Pulmonary arterial hypertension (PAH) is a progressive and ultimately fatal disease characterized by elevated blood pressure in the pulmonary artery1. The pathogenesis of PAH is driven by hyperproliferation of vascular smooth muscle cells in a hyperinflammatory environment, withlocal accumulation of innate immune cells, such as macrophages2, 3, and increased levels of inflammatory cytokines such as IL-6, IL-1β and IL-18. While we traditionally consider PAH a disease that selectively affects the pre-capillary arterioles, coronary artery disease (CAD) is reported to be 4-times more prevalent in patients with PAH, suggesting possible common mechanisms of disease or risk factors. Indeed, PAH is associated with a higher prevalence of the metabolic syndrome, an observation that is particularly notable in patients who develop pulmonary vascular remodeling in the setting of heart failure with preserved ejection fraction4.
In this issue of ATVB, Meloche and colleagues postulate that systemic inflammation could be a central mechanism linking the development of both coronary and pulmonary vascular smooth muscle cell proliferation. The authors provide rich experimental data demonstrating that, in rat models of PAH and in humans with PAH, there is evident vascular remodeling of the coronary arteries, characterized by the thickening of the arterial wall due to smooth muscle cell proliferation, and reduced cardiac perfusion. They also find elevated IL-6 mRNA levels in the human hearts of patients with PAH and stimulation of coronary artery smooth muscle cells in culture with IL-6 induces smooth muscle cell proliferation and DNA damage.
Investigating mechanisms that might tie vascular IL-6 mediated inflammation and secondary smooth muscle proliferation in PAH and CAD, Meloche et al. demonstrate that the bromodomain containing protein 4 (BRD4) is a key molecule regulating this observed PAH-associated distal coronary artery thickening. BRD4 is an epigenetic “reader” that recognizes histone modification patterns and binds to acetylated lysine residues of the histone tails. BRD4 is a cofactor facilitating transcriptional activation of target genes by stabilizing the recruitment of transcription factors and transcription elongation factors5, 6. The authors previously showed that BRD4 expression in pulmonary artery smooth muscle cells contributed to the development of experimental PAH7. Moreover, BRD4 inhibition with JQ1 inhibitor, which also suppresses BRD2 and BRD3, limits coronary artery atherogenic processes8. Based on these reports, Meloche et al., hypothesized that increased BRD4 expression in smooth muscle cells in the coronary vasculature of PAH patients triggers the development of CAD. Indeed, BRD4 expression was increased in both pulmonary artery and coronary artery smooth muscle cells in rat models of PAH and in humans with PAH. IL-6 increased BRD4 expression in smooth muscle cell cultures, which augmented cellular proliferation and suppressed apoptosis. Pharmacological inhibition of BRD4 with JQ1 reduced smooth muscle cell hyperproliferation and enhanced cellular apoptosis. In vivo studies using both a silencing RNA and JQ1 to inhibit BRD4 reduced coronary artery thickness in the sugen-hypoxia rat model of PAH. These studies suggest an important role for IL-6 driven BRD4 expression in the pro-proliferative and anti-apoptotic phenotype of coronary and pulmonary vascular smooth muscle cells in PAH models. It remains to be investigated if other inflammatory cytokines, in addition to IL-6, have an effect on the expression of the epigenetic reader.
The implication of epigenetic “readers” like BRD4 in the inflammation-mediated coronary artery remodeling associated with PAH is of particular interest since it draws a causal association between inflammation and epigenetic reprogramming, which is a new avenue of research in major diseases including cancer, metabolic syndrome and cardiovascular disease7–10. The nature and function of BRD4 as an enzymatically inactive epigenetic “reader” suggest that additional epigenetic and transcriptional mechanisms, such as DNA methylation, histone modification, imbalance of epigenetic “writers” (enzymes adding modifications) and/or epigenetic “erasers” (enzymes removing modifications), as well as microRNA, might be initiated or altered by inflammation. Additional studies are therefore required to delineate the ensemble of mechanisms involved in the inflammation-mediated epigenetic reprogramming in CAD. Importantly, BRD4 can be recruited to both promoter and super enhancer regions8. Super enhancers are genomic regulatory elements displaying distinct chromatin landscape and enriched in transcription binding sites. A lineage specific subset of distal enhancers is primed for activation in distinct cell types and there is evidence supporting a critical functional role of enhancers in controlling cell differentiation and cell lineage identity11, 12. This raises the question whetherBRD4 binds to specific subsets of enhancers and which target genes are upregulated in smooth muscle cells, endothelial cells and immune cells respectively.
The study by Meloche and colleagues focused on the role of BRD4 in proliferation and apoptosis of smooth muscle cells in the coronary artery. However, aside from smooth muscle cells, endothelial cells can contribute to vascular remodeling process in PAH and CAD. Endothelial dysfunction in CAD precedes adherence and extravasation of inflammatory monocytes, which differentiate into pathogenic foam cells in atherosclerotic plaques. Moreover, endothelial cells can indirectly aggravate disease pathogenesis by releasing inflammatory cytokines. Since endothelial dysfunction is a common pathogenic feature in PAH and CAD13, BRD4 signaling in endothelial cells may play a role in CAD development in PAH patients. Interestingly, a recent paper14 revealed the role of BRD4 in producing inflammatory cytokines, such and IL-6 and IL-8, by pulmonary endothelial cells. Clearly, future studies are warranted to investigate BRD4 signaling in coronary artery endothelial cells in patients with PAH and CAD. Moreover, since inflammatory macrophages and T cells are the major sources of IL-6 in tissues, it is possible that these inflammatory cells are recruited in the perivascular space and then induce smooth muscle cell proliferation by increasing BRD4 expression, suggesting a complex crosstalk between these cell types (Figure 1).
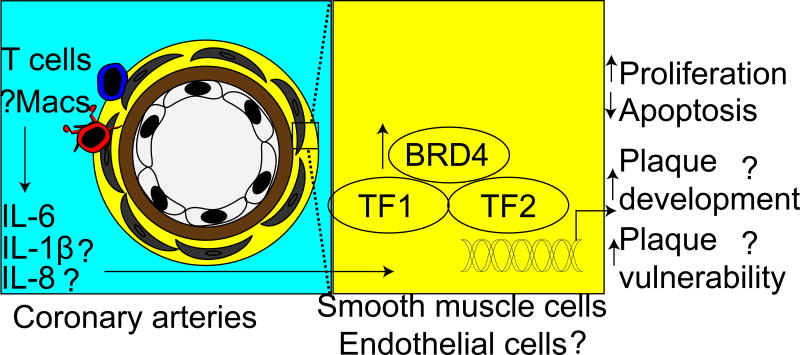
Figure 1.
Hypothesized schematic representation of increased CAD in PAH patients: Inflammatory cytokines, such as IL-6, IL-8 and IL-1β, produced by recruited macrophages and T cells increase BRD4 expression. Augmented bromodomain containing protein 4 (BRD4) expression in smooth muscle cells or endothelial cells in the coronary arteries facilitates transcription of genes involved in cell proliferation, apoptosis inhibition, plaque development and plaque vulnerability. Question marks indicate unanswered questions. TF, transcription factor.
Another hallmark of CAD is presence of atherosclerotic plaques, but it is not currently known if IL-6-mediated DNA damage and epigenetic modifications can mediate plaque development. It would also be interesting to know if the drugs commonly used in CAD, such as statins and aspirin, or anti-inflammatory therapies currently tested in clinical trial like methotrexate15 (decreasing IL-6 levels) or canakinumab16 (anti-IL-1β antibody) can reduce BRD4 expression. As suggested by the authors, smooth muscle cell proliferation directly contributes to distal CAD by inducing vasoconstriction and reducing coronary perfusion. It is also widely assumed that proliferation of smooth muscle cell and their participation in the formation of a protective smooth muscle cell-rich, collagen-rich fibrous cap protects against plaque rupture in coronary arteries17, 18. Whether BRD4 contributes to these disparate properties of the atherosclerotic lesion remains to be studied.
In conclusion, this interesting study provides new evidence suggesting that PAH is a systemic disease affecting other organs such as the systemic vasculature and heart. Future studies will be required to untangle the interactions and clearly define causality and new therapies that capitalize on this new knowledge.
References
- 1.Lai YC, Potoka KC, Champion HC, Mora AL, Gladwin MT. Pulmonary arterial hypertension: The clinical syndrome. Circ Res. 2014;115:115–130. doi: 10.1161/CIRCRESAHA.115.301146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El Kasmi KC, Pugliese SC, Riddle SR, et al. Adventitial fibroblasts induce a distinct proinflammatory/profibrotic macrophage phenotype in pulmonary hypertension. J Immunol. 2014;193:597–609. doi: 10.4049/jimmunol.1303048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sawada H, Saito T, Nickel NP, et al. Reduced bmpr2 expression induces gm-csf translation and macrophage recruitment in humans and mice to exacerbate pulmonary hypertension. J Exp Med. 2014;211:263–280. doi: 10.1084/jem.20111741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meng Q, Lai YC, Kelly NJ, Bueno M, Baust JJ, Bachman TN, Goncharov D, Vanderpool RR, Radder JE, Hu J, Goncharova E, Morris AM, Mora AL, Shapiro SD, Gladwin MT. Development of a mouse model of metabolic syndrome, pulmonary hypertension, and heart failure with preserved ejection fraction. Am J Respir Cell Mol Biol. 2017;56:497–505. doi: 10.1165/rcmb.2016-0177OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belkina AC, Denis GV. Bet domain co-regulators in obesity, inflammation and cancer. Nat Rev Cancer. 2012;12:465–477. doi: 10.1038/nrc3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanno T, Kanno Y, LeRoy G, Campos E, Sun HW, Brooks SR, Vahedi G, Heightman TD, Garcia BA, Reinberg D, Siebenlist U, O'Shea JJ, Ozato K. Brd4 assists elongation of both coding and enhancer rnas by interacting with acetylated histones. Nat Struct Mol Biol. 2014;21:1047–1057. doi: 10.1038/nsmb.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meloche J, Potus F, Vaillancourt M, et al. Bromodomain-containing protein 4: The epigenetic origin of pulmonary arterial hypertension. Circ Res. 2015;117:525–535. doi: 10.1161/CIRCRESAHA.115.307004. [DOI] [PubMed] [Google Scholar]
- 8.Brown JD, Lin CY, Duan Q, et al. Nf-kappab directs dynamic super enhancer formation in inflammation and atherogenesis. Mol Cell. 2014;56:219–231. doi: 10.1016/j.molcel.2014.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suzuki H, Toyota M, Kondo Y, Shinomura Y. Inflammation-related aberrant patterns of DNA methylation: Detection and role in epigenetic deregulation of cancer cell transcriptome. Methods Mol Biol. 2009;512:55–69. doi: 10.1007/978-1-60327-530-9_5. [DOI] [PubMed] [Google Scholar]
- 10.Raghuraman S, Donkin I, Versteyhe S, Barres R, Simar D. The emerging role of epigenetics in inflammation and immunometabolism. Trends Endocrinol Metab. 2016;27:782–795. doi: 10.1016/j.tem.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Hnisz D, Abraham BJ, Lee TI, Lau A, Saint-Andre V, Sigova AA, Hoke HA, Young RA. Super-enhancers in the control of cell identity and disease. Cell. 2013;155:934–947. doi: 10.1016/j.cell.2013.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Creyghton MP, Cheng AW, Welstead GG, et al. Histone h3k27ac separates active from poised enhancers and predicts developmental state. Proc Natl Acad Sci U S A. 2010;107:21931–21936. doi: 10.1073/pnas.1016071107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lundberg JO, Gladwin MT, Weitzberg E. Strategies to increase nitric oxide signalling in cardiovascular disease. Nat Rev Drug Discov. 2015;14:623–641. doi: 10.1038/nrd4623. [DOI] [PubMed] [Google Scholar]
- 14.Mumby S, Gambaryan N, Meng C, Perros F, Humbert M, Wort SJ, Adcock IM. Bromodomain and extra-terminal protein mimic jq1 decreases inflammation in human vascular endothelial cells: Implications for pulmonary arterial hypertension. Respirology. 2017;22:157–164. doi: 10.1111/resp.12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Everett BM, Pradhan AD, Solomon DH, Paynter N, Macfadyen J, Zaharris E, Gupta M, Clearfield M, Libby P, Hasan AA, Glynn RJ, Ridker PM. Rationale and design of the cardiovascular inflammation reduction trial: A test of the inflammatory hypothesis of atherothrombosis. Am Heart J. 2013;166:199–207. e115. doi: 10.1016/j.ahj.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1beta inhibition and the prevention of recurrent cardiovascular events: Rationale and design of the canakinumab anti-inflammatory thrombosis outcomes study (cantos) Am Heart J. 2011;162:597–605. doi: 10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Kolodgie FD, Virmani R, Burke AP, Farb A, Weber DK, Kutys R, Finn AV, Gold HK. Pathologic assessment of the vulnerable human coronary plaque. Heart. 2004;90:1385–1391. doi: 10.1136/hrt.2004.041798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davies MJ, Richardson PD, Woolf N, Katz DR, Mann J. Risk of thrombosis in human atherosclerotic plaques: Role of extracellular lipid, macrophage, and smooth muscle cell content. Br Heart J. 1993;69:377–381. doi: 10.1136/hrt.69.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]