Abstract
Platelet count has been shown to be lower and mean platelet volume (MPV) to be higher in acute myocardial infarction (MI). However, it is not known whether these changes persist post-MI or if these measures are able to distinguish between acute thrombotic and non-thrombotic MI. Platelet count and MPV were measured in 80 subjects with acute MI (thrombotic and non-thrombotic) and stable coronary artery disease (CAD) at cardiac catheterization (acute phase) and at >3-month follow-up (quiescent phase). Subjects were stratified using stringent clinical, biochemical, histological, and angiographic criteria. Outcome measures were compared between groups by analysis of variance. Forty-seven subjects met criteria for acute MI with clearly defined thrombotic (n = 22) and non-thrombotic (n = 12) subsets. Fourteen subjects met criteria for stable CAD. No significant difference was observed in platelet count between subjects with acute MI and stable CAD at the acute or quiescent phase. MPV was higher in acute MI (9.18 ± 1.21) compared to stable CAD (8.13 ± 0.66; P = 0.003) at the acute phase but not at the quiescent phase (8.48 ± 0.58 vs 8.94 ± 1.42; P = 0.19). No difference in platelet count or MPV was detected between thrombotic and non-thrombotic subsets at acute or quiescent phases. The power to detect differences in these measures between thrombotic and non-thrombotic subsets was 58%. Higher MPV at the time of acute MI is not observed by 3 months post-MI (quiescent phase). Platelet count and MPV do not differ in subjects with thrombotic versus non-thrombotic MI. Further investigation is warranted to evaluate the utility of these measures in the diagnosis of acute MI.
Keywords: platelet activation, vascular physiology, atherothrombosis, acute coronary syndrome
Introduction
Myocardial infarction (MI) is the most common cause of death and disability worldwide.1,2 Although significant advancements have been made in using biomarkers (eg, troponin) and electrocardiograms to diagnose MI, these methods measure myocardial necrosis, as opposed to the cause and therapeutic target—coronary thrombosis. Since myocardial necrosis follows thrombosis, these diagnostic criteria for acute MI may fail to identify patients before the induction of irreversible myocardial necrosis. Additionally, the current diagnostic methods do not target the differentiation of thrombotic and non-thrombotic causes of MI such as demand ischemia and stress cardiomyopathy, which has varying therapeutic and prognostic implications.3 While the third universal definition of acute MI identifies angiographic confirmation of coronary thrombus as a criterion for the diagnosis of acute MI and differentiating myocardial necrosis caused by thrombotic versus non-thrombotic etiologies,4 coronary angiography is an invasive procedure with significant side effects and often cannot differentiate between stable nonculprit lesions and lesions causing acute thrombotic MI. Management of thrombotic MI focuses on resolving the thrombotic obstruction to revive coronary blood flow with the use of antithrombotic, anticoagulant, and fibrinolytic drugs and procedural revascularization therapies. These therapies would be expected to have all of the same risks (eg, bleeding) without any potential benefit in patients who do not have an acute thrombus. Therefore, in patients presenting with symptoms suggestive of acute MI, the ability to identify an MI early on, prior to serum troponin elevation, and to discriminate thrombotic from non-thrombotic MI would significantly improve treatment outcomes.
Platelets have a primary function of stopping hemorrhage from vascular endothelium or tissue following an injury.5 In a pathological spin-off to their apportioned physiological function of plugging endothelial defects, platelet activation in response to plaque disruption, resulting in occlusive thrombosis, is the paradigm for acute MI. The process of plaque progression and disruption is triggered by inflammatory and immune changes that convert the surface endothelium into a pro-atherothrombotic surface via cell adhesion molecules, primarily P-selectin and E-selectin.6 Upon damage to the plaque, platelet activation is enhanced through the secretion of storage granules and adhesive ligands, which further promote platelet aggregation.7 In the event of atherosclerotic plaque disruption, elevated rates of platelet aggregation potentiate the release of larger, more reactive platelets from the bone marrow.8 The enhanced reactivity of these newly released platelets is attributed to elevated concentrations of active substances within microgranules (eg, thromboxane A2 and B2, platelet factor 4, P-selectin, platelet-derived growth factor), as well as increased expression of adhesive receptors (glycoprotein IIb/IIIa).9,10 Prior studies have demonstrated a significantly higher mean platelet volume (MPV) in patients presenting with acute coronary syndrome (ACS); however, the duration of MPV elevation post-ACS has not been delineated.11–17
The MPV is a measure of platelet size and activity,18 and the role of platelet activation is fundamental in the development of an acute MI.5 Thus, we hypothesize that platelet count and higher MPV could be used as surrogates for platelet consumption and activation and therefore aid in the diagnosis of an acute MI, specifically acute thrombotic MI. Therefore, we compared platelet count and MPV between subjects with stable coronary artery disease (CAD) and subjects with acute MI at the time of acute event (MI/cardiac catheterization) and after resolution of acute event (follow-up during the quiescent phase). A secondary analysis aims to assess the difference in platelet count and MPV in acute MI subtypes—thrombotic and non-thrombotic MI.
Methods
Study Design and Population
Following institutional review board approval, subjects were recruited from 2 hospitals in Louisville, Kentucky between March 2012 and August 2013. Two categories of subjects were sought for enrollment—those with suspected acute MI and those with suspected stable CAD. All subjects provided written informed consent. Enrollment criteria for both groups required that each participant should be aged >18 years and scheduled for coronary angiography within 48 hours. Subjects who received fibrinolytic therapy were not eligible. Enrollment criteria for suspected acute MI and suspected stable CAD are detailed in the supplemental material (Appendix Table 1).
Biochemical Analysis
Clinical laboratory data were tested at 2 independent clinical laboratory improvement amendments (CLIA) approved laboratories at the University of Louisville Hospital (ULH) and Ken-tuckyOne Health Jewish Hospital (JH). The MPV and platelet counts were tested using the impedance technique in a Sysmex XE-2100 analyzer (Sysmex, Kobe, Japan, MPV normal range: 8.7–12 fL; platelet count normal range: 140–370 × 103/mm3) at ULH and Beckman Coulter LH780 analyzer (Beckman Coulter, California, USA, MPV normal range: 7.4–10.4 fL; platelet count normal range: 150–390 × 103/mm3) at JH. Troponin criteria for outcomes and additional biochemical analysis are detailed in the supplemental material.
Coronary Angiography and Histological Analysis
Angiograms were systematically evaluated in all subjects by the Angiographic Core Laboratory at the Johns Hopkins University (Baltimore, Maryland) by a technician and physician blinded to all other participant data.
Coronary aspiration was left to the discretion of the treating interventional cardiologist. The standard of care at the enrollment sites for this study was to attempt thrombus aspiration in all ST-segment elevation myocardial infarction (STEMI) patients when coronary thrombus was thought to exist. If aspiration was attempted, the aspirate was immediately filtered and preserved in formalin for histological evaluation by a pathologist specializing in coronary thrombosis at CVPath, Inc. (Gaithersburg, Maryland).19 The pathologist was blinded to all other participant data, except the vessel from which the aspirate was obtained.
Sample Time Points
Samples were collected immediately upon arrival to cardiac catheterization laboratory (acute phase) prior to angiography or any coronary interventions and at follow-up (quiescent phase). Acute phase samples were collected from an arterial sheath after a 5 to 10 mL waste draw, whereas for the quiescent phase samples, virgin peripheral veins were phlebotomized with 5 to 10 mL waste draw.
Study Group Classification
Four subject groups were defined for this study a priori—stable CAD, acute MI, thrombotic MI, non-thrombotic MI (Appendix Table 2). Subjects with thrombotic MI and non-thrombotic MI were a subset of the acute MI group with borderline cases removed. We developed novel conservative criteria, a priori, eliminating borderline cases from analysis in order to limit confounding factors from misclassification and to produce an ideal cohort for discovering new biology related to acute MI (Appendix Table 2). Our criteria are a variation of criteria previously proposed by our group.10,20 We believe these criteria are more robust than any other published criteria for distinguishing between thrombotic and non-thrombotic MI.21–25
Statistical Analysis
Normally distributed outcome measures were compared between groups using Student or Welch t tests if the heterogeneity of variance assumption was violated. Outcome measures that were not approximately normal were compared using Wilcoxon rank sum tests. To check for the presence of confounding factors, the Pearson correlation coefficient was computed for each pair of outcome measure and risk factor (potential confounder). These associations were reported as signed r2 in order to summarize both the direction and strength of the association.
Analysis of Time Difference
Limited data suggest that 120 minutes from blood draw may be optimal for MPV assessment.26 Hence, to examine the effect of analysis time, the outcome measures for all subjects and time points were regressed on analysis time difference.
Outcome Measures
The primary outcome measures evaluated for differences between the stable CAD and acute MI groups were platelet count and MPV at the acute phase, absolute change from acute phase to follow-up in platelet count and MPV, and relative change from baseline to follow-up in platelet count and MPV. A subgroup analysis was conducted to evaluate the differences between the thrombotic and non-thrombotic subgroups. Inspection of histograms and Shapiro-Wilk tests were conducted in order to determine whether outcome measures were approximately normally distributed.
Calibration of Measurements
Subjects were enrolled from 2 different hospitals, both of which use different analysis techniques for platelet count and MPV measurement. A reliability study using biological samples independent of the study participants was conducted to determine whether platelet count and MPV measurements differed across the 2 hospitals. The effect of hospital was significant for both platelet count and MPV, necessitating calibration of both measurements (Appendix Figure 1). Each outcome measure was calibrated using the results of the reliability study to ensure consistency between the JH and ULH values. Specific details about the calibration study are included in the supplemental material.
Results
Of the 80 subjects enrolled, 19 subjects either did not meet inclusion criteria or had missing data and were hence removed from analysis. Study enrollment scheme is outlined in the supplemental material (Appendix Figure 2). Sixty-one valid subjects were included in the statistical analysis, of which 14 subjects met the phenotype criteria for stable CAD and 47 subjects for acute MI (Appendix Table 2). In the acute MI group, 22 subjects met criteria for thrombotic MI, 12 for non-thrombotic MI, and 13 had an indeterminate MI cause.
Current smoking, heart rate, diastolic blood pressure, and coronary stenosis were all higher as compared to subjects with stable CAD (Table 1). Past smoking, history of dyslipidemia, diabetes, and body mass index were lower in subjects with acute MI as compared to participants with stable CAD (Table 1).
Table 1.
Baseline Participant Characteristics.
| Variables | Acute MI Group (n = 47) | Stable CAD Group (n = 14) | P Value |
|---|---|---|---|
| Age (mean ± SD), years | 58.6 ± 14.4 | 61.3 ± 8.9 | 0.49 |
| Males (%) | 65.3 | 53.3 | 0.54 |
| Caucasian race (%) | 77.6 | 93.3 | 0.09 |
| Current smoker (%) | 53.1 | 20.0 | 0.04 |
| Former smoker (%) | 26.5 | 60.0 | 0.04 |
| Never consumed alcohol (%) | 55.1 | 40.0 | 0.54 |
| Currently consumes alcohol (%) | 32.7 | 46.7 | 0.37 |
| History of dyslipidemia (%) | 46.9 | 86.7 | 0.01 |
| History of diabetes mellitus (%) | 10.2 | 40.0 | 0.03 |
| History of hypertension (%) | 61.2 | 93.3 | 0.06 |
| History of atherosclerosis (%) (MI, CAD, PCI, CABG) | 38.8 | 100.0 | <0.0001 |
| History of congestive heart failure (%) | 8.2 | 6.7 | 1.00 |
| History of chronic renal failure (%) | 8.2 | 0.0 | 0.28 |
| History of stroke (%) | 10.2 | 0.0 | 0.33 |
| HR at the time of presentation (mean ± SD) | 85 | 66 | <0.0001 |
| SBP at the time of presentation (mean ± SD) | 137.1 ± 33.3 | 133.4 ± 16.5 | 0.56a |
| DBP at the time of presentation (mean ± SD) | 85.6 ± 21.4 | 70.3 ± 15.3 | 0.01 |
| MAP at the time of presentation (mean ± SD) | 102.8 ± 24.7 | 91.4 ± 14.3 | 0.09 |
| BMI at the time of presentation (mean ± SD) | 27.5 ± 7.24 | 33 ± 7.08 | 0.02 |
| Time (hours) elapsed from presentation to T0 (median ± IQR, range) | 3.5 ± 16.0, 36 | 2.0 ± 1.0, 3 | 0.21b |
| Time (hours) elapsed from symptoms to T0 (median ± IQR, range) | 16.0 ± 25.0, 156 | N/A | N/A |
| Time (hours) elapsed from blood draw to platelet analysis (median ± IQR, range) | 0.53 ± 0.65, 5.69 | 0.25 ± 0.72, 4.28 | 0.04b |
| Baseline troponin (mean ± SD, range), mg/dL | 5.59 ± 10.83, 55.3 | 0.009 ± 0.003, 0.01 | <0.0001b |
| Peak troponin (mean ± SD, range) | 33.5 ± 37.9, 150.3 | 0.008 ± 0.004, 0.01 | <0.0001b |
| Glucose at baseline (mean ± SD, range) | 142.1 ± 56.5, 285 | 131.6 ± 30.6, 107 | 0.35a |
| Creatinine at baseline (mean ± SD, range) | 1.13 ± 0.79, 4.34 | 0.92 ± 0.17, 0.66 | 0.10a |
| ST-segment elevation on ECG at baseline | 63.3 | 0.0 | <0.0001 |
| At least 1 vessel with ≥50% coronary stenosis on enrollment angiogram | 73.5 | 40.0 | 0.03 |
| Aspirin use at the time of enrollment (%) | 89.8 | 86.7 | 0.66 |
| P2Y12 inhibitors use at enrollment (%) | 53.1 | 50.0 | 0.77 |
Abbreviations: BMI, body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; DBP, diastolic blood pressure; ECG, electrocardiography; HR, heart rate; IQR, interquartile range; MAP, mean arterial pressure; MI, myocardial infarction; N/A, stable disease with no acute symptoms therefore unable to quantify time of onset or compute P Value; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; SD, standard deviation; T0, acute phase at cardiac catheterization.
Welch t test.
Wilcoxon rank sum test.
In the acute MI group, the time lapse from blood draw to platelet analysis was significantly higher (median ± inter-quartile range: 0.53 ± 0.65 hours) as compared to the stable CAD (0.25 ± 0.72 hours; P = 0.04). The coefficient for analysis time difference in the platelet count model was not significant. The coefficient for analysis time difference in the MPV model was significant (β = 0.00003; P = 0.005); however, analysis time difference explained only 3.58% of variance. In the regression analysis, the difference between when blood was drawn and when MPV was measured explained only 3.58% of the variation in MPV (R2 = 3.58%). Additional stratification by study group did not modify this trend (residual R2 = 2.91% attributed to time difference), indicating a lack of evidence that time from blood draw to MPV measurement impacted the study groups differently.
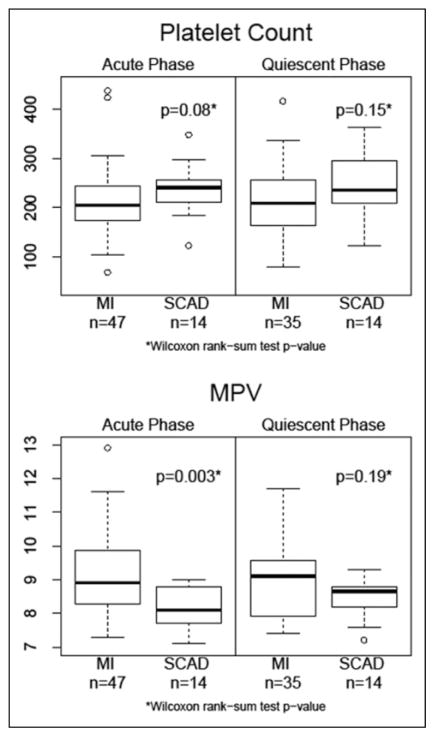
Significant differences were not observed in platelet count between subjects with acute MI and stable CAD at the acute phase (time of cardiac catheterization) or at the quiescent phase follow-up (median of 3.2 months post-MI/cardiac catheterization; Table 2 and Figure 1). The MPV was significantly higher in the acute MI group (9.18 ± 1.21) as compared to stable CAD (8.13 ± 0.66; P = 0.003) at the acute phase but not at the quiescent phase (Table 2 and Figure 1).
Table 2.
Comparison of PLT Counts and MPV Between Stable CAD and Acute MI Groups.
| Biomarkers | Time Point | Stable CAD (n = 14)
|
Acute MI (n = 47)
|
P Value | ||
|---|---|---|---|---|---|---|
| Mean ± SD | 95% CI | Mean ± SD | 95% CI | |||
| Platelet count | Acute phase | 237.3 ± 52.3 | 208.4–266.3 | 211.3 ± 69.4 | 191.4–231.3 | 0.08a |
| Quiescent phase | 241.1 ± 63.9 | 204.2–278.0 | 214.7 ± 65.3 | 192.6–236.7 | 0.15a | |
| Mean platelet volume | Acute phase | 8.13 ± 0.66 | 7.75–8.51 | 9.18 ± 1.21 | 8.82–9.53 | 0.003a |
| Quiescent phase | 8.48 ± 0.58 | 8.15–8.82 | 8.94 ± 1.42 | 8.55–9.34 | 0.19a | |
Abbreviations: CAD, coronary artery disease; CI, confidence interval; MI, myocardial infarction; MPV, mean platelet volume; PLT, platelet; SD, standard deviation.
Wilcoxon rank sum test P value.
Figure 1.
Comparison of platelet count and mean platelet volume between acute myocardial infarction and stable coronary artery disease groups. MI indicates myocardial infarction; MPV, mean platelet volume; SCAD, stable coronary artery disease.
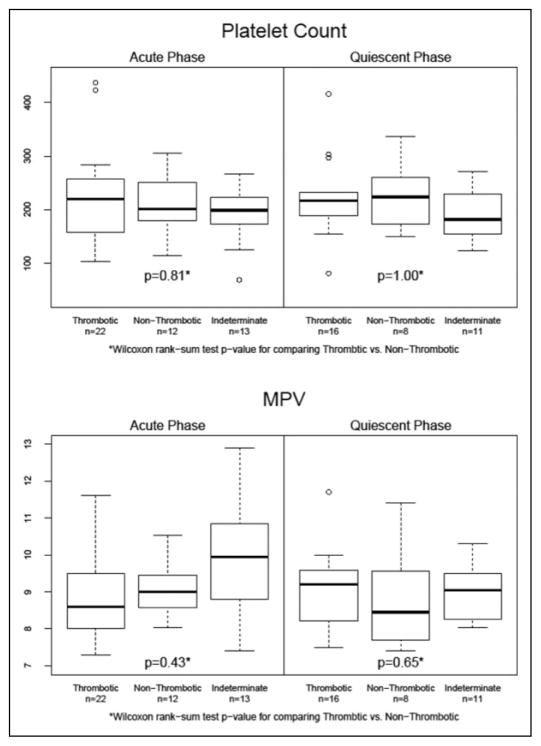
In the acute MI group, 22 subjects met criteria for thrombotic MI and 12 met criteria for non-thrombotic MI (Appendix Figure 2). Significant differences were not observed in platelet count between thrombotic and non-thrombotic subgroups at the acute phase (223.5 ± 84.4 vs 211.6 ± 56.3, P > 0.05) or at the quiescent phase follow-up (223.5 ± 75.3 vs 225.3 ± 63.6; P > 0.05; Table 3 and Figure 2). Significant differences were not observed in MPV between thrombotic and non-thrombotic subgroups at the acute phase (8.89 ± 1.15 vs 9 ± 0.72; P > 0.05) or at the quiescent phase follow-up (8.79 ± 1.32 vs 8.99 ± 0.8; P > 0.05; Table 3 and Figure 2). The power to detect a difference in MPV between thrombotic and non-thrombotic MI was 58%.
Table 3.
Comparison of PLT Count and MPV Between Thrombotic and Non-Thrombotic MI Groups.
| Biomarkers | Time Point | Thrombotic MI (n = 22)
|
Non-Thrombotic MI (n = 12)
|
P value | ||
|---|---|---|---|---|---|---|
| Mean ± SD | 95% CI | Mean ± SD | 95% CI | |||
| Platelet count | Acute phase | 223.5 ± 84.4 | 186.0–260.9 | 211.6 ± 56.3 | 175.8–247.4 | 0.81a |
| Quiescent phase | 223.5 ± 75.3 | 184.7–262.2 | 225.3 ± 63.6 | 172.1–278.4 | 1.00a | |
| Mean platelet volume | Acute phase | 8.89 ± 1.15 | 8.38–9.40 | 9.00 ± 0.72 | 8.54–9.45 | 0.43a |
| Quiescent phase | 8.79 ± 1.32 | 7.77–9.71 | 8.99 ± 0.80 | 8.32–9.66 | 0.41a | |
Abbreviations: CI, confidence interval; MI, myocardial infarction; MPV, mean platelet volume; PLT, platelet; SD, standard deviation.
Wilcoxon rank sum test P value.
Figure 2.
Comparison of platelet count and mean platelet volume between thrombotic and non-thrombotic myocardial infarction groups. MI indicates myocardial infarction; MPV, mean platelet volume; SCAD, stable coronary artery disease.
Discussion
In this prospective study of subjects with acute MI and stable CAD, there were 3 major findings with important clinical implications. First, MPV is significantly increased in subjects with acute MI as compared to subjects with stable CAD during the acute phase of an MI. Second, this difference is not observed during the quiescent phase (~3 months post-MI). Third, MPV does not differ significantly between thrombotic and non-thrombotic MI patients and is therefore not useful as an independent biomarker in distinguishing between these types of MI.
An association of increased MPV with acute MI has been previously observed9,27–29; however, the mechanism underlying this phenomenon has yet to be elucidated. One such explanation could be a global increase in serum thrombopoietin levels secondary to platelet consumption during acute MI, which would, in effect, stimulate megakaryocyte proliferation and enhance the production/liberation of larger, more hemostatically active immature platelets from the bone marrow.30–33 Consistent with this notion, in a previous study, Senaran and colleagues were able to establish a correlation between throm-bopoietin levels and MPV values in patients with acute MI.34 Therein they found patients with acute MI to exhibit elevated MPV and serum thrombopoietin levels compared to healthy controls.34 Although thrombopoiesis is a prominent mechanism influencing platelet volume,33 platelet consumption has also been shown to influence and increase MPV.35
Although there are conflicting opinions on the platelet turnover theory,36,37 our data are consistent with prior reports of higher MPV at the time of an acute MI.9,11,14,15,17,27–29 The second finding in our study is a novel observation wherein we found that during a quiescent phase of approximately 3 months post-MI, MPV in the acute MI group was no longer different from stable CAD. Plaque disruption triggers platelet activation resulting in thrombus formation/platelet consumption, which in turn stimulates production/release of immature platelets resulting in a sustained increase of MPV. The time period of MPV elevation post-MI has not been quantified in human subjects. Our study provides an insight to the duration of MPV elevation.
Martin et al found higher MPV 6 months post-MI in patients who had a second ischemic event 6 to 24 months postindex MI as compared to patients who did not experience any ischemic event 6 to 24 months postindex MI.38 Their findings do not identify a trajectory of MPV between 2 distinct time points (ie, acuity and quiescence) and differ from our study in that they did not compare the acute MI group with a non-MI control group (ie, stable CAD). Funck-Jensen et al found that indices of platelet turnover were higher in 48 hours following acute STEMI as compared to a stable (quiescent) phase 3 months later.39 Funck-Jensen et al did not have a non-MI comparator group. In both the above-mentioned studies, MPV increases are actually a deviation in values from the perceived norm rather than an observation of difference from a valid control group. Shah et al performed a retrospective analysis of MPV in 1512 patients undergoing percutaneous coronary intervention (PCI) and found increased risk of mortality among patients with increased MPV post-PCI.40 Therefore, MPV may be a modifiable prognostic factor post-MI/PCI.
Chu et al analyzed MPV of 282 consecutive patients arriving to the emergency department with acute chest pain, wherein they found increased MPV as an early and independent predictor of ACS.11 Similarly, Lippi et al reported patients with ACS to have higher MPV than patients without ACS.15 Furthermore, Yilmaz et al observed a stepwise decrease in MPV between patients with non-STEMI, unstable angina, and stable CAD, respectively.17 More importantly, Klovaite et al found that in the general population, the risk of MI was increased by 38% in individuals with elevated MPV (>7.4 fL) independently of known cardiovascular risk factors.14 Our third finding demonstrates that MPV and platelet count are not likely to be useful in differentiating thrombotic and non-thrombotic MIs as isolated diagnostic markers. Despite our limited power to conclude no difference in the mean MPV and platelet count in our thrombotic and non-thrombotic MI subgroups, the overlapping range of the values in these groups disqualifies these measures as diagnostic markers in isolation. Given the importance of the clinical distinction, further research is warranted to evaluate the utility of these analytes in combination with other factors for distinguishing thrombotic and nonatherothrombotic MI.
Limitations
Although this is the largest study of MPV and platelet count in subjects with acute thrombotic and non-thrombotic MI, the low sample and power of the study may have played a role in not finding significant differences between these groups. This study does not have long-term follow-up data on cardiovascular events and is therefore unable to evaluate these biomarkers as predictors of events in post-MI.
Conclusion
Higher MPV at the time of acute MI subjects resolves by 3 months. Platelet count and MPV measures overlap in subjects with thrombotic versus non-thrombotic MI, limiting the utility of these measures as individual diagnostic biomarkers. Further investigation is warranted to evaluate the utility of platelet count and MPV, in addition to other novel diagnostics, in the diagnosis of acute MI and the distinction between thrombotic and non-thrombotic MI.
Supplementary Material
Acknowledgments
The authors would like to thank the subjects who participated in this study. The authors also appreciate the support provided by the Diabetes and Obesity Center, University of Louisville; the University of Louisville Hospital, and the KentuckyOne Health Jewish Hospital.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: American Heart Association (11CRP7300003) and National Institutes of Health (1P20 GM103492).
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The online supplemental data are available at http://journals.sagepub.com/doi/suppl/10.1177/1076029616683804.
Authors’ Note
A. R. Amraotkar, D. D. Song, and A. P. DeFilippis contributed to conception and design. D. D. Song, A. R. Amraotkar, I. Ismail, V. Kothari, and A. Singh contributed to collection and assembly of data. A. R. Amraotkar, D. D. Song, A. P. DeFilippis, D. Otero, J. B. Moore IV, P. J. Trainor, I. Ismail, and V. Kothari drafted the article. A. R. Amraotkar, D. D. Song, A. P. DeFilippis, D. Otero, J. B. Moore IV, P. J. Trainor, I. Ismail, V. Kothari, A. Singh, and S. N. Rai contributed to critical revision for important intellectual content. A. P. DeFilippis and S. N. Rai contributed to administrative, technical, or logistic support.
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Executive summary: heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129(3):399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 3.Newby LK, Jesse RL, Babb JD, et al. ACCF 2012 expert consensus document on practical clinical considerations in the interpretation of troponin elevations: a report of the American College of Cardiology Foundation task force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2012;60(23):2427–2463. doi: 10.1016/j.jacc.2012.08.969. [DOI] [PubMed] [Google Scholar]
- 4.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Glob Heart. 2012;7(4):275–295. doi: 10.1016/j.gheart.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Davi G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357(24):2482–2494. doi: 10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- 6.Borissoff JI, Spronk HM, ten Cate H. The hemostatic system as a modulator of atherosclerosis. N Engl J Med. 2011;364(18):1746–1760. doi: 10.1056/NEJMra1011670. [DOI] [PubMed] [Google Scholar]
- 7.Ruggeri ZM. Platelets in atherothrombosis. Nat Med. 2002;8(11):1227–1234. doi: 10.1038/nm1102-1227. [DOI] [PubMed] [Google Scholar]
- 8.Ranjith MP, Divya R, Mehta VK, Krishnan MG, KamalRaj R, Kavishwar A. Significance of platelet volume indices and platelet count in ischaemic heart disease. J Clin Pathol. 2009;62(9):830–833. doi: 10.1136/jcp.2009.066787. [DOI] [PubMed] [Google Scholar]
- 9.Chu SG, Becker RC, Berger PB, et al. Mean platelet volume as a predictor of cardiovascular risk: a systematic review and meta-analysis. J Thromb Haemost. 2010;8(1):148–156. doi: 10.1111/j.1538-7836.2009.03584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeFilippis AP, Oloyede OS, Andrikopoulou E, et al. Thrombox-ane A(2) generation, in the absence of platelet COX-1 activity, in patients with and without atherothrombotic myocardial infarction. Circ J. 2013;77(11):2786–2792. doi: 10.1253/circj.cj-12-1421. [DOI] [PubMed] [Google Scholar]
- 11.Chu H, Chen WL, Huang CC, et al. Diagnostic performance of mean platelet volume for patients with acute coronary syndrome visiting an emergency department with acute chest pain: the Chinese scenario. Emerg Med J. 2011;28(7):569–574. doi: 10.1136/emj.2010.093096. [DOI] [PubMed] [Google Scholar]
- 12.Dehghani MR, Taghipour-Sani L, Rezaei Y, Rostami R. Diagnostic importance of admission platelet volume indices in patients with acute chest pain suggesting acute coronary syndrome. Indian Heart J. 2014;66(6):622–628. doi: 10.1016/j.ihj.2014.10.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khode V, Sindhur J, Kanbur D, Ruikar K, Nallulwar S. Mean platelet volume and other platelet volume indices in patients with stable coronary artery disease and acute myocardial infarction: a case control study. J Cardiovasc Dis Res. 2012;3(4):272–275. doi: 10.4103/0975-3583.102694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klovaite J, Benn M, Yazdanyar S, Nordestgaard BG. High platelet volume and increased risk of myocardial infarction: 39,531 participants from the general population. J Thromb Haemost. 2011;9(1):49–56. doi: 10.1111/j.1538-7836.2010.04110.x. [DOI] [PubMed] [Google Scholar]
- 15.Lippi G, Filippozzi L, Salvagno GL, et al. Increased mean platelet volume in patients with acute coronary syndromes. Arch Pathol Lab Med. 2009;133(9):1441–1443. doi: 10.5858/133.9.1441. [DOI] [PubMed] [Google Scholar]
- 16.Yaghoubi A, Golmohamadi Z, Alizadehasl A, Azarfarin R. Role of platelet parameters and haematological indices in myocardial infarction and unstable angina. J Pak Med Assoc. 2013;63(9):1133–1137. [PubMed] [Google Scholar]
- 17.Yilmaz MB, Cihan G, Guray Y, et al. Role of mean platelet volume in triagging acute coronary syndromes. J Thromb Thrombolysis. 2008;26(1):49–54. doi: 10.1007/s11239-007-0078-9. [DOI] [PubMed] [Google Scholar]
- 18.Park Y, Schoene N, Harris W. Mean platelet volume as an indicator of platelet activation: methodological issues. Platelets. 2002;13(5–6):301–306. doi: 10.1080/095371002220148332. [DOI] [PubMed] [Google Scholar]
- 19.CVPath Inc. package insert. Gaithersburg, MD: CVPath Inc; 2013. [Google Scholar]
- 20.DeFilippis AP, Chernyauskiy I, Amraotkar AR, et al. Circulating levels of plasminogen and oxidized phospholipids bound to plas-minogen distinguish between atherothrombotic and non-atherothrombotic myocardial infarction. J Thromb Thrombolysis. 2016;42(1):61–76. doi: 10.1007/s11239-015-1292-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ambrose JA, Loures-Vale A, Javed U, Buhari CF, Aftab W. Angiographic correlates in type 1 and 2 MI by the universal definition. JACC Cardiovasc Imaging. 2012;5(4):463–464. doi: 10.1016/j.jcmg.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 22.Javed U, Aftab W, Ambrose JA, et al. Frequency of elevated troponin I and diagnosis of acute myocardial infarction. Am J Cardiol. 2009;104(1):9–13. doi: 10.1016/j.amjcard.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Saaby L, Poulsen TS, Diederichsen AC, et al. Mortality rate in type 2 myocardial infarction: observations from an unselected hospital cohort. Am J Med. 2014;127(4):295–302. doi: 10.1016/j.amjmed.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 24.Saaby L, Poulsen TS, Hosbond S, et al. Classification of myocar-dial infarction: frequency and features of type 2 myocardial infarction. Am J Med. 2013;126(9):789–797. doi: 10.1016/j.amjmed.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 25.Stein GY, Herscovici G, Korenfeld R, et al. Type-II myocardial infarction—patient characteristics, management and outcomes. PLoS One. 2014;9(1):e84285. doi: 10.1371/journal.pone.0084285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lance MD, van Oerle R, Henskens YM, Marcus MA. Do we need time adjusted mean platelet volume measurements? Lab Hematol. 2010;16(3):28–31. doi: 10.1532/LH96.10011. [DOI] [PubMed] [Google Scholar]
- 27.Hendra TJ, Oswald GA, Yudkin JS. Increased mean platelet volume after acute myocardial infarction relates to diabetes and to cardiac failure. Diabetes Res Clin Pract. 1988;5(1):63–69. doi: 10.1016/s0168-8227(88)80080-9. [DOI] [PubMed] [Google Scholar]
- 28.Huczek Z, Kochman J, Filipiak KJ, et al. Mean platelet volume on admission predicts impaired reperfusion and long-term mortality in acute myocardial infarction treated with primary percutaneous coronary intervention. J Am Coll Cardiol. 2005;46(2):284–290. doi: 10.1016/j.jacc.2005.03.065. [DOI] [PubMed] [Google Scholar]
- 29.Yang A, Pizzulli L, Luderitz B. Mean platelet volume as marker of restenosis after percutaneous transluminal coronary angio-plasty in patients with stable and unstable angina pectoris. Thromb Res. 2006;117(4):371–377. doi: 10.1016/j.thromres.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Erhart S, Beer JH, Reinhart WH. Influence of aspirin on platelet count and volume in humans. Acta Haematol. 1999;101(3):140–144. doi: 10.1159/000040940. [DOI] [PubMed] [Google Scholar]
- 31.de Sauvage FJ, Hass PE, Spencer SD, et al. Stimulation of mega-karyocytopoiesis and thrombopoiesis by the c-Mpl ligand. Nature. 1994;369(6481):533–538. doi: 10.1038/369533a0. [DOI] [PubMed] [Google Scholar]
- 32.Kaushansky K. Thrombopoietin: the primary regulator of platelet production. Blood. 1995;86(2):419–431. [PubMed] [Google Scholar]
- 33.van der Loo B, Martin JF. A role for changes in platelet production in the cause of acute coronary syndromes. Arterioscler Thromb Vasc Biol. 1999;19(3):672–679. doi: 10.1161/01.atv.19.3.672. [DOI] [PubMed] [Google Scholar]
- 34.Senaran H, Ileri M, Altinbas A, et al. Thrombopoietin and mean platelet volume in coronary artery disease. Clin Cardiol. 2001;24(5):405–408. doi: 10.1002/clc.4960240511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gladwin AM, Martin JF. The control of megakaryocyte ploidy and platelet production: biology and pathology. Int J Cell Cloning. 1990;8(4):291–298. doi: 10.1002/stem.5530080414. [DOI] [PubMed] [Google Scholar]
- 36.Garg SK, Amorosi EL, Karpatkin S. Use of the megathrombocyte as an index of megakaryocyte number. N Engl J Med. 1971;284(1):11–17. doi: 10.1056/NEJM197101072840103. [DOI] [PubMed] [Google Scholar]
- 37.Martin JF, Plumb J, Kilbey RS, Kishk YT. Changes in volume and density of platelets in myocardial infarction. Br Med J (Clin Res Ed) 1983;287(6390):456–459. doi: 10.1136/bmj.287.6390.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin JF, Bath PM, Burr ML. Influence of platelet size on outcome after myocardial infarction. Lancet. 1991;338(8780):1409–1411. doi: 10.1016/0140-6736(91)92719-i. [DOI] [PubMed] [Google Scholar]
- 39.Funck-Jensen KL, Dalsgaard J, Grove EL, Hvas AM, Kristensen SD. Increased platelet aggregation and turnover in the acute phase of ST-elevation myocardial infarction. Platelets. 2013;24(7):528–537. doi: 10.3109/09537104.2012.738838. [DOI] [PubMed] [Google Scholar]
- 40.Shah B, Oberweis B, Tummala L, et al. Mean platelet volume and long-term mortality in patients undergoing percutaneous coronary intervention. Am J Cardiol. 2013;111(2):185–189. doi: 10.1016/j.amjcard.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.