Abstract
Background: Inconsistent evidence describes the association between dietary intake of dairy and milk-based products and type 2 diabetes (T2D) risk.
Objective: Our objective was to assess associations between consumption of milk-based products, incident prediabetes, and progression to T2D in the Framingham Heart Study Offspring Cohort.
Methods: Total dairy and milk-based product consumption was assessed by ≤4 food-frequency questionnaires across a mean of 12 y of follow-up in 2809 participants [mean ± SD age: 54.0 ± 9.7 y; body mass index (in kg/m2): 27.1 ± 4.7; 54% female]. Prediabetes was defined as the first occurrence of fasting plasma glucose ≥5.6 to <7.0 mmol/L (≥100 to <126 mg/dL), and T2D was defined as the first occurrence of fasting plasma glucose ≥7.0 mmol/L (≥126 mg/dL) or diabetes treatment. Proportional hazards models were used to estimate the risk of incident outcomes relative to dairy product intake in subsets of the cohort who were at risk of developing the outcomes. Spline regressions were used to examine potential nonlinear relations.
Results: Of 1867 participants free of prediabetes at baseline, 902 (48%) developed prediabetes. Total, low-fat, and high-fat dairy consumptions were associated with a 39%, 32%, and 25% lower risk of incident prediabetes, respectively, in the highest compared with the lowest intakes (≥14 compared with <4 servings/wk). Total, low-fat and skim milk, whole-milk, and yogurt intakes were associated nonlinearly with incident prediabetes; moderate intake was associated with the greatest relative risk reduction. Neither cheese nor cream and butter was associated with prediabetes. Of 925 participants with prediabetes at baseline, 196 (21%) developed T2D. Only high-fat dairy and cheese showed evidence of dose-response, inverse associations with incident T2D, with 70% and 63% lower risk, respectively, of incident T2D between the highest and lowest intake categories (≥14 compared with <1 serving/wk for high-fat dairy, ≥4 compared with <1 serving/wk for cheese).
Conclusion: Associations of dairy with incident prediabetes or diabetes varied both by dairy product and type and by baseline glycemic status in this middle-aged US population. Baseline glycemic status may partially underlie prior equivocal evidence regarding the role of dairy intake in diabetes.
Keywords: dairy, cheese, yogurt, prediabetes, diabetes
Introduction
Diabetes affects an estimated 1 in 11 people worldwide (1). In the United States in 2012, an estimated 29.1 million adults had diabetes, whereas prediabetes affected an estimated 86 million—over one-third of US adults (2). Diet modification is recommended as an important prevention strategy at any stage of progression from health to overt type 2 diabetes (T2D) (3).
US dietary guidelines have consistently recommended 2–3 servings dairy/d for adolescents and adults (4, 5). Many individuals may perceive dairy products as health foods, particularly reduced-fat products such as skim milk and low-fat yogurt. For some, certain dairy products may be an important source of protein and several shortfall nutrients (nutrients that may be underconsumed relative to the Dietary Reference Intakes), including calcium, magnesium, potassium, and vitamin D (6, 7). They may also be a source of potentially harmful saturated fats, although some research indicates that the effects of saturated fats, including those from dairy, are not universally harmful (8–11).
Existing observational evidence suggests a generally small, equivocal, or U-shaped relation between total dairy or specific dairy product consumption and the risk of T2D (11–14) and related risk factors (15–19). However, few prospective studies to our knowledge have examined dairy in relation to incident impaired glucose or hyperglycemic stages preceding T2D. A 2011 prospective study of metabolic syndrome in a French population observed that total dairy (except cheese) was associated with lower odds of hyperglycemia [defined as impaired fasting glucose (IFG) or T2D] over a 9-y follow-up, although its relation with T2D alone was not significant, and cheese was not associated with either hyperglycemia and T2D or T2D alone (17). Two recent cross-sectional studies reported inverse associations between total and fermented dairy intake and glucose traits, including T2D, although relations of other dairy products varied between the 2 studies and outcomes (13, 20). Thus questions remain regarding the point along the etiologic pathway, if any, at which dairy or specific dairy products may exert a potentially protective or deleterious role. In addition, given the high and largely unexplained heterogeneity of dairy-T2D associations across prior studies (12), glycemic status may be a potential source of the observed differences.
Therefore, the primary aim of this study was to investigate the relations between consumption of dairy and milk-based products and the long-term risk of prediabetes among initially healthy individuals, and the risk of T2D among individuals with prediabetes in a cohort of middle-aged adults, the Framingham Heart Study Offspring Cohort. As a secondary aim, we examined the effect modification of dairy intake and risk of T2D by baseline glycemic status in the combined study population. We hypothesize that associations with incident outcomes differ by dairy product and type, and that these associations may further differ in initially healthy compared with unhealthy participants.
Methods
Study participants.
The National Heart, Lung, and Blood Institute’s Framingham Heart Study Offspring Cohort is a community-based longitudinal study of cardiovascular disease that began in 1971 (21). In the fifth examination cycle (1991–1995) of the Offspring Cohort, 3799 participants underwent a standard medical examination consisting of laboratory and anthropometric as well as dietary intake assessments. In the study described here, participants were followed from the fifth exam (baseline) through the eighth exam (2005–2008). Individuals were excluded from this analysis if they had a history of diabetes or were identified as having diabetes at the baseline examination [fasting plasma glucose (FG) ≥7.0 mmol/L (≥126 mg/dL), or glucose ≥11.1 mmol/L (≥200 mg/dL) after a 2-h oral-glucose-tolerance test (OGTT), or use of an oral hypoglycemic drug or insulin; n = 375]; were missing blood glucose data (n = 101); had invalid dietary data at baseline (n = 318); were missing necessary covariates (n = 26); or had no follow-up data on diabetes status (n = 170). The final sample included 2809 participants (Supplemental Figure 1).
The original data collection protocols were approved by the institutional review board at Boston University Medical Center, and written informed consent was obtained from all participants. The protocol used in this study was reviewed and approved by the Tufts University Health Sciences Institutional Review Board.
Incident and prevalent prediabetes and incident diabetes.
At baseline and all subsequent exams, FG was measured in fresh plasma specimens with a hexokinase reagent kit (A-Gent glucose test; Abbot). Also at baseline, a 2-h OGTT was administered, and 2-h plasma glucose was measured in the same way as FG.
Participants were classified as having prediabetes at baseline if they had FG ≥5.6 to <7.0 mmol/L (≥100 to <126 mg/dL) or glucose ≥7.8 to <11.1 mmol/L (≥140 to <200 mg/dL) after a 2-h OGTT. Incident prediabetes was defined as the first incident measurement of FG ≥5.6 to <7.0 mmol/L (≥100 to <126 mg/dL). Incident T2D was defined by reported use of an oral hypoglycemic drug or insulin, or the first incident measurement of FG ≥7.0 mmol/L (≥126 mg/dL) (22).
Dairy and other dietary intake.
The Harvard semiquantitative, 126-item FFQ was used at each exam to assess dietary intake (23). The FFQ includes a list of foods for which participants were asked to report the frequency of consumption of standard serving sizes of each food item over the previous year. Eleven questions about products made from milk specifically inquired about consumption of whole milk, low-fat and skim milk, cream, sour cream, sherbet and ice milk, ice cream, yogurt, cottage and ricotta cheese, cream cheese, other cheese (e.g., American, cheddar), and butter. Possible response categories ranged from never or <1 time/mo to ≥6 times/d. Total dairy (servings per week) was calculated as the sum of each of the relevant individual line items, which corresponded to the USDA MyPlate definition of dairy as “foods made from milk that retain their calcium content,” including milk, sherbet and ice milk, ice cream, yogurt, cottage and ricotta cheese, and other cheese (https://www.choosemyplate.gov/dairy). Low-fat dairy was calculated as the sum of skim milk, sherbet and ice milk, and yogurt; high-fat dairy, the sum of whole milk, ice cream, cottage and ricotta cheese, and other cheese; total fluid milk, the sum of full-fat and skim and low-fat milk; total cheese, the sum of cottage and ricotta cheese and other cheese. We also assessed foods made from milk that do not retain calcium, including cream, sour cream, cream cheese, and butter; these were collapsed into a “cream and butter” category. One serving of each milk-based food can be converted to grams: skim milk, 245 g; whole milk, 245 g; cream, 15 g; sour cream, 12 g; sherbet and ice milk, 96 g; ice cream, 66 g; yogurt, 227 g; cottage and ricotta cheese, 105 g; cream cheese, 28 g; other cheese, 28 g; butter, 5 g. FFQs were defined as invalid if they estimated daily caloric intake as <600 kcal/d or as ≥4000 kcal/d for women and ≥4200 kcal/d for men, and if they had ≥12 blank items (23). The relative validity of the FFQ for measuring dairy intake has been previously reported, and it shows reasonable correlation with estimates from dietary records (e.g., highest for yogurt, r = 0.94–0.97; lowest for cheese, r = 0.38–0.57) (24).
Other dietary factors derived from the questionnaire—selected either because of previously shown relations with the outcome(s) or because they are markers of the healthfulness of the diet—included intake of coffee, nuts, fruits, vegetables, meats, alcohol, fish, and the glycemic index (used as a measure of carbohydrate quality). The Dietary Guidelines for Americans Index (DGAI) 2010 score, without its 2 dairy components, was calculated as previously described (25).
To account for long-term dietary exposure and to reduce within-person variability, intake is presented as the mean intake obtained from the dietary data available from each exam, averaged across all exams (5, 6, 7, and 8) for which dietary data were available up to the incident event, censoring, or end of follow-up.
Covariate assessment.
Potential confounders of the relation between diet and progression to prediabetes or diabetes, as well as other risk factors for these conditions, were considered as covariates. Covariates were assessed as follows: age (years); baseline BMI (kg/m2); parental history of diabetes (yes or no), based on self-reported history in one or both natural parents; hypertension (yes or no), defined as blood pressure ≥140/90 mm Hg, measured twice by a physician and averaged to calculate the systolic and diastolic pressures, or as receiving treatment for hypertension; dyslipidemia (yes or no), defined as plasma TGs ≥150 mg/dL, plasma HDL cholesterol <40 mg/dL in men or <50 mg/dL in women, total plasma cholesterol ≥200 mg/dL, or treatment for dyslipidemia; and regular smoking during the year before the examination (yes or no) as assessed via questionnaire. Weight change (kilograms) was calculated as the difference between the weight at the exam relevant to the incident event, censoring, or end of follow-up, and the baseline weight.
Statistical approach.
We created categories of averaged total dairy and milk-based product intake (servings per week) based on readily interpretable amounts and distributions of participants in these categories. Participant characteristics at baseline adjusted for age, sex, and energy (in the case of foods) are presented across categories of mean total dairy intake. Linear trends across increasing categories of intake were tested by assigning the median value of intake within each category and treating these as continuous variables.
Because we sought to characterize dairy’s associations with progression from normal to prediabetes, we assessed the prospective association of dairy intake with 1) incident prediabetes among participants free of prediabetes at baseline and 2) incident T2D among participants who had prediabetes at baseline. In sensitivity analyses, we repeated the healthy-to-prediabetes analyses including 17 incident T2D cases in the prediabetes category; these 17 cases had no observed instances of prediabetes. We conducted secondary analyses of the combined population for the outcome of incident T2D, wherein we tested for interaction between exposure and baseline glycemic status and to provide opportunity to compare results from the present cohort with those from the literature.
HRs and 95% CIs across categories of dairy intake were estimated from multivariable Cox proportional hazards models for incident prediabetes or diabetes. P-trend values were estimated using the median value in each intake category. Continuous values of weekly intake were assigned based on the value, or the midpoint value of the intake range, within the frequency response and used to assess per-serving associations. When categorical analyses of the final statistical model suggested nonlinear relations, these potential relations were assessed with restricted cubic splines (26) through use of the approach described by Li et al. (27). Four automatically selected knots at the 5th, 35th, 65th, and 95th percentiles with the use of continuous exposures were applied in all cases except for whole milk (1.0, 2.5, and 5.0 servings/wk) and yogurt (0.3, 1.7, and 4.0 servings/wk), for which we prespecified 3 knots based on the distribution of these data. Nonlinearity was tested through the use of the likelihood ratio test, comparing the model with only the linear term with the model with both the linear and the cubic spline terms.
For all outcomes, the initial model was adjusted for age, sex, and energy intake. Model 2 was adjusted as for model 1, plus parental history of diabetes, smoking status at baseline, dyslipidemia or treatment, and hypertension or treatment. In model 3, we further adjusted for other dietary characteristics, including mean intake of coffee, nuts, fruits, vegetables, meats, alcohol, and fish; the glycemic index (used as a measure of carbohydrate quality); and other dairy, as appropriate (for example, for associations of low-fat dairy intake, high-fat dairy intake was included in model 3). Model 4 was adjusted as for model 3, as well as for baseline BMI and weight change over follow-up; these traits are typically mediators of diet-diabetes associations. Models further adjusted for history of cardiovascular disease or cancer at baseline, interim cardiovascular events, DGAI 2010 score, sugar-sweetened beverage intake, or physical activity at baseline did not materially change results. Finally, using cross-product terms, we tested for statistical interaction between each dairy exposure as a continuous variable and baseline age, sex, and BMI in the final models.
All analyses were conducted in SAS software (version 9.4; SAS Institute). Statistical significance was set at the 0.05 level. All tests were 2-tailed.
Results
Baseline clinical and dietary characteristics of 2809 participants across categories of mean total dairy intake are presented in Table 1. The mean age of the population was 54.0 ± 9.7 y, 54% were women, 42% were overweight, and 22% were obese. Mean ± SD total dairy intake across follow-up was 10.8 ± 6.8 servings/wk; all participants reported consuming dairy at some point across follow-up. However, consumption varied by dairy product; for example, 36% of participants did not report consuming yogurt at any time across follow-up. In trends from the lowest to the highest category of total dairy intake, those in the highest category were more likely to be slightly younger, have a higher BMI, and have lower TG concentrations. They were less likely to have smoked regularly in the preceding year. Intake of dairy products, but not of cream and butter, was higher with higher total dairy intake. With increasing total dairy intake, intake of whole grains and DGAI 2010 score tended to be higher, whereas intake of alcohol, vegetables, meat, and sugar-sweetened beverages, and the glycemic index tended to be lower. Total dairy and milk-based product intakes were only weakly or not correlated with baseline BMI (range of partial r = −0.05 to 0.05) and weight change (range of partial r = −0.02 to 0.06).
TABLE 1.
Adjusted characteristics of 2809 participants of the Framingham Heart Study Offspring Cohort who were free of diabetes at exam 5 (baseline, 1991–1995), by category of mean total dairy intake1
| Categories of mean total dairy intake2 |
|||||
| Characteristics | 0 to <4 | 4 to <7 | 7 to <14 | ≥14 | P-trend |
| Servings/wk,2 median | 2.8 | 5.6 | 10.0 | 18.6 | |
| Participants, n | 355 | 583 | 1156 | 715 | |
| Age, y | 54.9 ± 0.5 | 54.2 ± 0.4 | 53.7 ± 0.3 | 53.9 ± 0.4 | <0.001 |
| Female sex, % | 52 ± 3 | 53 ± 2 | 54 ± 1 | 56 ± 2 | 0.06 |
| BMI, kg/m2 | 26.5 ± 0.3 | 26.9 ± 0.2 | 27.4 ± 0.1 | 27.1 ± 0.2 | 0.01 |
| Current smoker, % | 23 ± 2 | 20 ± 2 | 19 ± 1 | 14 ± 1 | <0.001 |
| Physical activity index, MET-h/wk | 34.4 ± 0.3 | 34.8 ± 0.3 | 35.0 ± 0.2 | 34.9 ± 0.2 | 0.46 |
| Plasma TGs, mg/dL | 147.3 ± 5.4 | 136.3 ± 4.2 | 145.2 ± 3.0 | 132.9 ± 3.8 | 0.03 |
| Plasma cholesterol, mg/dL | 205.8 ± 1.9 | 206.7 ± 1.5 | 205.0 ± 1.0 | 201.8 ± 1.3 | 0.07 |
| Plasma HDL cholesterol, mg/dL | 49.9 ± 0.7 | 51.4 ± 0.6 | 51.3 ± 0.4 | 50.4 ± 0.5 | 0.18 |
| BP, mm Hg | |||||
| Systolic | 125.9 ± 0.9 | 124.0 ± 0.7 | 124.8 ± 0.5 | 123.8 ± 0.6 | 0.17 |
| Diastolic | 74.5 ± 0.5 | 74.2 ± 0.4 | 74.5 ± 0.3 | 73.9 ± 0.4 | 0.62 |
| Plasma glucose, mg/dL | 94.7 ± 0.5 | 94.9 ± 0.4 | 95.0 ± 0.3 | 94.2 ± 0.3 | 0.24 |
| Milk-based dietary characteristics2 | |||||
| Total dairy | 3.5 ± 0.3 | 5.9 ± 0.2 | 9.8 ± 0.2 | 18.5 ± 0.2 | <0.001 |
| High-fat dairy | 2.6 ± 0.2 | 3.6 ± 0.2 | 4.3 ± 0.1 | 6.4 ± 0.2 | <0.001 |
| Low-fat dairy | 0.9 ± 0.3 | 2.3 ± 0.2 | 5.6 ± 0.2 | 12.1 ± 0.2 | <0.001 |
| Cheese | 1.7 ± 0.2 | 2.3 ± 0.1 | 2.6 ± 0.1 | 3.8 ± 0.1 | <0.001 |
| Cream and butter | 6.3 ± 0.5 | 6.6 ± 0.4 | 5.2 ± 0.3 | 4.2 ± 0.4 | <0.001 |
| Milk | |||||
| Total | 0.8 ± 0.3 | 2.1 ± 0.2 | 5.0 ± 0.2 | 11.4 ± 0.2 | <0.001 |
| Skim | 0.5 ± 0.3 | 1.5 ± 0.2 | 4.4 ± 0.2 | 10.2 ± 0.2 | <0.001 |
| Whole | 0.4 ± 0.1 | 0.5 ± 0.1 | 0.6 ± 0.1 | 1.2 ± 0.1 | <0.001 |
| Yogurt | 0.3 ± 0.1 | 0.6 ± 0.1 | 0.9 ± 0.1 | 1.3 ± 0.1 | <0.001 |
| Other dietary characteristics3 | |||||
| Whole grains | 15.5 ± 1.0 | 16.2 ± 0.8 | 19.8 ± 0.5 | 22.7 ± 0.8 | <0.001 |
| Fruit | 297.0 ± 11.1 | 279.7 ± 8.5 | 296.2 ± 6.0 | 291.7 ± 7.9 | 0.41 |
| Vegetables | 212.3 ± 6.5 | 223.8 ± 5.0 | 222.5 ± 3.5 | 211.4 ± 4.6 | 0.11 |
| Fish | 32.8 ± 1.5 | 33.4 ± 1.1 | 33.7 ± 0.8 | 31.8 ± 1.0 | 0.51 |
| Meat | 132.1 ± 3.0 | 127.8 ± 2.3 | 124.2 ± 1.6 | 116.2 ± 2.1 | <0.001 |
| Nuts | 5.7 ± 0.5 | 5.7 ± 0.4 | 6.1 ± 0.3 | 6.5 ± 0.4 | 0.47 |
| Coffee | 537.4 ± 20.3 | 540.8 ± 16.9 | 537.4 ± 10.1 | 520.5 ± 16.9 | 0.86 |
| Sugar-sweetened beverages | 203.4 ± 13.7 | 181.6 ± 10.1 | 136.4 ± 7.1 | 109.0 ± 9.6 | <0.001 |
| Alcohol | 7.3 ± 0.4 | 6.0 ± 0.3 | 5.0 ± 0.2 | 3.2 ± 0.3 | <0.001 |
| Glycemic index | 55.8 ± 0.2 | 54.9 ± 0.1 | 54.3 ± 0.1 | 53.0 ± 0.1 | <0.001 |
| DGAI 2010 score4 | 58.9 ± 0.6 | 61.1 ± 0.5 | 62.7 ± 0.3 | 62.5 ± 0.4 | <0.001 |
Values are means ± SDs, unless otherwise indicated. Characteristics are adjusted for age and sex, except for age (which is only adjusted for sex) and sex (which is only adjusted for age). Dietary characteristics are additionally adjusted for energy intake. BP, blood pressure; DGAI, Dietary Guidelines for Americans Index; MET-h, metabolic equivalent task hours.
Units are servings per week (unless otherwise indicated), with the following grams per serving: skim milk, 245 g; whole milk, 245 g; cream, 15 g; sour cream, 12 g; sherbet and ice milk, 96 g; ice cream, 66 g; yogurt, 227 g; cottage and ricotta cheese, 105 g; cream cheese, 28 g; other cheese, 28 g; butter, 5 g.
Units are grams per day except for coffee and sugar-sweetened beverages, which are both measured as milliliters per day, and the glycemic index and the DGAI 2010 score, which take no units.
The DGAI 2010 score presented does not include 2 dairy components of the total score (25).
Of the initial 2809 participants, 1884 (64.7%) had normoglycemia at baseline. They were statistically significantly different from the 925 participants with prediabetes at baseline with respect to several clinical and dietary characteristics, but they did not differ with respect to mean dairy intake (Supplemental Table 1).
Incident prediabetes among those with normal glucose status at baseline.
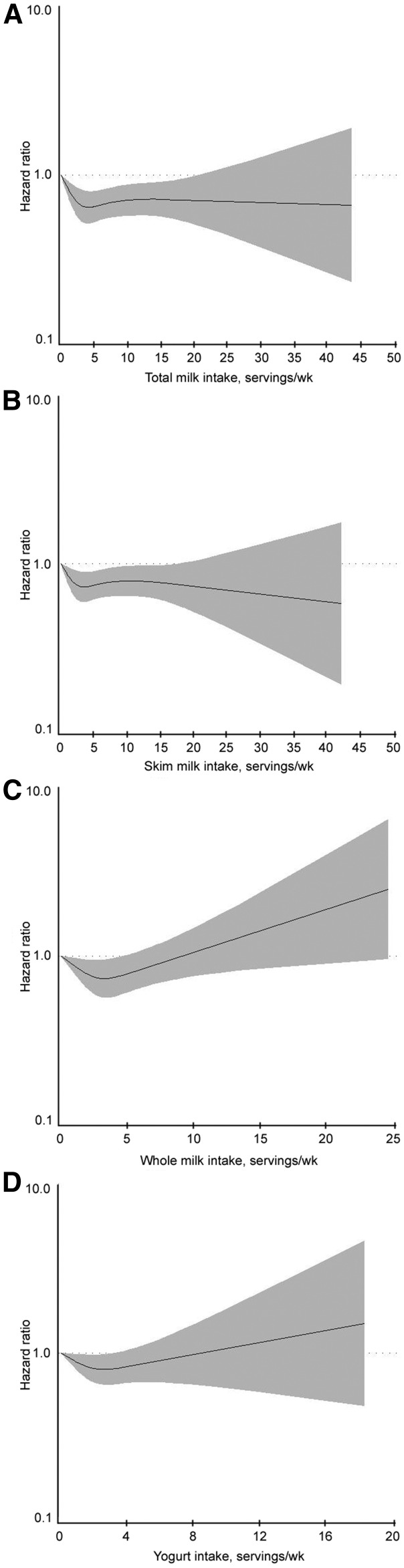
In the 1884 participants with normoglycemia at baseline, excluding those who developed T2D without captured prediabetes (n = 17), we identified 902 cases (48.3%) of incident prediabetes over a mean ± SD follow-up of 10.5 ± 4.1 y. Risks of incident prediabetes according to total dairy intake and intake of other products are presented in Table 2. In the basic model, adjusted for age, sex, and energy intake, increasing total dairy intake was associated with a 37% lower risk of incident prediabetes [lowest (reference) compared with highest intake, HR: 0.63 (95% CI: 0.49, 0.80); P-trend < 0.001]. This estimate remained after adjusting for risk factors, including other dietary factors, BMI at baseline, and weight change (fully adjusted model, model 4). In the fully adjusted model, low-fat dairy intake was linearly inversely associated with incident prediabetes [lowest compared with highest intake, HR: 0.68 (95% CI: 0.51, 0.92); P-trend = 0.03]. High-fat dairy intake was also significantly inversely associated with incident prediabetes, albeit more weakly [lowest compared with highest intake, HR: 0.75 (95% CI: 0.47, 1.17); P-trend = 0.03], and estimates suggested nonlinear associations; however, the P-nonlinearity value was not statistically significant (P-nonlinear trend = 0.13). Total milk, low-fat milk, and whole-milk intakes were not significantly associated with prediabetes in a dose-response fashion (P-trend > 0.05) in fully adjusted models, but significant nonlinear associations were present for all 3 milk categories (Figure 1A–C and Table 2). Yogurt intake also seemed to be significantly nonlinearly associated with prediabetes (P-nonlinear trend = 0.04); the lowest risk was observed for 1 to <3 servings/wk (median: 1.7 servings/wk) (Figure 1D and Table 2). Neither the cheese nor the cream and butter category was significantly associated, in either a linear or nonlinear fashion, with incident prediabetes among those with normoglycemia at baseline. Including 17 incident T2D cases with the prediabetes cases did not substantively alter these results (Supplemental Table 2). Interactions between exposures and age, sex, and BMI were not statistically significant (all P-interaction > 0.05).
TABLE 2.
Risk of incident prediabetes (n = 902 cases) among those initially healthy (n = 1867) at exam 5 (baseline, 1991–1995) in the Framingham Heart Study Offspring Cohort, by mean total dairy and milk-based product intakes1
| HR (95% CI) |
||||||||
| Intake, by category2 | Median intake2 | Respondents/cases, n | Person-years, n | Crude rate | Model 1 | Model 2 | Model 3 | Model 4 |
| Total dairy | ||||||||
| 0 to <4 | 2.7 | 258/135 | 2475.2 | 5.5 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| 4 to <7 | 5.7 | 378/179 | 3926.7 | 4.6 | 0.81 (0.64, 1.01) | 0.82 (0.65, 1.03) | 0.83 (0.66, 1.05) | 0.79 (0.63, 1.00) |
| 7 to <14 | 10.1 | 746/368 | 7962.2 | 4.6 | 0.76 (0.62, 0.94) | 0.77 (0.63, 0.95) | 0.79 (0.63, 0.99) | 0.74 (0.59, 0.93) |
| ≥14 | 18.4 | 485/220 | 5439.2 | 4.0 | 0.63 (0.49, 0.80) | 0.65 (0.51, 0.82) | 0.67 (0.50, 0.88) | 0.61 (0.46, 0.81) |
| P-trend | 0.0003 | 0.001 | 0.009 | 0.002 | ||||
| High-fat dairy | ||||||||
| 0 to <1 | 0.7 | 165/79 | 1581.3 | 5.0 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| 1 to <4 | 2.6 | 887/418 | 9353.4 | 4.5 | 0.89 (0.69, 1.13) | 0.85 (0.67, 1.09) | 0.87 (0.68, 1.11) | 0.81 (0.63, 1.04) |
| 4 to <7 | 5.2 | 514/262 | 5518.4 | 4.7 | 0.83 (0.64, 1.08) | 0.80 (0.61, 1.04) | 0.79 (0.60, 1.03) | 0.72 (0.55, 0.95) |
| 7 to <14 | 8.9 | 236/110 | 2676.7 | 4.1 | 0.67 (0.49, 0.92) | 0.62 (0.46, 0.85) | 0.60 (0.43, 0.83) | 0.56 (0.40, 0.78) |
| ≥14 | 17.5 | 65/33 | 673.4 | 4.9 | 0.77 (0.50, 1.18) | 0.81 (0.52, 1.24) | 0.80 (0.51, 1.25) | 0.75 (0.47, 1.17) |
| P-trend | 0.046 | 0.06 | 0.04 | 0.03 | ||||
| Low-fat dairy | ||||||||
| 0 to <1 | 0.2 | 330/181 | 3124.6 | 5.8 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| 1 to <4 | 2.5 | 497/237 | 5223.5 | 4.5 | 0.80 (0.66, 0.97) | 0.81 (0.67, 0.99) | 0.82 (0.67, 1.00) | 0.79 (0.65, 0.97) |
| 4 to <7 | 5.4 | 392/190 | 4289.9 | 4.4 | 0.77 (0.63, 0.95) | 0.80 (0.65, 0.98) | 0.81 (0.65, 1.00) | 0.75 (0.61, 0.94) |
| 7 to <14 | 9.2 | 447/204 | 4980.1 | 4.1 | 0.74 (0.60, 0.90) | 0.76 (0.62, 0.94) | 0.78 (0.62, 0.99) | 0.73 (0.58, 0.91) |
| ≥14 | 17.6 | 201/90 | 2185.2 | 4.1 | 0.73 (0.56, 0.95) | 0.75 (0.57, 0.97) | 0.76 (0.56, 1.02) | 0.68 (0.51, 0.92) |
| P-trend | 0.03 | 0.049 | 0.13 | 0.03 | ||||
| Total milk | ||||||||
| 0 to <1 | 0.3 | 364/197 | 3561.6 | 5.4 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| 1 to <4 | 2.5 | 575/277 | 6198.5 | 4.5 | 0.76 (0.64, 0.92) | 0.75 (0.63, 0.91) | 0.76 (0.63, 0.92) | 0.76 (0.63, 0.92) |
| 4 to <7 | 5.3 | 423/186 | 4607.6 | 4.0 | 0.69 (0.56, 0.85) | 0.68 (0.56, 0.84) | 0.70 (0.57, 0.87) | 0.70 (0.57, 0.86) |
| 7 to <14 | 9.3 | 334/157 | 3588.7 | 4.4 | 0.76 (0.62, 0.95) | 0.77 (0.62, 0.95) | 0.82 (0.65, 1.02) | 0.78 (0.62, 0.98) |
| ≥14 | 17.5 | 171/88 | 1846.7 | 4.8 | 0.82 (0.63, 1.07) | 0.80 (0.62, 1.04) | 0.81 (0.61, 1.08) | 0.79 (0.59, 1.05) |
| P-trend | 0.34 | 0.32 | 0.52 | 0.353 | ||||
| Skim milk | ||||||||
| 0 to <1 | 0.1 | 492/254 | 4868.0 | 5.2 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| 1 to <4 | 2.3 | 559/271 | 6049.1 | 4.5 | 0.83 (0.69, 0.98) | 0.82 (0.69, 0.98) | 0.82 (0.68, 0.98) | 0.81 (0.68, 0.97) |
| 4 to <7 | 5.2 | 361/161 | 3946.4 | 4.1 | 0.79 (0.65, 0.97) | 0.81 (0.66, 0.99) | 0.83 (0.67, 1.03) | 0.83 (0.67, 1.02) |
| 7 to <14 | 9.2 | 307/143 | 3336.4 | 4.3 | 0.83 (0.67, 1.02) | 0.85 (0.69, 1.04) | 0.89 (0.71, 1.11) | 0.83 (0.66, 1.04) |
| ≥14 | 17.5 | 148/73 | 1603.4 | 4.6 | 0.85 (0.65, 1.11) | 0.84 (0.64, 1.10) | 0.84 (0.62, 1.12) | 0.82 (0.61, 1.10) |
| P-trend | 0.24 | 0.29 | 0.49 | 0.293 | ||||
| Whole milk | ||||||||
| 0 to <1 | 0 | 1587/763 | 16797.8 | 4.5 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| ≥1 | 2.5 | 280/139 | 3005.5 | 4.6 | 0.88 (0.73, 1.06) | 0.84 (0.70, 1.01) | 0.80 (0.66, 0.98) | 0.84 (0.69, 1.01) |
| P-trend | 0.17 | 0.07 | 0.03 | 0.073 | ||||
| Cheese | ||||||||
| 0 to <1 | 0.6 | 401/199 | 4073.7 | 4.9 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| 1 to <4 | 2.3 | 1071/502 | 11372.6 | 4.4 | 0.88 (0.74, 1.04) | 0.87 (0.73, 1.03) | 0.87 (0.73, 1.03) | 0.83 (0.70, 0.98) |
| ≥4 | 5.5 | 395/201 | 4356.9 | 4.6 | 0.88 (0.71, 1.09) | 0.91 (0.73, 1.13) | 0.92 (0.74, 1.14) | 0.86 (0.69, 1.07) |
| P-trend | 0.37 | 0.64 | 0.71 | 0.41 | ||||
| Cream and butter | ||||||||
| 0 to <1 | 0.4 | 543/264 | 5540.8 | 4.8 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| 1 to <4 | 2.2 | 579/275 | 6289.4 | 4.4 | 0.91 (0.77, 1.08) | 0.88 (0.74, 1.05) | 0.85 (0.72, 1.01) | 0.82 (0.69, 0.98) |
| 4 to <7 | 5.4 | 246/115 | 2693.7 | 4.3 | 0.84 (0.68, 1.06) | 0.84 (0.67, 1.05) | 0.80 (0.64, 1.01) | 0.78 (0.62, 0.97) |
| 7 to <14 | 10.1 | 277/141 | 2986.8 | 4.7 | 0.98 (0.79, 1.21) | 0.94 (0.76, 1.16) | 0.88 (0.71, 1.10) | 0.88 (0.71, 1.10) |
| ≥14 | 19.7 | 222/107 | 2292.5 | 4.7 | 0.93 (0.73, 1.17) | 0.86 (0.68, 1.10) | 0.75 (0.58, 0.97) | 0.76 (0.59, 0.98) |
| P-trend | 0.84 | 0.48 | 0.09 | 0.18 | ||||
| Yogurt | ||||||||
| 0 | 0 | 642/346 | 6208.5 | 5.6 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| >0 to <1 | 0.3 | 554/265 | 5961 | 4.4 | 0.89 (0.76, 1.05) | 0.95 (0.81, 1.12) | 0.97 (0.82, 1.15) | 0.92 (0.78, 1.09) |
| 1 to <3 | 1.7 | 467/200 | 5336.6 | 3.7 | 0.77 (0.64, 0.92) | 0.81 (0.67, 0.97) | 0.80 (0.66, 0.98) | 0.76 (0.62, 0.92) |
| ≥3 | 4 | 204/91 | 2297.2 | 4.0 | 0.88 (0.69, 1.12) | 0.93 (0.73, 1.20) | 0.96 (0.73, 1.27) | 0.95 (0.72, 1.26) |
| P-trend | 0.12 | 0.23 | 0.35 | 0.333 | ||||
Cox proportional hazards models to estimate HRs (95% CIs) were adjusted as follows: Model 1 was adjusted for age, sex, and energy intake. Model 2 was adjusted as for model 1, plus parental history of diabetes, baseline smoking status, dyslipidemia or treatment, and hypertension or treatment. Model 3 was further adjusted for means of other dietary characteristics, including intake of coffee, nuts, fruits, vegetables, meats, alcohol, fish; the glycemic index (used as a measure of carbohydrate quality); and other dairy intake, as appropriate (for example, for associations of low-fat dairy intake, high-fat dairy intake was included in model 3). Model 4 was adjusted as for model 3, with further adjustment for baseline BMI and weight change over follow-up.
Units are servings per week, with the following grams per serving: skim milk, 245 g; whole milk, 245 g; cream, 15 g; sour cream, 12 g; sherbet and ice milk, 96 g; ice cream, 66 g; yogurt, 227 g; cottage and ricotta cheese, 105 g; cream cheese, 28 g; other cheese, 28 g; butter, 5 g.
Where Model 4 trends seemed to be potentially nonlinear, tests for nonlinearity were conducted using restricted cubic splines; these tests were statistically significant for total milk (P-nonlinear trend = 0.001), skim milk (P-nonlinear trend = 0.02), whole milk (P-nonlinear trend = 0.01), and yogurt (P-nonlinear trend = 0.04). Other potential nonlinear associations were not statistically significant.
FIGURE 1.
HRs of incident hyperglycemia (as impaired fasting glucose) among participants of the Framingham Heart Study Offspring Cohort with normoglycemic status at baseline, by weekly servings of total milk intake (P-nonlinear trend = 0.0008) (A), low-fat and skim milk intake (P-nonlinear trend = 0.02) (B), whole-milk intake (P-nonlinear trend = 0.01) (C), and yogurt intake (P-nonlinear trend = 0.04) (D). Spline models were developed because of potential nonlinear relations suggested by categorical analyses, and were adjusted for age, sex, energy intake, parental history of diabetes, baseline smoking status, dyslipidemia or treatment, hypertension or treatment, and the mean of other dietary characteristics (including intake of coffee, nuts, fruits, vegetables, meats, alcohol, fish; the glycemic index; and other dairy intake, as appropriate; for example, for associations of low-fat dairy intake, high-fat dairy intake was included in model 3), baseline BMI, and weight change over follow-up. The solid line indicates the estimates; the gray shaded areas represent the 95% CIs. Grams per serving are as follows: low-fat/skim milk, 245 g; whole milk, 245 g; yogurt, 227 g.
Incident T2D among those with prediabetes at baseline.
Among the 925 participants (31.8% of the total sample) with impaired fasting glucose or impaired glucose tolerance at baseline, 196 participants (21.2%) developed incident T2D over a mean ± SD follow-up of 11.5 ± 3.5 y. Only high-fat dairy and cheese intakes showed evidence of a dose-response association with incident T2D. In fully adjusted models, higher high-fat dairy intake was associated with a 70% lower risk of incident T2D [lowest compared with highest intake, HR: 0.30 (95% CI: 0.10, 0.92); P-trend = 0.03]; and higher cheese intake was associated with a 63% lower risk of incident T2D [HR: 0.37 (95% CI: 0.22, 0.62); P-trend < 0.001] (Table 3). Of the tested interactions of exposures with age, sex, and BMI, only that for cheese intake interacting with age was significant (P-interaction = 0.002). Stratifying by median age (57 y) resulted in a significant association between cheese intake and incident T2D for those >57 y old [per serving, HR: 0.79 (95% CI: 0.67, 0.91); P = 0.002] but not for those ≤57 y old [per serving, HR: 0.94 (95% CI: 0.87, 1.01); P = 0.11]. Otherwise we found no significant trends or nonlinear associations for intake of any other milk-based product with respect to risk of incident diabetes (all P-trend and P-nonlinear trend > 0.05).
TABLE 3.
Risk of incident diabetes (n = 196 cases) among those with prediabetes (n = 925) at exam 5 (baseline, 1991–1995) in the Framingham Heart Study Offspring Cohort, by mean total dairy and milk-based product intakes1
| HR (95% CI) |
||||||||
| Intake by category2 | Median intake2 | Respondents/cases, n | Person-years, n | Crude rate | Model 1 | Model 2 | Model 3 | Model 4 |
| Total dairy | ||||||||
| 0 to <4 | 2.8 | 117/30 | 1280.9 | 2.3 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| 4 to <7 | 5.6 | 206/33 | 2389.9 | 1.4 | 0.56 (0.34, 0.92) | 0.57 (0.35, 0.95) | 0.58 (0.35, 0.97) | 0.60 (0.36, 0.99) |
| 7 to <14 | 10 | 385/94 | 4431.9 | 2.1 | 0.92 (0.60, 1.41) | 0.93 (0.60, 1.43) | 0.93 (0.59, 1.46) | 0.84 (0.54, 1.33) |
| ≥14 | 18.5 | 217/39 | 2554.8 | 1.5 | 0.67 (0.40, 1.13) | 0.70 (0.41, 1.19) | 0.68 (0.37, 1.25) | 0.63 (0.34, 1.15) |
| P-trend | 0.59 | 0.69 | 0.65 | 0.39 | ||||
| High-fat dairy | ||||||||
| 0 to <1 | 0.7 | 73/22 | 751.1 | 2.9 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| 1 to <4 | 2.6 | 433/96 | 4965.7 | 1.9 | 0.63 (0.39, 1.00) | 0.63 (0.39, 1.01) | 0.57 (0.36, 0.92) | 0.56 (0.34, 0.90) |
| 4 to <7 | 5.1 | 274/53 | 3213.9 | 1.6 | 0.51 (0.30, 0.85) | 0.53 (0.32, 0.90) | 0.45 (0.26, 0.77) | 0.40 (0.23, 0.69) |
| 7 to <14 | 9 | 114/18 | 1357.8 | 1.3 | 0.41 (0.22, 0.79) | 0.45 (0.23, 0.86) | 0.39 (0.20, 0.78) | 0.36 (0.18, 0.72) |
| ≥14 | 18 | 31/7 | 369.1 | 1.9 | 0.58 (0.23, 1.47) | 0.53 (0.21, 1.36) | 0.29 (0.10, 0.86) | 0.30 (0.10, 0.92) |
| P-trend | 0.12 | 0.14 | 0.03 | 0.03 | ||||
| Low-fat dairy | ||||||||
| 0 to <1 | 0.3 | 182/46 | 1950.1 | 2.4 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| 1 to <4 | 2.5 | 257/47 | 3090.0 | 1.5 | 0.61 (0.41, 0.92) | 0.60 (0.40, 0.90) | 0.59 (0.39, 0.90) | 0.60 (0.39, 0.91) |
| 4 to <7 | 5.4 | 195/42 | 2294.4 | 1.8 | 0.81 (0.53, 1.23) | 0.79 (0.52, 1.21) | 0.80 (0.51, 1.24) | 0.73 (0.47, 1.14) |
| 7 to <14 | 9.4 | 196/42 | 2246.8 | 1.9 | 0.84 (0.55, 1.29) | 0.88 (0.57, 1.36) | 0.85 (0.53, 1.37) | 0.78 (0.48, 1.27) |
| ≥14 | 17.6 | 95/19 | 1076.3 | 1.8 | 0.74 (0.43, 1.28) | 0.73 (0.42, 1.28) | 0.72 (0.39, 1.35) | 0.75 (0.39, 1.41) |
| P-trend | 0.85 | 0.97 | 0.89 | 0.86 | ||||
| Total milk | ||||||||
| 0 to <1 | 0.2 | 183/41 | 1989.2 | 2.1 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| 1 to <4 | 2.5 | 300/62 | 3549.7 | 1.7 | 0.85 (0.58, 1.27) | 0.86 (0.58, 1.28) | 0.84 (0.56, 1.25) | 0.80 (0.53, 1.19) |
| 4 to <7 | 5.3 | 207/43 | 2462.6 | 1.7 | 0.91 (0.59, 1.40) | 0.90 (0.58, 1.40) | 0.90 (0.57, 1.41) | 0.72 (0.45, 1.13) |
| 7 to <14 | 9.3 | 153/33 | 1735.6 | 1.9 | 0.99 (0.62, 1.58) | 1.01 (0.63, 1.62) | 0.94 (0.57, 1.55) | 0.83 (0.50, 1.37) |
| ≥14 | 17.5 | 82/17 | 920.4 | 1.8 | 0.91 (0.51, 1.63) | 0.94 (0.52, 1.69) | 0.89 (0.47, 1.67) | 0.90 (0.47, 1.70) |
| P-trend | 0.95 | 0.86 | 0.93 | 0.89 | ||||
| Skim milk | ||||||||
| 0 to <1 | 0.1 | 247/58 | 2722.7 | 2.1 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| 1 to <4 | 2.4 | 281/58 | 3344.4 | 1.7 | 0.82 (0.57, 1.18) | 0.79 (0.55, 1.14) | 0.81 (0.55, 1.18) | 0.80 (0.54, 1.16) |
| 4 to <7 | 5.3 | 184/38 | 2169.6 | 1.8 | 0.91 (0.60, 1.38) | 0.88 (0.58, 1.34) | 0.86 (0.56, 1.33) | 0.73 (0.47, 1.14) |
| 7 to <14 | 9.3 | 138/26 | 1567.0 | 1.7 | 0.85 (0.53, 1.36) | 0.88 (0.54, 1.41) | 0.82 (0.49, 1.38) | 0.73 (0.43, 1.23) |
| ≥14 | 17.5 | 75/16 | 853.8 | 1.9 | 0.89 (0.51, 1.57) | 0.92 (0.52, 1.63) | 0.87 (0.47, 1.64) | 0.89 (0.47, 1.68) |
| P-trend | 0.76 | 0.93 | 0.73 | 0.64 | ||||
| Whole milk | ||||||||
| 0 to <1 | 0 | 802/163 | 9228.7 | 1.8 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| ≥1 | 2.3 | 123/33 | 1428.8 | 2.3 | 1.20 (0.82, 1.75) | 1.16 (0.79, 1.71) | 1.23 (0.82, 1.85) | 1.16 (0.77, 1.76) |
| P-trend | 0.35 | 0.45 | 0.33 | 0.48 | ||||
| Cheese | ||||||||
| 0 to <1 | 0.6 | 188/53 | 2025.4 | 2.6 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| 1 to <4 | 2.3 | 558/114 | 6466.6 | 1.8 | 0.66 (0.47, 0.92) | 0.69 (0.49, 0.97) | 0.68 (0.48, 0.96) | 0.64 (0.45, 0.90) |
| ≥4 | 5.5 | 179/29 | 2165.5 | 1.3 | 0.47 (0.29, 0.76) | 0.48 (0.30, 0.79) | 0.45 (0.27, 0.76) | 0.37 (0.22, 0.62) |
| P-trend | 0.003 | 0.004 | 0.003 | <0.001 | ||||
| Cream and butter | ||||||||
| 0 to <1 | 0.4 | 274/61 | 2999.1 | 2.0 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| 1 to <4 | 2.2 | 285/60 | 3378.9 | 1.8 | 0.84 (0.59, 1.20) | 0.86 (0.60, 1.23) | 0.89 (0.62, 1.29) | 0.88 (0.61, 1.28) |
| 4 to <7 | 5.2 | 124/27 | 1417.4 | 1.9 | 0.95 (0.60, 1.50) | 0.88 (0.55, 1.39) | 0.85 (0.53, 1.36) | 0.79 (0.49, 1.28) |
| 7 to <14 | 9.7 | 139/30 | 1666.1 | 1.8 | 0.89 (0.57, 1.39) | 0.89 (0.57, 1.40) | 0.89 (0.56, 1.42) | 1.00 (0.63, 1.61) |
| ≥14 | 19.8 | 103/18 | 1196.0 | 1.5 | 0.72 (0.42, 1.25) | 0.73 (0.42, 1.27) | 0.72 (0.41, 1.29) | 0.81 (0.45, 1.46) |
| P-trend | 0.36 | 0.35 | 0.34 | 0.68 | ||||
| Yogurt | ||||||||
| 0 | 0 | 362/81 | 4016.9 | 2.0 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| >0 to <1 | 0.4 | 299/69 | 3518.6 | 2.0 | 0.97 (0.70, 1.34) | 0.98 (0.70, 1.36) | 0.89 (0.64, 1.26) | 0.96 (0.68, 1.35) |
| 1 to <3 | 1.7 | 196/30 | 2353.2 | 1.3 | 0.61 (0.40, 0.94) | 0.63 (0.41, 0.96) | 0.63 (0.40, 1.00) | 0.65 (0.41, 1.02) |
| ≥3 | 4.2 | 68/16 | 768.8 | 2.1 | 1.05 (0.61, 1.82) | 1.15 (0.66, 1.99) | 1.07 (0.58, 1.98) | 1.24 (0.67, 2.29) |
| P-trend | 0.40 | 0.58 | 0.67 | 0.89 | ||||
Cox proportional hazards models to estimate HRs (95% CIs) were adjusted as follows: Model 1 was adjusted for age, sex, and energy intake. Model 2 was adjusted as for model 1, plus parental history of diabetes, baseline smoking status, dyslipidemia or treatment, and hypertension or treatment. Model 3 was further adjusted for means of other dietary characteristics, including intake of coffee, nuts, fruits, vegetables, meats, alcohol, and fish; the glycemic index (used as a measure of carbohydrate quality); and other dairy intake, as appropriate (for example, for associations of low-fat dairy intake, high-fat dairy intake was included in model 3). Model 4 was adjusted as for model 3, with further adjustment for baseline BMI and weight change over follow-up. Where Model 4 trends seemed to be potentially nonlinear, tests for nonlinearity were conducted using restricted cubic splines; no nonlinear associations were statistically significant (all P-nonlinear trend > 0.05).
Units are servings per week, with the following grams per serving: skim milk, 245 g; whole milk, 245 g; cream, 15 g; sour cream, 12 g; sherbet and ice milk, 96 g; ice cream, 66 g; yogurt, 227 g; cottage and ricotta cheese, 105 g; cream cheese, 28 g; other cheese, 28 g; butter, 5 g.
Incident T2D in the total population: impact of glycemic status at baseline.
Secondary analyses of the total population were conducted for incident T2D given the differences we observed between glycemic status at baseline and dairy-outcome relations. In addition, given the long follow-up, some initially healthy individuals did develop T2D after prediabetes (n = 40) or had no observed prediabetes stage (n = 17). The analysis thus combined the 196 incident cases in the initially impaired subset of the population with 57 cases in the initially normoglycemic subset of the population. We estimated proportional hazards in the total population for the outcome of T2D using the original models and an additional model that adjusted for baseline glycemic status (i.e., normal or impaired). In the latter we also included an interaction term between dairy exposure and baseline glycemic status. Results were most similar to results for the incident analysis in the population impaired at baseline, likely driven by the higher number of T2D outcomes in this subset of the population (Supplemental Table 3). High-fat dairy intake was modestly inversely associated with incident T2D in the fully adjusted model [model 4; lowest compared with highest category, HR: 0.49 (95% CI: 0.19, 1.24); P-trend = 0.06). After adjusting for glycemic status at baseline, the trend was weaker (P-trend = 0.09), although the interaction with baseline glycemic status was highly significant (P-interaction = 0.006). Cheese was strongly inversely associated with incident T2D, even after adjusting for glycemic status at baseline [HR: 0.51 (95% CI: 0.33, 0.78); P-trend = 0.005), and the interaction with baseline glycemic status was also highly significant (P-interaction ≤ 0.001).
Discussion
In these analyses, we observed that total, high-fat, and low-fat dairy intakes were inversely associated with the risk of developing prediabetes (or diabetes) among those with normoglycemia at baseline. Among those in an initially impaired glycemic state at baseline, cheese intake was inversely associated with incident diabetes, even after adjusting for BMI and other risk factors. This association between cheese intake and diabetes seemed to be the strongest dose-response association in these analyses. In addition, some protective associations we expected were not observed, such as that for yogurt intake, although in those with normoglycemia at baseline, we observed a nonlinear protective association at 1–3 servings/wk. In addition, we observed that associations between select dairy products and T2D differ according to glycemic status at baseline, which may underpin the equivocal relations observed across prior studies (12, 13, 28–30).
Given our deepening understanding of the differential handling of foods given pre-existing metabolic states (31–34), our results may help disentangle inconsistent evidence regarding the role of dairy in diabetes. Dairy intake, along with intake of other milk-based products, such as butter, has equivocal associations with incident diabetes, and meta-analyses of the topic have yielded relatively few insights into reasons for the high heterogeneity between studies (12, 28–30). Gijsbers et al. (12), in a recent meta-analysis of 22 prospective cohort studies of dairy intake and T2D, observed slightly protective linear associations for total and low-fat dairy intake (3% and 4% risk reduction, respectively, per 200 g/d increment), and a nonlinear association for yogurt intake; 80 g/d had the lowest risk (14% risk reduction relative to nonconsumers), and no additional benefit was observed above that amount. These results align with our observations for incident prediabetes among those with normoglycemia at baseline. We also observed lower risks of impaired fasting glucose for total and low-fat dairy intakes, along with a more modest trend for high-fat dairy intake, and our results for yogurt indicate that the lowest risk (25% risk reduction relative to nonconsumers) was observed at a median of 1.7 servings/wk (60.5 g/d at 244 g/serving); no benefit was observed above that amount. Among those with impaired glucose at baseline, we did not observe protective associations for total or low-fat dairy intake or yogurt intake against T2D, but we did observe favorable associations between cheese intake and incident T2D, which may be stronger among older individuals in our cohort. In general, prior prospective cohort studies have found nonsignificant associations of cheese with incident T2D (11, 12, 14, 17).
In analyses including our entire cohort for incident T2D, we observed significant interactions between total milk intake and, more notably, high-fat dairy and cheese intakes with baseline glycemic status. To our knowledge, no prior observational study or meta-analysis has accounted for the potentially modifying role of baseline glycemic status in associations of dairy products with incident prediabetes or diabetes. Therefore, baseline glycemic status may account for the heterogeneity observed across prior studies for dairy’s role in diabetes. A 2011 prospective study of the metabolic syndrome in a French population investigated the incidence of hyperglycemia (as IFG or T2D) and observed that total dairy intake, excluding cheese, was associated with lower odds of hyperglycemia over 9 y of follow-up (17). However, the relation between total dairy intake and T2D alone was not significant, and cheese was not associated with either hyperglycemia and T2D or T2D alone (17). The authors did not investigate hyperglycemia without T2D, nor did they assess possible differential associations by glycemic status at baseline.
The fat and protein components of traditionally high-fat dairy, possibly coupled with fermentation (e.g., in products such as cheese or whole-milk yogurt), might play a more important role for those experiencing a dysregulated metabolic state of hyperglycemia than for individuals with normoglycemia (35, 36). Several current lines of research are investigating the role of circulating biomarkers of dairy and dairy fat intake in cardiometabolic health (37, 38); some studies indicate benefits (8, 9, 39) whereas others report no associations (40) or report differential effects, depending on the type of FA (10, 41).
Beyond dairy fats, dairy proteins may also play a role in cardiometabolic health (42). For example, in a recent 12-wk trial of dairy protein (whey or casein) and milk fat (high or low medium-chain SFA content) supplementation in abdominally obese adults, whey protein, but not casein, decreased the postprandial chylomicron response (specifically apoB-48) compared with casein, whereas casein increased postprandial glucagon-like peptide 1 compared with whey (43). No differences were found on postprandial measures for the low compared with the high fat content. Another trial consisting of a whey protein load preceding a breakfast with a high glycemic index in Israeli adults with T2D found that postprandial glucose levels were reduced by 28%, whereas insulin was 105% higher and C-peptide was 43% higher with the whey protein load. In addition, glucagon-like peptide 1 levels (both total and intact) were also significantly higher with the whey protein load before the meal (44).
Finally, 2 recent cross-sectional studies showed that fermented dairy may be particularly beneficial in T2D and related outcomes. In addition to observing 25–29% lower odds of newly diagnosed T2D in those with the highest total dairy consumption, a 2015 cross-sectional study in nondiabetic Brazilian adults observed inverse associations between dairy intake and fasting glucose, 2-h OGTT glucose, glycated hemoglobin, insulin after a 2-h OGTT, and insulin resistance, with strong inverse associations between fermented dairy intake and glucose traits (20). A 2016 cross-sectional study in a middle-aged Dutch population found that both skim and fermented dairy products were inversely associated with impaired glucose metabolism (defined as impaired glucose tolerance or IFG), whereas total dairy was inversely and full-fat dairy was directly associated with T2D (13). Fermented dairy products are thought to promote gut microbial health, and while we observed dose-response relations for cheese intake in the impaired subset of our study population, we did not observe dose-response benefits of yogurt intake in either subset, which limits our ability to speculate on the relevance of our findings for fermented dairy as a singular class of dairy products.
Strengths and limitations.
We benefited from a large cohort followed for ≤17 y with repeated measures of exposures and outcomes. Although the FFQ has been widely used in epidemiologic studies, this method has limitations, notably its reliance on participant self-report and memory. However, dairy intake as assessed by FFQ has among the highest correlations with dietary records (23, 24). Although we maintained a strictly prospective analysis, some reverse causality may exist. However, “prediabetes” wasn’t defined as a risk factor for T2D until 1997 (45) and is still not considered a “clinical entity” in the most recent Standards of Medical Care of the American Diabetes Association (46). In addition, before 1997, the fasting glucose cutoff for T2D was 140 mg/dL (45), rather than the 126 mg/dL used in this study. Thus, behavior change in response to a prediabetes “diagnosis” was not likely in this cohort, especially during early follow-up. Although clinical diagnostic definitions of our outcomes usually stipulate 2 consecutive elevated measures, our approach of using a single measure is common in some epidemiologic approaches where consecutive exams occur far apart; nevertheless, our definition may have misclassified glycemic status in some individuals, resulting in biased estimates. We did not assess measures of dairy-related circulating lipids, which could have been useful as biomarkers of intake and confirmation of associations with the outcomes; this area merits further research. In addition, we tested associations between 9 dairy exposures and 2 outcomes in our primary hypotheses, and thus some of our findings could be due to chance. A strict Bonferroni correction would be too conservative, but even at a corrected α level (i.e., α = 0.05/18 = 0.003), P-trend values for total dairy intake in the initially healthy population and cheese intake in the initially impaired population would retain statistical significance. Finally, the Framingham Heart Study Offspring Cohort is a relatively homogenous Caucasian cohort, which may limit the generalizability of our findings.
In conclusion, in this long-term, population-based prospective cohort study in middle-aged Americans, we observed that dairy intake varies in its associations with T2D, depending on product type and glycemic status at baseline. Different types of dairy are associated with incident hyperglycemia in those with normoglycemia than are associated with T2D in those with existing impaired glycemic status. These observations regarding the impact of underlying metabolic states may partially underlie the heterogeneous evidence from prior studies regarding the role of dairy in incident diabetes.
Acknowledgments
The authors’ responsibilities were as follows—PFJ: designed the research; JBM: conducted the research (collected data); AH: analyzed the data and wrote the manuscript; GR and JM: reviewed the analysis; PFJ and AH: had primary responsibility for the final content; and all authors: interpreted the data, edited and reviewed the manuscript, and read and approved the final version of the manuscript.
Footnotes
Abbreviations used: DGAI, Dietary Guidelines for Americans Index; FG, fasting plasma glucose; IFG, impaired fasting glucose; OGTT, oral glucose tolerance test; T2D, type 2 diabetes.
References
- 1.International Diabetes Federation. IDF diabetes atlas [Internet]. 7th ed. Brussels, Belgium: International Diabetes Federation; 2015. [cited 2016 Sep 12]. Available from: http://www.diabetesatlas.org. [Google Scholar]
- 2.CDC. National diabetes statistics report: estimates of diabetes and its burden in the United States, 2014 [Internet]. Atlanta (GA): US Department of Health and Human Services; 2014. [cited 2016 Sep 12]. Available from: https://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. [Google Scholar]
- 3.American Diabetes Association. 4. Prevention or delay of type 2 diabetes. Diabetes Care 2016;39:S36–8. [DOI] [PubMed] [Google Scholar]
- 4.Office of Disease Prevention and Health Promotion, U.S. Department of Health and Human Services. Dietary guidelines for Americans 2015–2020 [Internet]. 8th ed. 2015. [cited 2016 Jan 7]. Available from: http://health.gov/dietaryguidelines/2015/guidelines/.
- 5.Office of Disease Prevention and Health Promotion, U.S. Department of Health and Human Services. 1990 Dietary guidelines [Internet]. 1990. [cited 12 Sep 2016]. Available from: https://health.gov/dietaryguidelines/1990.asp.
- 6.Wang H, Livingston KA, Fox CS, Meigs JB, Jacques PF. Yogurt consumption is associated with better diet quality and metabolic profile in American men and women. Nutr Res 2013;33:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quann EE, Fulgoni VL III, Auestad N. Consuming the daily recommended amounts of dairy products would reduce the prevalence of inadequate micronutrient intakes in the United States: diet modeling study based on NHANES 2007–2010. Nutr J 2015;14:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mozaffarian D, de Oliveira Otto MC, Lemaitre RN, Fretts AM, Hotamisligil G, Tsai MY, Siscovick DS, Nettleton JA. trans-Palmitoleic acid, other dairy fat biomarkers, and incident diabetes: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr 2013;97:854–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yakoob MY, Shi P, Willett WC, Rexrode KM, Campos H, Orav EJ, Hu FB, Mozaffarian D. Circulating biomarkers of dairy fat and risk of incident diabetes mellitus among men and women in the United States in two large prospective cohorts. Circulation 2016;133:1645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forouhi NG, Koulman A, Sharp SJ, Imamura F, Kröger J, Schulze MB, Crowe FL, Huerta JM, Guevara M, Beulens JW, et al. Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: the EPIC-InterAct case-cohort study. Lancet Diabetes Endocrinol 2014;2:810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guasch-Ferré M, Becerra-Tomás N, Ruiz-Canela M, Corella D, Schröder H, Estruch R, Ros E, Arós F, Gómez-Gracia E, Fiol M, et al. Total and subtypes of dietary fat intake and risk of type 2 diabetes mellitus in the Prevención con Dieta Mediterránea (PREDIMED) study. Am J Clin Nutr 2017;105:723–35. [DOI] [PubMed] [Google Scholar]
- 12.Gijsbers L, Ding EL, Malik VS, de Goede J, Geleijnse JM, Soedamah-Muthu SS. Consumption of dairy foods and diabetes incidence: a dose-response meta-analysis of observational studies. Am J Clin Nutr 2016;103:1111–24. [DOI] [PubMed] [Google Scholar]
- 13.Eussen SJ, van Dongen MC, Wijckmans N, den Biggelaar L, Oude Elferink SJ, Singh-Povel CM, Schram MT, Sep SJ, van der Kallen CJ, Koster A, et al. Consumption of dairy foods in relation to impaired glucose metabolism and type 2 diabetes mellitus: the Maastricht Study. Br J Nutr 2016;115:1453–61. [DOI] [PubMed] [Google Scholar]
- 14.Brouwer-Brolsma EM, van Woudenbergh GJ, Oude Elferink SJ, Singh-Povel CM, Hofman A, Dehghan A, Franco OH, Feskens EJ. Intake of different types of dairy and its prospective association with risk of type 2 diabetes: the Rotterdam Study. Nutr Metab Cardiovasc Dis 2016;26:987–95. [DOI] [PubMed] [Google Scholar]
- 15.Schwingshackl L, Hoffmann G, Schwedhelm C, Kalle-Uhlmann T, Missbach B, Knüppel S, Boeing H. Consumption of dairy products in relation to changes in anthropometric variables in adult populations: a systematic review and meta-analysis of cohort studies. PLoS One 2016;11:e0157461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eales J, Lenoir-Wijnkoop I, King S, Wood H, Kok FJ, Shamir R, Prentice A, Edwards M, Glanville J, Atkinson RL. Is consuming yoghurt associated with weight management outcomes? Results from a systematic review. Int J Obes (Lond) 2016;40:731–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fumeron F, Lamri A, Abi Khalil C, Jaziri R, Porchay-Baldérelli I, Lantieri O, Vol S, Balkau B, Marre M; Data from the Epidemiological Study on the Insulin Resistance Syndrome (DESIR) Study Group. Dairy consumption and the incidence of hyperglycemia and the metabolic syndrome: results from a French prospective study, Data from the Epidemiological Study on the Insulin Resistance Syndrome (DESIR). Diabetes Care 2011;34:813–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sayon-Orea C, Martínez-González MA, Ruiz-Canela M, Bes-Rastrollo M. Associations between yogurt consumption and weight gain and risk of obesity and metabolic syndrome: a systematic review. Adv Nutr 2017;8:146S–54S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim Y, Je Y. Dairy consumption and risk of metabolic syndrome: a meta-analysis. Diabet Med 2016;33:428–40. [DOI] [PubMed] [Google Scholar]
- 20.Drehmer M, Pereira MA, Schmidt MI, Molina MDCB, Alvim S, Lotufo PA, Duncan BB. Associations of dairy intake with glycemia and insulinemia, independent of obesity, in Brazilian adults: the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Am J Clin Nutr 2015;101:775–82. [DOI] [PubMed] [Google Scholar]
- 21.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study. Design and preliminary data. Prev Med 1975;4:518–25. [DOI] [PubMed] [Google Scholar]
- 22.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33:S62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–26. [DOI] [PubMed] [Google Scholar]
- 24.Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, Willett WC. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol 1989;18:858–67. [DOI] [PubMed] [Google Scholar]
- 25.Sauder KA, Proctor DN, Chow M, Troy LM, Wang N, Vita JA, Vasan RS, Mitchell GF, Jacques PF, Hamburg NM, et al. Endothelial function, arterial stiffness and adherence to the 2010 dietary guidelines for Americans: a cross-sectional analysis. Br J Nutr 2015;113:1773–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med 1989;8:551–61. [DOI] [PubMed] [Google Scholar]
- 27.Li R, Hertzmark E, Louie M, Chen L, Spiegelman D. The SAS LGTPHCURV9 macro [Internet]. 2011. [cited 2015 Feb 12]. Available from: https://cdn1.sph.harvard.edu/wp-content/uploads/sites/271/2012/09/lgtphcurv9_7-3-2011.pdf.
- 28.Aune D, Norat T, Romundstad P, Vatten LJ. Dairy products and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Am J Clin Nutr 2013;98:1066–83. [DOI] [PubMed] [Google Scholar]
- 29.Chen M, Sun Q, Giovannucci E, Mozaffarian D, Manson JE, Willett WC, Hu FB. Dairy consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. BMC Med 2014;12:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pimpin L, Wu JHY, Haskelberg H, Del Gobbo L, Mozaffarian D. Is butter back? A systematic review and meta-analysis of butter consumption and risk of cardiovascular disease, diabetes, and total mortality. PLoS One 2016;11:e0158118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yubero-Serrano EM, Delgado-Lista J, Tierney AC, Perez-Martinez P, Garcia-Rios A, Alcala-Diaz JF, Castaño JP, Tinahones FJ, Drevon CA, Defoort C, et al. Insulin resistance determines a differential response to changes in dietary fat modification on metabolic syndrome risk factors: the LIPGENE study. Am J Clin Nutr 2015;102:1509–17. [DOI] [PubMed] [Google Scholar]
- 32.Blanco-Rojo R, Alcala-Diaz JF, Wopereis S, Perez-Martinez P, Quintana-Navarro GM, Marin C, Ordovas JM, van Ommen B, Perez-Jimenez F, Delgado-Lista J, et al. The insulin resistance phenotype (muscle or liver) interacts with the type of diet to determine changes in disposition index after 2 years of intervention: the CORDIOPREV-DIAB randomised clinical trial. Diabetologia 2015. Oct 16 (Epub ahead of print; DOI:10.1007/s00125-015-3776-4). [DOI] [PubMed] [Google Scholar]
- 33.Morris C, O’Grada C, Ryan M, Roche HM, Gibney MJ, Gibney ER, Brennan L. Identification of differential responses to an oral glucose tolerance test in healthy adults. PLoS One 2013;8:e72890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim Y, Keogh J, Clifton P. Differential effects of red meat/refined grain diet and dairy/chicken/nuts/whole grain diet on glucose, insulin and triglyceride in a randomized crossover study. Nutrients 2016;8:687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Comerford KB, Pasin G. Emerging evidence for the importance of dietary protein source on glucoregulatory markers and type 2 diabetes: different effects of dairy, meat, fish, egg, and plant protein foods. Nutrients 2016;8 pii:E446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turner KM, Keogh JB, Clifton PM. Dairy consumption and insulin sensitivity: a systematic review of short- and long-term intervention studies. Nutr Metab Cardiovasc Dis 2015;25:3–8. [DOI] [PubMed] [Google Scholar]
- 37.Risérus U, Marklund M. Milk fat biomarkers and cardiometabolic disease. Curr Opin Lipidol 2017;28:46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drouin-Chartier J-P, Côté JA, Labonté M-È, Brassard D, Tessier-Grenier M, Desroches S, Couture P, Lamarche B. Comprehensive review of the impact of dairy foods and dairy fat on cardiometabolic risk. Adv Nutr 2016;7:1041–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mozaffarian D, Cao H, King IB, Lemaitre RN, Song X, Siscovick DS, Hotamisligil GS. Trans-palmitoleic acid, metabolic risk factors, and new-onset diabetes in U.S. adults: a cohort study. Ann Intern Med 2010;153:790–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen M, Li Y, Sun Q, Pan A, Manson JE, Rexrode KM, Willett WC, Rimm EB, Hu FB. Dairy fat and risk of cardiovascular disease in 3 cohorts of US adults. Am J Clin Nutr 2016;104:1209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Oliveira Otto MC, Nettleton JA, Lemaitre RN, Steffen LM, Kromhout D, Rich SS, Tsai MY, Jacobs DR, Mozaffarian D. Biomarkers of dairy fatty acids and risk of cardiovascular disease in the Multi-ethnic Study of Atherosclerosis. J Am Heart Assoc 2013;2:e000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bendtsen LQ, Lorenzen JK, Bendsen NT, Rasmussen C, Astrup A. Effect of dairy proteins on appetite, energy expenditure, body weight, and composition: a review of the evidence from controlled clinical trials. Adv Nutr 2013;4:418–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bohl M, Bjørnshave A, Rasmussen KV, Schioldan AG, Amer B, Larsen MK, Dalsgaard TK, Holst JJ, Herrmann A, O’Neill S, et al. Dairy proteins, dairy lipids, and postprandial lipemia in persons with abdominal obesity (DairyHealth): a 12-wk, randomized, parallel-controlled, double-blinded, diet intervention study. Am J Clin Nutr 2015;101:870–8. [DOI] [PubMed] [Google Scholar]
- 44.Jakubowicz D, Froy O, Ahrén B, Boaz M, Landau Z, Bar-Dayan Y, Ganz T, Barnea M, Wainstein J. Incretin, insulinotropic and glucose-lowering effects of whey protein pre-load in type 2 diabetes: a randomised clinical trial. Diabetologia 2014;57:1807–11. [DOI] [PubMed] [Google Scholar]
- 45.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183–97. [DOI] [PubMed] [Google Scholar]
- 46.American Diabetes Association. 2. Classification and diagnosis of diabetes. Diabetes Care 2017;40:S11–24. [DOI] [PubMed] [Google Scholar]