Abstract
OBJECTIVE
Assess patient-physician agreement on management goals for chronic musculoskeletal pain and its associations with patient and physician visit experiences.
METHODS
Pre- and post-visit questionnaires for 87 primary care visits involving patients taking opioids for chronic musculoskeletal pain and primary care residents. After each visit, patients and physicians independently ranked 5 pain treatment goals from most to least important.
RESULTS
48% of patients ranked reducing pain intensity as their top priority, while 22% ranked finding a diagnosis as most important. Physicians ranked improving function as the top priority for 41% of patients, and ranked reducing medication side effects as most important for 26%. The greatest difference between patient and physician rankings was for reducing pain intensity. In regression analyses, neither overall agreement on goals (i.e., the physician’s first or second priority included the patient’s top priority) nor difference in patient versus physician ranking of pain intensity was significantly associated with patient-reported visit experience (beta for overall agreement −0.08, 95%CI −0.45, 0.30; P = 0.69; beta for intensity −0.06, 95%CI −0.17, 0.04; P = 0.24) or physician-reported visit difficulty (beta for overall agreement 1.92, 95%CI −2.70, 6.55; P = 0.41; beta for intensity 0.42, 95%CI −0.87, 1.71, P = 0.53).
DISCUSSION
Patients and physicians prioritize substantially different goals for chronic pain management, but there is no evidence that agreement predicts patient experience or physician-reported visit difficulty. Primary care physicians may have adapted to new recommendations that emphasize functional goals and avoidance of long-term opioid therapy, while patients continue to focus on reducing pain intensity.
Keywords: primary care, opioid analgesics, goals, chronic pain, patient-physician relations, patient satisfaction
INTRODUCTION
Chronic musculoskeletal pain is a major cause of suffering and disability in the U.S. and a common reason for primary care visits.[1-4] Effective management of chronic pain requires a therapeutic relationship within which the patient and physician can assess pain, discuss goals of care, and make treatment decisions.[5] Patient-physician agreement about goals of care is considered an important component of patient-centered care,[6] particularly for chronic pain management, which typically requires a multi-pronged approach targeting different aspects of pain (e.g., functional limitation, emotional impact, pain intensity).[7] Both physicians and patients cite disagreements over goals of care – particularly around opioid analgesics – as a major reason that discussions about chronic pain are often unproductive and associated with negative patient experiences and the occurrence of “difficult” visits[8-10] (i.e. visits that result in physician frustration and negative countertransference[11]).
The dramatic rise in the use of opioids to treat chronic musculoskeletal pain and the subsequent increase in opioid overdose rates during the past 15 years have precipitated a shift in recommended clinical practice away from long-term opioid use.[12, 13] Clinical guidelines for using opioids to treat chronic pain have long endorsed collaborative goal setting,[14] but the putative benefits of agreement on goals for chronic pain have not been empirically investigated. Recent guidelines, including those published by the Medical Board of California[15] and the Centers for Disease Control and Prevention,[16] not only encourage collaborative goal setting but also recommend that in most cases reducing pain intensity should not be the primary treatment goal.[17, 18] These guidelines instead recommend prioritizing objective or observable goals, such as reducing pain-related functional impairment or minimizing risks of opioid-related harms. In contrast, recommendations during the late 1990s and 2000s emphasized reducing pain intensity (exemplified by the campaign to make pain assessment the “5th Vital Sign”[19]). These new recommendations may lead to greater disagreement about goals between patients and physicians. For example, if patients prioritize reducing pain intensity while their physicians prioritize improving function and reducing opioid use, their clinical interactions may be rife with opportunities for cross-purposes, misunderstandings, and rancor.
We know relatively little about how often primary care physicians and patients taking opioids for chronic musculoskeletal pain agree on pain management goals, or how this agreement relates to patients’ and physicians’ perceptions of their clinical interactions. A likely reason for this knowledge gap is that it is relatively difficult to study the sub-population of primary care patients with chronic pain who are also on long-term opioids. Many studies focus on pain clinics or patients with opioid use disorder; these populations are important but are highly selected groups and may not reflect the full range of patients on long-term opioids that primary care physicians (who write more than two-thirds of long-term opioid prescriptions[20, 21]) treat in everyday practice.
In this study we examined agreement on goals between primary care patients taking opioids for chronic musculoskeletal pain and their physicians after routine clinic visits. We addressed three research questions: RQ1: How do the pain management goals prioritized by primary care physicians compare to the goals prioritized by patients taking opioids for chronic musculoskeletal pain? RQ2: What is the correlation between patient-physician agreement on goals and physician-reported visit difficulty? RQ3: Does patient-physician agreement on goals predict patients’ self-reported visit experience?
Characterizing patient-physician agreement on goals around chronic pain is important for understanding the degree to which patients and physicians are on the same page when managing pain. If greater agreement is associated with better patient experience or fewer difficult visits, then efforts to foster agreement on goals holds promise as a strategy for improving chronic pain management in primary care.
MATERIALS AND METHODS
Participants
Patients and physicians were recruited from two hospital-based primary care resident clinics at the University of California, Davis Medical Center in Sacramento, California. Eligible physicians were internal medicine or family medicine residents who saw primary care patients at one of the study clinics and had completed ≥1 year of residency training. Eligible patients were established adult patients who were prescribed long-term opioids (defined as ≥1 opioid dose per day for ≥90 days) for chronic musculoskeletal pain by their primary care physician, reported at least moderate pain intensity, and planned to discuss pain management during a scheduled appointment with an enrolled physician. Patients were ineligible if they were getting active cancer treatment or palliative care, spoke a language other than English during clinic visits, or were being prescribed opioids by someone other than their primary care physician. The University of California, Davis Institutional Review Board approved this study; all participants provided written informed consent.
Recruitment
Physicians were recruited through email invitations and clinic presentations. Patients were recruited by reviewing clinic schedules of enrolled physicians to identify potentially eligible patients. A research assistant then contacted potentially eligible patients by either approaching them in clinic waiting rooms or mailing them a letter describing the study (including instructions for opting out of further contact) followed by a telephone call. For each contacted patient, the research assistant confirmed the patient’s eligibility and interest in the study and then asked the following three visit-specific screening questions:
How would you rate your average pain over the past week, with zero being no pain and 10 being the worst pain possible?
At your upcoming visit, how likely are you to talk about ways to get better control of your pain? (1-very unlikely to 5-very likely)
At your upcoming visit, how likely are you to talk about changing the dose or type of your pain medicine? (1-very unlikely to 5-very likely)
Patients were eligible to enroll if they rated their pain as ≥4 and answered “likely” or “very likely” to question 2 or 3. Questions 2 and 3 were designed to focus recruitment on clinic visits for which pain management was a substantive topic of discussion. Prior studies found that up to 25% of primary care patients reporting moderate to severe pain did not discuss pain during clinic visits,[9] and that nearly half of discussions about opioids were confined to routine refill requests.[22] Patients’ eligibility was confirmed within 48 hours of their scheduled appointment. Interested patients who reported being unlikely to discuss pain management were re-screened for eligibility prior to subsequent appointments. Each enrolled patient could only participate in a single visit; however, enrolled physicians could participate in multiple visits. Data collection took place from November 2014 through January 2016.
Baseline measures
Physicians provided demographic information at enrollment. Immediately before their clinic visits, patients completed questionnaires that included demographic information and several other pain-related measures. Physical and mental health were assessed using the Veterans RAND 12-item Health Survey (VR-12), a non-proprietary version of the SF-12.[23] Responses to the VR-12 were used to calculate physical health component scores (PCS) and mental health component scores (MCS). PCS and MCS range from 0 to 100 (with 100 indicating perfect health), are designed to be mutually orthogonal, and have been benchmarked against health status measures from nationally representative surveys.[23] Pain severity was assessed using the PEG (Pain intensity, Enjoyment, and General activity), a 3-item scale that performs well in primary care compared to longer measures.[24, 25] Pain catastrophizing was measured using the catastrophizing subscale from the coping strategies questionnaire.[26] Risk for opioid misuse was assessed using the 5-item Screener and Opioid Assessment for Patients with Pain (SOAPP-SF).[27]
Post-visit physician measures
Immediately after each visit, physicians completed the 10-item Difficult Doctor-Patient Relationship Questionnaire.[28] Physician-reported visit difficulty has been associated with worse patient satisfaction, greater symptom burden, and higher healthcare utilization.[11, 29] In addition, each physician was prompted “to think about which goals you would prioritize for this patient’s pain management” and then asked to rank the following five goals from first to fifth: “reduce this patient’s level of pain” (intensity), “decrease the effects of pain on this patient’s ability to perform daily activities” (function), “minimize harmful side effects from pain medications” (side effects), “decrease the effect of pain on this patient’s enjoyment of life” (enjoyment), and “determine what is causing this patient’s pain” (diagnosis).
Post-visit patient measures
Immediately after their clinic visits, patients completed several measures about their experience during the visit. Patient agreement with treatment plan was assessed using a 3-item scale developed by Staiger et al. that independently predicted long-term health outcomes in a previous study of patients with low back pain.[30] Patient appraisal of physicians’ communication skills was assessed using 6 items from the Consumer Assessment of Healthcare Providers and Systems (CAHPS) Adult Visit Survey.[31] Patient trust in physician was assessed using the short form of the Wake Forest trust in physician scale.[32] Patient assessment of visit difficulty was also assessed by rewording 5 items from the Difficult Doctor-Patient Relationship Questionnaire to assess the patient’s perception of visit difficulty. Patients also reported whether the visit was with their usual primary care physician and estimated the number of previous visits they had had with the physician they saw. Finally, patients were prompted to “think about which goals [for pain management] are most important to you,” and then asked to rank the same five goals that physicians ranked from first to fifth.
Chart abstraction
Data from patients’ electronic medical records were manually abstracted by a trained research assistant. One author (SGH) independently abstracted records for 23% of patients in order to check abstraction accuracy; he also reviewed and adjudicated ambiguous cases. Data abstracted included pain location, common physical, mental health, and substance use diagnoses, and patients’ baseline daily opioid dose measured in morphine milligram equivalents.[33] Diagnoses made after a patient’s clinic visit were not included.
Statistical analysis
To assess correlations and construct validity among the different measures of patient experience, product-moment correlations and exploratory factor analysis were conducted for the 4 measures of patients’ post-visit appraisal of the study encounter (agreement with treatment plan, assessment of physician communication skills, trust in physician, and patient assessment of visit difficulty). When multiple measures loaded onto a single factor (as determined by inspection of factor loadings, eigenvalues, and scree plots), we combined these measures to form a single, standardized measure of patient experience.[34]
For the first research question, patient-physician agreement on goals was evaluated both overall and for specific goals. Overall agreement was considered present if the physician’s first- or second-ranked priority for the patient included the patient’s top-ranked priority. Similar definitions have been used in prior studies comparing patient and physician priorities.[35] Goal-specific agreement was measured separately for each of the five ranked goals (intensity, function, side effects, enjoyment, and diagnosis) by calculating the difference between the patient and physician ranking for each goal.
For the second research question, which concerned the correlation between agreement on goals and physician-reported visit difficulty, we conducted regression analyses with physician-reported visit difficulty as the dependent variable and the agreement measures as the independent variables. We conducted separate regressions for overall agreement and for each measure of goal-specific agreement. Physician was analyzed as a random intercept to account for clustering within physicians.
For the third research question, which concerned the correlation between agreement on goals and patient experience, we conducted regression analyses with patient experience as the dependent variable and agreement on goals as the independent variable. We again conducted separate regressions for overall and goal-specific agreement and analyzed physician as a random intercept to account for clustering. Missing data was rare (0-2%) and was not imputed. Regression assumptions for RQ2 and RQ3 were checked by inspecting residual plots.
RESULTS
Seventy-five percent of eligible physicians (90 of 120) enrolled in the study. Of the 90 enrolled physicians, 49 saw at least one study patient and so were included in the final sample. Data from physicians who did not see any study patients were dropped from the final sample. The research assistant contacted and screened 194 patients and identified 134 eligible patients. Of these eligible patients, 113 (84%) were willing to enroll and 87 actually enrolled. The remaining 26 eligible and willing patients were unable to enroll due to scheduling conflicts (e.g., cancelled visits). Eight-six percent of patients reported that the study visit was with their primary care physician, and 92% reported at least one prior clinic visit with the physician they saw during their study visit.
Table 1 shows patient and physician demographics. Only 13% of patients reported working full or part time; 52% described themselves as unable to work and 34%, as retired. The median annual household income was between $10,001 and $20,000; 81% of patients reported incomes ≤ $60,000. For comparison, 2015 median household income in Sacramento County was $58,942.[36]
Table 1.
Participant Demographics
| Characteristic | Patients (n = 87) |
Physicians (n = 49) |
||
|---|---|---|---|---|
| Age, mean (SD) | 59.6 | (10.4) | 29.6 | (3.6) |
| Male sex, n (%) | 31 | (35.6%) | 12 | (24.5%) |
| Hispanic, n (%) | 12 | (13.8%) | 1 | (2.0%) |
| Race, n (%) | ||||
| White | 56 | (64.4%) | 24 | (49.0%) |
| Black | 24 | (27.6%) | 2 | (4.1%) |
| Asian | 0 | (0%) | 21 | (42.9%) |
| Native American | 2 | (2.3%) | 0 | (0%) |
| Multi-race / other | 5 | (5.8%) | 2 | (4.1%) |
| Clinic, n (%) | ||||
| Family practice | 23 | (26.4%) | 12 | (24.5%) |
| Internal medicine | 64 | (73.6%) | 37 | (75.5%) |
| Employment status, n (%) | ||||
| Working | 11 | (12.6%) | ||
| Not working | 1 | (1.2%) | ||
| Retired | 30 | (34.5%) | ||
| Disabled / unable to work | 45 | (51.7%) | ||
| Education, n (%) | ||||
| < High school | 16 | (18.4%) | ||
| High school graduate | 16 | (18.4%) | ||
| Some college | 30 | (34.5%) | ||
| Associates / technical degree | 11 | (12.6%) | ||
| Bachelor’s degree | 14 | (16.1%) | ||
| Annual household income*, n (%) | ||||
| ≤ $10, 000 | 21 | (24.4%) | ||
| $10,001 – $20,000 | 31 | (36.0%) | ||
| $20,001 – $40,000 | 11 | (12.8%) | ||
| $40,001 – $60,000 | 7 | (8.1%) | ||
| $60,001 – $80,000 | 9 | (10.5%) | ||
| > $80,000 | 7 | (8.1%) | ||
1 missing value
Table 2 shows patients’ clinical characteristics. Patients reported substantially lower physical and mental health than the general U.S. population. Patient median PCS was 22.8 (Interquartile range 18.0 – 29.9), which is 1.5 standard deviations worse than the U.S. median PCS of 40.7.[23] Patients’ median MCS (37.4, IQR 42.9 – 58.9) was 1.1 standard deviations worse than the U.S. median PCS of 53.1. Patients’ mean pain severity was 7.6; patients reported a mean of 2.4 (median 2) different pain sites. The most common pain locations were back (74%), lower limb (54%), upper limb (36%) and neck (21%). Common pain-related medical diagnoses included osteoarthritis (36%) and fibromyalgia (13%). A substantial proportion of patients also had mental health diagnoses, especially depression (54%) and anxiety (34%). Approximately two-fifths of patients had current or prior substance use disorder (excluding tobacco). Patients’ median daily opioid dose was 40 morphine milligram equivalents (IQR 20-80); 24% of patients were prescribed opioids and benzodiazepines concurrently.
Table 2.
Patients’ clinical characteristics, n = 87
| Self-reported measures | ||
|
| ||
| Health status, median (IQR) | ||
| VR-12 Physical component scorea | 22.8 | (17.9, 29.9) |
| VR-12 Mental component scorea | 37.4 | (31.6, 45.6) |
| Pain severityb, mean (SD) | 7.6 | (1.8) |
| Positive SOAPP-SF screenc, n (%) | 50 | (57.5%) |
| Pain catastrophizing, mean (SD) | 16.3 | (7.9) |
| Patient experience measuresd, median (IQR) | ||
| Agreement with treatment plan (range 3-21) | 20 | (17, 21) |
| Physician communication skills (range 0-12) | 12 | (11, 12) |
| Trust in physician (range 5-25) | 24 | (21, 25) |
| Patient-reported visit difficulty (range 5-30) | 7 | (5, 13) |
| Physician-reported visit difficultye, mean (SD) | 27.7 | (10.6) |
|
| ||
| Data from medical record review | ||
|
| ||
| Pain locationf, n (%) | ||
| Back | 64 | (73.6%) |
| Lower limb (e.g., knee, hip) | 47 | (54.0%) |
| Upper limb (e.g., shoulder) | 31 | (35.6%) |
| Neck | 18 | (20.7%) |
| Generalized pain | 17 | (19.5%) |
| Headache | 15 | (17.2%) |
| Abdomen / Pelvis | 10 | (11.5%) |
| Chest wall | 4 | (4.6%) |
| Other | 7 | (8.0%) |
| Medical diagnoses, n (%) | ||
| Hypertension | 61 | (70.1%) |
| Osteoarthritis | 31 | (35.6%) |
| COPD | 29 | (33.3%) |
| Diabetes | 24 | (27.6%) |
| Obstructive Sleep Apnea | 17 | (19.5%) |
| Coronary artery disease | 17 | (19.5%) |
| Fibromyalgia | 11 | (12.6%) |
| Neuropathy / Neuropathic pain | 7 | (8.0%) |
| Mental health diagnoses, n (%) | ||
| Depression | 47 | (54.0%) |
| Anxiety | 30 | (34.5%) |
| Insomnia | 10 | (11.5%) |
| Bipolar disorder | 7 | (8.0%) |
| Post-traumatic stress disorder | 3 | (3.4%) |
| Substance-related characteristics | ||
| Daily opioid doseg, (Median, IQR) | 40 | (20, 80) |
| Co-prescribed benzodiazepines, n (%) | 21 | (24.1%) |
| Any documented aberrant opioid behaviorh, n (%) | 19 | (21.8%) |
| Current or past substance use diagnosisi, n (%) | 35 | (40.2%) |
| Current tobacco use, n (%) | 24 | (27.6%) |
Veterans SF-12, range 0-100; higher score = better health
Measured using PEG, range 0-10
Range 0-20; higher score = greater risk of opioid misuse. Positive screen is score ≥4
Higher scores = more positive experience for agreement with treatment plan, communication skills, and trust in physician. Higher scores = worse experience for patient-reported visit difficulty. 1 missing value for trust and patient-reported visit difficulty
Range 10-60; higher scores = more difficult visit
Categories are not mutually exclusive
Morphine milligram equivalents
Includes early refill requests, taking more opioids than prescribed, lost or stolen prescriptions, using opioids for nonmedical purposes, abnormal urine drug screen, obtaining opioids from clinicians outside of UC Davis, and violation of signed patient-prescriber controlled substance agreement
Includes current or previous illegal drug use, prescription drug abuse, alcohol abuse as well as current marijuana use
All 4 post-visit measures of patient experience (agreement with treatment plan, assessment of physician communication skills, trust in physician, and patient assessment of visit difficulty) showed ceiling effects, with median values close to the best possible rating (Table 2). All 4 measures were also highly correlated with each other (mean | r | = 0.79, range 0.70 to 0.85). Exploratory factor analysis results indicated that all 4 variables loaded onto a single latent construct, so we combined all 4 variables into a standardized composite variable (i.e., summated rating scale) we called “patient experience.” This approach adjusts for the different ranges among the 4 variables and for the fact that patient-reported visit difficulty has a different polarity than the other 3 measures. Physician-reported visit difficulty scores were normally distributed, with a mean of 28 (SD 10.6). Physicians rated 41% of visits as difficult (defined as a visit difficulty score ≥30). In prior studies that examined all primary care visits, physicians rated only 15-18% of visits as difficult.[11, 28]
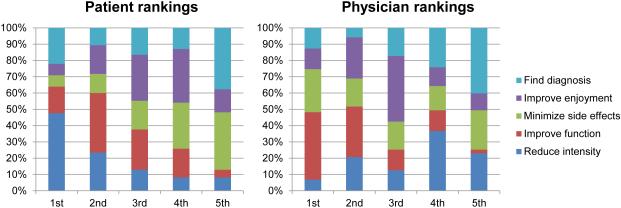
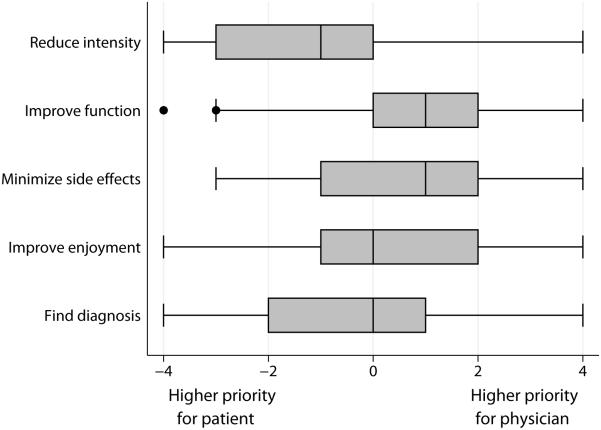
Figure 1 shows patients’ and physicians’ ranked priorities for pain management after each study visit. Nearly half (48%) of patients ranked reducing pain intensity as their top priority, followed by 22% who ranked diagnosing the cause of their pain as most important. In contrast, physicians ranked improving function as their top priority for 41% of patients, and ranked reducing medication side effects as most important for 26% of visits. Overall agreement on goals (i.e., the physician’s first- or second-ranked priority included the patient’s top-ranked priority) was present in 38% of visits. Figure 2 displays goal-specific agreement (i.e., the difference between patient and physician ranking for a specific goal) for each the 5 goals ranked by participants. By far, the greatest difference in ranking between patient and physician priorities was for reducing pain intensity. Patients nearly always ranked reducing pain intensity higher than did the physicians they saw; physicians ranked reducing pain intensity as higher priority than the patient in only 14% of visits. Patients typically ranked reducing side effects and improving function as lower priorities than the physicians they saw; however, the difference between patient and physician rankings had much more variation for both of these goals than was the case for reducing pain intensity
Figure 1. Patient and physician ranking of pain management goals.
1st indicates that the patient or physician ranked that goal as their top priority; 2nd indicates that the patient or physician ranked that goal as their second priority…etc. Patient ranking has 2 missing values.
Figure 2. Difference between patient and physician rankings of pain management goals.
Each row shows a box and whisker plot of the patient ranking less the physician ranking for one of the five ranked pain management goals. Negative values correspond to visits where the goal was ranked higher by the patient than the physician; positive values correspond to visits where the goal was ranked higher by the physician than the patient; and zero corresponds to visits in which the patient and physician ranked the goal equally. The middle line of each box indicates the median value. The left and right box edges indicate the 25th and 75th percentile, respectively. The left and right whiskers encompass values 1.5 IQR below the 25th percentile and 1.5 IQR above the 75th percentile, respectively. Solid circles indicate outlier values.
Because disagreement about goals was most pronounced for reducing pain intensity (and to reduce risk of type I error), we restricted statistical analyses for RQ2 and RQ3 to two measures: overall agreement on goals and intensity-specific agreement. Results of our regression analyses for RQ2 showed that neither overall agreement (beta = 1.92, 95%CI −2.70, 6.55; P = 0.41) nor intensity-specific agreement (beta = 0.42, 95%CI −0.87, 1.71, P = 0.53) were significantly correlated with physician-reported visit difficulty. Similarly, neither overall agreement (beta = −0.08, 95%CI −0.45, 0.30; P = 0.69) nor intensity-specific agreement (beta = −0.06, 95%CI −0.17, 0.04; P = 0.24) were significantly correlated with post-visit ratings of patient experience. Patient experience values were highly skewed, so we repeated analysis of RQ3 using generalized linear models with a log link.[37] Results did not meaningfully differ from those of our primary analysis.
DISCUSSION
In this study, we characterized patient-physician agreement about pain management goals after primary care visits that involved patients on long-term opioids for chronic musculoskeletal pain. To our knowledge, this is the first empirical analysis of patient-physician agreement about goals of chronic pain management. We found substantial differences in patients’ and physicians’ relative priorities for pain management. Patients’ most common top priority was reducing pain intensity, followed by diagnosing the cause of the pain. In contrast, physicians’ most common top priority was improving function, followed by reducing medication side effects.
Some of the observed disagreement is likely due to recent shifts in clinical practice. Physicians’ focus on functional goals and medication side effects (e.g., reducing opioid use) likely reflects recent recommendations to prioritize functional goals and to minimize opioid-related harms. In particular, the Medical Board of California guidelines were published just before data collection began, and physicians may have seen or read about these guidelines during the study period. In contrast, patients are likely more focused on symptom relief. New guideline recommendations may also reinforce some physicians’ tendency to avoid discussing diagnosis or physical symptoms when physicians perceive they have limited ability to reduce symptom severity.[38, 39] Patient-physician disagreement about pain management priorities may also reflect differences in how patients and physicians understand pain. Prior studies suggest that patients typically subscribe to a biomedical view of chronic pain, whereas most physicians subscribe to a more biopsychosocial view.[8, 40] Perhaps patients were more likely than physicians to prioritize diagnosing the cause of their pain because of a biomedical view of chronic pain, in which pain signifies undiagnosed mechanical dysfunction or injury. Another possible explanation is that patients may not readily distinguish between pain interference and pain intensity, or may view reducing pain intensity as a necessary antecedent to improved function. Further research is needed to corroborate our findings and investigate these potential explanations.
Pain management guidelines stress the importance of collaborative goal setting for chronic pain, so we were surprised to find no evidence that either overall agreement or intensity-specific agreement was associated with physician-reported visit difficulty or patient experience. Eighty-six percent of patients saw their regular physician, so one possible explanation is that disagreement about goals has relatively minor effects on patients’ and physicians’ experiences during a visit in the context of an established therapeutic relationship. A recent study found that veterans with chronic pain tended to evaluate physician recommendations in the context of their existing relationship, so that patients who have a positive therapeutic relationship with their physicians may accept recommendations or treatment plans even when they did not fully agree with them.[5] Another possibility is that patients and physicians may not have realized that they prioritized different goals, because goals were not explicitly discussed during the visit. Some patients may assume that their treatment priority and their physicians’ treatment priority are the same, even when they are not. Finally, disagreements about goals may only become relevant during visits where patients and physicians disagree about treatment plans (e.g., whether to prescribe opioids). These findings—and the absence of prior studies on this topic—underscore the need for additional empirical research on measuring patient-physician agreement about goals as well as on the relationships between agreement and visit outcomes related to chronic pain.
Patient characteristics in our study are consistent with prior research indicating that patients on long-term opioids for chronic pain constitute a vulnerable population who are poorer and sicker than the general population and have high rates of comorbid mental health and substance use disorders.[41, 42] Nearly a quarter (24%) of patients in our sample were co-prescribed benzodiazepines. This rate of co-prescribing is similar to rates reported in studies of veterans on long-term opioids;[20, 43] however, our data are more recent and were collected when the risks of co-prescribing were more widely appreciated. Therefore, the rate of co-prescribing in our sample may indicate that we recruited a patient population at relatively high risk for opioid-related harms. Most physicians in our sample were female and identified as either white or Asian. Physician gender, physician race and patient-physician concordance have important, complex associations with patient experience that require large samples to investigate.[44-46]
Although physicians rated a substantial proportion of visits as difficult (41% versus 15-18% for all primary care visits), our analyses suggest that disagreement about goals is apparently not a major driver of visit difficulty in this population. Most patients in our sample reported positive experiences (see Table 2), though a substantially lower proportion reported “top box” (i.e. best possible) scores compared to national samples of primary care. For example 72% of our sample gave “top box” scores for physician communication skills compared to 92% in national primary care samples.[47] Still, the positive measures in our sample indicate that even among patients taking opioids for chronic pain (and during visits where patients expected to discuss pain management), most patients have positive primary care experiences. As mentioned above, one likely explanation for these positive score is that most visits involved the patient’s regular physician.
Our study has several limitations. We studied visits in resident teaching clinics, so findings may not generalize to visits in community clinics or visits involving only attending physicians. We excluded residents with <1 year of experience, but residents may still be more likely to have poor communication skills than more experienced physicians. However, in our experience residents tend to develop communication styles early in training, and both good and bad communication habits are difficult to change over time. Resident clinics also typically care for a higher proportion of patients with high-risk opioid use than do community clinics,[48] and are also more likely to be “early adopters” of new recommendations for opioid prescribing. Thus, our results likely prefigure challenges that community clinics will face as new guidelines are adopted. Our sample is relatively small, largely because targeting specific visits during which patients planned to discuss pain management required resource-intensive recruitment methods. This approach was necessary to ensure that both patients and physicians could meaningfully rank pain treatment goals. This approach may have under-sampled patients with chronic pain who rarely discuss pain management or disagree with their physicians; however, comparison with national “top box” rates indicates that our sample was not unduly biased towards patients who have positive therapeutic relationships with their physicians. Finally, we assessed patient-physician agreement rather than patients’ and physicians’ perceptions of agreement.
Our findings suggest that patients and physicians prioritize substantially different management goals for chronic pain. In addition, recent clinical guideline changes may exacerbate differences in patient and physician treatment priorities, with patients focused on reducing pain intensity while physicians have shifted to emphasizing functional goals and avoidance of long-term opioid prescribing. Communication training initiatives and organizational changes (e.g., incorporating goals-of-care discussions into patient-provider prescribing agreements) may be needed to help primary care physicians ensure that their patients appreciate the importance of focusing on safe prescribing and functional goals when managing chronic musculoskeletal pain.
Supplementary Material
ACKNOWLEDGEMENTS
This work was funded by the National Institutes of Health (KL2TR000134/UL1TR000002) and the UC Davis Department of Internal Medicine. Dr. Kravitz is supported by National Institutes of Health (R01NR013938).
Footnotes
CONFLICTS OF INTEREST: No authors have any conflicts of interest to declare.
REFERENCES
- 1.Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Institute of Medicine; Washington, D.C.: 2011. [Google Scholar]
- 2.Brault M, Hootman J, Helmick C, Armour B. Prevalence and Most Common Causes of Disability Among Adults--United States, 2005. MWMR. 2009;58:421–6. [PubMed] [Google Scholar]
- 3.Gureje O, Von Korff M, Simon GE, Gater R. Persistent pain and well-being - A World Health Organization study in primary care. Jama-J Am Med Assoc. 1998;280:147–151. doi: 10.1001/jama.280.2.147. [DOI] [PubMed] [Google Scholar]
- 4.Deyo R, Mirza S, Martin B. Back pain prevalence and visit rates: estimates from U.S. national surveys. Spine. 2006;31:2724–2727. doi: 10.1097/01.brs.0000244618.06877.cd. [DOI] [PubMed] [Google Scholar]
- 5.Matthias MS, Krebs EE, Bergman AA, Coffing JM, Bair MJ. Communicating about opioids for chronic pain: A qualitative study of patient attributions and the influence of the patient-physician relationship. European journal of pain. 2014;18:835–843. doi: 10.1002/j.1532-2149.2013.00426.x. [DOI] [PubMed] [Google Scholar]
- 6.Street RL, Jr., Makoul G, Arora NK, Epstein RM. How does communication heal? Pathways linking clinician-patient communication to health outcomes. Patient Educ Couns. 2009;74:295–301. doi: 10.1016/j.pec.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 7.Mills S, Torrance N, Smith BH. Identification and Management of Chronic Pain in Primary Care: a Review. Curr Psychiatry Rep. 2016;18:22. doi: 10.1007/s11920-015-0659-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esquibel AY, Borkan J. Doctors and patients in pain: Conflict and collaboration in opioid prescription in primary care. Pain. 2014;155:2575–82. doi: 10.1016/j.pain.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 9.Matthias MS, Krebs EE, Collins LA, Bergman AA, Coffing J, Bair MJ. "I'm Not Abusing or Anything": Patient-physician communication about opioid treatment in chronic pain. Patient Educ Couns. 2013;93:197–202. doi: 10.1016/j.pec.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 10.Upshur CC, Bacigalupe G, Luckmann R. "They don't want anything to do with you": Patient views of primary care management of chronic pain. Pain Med. 2010;11:1791–8. doi: 10.1111/j.1526-4637.2010.00960.x. [DOI] [PubMed] [Google Scholar]
- 11.Hinchey SA, Jackson JL. A cohort study assessing difficult patient encounters in a walk-in primary care clinic, predictors and outcomes. J Gen Intern Med. 2011;26:588–94. doi: 10.1007/s11606-010-1620-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sullivan MD, Howe CQ. Opioid therapy for chronic pain in the United States: promises and perils. Pain. 2013;154(Suppl 1):S94–100. doi: 10.1016/j.pain.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudd R, Aleshire N, Zibbell JE, Gladden RM. Increases in Drug and Opioid Overdose Deaths — United States, 2000–2014. MMWR. 2016;64:1378–82. doi: 10.15585/mmwr.mm6450a3. [DOI] [PubMed] [Google Scholar]
- 14.Chou R, Fanciullo GJ, Fine PG, Adler JA, Ballantyne JC, Davies P, Donovan MI, Fishbain DA, Foley KM, Fudin J, Gilson AM, Kelter A, Mauskop A, O'Connor PG, Passik SD, Pasternak GW, Portenoy RK, Rich BA, Roberts RG, Todd KH, Miaskowski C, American Pain Society-American Academy of Pain Medicine Opioids Guidelines P Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113–30. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medical Board of California Guidelines for Prescribing Controlled Substances for Pain. 2014 [Google Scholar]
- 16.Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain-United States, 2016. JAMA. 2016 doi: 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ballantyne JC, Sullivan MD. Intensity of Chronic Pain--The Wrong Metric? N Engl J Med. 2015;373:2098–9. doi: 10.1056/NEJMp1507136. [DOI] [PubMed] [Google Scholar]
- 18.Lee TH. Zero Pain Is Not the Goal. Jama. 2016 doi: 10.1001/jama.2016.1912. [DOI] [PubMed] [Google Scholar]
- 19.Campbell J. American Pain Society. Washington, DC: 1996. Pain as the 5th vital sign [presidential address] [Google Scholar]
- 20.Dobscha S, Morasco B, Duckart J, Macey T, Deyo R. Correlates of Prescription Opioid Initiation and Long-term Opioid Use in Veterans With Persistent Pain. Clin J Pain. 2013;29:102–8. doi: 10.1097/AJP.0b013e3182490bdb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henry SG, Wilsey BL, Melnikow J, Iosif AM. Dose Escalation During the First Year of Long-Term Opioid Therapy for Chronic Pain. Pain Med. 2015;16:733–44. doi: 10.1111/pme.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hughes HK, Korthuis PT, Saha S, Eggly S, Sharp V, Cohn J, Moore R, Beach MC. A mixed methods study of patient-provider communication about opioid analgesics. Patient Educ Couns. 2015;98:453–61. doi: 10.1016/j.pec.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Selim AJ, Rogers W, Fleishman JA, Qian SX, Fincke BG, Rothendler JA, Kazis LE. Updated U.S. population standard for the Veterans RAND 12-item Health Survey (VR-12) Qual Life Res. 2009;18:43–52. doi: 10.1007/s11136-008-9418-2. [DOI] [PubMed] [Google Scholar]
- 24.Krebs EE, Lorenz KA, Bair MJ, Damush TM, Wu JW, Sutherland JM, Asch SM, Kroenke K. Development and initial validation of the PEG, a three-item scale assessing pain intensity and interference. J Gen Intern Med. 2009;24:733–738. doi: 10.1007/s11606-009-0981-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krebs EE, Bair MJ, Damush TM, Tu W, Wu J, Kroenke K. Comparative responsiveness of pain outcome measures among primary care patients with musculoskeletal pain. Med Care. 2010;48:1007–14. doi: 10.1097/MLR.0b013e3181eaf835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenstiel AK, Keefe FJ. The Use of Coping Strategies in Chronic Low-Back-Pain Patients - Relationship to Patient Characteristics and Current Adjustment. Pain. 1983;17:33–44. doi: 10.1016/0304-3959(83)90125-2. [DOI] [PubMed] [Google Scholar]
- 27.Koyyalagunta D, Bruera E, Aigner C, Nusrat H, Driver L, Novy D. Risk Stratification of Opioid Misuse among Patients with Cancer Pain Using the SOAPP-SF. Pain Med. 2013;14:667–75. doi: 10.1111/pme.12100. [DOI] [PubMed] [Google Scholar]
- 28.Hahn SR, Kroenke K, Spitzer RL, Brody D, Williams JBW, Linzer M, deGruy FV. The difficult patient: Prevalence, psychopathology, and functional impairment. J Gen Intern Med. 1996;11:1–8. doi: 10.1007/BF02603477. [DOI] [PubMed] [Google Scholar]
- 29.Jackson JL, Kay C. Heartsink Hotel, or "Oh No, Look Who's on My Schedule this Afternoon!". J Gen Intern Med. 2013;28:1385–6. doi: 10.1007/s11606-013-2447-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Staiger T, Jarvik J, Deyo R, Martin B, Braddock C. BRIEF REPORT: Patient-physician agreement as a predictor of outcomes in patients with back pain. J Gen Intern Med. 2005;20:935–937. doi: 10.1111/j.1525-1497.2005.0175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dyer N, Sorra JS, Smith SA, Cleary PD, Hays RD. Psychometric properties of the Consumer Assessment of Healthcare Providers and Systems (CAHPS(R)) Clinician and Group Adult Visit Survey. Med Care. 2012;50(Suppl):S28–34. doi: 10.1097/MLR.0b013e31826cbc0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dugan E, Trachtenberg F, Hall MA. Development of abbreviated measures to assess patient trust in a physician, a health insurer, and the medical profession. Bmc Health Serv Res. 2005;5:64. doi: 10.1186/1472-6963-5-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.PDMP Center of Excellence Calculating Daily Morphine Milligram Equivalents [Web Page] Available at http://www.pdmpassist.org/pdf/BJA_performance_measure_aid_MME_conversion.pdf. Accessed July 10 2016.
- 34.McIver JP, Carmines EG. Unidimensional scaling. Sage Publications; Beverly Hills: 1981. [Google Scholar]
- 35.Zulman DM, Kerr EA, Hofer TP, Heisler M, Zikmund-Fisher BJ. Patient-provider concordance in the prioritization of health conditions among hypertensive diabetes patients. J Gen Intern Med. 2010;25:408–14. doi: 10.1007/s11606-009-1232-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.US Census Bureau 2015 . American Community Survey 1-year estimates. US Census Bureau; [Google Scholar]
- 37.Nichols A. Stata Conference. Boston, MA: 2010. Regression for nonnegative skewed dependent variables. [Google Scholar]
- 38.Hahn SR. Physical symptoms and physician-experienced difficulty in the physician-patient relationship. Ann Intern Med. 2001;134:897–904. doi: 10.7326/0003-4819-134-9_part_2-200105011-00014. [DOI] [PubMed] [Google Scholar]
- 39.Epstein RM, Shields CG, Meldrum SC, Fiscella K, Carroll J, Carney PA, Duberstein PR. Physicians' responses to patients' medically unexplained symptoms. Psychosom Med. 2006;68:269–76. doi: 10.1097/01.psy.0000204652.27246.5b. [DOI] [PubMed] [Google Scholar]
- 40.Kenny DT. Constructions of chronic pain in doctor-patient relationships: Bridging the communication chasm. Patient Educ Couns. 2004;52:297–305. doi: 10.1016/S0738-3991(03)00105-8. [DOI] [PubMed] [Google Scholar]
- 41.Sullivan MD, Von Korff M, Banta-Green C, Merrill JO, Saunders K. Problems and concerns of patients receiving chronic opioid therapy for chronic non-cancer pain. Pain. 2010;149:345–53. doi: 10.1016/j.pain.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jensen MK, Thomsen AB, Hojsted J. 10-year follow-up of chronic non-malignant pain patients: opioid use, health related quality of life and health care utilization. European journal of pain. 2006;10:423–33. doi: 10.1016/j.ejpain.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 43.Park TW, Saitz R, Ganoczy D, Ilgen MA, Bohnert ASB. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. BMJ. 2015:1–8. doi: 10.1136/bmj.h2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cooper LA, Roter DL, Johnson RL, Ford DE, Steinwachs DM, Powe NR. Patient-centered communication, ratings of care, and concordance of patient and physician race. Annals of Internal Medicine. 2003;139:907–15. doi: 10.7326/0003-4819-139-11-200312020-00009. [DOI] [PubMed] [Google Scholar]
- 45.Hall JA, Blanch-Hartigan D, Roter DL. Patients' satisfaction with male versus female physicians: a meta-analysis. Med Care. 2011;49:611–7. doi: 10.1097/MLR.0b013e318213c03f. [DOI] [PubMed] [Google Scholar]
- 46.Bertakis KD, Franks P, Epstein RM. Patient-centered communication in primary care: physician and patient gender and gender concordance. Journal of women's health. 2009;18:539–45. doi: 10.1089/jwh.2008.0969. [DOI] [PubMed] [Google Scholar]
- 47.Agency for Healthcare Research and Quality CAHPS Clinician & Group Survey Database Comparative data [Web Page] Available at www.cahpsdatabase.ahrq.gov. Accessed 17 October 2016, 2016.
- 48.Colburn JL, Jasinski DR, Rastegar DA. Long-term opioid therapy, aberrant behaviors, and substance misuse: comparison of patients treated by resident and attending physicians in a general medical clinic. Journal of opioid management. 2012;8:153–60. doi: 10.5055/jom.2012.0111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.