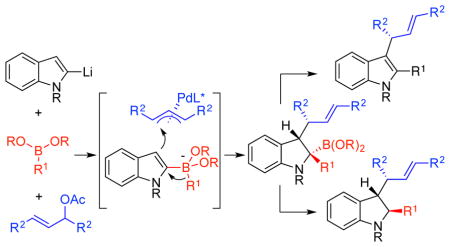
Abstract
Boronic esters react with 2-lithiated indoles to form boronate intermediates. The boronate reacts with allylic acetates in the presence of (BINAP)Pd catalysts to allylate C3 concurrent with alkyl migration from B to C2 of the indole. Overall, the process is a three-component coupling that joins an allylic acetate, and indole and an organo-B(pin) species to provide substituted indoles and indolines with high enantio-, regio-, and dia-stereo-selectivity.
Graphical Abstract
Multicomponent coupling reactions facilitate the synthesis of complex molecules through the formation of multiple bonds in a single operation. Enantioselective variants are even more valuable, especially when multiple stereocenters are generated.1,2 Within the field of multicomponent couplings, boron reagents have proven particularly useful, possibly because the open coordination site on boron can favor pre-assembly of reactants. Examples include 1) enantioselective addition of vinyl boronic esters to imines formed in situ (Petasis-type reaction),3 2) di-allylation with diboranes to effect conjunctive coupling of multiple aldehydes,4 and 3) iterative homologation using optically active boronic esters.5,6
We have been interested in developing asymmetric multicomponent coupling reactions to form nitrogen heterocycles. We7 and Aggarwal’s group8 independently reported a three-component dearomative addition to pyridines involving 1,2-metallate rearrangements.9 We hypothesized that this reactivity pattern could be extended to provide chiral non-racemic indoles and indolines by building on pioneering studies from Ishikura and coworkers.10 They coupled lithiated indoles (1) with trialkylboranes (2) and allylic acetates (3) in the presence of a palladium catalyst (Scheme 1a).11 We reasoned that outer sphere addition of an indole boronate (6) to a PdII(π-allyl) complex could activate the indole for 1,2-metallate rearrangement to yield the boronic ester 7. Oxidation could provide the indole 8 while replacement of the boronic ester could yield the indoline 9. The process could be rendered stereo selective with appropriate ligands on palladium to differentiate the faces of the indole nucleophile and the termini of the π-allyl electrophile.12
Scheme 1.
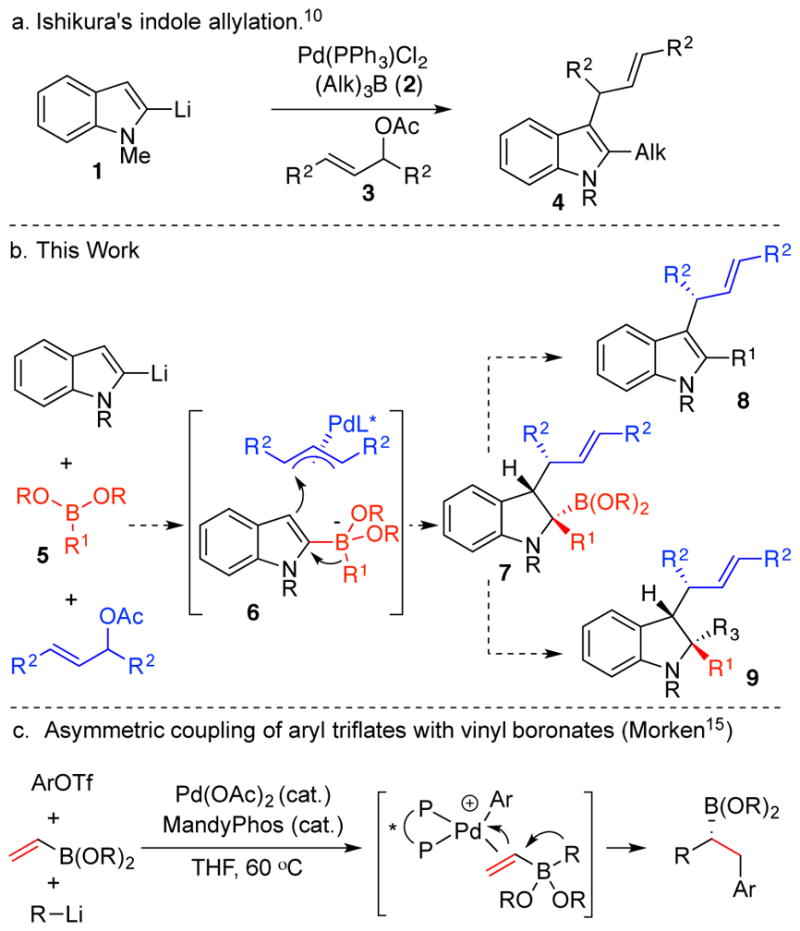
C-C Bond Formation via 1,2-Boronate Migration
Enantioselective 1,2-boronate rearrangements are rare. (Py-Box)Yb(OTf)3 catalyzes an asymmetric Matteson homologation with (Pin)BCHCl2.13 Of more relevance to work described here, Morken and co-workers recently described an enantioselective coupling of vinyl boronic esters, alkyl/aryl lithium reagents and aryl triflates.14 In this coupling, an ArPd(II) intermediate coordinates to the olefin moiety of a vinyl boronate species and thereby promotes facially selective 1,2-migration (Scheme 1c).
The asymmetric three-component coupling involving indoles would provide valuable heterocyclic products and could generate up to three new, contiguous stereocenters.15,16 Indoles and indolines are prized substructures in drug discovery and natural products research.17 A Reaxys search revealed over 6500 naturally occurring indoles, and 143 indoline natural products. Likewise, a DrugBank search revealed 270 indoles in preclinical development and 69 approved drugs.18 Prior work has documented enantioselective functionalization of indoles through allylation and 1,4-conjugate addition;16,17 likewise, chiral non-racemic indolines have been synthesized via asymmetric reduction19 and kinetic resolutions.20 Most existing methods introduce substituents only at C2 or C3, or they require substrates that are pre-functionalized at one or both of those positions. Herein, we report what is, to the best of our knowledge, the first enantioselective three component coupling that gives rise to indoles and indolines featuring concurrent C-C bond formation at both C2 and C3. This enantioselective version of Ishikura’s reaction extends the reactivity profile substantially. It provides access to both indoles and indolines in optically active form. We expand the coupling from trialkyl boranes to boronic esters, which allows for the introduction of aryl and vinyl groups. Moreover, the use of boronic esters facilitates isolation and protodeborylation of the intermediate tertiary boronic ester (7), and it allows us to access boronate 6 from either lithiated indoles or from indole boronic esters. Finally, in contrast to prior asymmetric 1,2-boronate rearrangements, the present work constructs up to three contiguous stereocenters.
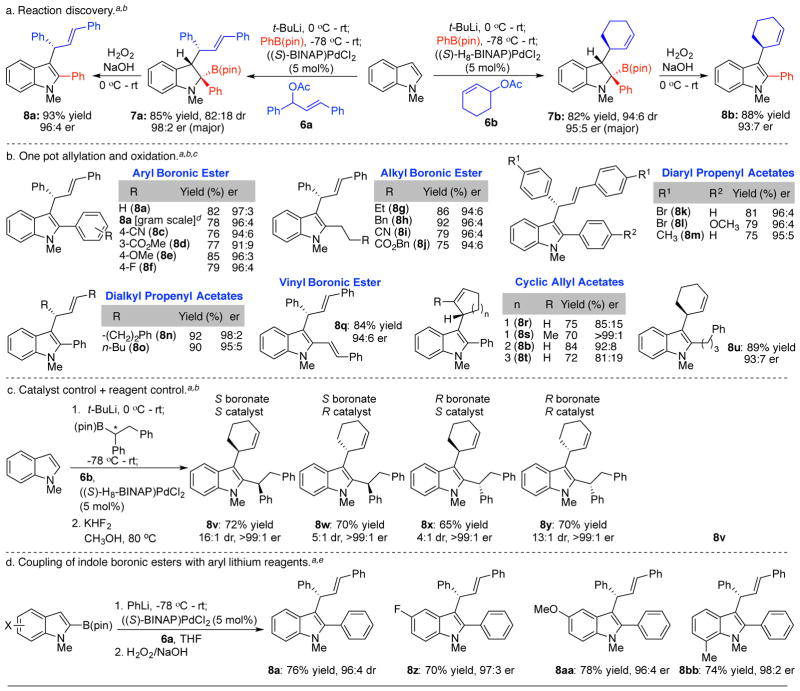
We initially focused on the formation of optically active tertiary boronates 7 (Scheme 2a). Under optimized conditions, N-methyl indole was lithiated at C2 and combined with PhB(pin) (4a). The resulting boronate was exposed to catalytic ((S)-BINAP)PdCl2 and diphenyl propenyl acetate (6a).21 Boronic ester 7a was isolated in 85% yield and 4:1 dr, with the major diastereomer displaying 98:2 er. The minor diastereomer was epimeric at both C2 and C3 of the indoline.22 The other two possible diastereomers were not observed. The mixture of diastereomers was oxidized to indole 8a, which was isolated in 96:4 er, demonstrating that both diastereomers had the same configuration at the allylic stereocenter (see below). For cyclic allylic acetates, ((S)-H8-BINAP)PdCl2 proved optimal, and boronic ester 7b was isolated with high dr and er (94:6, 95:5, respectively). Again, oxidation of the mixture of diastereomers proceeded without erosion of optical purity. Alternative catalysts, solvents and allylic electrophiles were less effective.22
Scheme 2. Enantioselective Three Component Coupling to Synthesize Substituted Indoles.
aIsolated yields on a 0.2 mmol scale unless otherwise noted. Er determined by HPLC. Dr determined by 1H NMR. bt-BuLi (1.1 equiv), N-methyl indole (1 equiv), RB(Pin) (1 equiv), 6 (1.2 equiv with acyclic, 2.0 equiv with cyclic substrates) in THF. cCrude boronic ester 7 was oxidized with H2O2/NaOH. Yield over 2 steps. d1.0 g N-methylindole. eIndole (1.0 equiv), PhLi (1.1 equiv), 6a (1.2 equiv) in THF.
To evaluate the scope of the reaction, we directly oxidized the crude boronic ester to the allylated indole 8 (Scheme 2b). A series of aryl boronic esters coupled successfully, highlighting the compatibility with a nitrile (8c), ester (8d), methoxy (8e) and halide (8f). Both electron rich and electron deficient aryl boronic esters coupled with around 95:5 er. Likewise, both alkyl (8g–8j) and vinyl boronic esters (8q) participated successfully. Other diaryl (8k–8m) and dialkyl (8n, 8o) allylic acetates provided similar enantioselectivities. Several cyclic allylic acetates were also evaluated. Five, six and seven membered rings were introduced in the coupling, with the highest enantioselectivity being observed with the cyclohexenyl acetate (8b, 8u) and 2-methyl cyclopentenyl acetate (8s). Using optically active boronic esters, we accessed all four diastereomers 8v–8y with high enantio- and diastereoselectivity by independently altering the chirality of the reagent and catalyst (Scheme 2c).23 In these cases, oxidation of the tertiary boronic ester with H2O2 proceeded slowly, perhaps due to steric hindrance. However, the corresponding BF3 salt smoothly oxidized under aerobic conditions. Finally, we were able to introduce substitution on the indole ring and demonstrate reversed polarity as shown in Scheme 2d. Specifically, substituted indole boronic esters24 were complexed with phenyl lithium to form the analogous boronate as obtained previously. Reaction with allylic acetates yielded the allylated indoles 8a, 8z–8bb with uniformly high enantioselectivities.
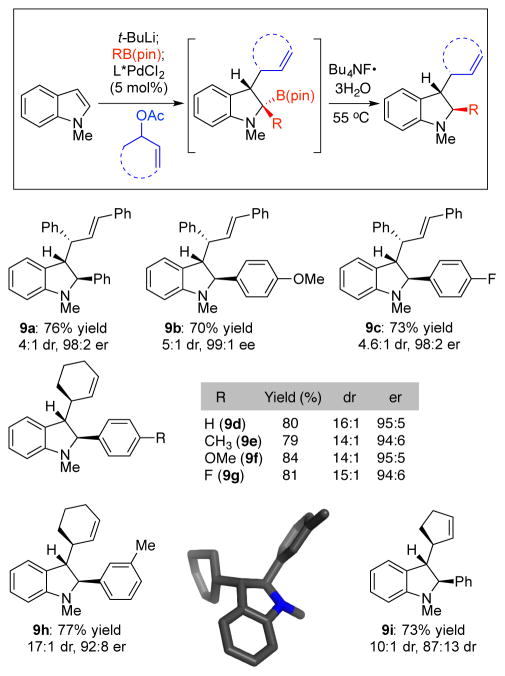
To synthesize optically active indolines, the boronic esters were deborylated using Bu4NF·3H2O (Scheme 3).25 X-ray crystallography confirmed that the deborylation occurred stereospecifically with retention of stereochemistry to yield the indolines 9a–9g with three contiguous stereocenters in high optical purity. Diastereoselectivities were generally >10:1 with cyclic allylic acetates and around 4:1 with acyclic allylic acetates. The diasteroeselectivity in this reaction is established in the addition to the Pd(π-allyl) species rather than in the protodeborylation step,26 and we only observe two of the four possible diastereomers. 1H nuclear Overhauser effect experiments revealed that the minor diastereomer retains the trans relationship between the C2 and C3 substituents on the indoline ring. It arises from addition to the opposite face of the indole compared to the major diastereomer. Indolines with variously substituted aryl rings could be prepared with this method. However, alkyl-substituted boronic esters yielded the indole or recovered starting materials under these conditions.
Scheme 3. Protodeborylation to form Trans-substituted Indolinesa.
aN-Methyl indole (1.0 equiv), t-BuLi (1.1 equiv), RB(pin) (1.0 equiv), allyl acetate (1.2 equiv with acyclic, 2.0 equiv with cyclic substrates) in THF. L* = (S)-BINAP for diphenylpropenyl acetate, (S)-H8-BINAP for cyclic substrates. Isolated yields. Dr determined by 1H NMR. Er determined by HPLC.
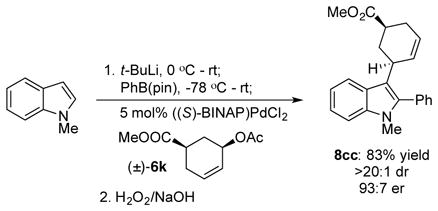
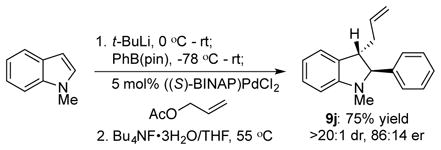
Several observations provide support for the mechanism outlined in Scheme 1b above. First, reaction with the cis-disubstituted allylic acetate 6k provided the cis-substituted product 8cc, consistent with an outer sphere attack on a Pd(π-allyl) species (eq 1).27 Second, the relative stereochemistry of the boronates 7 and indolines 9 are consistent with a trans addition across the indole. We cannot distinguish between a concerted allylation/boronate rearrangement and a stepwise process, but the absence of any cis-substituted products suggests a concerted addition. Finally, diastereoselectivity in the reaction reflects the catalyst’s ability to distinguish the faces of the indole. In an extension of this concept, we found that unsubstituted allyl acetate provided indoline 9j with >20:1 diastereoselectivity and a promising 72% ee. This is an unusual case of an asymmetric allylation in which chirality is introduced only on the nucleophile.
 |
(1) |
 |
(2) |
1,2-Boronate rearrangements have been known since early reports on the oxidation of alkyl boranes with peroxides,28 and they have been useful for C-C bond formation since Zweifel and Matteson described the olefination and homologations that bear their names.29 More recent studies have extended this reactivity to asymmetric synthesis.5,8,15 Here, we describe an enantioselective 1,2-boronate rearrangement in the context of a three component coupling to provide indoles and indolines. Future research may extend the modes of indole activation beyond π-allyl chemistry, harness the tertiary boronic ester for C-C bond formation, and extend the reactivity beyond the indole nucleus.
Supplementary Material
Acknowledgments
Financial support provided by the NIH (R01GM102403) and the Welch Foundation (I-1612). X-ray crystallography performed by Dr. V. Lynch (UT Austin).
Footnotes
ASSOCIATED CONTENT
Full experimental procedures and compound characterization is available free of charge via the internet at http://pubs.acs.org
Notes
The authors declare no competing financial interests.
References
- 1.(a) de Graaff C, Ruijter E, Orru RVA. Chem Soc Rev. 2012;41:3969–4009. doi: 10.1039/c2cs15361k. [DOI] [PubMed] [Google Scholar]; (b) Slobbe P, Ruijter E, Orru RVA. MedChemComm. 2012;3:1189–1218. [Google Scholar]; (c) Vetica F, de Figueiredo RM, Orsini M, Tofani D, Gasperi T. Synthesis. 2015;47:2139–2184. [Google Scholar]; (d) Eppe G, Didier D, Marek I. Chem Rev. 2015;115:9175–9206. doi: 10.1021/cr500715t. [DOI] [PubMed] [Google Scholar]
- 2.(a) Mizutani H, Degrado SJ, Hoveyda AH. J Am Chem Soc. 2002;124:779–781. doi: 10.1021/ja0122849. [DOI] [PubMed] [Google Scholar]; (b) Yoshida K, Ogasawara M, Hayashi T. J Am Chem Soc. 2002;124:10984–10985. doi: 10.1021/ja0271025. [DOI] [PubMed] [Google Scholar]; (c) Huang Y, Walji AM, Larsen CH, MacMillan DWC. J Am Chem Soc. 2005;127:15051–15053. doi: 10.1021/ja055545d. [DOI] [PubMed] [Google Scholar]; (d) Howell GP, Fletcher SP, Geurts K, ter Horst B, Feringa BL. J Am Chem Soc. 2006;128:14977–14985. doi: 10.1021/ja0651862. [DOI] [PubMed] [Google Scholar]; (e) Enders D, Huttl MRM, Grondal C, Raabe G. Nature. 2006;441:861–863. doi: 10.1038/nature04820. [DOI] [PubMed] [Google Scholar]; (f) Guo S, Xie Y, Hu X, Xia C, Huang H. Angew Chem Int Ed. 2010;49:2728–2731. doi: 10.1002/anie.200907320. [DOI] [PubMed] [Google Scholar]
- 3.(a) Candeias NR, Montalbano F, Cal PMSD, Gois PMP. Chem Rev. 2010;110:6169–6193. doi: 10.1021/cr100108k. [DOI] [PubMed] [Google Scholar]; (b) Jiang Y, Diagne AB, Thomson RJ, Schaus SE. J Am Chem Soc. 2017;139:1998–2005. doi: 10.1021/jacs.6b11937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Flamme EM, Roush WR. J Am Chem Soc. 2002;124:13644–13645. doi: 10.1021/ja028055j. [DOI] [PubMed] [Google Scholar]; (b) González AZ, Román JG, Alicea E, Canales E, Soderquist JA. J Am Chem Soc. 2009;131:1269–1273. doi: 10.1021/ja808360z. [DOI] [PubMed] [Google Scholar]
- 5.(a) Watson CG, Balanta A, Elford TG, Essafi S, Harvey JN, Aggarwal VK. J Am Chem Soc. 2014;136:17370–17373. doi: 10.1021/ja509029h. [DOI] [PubMed] [Google Scholar]; (b) Burns M, Essafi S, Bame JR, Bull SP, Webster MP, Balieu S, Dale JW, Butts CP, Harvey JN, Aggarwal VK. Nature. 2014;513:183–188. doi: 10.1038/nature13711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.See also: Nelson HM, Williams BD, Miró J, Toste FD. J Am Chem Soc. 2015;137:3213–3216. doi: 10.1021/jacs.5b00344.Hall DG, Rybak T, Verdelet T. Accts Chem Res. 2016;49:2489–2500. doi: 10.1021/acs.accounts.6b00403.
- 7.Panda S, Coffin A, Nguyen QN, Tantillo DJ, Ready JM. Angew Chem Int Ed. 2016;55:2205–2209. doi: 10.1002/anie.201510027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Llaveria J, Leonori D, Aggarwal VK. J Am Chem Soc. 2015;137:10958–10961. doi: 10.1021/jacs.5b07842. [DOI] [PubMed] [Google Scholar]
- 9.Aggarwal VK, Fang GY, Ginesta X, Howells DM, Zaja M. Pure Appl Chem. 2006;78:215–229. [Google Scholar]
- 10.(a) Ishikura M, Terashima M. J Chem Soc Chem Commun. 1991:1219–1221. [Google Scholar]; (b) Ishikura M, Agata I. Heterocycles. 1996;43:1591–1595. [Google Scholar]; (c) Ishikura M, Kato H. Tetrahedron. 2002;58:9827–9838. [Google Scholar]; (d) Ishikura M, Ida W, Yanada K. Tetrahedron. 2006;62:1015–1024. [Google Scholar]
- 11.Chen Y, Li NS, Deng MZ. Tetrahedron Lett. 1990;31:2405–2406. [Google Scholar]
- 12.(a) Trost BM, Crawley ML. Chem Rev. 2003;103:2921–2944. doi: 10.1021/cr020027w. [DOI] [PubMed] [Google Scholar]; (b) Butt NA, Zhang W. Chem Soc Rev. 2015;44:7929–7967. doi: 10.1039/c5cs00144g. [DOI] [PubMed] [Google Scholar]
- 13.Jadhav PK, Man H-W. J Am Chem Soc. 1997;119:846–847. [Google Scholar]
- 14.(a) Zhang L, Lovinger GJ, Edelstein EK, Szymaniak AA, Chierchia MP, Morken JP. Science. 2016;351:70–74. doi: 10.1126/science.aad6080. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lovinger GJ, Aparece MD, Morken JP. J Am Chem Soc. 2017;139:3153–3160. doi: 10.1021/jacs.6b12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Enantioselective indole allylations: Trost BM, Xie J, Sieber JD. J Am Chem Soc. 2011;133:20611–20622. doi: 10.1021/ja209244m.Cao Z, Liu Y, Liu Z, Feng X, Zhuang M, Du H. Org Lett. 2011;13:2164–2167. doi: 10.1021/ol200602x.Cao Z, Liu Y, Liu Z, Feng X, Zhuang M, Du H. Org Lett. 2011;13:2164–2167. doi: 10.1021/ol200602x.Liu Y, Du H. Org Lett. 2013;15:740–743. doi: 10.1021/ol3032736.Xu JX, Ye F, Bai XF, Zhang J, Xu Z, Zheng ZJ, Xu LW. RSC Advances. 2016;6:45495–45502.Feng B, Pu XY, Liu ZC, Xiao WJ, Chen JR. Org Chem Front. 2016;3:1246–1249.Zhang ZX, Chen SC, Jiao L. Angew Chem Int Ed. 2016;55:8090–8094. doi: 10.1002/anie.201602771.
- 16.Bandini M, Eichholzer A. Angew Chem Int Ed. 2009;48:9608–9644. doi: 10.1002/anie.200901843. [DOI] [PubMed] [Google Scholar]
- 17.(a) Kochanowska-Karamyan AJ, Hamann MT. Chem Rev. 2010;110:4489–4497. doi: 10.1021/cr900211p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kaushik N, Kaushik N, Attri P, Kumar N, Kim C, Verma A, Choi E. Molecules. 2013;18:6620. doi: 10.3390/molecules18066620. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Vitaku E, Smith DT, Njardarson JT. J Med Chem. 2014;57:10257–10274. doi: 10.1021/jm501100b. [DOI] [PubMed] [Google Scholar]
- 18.Wishart DS, Knox C, Guo AC, Shrivastava S, Hassanali M, Stothard P, Chang Z, Woolsey J. Nucleic Acids Res. 2006;34:D668–672. doi: 10.1093/nar/gkj067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.(a) Wang DS, Chen QA, Li W, Yu CB, Zhou YG, Zhang X. J Am Chem Soc. 2010;132:8909–8911. doi: 10.1021/ja103668q. [DOI] [PubMed] [Google Scholar]; (b) Xiao YC, Wang C, Yao Y, Sun J, Chen YC. Angew Chem Int Ed. 2011;50:10661–10664. doi: 10.1002/anie.201105341. [DOI] [PubMed] [Google Scholar]; (c) Duan Y, Li L, Chen MW, Yu CB, Fan HJ, Zhou YG. J Am Chem Soc. 2014;136:7688–7700. doi: 10.1021/ja502020b. [DOI] [PubMed] [Google Scholar]; (d) Ascic E, Buchwald SL. J Am Chem Soc. 2015;137:4666–4669. doi: 10.1021/jacs.5b02316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.(a) Arp FO, Fu GC. J Am Chem Soc. 2006;128:14264–14265. doi: 10.1021/ja0657859. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Saito K, Shibata Y, Yamanaka M, Akiyama T. J Am Chem Soc. 2013;135:11740–11743. doi: 10.1021/ja406004q. [DOI] [PubMed] [Google Scholar]
- 21.(BINAP)Pd in asymmetric allylic alkylation: Trost BM, Murphy DJ. Organometallics. 1985;4:1143–1145.Fuji K, Kinoshita N, Tanaka K. Chemical Commun. 1999:1895–1896.Wang Y, Xu Y-N, Fang G-S, Kang H-J, Gu Y, Tian S-K. Org Biomol Chem. 2015;13:5367–5371. doi: 10.1039/c5ob00671f.Ikeda K, Futamura T, Hanakawa T, Minakawa M, Kawatsura M. Org Biomol Chem. 2016;14:3501–3505. doi: 10.1039/c6ob00449k.
- 22.See supporting information for details.
- 23.Noh D, Yoon SK, Won J, Lee JY, Yun J. Chem Asian J. 2011;6:1967–1969. doi: 10.1002/asia.201100146. [DOI] [PubMed] [Google Scholar]
- 24.Furukawa T, Tobisu M, Chatani N. Chemical Commun. 2015;51:6508–6511. doi: 10.1039/c5cc01378j. [DOI] [PubMed] [Google Scholar]
- 25.Nave S, Sonawane RP, Elford TG, Aggarwal VK. J Am Chem Soc. 2010;132:17096–17098. doi: 10.1021/ja1084207. [DOI] [PubMed] [Google Scholar]
- 26.Protodeborylation of a diastereomerically pure sample of the boronate precursor to 9a (dr >50:1) provided 9a with dr >50:1.
- 27.(a) Trost BM, Verhoeven TR. J Am Chem Soc. 1980;102:4730–4743. [Google Scholar]; (b) Watson IDG, Yudin AK. J Am Chem Soc. 2005;127:17516–17529. doi: 10.1021/ja055288c. [DOI] [PubMed] [Google Scholar]
- 28.(a) Johnson JR, Van Campen MG., Jr J Am Chem Soc. 1938;60:121–124. [Google Scholar]; (b) Brown HC, Rao BCS. J Am Chem Soc. 1956;78:5694–5695. [Google Scholar]
- 29.(a) Zweifel G, Arzoumanian H, Whitney CC. J Am Chem Soc. 1967;89:3652–3653. [Google Scholar]; (b) Matteson DS. J Org Chem. 2013;78:10009–10023. doi: 10.1021/jo4013942. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.