Abstract
OBJECTIVE
Increase of subchondral bone area (tAB) in OA has been reported, but it remains unclear if this is specific to OA. We investigated differences in knee tAB after one year in healthy subjects and in those with radiographic OA (rOA).
METHOD
MR images of 899 right knees from the OA Initiative were acquired at baseline and one year follow-up (year-1). Medial and lateral tibial cartilage (MT and LT) and weight-bearing femoral cartilage (cMF and cLF) were segmented and tAB computed. Subjects were stratified into: healthy controls, pre-rOA (K&L grades 0 and 1, with OA risk factors), established rOA (K&L grades 2–4), and independently with regards to joint space narrowing (without, with medial, lateral and bilateral JSN). Primary analysis tested if tAB was different between baseline and year-1 in rOA. Exploratory analyses investigated whether: 1) tAB changes differed between healthy controls and those with rOA; 2) tAB differences were greater in higher K&L grades; and 3) tAB was different between baseline and year-1 in JSN. Significance was set at p<0.0125.
RESULTS
Differences in tAB were found in rOA in MT, cMF and cLF (ranging from +0.2% to +0.4%; p<0.001), but not in healthy controls or pre-rOA. Rates of change did not differ between groups. Within the JSN groups differences of 0.2% to 0.4% were found in the femur (p<0.05).
CONCLUSION
We find that knee tABs differ in rOA between baseline and year-1, but the change was not greater than in healthy knees, and is restricted to the femur in JSN.
Keywords: Osteoarthritis, subchondral bone size, sensitivity to change, magnetic resonance imaging
INTRODUCTION
Bone remodeling is an ongoing process in which bone adapts to its mechanical environment through addition, removal and rearrangement of bone mass. Importantly, bone remodeling is thought to play a role in disease progression of osteoarthritis (OA)1–3. An increase of subchondral bone area (tAB) in knee OA has been suggested4–15, but it remains unclear if this is specific to OA, or occurs independently of OA, possibly due to physiological aging16 or an altered biomechanical environment17–19.
Several studies support the hypothesis that the increase in tAB in the course of OA is primarily associated with the principal pathological processes of OA such as osteophytosis, cartilage defects, and general cartilage thinning, represented by joint space narrowing (JSN). Cross-sectionally, an association between larger tibial tAB and osteophytosis of the medial tibia (MT) was reported in early OA4, and it was found that Kellgren and Lawrence (K&L) grade explains approximately 20% of medial tAB variance20. A longitudinal study found an incremental increase in tAB for each grade of osteophytosis and JSN8. Other prospective evaluations have observed an association between both male sex and higher baseline medial JSN grade with an increase in medial tibial tAB in knee OA7. It has been suggested that the increase of tibial tAB may precede cartilage defects5 and cartilage swelling11, and may be predictive for the incidence of OA9,15.
Other studies have found an association between tAB increase in OA and mechanical factors. For example, Howell et al observed that in OA subjects with varus malalignment, the radius of the lateral femoral condyle (a surrogate measure of bone size) is higher than that of the medial condyle, whereas in valgus knees, the relation is reversed13. In similar subjects, the annual tAB increase has been reported to range from 0.1% to 0.3%, depending on the compartment with regards to alignment12. Also, the size of medial tibial plateau bone area has been shown to positively correlate with the knee adduction moment in medial femorotibial OA14. Similarly, in 52 young subjects an increase in the medial and a decrease in the lateral tibial bone area were found seven years after anterior cruciate ligament reconstruction19.
Evidence exists that remodeling of tAB also occurs in subjects without OA, potentially as a result of physiological aging, or an adaptation to the mechanical environment. In 50–76 year old healthy females, an increase of approximately 1% per annum in tibial tAB has been reported, with a negative association between baseline tAB and age16. In comparison, a positive association between age and medial/lateral tibial tAB and patellar volume has been found in a cross-sectional convenience sample of 372 subjects21. In postmenopausal women, an increase of tAB over two years in the dominant knee has been shown to positively correlate with the prevalence of meniscal tears18. In elderly healthy women, the size of the medial, but not of the lateral tibial subchondral bone area has been shown to positively correlate with the knee adduction moment17.
In summary, it remains unclear whether increasing subchondral bone area is specific to OA, or occurs in association with biomechanical factors or age, independently of OA. The purpose of the current study was to examine differences in tAB between baseline and year-1 in healthy knees and in those with different grades of rOA, and to explore the correlation with JSN as a surrogate marker for malalignment and therefore localization of joint loads. Specifically, the primary hypothesis was:
Knees with definite rOA show significant larger tABs over one year
Further exploratory analyses tested whether:
The rate of change in tAB over one year is greater in knees with definite rOA than in healthy reference knees
Differences in tAB over one year are greater in knees with higher grades of rOA
Differences in tAB over one year are greater in compartments with JSN than in those without.
METHOD
OAI cohort and MRI sequences
Participants in the OAI (http://oai.epi-ucsf.org/datarelease/) were 45–79 years old (2804 females, 1992 males). General exclusion criteria were rheumatoid or inflammatory arthritis, bilateral end-stage knee OA, inability to walk without aids, and MRI contraindications. The healthy, “non-exposed” control subcohort (n=122) met the following inclusion criteria: 1) no pain, aching or stiffness in either knee in the past year; 2) no femorotibial rOA in either knee using the clinical site readings of the baseline fixed flexion radiographs22; 3) no risk factors for OA, including obesity, knee injury, knee surgery, a family history of knee replacement in a biological parent or sibling, Heberden’s nodes, or repetitive knee bending. The pre-rOA and rOA cohorts were sampled from the combined “incidence” and “progression” subcohorts of the OAI, following self-defined inclusion criteria. Both groups could have risk factors like frequent knee symptoms, overweight (BMI <= 25), history of knee injury or surgery, family history of knee replacement, and hand OA. The pre-rOA subjects had no symptoms or definite signs of rOA in the target (right), but possibly in the contralateral knee, represented by K&L grades 0 and 1, while the rOA subjects had definite OA (K&L grades 2 to 4) in the target knee in addition to being symptomatic (pain, aching or stiffness on most days of a month in past year). Please refer to the OAI webpage for more detailed information: http://oai.epi-ucsf.org/datarelease/docs/StudyDesignProtocol.pdf.
The datasets for the current longitudinal analysis were drawn from a subsample of 1003 right knees, which were analyzed by a consortium of industry partners (see acknowledgments), the OAI Coordinating Center at University of California San Francisco (UCSF), and an image analysis company (Chondrometrics GmbH) as described previously23. From this group, 96 datasets were excluded, as they were acquired with a double-echo steady-state MRI sequence, and not with a double oblique coronal FLASH water excitation sequence at 3Tesla according to a standardized protocol24,25. One dataset was excluded because central rOA grading was missing, and 47 datasets were excluded because they were left knees (some overlap with the 96 from above), leading to a final sub sample of 899 right knees.
Radiographic grading and sample selection
The rOA grading was derived from the central X-ray readings provided by the OAI, which were performed at Boston University by two expert readers, who independently assessed each image, blinded to each other’s reading and to clinical data. Discrepancies between readers were adjudicated in a consensus session with blinded images viewed simultaneously and a third reader participating. The readers assessed each knee for K&L grades (Figure 1)26, and other individual radiographic features27,28, including medial and lateral JSN grades (Figure 2). Based on these readings, the analyzed sample was stratified into the following cohorts, based on OA status:
Figure 1.
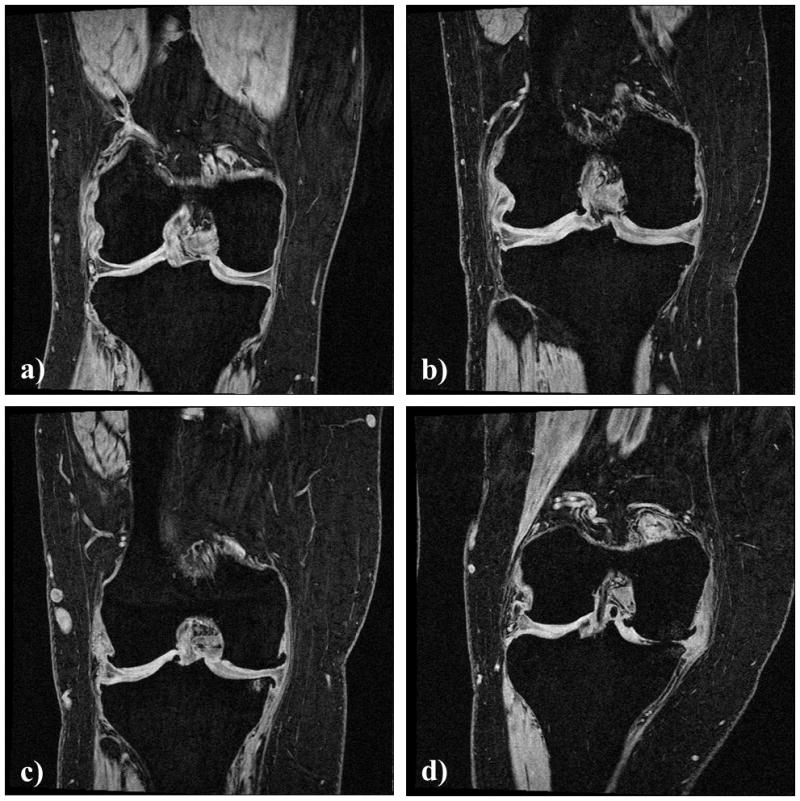
Coronal spoiled gradient echo (FLASH) images of the right knee of four subjects from cohorts with different Kellgren and Lawrence (K&L) grades of radiographic OA: a) K&L grade 0, b) K&L grade 2, c) K&L grade 3, and d) K&L grade 4.
Figure 2.
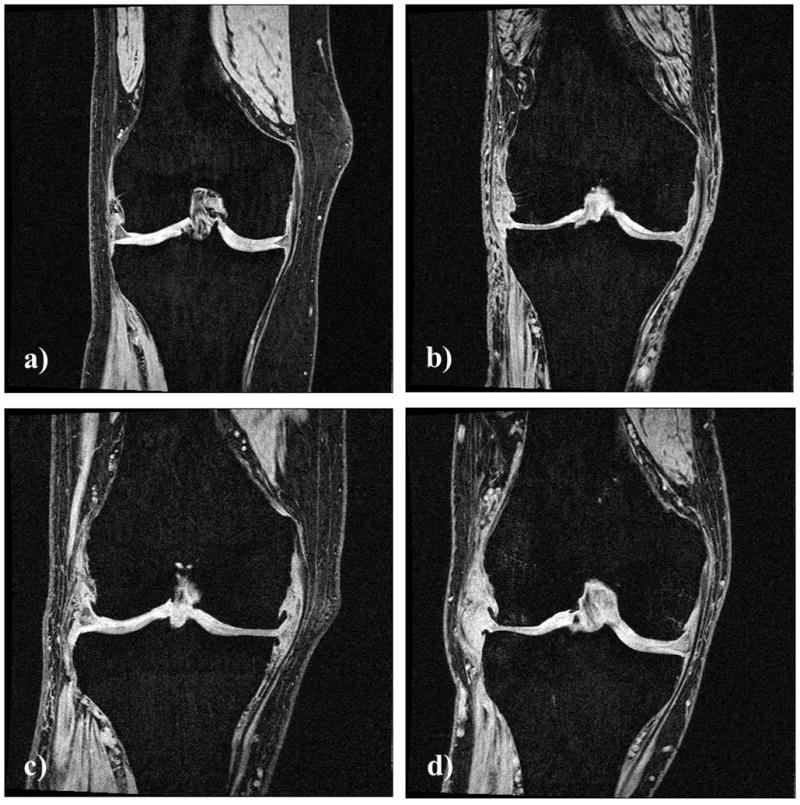
Coronal spoiled gradient echo (FLASH) images of the right knee of four subjects from different cohorts with joint space narrowing (JSN): a) without JSN, b) with bilateral JSN grade 2, c) with medial unilateral JSN grade 3, and d) with lateral unilateral JSN grade 2.
Healthy = non-exposed control cohort without rOA in either knee and without risk factors
Pre-rOA = cohort without signs of rOA in the target knee (K&L grades 0 and 1), but with risk factors and/or rOA in the contralateral knee
-
rOA = cohort with established rOA (K&L grades 2 to 4) in the investigated knee
KLG 2 = cohort with K&L grade 2 (the KLG cohorts are sub-cohorts of the rOA cohort)
KLG 3 = cohort with K&L grade 3
KLG 4 = cohort with K&L grade 4
For the exploratory analyses, stratification was also performed based on JSN rather than OA status:
M0 / L0 = medial and lateral JSN grade 0 (including healthy, pre rOA and rOA cases)
M0 / L1–3 = medial JSN grade 0 and lateral JSN grades 1 to 3
M1–3 / L0 = medial JSN grades 1 to 3 and lateral JSN grade 0
M1–3 / L1–3 = bicompartimental JSN, with medial and lateral JSN grades 1 to 3
Table 1 gives a detailed breakdown of the number of subjects in each of the strata, and the basic demographic variables.
TABLE 1.
Demographic variables for the study sample, the healthy group without risk factors (Healthy Control), the group with risk factors for knee osteoarthritis (pre-rOA), groups with different grades of radiographic osteoarthritis (rOA: KLG2 through KLG4), and independently groups with different configurations of joint space narrowing (without, medial, lateral, and bilateral).
| Females | Males | Age | Height[cm] | Weight[kg] | BMI | |
|---|---|---|---|---|---|---|
| Total | 539 | 360 | 61.6±9.5 | 167.7±9.0 | 81.5±16.1 | 28.9±4.8 |
| Healthy Control | 61 | 40 | 55.0±7.6 | 167.8±8.7 | 69.0±11.9 | 24.4±3.0 |
| Pre-rOA | 137 | 117 | 60.7±9.5 | 168.0±9.3 | 80.7±15.7 | 28.4±4.3 |
| rOA | 341 | 203 | 63.2±9.2 | 167.5±8.9 | 84.3±15.9 | 30.0±4.8 |
| KLG2 | 220 | 96 | 62.4±9.1 | 166.7±8.5 | 83.5±16.0 | 30.1±4.8 |
| KLG3 | 94 | 73 | 64.9±9.1 | 168.0±9.3 | 85.3±15.8 | 30.1±4.7 |
| KLG4 | 27 | 34 | 62.7±9.5 | 171.0±9.0 | 85.4±16.1 | 29.0±4.6 |
|
| ||||||
| M0 / L0 | 198 | 158 | 59.1±9.2 | 167.3±8.9 | 77.5±15.5 | 27.6±4.6 |
| M0 / L1–3 | 68 | 36 | 63.8±9.5 | 168.2±9.8 | 82.6±16.9 | 29.1±4.8 |
| M1–3 / L0 | 225 | 156 | 63.5±9.2 | 168.0±8.9 | 85.2±15.5 | 30.2±4.8 |
| M1–3 / L1–3 | 3 | 10 | 62.4±10.8 | 169.6±9.8 | 88.9±18.8 | 30.0±3.9 |
Pre-rOA = Kellgren & Lawrence grades 1 and 2, rOA = Kellgren & Lawrence Grades 2 to 4, M0 = no medial joint space narrowing, M1–3 = medial joint space narrowing grade 1–3, L0 = no lateral joint space narrowing, L1–3 = lateral joint space narrowing grade 1–3
MRI analysis
Acquisition of MRI datasets, transfer logistics and segmentation were performed as described previously29,30. The total area of subchondral bone (tAB), represented by the tidemark between calcified and non-calcified cartilage, was segmented manually in the medial (MT) and lateral tibiae (LT), and in the weight-bearing (central) part of the medial (cMF) and lateral femoral condyles (cLF). Osteophytes were excluded from tAB by taking into account the borders of the tibial and femoral condyles. The posterior aspects of the femoral condyles were not included, as segmentation in these areas is not supported by the coronal imaging protocol, and because the sensitivity to change in these regions has been shown to be unfavourable31. To minimize segmentation errors and deviations between readers, all segmentations were quality controlled by one expert (S.M.), and corrected if necessary. The segmentations were used to generate virtual 3D-reconstructions, from which the size of the tAB was computed by triangulation32. The test-retest precision of the method has been reported as good to excellent (precision error ranging from 0.7–1.7%) previously33.
Statistical analysis
The primary analysis focused on differences in tAB size in the four femorotibial cartilage plates after one year in the cohort with rOA. The exploratory analyses were to test whether the changes in tAB size over one year were different between rOA and healthy knees, and to compare tAB differences between groups with pre-rOA, different K&L and JSN grades.
The mean change (MC) and standard deviation of change in tAB [mm2] between baseline and year-1 follow-up were determined for all cartilage plates of the femorotibial joint (MT, cMF, LT, cLF). Percent changes were derived by dividing the MC in a group by the mean tAB at baseline for the same cohort. The standardized response mean (SRM defined as mean change divided by the standard deviation of change) was used as a measure of sensitivity to change. Double-sided paired student’s t-test was employed to detect significant differences in tAB between baseline and year-1 follow-up and one-sided non-paired t-tests were used to check whether baseline values or rates of change differed between groups. Double-sided paired t-tests were used to test whether tAB differences were greater in compartments affected by JSN rather than in the non-JSN compartment. The required p-value was set to ≤0.0125 to correct for multiple testing of the four cartilage plates in each analysis at a global error level of 5%. Changes where single comparisons did not reach the global significance level but were p ≤0.05 were deemed trends.
RESULTS
Figure 3 gives an overview of absolute changes in tAB size for each analyzed cartilage plate in the various cohorts. Baseline values of tAB differed significantly between healthy controls, pre-rOA and rOA for all cartilage plates (unpaired t-test). Only the cMF tAB differed between pre-rOA and rOA.
Figure 3.
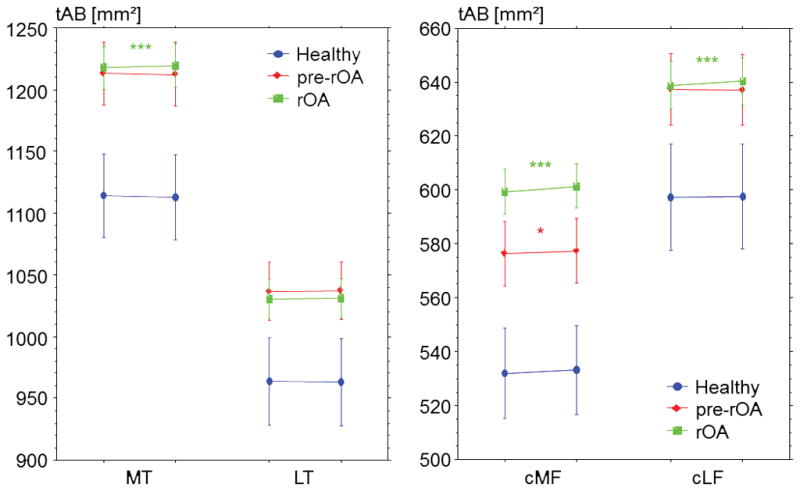
Graph showing the size of subchondral bone area (tAB) in the medial tibia (MT), lateral tibia (LT), central medial femur (cMF), and central lateral femur (cLF) at baseline (left side of each pair) and at one-year follow up (right side of each pair), for healthy controls, pre-rOA (Kellgren and Lawrence grades 0 and 1) and rOA (Kellgren and Lawrence grades 2 to 4) cohorts. Error bars indicate 95% confidence intervals, *** p<0.001, * p<0.05, please note that only p<0.0125 is considered significant for the primary analysis (Bonferroni correction).
tAB differences and changes in rOA
Between baseline and year-1, the entire rOA cohort displayed significant (p<0.001) differences in tAB of 0.2% in MT, 0.4% in cMF, and 0.2% in cLF. tAB of the LT did not significantly change (p=0.437) (Table 2). The rates of change were not different from those found in the healthy reference cohort.
TABLE 2.
Absolute size and change over one year of the bone-cartilage-interface area (tAB) for the entire study sample, a healthy group without risk factors (Healthy Control), a group the group with risk factors for knee osteoarthritis (pre-rOA), and groups with different grades of radiographic osteoarthritis (rOA: KLG2 through KLG4). p is given for a paired t-test between baseline and 1 year follow-up.
| Area[mm2] | Change[mm2] | Change[%] | 95%CI[mm2] | SRM | p | |
|---|---|---|---|---|---|---|
| Medial Tibia (MT) | ||||||
| Total | 1204.6 | 0.8 | 0.1 | 0.85 | 0.06 | 0.062 |
| Healthy Control | 1114.3 | −1.2 | −0.1 | 2.38 | −0.10 | 0.309 |
| Pre-rOA | 1212.8 | −0.5 | 0.0 | 1.54 | −0.04 | 0.490 |
| rOA | 1217.6 | 1.8 | 0.2 | 1.12 | 0.14 | 0.002 |
| KLG2 | 1195.0 | 1.0 | 0.1 | 1.37 | 0.09 | 0.133 |
| KLG3 | 1231.6 | 2.4 | 0.2 | 2.07 | 0.18 | 0.023 |
| KLG4 | 1296.6 | 4.2 | 0.3 | 4.25 | 0.25 | 0.055 |
| Central Medial Femur (cMF) | ||||||
| Total | 585.3 | 1.7 | 0.3 | 0.65 | 0.17 | <0.001 |
| Healthy Control | 532.0 | 1.2 | 0.2 | 2.11 | 0.11 | 0.279 |
| Pre-rOA | 576.4 | 0.9 | 0.2 | 1.15 | 0.10 | 0.099 |
| rOA | 599.3 | 2.1 | 0.4 | 0.85 | 0.21 | <0.001 |
| KLG2 | 589.3 | 1.7 | 0.3 | 1.02 | 0.18 | 0.001 |
| KLG3 | 608.6 | 3.0 | 0.5 | 1.67 | 0.27 | 0.001 |
| KLG4 | 626.2 | 1.9 | 0.3 | 3.03 | 0.16 | 0.223 |
| Lateral Tibia (LT) | ||||||
| Total | 1024.9 | 0.4 | 0.1 | 0.81 | 0.03 | 0.382 |
| Healthy Control | 964.0 | −0.7 | −0.1 | 2.36 | −0.05 | 0.577 |
| Pre-rOA | 1036.6 | 0.6 | 0.1 | 1.44 | 0.05 | 0.391 |
| rOA | 1030.7 | 0.4 | 0.0 | 1.08 | 0.03 | 0.437 |
| KLG2 | 1017.1 | 0.6 | 0.1 | 1.38 | 0.05 | 0.374 |
| KLG3 | 1038.5 | 0.5 | 0.1 | 1.95 | 0.04 | 0.583 |
| KLG4 | 1079.5 | −0.9 | −0.1 | 3.84 | −0.06 | 0.638 |
| Central Lateral Femur (cLF) | ||||||
| Total | 633.7 | 0.8 | 0.1 | 0.59 | 0.09 | 0.006 |
| Healthy Control | 597.4 | 0.2 | 0.0 | 1.72 | 0.02 | 0.828 |
| Pre-rOA | 637.3 | −0.1 | 0.0 | 1.07 | −0.02 | 0.816 |
| rOA | 638.8 | 1.4 | 0.2 | 0.79 | 0.15 | <0.001 |
| KLG2 | 630.1 | 1.4 | 0.2 | 1.03 | 0.15 | 0.010 |
| KLG3 | 645.9 | 1.3 | 0.2 | 1.53 | 0.13 | 0.103 |
| KLG4 | 664.7 | 2.2 | 0.3 | 2.13 | 0.27 | 0.042 |
SEM = standard error of the mean, SRM = standardized response mean, Pre-ROA = Kellgren & Lawrence Grades 1 and 2, rOA = Kellgren & Lawrence Grades 2 to 4, KLG = Kellgren & Lawrence Grade
tAB differences and changes in KLG
The healthy reference cohort and the pre-rOA cohort (K&L grades 0 and 1) showed no significant differences in tAB in any of the cartilage plates between baseline and year-1. In the KLG2 sub-cohort differences were seen in cMF (0.3%, p=0.001) and a trend in cLF (0.2%, p=0.010), but not in the tibia. The KLG3 cohort showed tAB differences in cMF (0.5%, p=0.001), and a trend in MT (0.2%, p=0.023), but not in the lateral compartment (Table 2). In the KLG4 cohort trends were found in cMF (0.3%, p = 0.027) and cLF (0.3%, p=0.042). The SRMs for significant tAB changes in these cohorts ranged from 0.14 to 0.27 (Table 2). The rate of change was significantly different in MT between the pre-rOA and the rOA cohort (p=0.009), and a trend existed for MT between the healthy and the rOA cohort (p=0.016).
tAB differences and changes in JSN
The cohort without JSN (M0 / L0), including healthy, pre-rOA and rOA knees, showed significant (p=0.003) tAB differences of 0.3% in cMF between baseline and year-1, and a trend in LT (0.1%, p=0.047). In the cohort with lateral JSN (M0 / L1–3) a tAB difference of 0.4% was found in cLF (p=0.003), and a trend in cMF (0.3%, p=0.043). The cohort with medial JSN (M1–3 / L0) displayed larger femoral cartilage plates at year-1 (cMF: 0.3%, p=0.002 and cLF: 0.2%, p=0.011). In the cohort with bilateral JSN (M1–3 / L1–3) a tAB difference was found in cMF (1.0%, p=0.018), but not in the other cartilage plates. The SRMs for significant tAB changes between baseline and year-1 in unicompartimental JSN ranged from 0.13 to 0.30, being in the same range as for the different OA specific strata (Table 3). The rates of change in tAB in unicompartimental JSN were not significantly different between compartments with and without JSN.
TABLE 3.
Absolute size and rate of change over one year of the bone-cartilage-interface area (tAB) in a group without joint space narrowing (JSN, M0 / L0), groups with unilateral JSN (M1–3 / L0 and L1–3 / M0) and a group with bilateral JSN (M1–3 / L1–3).
| Area[mm2] | Change[mm2] | Change[%] | 95%CI[mm2] | SRM | p | |
|---|---|---|---|---|---|---|
| Medial Tibia (MT) | ||||||
| M0 / L0 | 1169.4 | 0.2 | 0.0 | 1.19 | 0.02 | 0.727 |
| M0 / L1–3 | 1221.3 | 1.7 | 0.1 | 2.63 | 0.13 | 0.193 |
| M1–3 / L0 | 1235.1 | 1.2 | 0.1 | 1.38 | 0.09 | 0.095 |
| M1–3 / L1–3 | 1266.3 | 0.9 | 0.1 | 6.82 | 0.08 | 0.786 |
| Central Medial Femur (cMF) | ||||||
| M0 / L0 | 559.7 | 1.4 | 0.3 | 0.92 | 0.15 | 0.003 |
| M0 / L1–3 | 593.3 | 2.0 | 0.3 | 1.98 | 0.20 | 0.043 |
| M1–3 / L0 | 608.6 | 1.7 | 0.3 | 1.05 | 0.16 | 0.002 |
| M1–3 / L1–3 | 626.9 | 6.2 | 1.0 | 4.94 | 0.76 | 0.018 |
| Lateral Tibia (LT) | ||||||
| M0 / L0 | 1003.4 | 1.2 | 0.1 | 1.14 | 0.01 | 0.047 |
| M0 / L1–3 | 1061.1 | 1.9 | 0.2 | 2.63 | 0.14 | 0.162 |
| M1–3 / L0 | 1034.0 | −0.8 | −0.1 | 1.29 | −0.07 | 0.206 |
| M1–3 / L1–3 | 1130.4 | −1.3 | −0.1 | 5.64 | −0.14 | 0.633 |
| Central Lateral Femur (cLF) | ||||||
| M0 / L0 | 617.9 | 0.1 | 0.0 | 0.89 | 0.01 | 0.873 |
| M0 / L1–3 | 661.2 | 2.6 | 0.4 | 1.68 | 0.30 | 0.003 |
| M1–3 / L0 | 641.4 | 1.2 | 0.2 | 0.92 | 0.13 | 0.011 |
| M1–3 / L1–3 | 675.9 | 1.0 | 0.1 | 6.82 | 0.09 | 0.764 |
SEM = standard error of the mean, SRM = standardized response mean, M0 = no medial joint space narrowing, M1–3 = medial joint space narrowing grade 1 to 3, L0 = no lateral joint space narrowing, L1–3 = lateral joint space narrowing grade 1–3
DISCUSSION
The purpose of the current study was to test the primary hypothesis that knees with definite radiographic osteoarthritis (rOA) show significant differences in subchondral bone size (tAB) over one year. Exploratory analyses investigated whether the rate of change in tAB was greater in knees with definite rOA than in healthy reference knees, and compared differences in tAB after one year in various groups with different K&L grades and configurations of joint space narrowing (JSN). The knees with rOA showed significant tAB differences in the medial tibia (MT) and in the central femur (cMF and cLF). The tAB increase in rOA was not significantly different (greater) than in healthy reference knees. Differences in tAB over one year tended to be greater in knees with higher grades of rOA, but differences were not generally greater in compartments with JSN rather than those without.
A limitation of the study is that the differences/changes observed here are smaller, albeit only slightly smaller, than the precision errors that have been reported for MRI-based measures of tAB with this technique33. However, the analysis was performed in a large cohort, and results were generally consistent between different rOA grades. Future studies with observation periods of more than one year may observe differences/changes that are greater than the reported precision errors. It is also important to be aware of the sample size differences between groups (Table 1); the p-values should not be compared between groups, as these become intrinsically smaller (i.e. reach higher levels of significance) with larger group sizes. The large differences in group sizes are due to the numbers enrolled in each group of the OAI (i.e. the healthy control group was deliberately chosen to be roughly 100 subjects), and factors intrinsic to OA (i.e. medial JSN is more common than lateral34). However, to maximize study power, all eligible participants for each group were included. It should also be noted that the groups differ in age and BMI; the healthy control group is younger and has a lower BMI than other groups. We have shown previously that knee tAB does not differ between younger and older healthy subjects35, which is supported by the lack of difference between baseline and year-1 in the healthy control group. It is possible that increased load due to high BMI is a driving factor behind changes in tAB size in knee OA (see also below), and could possibly also have been used as a surrogate marker for mechanical load, as JSN was in this study. However, both BMI and tAB20 are strongly associated with body height, whereas JSN is probably not. Another limitation is that the association with standard biomechanical factors, such as knee alignment or quadriceps strength was not investigated, because these have not been reported for all of the investigated subjects in the OAI database. However, unicompartimental JSN may be an indicator of the side with greater mechanical stress, as varus and valgus alignments are known to increase load36, cartilage loss37, JSN and osteophyte growth38 in the affected compartment. It is important to note that bias from osteophytes has been minimized in the current study, as the segmentation process explicitly excluded osteophytes from the tAB measurements. Although the change of tAB was not significantly different between the various K&L grades, the amounts and SRMs seem to generally increase with K&L grade in MT and cLF, and stay even in LT and cMF. These findings imply that the tAB differences are slightly more pronounced in MT and in higher grades of rOA, which has also been suggested by others8,12,14.
A strength of this study is the inclusion of knees from the non-exposed healthy reference cohort free of symptoms femorotibial rOA, and without risk factors for knee OA. The healthy controls and the pre-rOA cohort (K&L grades 0 and 1) displayed no significant differences in tAB over one year, providing evidence that the differences measured in the other groups are associated with rOA, and not scanner drift or other systematic bias. The size of the tAB after one year was lower, albeit non-significant, in the healthy reference cohort in all cartilage plates, except for the medial femur, where a slight but non-significant higher tAB (0.2%) was found. This seems to contradict the reported association between age and bone-size in a mixed sample of people with a family history of knee replacement and a random population based selection21. The association remained significant, but decreased after adjusting for rOA status, supporting the hypothesis that a tAB increase might not be linked to rOA directly, but to accompanying or preluding factors like changes in biomechanics or age.
Similar to a previous cross-sectional study23 the tAB size at baseline in the current study was significantly smaller (7–12%) in the healthy reference cohort than the pre-rOA or rOA cohorts. When considering that the yearly amount of increase of tAB ranges between 0.1% and 0.5%, it is possible that these groups experienced several years of small yearly increases in tAB, while shifting from healthy to pre-rOA or rOA. This would lead to the assumption that the tAB increases due to factors that do not immediately lead to rOA, but may facilitate the pathogenic process, which is supported by several studies suggesting that an increase in tAB is mainly associated with early rOA and may precede more severe pathologies4,5,7. It is reasonable to think that mechanical loading is at least one of the factors influencing tAB, as some studies imply that mechanical loading correlates with tAB12 or bone size in general13. The differences or changes in tAB or bone size in these studies were also very small but significant, which may be the pattern after which subchondral bone adapts (to the mechanical environment) in rOA, which makes it difficult to detect.
In the current study, the increase of tAB in rOA was predominantly found in the medial tibia and femur, which might represent the high prevalence of varus malalignment in rOA39. However, the tAB increase was not confined to medial unicompartimental JSN (surrogate marker for varus malalignment), and was also found in cohorts with bicompartimental JSN or those without JSN. Only unicompartimental lateral JSN did not account for medial tAB increases, but instead accounted for an increase in the cLF. There is an intriguing possible explanation of the medial dominance of tAB increases in rOA: although it is reasonable that in varus malalignment loads are higher medially than laterally40, it has been shown that medial loading is also higher in healthy controls40, and even in valgus malalignment41. A possible connection between tAB increase (and osteophyte growth) on the one hand, and mechanical loading on the other hand, may be a mechanism by which the subchondral bone copes with increased mechanical loads42, probably through an increase of the load transferring area.
In conclusion, we find larger subchondral bone areas after one year in the medial tibia and the central femur of subjects with radiographic OA, but not in healthy controls or subjects with pre-radiographic OA. The differences in subchondral bone size tend to increase in the femur with higher grades of radiographic OA, but were not consistently associated with joint space narrowing. These results suggest that an increasing subchondral bone area is associated with radiographic OA in general, but not so much with OA grading and even less with joint space narrowing. These findings may prove useful for understanding the pathogenesis of osteoarthritis.
Acknowledgments
Funding Source: The study and image acquisition were funded by the Osteoarthritis Initiative (OAI), a public-private partnership comprised of five contracts (N01-AR-2-2258, N01-AR-2-2259, N01-AR-2-2260, N01-AR-2-2261, N01-AR-2-2262). The image analysis of this study was funded by an industry consortium consisting of Pfizer Inc., Eli Lilly & Co, Merck Serono SA, Glaxo Smith Kline Inc., Wyeth Research, Centocor Research and Development, Inc., and Novartis Pharma AG and by the OAI coordinating center (UCSF).
We would like to thank the following readers: Gudrun Goldmann, Linda Jakobi, Manuela Kunz, Dr. Susanne Maschek, Jana Matthes, Sabine Mühlsimer, Annette Thebis, and Dr. Barbara Wehr for dedicated data segmentation.
FUNDING
The image analysis of this study was funded by an industry consortium consisting of Pfizer Inc., Eli Lilly & Co, Merck Serono SA, Glaxo Smith Kline Inc., Wyeth Research, Centocor Research and Development Inc., and Novartis Pharma AG, and by the coordinating center of the OAI at UCSF and Chondrometrics GmbH.
The OAI is a public-private partnership comprised of five contracts (N01-AR-2-2258, N01-AR-2-2259, N01-AR-2-2260, N01-AR-2-2261, N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories, Novartis Pharmaceuticals Corporation, GlaxoSmithKline, and Pfizer Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript has received the approval of the OAI Publications Committee based on a review of its scientific content and data interpretation.
Footnotes
CONFLICT OF INTEREST
Wolfgang Wirth and Martin Hudelmaier have part time appointments with Chondrometrics GmbH.
AUTHORS CONTRIBUTION
Martin Hudelmaier and Wolfgang Wirth have both contributed to the analysis and interpretation of the data, the drafting of the article and critical revision of the article for important intellectual content.
References
- 1.Buckland-Wright C. Subchondral bone changes in hand and knee osteoarthritis detected by radiography. Osteoarthritis Cartilage. 2004;12(Suppl A):S10–9. S10–S19. doi: 10.1016/j.joca.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Burr DB. Anatomy and physiology of the mineralized tissues: role in the pathogenesis of osteoarthrosis. Osteoarthritis Cartilage. 2004;12(Suppl A):S20–30. S20–S30. doi: 10.1016/j.joca.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 3.Messent EA, Ward RJ, Tonkin CJ, Buckland-Wright C. Cancellous bone differences between knees with early, definite and advanced joint space loss; a comparative quantitative macroradiographic study. Osteoarthritis Cartilage. 2005;13:39–47. doi: 10.1016/j.joca.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Jones G, Ding C, Scott F, Glisson M, Cicuttini F. Early radiographic osteoarthritis is associated with substantial changes in cartilage volume and tibial bone surface area in both males and females. Osteoarthritis Cartilage. 2004;12:169–74. doi: 10.1016/j.joca.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Ding C, Garnero P, Cicuttini F, Scott F, Cooley H, Jones G. Knee cartilage defects: association with early radiographic osteoarthritis, decreased cartilage volume, increased joint surface area and type II collagen breakdown. Osteoarthritis Cartilage. 2005;13:198–205. doi: 10.1016/j.joca.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Messent EA, Ward RJ, Tonkin CJ, Buckland-Wright C. Cancellous bone differences between knees with early, definite and advanced joint space loss; a comparative quantitative macroradiographic study. Osteoarthritis Cartilage. 2005;13:39–47. doi: 10.1016/j.joca.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Wluka AE, Cicuttini FM. The determinants of change in tibial plateau bone area in osteoarthritic knees: a cohort study. Arthritis Res Ther. 2005;7:R687–R693. doi: 10.1186/ar1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wluka AE, Wang Y, Davis SR, Cicuttini FM. Tibial plateau size is related to grade of joint space narrowing and osteophytes in healthy women and in women with osteoarthritis. Ann Rheum Dis. 2005;64:1033–7. doi: 10.1136/ard.2004.029082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding C, Cicuttini F, Jones G. Tibial subchondral bone size and knee cartilage defects: relevance to knee osteoarthritis. Osteoarthritis Cartilage. 2007;15:479–86. doi: 10.1016/j.joca.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Messent EA, Ward RJ, Tonkin CJ, Buckland-Wright C. Osteophytes, juxta-articular radiolucencies and cancellous bone changes in the proximal tibia of patients with knee osteoarthritis. Osteoarthritis Cartilage. 2007;15:179–86. doi: 10.1016/j.joca.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 11.Ding C, Martel-Pelletier J, Pelletier JP, Abram F, Raynauld JP, Cicuttini F, et al. Two-year prospective longitudinal study exploring the factors associated with change in femoral cartilage volume in a cohort largely without knee radiographic osteoarthritis. Osteoarthritis Cartilage. 2008;16:443–9. doi: 10.1016/j.joca.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 12.Eckstein F, Hudelmaier M, Cahue S, Marshall M, Sharma L. Medial-to-lateral ratio of tibiofemoral subchondral bone area is adapted to alignment and mechanical load. Calcif Tissue Int. 2009;84:186–94. doi: 10.1007/s00223-008-9208-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howell SM, Howell SJ, Hull ML. Assessment of the radii of the medial and lateral femoral condyles in varus and valgus knees with osteoarthritis. J Bone Joint Surg Am. 2010;92:98–104. doi: 10.2106/JBJS.H.01566. [DOI] [PubMed] [Google Scholar]
- 14.Creaby MW, Wang Y, Bennell KL, Hinman RS, Metcalf BR, Bowles KA, et al. Dynamic knee loading is related to cartilage defects and tibial plateau bone area in medial knee osteoarthritis. Osteoarthritis Cartilage. 2010;18:1380–5. doi: 10.1016/j.joca.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 15.Antony B, Ding C, Stannus O, Cicuttini F, Jones G. Association of baseline knee bone size, cartilage volume, and body mass index with knee cartilage loss over time: a longitudinal study in younger or middle-aged adults. J Rheumatol. 2011;38:1973–80. doi: 10.3899/jrheum.101309. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Wluka AE, Davis S, Cicuttini FM. Factors affecting tibial plateau expansion in healthy women over 2. 5 years: a longitudinal study. Osteoarthritis Cartilage. 2006;14:1258–64. doi: 10.1016/j.joca.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 17.Jackson BD, Teichtahl AJ, Morris ME, Wluka AE, Davis SR, Cicuttini FM. The effect of the knee adduction moment on tibial cartilage volume and bone size in healthy women. Rheumatology (Oxford) 2004;43:311–4. doi: 10.1093/rheumatology/keh002. [DOI] [PubMed] [Google Scholar]
- 18.Davies-Tuck ML, Martel-Pelletier J, Wluka AE, Pelletier JP, Ding C, Jones G, et al. Meniscal tear and increased tibial plateau bone area in healthy post-menopausal women. Osteoarthritis Cartilage. 2008;16:268–71. doi: 10.1016/j.joca.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 19.Andreisek G, White LM, Sussman MS, Kunz M, Hurtig M, Weller I, et al. Quantitative MR imaging evaluation of the cartilage thickness and subchondral bone area in patients with ACL-reconstructions 7 years after surgery. Osteoarthritis Cartilage. 2009;17:871–8. doi: 10.1016/j.joca.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 20.Barr AJ, Dube B, Hensor EM, Kingsbury SR, Peat G, Bowes MA, et al. The relationship between clinical characteristics, radiographic osteoarthritis and 3D bone area: data from the osteoarthritis initiative. Osteoarthritis Cartilage. 2014;22:1703–9. doi: 10.1016/j.joca.2014.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding C, Cicuttini F, Scott F, Cooley H, Jones G. Association between age and knee structural change: a cross sectional MRI based study. Ann Rheum Dis. 2005;64:549–55. doi: 10.1136/ard.2004.023069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peterfy C, Li J, Zaim S, Duryea J, Lynch J, Miaux Y, et al. Comparison of fixed-flexion positioning with fluoroscopic semi-flexed positioning for quantifying radiographic joint-space width in the knee: test-retest reproducibility. Skeletal Radiol. 2003;32:128–32. doi: 10.1007/s00256-002-0603-z. [DOI] [PubMed] [Google Scholar]
- 23.Frobell RB, Nevitt MC, Hudelmaier M, Wirth W, Wyman BT, Benichou O, et al. Femorotibial subchondral bone area and regional cartilage thickness: a cross-sectional description in healthy reference cases and various radiographic stages of osteoarthritis in 1,003 knees from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken ) 2010;62:1612–23. doi: 10.1002/acr.20262. [DOI] [PubMed] [Google Scholar]
- 24.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16:1433–41. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneider E, NessAiver M, White D, Purdy D, Martin L, Fanella L, et al. The osteoarthritis initiative (OAI) magnetic resonance imaging quality assurance methods and results. Osteoarthritis Cartilage. 2008;16:994–1004. doi: 10.1016/j.joca.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.KELLGREN JH, LAWRENCE JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altman RD, Hochberg M, Murphy WA, Jr, Wolfe F, Lequesne M. Atlas of individual radiographic features in osteoarthritis. Osteoarthritis Cartilage. 1995;3(Suppl A):3–70. 3–70. [PubMed] [Google Scholar]
- 28.Altman RD, Gold GE. Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthritis Cartilage. 2007;15(Suppl A):A1–56. A1–56. doi: 10.1016/j.joca.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Eckstein F, Maschek S, Wirth W, Hudelmaier M, Hitzl W, Wyman B, et al. One year change of knee cartilage morphology in the first release of participants from the Osteoarthritis Initiative progression subcohort: association with sex, body mass index, symptoms and radiographic osteoarthritis status. Ann Rheum Dis. 2009;68:674–9. doi: 10.1136/ard.2008.089904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frobell RB, Wirth W, Nevitt M, Wyman BT, Benichou O, Dreher D, et al. Presence, location, type and size of denuded areas of subchondral bone in the knee as a function of radiographic stage of OA - data from the OA initiative. Osteoarthritis Cartilage. 2010;18:668–76. doi: 10.1016/j.joca.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eckstein F, Benichou O, Wirth W, Nelson DR, Maschek S, Hudelmaier M, et al. Magnetic resonance imaging-based cartilage loss in painful contralateral knees with and without radiographic joint space narrowing: Data from the Osteoarthritis Initiative. Arthritis Rheum. 2009;61:1218–25. doi: 10.1002/art.24791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hohe J, Ateshian G, Reiser M, Englmeier KH, Eckstein F. Surface size, curvature analysis, and assessment of knee joint incongruity with MRI in vivo. Magn Reson Med. 2002;47:554–61. doi: 10.1002/mrm.10097. [DOI] [PubMed] [Google Scholar]
- 33.Eckstein F, Buck RJ, Burstein D, Charles HC, Crim J, Hudelmaier M, et al. Precision of 3. 0 Tesla quantitative magnetic resonance imaging of cartilage morphology in a multicentre clinical trial. Ann Rheum Dis. 2008;67:1683–8. doi: 10.1136/ard.2007.076919. [DOI] [PubMed] [Google Scholar]
- 34.Wise BL, Niu J, Yang M, Lane NE, Harvey W, Felson DT, et al. Patterns of compartment involvement in tibiofemoral osteoarthritis in men and women and in whites and African Americans. Arthritis Care Res (Hoboken ) 2012;64:847–52. doi: 10.1002/acr.21606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hudelmaier M, Glaser C, Hohe J, Englmeier KH, Reiser M, Putz R, et al. Age-related changes in the morphology and deformational behavior of knee joint cartilage. Arthritis Rheum. 2001;44:2556–61. doi: 10.1002/1529-0131(200111)44:11<2556::aid-art436>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 36.Hsu HP, Garg A, Walker PS, Spector M, Ewald FC. Effect of knee component alignment on tibial load distribution with clinical correlation. Clin Orthop Relat Res. 1989:135–44. [PubMed] [Google Scholar]
- 37.Eckstein F, Wirth W, Hudelmaier M, Stein V, Lengfelder V, Cahue S, et al. Patterns of femorotibial cartilage loss in knees with neutral, varus, and valgus alignment. Arthritis Rheum. 2008;59:1563–70. doi: 10.1002/art.24208. [DOI] [PubMed] [Google Scholar]
- 38.Teichtahl AJ, Cicuttini FM, Janakiramanan N, Davis SR, Wluka AE. Static knee alignment and its association with radiographic knee osteoarthritis. Osteoarthritis Cartilage. 2006;14:958–62. doi: 10.1016/j.joca.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 39.Laxafoss E, Jacobsen S, Gosvig KK, Sonne-Holm S. The alignment of the knee joint in relationship to age and osteoarthritis : The Copenhagen Osteoarthritis Study. Skeletal Radiol. 2013;42:531–40. doi: 10.1007/s00256-012-1509-z. [DOI] [PubMed] [Google Scholar]
- 40.Kumar D, Manal KT, Rudolph KS. Knee joint loading during gait in healthy controls and individuals with knee osteoarthritis. Osteoarthritis Cartilage. 2013;21:298–305. doi: 10.1016/j.joca.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson F, Leitl S, Waugh W. The distribution of load across the knee. A comparison of static and dynamic measurements. J Bone Joint Surg Br. 1980;62:346–9. doi: 10.1302/0301-620X.62B3.7410467. [DOI] [PubMed] [Google Scholar]
- 42.Goldring MB, Goldring SR. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann N Y Acad Sci. 2010;1192:230–7. doi: 10.1111/j.1749-6632.2009.05240.x.:230-7. [DOI] [PubMed] [Google Scholar]


