Abstract
Objective
Venous malformations (VM) are slow-flow vascular malformations present at birth. Localized intravascular coagulopathy (LIC) causes pain and thrombosis within a lesion and severe bleeding during surgical procedures. This study aimed to determine which venous malformations are at risk for coagulopathy.
Design
Prospective convenience sample accrued from 2 multidisciplinary sites in Brussels, Belgium and Caen, France.
Participants
140 patients with clinical data and coagulation parameters. Magnetic resonance imaging was performed for 110 patients.
Results
Forty two per cent of patients showed high D-dimers, 61% of which had more than 1000ng/ml. Six of them had low fibrinogen levels. In univariate analysis, large surface, presence of palpable phleboliths, and truncal localization were associated with high D-dimer levels. In the multivariate analysis, only large surface area, and presence of phleboliths remained independently associated with high D-dimer levels. Severe LIC, characterized by concomitant low fibrinogen, was associated with extensive VM of extremities.
Conclusion
Localized intravascular coagulopathy is statistically associated with large and/or deep venous malformations that affect any location, which can have a palpable phlebolith. These patients are at risk of local pain due to thrombosis. Lesions with elevated D-dimer levels associated with low fibrinogen levels (severe LIC) commonly affect an extremity, and have a high risk of hemorrhage. Low molecular weight heparin can be used both to treat the pain caused by LIC and to prevent decompensation of severe LIC to DIC.
Keywords: vascular anomaly, low molecular weight heparin (LMWH), D-dimer, localized intravascular coagulopathy (LIC), disseminated intravascular coagulopathy (DIC), fibrinogen, surgery, sclerotherapy, treatment
INTRODUCTION
Vascular malformations are localized or diffuse lesions caused by errors in embryonic development. They are subdivided anatomically and rheologically into slow-flow and fast-flow lesions. Venous malformations represent more than 50% of patients referred to centers for vascular anomalies (1, 2, 3, 4). These slow-flow lesions present as bluish or purple lesions which are mainly localized on the skin and mucosa, but can present in any anatomic location, tissue or organ (5, 6, 7). Most of them are asymptomatic, but swelling and pain are common. Due to blood stagnation, thrombosis can occur leading to phlebolith formation (8, 9).
In a retrospective study, a coagulation disorder named Localized Intravascular Coagulopathy (LIC), characterized by elevated D-dimers, was observed in 19 out of 24 patients with extensive and painful venous malformation of limbs (10,11). Six of them had severe LIC characterized by concomitant low fibrinogen level and variable platelet count (11). LIC needs to be distinguished from Kasabach-Merritt phenomenon characterized by a predominant platelet consumption (11).
Our experience with the index case of a child, who was operated 3 times for functional impairment due to her VM, led to this study of the frequency of blood coagulation disturbance among VMs and the type of VMs that are associated with it. The first procedure, which was performed without Low Molecular Weight Heparin (LMWH), was complicated by peri-operative Disseminated Intravascular Coagulopathy (DIC) marked by bleeding and consumption of platelets and coagulation factors. During the second procedure, fibrinogen was injected before the surgical incision was made and helped to control DIC. Pre- and postoperative treatment with LMWH prevented DIC during the third procedure.
PATIENTS AND METHODS
We conducted a prospective study from January 2004 to December 2005 in 2 multidisciplinary centers for vascular anomalies (Brussels, Belgium and Caen, France). This study was approved by the ethics committee of Université catholique de Louvain. All participants signed an informed consent.
Patients
All 141 patients presenting with a mucosal or cutaneous/subcutaneous venous malformation diagnosed by either multidisciplinary team were included. The diagnosis was made by clinical evaluation and confirmed by Doppler ultrasonography, if necessary. Magnetic resonance imaging (MRI) with T1 and T2-weighted and fat-saturated sequences was performed on 110 patients to evaluate the involvement of skin, muscles and joints.
One patient with concomitant inflammatory disease was excluded. None had a malignant tumor, a past history of thrombosis, known thombophilia or antithrombotic therapy. Patients with other vascular malformations were not included.
The remaining 140 patients were evaluated by LMB (Brussels) and AD (Caen) and the following nine datapoints were recorded :
clinical criteria : age ; sex ; number (unique, multifocal) ; location (limbs, head and neck, trunk) ; size (< or >10cm2) ; aspect (flat or raised) ; color (blue or skin color) ; palpable phleboliths.
radiological criterion : deepness of involvement (subcutis, muscles, joints).
Despite the use of aspirin, analgesic or non steroidal anti-inflammatory drugs, 22 of the 140 subjects had elevated D-Dimers and pain impairing their daily life. These patients were treated by subcutaneous injections of enoxoparine (100 anti-Xa/kg) once a day during 20 days.
Methods
At the initial examination and at follow-up every 1–3 months for 1–2 years, blood was drawn from a peripheral vein not involved by the venous malformation for coagulation tests. Platelets (normal: 150 000 – 400 000/ml) were counted in an EDTA sample using an automated instrument (XE-2100 Roche). Prothrombin time (PT) (Thromborel S DadeBehring), activated partial prothrombin time (APTT) (Platelin L BioMérieux), and fibrinogen level (normal: 2,0g/l–4,5g/l) (Fibriquick, BioMérieux France) were measured in a tube containing 0.129M of trisodium citrate and determined using a coagulation device (MDA 2 BioMérieux). Plasma D-dimers (normal<500ng/ml) were determined using ELISA VIDASÒ D-Dimer New DD2. For the 22 patients with painful VM treated with LMWH for 20 days, another sample for D-dimers and fibrinogen was drawn 10–21 days after beginning of treatment, and one month after therapy. Treatment was re-started if pain re-appeared, and the patient was re-evaluated every 20 days.
The statistical analysis was performed using the SAS software (version 9). Percentages of positive and negative D-dimers for each item (age, sex, number, location, size, aspect, color, phleboliths and deepness of involvement) were compared by univariate analysis using the Chi2 or Fischer test. For quantitative variables, the Student test was used. Logistic regression model was used for the variables significantly associated with positive D-dimers in the univariate analysis. The threshold of significancy was less or equal to 0,20. The comparison between D-Dimer levels before and after treatment was done using the Wilcoxon test. In all the statistical analysis, p<0.05 was considered as significant.
RESULTS
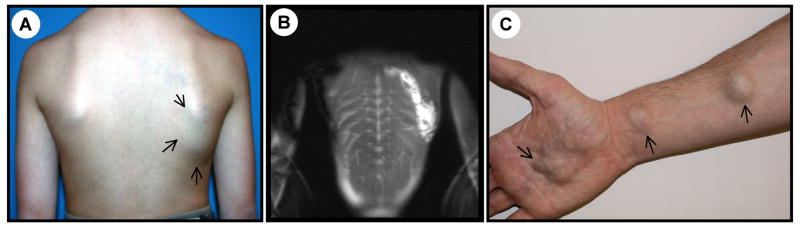
Within the 140 patients, 44 were males and 96 were females, aged 10 months to 78 years with a mean age of 26.8 years (SD=16.4) and a median age of 22 years. Most of the VMs were limited to a single anatomical region, such as a limb, trunk or head and neck (Figure 1A, 1B) (n=129/ 140), yet 6 extended to 2 regions (Table 1). Patients with small, numerous and disseminated lesions (multifocal) (Figure 1C) were rare (n=5).
Figure 1.
A) Large, unifocal, trunkal, skin-color, flat venous malformation of a 13-year-old male associated with high D-dimers (1000ng/ml) with palpable phleboliths B) MRI shows muscle involvement of this deep VM C) Multifocal VM on right arm of a 28-year-old male with high D-dimer (7818ng/ml). Additional lesions are present on trunk and other limbs.
Table 1.
Localization of venous malformations in the 140 patients
including 6 extensive VMs with more than one location
Forty two per cent (n=59/140) had repeatedly elevated D-dimers, and 36 of them (61%) had more than 1000 ng/ml. Fibrinogen levels were normal in 134 patients with normal prothrombin time, APTT and platelet counts. Six patients had low fibrinogen levels (0.85g/l–1.76 g/l), only one of which below 1g/l. These patients had very high D-dimer levels (1899–9040ng/ml) and one had low platelet count (114 000/ml).
Due to the low number of multifocal VMs, these five patients were excluded from the statistical analysis, resulting in a final sample size of 135 subjects. There was no statistical difference between the D-dimer positive and negative populations for age, sex, aspect or color of the VM (Table 2). Elevated D-dimer levels were statistically associated with truncal localization (74 %), in contrast to head and neck VMs (31 %). Small size (<10cm2) was associated with head and neck location (Chi2 = 7,78 p<0.01). Large size (>10cm2) was statistically associated with positive D-dimers, independently of the localization, as 59% of the large VMs (>10cm2) had positive D-dimers versus 23% of small VMs (p= 0.0001). Interestingly, 40/45 (89%) of the diffuse and extensive VM (half or more of a limb, hemitruncal or hemifacial) had elevated D-dimers. Sixty one per cent of patients presenting with palpable phleboliths had positive D-dimers versus 27% of those without palpable phleboliths (p = 0.0001).
Table 2.
Univariate analysis performed on 135 VM patients. N.B. Localization was studied for 141 VMs, as 6 patients had VMs affecting 2 localizations
| DD*<500 | DD*>500 | p-value | |
|---|---|---|---|
| Sex | |||
|
|
|||
| Male | 22(51%) | 21(49%) | 0.190 |
| Female | 58(63%) | 34(37%) | |
|
|
|||
| Medium age (± st dev) | 28(±16) | 25(±17) | 0.480 |
|
|
|||
| Localization | |||
|
|
|||
| Head and neck | 36(69%) | 16(31%) | 0.060 |
| Trunk | 5(26%) | 14(74%) | 0.002 |
| Limbs | 39(56%) | 31(44%) | 0.380 |
|
|
|||
| Surface | <0.001 | ||
|
|
|||
| <10cm2 | 53(77%) | 16(23%) | |
| >10cm2 | 27(41%) | 39(59%) | |
|
|
|||
| Aspect | 0.340 | ||
|
|
|||
| Flat | 53(62%) | 32(38%) | |
| Raised | 27(54%) | 23(46%) | |
|
|
|||
| Color | 0.458 | ||
|
|
|||
| Blue | 53(62%) | 33(38%) | |
| Skin color | 27(55%) | 22(45%) | |
|
|
|||
| Palpable phlebolith(s) | <0.001 | ||
|
|
|||
| No | 58(73%) | 21(27%) | |
| Yes | 22(39%) | 34(61%) |
The comparison between VM depth and D-dimer levels was done for the 110 patients who had MRI. In this population, we noted a strong correlation between large surface size ( >10cm2) and deep involvement (at least one muscle), as 62% of VM with muscle involvement had a surface area >10cm2, versus 24% of (sub)cutaneous VMs with a surface area > 10cm2) (p = 0.003). Large surface area and deep involvement were both statistically associated with localized intravascular coagulopathy.
In the multivariate analysis (Table 3), the effect of deep involvement and surface area in regard to the D-dimer levels, could not be studied in the same model, because of their high correlation. Thus, they were compared in 2 different models to the presence of palpable phleboliths and truncal localization. Surface area and palpable phleboliths remained independently associated with elevated D-dimer levels with an Odds Ratio (OR) of 2.82 (95% CI : 1.24–6.39) and 3.16 (95% CI : 1.40–7.09), respectively. The effect of truncal location was not found. When examining the effect of deep involvement, the adjusted OR was 3.09 (CI 1.21–7.87). The other results were very similar to those of the model including VM surface area. This means that VM with deep involvement or large surface area or with palpable phleboliths has 3 times higher risk to be coupled with elevated D-dimer levels. In consequence, a patient who has a deep or a large VM with palpable phleboliths, has 9 times higher risk for LIC.
Table 3.
Multivariate analysis performed on 135 VM patients
| Adjusted OR* | p-value | |
|---|---|---|
| Sex (Male vs Female) | 1.66[0.72–3.87] | 0.240 |
|
|
||
| Localization: trunk | 2.79[0.81–9.55] | 0.100 |
|
|
||
| Surface > 10cm2 | 2.82[1.24–6.39] | 0.010 |
|
|
||
| Palpable phlebolith(s) | 3.16[1.40–7.09] | 0.005 |
OR = Odds Ratio
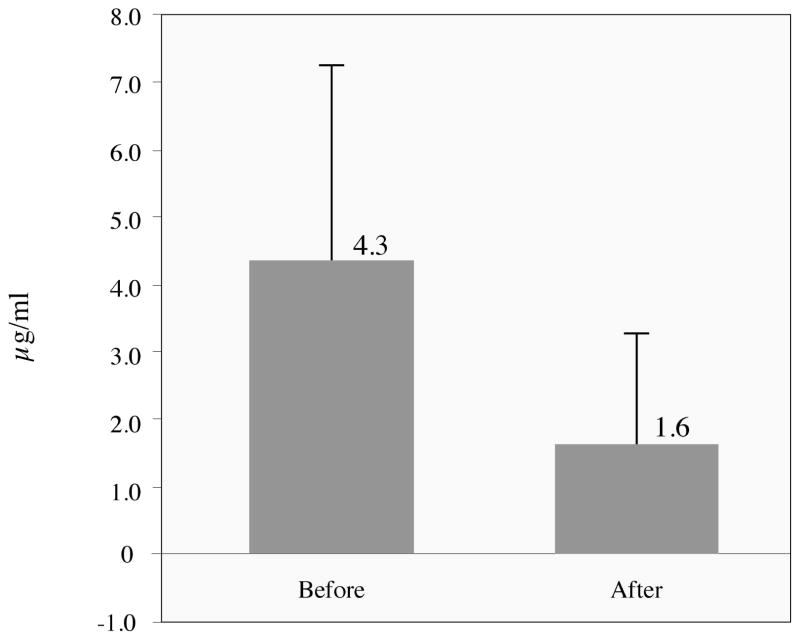
Mean D-dimer levels for the 22 patients treated with LMWH was 4339 ng/ml (sd 2886 ng/ml) before treatment and 1639 ng/ml (sd 1643 ng/ml) after treatment. This significant decrease in D-Dimer levels (p<0.001) was associated with rapid relieve in pain (Table 4). Fibrinogen levels did not change in a statistically significant manner.
Table 4.
D-dimer levels, with means and standard deviations, before and after LMWH treatment of painful venous malformation
DISCUSSION
In this prospective study, we demonstrated that coagulation abnormalities are frequent among patients with VM, as nearly half of our 140 patients (42%) had elevated D-dimers with 25% (36/140) having D-dimers above 1000ng/ml. In our study, elevated D-dimers were not associated with limb lesions, whereas truncal localization was a predictable factor in the univariate analysis. However, truncal localization was not an independent criterion in the multivariate analysis. Cervicofacial localization was not statistically associated with elevated D-dimers either. In fact, 69% of cervicofacial VMs (n=36) had low D-dimer levels and were statistically associated with small size, which is probably a reflection of facial lesions causing earlier consultation due to esthetic concerns. Large surface and deep involvement were statistically associated with elevated D-dimers. Thus, the univariate association observed for trunkal localization could be due to the large size and/or deep involvement of such lesions (Figure 1A, 1B). The strong statistical correlation between large surface area and deep involvement highlights the fact that large cutaneous VMs involve underlying structures.
Palpable phlebolith was an independant criterion also associated with positive D-dimers. However, the incidence of phleboliths is underestimated because deep phleboliths are only seen on radiological examination. The presence of palpable phleboliths in a large or deep VM increases the risk for LIC to 9-fold. None of our patients exhibited pulmonary embolism, which can be observed in Klippel-Trenaunay syndrome but has never been reported in a patient with an isolated venous malformation (12).
Constant activation of coagulation due to blood stagnation within the distorted and enlarged slow-flow venous channels leads to the production of thrombin and the conversion of fibrinogen into fibrin. The subsequent fibrinolysis is reflected by elevated levels of fibrin degradation products (FDP), such as D-dimer epitopes. Such activation was named localized intravascular coagulopathy (LIC) (10). It can sometimes cause localized hemorrhage and/or thrombosis (phleboliths) with normal coagulation factor (PT, APPT) levels and platelet count.. Interestingly, 80% of our multifocal VMs (n=4/5) were associated with elevated D-dimer levels although the lesions were small (<10cm2) (Figure 1C). This could be due to the combined lesional volume, or indicates that blood stagnation may not be the only etiological factor of the activation of coagulation. The intrinsic nature of increased coagulation in venous malformations is underscored by the fact that LIC can be induced by sclerotherapy with dehydrated Ethanol or Sodium Tetradecyl Sulfate in VM patients with normal preoperative D-dimer levels (13).
We were surprised by the extremely high level of D-dimers in 25% of our patients. To our knowledge, VM is the only disease, which can dramatically increase D-dimer levels in otherwise healthy patients. Similar levels are only seen in life-threatening disorders, such as pulmonary embolism, malignant tumors and inflammatory diseases. Although LIC is well tolerated in everyday life, systemic activation of coagulation can occur during surgical resection as disseminated intravascular coagulopathy (DIC), which is marked by consumption of platelets, fibrinogen and coagulation factors, with increased prothrombin time, and dramatic peri-operative bleeding. Remarkably, 6 patients had extensive, diffuse, painful VMs involving muscles, localized on the limbs (n=4), the trunk (n=1) or multifocally (limb + trunk: n=1). Mazoyer and co-authors noted that 6/24 patients with limb VM had high D-dimers and low fibrinogen, reinforcing the observation that VMs with severe LIC often affect an extremity.
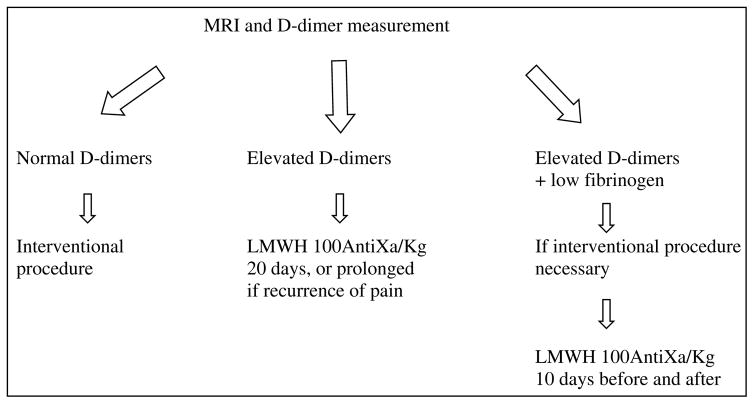
If fibrinogen level is normal, it is not necessary to treat this chronic coagulopathy; however, painful lesions need surgical management (Table 5). Elastic compression, when possible, is useful to minimize blood stasis. Aspirin as published by others (10), and in our experience, is poorly effective because, contrary to Kasabach-Merritt phenomenon, platelets are not involved in LIC (14–17). Moreover, aspirin increases bleeding during surgical procedures. Oral vitamin K antagonists decrease the level of coagulation factors, but are not sufficient to prevent thrombin formation in this coagulation activation. Therefore, the only efficient treatment is Low Molecular Weight Heparin, which can stop the pain due to thrombosis, as we observed in all our 22 patients with painful VM associated with elevated D-dimers, who were treated with LMWH (18). In these patients, D-dimer levels were lowered, although not normalized. In contrast, the low fibrinogen level observed in two, was normalized. The low fibrinogen reflects high consumption due to clotting associated with high fibrinolysis, and increased risk for bleeding. Therefore, these patients need careful management of LIC before any interventional procedure, such as surgery or sclerotherapy, to improve the hematological status and prevent DIC. We currently start preventive subcutaneous enoxoparine 10 days before any surgical procedure for a total of 20 days.
Table 5.
Management scheme for painful venous malformations
Our study demonstrates that LIC is frequently associated with VM, and that large surface area, muscle involvement and palpable phleboliths are strong predictable criteria for coagulation anomaly. This is not only true for large venous malformations, but also for multifocal VMs. D-dimer and fibrinogen measurements, simple and cheap tests, must be performed as part of the medical evaluation of VMs, no matter of their size and location. When elevated D-Dimers are associated with pain, LMWH is the treatment of choice. When fibrinogen is low, most often occurring in extensive VMs affecting at least partially an extremity, the potential aggravation of LIC to DIC needs preventive management by LMWH.
Acknowledgments
These studies were partially supported by the Interuniversity Attraction Poles initiated by the Belgian Federal Science Policy network 5/25 and 6/05; concerted Research Actions (A.R.C) - Convention N° 02/07/276 of the Belgian French Community Ministry ; the National Institute of Health Program Project P01 AR048564-01A1 ; EU FW6 Integrated project LYMPHANGIOGENOMICS, LSHG-CT-2004-503573 ; and the FNRS (Fonds national de la recherche scientifique) (to M.V., a “Maitre de recherches du F.N.R.S.”). The authors thank Dr Guillaume Acher for statistical help and Ms Liliana Niculescu for secretarial work.
Footnotes
Author Contributions: Dr. Boon had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Boon, Dompmartin, Vikkula
Acquisition of data: Acher, Boon, Deneys, Dompmartin, Hermans, Lequerrec, Pocock, Tourbach
Analysis and interpretation of data: Acher, Boon, Dompmartin, Thibon, Vanwijck, Vikkula
Drafting of the manuscript: Boon, Dompmartin, Vikkula
Critical revision of the manuscript for important intellectual content: Acher, Barrellier, Boon, Deneys, Dompmartin, Hermans, Labbé, Lequerrec, Pocock, Thibon, Tourbach, Vanwijck, Vikkula
Statistical analysis: Acher, Thibon
Obtained funding: Vikkula
Administrative, technical, or material support: Vikkula
Study supervision: Boon, Vikkula
Financial support and conflict of interest: I certify that all my affiliations with or financial involvement, from the conception of the study until the publication of the manuscript with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript are completely disclosed.
References
- 1.Mulliken JB, Glowaki J. Hemangiomas and vascular malformations in infants and children : a classification based on endothelial characteristics. Plast Reconstr Surg. 1982;69:412–420. doi: 10.1097/00006534-198203000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Boon LM, Mulliken JB, Enjolras O, Vikkula M. Glomuvenous malformations (glomangioma) and venous malformation Distinct clinicopathologic and genetic entities. Arch Dermatol. 2004;140:971–976. doi: 10.1001/archderm.140.8.971. [DOI] [PubMed] [Google Scholar]
- 3.Vikkula M, Boon LM, Mulliken JB. Molecular basis of vascular anomalies. Trends Cardiovasc Med. 1998;8:281–292. doi: 10.1016/s1050-1738(98)00024-3. [DOI] [PubMed] [Google Scholar]
- 4.Brouillard P, Vikkula M. Vascular malformations : localized defects in vascular morphogenesis. Clin Genet. 2003 May;63(5):340–51. doi: 10.1034/j.1399-0004.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- 5.Enjolras O, Mulliken JB. The current management of vascular birthmarks. Pediatr Dermatol. 1993;10:311–333. doi: 10.1111/j.1525-1470.1993.tb00393.x. [DOI] [PubMed] [Google Scholar]
- 6.Buckmiller LM. Update on hemangiomas and vascular malformations. Curr Opin Otolaryngol Head Neck Surg. 2004;12(6):476–487. doi: 10.1097/01.moo.0000145946.67222.01. [DOI] [PubMed] [Google Scholar]
- 7.Casanova D, Boon LM, Vikkula M. Venous malformations: clinical characteristics and differential diagnosis. Ann Chir Plast Esthet. 2006;51(4–5):373–87. doi: 10.1016/j.anplas.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 8.Hein KD, Mulliken JB, Kozakewich HP, Upton J, Burrow P. Venous malformations of skeletal muscle. Plast Recontr Surg. 2002;110:1625–1635. doi: 10.1097/01.PRS.0000033021.60657.74. [DOI] [PubMed] [Google Scholar]
- 9.Hermans C, Dessomme B, Lambert C, Deneys V. Venous malformations and coagulopathy. Ann Chir Plast Esthet. 2006;51(4–5):388–93. doi: 10.1016/j.anplas.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Enjolras O, Ciabrini D, Mazoyer E, Laurian C, Herbreteau D. Extensive pure venous malformations in the upper or lower limb : a review of 27 cases. J Am Acad Dermatol. 1997;36:219–225. doi: 10.1016/s0190-9622(97)70284-6. [DOI] [PubMed] [Google Scholar]
- 11.Mazoyer E, Enjolras O, Laurian C, Houdart E, Drouhet L. Coagulation abnormalities associated with extensive venous malformations of the limbs : differentiation from Kasabach-Merritt syndrome. Clin Lab Haem. 2002;24:243–251. doi: 10.1046/j.1365-2257.2002.00447.x. [DOI] [PubMed] [Google Scholar]
- 12.Huiras E, barnes C, Eichenfield L, et al. Pulmonarry embolism associated with Klippel Trenaunay Syndrome. Pediatrics. 2005;116(4) doi: 10.1542/peds.2004-1607. [DOI] [PubMed] [Google Scholar]
- 13.Mason KP, Neufeld EJ, Karian VE, et al. Coagulation abnormalities in pediatric and adult patients after sclerotherapy or embolization of vascular anomalies. AJR. 2001;(177):1359–63. doi: 10.2214/ajr.177.6.1771359. [DOI] [PubMed] [Google Scholar]
- 14.Enjolras O, Wassef M, mazoyer E, et al. Infants with Kasabach merrit syndrome do not have « true » hemangiomas. J Pediatr. 1997;130:631–640. doi: 10.1016/s0022-3476(97)70249-x. [DOI] [PubMed] [Google Scholar]
- 15.Sarkar M, Mulliken J, Kozakewich HP, et al. Thrombocytopenic coagulopathy Kasabach Merrit Phenomenom is associated with hemangioendothelioma and not with common infantile hemangioma. Plast Recontr Surg. 1997;100:1377–1385. doi: 10.1097/00006534-199711000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Hall GW. Kasabach-Merritt syndrome : pathogenesis and management. Br J of Haematol. 2001;112:851–862. doi: 10.1046/j.1365-2141.2001.02453.x. [DOI] [PubMed] [Google Scholar]
- 17.Ezkowitz RA, Mulliken JB, Folkman J. Interferon alpha 2a therapy in life threatening hemangioma in infancy. N Engl J Med. 1992;326(22):1456–63. doi: 10.1056/NEJM199205283262203. [DOI] [PubMed] [Google Scholar]
- 18.Kelly GL. Heparin therapy for bleeding associated with hemangioma. Surgery. 1969;65(6):894–89. [PubMed] [Google Scholar]