Abstract
SUMMARY
In animals, defective brown adipogenesis leads to bone loss. Whether brown adipose tissue (BAT) mass relates to bone mineral density (BMD) in humans is unclear. We determined the relationship between BAT mass and BMD using cold-stimulated Positron Emission Tomography (PET) and dual-energy X-ray absorptiometry (DXA) in healthy volunteers. Higher BAT mass was associated with higher BMD in healthy women, but not men, independent of age and body composition.
INTRODUCTION
Contrary to traditional belief that BAT is present only in infants, recent studies revealed significant depots of BAT present in adult humans. In animals, defective brown adipogenesis leads to bone loss. While white adipose tissue mass is a known determinant of BMD in humans, the relationship between BAT and BMD in humans is unclear. We thus examined the relationship between BAT and BMD in healthy adults.
METHODS
BAT volume (ml) and activity (standard uptake value (SUV)) were determined by 18F-Fluorodeoxyglugose (18F-FDG) Positron Emission Tomography (PET) after overnight mild cold exposure at 19°C and BMD by DXA.
RESULTS
Among 24 healthy adults (age 28±1 yr, F=10), BAT volumes were 82.4±99.5 in women and 49.7±54.5 ml in men. Women manifested significantly higher BAT activity, by 9.4±8.1% (p=0.03), than in men. BAT volume correlated positively with total and spine BMD (r2=0.40 and 0.49, respectively, p<0.02) in women and remained a significant predictor after adjustment for age, fat and lean body mass (p<0.05). Total and spine BMD was higher in women who harbored visually detectable BAT on PET images than those without by 11±2% (p=0.02) and 22±2% (p<0.01), respectively. No associations were observed between BAT parameters and BMD in men.
CONCLUSIONS
This study demonstrated higher BMD among healthy women with more abundant BAT, independent of age and other body compositional parameters. This was not observed in men. The data suggest that brown adipogenesis may be physiologically related to modulation of bone density.
Keywords: bone mineral density, brown adipose tissue, human, thermogenesis, metabolism
Introduction
There are two kinds of adipose tissues. White adipose tissue specializes in energy storage and is in excess in obesity. In contrast, brown adipose tissue (BAT) regulates adaptive thermogenesis and energy homeostasis [1]. Through the action of uncoupling protein-1 (UCP1), BAT dissipates the electrochemical energy in the mitochondrial respiratory chain as heat. Animals with abundant BAT are protected from diet-induced obesity while UCP1 knock-out animals become obese on a normal diet at thermoneutrality [2]. Although BAT was traditionally believed to disappear after infancy in humans, recent evidence has revealed significant depots of BAT in the cervico-supraclavicular region of adults [3–6]. Consistent with its energy-dissipating function, BAT abundance is associated with reduced fat mass (FM) in humans [3, 6].
Body composition is an important predictor of bone mineral density (BMD). Greater lean body mass (LBM) predicts higher BMD [7], and has been attributed to the stimulatory effects of mechanical stress on osteoblastogenesis. Conversely, some [8, 9] but not all [10, 11] studies showed positive correlations between higher FM and BMD, with variations apparently arising from differences in age, gender, health status and/or adipose distribution [12]. For example, excess visceral adipose tissue correlates negatively with BMD, attributable to actions of inflammatory cytokines on bone modeling [12].
These apparently conflicting data beg the question as to whether adiposity exerts differential impact on bone in an adipose composition-specific manner and a causal relationship between adiposity and BMD may reside within BAT, at least in some humans. This may be supported by recent studies that revealed CCAAT/enhancer binding protein β (C/EBPβ) to be a regulator of bone remodeling and adipogenesis. Animals with C/EBPβ deficiency demonstrate both bone loss and impaired BAT formation [13, 14], reminiscent of age-related decline in bone mass and BAT activity observed in humans. Whether BAT impacts BMD in humans is unclear. Given these findings, we hypothesize greater BAT abundance may be associated with higher BMD in humans. To investigate this we capitalized our recently developed technique for quantifying BAT volume and correlate it with BMD in healthy adults.
Subjects and Methods
Subjects
Study participants consisted of 24 volunteers (10 women) recruited through local advertisement to participate in energy metabolism-related protocol at the National Institutes of Health (ClinicalTrials.gov identifier number NCT00521729). All subjects were healthy and on no medications. Women were studied during the follicular phase of the menstrual cycle. Volunteers were admitted to the Metabolic Clinical Research Unit of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) in Bethesda, Maryland for assessment of body composition, BMD and cold-stimulated BAT. The protocol was approved by the NIDDK-NIAMS institutional review board and informed consent obtained from all subjects.
BMD and body composition
BMD and body composition were measured by dual-energy x-ray absorptiometry (Lunar iDXA; GE Healthcare, Madison, WI). Scan analysis was performed using GE Encore 11.10 software. The NHANES database was used as reference for body Z scores according to manufacturer. The following parameters were measured in each volunteer: total body, total arm, total leg, spine and pelvis BMD, fat mass (FM), trunk fat and LBM. The coefficient of variation of BMD, FM and LBM were 0.5%, 1.0%, and 0.5%, respectively.
Cold-stimulated BAT
As BAT functions as a thermogenic organ, cold exposure increases its activity while thermoneutrality mutes it [3, 4]. The current standard for BAT detection is by Positron Emission Tomography (PET) scanning following cold exposure. This method utilizes 18flurodeoxyglucose (FDG) as a tracer to quantify overall BAT metabolic activity. Previous studies showed reliable detection and estimation of BAT volume in humans using FDG-PET following cold exposure [3, 4]. We therefore employed cold-stimulated PET scanning, an established and validated technique, to measure BAT volume and activity for correlation with BMD.
Volunteers wearing standardized hospital gowns slept for 12 hours in a room where the temperature was set at 19°C. A 15-mCi injection of FDG was given one hour prior to the end of the cold exposure, which continued during the hour of FDG equilibration. PET scanning was performed immediately thereafter and completed within 30 minutes.
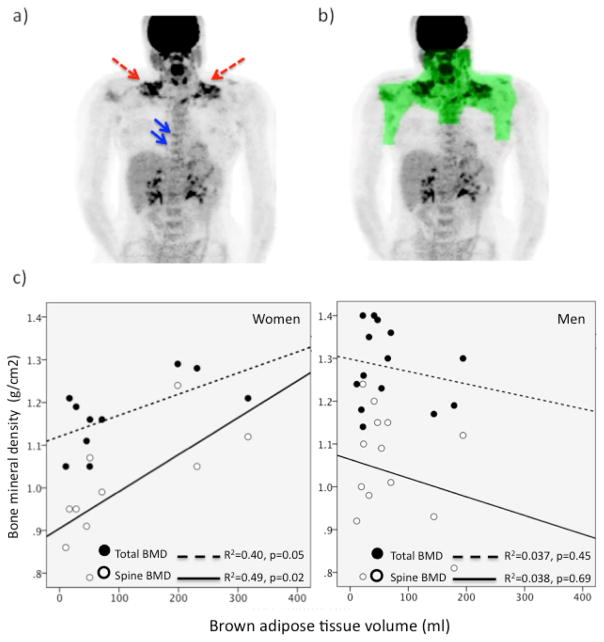
Each scan was first examined for visually detectable BAT in the ventral neck, superficial and lateral to the sternocleidomastoid muscles, the established common reservoir of BAT in humans [3, 6]. However BAT activity varies between individuals and previous histology-imaging correlative studies showed that FDG uptake can be below the visually detectable threshold on PET scans obtained from individuals who harbor lower abundance of BAT [5]. In other words, low grade BAT activity is visually undetectable but histologically present in some humans [5]. We therefore constructed a torso-mantle to define a region of interest (ROI) to capture the cervico-supraclavicular-thoracic BAT depot using a customized program (IDL software, Exelis Visual Information Solutions, Inc., Boulder, CO). Mean standardized uptake values (SUVs), which represent the activity per milliliter within the ROI divided by the injected dose in megabecquerels per gram of body weight, were determined within the torso-mantle. The volume of BAT, defined as the sum of the volume of all voxels with SUV values greater or equal to 2.0 g/ml, was quantified within the torso-mantle. Quantification of mean SUV within the BAT-specific torso mantle allows a systematic and objective estimation of BAT volume and activity, more sensitive than visual examination alone. A representative image of BAT uptake and the three-dimensional reconstruction of the ROI are shown in Figure 1.
Figure 1.
a) PET image of a 20 year old woman demonstrating FDG uptake in cervical-supraclavicular BAT depots (red arrows). Uptake was also evident within vertebra (blue arrows) that harbored bone marrow with BAT characteristics [18]; b) a representative image of BAT uptake and the three-dimensional reconstruction of the torso-mantle (green) that was constructed to allow quantification of FDG uptake within BAT-specific region; c) positive correlations between BAT volume with total and spine BMD in women (left) but not men (right).
Statistical analysis
Statistical analysis was performed using SPSS 17.0 (SPSS, Inc., Chicago, IL). Data are expressed as mean±standard deviation for normally-distributed variables. Comparisons between results were performed using the un-paired t-test. Multivariate linear regression models using analysis of variance were constructed to determine the predictors of BMD, including age, gender, FM and LBM. An α error of 0.05 was considered the threshold for statistical significance.
Results
Clinical characteristics
Table 1 summarizes clinical characteristics of participating subjects. All subjects had normal BMD. BAT was visually detectable in 6/24 (3 females and 3 males) individuals following cold exposure. Using the BAT-specific torso-mantle quantification method, the amount of BAT ranged from 10.4 to 316.9 ml in the entire cohort, with mean torso-mantle SUV between 0.74–1.10.
Table 1.
Clinical characteristics
| All | Men | Women | |
|---|---|---|---|
| N | 24 | 14 | 10 |
| Age (years) | 28±7 | 28±7 | 28±8 |
| Weight (kg) | 70.2±11.1 | 74.8±2.8 | 64.4±2.8 |
| Body mass index (kg/m2) | 22.5±2.1 | 22.7±2.2 | 22.3±2.1 |
| Fat mass (kg) | 16.0±6.1 | 13.5±5.4 | 19.2±5.6* |
| Trunk fat mass (kg) | 67.7±27.3 | 63.9±29.2 | 74.5±23.2 |
| Fat-free mass (kg) | 54.2±10.6 | 61.4±7.8 | 45.2±5.6* |
| Lean body mass (kg) | 51.0±10.2 | 57.9±73.4 | 42.2±53.3* |
| Total BMD (g/cm2) | 1.23±1.10 | 1.28±0.09 | 1.17±0.08* |
| Total Z-score | 0.77±0.64 | 0.59±0.55 | 1.04±0.64 |
| Spine BMD (g/cm2) | 1.01±0.13 | 1.04±0.14 | 0.99±0.13* |
| Pelvis BMD (g/cm2) | 1.10±0.15 | 1.15±0.17 | 1.04±0.11* |
| Leg BMD (g/cm2) | 1.34±0.16 | 1.44±0.13 | 1.22±0.10* |
| Arm BMD (g/cm2) | 0.98±0.13 | 1.07±0.10 | 0.86±0.08* |
| BAT volume (ml) | 81.0±83.1 | 49.7±54.5 | 82.4±99.5 |
| Mean torso-mantle SUV | 0.85±0.1 | 0.82±0.06 | 0.90±0.10* |
p<0.05 compared with men
BMD=bone mineral density, BAT=brown adipose tissue, SUV=standard uptake values
As expected, men had significantly higher LBM, total and regional BMD, but lower FM, than women [Table 1]. Z-scores were within the normal range for all subjects. There was a trend for higher BAT volume in women. BAT activity, as quantified by SUV, was 9.4±8.1% higher in women than in men (p=0.03).
BMD and body composition
Total BMD correlated positively with LBM (r=0.75, p<0.001) but not FM (p=0.8). Positive correlation between BMD and LBM was seen in men (r=0.62, p=0.02) and similar positive correlation approached significance in women (r=0.59, p=0.06).
BAT and body composition
As BMD, LBM and FM were different between men and women, we next investigated the relationship between BAT and body composition in each gender. In men, there was no association between BAT parameters, LBM or FM. In contrast, BAT volume correlated positively with FM (r=0.74, p=0.02) in women, and a trend for a positive correlation between mean BAT SUV and FM (r=0.58, p=0.08) was also observed. There was no association between BAT parameters and LBM.
BAT and BMD
Figure 1 depicts the relationship between BAT and BMD. There were no associations between BAT parameters with total or regional BMD in men. However, in women, BAT volume correlated positively with total and spine BMD. In a forward step-wise multiple regression analysis controlling for age, FM and LBM, BAT volume was the independent predictor of total (β=0.63, p=0.049) and spine BMD (β=0.70, p=0.02) [Supplementary Tables 1 and 2]. Total and spine BMD were higher in women with visually detectable BAT activity than those without by 11±2% (p=0.02) and 22±2% (p<0.01), respectively.
Discussion
This is the first study investigating the relationship between cold-activated BAT and BMD in healthy men and women. Our findings revealed a positive correlation between BAT volume with total and spine BMD in women, independent of FM and LBM. These findings suggest a possible regulatory link between brown adipogenesis and bone density in humans.
While numerous studies have demonstrated association of higher body mass on BMD, little is known about whether adipose sub-types relate to bone mass. This is due to the absence of a sensitive BAT detection method, rendering research into BAT and bone mass difficult. The recent re-discovery of BAT in adulthood by PET scanning has made such research pursuit feasible for the first time. However, given the protective role of BAT in diet-induced obesity, all studies to-date have focused on its potential anti-obesity property. Animals with defective BAT activity have been evaluated for their metabolic vulnerability to obesity. Interestingly, animal models with defective brown adipogenesis also manifest reduced bone mass [13, 15], suggesting mechanistic links between BAT function and bone mass regulation [16]. This is concordant with findings in a recent study revealing lower BMD and BAT mass in women with anorexia nervosa [17]. Our study further uncovers a positive correlation between cold-activated BAT mass and BMD in healthy women, but not in men. Collectively, these findings suggest a previously unrecognized regulatory role of BAT on bone mass in humans, which appears to be gender-specific.
The mechanisms underlying the positive association between BAT activity and bone mass are unknown. Recent studies have identified a brown-adipocyte like phenotype in marrow adipocytes [18]. Bone marrow is a unique microenvironment, where cellular and stromal-vascular components of adipose and bone juxtapose. As brown adipogenesis is capable of generating a hypoxic gradient, which fuels chondrocytogenesis and bone formation [19], we speculate individuals with higher BAT mass harbor a more osteoblastogenic bone marrow microenvironment, leading to greater BMD. Consistent with this hypothesis, brown adipocyte genes in marrow fat are reduced in ageing and diabetes, conditions which also exhibit bone loss [16]. As shown in Figure 1, FDG uptake was evident within vertebra following cold stimulation, suggestive of the presence of BAT-enriched bone marrow. Given such uptake was not observed in other bones, it is our speculation that it may explain our data showing BAT volume correlates strongest with spine BMD. It is also possible that BAT regulates bone mass through the local milieu of adipokines and bone growth factors, given recent discoveries of the role of bone morphogenic proteins on bone remodeling and brown adipogenesis [20].
It is uncertain why correlations between BMD and BAT were only seen in women but not men. While this could be a type 2 error, sexual dimorphism in brown adipogenesis cannot be excluded given women harbor more BAT than men [3]. Indeed, women tended to have higher total Z-scores than men, supportive of a positive effect of BAT on age-adjusted BMD in women, who also tended to have more abundant BAT than men in our study. It is possible that the impact of BAT on BMD is magnified in women due to their overall lower LBM than men. Collectively, these findings suggest complex interplay between BMD, BAT, LBM, age and gender. Future studies should include subjects with wider range of body composition and BMD to decipher these relationships.
The cross-sectional design and small sample size are limitations of the current study. Despite careful adjustment for potential confounders, a causal relationship between brown adipogenesis and higher BMD cannot be established. We cannot exclude the possibility that the observed relationship between BAT and BMD represents a type 1 error and larger confirmatory studies are required. However, validity of our data is supported by their demonstration of the expected relationship between LBM and BMD. We did not perform regional BMD measurement, which precludes the assessment of association between BAT parameters and clinically relevant regions such as femoral neck. The positive relationship observed between BAT volume and FM contrasts with previous studies [3, 6], which may be explained by our inclusion of only lean individuals. Whether the interaction between BAT and FM is dependent on absolute adiposity merits further studies in future.
Our study provides exploratory, hypothesis-generating insights into a novel interactive domain of adipose-bone biology. The reason for bone loss beginning in the second decade in women, long before onset of estrogen deficiency is unclear. We speculate BAT-decline related bone loss may be a contributing mechanism as BAT abundance falls rapidly after early adulthood [3, 6]. Given bone loss results in osteoporosis, a highly prevalent condition with significant morbidity and mortality, identification of novel positive regulator of bone-formation may shed therapeutic insight into treatment of osteoporosis in the future.
In summary, the current study reveals healthy women with more abundant BAT to have higher BMD, independent of age and other body compositional parameters. These positive correlations are absent in men. While we emphasize causality cannot be proven in our associative study, we hypothesize BAT may be a novel regulator of bone mass in humans. Whether age-related BAT atrophy contributes to bone loss merits future longitudinal studies.
Supplementary Material
Acknowledgments
Paul Lee was supported by an Australian National Health Medical Research Council Early Career Fellowship, the Diabetes Australia Fellowship and Bushell Travelling Fellowship.
Footnotes
Conflict of interest: None of the authors have any conflicts of interest to disclose
References
- 1.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293:E444–452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 2.Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009;9:203–209. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 3.Saito M, Okamatsu-Ogura Y, Matsushita M, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 5.Lee P, Zhao JT, Swarbrick MM, Gracie G, Bova R, Greenfield JR, Freund J, Ho KK. High prevalence of brown adipose tissue in adult humans. J Clin Endocrinol Metab. 2011;96:2450–2455. doi: 10.1210/jc.2011-0487. [DOI] [PubMed] [Google Scholar]
- 6.Lee P, Greenfield JR, Ho KK, Fulham MJ. A critical appraisal of the prevalence and metabolic significance of brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2010;299:E601–606. doi: 10.1152/ajpendo.00298.2010. [DOI] [PubMed] [Google Scholar]
- 7.Salamone LM, Glynn N, Black D, Epstein RS, Palermo L, Meilahn E, Kuller LH, Cauley JA. Body composition and bone mineral density in premenopausal and early perimenopausal women. J Bone Miner Res. 1995;10:1762–1768. doi: 10.1002/jbmr.5650101120. [DOI] [PubMed] [Google Scholar]
- 8.Albala C, Yanez M, Devoto E, Sostin C, Zeballos L, Santos JL. Obesity as a protective factor for postmenopausal osteoporosis. Int J Obes Relat Metab Disord. 1996;20:1027–1032. [PubMed] [Google Scholar]
- 9.Reid IR, Plank LD, Evans MC. Fat mass is an important determinant of whole body bone density in premenopausal women but not in men. J Clin Endocrinol Metab. 1992;75:779–782. doi: 10.1210/jcem.75.3.1517366. [DOI] [PubMed] [Google Scholar]
- 10.Janicka A, Wren TA, Sanchez MM, Dorey F, Kim PS, Mittelman SD, Gilsanz V. Fat mass is not beneficial to bone in adolescents and young adults. J Clin Endocrinol Metab. 2007;92:143–147. doi: 10.1210/jc.2006-0794. [DOI] [PubMed] [Google Scholar]
- 11.Gilsanz V, Chalfant J, Mo AO, Lee DC, Dorey FJ, Mittelman SD. Reciprocal relations of subcutaneous and visceral fat to bone structure and strength. J Clin Endocrinol Metab. 2009;94:3387–3393. doi: 10.1210/jc.2008-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russell M, Mendes N, Miller KK, Rosen CJ, Lee H, Klibanski A, Misra M. Visceral fat is a negative predictor of bone density measures in obese adolescent girls. J Clin Endocrinol Metab. 2010;95:1247–1255. doi: 10.1210/jc.2009-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Motyl KJ, Raetz M, Tekalur SA, Schwartz RC, McCabe LR. CCAAT/enhancer binding protein beta-deficiency enhances type 1 diabetic bone phenotype by increasing marrow adiposity and bone resorption. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1250–1260. doi: 10.1152/ajpregu.00764.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carmona MC, Hondares E, Rodriguez de la Concepcion ML, Rodriguez-Sureda V, Peinado-Onsurbe J, Poli V, Iglesias R, Villarroya F, Giralt M. Defective thermoregulation, impaired lipid metabolism, but preserved adrenergic induction of gene expression in brown fat of mice lacking C/EBPbeta. Biochem J. 2005;389:47–56. doi: 10.1042/BJ20050009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zanotti S, Stadmeyer L, Smerdel-Ramoya A, Durant D, Canalis E. Misexpression of CCAAT/enhancer binding protein beta causes osteopenia. J Endocrinol. 2009;201:263–274. doi: 10.1677/JOE-08-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Motyl KJ, Rosen CJ. Temperatures rising: brown fat and bone. Discov Med. 2011;11:179–185. [PMC free article] [PubMed] [Google Scholar]
- 17.Bredella MA, Fazeli PK, Freedman LM, Calder G, Lee H, Rosen CJ, Klibanski A. Young Women with Cold-Activated Brown Adipose Tissue Have Higher Bone Mineral Density and Lower Pref-1 than Women without Brown Adipose Tissue: A Study in Women with Anorexia Nervosa, Women Recovered from Anorexia Nervosa, and Normal-Weight Women. J Clin Endocrinol Metab. 2012 doi: 10.1210/jc.2011-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krings A, Rahman S, Huang S, Lu Y, Czernik PJ, Lecka-Czernik B. Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes. Bone. 2012;50:546–552. doi: 10.1016/j.bone.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olmsted-Davis E, Gannon FH, Ozen M, et al. Hypoxic adipocytes pattern early heterotopic bone formation. Am J Pathol. 2007;170:620–632. doi: 10.2353/ajpath.2007.060692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang H, Schulz TJ, Espinoza DO, Huang TL, Emanuelli B, Kristiansen K, Tseng YH. Cross talk between insulin and bone morphogenetic protein signaling systems in brown adipogenesis. Mol Cell Biol. 2010;30:4224–4233. doi: 10.1128/MCB.00363-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.