Abstract
Objectives
To assess the association between biomarkers of thyroid status and 5α-stanols in sitosterolemia (STSL) patients treated with ezetimibe (EZE).
Study design
Eight STSL patients (16-56 years) were studied during 14 weeks off EZE therapy (OFF EZE) and 14 weeks on EZE (10 mg/d; ON EZE). Serum thyroid biomarkers [free triiodothyronine (FT3), free thyroxine (FT4), FT3/FT4 ratio, thyroid stimulating hormone], 5α-stanols (sitostanol and cholestanol), and cholestanol precursors [total cholesterol (TC) and its synthesis marker lathosterol, and 7α-hydroxy-4-cholesten-3-one cholestanol (7α-H-C4)] were measured at baseline and during the 14 weeks off and on EZE.
Results
EZE increased FT3/FT4 (10% ± 4%, P = .02). EZE reduced plasma and red blood cells sitostanol (−38% ± 6% and −20% ± 4%, all P < .05) and cholestanol (−18% ± 6% and −13% ± 3%, all P < .05). The change in plasma cholestanol level ON EZE inversely correlated with the change in FT3/FT4 (r = −0.86, P = .01). EZE lowered TC (P <.0001) and did not affect 7α-H-C4. EZE increased (P <.0001) lathosterol initially but the level was not sustained, resulting in similar levels at week 14 OFF and ON EZE.
Conclusion
In STSL patients, 5α-stanols levels might be associated with thyroid function. EZE reduces circulating 5α-stanols while increasing FT3/FT4, implying increased conversion of T4 to T3, thus possibly improving thyroid hormone status.
Trial registration: ClinicalTrials.gov NCT01584206
Keywords: Cholestanol, sitostanol, total cholesterol, lathosterol, 7α-hydroxy-4-cholesten-3-one, phytosterolemia
Sitosterolemia (STSL) is a rare disease caused by mutations in either of the ATP-binding cassette transporter genes, ABCG5 or ABCG8, that result in accumulation of plant sterols and their corresponding saturated 5α-stanols in the body.1, 2 Clinical features of STSL include xanthomas, premature atherosclerosis and macrothrombocytopenia.1 Endocrine disruption has also been reported.3 Synthesis of thyroid hormones appear to be deranged in cerebrotendinous xanthomatosis (CTX), a disorder of bile acid synthesis,4–6 and STSL.7 Both disorders have elevated plasma and tissue cholestanol levels that might contribute to thyroid imbalance. Specifically, concurrent high levels of cholestanol, a 5α-stanol saturated derivative of cholesterol,8 and hypothyroidism have been observed in CTX,4–6 and STSL,7 suggesting a link between underactive thyroid and 5α-stanols. However, the association between thyroid function and 5α-stanols has not, to our knowledge, been further elucidated.
For most individuals on a typical Western diet, 5α-stanols (cholestanol and sitostanol) are almost absent from the diet thus their presence in the body is mostly via endogenous production from cholesterol and sitosterol, respectively.9–11 Cholestanol is biosynthesized from cholesterol12,13 or its metabolite 7α-hydroxy-4-cholesten-3-one (7α-H-C4), which is also involved in bile acid synthesis.14, 15 Plasma cholestanol levels are normally low, but high in STSL16 and CTX.17 Biosynthesis of cholestanol precursors, including 7α-H-C4 and cholesterol (reflected by its synthesis marker lathosterol), are low in STSL,18–20 so it is unclear if cholestanol accumulation arises from the diet or endogenously. Ezetimibe (EZE), the primary treatment for STSL that works by reducing plant sterol absorption, reduces intestinal sterol uptake,21, 22 but its effect on circulating 5α-stanols has not yet been examined. This study aimed to explore the nature of the relationship between 5α-stanols and thyroid hormones, and determine the effects of EZE on thyroid hormones as well as blood levels of sitostanol, and cholestanol and its precursors (total cholesterol (TC) and its synthesis marker lathosterol, and 7α-H-C4) in STSL patients.
Methods
The study design from which these data were taken was previously reported.23 This report was part of a much larger pilot, interventional trial (ClinicalTrials.gov: NCT01584206) investigating the effect of EZE on sterol metabolism in STSL patients. In summary, eight patients (5 males and 3 females, between 16 and 56 years of age) with homozygous ABCG8 S107X mutation (NM_022437.2:c.320C>G) were recruited from Hutterite communities. The study was approved by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the University of Manitoba Biomedical Ethics board, and written informed consent was obtained from all patients. After enrollment, patients were taken off their EZE treatment for 14 weeks (OFF EZE). For the full study design, blood was collected at week 2, 4, 6, 8, 10, 12 and 14. After 14 weeks OFF EZE, patients were instructed to take EZE (10 mg/day) for 14 weeks (ON EZE). Blood was collected following the same schedule as in the OFF EZE phase. Note that only selected samples from the blood collection protocol were used for assessment of thyroid hormones (see below) due to limited availability of serum samples required. Serum, plasma, and red blood cells (RBC) fractions were separated by centrifugation at 3000 rpm for 20 min at 4°C, and stored at −80°C until analysis.
The concentrations of serum thyroid hormones: free triiodothyronine (FT3), free thyroxine (FT4) and thyroid-stimulating hormone (TSH) were measured at baseline (beginning of the OFF EZE phase), 8 and 14 weeks OFF and ON EZE by an outsourced laboratory (Gamma-Dynacare Medical Laboratories, Winnipeg, MB, Canada) using automated immunoassays (Abbott Laboratories, Abbott Park, IL, US). The co-efficient of variation (CV) for the assays were: FT4: intra-CV <2.2%; inter-CV <4.9%. FT3: intra-CV <3.2%; inter-CV <5.9%. TSH: intra-CV <1.2%; inter-CV <2.8%.The ratio of FT3 to FT4 (FT3/FT4) was calculated as an indirect index of deiodinase activity.24
Plasma and RBC 5α-stanols, TC and lathosterol levels were measured using gasliquid chromatography equipped with a flame ionization detector (Varian 430-GC; Agilent Technologies, Santa Clara, CA, US) as published previously.23 Measurement of RBC sterol levels indicates a longer-term average of plasma levels and a better reflection of tissue stores. Plasma sterol and 5α-stanol levels were determined at baseline and biweekly up to 14 weeks OFF and ON EZE, while those in RBC were measured at baseline, 4, 8, 10 and 14 weeks OFF and ON EZE.
Serum 7α -H-C4 levels were measured at baseline, 4, 8, 10 and 14 weeks OFF and ON EZE using ultra-performance liquid chromatography tandem mass spectrometry.25 Serum (50 µL) was diluted with 100 µL of double distilled water (ddH2O), and 50 µL of the 40 ng/mL deuterated internal standard 7-hydroxy-4-cholesten-3-one-25,26,26,26,27,27,27-d7 (C4-d7) (Avanti Polar Lipids Inc, Alabaster, AL, US) and 0.2 mL of methanol were added. The mixture was applied to a Bond Elut C18 cartridge (Agilent Technologies Inc, Mississauga, ON, Canada) preconditioned with 2 mL methanol followed by 2 mL ddH2O. The cartridge was washed twice with 2 mL ddH2O and 2 mL of methanol. After evaporation, the residue was dissolved in 80 µL methanol and 3 µL was injected into the system. The separation was performed using a Kinetix XB-C18 column (2.1 × 100 mm, particle size 1.7 µm; Phenomenex, Torrance, CA, US) at 35°C. The mobile phases were A (0.1% formic acid in ddH2O) and B (0.1% formic acid in acetonitrile) and used at a flow rate of 0.20 mL/min. The gradient program was started at 10% phase A and 90% phase B for 6 min, increased linearly to 100% phase B for 4 min, held at 100% phase B for 4 min, then returned to initial conditions and reequilibrated for 4 min. The total run time for each sample analysis was 16 min. The quantitative data were acquired using multi reaction- monitoring (MRM) mode. The MRM transitions for 7α-H-C4 were 401.4 > 383.4 m/z and for C4-d7 were 408.4 > 390.4 m/z. The following settings were applied during each run: capillary voltage 3.50 kV; source temperature 100°C; desolvation temperature 400°C; nitrogen gas with flow rates of desolvation and cone gas of 400 and 50 L/hr, respectively; argon was used as the collision gas; cone voltage was 20V; collision energy was 20 eV.
Statistical Analyses
Statistical analyses were performed using SPSS 21.0 (SPSS, Inc, Chicago, IL, US). All data are presented as mean ± SEM. Statistical significance was set at P < .05. Linear mixed-model analysis was used where treatment and time were specified as fixed factors, and age and body weight were specified as a covariate in the model. Significant treatment effects were examined with Bonferroni adjustment for multiple comparisons. Both treatment and time (with time representing the different time periods) were entered into the model. When a significant treatment effect, but no significant treatment-by-time interaction, was observed, the interpretation was that the treatment effect was consistent over the different time periods. Relationships between 2 variables were assessed with stepwise multiple linear regression analysis. Percentage change from baseline for each phase was analyzed using 2-tailed paired Student’s t-test. Data that were not normally distributed, as determined by a Shapiro-Wilk test, were log or inverse transformed before statistical analysis.
Results
Baseline characteristics of the study patients are presented in Table I. All patients were euthyroid based on serum TSH. Mean FT3 concentration was at the lower limit of the normal range (0.3 – 0.7 ng/dL) with one patient at 0.4 ng/dL, 5 patients at 0.3 ng/dL and one having a subnormal level of 0.2 ng/dL. Mean serum FT4 concentration was within the normal range (0.7 – 1.8 ng/dL), although two patients were near the lower limit of normal (0.7 – 0.9 ng/dL). Serum FT3/FT4 range was within the reference range reported in euthyroid adults (0.27 – 0.37).26 All patients had high plasma and RBC cholestanol and sitostanol levels. Plasma and RBC levels of cholestanol were similar (P = .20) while sitostanol tended to be higher in plasma than in RBC (P = .07). Lathosterol and its ratio to TC (lathosterol/TC) were higher (P < .05) in RBC relative to plasma. Mean serum 7α-H-C4 concentration was 16 ± 4 ng/mL; normal range of 7α-H-C4 has not been established. Two subjects had Achilles tendon xanthomas, which regressed with EZE (data not shown). Assessment of the subjects by their physicians prior to enrollment in the study were carried out using non-invasive imaging to exclude coronary and carotid plaque, and found no evidence of atherosclerotic manifestations (i.e. heart murmurs and vascular bruits) in all 8 subjects. There were no cases of myocardial infarction, atrial fibrillation, diabetes, heart failure, hypertension, stroke or any other comorbidity among the 8 patients.
Table I.
Baseline characteristics of patients with STSL
| Variables (n = 7)* | Mean ± SEM | Range |
|---|---|---|
| FT3 (ng/dL) | 0.3 ± 0.02 | (0.22 – 0.36) |
| FT4 (ng/dL) | 1.0 ± 0.06 | (0.70 – 1.22) |
| FT3/FT4 | 0.3 ± 0.01 | (0.26 – 0.36) |
| TSH (mU/L) | 2.9 ± 0.3 | (1.61 – 4.15) |
| Plasma sitostanol (mg/dL) | 1.1 ± 0.1 | (0.5 – 1.6) |
| Plasma sitostanol/TC | 0.01 ± 0.0 | (0.003 – 0.01) |
| RBC sitostanol (mg/dL) | 0.9 ± 0.1 | (0.5 – 1.3) |
| RBC sitostanol/TC | 0.01 ± 0.0 | (0.004 – 0.02) |
| Plasma cholestanol (mg/dL) | 1.4 ± 0.1 | (1.2 – 2.0) |
| Plasma cholestanol/TC | 0.01 ± 0.0 | (0.006 – 0.01) |
| RBC cholestanol (mg/dL) | 1.2 ± 0.1 | (0.74 – 1.5) |
| RBC cholestanol/TC | 0.01 ± 0.0 | (0.01 – 0.02) |
| Plasma TC (mg/dL) | 155.8 ± 12.7 | (116.7 – 211.8) |
| RBC TC (mg/dL) | 100.8 ± 6.2 | (72.3 – 121.4) |
| Plasma lathosterol (ug/dL) (n=5) | 325.8 ± 156.1 | (95.6 – 912.0) |
| Plasma lathosterol/TC | 1.9 ± 0.6 | (0.7 – 4.3) |
| RBC lathosterol (ug/dL) | 766.2 ± 86.6 | (329.3 – 1027.8) |
| RBC lathosterol/TC | 7.9 ± 1.0 | (4.0 – 11.3) |
| 7α-H-C4 (ng/mL) | 16.2 ± 4.4 | (5.2 – 40.4) |
| 7α-H-C4/TC | 0.10 ± 0.02 | (0.03 – 0.19) |
n = 7, variables data was not available for 1 patient
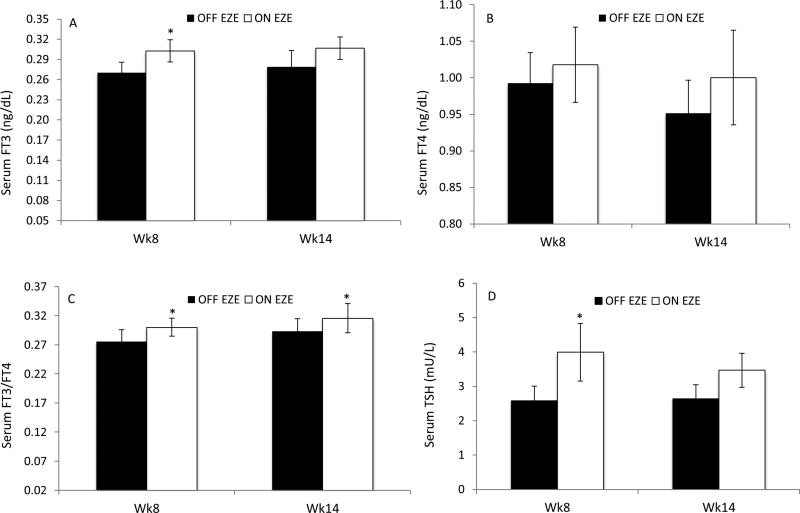
Serum FT3 concentrations did not change over time (P = .56), but were higher generally ON EZE than OFF EZE (Figure 1, A, Table II; Table II available at www.jpeds.com). At week 8 OFF EZE, serum FT3 levels tended to decrease (−10%, 0.27 ± 0.02 vs 0.30 ± 0.02 ng/dL, P = .08) compared with baseline. Although the decrease in FT3 level was evident in 5 patients (−23 ± 6.0%), one patient had no change, and the remaining two patients had an increase of 16±1.0% compared with baseline. At week14 the same 5 patients had an average decrease of 25±6.0% in FT3 levels compared with baseline but the remaining three patients had an increase of 22±5.0%; therefore on average the change in FT3 from baseline at week 14 OFF EZE was not significant (P = .18) (Table III; available at www.jpeds.com). At week 14 OFF EZE, the percentage changes in FT3 from baseline negatively correlated with the percentage changes in RBC cholestanol from baseline (r= −0.74, P= .04) but not with plasma cholestanol (r= −0.36, P=.38), suggesting that increases in cholestanol levels may contribute to decrease in FT3 observed during OFF EZE phase. In contrast, ON EZE FT3 concentrations were about 13% higher at week 8 compared with week 8 OFF EZE (P = .04), whereas at week 14 the difference was no longer significant (0.31 ± 0.02 vs 0.28 ± 0.02 ng/dL, P = .07) (Figure 1, A).
Figure 1.
A, Serum FT3, B, FT4, C, FT3/FT4 and D, TSH levels in patients with STSL (n = 8) at 8 and 14 weeks off EZE (OFF EZE) and on EZE (ON EZE). Data are mean ± SEM. *P < .05; Wk, week.
Table II.
Mixed linear model showing effect of time, treatment and their interactions
| Parameter | Time P value |
Treatment P value |
Interaction P value |
|---|---|---|---|
| FT3 | .56 | .01 | .82 |
| FT4 | .50 | .25 | .67 |
| FT3/FT4 | .17 | .001 | .87 |
| TSH | .42 | .008 | .36 |
| Plasma sitostanol | .002 | <.0001 | .02 |
| Plasma sitostanol/TC | .005 | .31 | <.0001 |
| RBC sitostanol | <.0001 | .001 | .06 |
| RBC sitostanol/TC | .07 | .03 | .001 |
| Plasma cholestanol | <.0001 | <.0001 | .003 |
| Plasma cholestanol/TC | .005 | .03 | .02 |
| RBC cholestanol | .30 | <.0001 | .08 |
| RBC cholestanol/TC | .009 | .006 | <.0001 |
| Plasma TC | <.0001 | <.0001 | .004 |
| RBC TC | .01 | .006 | .002 |
| Plasma lathosterol | .24 | .04 | .01 |
| Plasma lathosterol/TC | .13 | <.0001 | .004 |
| RBC lathosterol | <.0001 | <.0001 | .26 |
| RBC lathosterol/TC | <.0001 | <.0001 | .43 |
| Serum 7α-H-C4 | .49 | .32 | .31 |
| Serum 7α-H-C4/TC | .45 | .63 | .26 |
Table III.
Absolute and percentage changes in thyroid hormones at week 8 and week 14 OFF and ON ezetimibe compared with baseline (beginning of OFF ezetimibe phase).
| Parameter | Absolute change at week 8 |
Percentage change at week 8 |
P value vs baseline |
Absolute change at week 14 |
Percentage change at week 14 |
P value vs baseline |
|---|---|---|---|---|---|---|
|
| ||||||
| FT3 | ||||||
| OFF EZE | −0.04 ± 0.02 ng/dL | −10.2 ± 7.3 | .08 | −0.03 ± 0.03 ng/dL | −7.4 ± 9.4 | .18 |
| ON EZE | −0.01± 0.01 ng/dL | −0.9 ± 4.8 | .35 | +0.002 ± 0.02 ng/dL | +1.1 ± 6.1 | .46 |
|
| ||||||
| FT4 | ||||||
| OFF EZE | −0.02 ± 0.04 ng/dL | −0.54 ± 3.9 | .36 | −0.06 ± 0.06 ng/dL | −4.2 ± 5.7 | .19 |
| ON EZE | +0.01 ± 0.05 ng/dL | +2.0 ± 5.1 | .44 | −0.01 ± 0.03 ng/dL | −1.0 ± 3.3 | .40 |
|
| ||||||
| FT3/FT4 | ||||||
| OFF EZE | −0.03 ± 0.02 | −10.3 ± 5.2 | .05 | −0.01 ± 0.02 | −4.5 ± 5.4 | .21 |
| ON EZE | +0.01 ± 0.05 | −2.1 ± 4.2 | .59 | +0.01 ± 0.02 | + 2.4 ± 6.4 | .35 |
|
| ||||||
| TSH | ||||||
| OFF EZE | −0.15 ± 0.25 (mU/L) | −4.7 ± 11.6 | .29 | −0.09 ± 0.22 (mU/L) | −1.5 ± 9.2 | .34 |
| ON EZE | +1.26 ± 0.63 (mU/L) | +45.9 ± 16.0 | .04 | +0.74 ± 0.29 (mU/L) | +31 ± 10.7 | .02 |
Serum FT4 concentrations did not change over 14 weeks of EZE (P = .50; Table II), and were similar (P = .24) between OFF and ON EZE (Figure 1, B). Likewise, OFF EZE FT4 levels did not change either at week 8 or 14 compared with baseline (Table III). Serum FT3/FT4 did not change over time (P= .17), but was higher on average ON EZE than OFF EZE (P = .001; Table II). At 8 and 14 weeks OFF EZE, the changes in FT3/FT4 compared with baseline were the same as those of FT3 (Table III).ON EZE, serum FT3/FT4 was higher (10% ± 4%, P = .02) at week 8 compared with week 8 OFF EZE and remained high at week 14 (0.32 ± 0.03 vs 0.29 ± 0.02, P = .02) (Figure 1, C). Serum TSH concentrations did not change over time (P = .42) but were higher on average ON EZE than OFF EZE (P = .008; Table II). TSH levels did not change at weeks 8 and 14 OFF EZE compared with baseline (Table III). Percentage changes in TSH at week 14 OFF EZE from baseline negatively correlated with those in plasma cholestanol (r= −0.81, P = .02), sitostanol (r=−0.82, P = .01), and TC (r=−0.65, P = .08). Likewise, OFF EZE TSH levels at week 14 negatively correlated with cholestanol concentrations in RBC (r= −0.78, P= .02) but not plasma (r= −0.56, P =.15), suggesting that long-term cholestanol levels may negatively affect TSH levels. In contrast, ON EZE TSH concentrations were 2-fold higher at week 8 compared with week 8 OFF EZE (4.0 ± 0.8 vs. 2.6 ± 0.4 mU/L, P = .008) but the difference was not significant at week 14 (P = .09) (Figure 1, D). Moreover, correlations between TSH and cholestanol levels in plasma (r= −0.26, P= .54) and RBC (r= −0.64, P= .10) were insignificant by 14 week ON EZE.
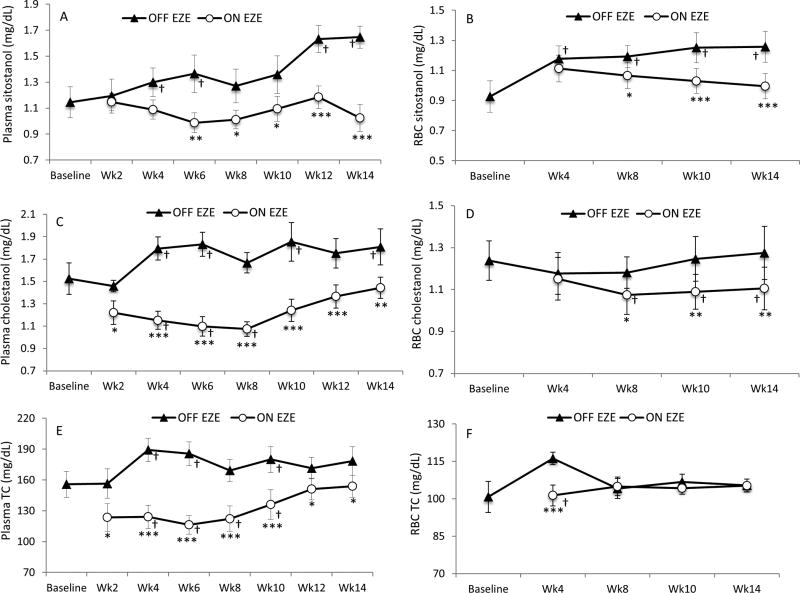
EZE lowered plasma (P = .002) and RBC (P <.0001) sitostanol concentrations over 14 weeks (Table II) with substantial decreases (38% ± 6% and 21% ± 2%, P <.0001) noted at week 14 compared with OFF EZE (Figure 2, A and B). These decreases (28% ± 7% and 21% ± 2%, all P <.0001) were sustained over time when ON EZE plasma and RBC sitostanol concentrations at week 14 were adjusted for TC (P = .005 and P = .07, respectively; Table II). EZE time-dependently lowered plasma cholestanol concentrations (P <.0001; Table II), and largely decreased cholestanol at week 6 (40% ± 3%, P <.0001) but the difference at week 14 was only 18% ± 6% (P = .001) compared with OFF EZE (Figure 2, C). When adjusted for TC, plasma cholestanol changed over time (P = .005; Table II) with lower ON EZE concentrations (6% ± 2%, P = .05) noted at week 14. EZE time-independently lowered RBC cholestanol concentrations (Table II), with a decrease of 13% (P <.0001) noted at week 14 (Figure 2, D) and when adjusted for TC (P = .006).
Figure 2.
A and B, Plasma and RBC sitostanol, C and D, plasma and RBC cholestanol, and E and F, plasma and RBC TC levels in patients with STSL (n = 8) throughout 14 weeks off EZE (OFF EZE) and on EZE (ON EZE). Data are mean ± SEM. *P < .05, **P < .01, ***P <.0001 vs OFF EZE. † P < .05 vs baseline; Wk, week.
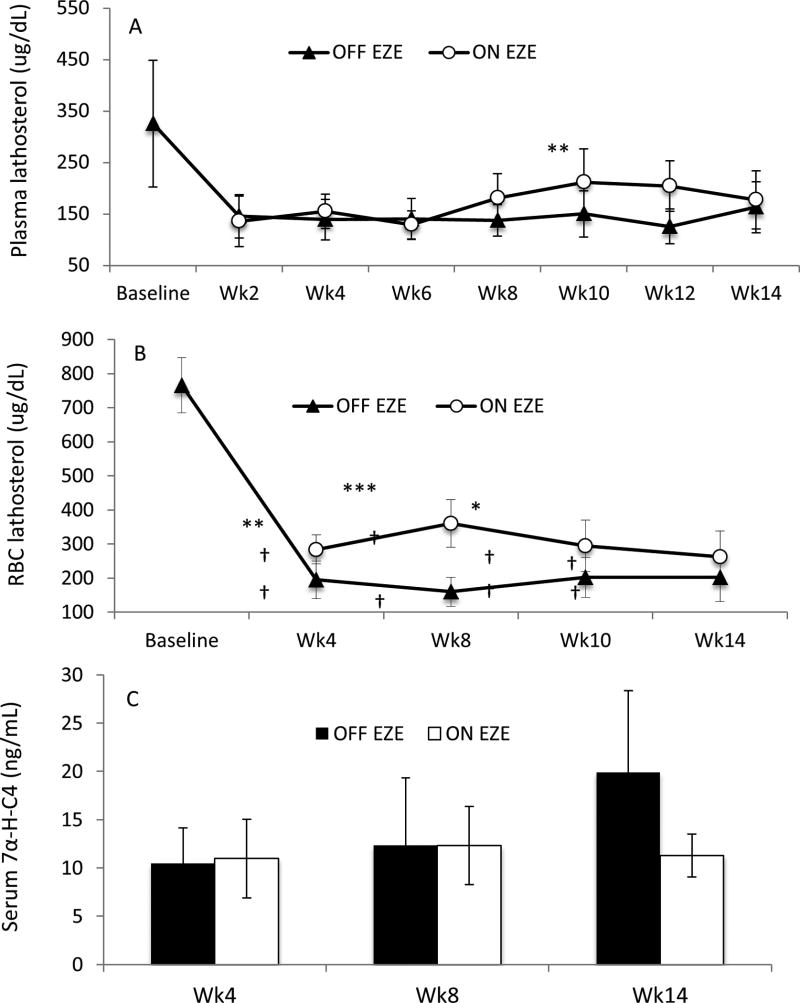
EZE time-dependently lowered plasma TC concentrations (P <.0001; Table II). EZE substantially decreased (37%, P < .001) TC at week 6 compared with OFF EZE; however, at week 14 the difference was only 12% (P = .01) (Figure 2, E). EZE-induced changes in plasma TC strongly correlated (r = 0.94, P = .001) with those in plasma cholestanol. RBC TC concentrations changed over time (P = .01; Table II). However, ON EZE, RBC TC levels were decreased (13% ± 1.0%, P <.0001) at week 4 but were similar (P = 1.0) to OFF EZE at week 14 (Figure 2, F). EZE time-dependently increased plasma lathosterol (Table II) with increases of 84% (P = .004) noted at week 12 but at week 14 the difference was not significant compared with OFF EZE (P = .91) (Figure 3, A). Similar results were observed when lathosterol levels were adjusted for TC (Table II). EZE increased RBC lathosterol concentrations but the effect was not time-dependent (Table II), suggesting a consistent effect of EZE on cholesterol synthesis in RBC. EZE increased (2-fold, P < .01) RBC lathosterol levels at week 4 but at week 14 they were comparable (P = .12) to OFF EZE (Figure 3, B). Similar results were observed in TC-adjusted RBC lathosterol (Table II). EZE did not affect absolute and TC-adjusted serum 7α-H-C4 levels (Table II, Figure 3, C). Changes in serum 7α-H-C4 after EZE did not correlate (r=−0.02, P = .97) with those in plasma cholestanol.
Figure 3.
A and B, Plasma and RBC lathosterol levels and C, serum 7α-H-C4 in patients with STSL (n = 8) throughout 14 weeks off EZE (OFF EZE) and on EZE (ON EZE). Data are mean ± SEM. *P < .05, **P < .01, ***P <.0001 vs OFF EZE. † P < .05 vs baseline; Wk, week.
OFF EZE plasma and RBC cholestanol levels inversely correlated with FT3 and FT3/FT4. However, only OFF EZE plasma sitostanol levels significantly inversely correlated with FT3 and FT3/FT4. Likewise, only EZE-induced changes in plasma cholestanol significantly inversely correlated with those in FT3/FT4 while those in sitostanol did not reach statistical significance (Table IV; available at www.jpeds.com).
Table IV.
Correlations between 5α-stanols and thyroid hormones 14 weeks off ezetimibe (OFF EZE) and on ezetimibe (ON EZE)
| Serum FT3 | Serum FT4 | Serum FT3/FT4 | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| r value | P value | r value | P value | r value | P value | |
| OFF EZE | ||||||
| Plasma cholestanol | − 0.92 | .001 | − 0.51 | .20 | − 0.74 | .04 |
| RBC cholestanol | − 0.62 | .06 | − 0.08 | .86 | − 0.75 | .03 |
| ON EZE | ||||||
| Plasma cholestanol | − 0.41 | .32 | 0.58 | .14 | − 0.79 | .02 |
| RBC cholestanol | − 0.27 | .52 | 0.52 | .19 | − 0.64 | .09 |
| EZE-induced changes | ||||||
| Plasma cholestanol | − 0.52 | .19 | − 0.25 | .56 | − 0.86 | .01 |
| RBC cholestanol | − 0.41 | .31 | − 0.38 | .35 | − 0.40 | .37 |
| OFF EZE | ||||||
| Plasma sitostanol | − 0.65 | .08 | 0.13 | .77 | − 0.89 | .003 |
| RBC sitostanol | 0.04 | .94 | 0.60 | .12 | − 0.52 | .19 |
| ON EZE | ||||||
| Plasma sitostanol | − 0.16 | .70 | 0.78 | .02 | − 0.69 | .06 |
| RBC sitostanol | 0.02 | .97 | 0.60 | .11 | − 0.59 | .13 |
| EZE-induced changes | ||||||
| Plasma sitostanol | 0.09 | .84 | 0.22 | .60 | − 0.34 | .46 |
| RBC sitostanol | 0.16 | .70 | − 0.30 | .94 | − 0.39 | .45 |
Discussion
STSL is an autosomal recessive disorder caused by mutation in the ABCG5 or ABCG8 genes, and characterized by increased plasma and tissue levels of plant sterols and 5α-stanols.1, 2 These compounds have been shown to disrupt the endocrine system in mice with STSL,32 although in humans endocrine insufficiency has only been reported once.3 Plasma cholestanol, a 5α-stanol, is generally low in healthy subjects (<0.4 mg/dL)16 but high in STSL (0.5 – 2 mg/dL)16 and CTX (1 – 4 mg/dL), a disorder of bile acid synthesis caused by mutations in CYP27A1 gene that codes for sterol 27-hydroxylase, an essential enzyme for bile acids synthesis.17 Increased cholestanol levels in CTX have been associated with hypothyroidism.4
The current results are in accordance with previous reports,19,11 showing increased plasma and RBC sitostanol and cholestanol levels in STSL. These levels were increased by at least 30% when EZE was discontinued, and inversely correlated with serum FT3 and FT3/FT4, suggesting that 5α-stanols may modulate thyroid function.
It is possible that when the patients were taken off EZE accumulation of cholesterol, plant sterols and 5α-stanols increased in the blood and tissues, which may induce inflammation, and in turn may influence deiodination of T4 to T3 and cause the low FT3 levels observed at week 8 and week 14 OFF EZE. Cytokines have been shown to inhibit sodium iodide transporter mRNA expression and iodide uptake activity in human and rat thyroid cells33 and alter serum thyroid hormone levels directly or indirectly by impairing the hypothalamic-pituitary-thyroid axis,34 reducing the activity or release of TSH,35, 36 inhibiting the synthesis or conversion of thyroid hormones and regulating their protein binding.34, 37
EZE, the primary treatment for STSL, reduces intestinal uptake of sterols by inhibiting the transport function of Niemann-Pick C 1-like 1 protein.21, 22 EZE has no effect on thyroid hormone levels in healthy subjects,44, 45 where the plasma 5α-stanols are low. The current patients were on average euthyroid, although baseline serum FT3 concentrations were within the lower levels of the reference range for six patients, and one patient had subnormal level of 0.2 ng/dL. FT4 levels were near the lower limit of normal in two patients. In the current study, EZE did not significantly change serum thyroid hormones but increased serum FT3/FT4, reflecting increased peripheral conversion of the less metabolically potent T4 to the stronger T3, which is catalyzed by 5’-deiodinase. Increased FT3/FT4 with EZE suggests increased 5’-deiodinase activity to provide sufficient T3 and maintain euthyroidism.
TSH is important for the conversion of T4 to FT3, and its release is under negative feedback regulation by thyroid hormone at the pituitary and hypothalamic levels. Approximately 25% increase in serum TSH was reported after 4 weeks of EZE compared with baseline in high cardiovascular risk subjects.49 In the current study, EZE increased FT3 and TSH at week 8 and then levels returned to similar levels at week14 ON EZE while FT3/FT4 ratio at week14 remained high. The mechanism mediating the increased TSH concentrations in response to EZE is not clear.
The current results fit well with the studies outside of STSL, reporting reduced peripheral conversion of T4 to T3 and circulating T3 levels in hepatic cirrhosis patients, 50, 51 where the serum 5α-stanols and plant sterols levels were elevated due to impaired biliary elimination.52–54 In addition, we observed substantial decreases in plasma cholestanol levels with EZE at week 6, suggesting reduced intestinal absorption of cholestanol and its precursors. However, the levels rose during the last 4 weeks of the phase, and only a 20% decrease in cholestanol was noted at week 14. This suggests increases in precursor availability for synthesis of cholestanol, which potentially would attenuate decreases in plasma cholestanol. In contrast, decreases in RBC cholestanol levels were consistent over time, due to the slower incorporation rate of cholestanol from plasma into RBC membrane because RBC do not make cholestanol.55 Unlike cholestanol, decreases in plasma and RBC sitostanol levels with EZE were progressive over 14 weeks, suggesting that time may be required for further reducing sitostanol levels.
The results of this study showed evidence for altered thyroid hormone metabolism in STSL. An inverse relationship between plasma 5α-stanols levels and FT3/FT4 in STS was demonstrated. EZE, the treatment for STSL, seems to be as effective in reducing 5α-stanol concentrations in STSL as in hypercholesterolemia. EZE reduces blood levels of sitostanol, cholestanol and their ratios to TC, while increasing serum FT3/FT4, thereby potentially enhancing peripheral conversion of T4 to T3, and reducing the risk of developing hypothyroidism in STSL patients. Further studies are needed to elucidate a potential direct action of 5α-stanols on the T4-to-T3 deiodination process in peripheral tissues.
Acknowledgments
Supported by the Canadian Institutes of Health Research (MOP12339). The Sterol and Isoprenoid Research (STAIR; U54HD061939) Consortium is a part of the National Institutes of Health (NIH) Rare Diseases Clinical Research Network, supported through collaboration between the NIH Office of Rare Diseases Research at the National Center for Advancing Translational Science, and National Institute of Child Health and Human Development. R.O. was supported by the Libyan Scholarship Program and Research Manitoba Fellowship.
We are grateful to the patients for their loyal support.
List of abbreviations
- 7α-H-C4
7α-hydroxy-4-cholesten-3-one
- CTX
cerebrotendinous xanthomatosis
- EZE
ezetimibe
- RBC
red blood cells
- STSL
sitosterolemia
- FT3
free triiodothyronine
- FT4
free thyroxine
- FT3/FT4
free triiodothyronine/free thyroxine ratio
- TC
total cholesterol
- TSH
thyroid-stimulating hormone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Othman RA, Myrie SB, Jones PJ. Non-cholesterol sterols and cholesterol metabolism in sitosterolemia. Atherosclerosis. 2013;231:291–9. doi: 10.1016/j.atherosclerosis.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 2.Wang C, Lin HJ, Chan TK, Salen G, Chan WC, Tse TF. A unique patient with coexisting cerebrotendinous xanthomatosis and beta-sitosterolemia. The American journal of medicine. 1981;71:313–9. doi: 10.1016/0002-9343(81)90134-0. [DOI] [PubMed] [Google Scholar]
- 3.Mushtaq T, Wales JK, Wright NP. Adrenal insufficiency in phytosterolaemia. European journal of endocrinology / European Federation of Endocrine Societies. 2007;157(Suppl 1):S61–5. doi: 10.1530/EJE-07-0222. [DOI] [PubMed] [Google Scholar]
- 4.Idouji K, Kuriyama M, Fujiyama J, Osame M, Hoshita T. [Hypothyroidism with increased serum levels of cholestanol and bile alcohol--analogous symptoms to cerebrotendinous xanthomatosis] Rinsho shinkeigaku = Clinical neurology. 1991;31:402–6. [PubMed] [Google Scholar]
- 5.Bouwes Bavinck JN, Vermeer BJ, Gevers Leuven JA, Koopman BJ, Wolthers BG. Capillary gas chromatography of urine samples in diagnosing cerebrotendinous xanthomatosis. Archives of dermatology. 1986;122:1269–72. doi: 10.1001/archderm.122.11.1269. [DOI] [PubMed] [Google Scholar]
- 6.Philippart M, Van Bogaert L. Cholestanolosis (cerebrotendinous xanthomatosis). A follow-up study on the original family. Archives of neurology. 1969;21:603–10. doi: 10.1001/archneur.1969.00480180059004. [DOI] [PubMed] [Google Scholar]
- 7.Wang J, Joy T, Mymin D, Frohlich J, Hegele RA. Phenotypic heterogeneity of sitosterolemia. J Lipid Res. 2004;45:2361–7. doi: 10.1194/jlr.M400310-JLR200. [DOI] [PubMed] [Google Scholar]
- 8.Seyama Y. Cholestanol metabolism, molecular pathology, and nutritional implications. Journal of medicinal food. 2003;6:217–24. doi: 10.1089/10966200360716634. [DOI] [PubMed] [Google Scholar]
- 9.Salen G, Kwiterovich PO, Jr, Shefer S, Tint GS, Horak I, Shore V, et al. Increased plasma cholestanol and 5 alpha-saturated plant sterol derivatives in subjects with sitosterolemia and xanthomatosis. J Lipid Res. 1985;26:203–9. [PubMed] [Google Scholar]
- 10.Ilias AM, Connor WE, Cory HT, Lin DS, Daves GD, Jr, Krippaehne WW. Sterols of human gallstones: the recent identification of eight different digitonin precipitable sterols. Gastroenterology. 1980;79:539–44. [PubMed] [Google Scholar]
- 11.Connor WE, Lin DS, Pappu AS, Frohlich J, Gerhard G. Dietary sitostanol and campestanol: accumulation in the blood of humans with sitosterolemia and xanthomatosis and in rat tissues. Lipids. 2005;40:919–23. doi: 10.1007/s11745-005-1452-7. [DOI] [PubMed] [Google Scholar]
- 12.Salen G, Shefer S, Tint GS. Transformation of 4-cholesten-3-one and 7 alpha-hydroxy-4-cholesten-3-one into cholestanol and bile acids in cerebrotendinous xanthomatosis. Gastroenterology. 1984;87:276–83. [PubMed] [Google Scholar]
- 13.Tint GS, Salen G. Transformation of 5 alpha-cholest-7-en-3 beta-ol to cholesterol and cholestanol in cerebrotendinous xanthomatosis. J Lipid Res. 1974;15:256–62. [PubMed] [Google Scholar]
- 14.Skrede S, Bjorkhem I, Buchmann MS, Hopen G, Fausa O. A novel pathway for biosynthesis of cholestanol with 7 alpha-hydroxylated C27-steroids as intermediates, and its importance for the accumulation of cholestanol in cerebrotendinous xanthomatosis. J Clin Invest. 1985;75:448–55. doi: 10.1172/JCI111719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bjorkhem I, Skrede S, Buchmann MS, East C, Grundy S. Accumulation of 7 alpha-hydroxy-4-cholesten-3-one and cholesta-4,6-dien-3-one in patients with cerebrotendinous xanthomatosis: effect of treatment with chenodeoxycholic acid. Hepatology. 1987;7:266–71. doi: 10.1002/hep.1840070210. [DOI] [PubMed] [Google Scholar]
- 16.Miettinen TA, Tilvis RS, Kesaniemi YA. Serum plant sterols and cholesterol precursors reflect cholesterol absorption and synthesis in volunteers of a randomly selected male population. American journal of epidemiology. 1990;131:20–31. doi: 10.1093/oxfordjournals.aje.a115479. [DOI] [PubMed] [Google Scholar]
- 17.Monson DM, DeBarber AE, Bock CJ, Anadiotis G, Merkens LS, Steiner RD, et al. Cerebrotendinous xanthomatosis: a treatable disease with juvenile cataracts as a presenting sign. Archives of ophthalmology. 2011;129:1087–8. doi: 10.1001/archophthalmol.2011.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lutjohann D, von Bergmann K, Sirah W, Macdonell G, Johnson-Levonas AO, Shah A, et al. Long-term efficacy and safety of ezetimibe 10 mg in patients with homozygous sitosterolemia: a 2-year, open-label extension study. Int J Clin Pract. 2008;62:1499–510. doi: 10.1111/j.1742-1241.2008.01841.x. [DOI] [PubMed] [Google Scholar]
- 19.Salen G, Batta AK, Tint GS, Shefer S, Ness GC. Inverse relationship between plasma cholestanol concentrations and bile acid synthesis in sitosterolemia. J Lipid Res. 1994;35:1878–87. [PubMed] [Google Scholar]
- 20.Shefer S, Salen G, Nguyen L, Batta AK, Packin V, Tint GS, et al. Competitive inhibition of bile acid synthesis by endogenous cholestanol and sitosterol in sitosterolemia with xanthomatosis. Effect on cholesterol 7 alpha-hydroxylase. J Clin Invest. 1988;82:1833–9. doi: 10.1172/JCI113799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Heek M, Farley C, Compton DS, Hoos L, Alton KB, Sybertz EJ, et al. Comparison of the activity and disposition of the novel cholesterol absorption inhibitor, SCH58235, and its glucuronide, SCH60663. British journal of pharmacology. 2000;129:1748–54. doi: 10.1038/sj.bjp.0703235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salen G, von Bergmann K, Lutjohann D, Kwiterovich P, Kane J, Patel SB, et al. Ezetimibe effectively reduces plasma plant sterols in patients with sitosterolemia. Circulation. 2004;109:966–71. doi: 10.1161/01.CIR.0000116766.31036.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Othman RA, Myrie SB, Mymin D, Merkens LS, Roullet JB, Steiner RD, et al. Ezetimibe reduces plant sterol accumulation and favorably increases platelet count in sitosterolemia. The Journal of pediatrics. 2015;166:125–31. doi: 10.1016/j.jpeds.2014.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Pergola G, Ciampolillo A, Paolotti S, Trerotoli P, Giorgino R. Free triiodothyronine and thyroid stimulating hormone are directly associated with waist circumference, independently of insulin resistance, metabolic parameters and blood pressure in overweight and obese women. Clinical endocrinology. 2007;67:265–9. doi: 10.1111/j.1365-2265.2007.02874.x. [DOI] [PubMed] [Google Scholar]
- 25.Steiner C, von Eckardstein A, Rentsch KM. Quantification of the 15 major human bile acids and their precursor 7alpha-hydroxy-4-cholesten-3-one in serum by liquid chromatography-tandem mass spectrometry. Journal of chromatography B, Analytical technologies in the biomedical and life sciences. 2010;878:2870–80. doi: 10.1016/j.jchromb.2010.08.045. [DOI] [PubMed] [Google Scholar]
- 26.Gullo D, Latina A, Frasca F, Le Moli R, Pellegriti G, Vigneri R. Levothyroxine monotherapy cannot guarantee euthyroidism in all athyreotic patients. PLoS One. 2011;6:e22552. doi: 10.1371/journal.pone.0022552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kahaly GJ. Cardiovascular and atherogenic aspects of subclinical hypothyroidism. Thyroid : official journal of the American Thyroid Association. 2000;10:665–79. doi: 10.1089/10507250050137743. [DOI] [PubMed] [Google Scholar]
- 28.Abrams JJ, Grundy SM. Cholesterol metabolism in hypothyroidism and hyperthyroidism in man. J Lipid Res. 1981;22:323–38. [PubMed] [Google Scholar]
- 29.Schmidt-Ott UM, Ascheim DD. Thyroid hormone and heart failure. Current heart failure reports. 2006;3:114–9. doi: 10.1007/s11897-006-0010-1. [DOI] [PubMed] [Google Scholar]
- 30.Brozaitiene J, Mickuviene N, Podlipskyte A, Burkauskas J, Bunevicius R. Relationship and prognostic importance of thyroid hormone and N-terminal pro-B-Type natriuretic peptide for patients after acute coronary syndromes: a longitudinal observational study. BMC cardiovascular disorders. 2016;16:45. doi: 10.1186/s12872-016-0226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kozdag G, Ural D, Vural A, Agacdiken A, Kahraman G, Sahin T, et al. Relation between free triiodothyronine/free thyroxine ratio, echocardiographic parameters and mortality in dilated cardiomyopathy. European journal of heart failure. 2005;7:113–8. doi: 10.1016/j.ejheart.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 32.Solca C, Tint GS, Patel SB. Dietary xenosterols lead to infertility and loss of abdominal adipose tissue in sterolin-deficient mice. Journal of lipid research. 2013;54:397–409. doi: 10.1194/jlr.M031476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pekary AE, Hershman JM. Tumor necrosis factor, ceramide, transforming growth factor-beta1, and aging reduce Na+/I- symporter messenger ribonucleic acid levels in FRTL-5 cells. Endocrinology. 1998;139:703–12. doi: 10.1210/endo.139.2.5760. [DOI] [PubMed] [Google Scholar]
- 34.Imura H, Fukata J, Mori T. Cytokines and endocrine function: an interaction between the immune and neuroendocrine systems. Clinical endocrinology. 1991;35:107–15. doi: 10.1111/j.1365-2265.1991.tb03506.x. [DOI] [PubMed] [Google Scholar]
- 35.Dubuis JM, Dayer JM, Siegrist-Kaiser CA, Burger AG. Human recombinant interleukin-1 beta decreases plasma thyroid hormone and thyroid stimulating hormone levels in rats. Endocrinology. 1988;123:2175–81. doi: 10.1210/endo-123-5-2175. [DOI] [PubMed] [Google Scholar]
- 36.Poth M, Tseng YC, Wartofsky L. Inhibition of TSH activation of human cultured thyroid cells by tumor necrosis factor: an explanation for decreased thyroid function in systemic illness? Thyroid : official journal of the American Thyroid Association. 1991;1:235–40. doi: 10.1089/thy.1991.1.235. [DOI] [PubMed] [Google Scholar]
- 37.Ongphiphadhanakul B, Fang SL, Tang KT, Patwardhan NA, Braverman LE. Tumor necrosis factor-alpha decreases thyrotropin-induced 5’-deiodinase activity in FRTL-5 thyroid cells. European journal of endocrinology / European Federation of Endocrine Societies. 1994;130:502–7. doi: 10.1530/eje.0.1300502. [DOI] [PubMed] [Google Scholar]
- 38.Plat J, Brzezinka H, Lutjohann D, Mensink RP, von Bergmann K. Oxidized plant sterols in human serum and lipid infusions as measured by combined gas-liquid chromatography-mass spectrometry. J Lipid Res. 2001;42:2030–8. [PubMed] [Google Scholar]
- 39.MacLatchy DL, Van Der Kraak GJ. The phytoestrogen beta-sitosterol alters the reproductive endocrine status of goldfish. Toxicology and applied pharmacology. 1995;134:305–12. doi: 10.1006/taap.1995.1196. [DOI] [PubMed] [Google Scholar]
- 40.Malini T, Vanithakumari G. Effect of beta-sitosterol on uterine biochemistry: a comparative study with estradiol and progesterone. Biochemistry and molecular biology international. 1993;31:659–68. [PubMed] [Google Scholar]
- 41.Mellanen P, Petanen T, Lehtimaki J, Makela S, Bylund G, Holmbom B, et al. Wood-derived estrogens: studies in vitro with breast cancer cell lines and in vivo in trout. Toxicology and applied pharmacology. 1996;136:381–8. doi: 10.1006/taap.1996.0046. [DOI] [PubMed] [Google Scholar]
- 42.Gutendorf B, Westendorf J. Comparison of an array of in vitro assays for the assessment of the estrogenic potential of natural and synthetic estrogens, phytoestrogens and xenoestrogens. Toxicology. 2001;166:79–89. doi: 10.1016/s0300-483x(01)00437-1. [DOI] [PubMed] [Google Scholar]
- 43.Chen HJ, Walfish PG. Effects of estradiol benzoate on thyroid-pituitary function in female rats. Endocrinology. 1978;103:1023–30. doi: 10.1210/endo-103-4-1023. [DOI] [PubMed] [Google Scholar]
- 44.John-Kalarickal J, Pearlman G, Carlson HE. New medications which decrease levothyroxine absorption. Thyroid : official journal of the American Thyroid Association. 2007;17:763–5. doi: 10.1089/thy.2007.0060. [DOI] [PubMed] [Google Scholar]
- 45.Ananthakrishnan S, Braverman LE, Levin RM, Magnani B, Pearce EN. The effect of famotidine, esomeprazole, and ezetimibe on levothyroxine absorption. Thyroid : official journal of the American Thyroid Association. 2008;18:493–8. doi: 10.1089/thy.2007.0381. [DOI] [PubMed] [Google Scholar]
- 46.Turfaner N, Uzun H, Balci H, Ercan MA, Karter YH, Caner M, et al. Ezetimibe therapy and its influence on oxidative stress and fibrinolytic activity. Southern medical journal. 2010;103:428–33. doi: 10.1097/SMJ.0b013e3181d83374. [DOI] [PubMed] [Google Scholar]
- 47.Sternberg Z, Chichelli T, Sternberg D, Hojnacki D, Drake A, Liu S, et al. Quantitative and qualitative pleiotropic differences between Simvastatin single and Vytorin combination therapy in hypercholesterolemic subjects. Atherosclerosis. 2013;231:411–20. doi: 10.1016/j.atherosclerosis.2013.09.031. [DOI] [PubMed] [Google Scholar]
- 48.Reinehr T, Isa A, de Sousa G, Dieffenbach R, Andler W. Thyroid hormones and their relation to weight status. Hormone research. 2008;70:51–7. doi: 10.1159/000129678. [DOI] [PubMed] [Google Scholar]
- 49.Barbosa SP, Lins LC, Fonseca FA, Matos LN, Aguirre AC, Bianco HT, et al. Effects of ezetimibe on markers of synthesis and absorption of cholesterol in high-risk patients with elevated C-reactive protein. Life Sci. 2013;92:845–51. doi: 10.1016/j.lfs.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 50.Nomura S, Pittman CS, Chambers JB, Jr, Buck MW, Shimizu T. Reduced peripheral conversion of thyroxine to triiodothyronine in patients with hepatic cirrhosis. J Clin Invest. 1975;56:643–52. doi: 10.1172/JCI108134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamanaka T, Ido K, Kimura K, Saito T. Serum levels of thyroid hormones in liver diseases. Clinica chimica acta; international journal of clinical chemistry. 1980;101:45–55. doi: 10.1016/0009-8981(80)90054-6. [DOI] [PubMed] [Google Scholar]
- 52.Gylling H, Vuoristo M, Farkkila M, Miettinen TA. The metabolism of cholestanol in primary biliary cirrhosis. Journal of hepatology. 1996;24:444–51. doi: 10.1016/s0168-8278(96)80165-6. [DOI] [PubMed] [Google Scholar]
- 53.Nikkila K, Miettinen TA. Serum cholesterol precursors, cholestanol, and plant sterols in primary biliary cirrhosis. Scandinavian journal of gastroenterology. 1988;23:967–72. doi: 10.3109/00365528809090155. [DOI] [PubMed] [Google Scholar]
- 54.Miettinen TA, Farkkila M, Vuoristo M, Karvonen AL, Leino R, Lehtola J, et al. Serum cholestanol, cholesterol precursors, and plant sterols during placebo-controlled treatment of primary biliary cirrhosis with ursodeoxycholic acid or colchicine. Hepatology. 1995;21:1261–8. [PubMed] [Google Scholar]
- 55.Bruckdorfer KR, Demel RA, De Gier J, van Deenen LL. The effect of partial replacements of membrane cholesterol by other steroids on the osmotic fragility and glycerol permeability of erythrocytes. Biochimica et biophysica acta. 1969;183:334–45. doi: 10.1016/0005-2736(69)90089-3. [DOI] [PubMed] [Google Scholar]
- 56.Miettinen TA, Tilvis RS, Kesaniemi YA. Serum cholestanol and plant sterol levels in relation to cholesterol metabolism in middle-aged men. Metabolism: clinical and experimental. 1989;38:136–40. doi: 10.1016/0026-0495(89)90252-7. [DOI] [PubMed] [Google Scholar]
- 57.Yang C, Yu L, Li W, Xu F, Cohen JC, Hobbs HH. Disruption of cholesterol homeostasis by plant sterols. J Clin Invest. 2004;114:813–22. doi: 10.1172/JCI22186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.DeBarber AE, Connor WE, Pappu AS, Merkens LS, Steiner RD. ESI-MS/MS quantification of 7alpha-hydroxy-4-cholesten-3-one facilitates rapid, convenient diagnostic testing for cerebrotendinous xanthomatosis. Clinica chimica acta; international journal of clinical chemistry. 2010;411:43–8. doi: 10.1016/j.cca.2009.09.036. [DOI] [PubMed] [Google Scholar]
- 59.Hahn C, von Bergmann K. Relationship between the serum concentration of 7 alpha-hydroxycholesterol and fecal bile acid excretion in humans. Scandinavian journal of gastroenterology. 1996;31:804–8. doi: 10.3109/00365529609010356. [DOI] [PubMed] [Google Scholar]
- 60.Hirayama S, Nakagawa S, Soda S, Kamimura Y, Nishioka E, Ueno T, et al. Ezetimibe decreases serum oxidized cholesterol without impairing bile acid synthesis in Japanese hypercholesterolemic patients. Atherosclerosis. 2013;230:48–51. doi: 10.1016/j.atherosclerosis.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 61.Li Y, Salfelder A, Schwab KO, Grunert SC, Velten T, Lutjohann D, et al. Against all odds: blended phenotypes of three single-gene defects. European journal of human genetics : EJHG. 2016;24:1274–9. doi: 10.1038/ejhg.2015.285. [DOI] [PMC free article] [PubMed] [Google Scholar]