversial. In general, epidemiologic studies have found weak inverse associations between calcium intake (through diet, supplements, or both) and reductions in BP (7, 8). In the large Women’s Health Initiative Calcium/Vitamin D Supplementation Study, no significant differences were noted in BP changes over time among postmenopausal women randomly assigned to supplements or placebo (9). But in a meta-analysis of randomized controlled trials, calcium supplementation had a stronger effect in lowering blood pressure among populations with inadequate dietary intake of calcium (8). Further, evidence shows that calcium may be more beneficial in salt-sensitive hypertension (10, 11). Also, in the Nurses’ Health Study, calcium intake >800 mg/d compared with intake <400 mg/d was associated prospectively with a reduced risk of hypertension [HR: 0.78 (95% CI: 0.69, 0.88%)] (12). In another study, intake of low-fat dairy foods protected against hypertension in a large prospective cohort of middle-aged and older women (13). Higher calcium intakes may downregulate the activity of the renin-angiotensin system and improve sodium-potassium balance (11, 14). Furthermore, high calcium intake may reduce vasoconstriction in vascular smooth muscle cells and thereby reduce vascular resistance (15). Also, calcium intake is often correlated with magnesium intake, which has known beneficial effects on BP regulation (16).
To our knowledge, no study has evaluated whether dietary assessments of calcium intake during pregnancy identify women at risk of developing hypertension after pregnancy, or whether nutritional risk factors for hypertension after pregnancy are similar between women with and women without gestational hypertension or preeclampsia. Given that pregnancy represents a period of repeated interaction with the health care system, pregnancy is an ideal opportunity to monitor and engage women in primary prevention programs.
We hypothesized that low calcium intake assessed during midpregnancy would associate with the development of hypertension within 10 y after pregnancy, and that associations would be stronger among those with a high sodium-to-potassium ratio and among high-risk women who had gestational hypertension. Further, we explored the associations between dietary and supplemental magnesium intake as it relates to hypertension risk in the study population.
Methods
This study uses the Norwegian Mother and Child Cohort Study (MoBa), which is a prospective, population-based pregnancy cohort study conducted by the Norwegian Institute of Public Health (17). Participants were recruited from all regions in Norway from 1999 through 2008 (i.e., deliveries included those in 2009). The women consented to participation in 41% of the pregnancies. The cohort includes 114,500 children, 95,200 mothers, and 75,500 fathers. The current research project was approved by the Regional Ethics Committee (Region West: 2013/740), the Data Inspectorate, MoBa, and the Norwegian Prescription Database (NPD). Women provided informed consent, which included subsequent record linkages.
MoBa was linked to the Medical Birth Registry of Norway (MBRN) (18) to ascertain pregnancy-related complications, and to the NPD, 2004–2013 (19), to ascertain antihypertensive medication use after delivery. Given that the prescription database was not established until 2004, and given the early changes in the nutritional assessments, the study was restricted to participants who delivered in 2004–2009 (n = 75,906 deliveries). In the event that the mother participated more than once, only the last pregnancy in which the mother participated in MoBa was included in this analysis (n = 62,746 mothers). Women who had a fetal loss (i.e., a nonviable fetus weighing <500 g or delivered at <22 wk of gestation) or who had hypertension before pregnancy (n = 1414) were excluded from the analyses.
Dietary intake and use of supplements.
Dietary intake was estimated using a semiquantitative FFQ developed specifically for MoBa and was administered in the 22nd week of pregnancy (20). The MoBa FFQ had been validated against 4-d weighted food diary, motion sensors, and biological markers (21, 22). Those with missing or unreasonably low or high energy intakes (<1070 or >4400 kcal/d, respectively; n = 1305) were excluded, resulting in 60,027 women for the current analyses. Supplements (brand names) containing calcium, magnesium, or both were identified from the same questionnaire, and intakes (milligrams per day) were calculated through the use of a supplement database (23).
Outcome.
Hypertension after pregnancy was ascertained through the NPD, which contains information regarding dispensed drugs for noninstitutionalized individuals (19). Antihypertensive medications are generally prescribed according to guidelines requiring a BP cutoff (≥140/90 mm Hg) measured at ≥2 physician visits (24, 25). Hypertension was considered present if it was listed as the underlying indication for treatment for any of the following dispensed Anatomical Therapeutic Chemical classification system prescriptions: antihypertensives (Anatomical Therapeutic Chemical classification code C02), diuretics (C03), β-blockers (C07), calcium channel blockers (C08), and renin-angiotensin system medications (C09) (26). In addition, prescriptions had to be dispensed >90 d after delivery in order for women to be considered hypertensive. If medication was not used after 90 d but was used during the immediate postpartum period (<90 d from the date of delivery), the women were coded as nonhypertensive.
Covariates.
The first MoBa questionnaire, administered at 15–17 wk of gestation, was used to ascertain prepregnancy characteristics: smoking status, leisure-time physical activity (27), BMI (kg/m2), alcohol consumption frequency, maternal education, and duration of oral contraceptive use. The fourth questionnaire, administered 6 mo after delivery, was used to ascertain weight gain and breastfeeding status. Very few data were missing (0–3.2%) for the majority of parameters, with the exception of alcohol frequency (4.3%), weight gain (17.9%), and breastfeeding (14.3%) 6 mo postpartum.
Pregnancy outcomes.
The MBRN (18) was used to ascertain pregnancy characteristics of potential importance as covariates for the current analyses. Parameters of interest included maternal age at delivery, parity, multiple-birth pregnancy, gestational hypertension (without proteinuria), preeclampsia, prepregnancy and gestational diabetes mellitus (including unspecified), preterm deliveries (<37 wk or, when gestational age was missing, a birth weight <2500 g), and stillbirths. Furthermore, the MBRN was used to ascertain the existence of chronic hypertension before pregnancy, which was then combined with information from MoBa and the NPD to identify and exclude women with hypertension before the indexed pregnancy. Gestational hypertension was defined as a diagnosis of hypertension after 20 wk of gestation (systolic BP ≥140 mm Hg, a diastolic BP ≥90 mm Hg, or both). Preeclampsia diagnoses included gestational hypertension with the additional requirement of proteinuria (≥0.3 g in 24-h urine collection or ≥1-point increase on a urinary dipstick) (28).
Statistical analyses.
Demographic and dietary characteristics of study participants were evaluated by approximate quartile groupings of calcium intake. Dose-response relationships between calcium intake and hypertension were explored using cubic spline functions with 5 knots (29); the reference value selected for the spline reflected the dietary adequacy intake cutoff of 1000 mg/d for pregnant women (30). The cubic spline analyses adjusted for maternal age and total energy intake, and included all study participants. Because the spline analyses identified a nonlinear relation between calcium intake and subsequent hypertension, further modeling used quartile groups of calcium intake in Cox proportional hazard analyses where we stratified by the presence or absence of gestational hypertension (i.e., with or without proteinuria). The proportional hazards assumption was evaluated with the Schoenfeld test. Dietary calcium intake quartiles were evaluated separately in several Cox proportional hazard models. Model 1 adjusted for 2 covariates: maternal age (years) and energy intake (kilocalories per day); model 2 adjusted for model 1 covariates plus the sodium-to-potassium dietary intake ratio. Model 3 adjusted for 7 covariates: the 3 covariates from models 1 and 2, leisure-time physical activity before pregnancy (<3 or ≥3 times/wk, or missing), prepregnancy BMI, smoking before pregnancy (none, occasional, or daily), and maternal education (primary, secondary or vocational, or any college or university). An additional model (model 4) adjusted for model 3 covariates and magnesium supplements (none, <75 mg/d, ≥75 mg/d), and dietary magnesium (per 100 mg/d units).
Additional multivariable models evaluated the following variables: alcohol intake frequency before pregnancy (none or rare, monthly, or weekly), oral contraceptive use (none, <4 y, or ≥4 y), multiple-birth pregnancy, stillbirth delivery, preterm delivery, parity (0, 1, or ≥2), pregestational or gestational diabetes mellitus, total vitamin D intake, long-chain n–3 FA supplement intake, weight gain (≥7 compared with <7 kg), and breastfeeding at 6 mo. Because these results did not alter the HRs associated with the calcium and magnesium parameters reported, they were not included in the models presented.
Stata 14 (StataCorp LP) was used for all analyses. Statistical significance was determined at P < 0.05.
Additional analyses.
We evaluated the consistency of the calcium intake quartile results in analyses stratified by a low and high sodium-to-potassium intake ratio using the median cutoff value of the ratio (<0.76 compared with ≥0.76) in order to define the 2 groups. The sodium/potassium ratio was not highly related to dietary calcium (r = −0.32) or magnesium (r = −0.30), but dietary calcium and magnesium were correlated (r = 0.73). Further, magnesium residuals were even more highly correlated with calcium than the absolute values, given that residuals were smaller at the lower end of the calcium intake range than at the higher end. Therefore, we used observed magnesium intake rather than magnesium residuals in the final model of the multivariable analyses. Supplementary analyses evaluated midpregnancy magnesium quartiles and subsequent risk of hypertension and are presented here.
Results
Participating women had a mean ± SD age of 30.5 ± 4.6 y and a BMI of 24.0 ± 4.3 before pregnancy. The mean duration of follow-up of was 7.1 ± 1.6 y, with a maximum follow-up of 10 y. A total of 97% of women were married or lived with a partner, 43% were nulliparous, and 28% had a higher education (any college or university). Before pregnancy, 16.5% smoked daily and 48% engaged in leisure-time physical activity ≥3 times/wk. The majority of demographic and pregnancy-related characteristics showed little variability across the quartiles of calcium intake (Table 1). However, energy intake, physical activity, and having a higher education increased, whereas the sodium-to-potassium ratio and prepregnancy BMI decreased, with increasing quartiles of calcium intake (P < 0.001). The mean ± SD percentage of energy from the macronutrients was stable across intake quartiles and was within acceptable ranges: protein, 15.4% ± 2.1%; fat, 31.4% ± 4.5%; and carbohydrates, 53.2% ± 4.7%. The percentage reporting any intake of calcium from a supplement was slightly but significantly inversely related to quartiles of dietary calcium intake (P < 0.001; given the large sample size).
TABLE 1.
Baseline demographic characteristics by dietary calcium quartiles in the Norwegian Mother and Child Cohort Study (n = 60,027)1
| Calcium quartiles |
|||||
| FFQ, mg/d | Q1 (≤738 mg/d)(n = 14,064) | Q2 (739–966 mg/d)(n = 15,029) | Q3 (967–1253 mg/d)(n = 15,598) | Q4 (≥1254 mg/d)(n = 15,336) | P-trend |
| Maternal age, y | 30.4 ± 4.7 | 30.6 ± 4.5 | 30.7 ± 4.5 | 30.3 ± 4.7 | |
| BMI,2 kg/m2 | 24.4 ± 4.5 | 24.0 ± 4.2 | 23.8 ± 4.1 | 23.9 ± 4.3 | * |
| Nulliparous | 43.8 | 43.7 | 42.2 | 43.7 | |
| Daily smoking2 | 17.1 | 15.8 | 15.1 | 18.1 | |
| Married or with partner | 96.7 | 97.4 | 97.1 | 96.6 | |
| Diabetes mellitus3 | 1.7 | 1.5 | 1.4 | 1.6 | |
| Preterm4 | 5.6 | 5.6 | 5.6 | 5.5 | |
| Gestational hypertension5 | 2.1 | 1.9 | 2.1 | 2.1 | |
| Preeclampsia6 | 3.9 | 3.5 | 3.4 | 3.5 | |
| Physical activity ≥3 times/wk2 | 42.3 | 47.3 | 50.6 | 51.0 | * |
| High education7 | 24.9 | 29.5 | 30.2 | 27.3 | * |
| Years of follow-up | 7.1 ± 1.7 | 7.1 ± 1.6 | 7.1 ± 1.6 | 7.1 ± 1.6 | |
| Calcium,8 mg/d | 588 ± 107 | 854 ± 65 | 1097 ± 82 | 1606 ± 356 | |
| Magnesium,8 mg/d | 302 ± 70 | 364 ± 71 | 420 ± 80 | 517 ± 106 | * |
| Sodium-to-potassium ratio8 | 0.85 ± 0.20 | 0.80 ± 0.17 | 0.76 ± 0.16 | 0.70 ± 0.15 | * |
| Energy intake, kcal/d | 1804 ± 389 | 2099 ± 404 | 2378 ± 440 | 2843 ± 562 | * |
| Ca supplements | 17 | 14 | 13 | 12 | * |
| Mg supplements | 25 | 24 | 25 | 25 | |
| Weight gain ≥7 kg | 9 | 9 | 10 | 12 | * |
Values are means ± SDs or percentages. *P-trend < 0.001, by increasing calcium quartiles (1–4) as a continuous variable. Q, quartile.
Before pregnancy.
Pregestational, gestational, or unspecified.
Gestational age <37 wk or, when gestational age was missing, birth weight <2500 g.
Diagnosed after 20 wk of gestation, when systolic blood pressure was ≥140 mm Hg or diastolic blood pressure was ≥90 mm Hg, or both.
Gestational hypertension with proteinuria (≥0.3 g in 24-h urine collection or ≥1-point increase on a urinary dipstick).
Any college or university education.
Intake from dietary sources only (excludes supplements) as estimated by a semiquantitative FFQ.
Of the women who were normotensive during pregnancy (n = 56,646), 1039 (1.8%) developed hypertension after pregnancy. A total of 3381 women had gestational hypertension in a MoBa pregnancy of whom 1235 had gestational hypertension without proteinuria, 1642 had preeclampsia at term, and 504 had preterm preeclampsia. Of the women who had any gestational hypertension (n = 3381), 441 (13.0%) developed hypertension after pregnancy [maternal age-adjusted HR: 8.1 (95% CI: 7.27, 9.09)]. No significant differences were found between gestational hypertension without proteinuria [HR: 8.93 (95% CI: 7.61, 10.49)] and eclampsia or preeclampsia [HR: 7.67 (95% CI: 6.69, 8.80)] with regard to the magnitude of the HRs for subsequent hypertension relative to women who were normotensive during pregnancy.
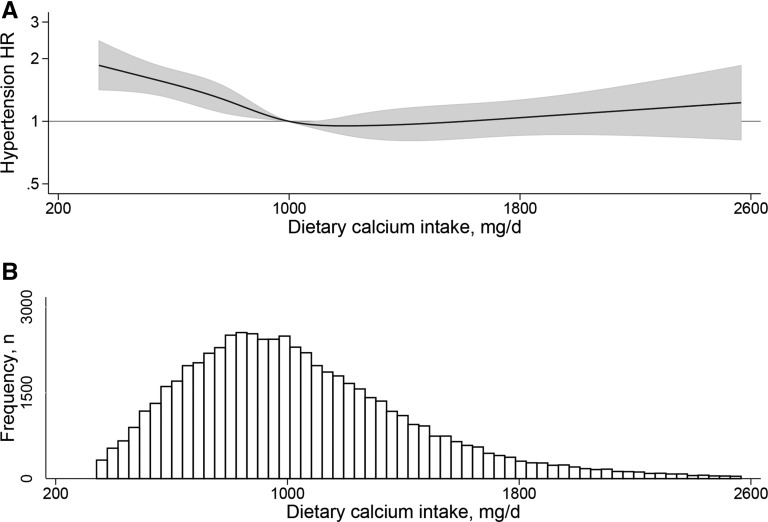
The cubic spline graph for dietary calcium (adjusted for maternal age and energy intake) indicated a threshold effect, whereby low intakes (<1000 mg/d) were associated with excess risk for hypertension (Figure 1).
FIGURE 1.
Cubic spline graph of dietary calcium intake (milligrams per day) during midpregnancy and associated HRs (line) and 95% CIs (shaded region) for the development of hypertension after pregnancy (A), and the distribution of dietary calcium intake (milligrams per day) (B) among participants (n = 60,027) in the Norwegian Mother and Child Cohort Study.
In the evaluation of women who were normotensive during their MoBa pregnancy, those in the lowest quartile of dietary calcium intake had a 34% greater risk of hypertension after pregnancy than women in the highest quartile of calcium intake, adjusting for the 7 covariates of model 3 [HR: 1.34 (95% CI: 1.05, 1.70)] (Table 2). Among women with a pregnancy affected by gestational hypertension, those in the lowest quartile of calcium intake had a 64% greater risk of hypertension than women in the highest intake quartile, adjusting for the 7 model 3 covariates [HR: 1.64 (95% CI: 1.14, 2.35)]. The marked increased risk of subsequent hypertension associated with the lowest quartile of dietary calcium intake remained significant after further adjustment for dietary and supplemental magnesium among women who had gestational hypertension during their MoBa pregnancy. All other parameters evaluated had no effect on the significance or magnitude of the HRs presented (≤3% change in HRs).
TABLE 2.
Midpregnancy dietary calcium intake and HRs for development of hypertension after pregnancy during the Norwegian Mother and Child Cohort Study1
| Dietary calcium quartiles | Participants, N | Cases, n2 | Model 13 | Model 24 | Model 35 | Model 46 |
| Normotensive during pregnancy | 56,646 | 1039 | ||||
| Q1 (≤738 mg/d) | 13,223 | 292 | 1.45 (1.16, 1.80) | 1.31 (1.04, 1.65) | 1.34 (1.05, 1.70) | 1.26 (0.99, 1.61) |
| Q2 (739–966 mg/d) | 14,211 | 259 | 1.13 (0.99, 1.38) | 1.06 (0.86, 1.30) | 1.14 (0.92, 1.41) | 1.09 (0.88, 1.35) |
| Q3 (967–1253 mg/d) | 14,745 | 233 | 0.94 (0.78, 1.14) | 0.91 (0.75, 1.10) | 0.98 (0.80, 1.20) | 0.95 (0.78, 1.17) |
| Q4 (≥1254 mg/d) | 14,467 | 255 | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) |
| Gestational hypertension7 | 3381 | 441 | ||||
| Q1 (≤738 mg/d) | 841 | 133 | 1.88 (1.34, 2.64) | 1.79 (1.25, 2.56) | 1.64 (1.14, 2.35) | 1.66 (1.14, 2.42) |
| Q2 (739–966 mg/d) | 818 | 99 | 1.27 (0.92, 1.75) | 1.23 (0.89, 1.70) | 1.25 (0.90, 1.75) | 1.26 (0.90, 1.78) |
| Q3 (967–1253 mg/d) | 853 | 109 | 1.24 (0.93, 1.65) | 1.21 (0.90, 1.62) | 1.20 (0.88, 1.63) | 1.20 (0.88, 1.64) |
| Q4 (≥1254 mg/d) | 869 | 100 | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) |
Values are HRs (95% CIs), unless otherwise indicated. Q, quartile.
Use of antihypertensive medications >3 mo after pregnancy, where hypertension was listed as the indication for treatment.
Adjusted for maternal age (years) and energy intake (kilocalories per day).
Adjusted for model 1 covariates plus sodium-to-potassium ratio.
Adjusted for model 1 and 2 covariates plus prepregnancy smoking status (none, occasional, or daily), prepregnancy physical activity (<3 or ≥3 times/wk, or missing), prepregnancy BMI (kg/m2), and maternal education (primary, secondary or vocational, or any college or university).
Adjusted for all covariates from models 1–3 plus magnesium supplements (none, low, or high) and magnesium (per 100-mg units).
Gestational hypertension with or without proteinuria.
Calcium and magnesium supplements.
Of the 13.7% (n = 8246) who reported taking a supplement containing any amount of calcium, the median supplemental calcium intake was 229 mg/d (IQR: 143–457 mg/d). Intake of calcium supplements evaluated as a tertile dose or as any amount of supplemental calcium intake compared with none was not associated with risk of hypertension (Supplemental Table 1). Further, given the small percentage who used calcium supplements, the results of the total calcium intake quartiles (diet and supplement sources combined) was very similar to the results of the dietary calcium quartiles presented in Table 2, and are therefore not presented separately.
Supplements containing magnesium were taken by 25% of the study population, but only 9% of the women took supplements that provided ≥75 mg Mg/d.
Magnesium supplement intake ≥75 mg/d was protective of subsequent hypertension among women with a history of gestational hypertension, whereas quartiles of dietary magnesium intake were inversely related to subsequent risk of hypertension among women who were normotensive during their MoBa pregnancy (Supplemental Tables 1 and 2).
Stratification by low and high sodium-to-potassium ratio.
In the analyses stratified by a low and high sodium-to-potassium ratio and adjusting for maternal age and energy intake, the lowest calcium quartile was associated with significantly elevated HRs, compared with the highest quartile, when the sodium-to-potassium ratio was high, but not when the ratio was low (Table 3). For women who were normotensive during their MoBa pregnancy, the age- and energy-adjusted HR for the lowest calcium intake quartile relative to the highest quartile was 1.57 (95% CI: 1.14, 2.17) in the group with a high sodium-to-potassium ratio and 1.19 (95% CI: 0.85, 1.67) in the group with a low sodium-to-potassium ratio. For women with gestational hypertension during pregnancy, the age- and energy-adjusted HR for the lowest calcium intake quartile relative to the highest was 2.15 (95% CI: 1.29, 3.59) in the group with a high sodium-to-potassium ratio and 1.52 (95% CI: 0.93, 2.47) in the group with a low sodium-to-potassium ratio. In analyses adjusting for all covariates, however, the lowest calcium quartile was significantly predictive of hypertension risk only among women with gestational hypertension in the group with a high sodium-to-potassium ratio [HR: 1.92 (95% CI: 1.09, 3.39)] (Table 3). In subsequent sensitivity analyses, similar HRs were obtained when separately evaluating women with preeclampsia and women with gestational hypertension without proteinuria (data not shown).
TABLE 3.
Midpregnancy dietary calcium intake by dietary sodium-to-potassium ratio and HRs (95% CIs) for the development of hypertension after pregnancy in the Norwegian Mother and Child Cohort Study1
| Midpregnancy calcium intake in Q1 vs. in Q4, by sodium-to-potassium ratio |
||
| Low ratio (<0.76) | High ratio (≥0.76) | |
| Normotensive (n = 56,646) | ||
| Cases,2 n | 475 | 564 |
| Q1 (cases/total) | 88/4352 | 204/8871 |
| Q4 (cases/total) | 168/9828 | 87/4639 |
| Model 13 | 1.19 (0.85, 1.67) | 1.57 (1.14, 2.17) |
| Model 34 | 1.34 (0.95, 1.88) | 1.43 (1.03, 2.00) |
| Model 45 | 1.31 (0.93, 1.87) | 1.24 (0.87, 1.78) |
| Gestational hypertension (n = 3381)6 | ||
| Cases,2 n | 205 | 236 |
| Q1 (cases/total) | 43/288 | 90/553 |
| Q4 (cases/total) | 69/604 | 31/265 |
| Model 13 | 1.52 (0.93, 2.47) | 2.15 (1.29, 3.59) |
| Model 34 | 1.53 (0.93, 2.53) | 1.70 (1.01, 2.86) |
| Model 45 | 1.52 (0.90, 2.57) | 1.92 (1.09, 3.39) |
Values are HRs (95% CIs) unless otherwise indicated. Q, quartile.
Use of antihypertensive medications >3 mo after pregnancy, where hypertension was listed as the indication for treatment.
Adjusted for maternal age (years) and energy intake (kilocalories per day).
Adjusted for model 1 covariates plus prepregnancy smoking status (none, occasional, or daily), prepregnancy physical activity (<3 or ≥3 times/wk, or missing), prepregnancy BMI (kg/m2), and maternal education (primary, secondary or vocational, or any college or university).
Adjusted for model 1 and 3 covariates plus sodium-to-potassium ratio, magnesium supplements (none, low, or high), and magnesium (per 100-mg units).
Gestational hypertension with or without proteinuria.
Discussion
These results provide some evidence of the importance of low dietary calcium intake in the pathogenesis of hypertension in women after pregnancy, particularly for women who had a history of gestational hypertension. Interestingly, Figure 1 is very similar to a figure presented in meta-analyses of the association of dietary calcium intake with stroke in prospective studies, where only inadequate dietary intakes were related to elevated risk (31). Given that hypertension is a major and modifiable risk factor for stroke, the similarities in the graphs are noteworthy. One possible explanation of the results is that low calcium intake might predispose to salt-sensitive hypertension (10, 11, 32, 33). In our study, low calcium intake had a more pronounced effect on the risk of hypertension in women with gestational hypertension when their sodium-to-potassium intake ratio was high than when it was low. Salt sensitivity is the inability to excrete a sodium load without increasing BP (34), and may be present in ~50% of hypertensive Caucasian patients (35). Two studies have shown that women with previous gestational hypertension have greater salt sensitivity than women with normotensive pregnancies (36, 37). Further, the nighttime dip in BP that is normal in healthy individuals is blunted in women with a history of preeclampsia who consume a high-salt diet (37), which is also the case in salt-sensitive hypertensive patients who consume a high-salt diet (38). Nighttime increases in BP may reflect a compensatory mechanism to achieve sodium balance. Subtle injury to the kidneys was hypothesized to increase susceptibility to salt-sensitive hypertension (39). The increased risk of hypertension in women who have had a hypertensive pregnancy (5) could potentially be explained by greater salt sensitivity as a result of subtle kidney injury; however, studies of kidney function after preeclamptic pregnancies have shown that glomerular filtration rate seems to return to normal in the early postpartum period (40), and long-term follow-up studies of microalbuminuria have provided mixed results (41, 42), possibly a reflection of differences in the extent of comorbidities, characteristics before pregnancy, severity of preeclampsia, and ethnicity. However, one study showed a greater glomerular filtration fraction response to a high sodium intake relative to a low sodium intake over 7 d in women with a previous preeclamptic pregnancy compared with women with a normal pregnancy (43), suggesting a higher glomerular capillary pressure, a marker for future renal damage over the long term (44).
In the Nurses’ Health Studies I and II, women in the highest 3 quintiles of total and dietary calcium intakes had significantly lower risk of total and ischemic stroke than did women in the lowest quintiles of total and dietary calcium intakes, which represented inadequate intakes in age-adjusted analyses (45). However, the findings were no longer significant in multivariate analyses that adjusted for a history of hypertension along with numerous other covariates. By contrast, in our study, low calcium intake persisted as being associated with increased risk of hypertension among women who had gestational hypertension in the full multivariable analyses.
Our study findings are also consistent with the known inverse association of magnesium with risk of hypertension (16, 30). We found that the highest 2 quartiles of dietary magnesium intake were associated with lower subsequent risk of hypertension for women with normotensive MoBa pregnancies, and that supplemental magnesium intake was associated with lower risk for women with gestational hypertension. Further work is required to identify threshold levels of the effect for hypertension associated with dietary and supplemental magnesium intakes.
Limitations.
The low prevalence of calcium supplement use (13%) limited our statistical power to evaluate a putative antihypertensive effect in the study population. Also, the sodium-to-potassium intake ratio likely underestimated the true ratio, given the known difficulties in documenting all sodium intake in observational studies. Despite this limitation, the reported ratio correlated positively and significantly with risk of hypertension, as expected. Another limitation is the lack of information on gestational hypertension in pregnancies before or after the indexed MoBa pregnancy, as a result of limitations imposed by consent forms and research ethics. While some normotensive women may have had gestational hypertension before or after their MoBa pregnancy, this limitation would not bias the HRs, given the large size of the normotensive group. We had no information on changes in risk factors over time. Another limitation is the lack of more detailed, clinically relevant information and biomarkers that could help characterize underlying pathologies. Given the homogenous study population, the results may not be generalizable to ethnically diverse populations. Finally, our findings regarding threshold effects should be interpreted with caution, given that the absolute intake of nutrients are difficult to assess based on FFQs and because we cannot rule out that the observed associations may be explained by other dietary factors or by calcium intake in combination with other dietary factors.
Strengths of the study include the prospective design, the ability to control for a wide range of sociodemographic and pregnancy-related determinants of hypertension, and the relatively homogeneous study population with uniform access to health care through a national health care system. Finally, a key strength of this study is the evaluation of the importance of calcium intake within the context of sodium, potassium, and magnesium intakes and women’s reproductive histories.
Summary.
Our results suggest that low calcium intake may be a risk factor, or at least a risk marker, for hypertension, particularly for women with a history of gestational hypertension. Further, our observed association between calcium intake and treated hypertension is compatible with the observed threshold effect for calcium as it relates to stroke (31) and BP (46). Interventions designed to achieve adequate dietary intake of minerals important in regulating BP could be an effective primary prevention approach at the population level. Also, because previous research indicates that BP is elevated before pregnancy among women who develop gestational hypertension (47), routine BP monitoring of women of reproductive age may identify at-risk women who require dietary or pharmacological interventions, or both. Given the long-term cardiovascular sequelae of gestational hypertension, further research exploring the nutritional determinants of hypertension in women with a history of gestational hypertension is important.
Acknowledgments
The authors’ responsibilities were as follows—GME and MH: designed the research; MH: helped design the cohort study and the original research on nutrient intakes; GME: performed the statistical analyses and wrote the manuscript; S Skurtveit and S Sakshaug: analyzed the NPD to determine the outcomes of hypertension; S Skurtveit, S Sakshaug, AKD, BEV, and MH: provided critical feedback on the manuscript; and all authors: read and approved the final manuscript.
Footnotes
Abbreviations used: BP, blood pressure; MBRN, Medical Birth Registry of Norway; MoBa, Norwegian Mother and Child Cohort Study; NPD, Norwegian Prescription Database.
References
- 1.Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ. Selected major risk factors and global and regional burden of disease. Lancet 2002;360:1347–60. [DOI] [PubMed] [Google Scholar]
- 2.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet 2005;365:217–23. [DOI] [PubMed] [Google Scholar]
- 3.Skjærven R, Wilcox AJ, Klungsøyr K, Irgens LM, Vikse BE, Vatten LJ, Lie RT. Cardiovascular mortality after pre-eclampsia in one child mothers: prospective, population based cohort study. BMJ 2012;345:e7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith GC, Pell JP, Walsh D. Pregnancy complications and maternal risk of ischaemic heart disease: a retrospective cohort study of 129,290 births. Lancet 2001;357:2002–6. [DOI] [PubMed] [Google Scholar]
- 5.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ 2007;335:974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vikse BE, Irgens LM, Leivestad T, Skjaerven R, Iversen BM. Preeclampsia and the risk of end-stage renal disease. N Engl J Med 2008;359:800–9. [DOI] [PubMed] [Google Scholar]
- 7.Chan Q, Stamler J, Griep LMO, Daviglus M, Van Horn L, Elliott P. An update on nutrients and blood pressure: summary of INTERMAP study findings. J Atheroscler Thromb 2016;23:276–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Mierlo LA, Arends LR, Streppel MT, Zeegers MP, Kok FJ, Grobbee DE, Geleijnse JM. Blood pressure response to calcium supplementation: a meta-analysis of randomized controlled trials. J Hum Hypertens 2006;20:571–80. [DOI] [PubMed] [Google Scholar]
- 9.Hsia J, Heiss G, Ren H, Allison M, Dolan NC, Greenland P, Heckbert SR, Johnson KC, Manson JE, Sidney S, et al. Calcium/vitamin D supplementation and cardiovascular events. Circulation 2007;115:846–54. [DOI] [PubMed] [Google Scholar]
- 10.Sowers JR, Zemel MB, Zemel PC, Standley PR. Calcium metabolism and dietary calcium in salt sensitive hypertension. Am J Hypertens 1991;4:557–63. [DOI] [PubMed] [Google Scholar]
- 11.Resnick LM. The role of dietary calcium in hypertension: a hierarchical overview. Am J Hypertens 1999;12:99–112. [DOI] [PubMed] [Google Scholar]
- 12.Witteman JC, Willett WC, Stampfer MJ, Colditz GA, Sacks FM, Speizer FE, Rosner B, Hennekens CH. A prospective study of nutritional factors and hypertension among US women. Circulation 1989;80:1320–7. [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Manson JE, Buring JE, Lee IM, Sesso HD. Dietary intake of dairy products, calcium, and vitamin D and the risk of hypertension in middle-aged and older women. Hypertension 2008;51:1073–9. [DOI] [PubMed] [Google Scholar]
- 14.Resnick LM, Laragh JH, Sealey JE, Alderman MH. Divalent cations in essential hypertension. Relations between serum ionized calcium, magnesium, and plasma renin activity. N Engl J Med 1983;309:888–91. [DOI] [PubMed] [Google Scholar]
- 15.Centeno V, Díaz de Barboza G, Marchionatti A, Rodríguez V, Tolosa de Talamoni N. Molecular mechanisms triggered by low-calcium diets. Nutr Res Rev 2009;22:163–74. [DOI] [PubMed] [Google Scholar]
- 16.Sontia B, Touyz RM. Role of magnesium in hypertension. Arch Biochem Biophys 2007;458:33–9. [DOI] [PubMed] [Google Scholar]
- 17.Magnus P, Birke C, Vejrup K, Haugan A, Alsaker E, Daltveit AK, Handal M, Haugen M, Høiseth G, Knudsen GP, et al. Cohort profile update: the Norwegian Mother and Child Cohort study (MoBa). Int J Epidemiol 2016;45:382–8. [DOI] [PubMed] [Google Scholar]
- 18.Irgens LM. The Medical Birth Registry of Norway. Epidemiological research and surveillance throughout 30 years. Acta Obstet Gynecol Scand 2000;79:435–9. [PubMed] [Google Scholar]
- 19.Furu K. Establishment of the nationwide Norwegian Prescription Database (NorPD) – new opportunities for research in pharmacoepidemiology in Norway. Nor J Epidemiol 2008;18:129–36. [Google Scholar]
- 20.Meltzer HM, Brantsæter AL, Ydersbond TA, Alexander J, Haugen M. Methodological challenges when monitoring the diet of pregnant women in a large study; experiences from the Norwegian Mother and Child Cohort Study (MoBa). Matern Child Nutr 2008;4:14–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brantsæter AL, Haugen M, Alexander J, Meltzer HM. Validity of a new food frequency questionnaire for pregnant women in the Norwegian Mother and Child Cohort Study (MoBa). Matern Child Nutr 2008;4:28–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brantsaeter AL, Haugen M, Julshamn K, Alexander J, Meltzer HM. Evaluation of urinary iodine excretion as a biomarker for intake of milk and dairy products in pregnant women in the Norwegian Mother and Child Cohort Study (MoBa). Eur J Clin Nutr 2009;63:347–54. [DOI] [PubMed] [Google Scholar]
- 23.Brantsaeter AL, Haugen M, Hagve TA, Aksnes L, Rasmussen SE, Julshamn K, Alexander J, Meltzer HM. Self-reported dietary supplement use is confirmed by biological markers in the Norwegian Mother and Child Cohort Study (MoBa). Ann Nutr Metab 2007;51:146–54. [DOI] [PubMed] [Google Scholar]
- 24.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure. Hypertension 2003;42:1206–52. [DOI] [PubMed] [Google Scholar]
- 25.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, et al. 2013 ESH/ESC practice guidelines for the management of arterial hypertension. Blood Press 2014;23:3–16. [DOI] [PubMed] [Google Scholar]
- 26.WHO Collaborating Centre for Drug Statistics Methodology [Internet]. [updated 2016 Dec 19; cited 2017 Jul 5]. Available from: https://www.whocc.no/atc_ddd_index/.
- 27.Brantsaeter AL, Owe KM, Haugen M, Alexander J, Meltzer HM, Longnecker MP. Validation of self-reported recreational exercise in pregnant women in the Norwegian Mother and Child Cohort Study. Scand J Med Sci Sports 2010;20:e48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klungsøyr K, Harmon QE, Skard LB, Simonsen I, Austvoll ET, Alsaker ER, Starling A, Trogstad L, Magnus P, Engel SM. Validity of pre-eclampsia registration in the medical birth registry of Norway for women participating in the Norwegian Mother and Child Cohort Study, 1999–2010. Paediatr Perinat Epidemiol 2014;28:362–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med 2010;29:1037–57. [DOI] [PubMed] [Google Scholar]
- 30.Institute of Medicine. Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington (DC): National Academies Press; 1997. [PubMed] [Google Scholar]
- 31.Larsson SC, Orsini N, Wolk A. Dietary calcium intake and risk of stroke: a dose-response meta-analysis. Am J Clin Nutr 2013;97:951–7. [DOI] [PubMed] [Google Scholar]
- 32.Hamet P. The evaluation of the scientific evidence for a relationship between calcium and hypertension. J Nutr 1995;125(2 Suppl):311S–400S. [DOI] [PubMed] [Google Scholar]
- 33.Zemel MB, Gualdoni SM, Sowers JR. Reductions in total and extracellular water associated with calcium-induced natriuresis and the antihypertensive effect of calcium in blacks. Am J Hypertens 1988;1:70–2. [DOI] [PubMed] [Google Scholar]
- 34.Elijovich F, Weinberger MH, Anderson CAM, Appel LJ, Bursztyn M, Cook NR, Dart RA, Newton-Cheh CH, Sacks FM, Laffer CL; on behalf of the American Heart Association Professional and Public Education Committee of the Council on Hypertension, Council on Functional Genomics and Translational Biology, and Stroke Council. Salt sensitivity of blood pressure: a scientific statement from the American Heart Association. Hypertension 2016;68:e7–46. [DOI] [PubMed] [Google Scholar]
- 35.Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension 1996;27:481–90. [DOI] [PubMed] [Google Scholar]
- 36.Saxena AR, Karumanchi A, Brown NJ, Royle CM, McElrath TF, Seely EW. Increased sensitivity to antiotensin II is present portpartum in women with a history of hypertensive pregnancy. Hypertension 2010;55:1239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martillotti G, Ditisheim A, Burnier M, Wagner G, Boulvain M, Irion O, Pechère-Bertschi A. Increased salt sensitivity of ambulatory blood pressure in women with a history of severe preeclampsia. Hypertension 2013;62:802–8. [DOI] [PubMed] [Google Scholar]
- 38.Uzu T, Kimura G, Yamauchi A, Kanasaki M, Isshiki K, Araki S, Sugiomoto T, Nishio Y, Maegawa H, Koya D, et al. Enhanced sodium sensitivity and disturbed circadian rhythm of blood pressure in essential hypertension. J Hypertens 2006;24:1627–32. [DOI] [PubMed] [Google Scholar]
- 39.Johnson RJ, Herrera-Acosta J, Schreiner GF, Rodríguez-Iturbe B. Subtle acquired renal injury as a mechanism of salt-sensitive hypertension. N Engl J Med 2002;346:913–23. [DOI] [PubMed] [Google Scholar]
- 40.Stillman IE, Karumanchi SA. The glomerular injury of preeclampsia. J Am Soc Nephrol 2007;18:2281–4. [DOI] [PubMed] [Google Scholar]
- 41.McDonald SD, Han Z, Walsh MW, Gerstein HC, Devereaux PJ. Kidney disease after preeclampsia: a systematic review and meta-analysis. Am J Kidney Dis 2010;55:1026–39. [DOI] [PubMed] [Google Scholar]
- 42.Sandvik MK, Hallan S, Svarstad E, Vikse BE. Preeclampsia and prevalence of microalbuminuria 10 years later. Clin J Am Soc Nephrol 2013;8:1126–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toering TJ, van der Graaf AM, Visser FW, Groen H, Faas MM, Navis G, Lely AT. Higher filtration fraction in formerly early-onset preeclamptic women without comorbidity. Am J Physiol Renal Physiol 2015;308:F824–31. [DOI] [PubMed] [Google Scholar]
- 44.Helal I, Fick-Brosnahan GM, Reed-Gitomer B, Schrier RW. Glomerular hyperfiltration: definitions, mechanisms and clinical implications. Nat Rev Nephrol 2012;8:293–300. [DOI] [PubMed] [Google Scholar]
- 45.Adebamowo SN, Spiegelman D, Willett WC, Rexrode KM. Association between intakes of magnesium, potassium, and calcium and risk of stroke: 2 cohorts of US women and updated meta-analyses. Am J Clin Nutr 2015;101:1269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gruchow HW, Sobocinski KA, Barboriak JJ. Threshold effects of dietary calcium on blood pressure. J Hypertens 1986;4 Suppl 5:S355–7. [Google Scholar]
- 47.Egeland GM, Klungsøyr K, Øyen N, Tell GS, Næss Ø, Skjærven R. Preconception cardiovascular risk factor differences between gestational hypertension and preeclampsia: Cohort Norway Study. Hypertension 2016;67:1173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]