Abstract
We evaluated the epidemiology of Candida bloodstream infections in the neonatal intensive care unit (NICU) of an Italian university hospital during a 9-year period as a means of quantifying the burden of infection and identifying emerging trends. Clinical data were searched for in the microbiological laboratory database. For comparative purposes, we performed a review of NICU candidemia. Forty-one candidemia cases were reviewed (overall incidence, 3.0 per 100 admissions). Candida parapsilosis sensu stricto (58.5%) and C. albicans (34.1%) were the most common species recovered. A variable drift through years was observed; in 2015, 75% of the cases were caused by non-albicans species. The duration of NICU hospitalization of patients with non-albicans was significantly longer than in those with C. albicans (median days, 10 versus 12). Patients with non-albicans species were more likely to have parenteral nutrition than those with C. albicans (96.3% versus 71.4%). Candida albicans was the dominant species in Europe and America (median, 55% and 60%; resp.); non-albicans species predominate in Asia (75%). Significant geographic variation is evident among cases of candidemia in different parts of the world, recognizing the importance of epidemiological data to facilitate the treatment.
1. Introduction
Although blood stream infection (BSI) due to Candida species (spp.) in the neonatal intensive care unit (NICU) is less frequent than that due to Gram-positive or Gram-negative bacteria, it has higher morbidity and mortality rates. In particular, among newborns with a birth weight < 1000 g, 4–8% will develop candidemia, which has a 30% mortality in this group of patients [1]. Newborns who survive frequently have long-term neurological impairment, including cerebral palsy, blindness, hearing impairment, cognitive deficits, and periventricular leukomalacia [2]. Risk factors for neonatal candidemia include prematurity, use of central venous lines, endotracheal tubes, parenteral nutrition, broad-spectrum antibiotic administration (especially third-generation cephalosporins), prolonged hospitalization, abdominal surgery, exposure to H2 blockers, and Candida colonization. Although Candida albicans is the most prevalent yeast pathogen, BSIs caused by Candida non-albicans, particularly Candida parapsilosis complex and Candida glabrata complex, have increased in recent years [2, 3].
This study aimed (i) to determine the epidemiology of Candida BSIs in the NICU of an Italian university hospital during 9 years of observation; (ii) to analyze the trend in species distribution; and (iii) to examine in vitro susceptibility to common antifungal drugs. Furthermore, for comparative purposes, a systematic review of studies concerning the distribution of Candida spp. causing candidemia in NICU patients is presented.
2. Materials and Methods
2.1. Study Design
A retrospective, observational survey of all consecutive cases of candidemia was conducted at the NICU (capacity of 8 beds; level III) of a university hospital in Southern Italy, from January 1, 2007, to December 31, 2015. The number of annual admissions ranged from 135 to 169, with no significant variation during the period of study. All of the neonates who had at least one positive blood culture for Candida spp. and signs or symptoms of infection were considered in this study. Only the first episode of candidemia was reported for patients with recurrent or subsequent episodes. Clinical data were searched for in the microbiological laboratory database and included sex, gestational age, birth weight, and predisposing risk factors for Candida BSI (i.e., intravascular devices, prolonged antibiotics, administration of total parental nutrition, and prolonged hospitalization).
2.2. Definitions
Extremely low birth weight (ELBW) infants were defined as those with a birth weight ≤ 1000 g, very low birth weight (VLBW) infants were those with a birth weight <1500 g, and low birth weight infants were those with a birth weight < 2500 g. Prolonged antibiotic use was defined as >14 days of continuous administration. Late-onset sepsis (LOS) was defined as infection occurring for >48 h of life. Candidemia was considered as probably catheter-related when semiquantitative culture of the catheter tip yielded >15 colony-forming units of Candida.
2.3. Laboratory Procedures
Blood cultures were performed using a lysis-centrifugation system (Isolator; DuPont Co., Wilmington, DE, USA). The samples were cultured on two plates of Sabouraud dextrose agar with 0.05% chloramphenicol (BioRad, Marnes-la-Coquette, France) and then incubated at 36°C (±1) and 28°C (±1). The samples were examined daily for 10 days. The isolates were identified using standard procedures (morphology on cornmeal agar plates, germ-tube production in serum, and ability to grow at 37°C and 42°C) and biochemical analysis using two methods, the Vitek 2 system and ID 32C panels (Bio-Merieux, Rome, Italy), to obtain accurate results. All strains were frozen at −70°C until further investigations [4]. Candida parapsilosis complex genotyping was performed by PCR amplification as reported previously [5, 6].
Antifungal susceptibility tests to five antifungal drugs (anidulafungin, fluconazole, caspofungin, micafungin, and amphotericin B) were performed for all Candida spp., using the Sensititre YeastOne technique (SYO-09 panel; Trek Diagnostic Systems, Ltd., East Grinstead, England).
The susceptibility values were interpreted taking into account the species-specific clinical breakpoints (CBPs) suggested by the Clinical Laboratory Standards Institute (CLSI) subcommittee for the most common species of Candida [7]. The epidemiological cut-off values were used to define wild-type and non-wild-type isolates if no CBPs were available from the CLSI [8, 9]. Minimum inhibitory concentration (MIC) data are presented as MIC50 (MIC causing inhibition of 50% of isolates) and MIC90 (MIC causing inhibition of 90% of isolates).
2.4. Statistical Analysis
The Shapiro–Wilk test was used to test the normal distribution of data. Non-normally distributed data are expressed as median and interquartile range (IQR) and were compared using the Mann–Whitney U test. Categorical data are expressed as number and percentage and were compared using χ2 or Fisher's exact test. All p values are two-tailed, and statistical significance was defined as p < 0.05 (Social Sciences (SPSS) software 10 for Mac OS X; SPSS Inc., Chicago, IL, USA).
2.5. Literature Review
A review of full-text articles that were published in English from January 2000 to February 2015 was performed. The MEDLINE database was used for the bibliographic research, using the following key words: “neonatal candidemia”, “candidemia neonatal intensive care unit”, “Candida neonatal intensive care unit, and “NICU candidemia”. Additionally, the bibliographies of the selected articles were reviewed for relevant publications.
The exclusion criteria were as follows: articles that reported a period of study prior to 2000; letters, randomized, controlled trials; and studies that reported a total number of Candida BSIs less than five. The following data were collected from each selected study: geographic location, year of publication, study period, type of study, incidence, influencing factors candidemia, total number of isolated Candida spp., and relative proportion of each of the Candida spp.
3. Results
3.1. Analysis of Cases in the NICU
A total of 41 infants with Candida infection were reviewed. The overall incidence of candidemia was 3.0 per 100 NICU admissions (range, 2.2–3.0). The male : female ratio was 1.6 : 1. The cohort had a median gestational age of 30 weeks (29–31 weeks) and a median birth weight of 1110 g (900–1345 g). The majority of candidemia episodes occurred in VLBW infants (56.1%). The median duration of the total hospital stay was 11 days (8–14 days). Candidemia was catheter-related in 23 cases (56.1%). All Candida infections were classified as LOS. At the moment of candidemia, only ELBW infants were receiving antifungal prophylaxis with fluconazole (3 mg/kg/day).
Candida parapsilosis sensu stricto was isolated with the highest frequency (58.5%), followed by C. albicans (34.1%), C. glabrata complex, C. guilliermondii, and C. orthopsilosis (2.4% for each). Therefore, 65.9% of candidemia episodes were caused by Candida non-albicans. With regard to the temporal trend of C. albicans and Candida non-albicans, a variable drift from 2007–2015 was observed, with a considerable percentage (75%) increase in non-albicans species in 2015 (Figure 1). Predisposing factors associated with C. albicans and non-albicans are listed in Table 1. The duration of NICU hospitalization of patients with C. non-albicans was significantly longer than that in those with C. albicans (median days, 10 [7.5–12] versus 12 [10–15], p = 0.045). Patients with C. non-albicans were more likely to have parenteral nutrition than those with C. albicans (96.3% versus 71.4%, p = 0.039).
Figure 1.
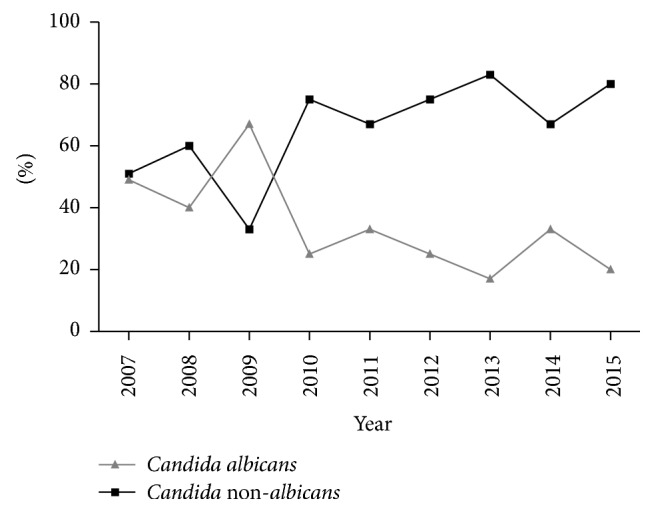
Temporal trend of Candida albicans and Candida non-albicans during a 9-year period.
Table 1.
Clinical characteristics of the patients with candidemia by species.
| Characteristics | Candida albicans (n = 14) | Candida non-albicans (n = 27) | p value |
|---|---|---|---|
| Low gestational age ≤ 32 wk, n (%) | 11 (78.6) | 25 (92.6) | 0.317 |
| Gestational age‡ | 31 (29.5–31.5) | 30 (29–31) | 0.193 |
| Birth weight ≤ 1500 g, n (%) | 11 (78.6) | 25 (92.6) | 0.317 |
| Birth weight (g)‡ | 1200 (1013–1625) | 1200 (900–1380) | 0.573 |
| Stay in NICU ≤ 7 days, n (%) | 12 (85.7) | 27 (100) | 0.111 |
| Length of stay before candidemia (days)‡ | 10 (7.5–12) | 12 (10–15) | 0.045 |
| Presence of CVC, n (%) | 13 (92.8) | 27 (100) | 0.342 |
| TPN, n (%) | 10 (71.4) | 26 (96.3) | 0.039 |
| Mechanical ventilation, n (%) | 11 (78.6) | 26 (96.3) | 0.107 |
| Prolonged antibiotic therapy, n (%) | 12 (85.7) | 24 (92.3) | 1.000 |
‡Median (interquartile range). CVC: central venous catheter; TPN: total parenteral nutrition. Bold values are significant.
Results of antifungal susceptibility are shown in Table 2. All of the strains were sensitive to tested drugs. Overall, the MIC50/MIC90 values (mg/L) were as follows: amphotericin B, 0.25/0.5; anidulafungin, 1/2; caspofungin, 0.25/0.5; fluconazole, 0.5/2; and micafungin, 1/1.
Table 2.
Cumulative distribution of the MICs of 41 clinical Candida isolates.
| Isolates (number) | Antifungal drugs | Cumulative % of strains inhibited at the indicated concentrations (mg/L) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.008 | 0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | ||
| Candida parapsilosis complex (25) | Fluconazole | 4 | 38 | 77 | 92 | 100 | ||||||
| Amphotericin B | 4 | 58 | 100 | |||||||||
| Anidulafungin | 12 | 65 | 100 | |||||||||
| Caspofungin | 12 | 58 | 100 | |||||||||
| Micafungin | 19 | 92 | 100 | |||||||||
| Candida albicans (14) | Fluconazole | 17 | 75 | 83 | 100 | |||||||
| Amphotericin B | 8 | 17 | 75 | 100 | ||||||||
| Anidulafungin | 33 | 67 | 100 | |||||||||
| Caspofungin | 12 | 33 | 83 | 100 | ||||||||
| Micafungin | 42 | 100 | ||||||||||
| All species (41) | Fluconazole | 5 | 25 | 50 | 83 | 93 | 98 | 100 | ||||
| Amphotericin B | 5 | 8 | 65 | 100 | ||||||||
| Anidulafungin | 10 | 20 | 33 | 40 | 75 | 100 | ||||||
| Caspofungin | 5 | 10 | 25 | 33 | 70 | 98 | 100 | |||||
| Micafungin | 13 | 30 | 33 | 45 | 93 | 100 | ||||||
3.2. Literature Review
A total of 45 articles were selected (Tables 3 and 4). Thirty-two studies reported data from a single hospital and 27 were retrospective studies. Seventeen studies were conducted in Asia, 13 in Europe, 11 in North and South America, and 2 in South Africa. Finally, one cohort was carried out in Australia.
Table 3.
Distribution of Candida spp. from bloodstream infections in NICU patients from 2000–2015 in various studies.
| Reference | Country/observation time | Study design | Number of isolatesa | Distribution of Candida spp. (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CA | CP | CG | CT | CGU | CF | CK | CL | CD | CLI | CST | CKE | Candida spp.b | ||||
| Europe | ||||||||||||||||
| Presterl et al., 2007 [10] | Austria/January 2001 to December 2006 | Retrospective/single hospital | 16 | 93.8 | 6.2 | |||||||||||
| Lagrou et al., 2007 [11] | Belgium/January 2001 to December 2005 | Retrospective, data from the hospital information system/single hospital | 9 | 88.9 | 11.1 | |||||||||||
| Sarvikivi et al., 2005 [12] | Finland/January 2000 to December 2002 (original period: 1991–2002) | Retrospective, data were laboratory-based/single hospital | 25 | 32 | 68 | |||||||||||
| Spiliopoulou et al., 2012 [13] | Greece/January 2005 to December 2009 | Retrospective/single hospital | 40 | 67.5 | 25 | 2.5 | 5 | |||||||||
| Lovero et al., 2016 [14] | Italy/January 2000 to December 2014 | Retrospective, data were laboratory-based/single hospital | 57 | 47 | 44 | 4 | 5 | |||||||||
| Montagna et al., 2010 [15] | Italy/February 2007 to August 2008 | Prospective (Aurora), data were web database-based/6 neonatal units | 21 | 35 | 60 | 5 | ||||||||||
| Tortorano et al., 2013 [16] | Italy/January 2009 to December 2009 | Prospective, data were laboratory-based/34 hospitals | 17 | 58.8 | 35.3 | 5.9 | ||||||||||
| Rodriguez et al., 2006 [17] | Spain/January 2002 to December 2003 | Prospective, data were laboratory-based/5 hospitals | 24 | 29.2 | 66.7 | 4.1 | ||||||||||
| Pemán et al., 2011 [18] | Spain/January 2009 to February 2010 | Prospective (FUNGEMYCA)/30 hospitals | 27 | 51.9 | 33.3 | 3.7 | 3.7 | 3.7 | 3.7 | |||||||
| Yalaz et al., 2006 [19] | Turkey/January 2000 to December 2002 | Retrospective, review of medical records/single hospital | 14 | 100 | ||||||||||||
| Celebi et al., 2012 [20] | Turkey/January 2000 to December 2007 | Prospective/single hospital | 28 | 42.9 | 57.1 | |||||||||||
| Ozkan et al., 2014 [21] | Turkey/January 2003 to December 2010 | Prospective/single hospital | 24 | 33.3 | 66.7 | |||||||||||
| Clerihew et al., 2006 [22] | United Kingdom/February 2003 to February 2004 | Prospective (British Paediatric Surveillance Unit)/56 neonatal units | 67 | 55.2 | 32.8 | 12 | ||||||||||
| Vergnano et al., 2011 [23] | United Kingdom/January 2006 to December 2008 | Prospective (NeonIN), data were web database-based/12 neonatal units | 37 | 73 | 27 | |||||||||||
|
| ||||||||||||||||
| North and South America | ||||||||||||||||
| Aziz et al., 2010 [24] | USA/January 2000 to December 2006 | Retrospective, review of medical records/single hospital | 10 | 40 | 40 | 10 | 10 | |||||||||
| Feja et al., 2005 [25] | USA/March 2001 to January 2003 | Prospective/2 neonatal units | 45 | 62 | 31 | 2 | 2 | 2 | ||||||||
| Horn et al., 2009 [26] | USA/July 2004 to March 2008 | Prospective (PATH Alliance), data were web database-based/23 hospitals | 26 | 69.2 | 26.9 | 3.8 | ||||||||||
| Pfaller et al., 2012 [27] | USA-Canada/July 2004 to December 2008 | Prospective (PATH Alliance), data were web database-based/23 medical centers in the USA and two in Canada | 62 | 54.8 | 30.6 | 1.6 | 6.5 | 6.5 | ||||||||
| Bizzarro et al., 2015 [28] | USA/January 2004 to December 2013 | Retrospective, review of medical records/single hospital | 20 | 50 | 35 | 5 | 5 | 5 | ||||||||
| Natarajan et al., 2009 [29] | USA/January 2006 to December 2007 | Retrospective, review of medical records/single hospital | 29 | 58.6 | 27.6 | 6.9 | 3.4 | 3.4 | ||||||||
| Robinson et al., 2012 [30] | USA/January 2000 to December 2010 | Retrospective, data from the hospital information system/single hospital | 37 | 59.5 | 24.3 | 8.1 | 5.4 | 2.7 | ||||||||
| Batista et al., 2014 [31] | Brazil/October 2006 to March 2007 | Prospective/single hospital | 10 | 60 | 40 | |||||||||||
| Hoffmann-Santos et al., 2013 [32] | Brazil/January 2006 to December 2011 | Retrospective, data were laboratory-based/2 hospitals | 45 | 33.3 | 48.9 | 11.2 | 6.7 | |||||||||
| Cortés et al., 2011 [33] | Colombia/January 2001 to December 2007 | Prospective, data were laboratory-based/27 hospitals | 143 | 61 | 15 | 5 | 19 | |||||||||
| Cortés et al., 2014 [34] | Colombia/March 2008 to March 2009 | Prospective/7 hospitals | 15 | 60 | 13.3 | 13.3 | 13.3 | |||||||||
|
| ||||||||||||||||
| Asia | ||||||||||||||||
| Hua et al., 2012 [35] | China/February 2008 to February 2010 | Retrospective, review of medical records/single hospital | 34 | 38.2 | 32.4 | 2.9 | 5.9 | 5.9 | 11.8 | 2.9 | ||||||
| Wu et al., 2014 [36] | China/January 2009 to December 2011 | Retrospective, review of medical records/single hospital | 37 | 16.2 | 54.1 | 29.7 | ||||||||||
| Chen et al., 2015 [37] | China/January 2010 to December 2013 | Retrospective, data from the hospital information system/single hospital | 43 | 14 | 39.5 | 32.6 | 14 | |||||||||
| Rani et al., 2002 [38] | India/January 2000 to June 2000 | Prospective/single hospital | 50 | 4 | 92 | 4 | ||||||||||
| Agarwal et al., 2004 [39] | India/August 2002 to April 2003 | Prospective/single hospital | 90 | 15.6 | 84.4 | |||||||||||
| Femitha et al., 2013 [40] | India/October 2009 and July 2011 | Prospective/single hospital | 36 | 25 | 44.4 | 30.6 | ||||||||||
| Mehara et al., 2013 [41] | India/January 2012 to September 2012 | Retrospective, review of medical records/single hospital | 9 | 44.4 | 22.2 | 33.3 | ||||||||||
| Juyal et al., 2013 [42] | India/January 2012 to December 2012 | Prospective, data were laboratory-based/single hospital | 132 | 19.7 | 25 | 14.4 | 24 | 10.6 | 8.3 | |||||||
| Chaurasia et al., 2015 [43] | India/January 2013 to June 2013 | Retrospective, review of medical records/single hospital | 30 | 20 | 23.3 | 10 | 36.7 | 10 | ||||||||
| Wadile and Bhate, 2015 [44] | India/January 2014 to December 2014 | Retrospective, review of medical records/single hospital | 20 | 65 | 15 | 10 | 5 | 5 | ||||||||
| Al-Sweih et al., 2009 [45] | Kuwait/January 2000 to December 2006 (original period: 1995–2006) | Retrospective, review of medical records/single hospital | 108 | 41.7 | 45.4 | 12.9 | ||||||||||
| Hammoud et al., 2013 [46] | Kuwait/January 2007 to December 2010 | Retrospective, review of medical records/single hospital | 89 | 47.2 | 38.2 | 6.7 | 1.1 | 4.5 | 2.2 | |||||||
| Khan et al., 2015 [47] | Pakistan/January 2009 to January 2014 | Retrospective, data were laboratory-based/single hospital | 41 | 26 | 74 | |||||||||||
| Wu et al., 2009 [48] | Taiwan/January 2001 to December 2006 | Retrospective, review of medical records/single hospital | 13 | 23.1 | 69.2 | 7.7 | ||||||||||
| Tsai et al., 2014 [49] | Taiwan/January 2004 to December 2011 | Retrospective, review of medical records and administrative databases/single hospital | 52 | 61.5 | 30.8 | 7.7 | ||||||||||
| Lim et al., 2012 [50] | Taiwan/January 2005 to December 2009 | Retrospective, review of medical records and administrative database/single hospital | 6 | 66.7 | 33.3 | |||||||||||
| Chen et al., 2015 [37] | Taiwan/January 2008 to December 2013 | Retrospective, review of medical records/single hospital | 9 | 22.2 | 77.8 | |||||||||||
| Africa | ||||||||||||||||
| Motara et al., 2005 | South Africa/July 2002 to July 2003 | Retrospective, data were laboratory-based/single hospital | 10 | 80 | 20 | |||||||||||
| Ballot et al., 2013 [51] | South Africa/January 2007 to December 2011 | Retrospective/single hospital | 57 | 28.1 | 56.1 | 3.5 | 8.8 | 1.8 | 1.8 | |||||||
|
| ||||||||||||||||
| Oceania | ||||||||||||||||
| Chen et al., 2006 [52] | Australia/August 2001 to July 2004 | Retrospective, data were laboratory-based/50 microbiology laboratories | 35 | 42 | 43 | 9 | 2 | 2 | 2 | |||||||
CA: Candida albicans; CP: C. parapsilosis; CG: C. glabrata; CT: C. tropicalis; CGU: C. guilliermondii; CF: C. famata; CK: C. krusei; CL: C. lusitaniae; CD: C. dubliniensis; CLI: C. lipolytica; CST: C. stelloidea; CKE: C. kefyr. aTotal number of Candida isolates from blood (or the total number of candidemia episodes when the number of isolates was not available from the original study). bIncluding Candida spp. not depicted in the table and Candida spp. not identified at the species level.
Table 4.
Main candidemia finding in the NICU as reported in various studies.
| Reference | Main candidemia finding in the NICU |
|---|---|
| Lagrou et al., 2007 [11] | Annual incidence: 0.30 episodes per 10,000 patient-days. |
| Sarvikivi et al., 2005 [12] | Fluconazole prophylaxis contributed to the emergence of C. parapsilosis with decreased susceptibility to fluconazole. |
| Spiliopoulou et al., 2012 [13] | Candidemia incidence decreased. C. albicans was most frequently isolated from ELBW infants. Mortality (35.7%) was associated with low gestational age and low birth weight. |
| Lovero et al., 2016 [14] | Incidence rate of Candida non-albicans increased from 46% in 2000–2004 to 71% in 2010–2014. |
| Montagna et al., 2010 [15] | Overall incidence: 1.3 per 100 NICU discharges. The incidence in ELBW infants was 4.3% versus 0.2% in LBW infants. |
| Rodriguez et al., 2006 [17] | Annual incidence: 1.1 per 100 NICU discharges and 1.08 per 1000 patient-days. Low mortality (21%) rate may have been caused by a high prevalence of C. parapsilosis fungemia. |
| Pemán et al., 2011 [18] | C. albicans was more common in the NICU setting than in the pediatric ICU. |
| Yalaz et al., 2006 [19] | Candidemia markedly increased in 2002 compared with previous years. A significant association was found between Candida infection and the duration of antibiotic therapy. |
| Celebi et al., 2012 [20] | Overall incidence: 11.5 per 1000 NICU admissions. The mortality rate was 42.8%. |
| Ozkan et al., 2014 [21] | Gram-positive sepsis (67.6%) was more common than Gram-negative bacteremia (16.6%) and candidemia (15.8%). Candida spp. caused LOS (58.3%), VLOS (41,7%), and no EOS sepsis. |
| Clerihew et al., 2006 [22] | C. parapsilosis was associated with fewer deep-seated infections than C. albicans, but mortality was similar. |
| Vergnano et al., 2011 [23] | A decrease in candidemia was observed: 1.8% in 2006, 1.2% in 2007, and 1.3% in 2008. Candida spp. were more common in LOS (97%) than in EOS (3%) sepsis. |
| Aziz et al., 2010 [24] | Fluconazole prophylactic administration to ELBW infants was associated with a decreased rate of candidemia. |
| Feja et al., 2005 [25] | Overall incidence: 1.6 per 100 NICU discharges. Catheter use, previous bacterial sepsis, and GI pathology were significantly associated with candidemia. |
| Bizzarro et al., 2015 [28] | Candida spp. were more common in LOS than in EOS sepsis. |
| Natarajan et al., 2009 [29] | Candidemia refractory to conventional antifungals was associated with prolonged antibiotic use and Candida non-albicans infection. |
| Robinson et al., 2012 [30] | Overall incidence: 0.45 per 100 NICU discharges. An increased time between blood culture draw and initial antifungal therapy was associated with an increased incidence of persistent candidemia. |
| Batista et al., 2014 [31] | Oral colonization should be considered as a risk factor for candidemia. |
| Hua et al., 2012 [35] | Patients with C. parapsilosis had a significantly longer hospital stay than those with C. albicans sepsis. |
| Wu et al., 2014 [36] | C. guilliermondii was associated with preterm infants and with low birth weight. |
| Chen et al., 2015 [37] | Fluconazole prophylaxis alone was not efficacious; it had to be combined with reinforcement of management and supervision of hand hygiene to effectively prevent invasive candidiasis. |
| Rani et al., 2002 [38] | Candida non-albicans accounted for 96% of the cases of neonatal candidemia. |
| Agarwal et al., 2004 [39] | Overall incidence: 77 per 1000 NICU discharges. Candida non-albicans is gaining importance as a cause of neonatal septicemia. |
| Femitha et al., 2013 [40] | Overall incidence: 0.82 cases per 100 NICU discharges. Mortality was 44.4%. Presence of candiduria was a significant riskfactor for death. |
| Mehara et al., 2013 [41] | Candida spp. were more common in LOS than in EOS sepsis. |
| Juyal et al., 2013 [42] | Candida non-albicans accounted for 80.30% of the cases of neonatal candidemia. The crude mortality was 34.85%. |
| Chaurasia et al., 2015 [43] | Clinical features in neonates with candida sepsis were nonspecific. A common laboratory feature was thrombocytopenia. |
| Al-Sweih et al., 2009 [45] | Overall incidence: 4 per 100 NICU discharges. |
| Hammoud et al., 2013 [46] | C. albicans was the most prevalent species in nonpersistent candidemia. C. parapsilosis was more common among infants with persistent candidemia. Persistent candidemia was associated with an increased risk of mortality. |
| Wu et al., 2009 [48] | The most common causative microorganisms of LOS sepsis were CONS and Candida spp. C. parapsilosis was associated with a high mortality rate. |
| Tsai et al., 2014 [49] | Candidemia had a significantly higher rate of infectious complications, persistent bloodstream infection, and sepsis-attributable mortality than Gram-negative and Gram-positive bacteremia. |
| Lim et al., 2012 [50] | Sepsis by Gram-negative bacteria or Candida spp. presented with more severe clinical symptoms and was associated with a higher mortality rate compared with that by Gram-positive bacteria. |
| Chen et al., 2015 [37] | Decrease incidence of candidemia during the study period. |
| Ballot et al., 2013 [51] | Increased incidence of Candida non-albicans during the study period. |
CONS: coagulase-negative staphylococci; ELBW: extremely low birth weight; VLBW: very low birth weight; GI: gastrointestinal; EOS: early-onset sepsis; LOS: late-onset sepsis; VLOS: very late-onset sepsis; NICU: neonatal intensive care unit; ICU: intensive care unit.
The distribution of Candida spp. varied according to the different geographical areas. Candida albicans was the dominant species in Europe with proportions ranging from 47 to 100% [10, 11, 13, 14, 16, 18, 19, 22, 23, 53] and in North and South America with proportions ranging from 40 to 69.2% [24–31, 33, 34]. Candida non-albicans species were predominant in Asia [36–40, 42, 43, 45, 47, 48], with proportions ranging from 25 to 92%, with a median of 75% (Figure 2). In Australia, C. albicans and C. non-albicans were equally distributed (42% and 43%, resp.) [52].
Figure 2.
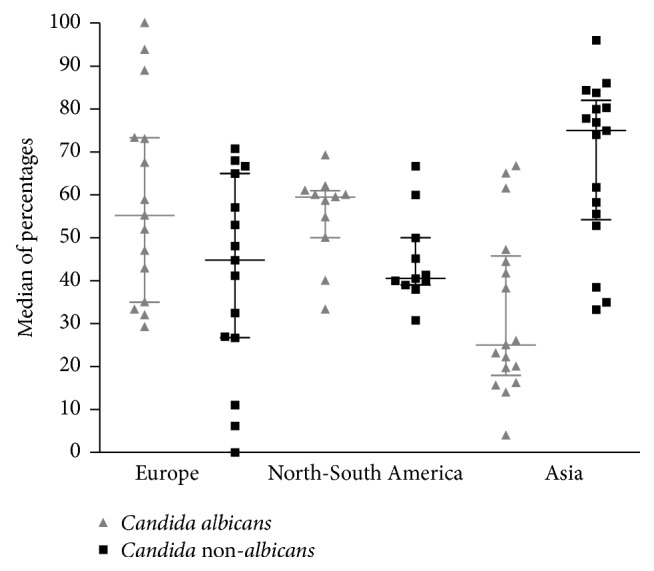
Distribution of Candida spp. according to the different geographical areas.
For C. non-albicans, the three most prevalent species were C. parapsilosis complex, C. glabrata complex, and C. tropicalis. Generally, C. parapsilosis complex was the second most common pathogen (range, 6.2–77.8%). C. parapsilosis complex was the predominant species in some studies from Europe [12, 15, 17, 20, 21] and Asia [37, 40, 42, 45, 48]. The highest proportions of C. glabrata complex were reported in studies that were conducted in the central part of India (range, 22.2–44.4%), while the lowest proportions were observed in European countries (range, 2.5–5.9%). No cases due to C. glabrata complex were reported in South America. The highest frequency of C. tropicalis was found in South India (36.7–92%), followed by studies from South America (11.2–13.3%) and South Africa (8.8%). The lowest frequencies were observed in Europe (3.7–5%) and Australia (2%). There were no reports of C. tropicalis in North America.
4. Discussion
This study aimed to describe the epidemiology and drug susceptibility of Candida isolates causing candidemia in a NICU of an Italian university hospital over 9 years. Our survey showed that candidemia is a common problem among critically ill neonates, with an overall incidence of 3%. This finding is higher than data reported in a literature review from Europe (1.1–1.3%) [15, 17] and the North and South America (0.5–1.6%) [25, 30], but lower than that reported in Asia (4–7.7%) [39, 45]. This variability may reflect differences in health care practices among countries, as well as the study design adopted, including differences in the examined population.
VLBW infants are known to be at a high risk of candidemia because of more aggressive and invasive therapies, such as indwelling central lines, mechanical ventilation, parenteral hyperalimentation, and longer hospital stay [1–3]. The majority of infected neonates have a gestational age at birth of 30 weeks or earlier and birth weight is ≤1500 g (87.8%, each one). Intravenous catheters are risk factors for Candida BSI in critically ill infants. We found that all patients had intravenous catheter placement and that candidemia was catheter-related in 56.1% of cases. This finding is not surprising because Candida spp. can adhere to platelets and fibrinogen on the surface of catheters and form biofilms that may become a reservoir for systemic spread [1–3].
In our systematic review, we found that only four species (C. albicans, C. parapsilosis complex, C. tropicalis, and C. glabrata complex) accounted for 95.4% of cases of candidemia. However, the ranking of these four species was variable. Generally, C. albicans was the predominant isolated spp. in Europe [10, 11, 13, 14, 16, 18, 19, 22, 23, 53] and North and South America [24–31, 33, 34]. However, non-albicans species were predominant in Asia [36–40, 42, 43, 45, 47, 48].
Moreover, data regarding changes in the relative frequencies of isolated Candida spp. showed a shift toward Candida non-albicans, with a frequency higher than 50% in some NICUs. This, in part, is attributed to the increased use of azole prophylaxis and therapy [12]. However, in a recent study, where fluconazole was rarely used for prophylaxis and therapy, a high incidence of non-albicans (60.8% of all candidemia episodes) was found [20]. Similarly, our study showed a higher percentage of C. non-albicans (66%) than C. albicans and a variable drift through 9 years. In 2015, 75% of the cases were caused by non-albicans species.
In our study, appearance of C. parapsilosis complex as the predominant fungal pathogen (61% of all isolates) was consistent with the pattern seen in some hospitals in Europe, Asia, and Africa [12, 15, 17, 20, 37, 42, 45, 48, 51].
Main risk factors for C. parapsilosis complex infection were the presence of indwelling vascular catheters and parenteral nutrition, both of which predispose to formation of biofilms. Morphogenesis from yeast cells to pseudohyphae is essential for biofilm formation and virulence in C. parapsilosis complex. Amino acids mediate cell differentiation, and this could explain the high incidence of this yeast in catheterized neonates who receive amino acid-rich parenteral nutrition solutions [54]. Our data highlights an association between parenteral nutrition and non-albicans spp. The high proportion of C. parapsilosis complex may explain this finding. Notably, we observed that NICU patients were more likely to develop C. parapsilosis sensu stricto (58.5%) than C. orthopsilosis (2.4%) candidemia. This finding may be explained by the greater capacity of C. parapsilosis sensu stricto to adhere to central lines compared with closely related species [55].
In agreement with other studies [13–15, 17, 18], none of the isolated strains showed resistance to fluconazole and amphotericin B. These are the antifungal drugs of choice that are used in prophylaxis and treatment of Candida BSI in neonates [56]. No fluconazole resistance may be related to the treatment policy in use at our hospital, where systemic antifungal prophylaxis with fluconazole was used only in ELBW infants. In neonates, fluconazole prophylaxis has been linked to the emergence of azole resistance [12, 57].
5. Conclusions
Limitations of the present study are mainly related to its retrospective nature with limited follow-up data. Although all of the data were prospectively collected, some variables could not be examined because of missing data. Furthermore, we did not have data on specific characteristics of noninfected patients in our NICU. Therefore, we were not able to risk-adjust our rates to compare with incidences from other reports.
Nevertheless, this study shows that C. non-albicans candidemia is increasing, despite limited use of fluconazole for prophylaxis/empiric therapy in our unit. Our results also confirm that candidemia plays an important pathogenic role in NICU patients. There is a significant variation in cases of candidemia in different geographic regions, even within the same continent. Therefore, monitoring epidemiological data to facilitate the choice of treatment is important.
Ethical Approval
The study protocol was approved by the Ethics Committee of the Azienda Ospedaliero-Universitaria Policlinico of Bari, Italy (Application no. 1321, 2007). Registered data were managed in accordance with the Italian data protection laws (privacy law).
Consent
Written informed consent was obtained from patient parents or their legal guardians.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Testoni D., Hayashi M., Cohen-Wolkowiez M., et al. Late-onset bloodstream infections in hospitalized term infants. Pediatric Infectious Disease Journal. 2014;33(9):920–923. doi: 10.1097/INF.0000000000000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelly M. S., Benjamin D. K., Smith P. B. The epidemiology and diagnosis of invasive candidiasis among premature infants. Clinics in perinatology. 2015;42(1):17–105. doi: 10.1016/j.clp.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leibovitz E. Strategies for the prevention of neonatal candidiasis. Pediatrics and Neonatology. 2012;53(2):83–89. doi: 10.1016/j.pedneo.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Caggiano G., Coretti C., Bartolomeo N., Lovero G., De Giglio O., Montagna M. T. Candida bloodstream infections in Italy: Changing epidemiology during 16 years of surveillance. BioMed Research International. 2015;2015 doi: 10.1155/2015/256580.256580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tavanti A., Davidson A. D., Gow N. A. R., Maiden M. C. J., Odds F. C. Candida orthopsilosis and Candida metapsilosis spp. nov. to replace Candida parapsilosis groups II and III. Journal of Clinical Microbiology. 2005;43(1):284–292. doi: 10.1128/JCM.43.1.284-292.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borghi E., Sciota R., Iatta R., Biassoni C., Montagna M. T., Morace G. Characterization of Candida parapsilosis complex strains isolated from invasive fungal infections. European Journal of Clinical Microbiology and Infectious Diseases. 2011;30(11):1437–1441. doi: 10.1007/s10096-011-1242-x. [DOI] [PubMed] [Google Scholar]
- 7.Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of yeasts; fourth informational supplement, M27-S4. Wayne, PA, USA: CLSI; 2012. [Google Scholar]
- 8.Espinel-Ingroff A., Pfaller M. A., Bustamante B., et al. Multilaboratory study of epidemiological cutoff values for detection of resistance in eight Candida species to fluconazole, posaconazole, and voriconazole. Antimicrobial Agents and Chemotherapy. 2014;58(4):2006–2012. doi: 10.1128/AAC.02615-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfaller M. A., Castanheira M., Messer S. A., Jones R. N. In vitro antifungal susceptibilities of isolates of Candida spp. and Aspergillus spp. from China to nine systemically active antifungal agents: Data from the SENTRY antifungal surveillance program, 2010 through 2012. Mycoses. 2015;58(4):209–214. doi: 10.1111/myc.12299. [DOI] [PubMed] [Google Scholar]
- 10.Presterl E., Daxböck F., Graninger W., Willinger B. Changing pattern of candidaemia 2001-2006 and use of antifungal therapy at the University Hospital of Vienna, Austria. Clinical Microbiology and Infection. 2007;13(11):1072–1076. doi: 10.1111/j.1469-0691.2007.01812.x. [DOI] [PubMed] [Google Scholar]
- 11.Lagrou K., Verhaegen J., Peetermans W. E., De Rijdt T., Maertens J., Van Wijngaerden E. Fungemia at a tertiary care hospital: Incidence, therapy, and distribution and antifungal susceptibility of causative species. European Journal of Clinical Microbiology and Infectious Diseases. 2007;26(8):541–547. doi: 10.1007/s10096-007-0339-8. [DOI] [PubMed] [Google Scholar]
- 12.Sarvikivi E., Lyytikäinen O., Soll D. R., et al. Emergence of fluconazole resistance in a Candida parapsilosis strain that caused infections in a neonatal intensive care unit. Journal of Clinical Microbiology. 2005;43(6):2729–2735. doi: 10.1128/JCM.43.6.2729-2735.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spiliopoulou A., Dimitriou G., Jelastopulu E., Giannakopoulos I., Anastassiou E. D., Christofidou M. Neonatal Intensive Care Unit Candidemia: Epidemiology, Risk Factors, Outcome, and Critical Review of Published Case Series. Mycopathologia. 2012;173(4):219–228. doi: 10.1007/s11046-011-9498-3. [DOI] [PubMed] [Google Scholar]
- 14.Lovero G., De Giglio O., Montagna O., et al. Epidemiology of candidemia in neonatal intensive care units: A persistent public health problem. Annali di Igiene. 2016;28(4):282–287. doi: 10.7416/ai.2016.2107. [DOI] [PubMed] [Google Scholar]
- 15.Montagna M. T., Lovero G., De Giglio O., Iatta R., Caggiano G., Montagna O., et al. Invasive fungal infections in neonatal intensive care units of Southern Italy: a multicentre regional active surveillance (AURORA project). Journal of Preventive Medicine and Hygiene. Vol. 51. 125: 130; 2010. [PubMed] [Google Scholar]
- 16.Tortorano A. M., Prigitano A., Lazzarini C., et al. A 1-year prospective survey of candidemia in Italy and changing epidemiology over one decade. Infection. 2013;41(3):655–662. doi: 10.1007/s15010-013-0455-6. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez D., Almirante B., Park B. J., et al. Candidemia in neonatal intensive care units: Barcelona, Spain. Pediatric Infectious Disease Journal. 2006;25(3):224–229. doi: 10.1097/01.inf.0000202127.43695.06. [DOI] [PubMed] [Google Scholar]
- 18.Pemán J., Cantón E., Linares-Sicilia M. J., et al. Epidemiology and antifungal susceptibility of bloodstream fungal isolates in pediatric patients: A Spanish multicenter prospective survey. Journal of Clinical Microbiology. 2011;49(12):4158–4163. doi: 10.1128/JCM.05474-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yalaz M., Çetin H., Akisu M., Aydemir Ş., Tunger A., Kültürsay N. Neonatal nosocomial sepsis in a level-III NICU: Evaluation of the causative agents and antimicrobial susceptibilities. Turkish Journal of Pediatrics. 2006;48(1):13–18. [PubMed] [Google Scholar]
- 20.Celebi S., Hacimustafaoglu M., Koksal N., Ozkan H., Cetinkaya M., Ener B. Neonatal candidiasis: Results of an 8 year study. Pediatrics International. 2012;54(3):341–349. doi: 10.1111/j.1442-200X.2012.03574.x. [DOI] [PubMed] [Google Scholar]
- 21.Ozkan H., Cetinkaya M., Koksal N., Celebi S., Hacimustafaoglu M. Culture-proven neonatal sepsis in preterm infants in a neonatal intensive care unit over a 7 year period: coagulase-negative Staphylococcus as the predominant pathogen. Pediatrics International. 2014;56(1):60–66. doi: 10.1111/ped.12218. [DOI] [PubMed] [Google Scholar]
- 22.Clerihew L., Lamagni T. L., Brocklehurst P., McGuire W. Invasive fungal infection in very low birthweight infants: National prospective surveillance study. Archives of Disease in Childhood: Fetal and Neonatal Edition. 2006;91(3):F188–F192. doi: 10.1136/adc.2005.082024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vergnano S., Menson E., Kennea N., et al. Neonatal infections in England: The neonIN surveillance network. Archives of Disease in Childhood: Fetal and Neonatal Edition. 2011;96(1):F9–F14. doi: 10.1136/adc.2009.178798. [DOI] [PubMed] [Google Scholar]
- 24.Aziz M., Patel A. L., Losavio J., et al. Efficacy of fluconazole prophylaxis for prevention of invasive fungal infection in extremely low birth weight infants. Pediatric Infectious Disease Journal. 2010;29(4):352–356. doi: 10.1097/INF.0b013e3181bf8eb1. [DOI] [PubMed] [Google Scholar]
- 25.Feja K. N., Wu F., Roberts K., et al. Risk factors for candidemia in critically ill infants: a matched case-control study. Journal of Pediatrics. 2005;147(2):156–161. doi: 10.1016/j.jpeds.2005.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horn D. L., Neofytos D., Anaissie E. J., et al. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clinical Infectious Diseases. 2009;48(12):1695–1703. doi: 10.1086/599039. [DOI] [PubMed] [Google Scholar]
- 27.Pfaller M., Neofytos D., Diekema D., et al. Epidemiology and outcomes of candidemia in 3648 patients: data from the Prospective Antifungal Therapy (PATH Alliance) registry, 2004–2008. Diagnostic Microbiology and Infectious Disease. 2012;74(4):323–331. doi: 10.1016/j.diagmicrobio.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Bizzarro M. J., Shabanova V., Baltimore R. S., Dembry L.-M., Ehrenkranz R. A., Gallagher P. G. Neonatal sepsis 2004-2013: The rise and fall of coagulase-negative staphylococci. Journal of Pediatrics. 2015;166(5):1193–1199. doi: 10.1016/j.jpeds.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Natarajan G., Lulic-Botica M., Aranda J. V. Refractory neonatal candidemia and high-dose micafungin pharmacotherapy. Journal of Perinatology. 2009;29(11):738–743. doi: 10.1038/jp.2009.75. [DOI] [PubMed] [Google Scholar]
- 30.Robinson J. A., Pham H. D., Bloom B. T., Wittler R. R. Risk factors for persistent candidemia infection in a neonatal intensive care unit and its effect on mortality and length of hospitalization. Journal of Perinatology. 2012;32(8):621–625. doi: 10.1038/jp.2011.162. [DOI] [PubMed] [Google Scholar]
- 31.Batista G. C. M., Krebs V. L. J., Ruiz L. S., Auler M. E., Hahn R. C., Paula C. R. Oral colonization: A possible source for candidemia in low-weight neonates. Journal de Mycologie Medicale. 2014;24(2):81–86. doi: 10.1016/j.mycmed.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Hoffmann-Santos H. D., Paula C. R., Yamamoto A. C. A., Tadano T., Hahn R. C. Six-Year Trend Analysis of Nosocomial Candidemia and Risk factors in Two Intensive Care Hospitals in Mato Grosso, Midwest Region of Brazil. Mycopathologia. 2013;176(5-6):409–415. doi: 10.1007/s11046-013-9705-5. [DOI] [PubMed] [Google Scholar]
- 33.Cortés J. A., Reyes P., Gómez C., Buitrago G., Leal A. L. Fungal bloodstream infections in tertiary care hospitals in Colombia. Revista Iberoamericana de Micología. 2011;28(2):74–78. doi: 10.1016/j.riam.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Cortés J. A., Reyes P., Gómez C. H., et al. Clinical and epidemiological characteristics and risk factors for mortality in patients with candidemia in hospitals from Bogotá, Colombia. Brazilian Journal of Infectious Diseases. 2014;18(6):631–637. doi: 10.1016/j.bjid.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hua S., Huang J., Wu Z., Feng Z. A comparison study between Candida parapsilosis sepsis and Candida albicans sepsis in preterm infants. Turkish Journal of Pediatrics. 2012;54(5):502–508. [PubMed] [Google Scholar]
- 36.Wu Z., Liu Y., Feng X., et al. Candidemia: Incidence rates, type of species, and risk factors at a tertiary care academic hospital in China. International Journal of Infectious Diseases. 2014;22:e4–e8. doi: 10.1016/j.ijid.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 37.Chen I., Chiu N., Chi H., et al. Changing of bloodstream infections in a medical center neonatal intensive care unit. Journal of Microbiology, Immunology and Infection. 2015 doi: 10.1016/j.jmii.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 38.Rani R., Mohapatra N. P., Mehta G., Randhawa V. S. Changing trends of Candida species in neonatal septicaemia in a tertiary North Indian hospital. Indian Journal of Medical Microbiology. 2002:2042–2044. [PubMed] [Google Scholar]
- 39.Agarwal J., Bansal S., Malik G. K., Jain A. Trends in neonatal septicemia: Emergence of non-albicans Candida. Indian Pediatrics. 2004;41(7):712–715. [PubMed] [Google Scholar]
- 40.Femitha P., Joy R., Adhisivam B., et al. Candidemia in neonatal ICU- experience from a tertiary care hospital. Current Pediatric Research. 2013;17(1):44–48. [Google Scholar]
- 41.Mehara V., Yadava D., Somania P., Bhatambareb G., Mulyea S., Singh K. Neonatal sepsis in a tertiary care center in central India: microbiological profile, antimicrobial sensitivity pattern and outcome. Journal of Neonatal-Perinatal Medicine. 2013;6:165–172. doi: 10.3233/NPM-1367312. [DOI] [PubMed] [Google Scholar]
- 42.Juyal D., Sharma M., Pal S., Rathaur V. K., Sharma N. Emergence of non-albicans Candida species in neonatal candidemia. North American Journal of Medical Sciences. 2013;5(9):541–545. doi: 10.4103/1947-2714.118919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chaurasia D., Goel M., Dhruw S., Dubey D., Dwivedi R. Changing Pattern of Neonatal Fungal Sepsis: A Matched Case – Control Study. National Journal of Medical and Allied Sciences. 2015;4(1) Online first. [Google Scholar]
- 44.Wadile R., Bhate V. Study of clinical spectrum and risk factors of neonatal candidemia. Indian Journal of Pathology and Microbiology. 2015;58(4):472–474. doi: 10.4103/0377-4929.168888. [DOI] [PubMed] [Google Scholar]
- 45.Al-Sweih N., Khan Z., Khan S., Devarajan L. V. Neonatal candidaemia in Kuwait: A 12-year study of risk factors, species spectrum and antifungal susceptibility. Mycoses. 2009;52(6):518–523. doi: 10.1111/j.1439-0507.2008.01637.x. [DOI] [PubMed] [Google Scholar]
- 46.Hammoud M. S., Al-Taiar A., Fouad M., Raina A., Khan Z. Persistent candidemia in neonatal care units: Risk factors and clinical significance. International Journal of Infectious Diseases. 2013;17(8):e624–e628. doi: 10.1016/j.ijid.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 47.Khan E. A., Choudhry S., Fatima M., Batool Z. Clinical spectrum, management and outcome of neonatal candidiasis. Journal of the Pakistan Medical Association. 2015;65:1206–1209. [PubMed] [Google Scholar]
- 48.Wu J.-H., Chen C.-Y., Tsao P.-N., Hsieh W.-S., Chou H.-C. Neonatal sepsis: a 6-year analysis in a neonatal care unit in Taiwan. Pediatrics & Neonatology. 2009;50(3):88–95. doi: 10.1016/S1875-9572(09)60042-5. [DOI] [PubMed] [Google Scholar]
- 49.Tsai M.-H., Hsu J.-F., Chu S.-M., et al. Incidence, clinical characteristics and risk factors for adverse outcome in neonates with late-onset sepsis. Pediatric Infectious Disease Journal. 2014;33(1):e7–e13. doi: 10.1097/INF.0b013e3182a72ee0. [DOI] [PubMed] [Google Scholar]
- 50.Lim W. H., Lien R., Huang Y.-C., et al. Prevalence and pathogen distribution of neonatal sepsis among very-low-birth-weight infants. Pediatrics and Neonatology. 2012;53(4):228–234. doi: 10.1016/j.pedneo.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 51.Ballot D. E., Bosman N., Nana T., Ramdin T., Cooper P. A. Background changing patterns of neonatal fungal sepsis in a developing country. Journal of Tropical Pediatrics. 2013;59(6):460–464. doi: 10.1093/tropej/fmt053.fmt053 [DOI] [PubMed] [Google Scholar]
- 52.Chen S., Slavin M., Nguyen Q., et al. Active Surveillance of Candidemia, Australia. Emerging Infectious Diseases. 2006;12(10):1508–1516. doi: 10.3201/eid1210.060389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clerihew L., Lamagni T. L., Brocklehurst P., McGuire W. Candida parapsilosis infection in very low birthweight infants. Archives of Disease in Childhood: Fetal and Neonatal Edition. 2007;92(2):F127–F129. doi: 10.1136/fnn.2006.097758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim S.-K., El Bissati K., Mamoun C. B. Amino acids mediate colony and cell differentiation in the fungal pathogen Candida parapsilosis. Microbiology. 2006;152(10):2885–2894. doi: 10.1099/mic.0.29180-0. [DOI] [PubMed] [Google Scholar]
- 55.Lattif A. A., K. Mukherjee P., Chandra J., et al. Characterization of biofilms formed by Candida parapsilosis, C. metapsilosis, and C. orthopsilosis. International Journal of Medical Microbiology. 2010;300(4):265–270. doi: 10.1016/j.ijmm.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 56.Pappas P. G., Kauffman C. A., Andes D. R., Clancy C. J., Marr K. A., Ostrosky-Zeichner L., et al. Clinical practice guideline for the management of candidiasis: 2016 update by the infectious diseases society of America. Clinical Infectious Diseases. 2016;62:e1–50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brion L. P., Uko S. E., Goldman D. L. Risk of resistance associated with fluconazole prophylaxis: Systematic review. Journal of Infection. 2007;54(6):521–529. doi: 10.1016/j.jinf.2006.11.017. [DOI] [PubMed] [Google Scholar]


