Abstract
Hematopoietic stem/progenitor cells (HSPCs) respond robustly to α-chemokine stromal derived factor-1 (SDF-1) gradients and blockage of CXCR4, a seven-transmembrane-spanning GαI protein-coupled SDF-1 receptor, mobilizes HSPCs into peripheral blood (PB). While the SDF-1–CXCR4 axis plays an unquestionably important role in the retention of HSPCs in bone marrow (BM), new evidence shows that, in addition to SDF-1, the migration of HSPCs is directed by gradients of the bioactive lipids sphingosine-1 phosphate (S1P) and ceramide 1-phosphate (C1P). Furthermore, the SDF-1 gradient may be positively primed/modulated by cationic peptides (C3a anaphylatoxin and cathelicidin) and, as previously demonstrated, HSPCs respond robustly, even to very low SDF-1 gradients in the presence of priming factors. In this review, we discuss the role of bioactive lipids in stem cell trafficking and the consequences of HSPC priming by cationic peptides. Together, these phenomena support a picture in which the SDF-1–CXCR4 axis modulates homing, BM-retention, and mobilization of HSPCs in a more complex way than previously envisioned.
Keywords: S1P, C1P, SDF-1, CXCR4, C3a, cathelicidin
Introduction
Hematopoietic stem progenitor cells (HSPCs) are retained in bone marrow (BM) niches due to the stromal-derived growth factor-1 (SDF-1)–CXCR4 receptor axis and interactions between Very Late Antigen-4 (VLA-4, also known as α4β1 integrin) and its ligand, Vascular Adhesion Molecule-1 (VCAM-1, also known as CD106). While HSPCs express CXCR4 and VLA-4, their corresponding ligands, SDF-1 and VCAM-1, are expressed by cells in the BM microenvironment (e.g., osteoblasts and fibroblasts).1,2 In addition, some other adhesion molecules, as well as growth-factor–growth-factor receptor axes, e.g., kit ligand (KL)–c-kit receptor, play an additional role in retention of HSPCs in BM.3 It is significant that all of these ligand-receptor axes are susceptible to degradation by proteolytic enzymes secreted in BM during both myeloablative conditioning for transplantation, as well as during stem cell mobilization.4–6
While a role for the SDF-1–CXCR4 axis in retention of HSPCs in BM under steady state conditions is undisputed, its role in stem cell trafficking (homing and mobilization) needs further clarification. For many years a “tug of war” hypothesis has been proposed to explain how a chemotactic SDF-1 gradient across the BM–peripheral blood (PB) barrier determines whether cells will be released and mobilized from BM into PB or home back from PB into the BM microenvironment.7–9
However, this simple explanation has been challenged by several observations10–13 supporting SDF-1–CXCR4-independent homing and mobilization. In particular, i) CXCR4−/− fetal liver HSPCs may home to BM in an SDF-1-independent manner,10 ii) homing of murine HSPCs made refractory to SDF-1 by incubation and co-injection with a CXCR4 receptor antagonist (AMD3100) is normal or only mildly reduced,11 iii) HSPCs in which CXCR4 has been knocked down by means of an SDF-1 intrakine strategy are able to engraft, even in lethally irradiated recipients,12 and iv) myeloablative conditioning for transplantation induces a highly proteolytic microenvironment in BM that leads to proteolytic degradation of SDF-1.13 Furthermore, as reported recently in HSPC mobilization studies,9,14,15 SDF-1 does not increase significantly during mobilization into PB and changes in plasma SDF-1 level correlate poorly with egress of HSPCs from BM.
All this evidence strongly suggests the involvement of other factors and/or the existence of mechanisms besides changes in the SDF-1 gradient between BM and PB in the mobilization and homing of HSPCs. In this review, we will present the cumulative evidence that gradients of the bioactive sphingophospholipids, such as sphingosine-1-phosphate (S1P)7–9,16 and ceramide-1-phosphate (C1P),13,17,18 which are products of membrane lipid metabolism, are involved in stem cell trafficking. In addition, we discuss the “priming effect” which is another important mechanism that enhances stem cell response to an SDF-1 gradient. This mechanism results from a phenomenon in which some molecules significantly increase the chemotactic responsiveness of HSPCs to very low SDF-1 gradients.19 At the molecular level, this sensitization of the responsiveness to SDF-1 depends on incorporation of the CXCR4 receptor into membrane lipid rafts, which is promoted, for example, by cationic peptides, such as C3a anaphylatoxin and cathelicidin (LL-37).19–22
All these changes in SDF-1, S1P, and C1P chemotactic gradients and the appearance of priming factors in the BM microenvironment and PB plasma are triggered by the induction of a proteolytic microenvironment in BM and activation of the complement cascade (CC), which occurs during conditioning for transplantation by radio-chemotherapy13 or after administration of drugs that mobilize HSPCs (e.g., by granulocyte-colony stimulating factor [G-CSF]).4
Novel Chemoattractants for HSPCs beyond SDF-1
HSPCs respond to chemotactic gradients, migrating during ontogenesis to populate different anatomical sites of active lympho-hematopoiesis (e.g., fetal liver, spleen, thymus, and BM), as well as in adult life, and can be detected in PB even under steady-state conditions.23 It is accepted that HSPCs circulating in PB are involved in immune surveillance, acting as “paramedics” that can, for example, proliferate locally in infected tissues to supply granulocytes, monocytes and dendritic cells.14,15 In adult organisms, circulating stem cells i) show a circadian rhythm in the circulation, with the peak occurring early in the morning and the nadir at night,24 ii) are mobilized in response to strenuous exercise,25 inflammation,14,26 and tissue/organ injury (for example, heart infarct or stroke),27–30 and iii) may increase in number up to 100-fold after the administration of certain cytokines and pharmacological drugs.30,31
It is well known that migration of stem cells is directed by the family of cytokines with chemotactic properties, which are called chemokines. While more than 50 different chemokines and more than 20 G protein-coupled seven-transmembrane-spanning chemokine receptors have been cloned, of all the known chemokine–chemokine receptor axes, only the SDF-1–CXCR4 axis has been shown to direct migration of HSPCs.32,33 For many years in the chemokine field, this axis was believed to be a unique exception to the rule that different chemokine receptors are engaged by more than one chemokine and, vice versa, that chemokines bind to more than one receptor.32 It was therefore believed that SDF-1 is the only ligand for CXCR4 and that CXCR4 binds exclusively to SDF-1.33,34 This concept has been recently challenged35 by the demonstration that SDF-1 binds to CXCR735 and CXCR4 may also bind to another chemokine, macrophage migration inhibitory factor (MIF).36 However, since the SDF-1–CXCR7 and MIF–CXCR4 axes are not involved in chemotaxis of HSPCs,35,36 the SDF-1–CXCR4 axis remains, so far, as the only one directly involved in stem cell trafficking.
While this axis can drive stem cell chemotaxis, most of the studies on the chemotactic responsiveness of HSPCs to SDF-1 employed SDF-1 at supra-physiological concentrations (100–300 ng/ml).11,37 However, direct measurement of the SDF-1 level in PB plasma revealed that, in both humans and mice, its concentration in biological fluids is ~100 times lower (~1–3 ng/ml).38 It is also known that the promoter of the SDF-1 gene contains hypoxia inducible factor-1α (HIF-1α) binding sites, and that SDF-1 is upregulated in situations of tissue injury as a result of local hypoxia.39 To some degree, this occurs in the BM microenvironment during, for example, conditioning for transplantation by radio-chemotherapy or after administration of pharmacological agents that promote mobilization of HSPCs (e.g., G-CSF).5,13,40 However, since the same conditions induce a proteolytic microenvironment in BM due to the release of several proteolytic enzymes from myeloid cells and stromal fibroblasts. Both SDF-1 and CXCR4 contain peptide bonds and are susceptible to proteolytic degradation.5,6,13 On the one hand, induction of a proteolytic environment in BM facilitates the mobilization process (decreases SDF-1–CXCR4-mediated retention of HSPCs in BM)5,6 and, on the other hand, it impairs the SDF-1 homing gradient in BM after conditioning for transplantation.13 These observations imply that other factors somehow compensate for a decrease in the SDF-1 gradient between BM and plasma.
In support of this notion, new potent chemoattractants for HSPCs, such as proteolytic enzyme-resistant sphingophospholipids, have been identified.8,9 Sphingophospholipids are important components of cell membranes; they are derived from the aliphatic amino alcohol sphingosine and its acylated derivative ceramide which are precursors for the bioactive derivatives sphingosine-1-phosphate (S1P) and ceramide-1-phosphate (C1P)13 that strongly chemoattract HSPCs. S1P is a product of two sphingosine phosphatases (SK1 and SK2), is released from cells by a transporter-facilitated process, and interacts with at least five Gαi protein-coupled seven-transmembrane-spanning receptors, S1P1–5, on the surface of target cells.41–43 While S1P1 and S1P3 receptors are most important in promoting the migration of HSPCs,14,44,45 S1P2 may have opposing function.46 Of note, S1P1–5 receptors are rapidly internalized from the cell surface after binding S1P, which is similar to the internalization of CXCR4 after binding SDF-1 (Figure 1).47 Structurally related to S1P, C1P is another bioactive lipid that can be generated by phosphorylation of ceramide (N-acyl sphingosine) by ceramide kinase (CERK).48 Unlike ceramide (which is often pro-apoptotic),49,50 C1P has been reported to promote cell growth, survival, and migration through an unknown receptor-initiated signaling pathway that is pertussis toxin-sensitive and therefore likely to involve a Gαi protein-coupled seven-transmembrane-spanning receptor.18,51 The receptor/s for C1P, however have not yet been identified although these are clearly distinct from the known S1P receptors.
Figure 1. S1P receptors on BM HSPCs are downregulated by S1P exposure.
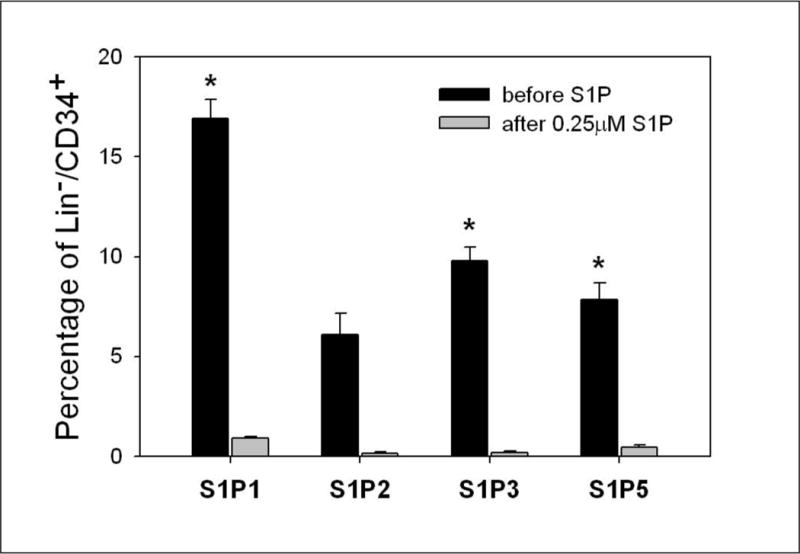
BM-derived stem/progenitor cells were identified by their expression of the CD34 marker and the lack of expression of the committed lineage (Lin) markers. At baseline, Lin−CD34+ cells isolated from the BM express S1P receptors, with the highest percentage of cells expressing S1P receptor-1. After incubation with S1P at 0.25 μM for 2 hours, the expression of S1P receptors is reduced dramatically due to the internalization of the occupied receptors (*P < 0.05).
Since the plasma S1P level is relatively high (~1 μM) in mobilized PB (mPB) and umbilical cord blood (UCB), causing the S1P1–5 receptors to become internalized, mPB- and UCB-derived HSPCs, in contrast to BM-derived HSPCs, respond weakly to bioactive lipid gradients (Figure 2). However, the expression of these receptors on HSPCs and their responsiveness to S1P and C1P gradients is reestablished in culture medium free of both bioactive lipids.
Figure 2. Differences in responsiveness of BM-, mPB-, and UCB-derived HSPCs to SDF-1, S1P, and C1P gradients.
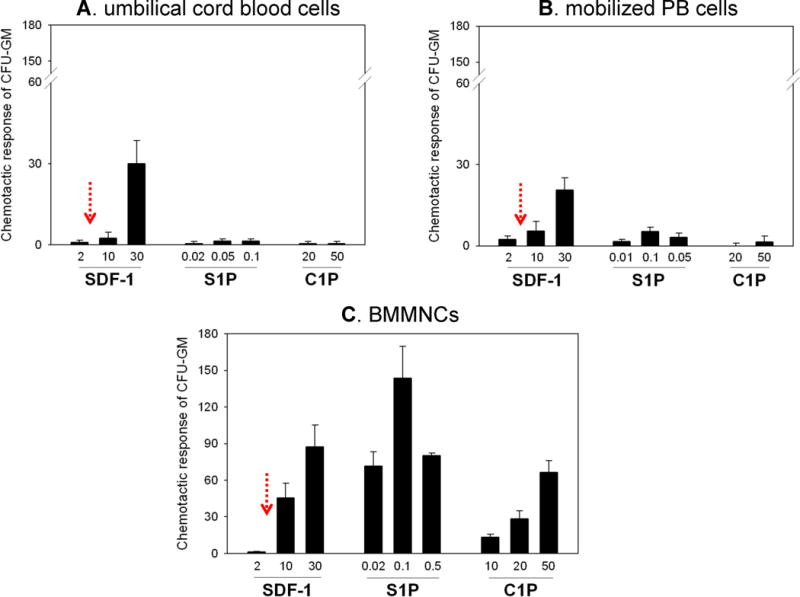
Mononuclear cells isolated from UCB (Panel A), murine mPB (Panel B), and murine BM (Panel C) were evaluated for the chemotactic activity of CFU-GM to SDF-1, S1P, and C1P gradients. The physiological concentration of SDF-1, as measured in plasma by ELISA, is indicated by red arrows. In contrast to their BM-derived counterparts, CFU-GM from UCB and mPB respond poorly to bioactive lipids. This is explained by downregulation of S1P receptors in the presence of elevated levels of S1P in mPB and UCB plasma. The data shown represent the combined results from three independent experiments carried out in triplicate per group (n=9).
Sphingolipids have well-characterized roles in intracellular membrane function, but it is now widely appreciated that extracellularly secreted S1P, and possibly C1P, are specific cell-surface receptor-directed bioactive lipids involved in apoptosis and survival, proliferation, stress responses, and cell trafficking.18 Accordingly, S1P, has been identified as a chemottractant for hematopoietic progenitor cells,8,9,15 a regulator of trafficking of T lymphocytes between lymphoid organs and PB,52–54 a factor involved in egress of early B-lymphoid cell progenitors from BM,54,55 and a regulator in trafficking of myeloid progenitors between BM and peripheral organs.56 While S1P is released from cells as an important signaling molecule and in PB is transported by erythrocytes, platelets, albumin and high density lipoproteins (HDL), C1P is an intracellular second messenger released from “damaged leaky cells”, for example, by cells in the BM microenvironment, after myeloablative conditioning of BM for transplantation.13 It is also abundant in plasma in fraction of HDLs. C1P was initially identified as a chemottractant for monocytes17 and, as recently demonstrated, is also an important and underappreciated chemotactic factor involved in the homing of HSPCs to BM.13 While considering chemotactic gradients of S1P and C1P one has to remember that both bioactive lipids in order to reveal chemotactic potential have to be present in biological fluids as free “unbound” molecules.
Accordingly, our recent mass spectrometry (MS) analysis revealed that the major isoforms of C1P and S1P were detected at higher concentrations in supernatants harvested from irradiated BM than supernatants from non-irradiated BM and that free S1P concentration increases in PB plasma isolated from mice mobilized by G-CSF.13 Taken together with their potent chemotactic effects, these changes in concentration of bioactive lipids between BM and PB during homing and mobilization suggest that these factors play an important role in trafficking of HSPCs.13 However, both bioactive lipids have a limited half-life, and, while S1P is degraded by several enzymes, such as S1P lyase (SPL), lipid phosphate phosphatases (LPP1–3), and S1P-specific phosphatases (SPP1 and SPP2),57–65 C1P is degraded by LPP1–3.57,58 These pathways may terminate the effects of S1P and C1P on HSPC migration. Furthermore, as we mentioned above, HSPCs harvested from mPB or UCB that were previously exposed to high S1P concentrations in plasma internalize S1P receptors and need some time to re-express them on the cell surface to recover responsiveness to an S1P gradient. Of note, we observed that, in contrast to S1P and C1P, other members of the bioactive lipid family, such as lysophosphatidic acid (LPA) and lysophosphatidylcholine (LPC), do not show chemotactic activity against HSPCs.
More importantly, since several agonists and inhibitors of S1P receptors and enzymes involved in synthesis or degradation of S1P and C1P are available, this opens up new possibilities for positively modulating the responsiveness of HSPCs to S1P and C1P gradients. These tools may lead to more efficient strategies to improve both homing and mobilization of HSPCs, and these approaches are currently being tested in several murine models. Other convenient research tools are mice with various gene knockouts of S1P receptors and S1P- and C1P-processing enzymes.66–70
Activation of the complement cascade during conditioning for transplantation and stem cell mobilization leads to induction of a proteolytic microenvironment in BM
The complement cascade (CC) is an arm of innate immunity that senses tissue/organ injury and becomes activated, both in the BM microenvironment and in BM sinusoid plasma, in response to factors that perturb BM homeostasis, such as irradiation, cytostatics, and mobilizing drugs. The activated CC releases several bioactive cleavage fragments (e.g., anaphylatoxins C3a and C5a and soluble membrane attack complex C5b-C9). Next, in response to C5a and desArgC5a, BM fibroblasts and cells from granulo-monocytic lineages release several proteolytic enzymes into the BM microenvironment that attenuate SDF-1–CXCR4, VLA-4–VCAM1, and KL–c-kit interactions.3,71 As observed during G-CSF- or cyclophosphamide-induced mobilization, this promotes detachment of HSPCs from their osteoblastic and endothelial stem cell niches.40,71 The same mechanism, however, negatively affects the SDF-1 homing gradient, as observed following conditioning for transplantation by radio-chemotherapy.4,5,13
Besides promoting a proteolytic microenvironment in BM, activation of the CC also has other important implications for homing and mobilization. These effects are summarized in Table I. First, activation of the CC in the BM microenvironment and release of soluble fragments of C3 cleavage, C3a and its derivative desArgC3a, sensitizes the responsiveness of HSPCs to an SDF-1 gradient.73,74 Thus, during mobilization when SDF-1 becomes degraded by proteolytic enzymes, both soluble cleavage fragments of C3 (C3a and desArgC3a) can be considered as a last check-point to prevent uncontrolled release of HSPCs into PB. Second, a solid-phase cleavage fragment of C3, iC3b, which is released during CC activation in the BM microenvironment, becomes deposited on BM endothelium and stromal cells, tethers CR3 receptor (CD11b/CD18)-positive HSPCs, and facilitates their retention in the BM microenvironment. A distal product of CC activation, C5b-C9 (also known as the membrane attack complex [MAC]), also plays an important role in homing. In its non-lytic soluble form, MAC promotes engraftment of HSPCs by preventing a decrease in SDF-1 expression by BM fibroblasts and facilitates retention interactions between iC3b deposits and CR3 receptors on HSPCs.22
Table I.
The effects of CC cleavage fragments on stem cell trafficking
| Cleavage fragments | Released in BM microenvironment | Released in BM sinusoid plasma |
|---|---|---|
| C3a, desArgC3a | Increase responsiveness to BM SDF-1 gradients | Increase chemotactic responsiveness to BM sinusoid plasma SDF-1 gradients |
| C5a, desArgC5a | Stimulate release of proteolytic enzymes by BM fibroblasts and cells from the granulo-monocytic lineage | Induce egress of granulocytes and monocytes from BM to pave the way for subsequent egress of HSPCs |
| iC3b | Tethers CR3+ HSPCs in the BM microenvironment | Tethers CR3+ HSPCs in the BM microenvironment |
| Soluble C5b-C9 (MAC) | Increases secretion of SDF-1 by BM stromal cells and increases iC3b–CR3 tethering | Induces release of S1P from erythrocytes and platelets |
At the same time, activation of CC in BM sinusoid plasma leads to other phenomena. While C3 cleavage fragments (C3a and desArgC3a) sensitize the responsiveness of HSPCs to SDF-1 present in PB, C5 cleavage fragments, anaphylatoxins C5a and desArgC5a, are potent chemoattractants for BM granulocytes and monocytes,19 which are enriched for proteolytic enzymes and play an important role in proteolytic disintegration of the BM–PB sinusoid barrier. The granulocytes and monocytes that egress from BM into PB in response to a C5a/desArgC5a gradient “pave the way” across the endothelial barrier for HSPCs.19 Finally, soluble C5b-C9 (sMAC), as the final product of CC activation in sinusoid plasma, stimulates release of S1P from its principal reservoir in PB, which is erythrocytes.9 As reported previously, this free plasma S1P is a major chemottractant that directs egress of HSPCs from BM into PB.8,9
These important interactions between two ancient, evolutionarily conserved systems, activation of the CC and release of bioactive lipids in the context of HSPC trafficking, are discussed in more detail below.
Bioactive lipids, the complement cascade, and stem cell homing
Homing is the process in which HSPCs circulating in PB lodge in their niches including the BM microenvironment. Lodging of HSPCs after transplantation is followed by their engraftment in the BM microenvironment, which leads to reestablishment of new hematopoiesis.10,23,75 If HSPCs are to be infused intravenously in large numbers, as seen, for example, during allogeneic transplantation, an important preceding step is myeloblative conditioning of the recipient by radio-chemotherapy i) to destroy old pathological hematopoiesis and ii) to empty stem cell niches to accommodate newly transplanted HSPCs. As mentioned above, this step activates the CC, which induces a proteolytic microenvironment in BM.13 The importance of the CC in homing of HSPCs has been demonstrated in complement component-deficient mice. In particular, while mice that are deficient in C3 and C5 engraft less successfully with HSPCs from wild type (wt) animals,19,22,76 HSPCs from C3a receptor (C3aR)-deficient mice show defective engraftment in wt littermates.76
As mentioned above, activation of the CC in BM induces a highly proteolytic microenvironment that degrades SDF-1, which has been accepted for many years as the only major homing factor for HSPCs.13 However, in the introductory section we mentioned that doubts have accumulated about whether SDF-1 is the only homing factor responsible for HSPC lodgment into BM.5,13
In further support of these doubts, we found that media conditioned by cells recovered from murine long bones 24 hours after lethal irradiation strongly chemoattract HSPCs in an SDF-1-independent manner.13 In particular, we observed that i) chemotaxis occurred in the presence of the CXCR4 antagonist AMD3100 and ii) it was resistant to heat inactivation.9,13 Based on these findings and data from the literature,8,9,13,17 we became interested in bioactive lipids. Subsequently, we found that S1P and C1P are upregulated in BM conditioned for transplantation and are present at biologically relevant concentrations in conditioned media harvested from irradiated BM that chemoattracts HSPCs.13 Based on these findings, we proposed that S1P and C1P are able to support the SDF-1 homing gradient, which decreases after induction of a proteolytic microenvironment by conditioning for transplantation (Figure 3).13 We also postulated that S1P and C1P explain SDF-1-independent homing of transplanted HSPCs.9,13
Figure 3. The concept of a chemotactic tug-of-war gradient between BM and PB explains mobilization and homing of HSPCs.
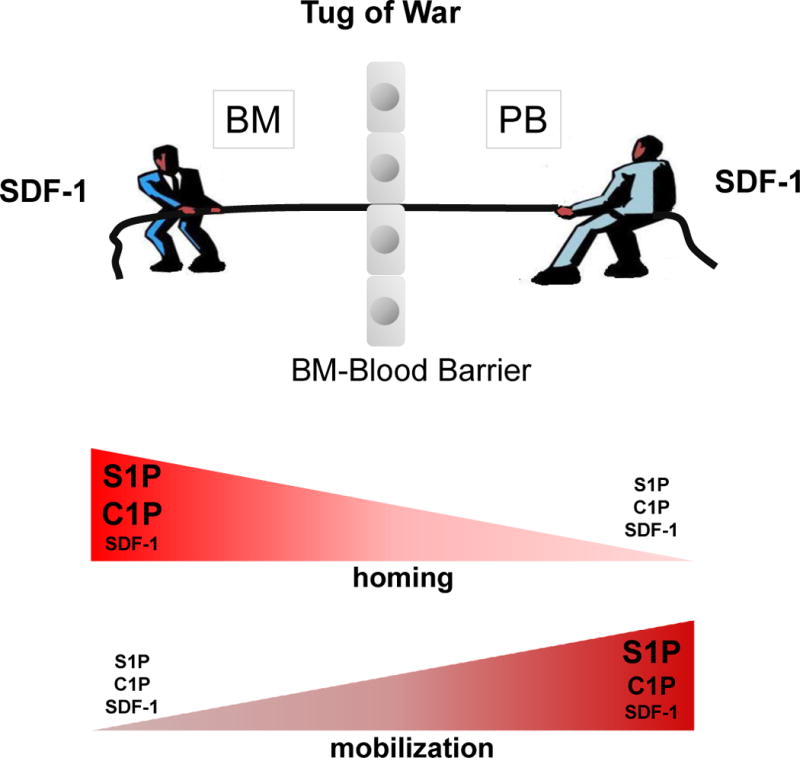
It has been postulated that an SDF-1 gradient between BM and PB regulates trafficking of HSPCs (homing vs. mobilization). Under steady-state conditions, this gradient should be in balance. New evidence indicates that, rather than changes in the SDF-1 gradient across the BM–PB barrier, upregulation of the S1P and C1P concentrations on either side of the barrier play an important role in homing or mobilization of HSPCs. While considering chemotactic gradients of S1P and C1P one has to remember that both bioactive lipids in order to reveal chemotactic potential i) have to be present in biological fluids as free “unbound” molecules and ii) and their gradient is also influenced by degrading enzymes. Not shown in this figure is that, in addition to upregulation of bioactive lipids during mobilization or homing, some priming molecules related to CC activation (e.g., C3a), as well as granulocyte-derived cationic peptides (e.g., cathelicidin/LL-37 and β2-defensin) may also sensitize the responsiveness of HSPCs to low SDF-1 levels and thus powerfully modulate trafficking of HSPCs.
Based on these findings, a new paradigm is emerging in which bioactive lipids play a crucial role in homing of transplanted HSPCs.9,13 Further studies are needed to confirm this phenomenon in (i) S1P- and C1P-deficient mice, (ii) transplants where S1P1 or S1P3 receptors are blocked on wt HSPCs by small molecule inhibitors, or (iii) HSPCs from S1P1−/− and S1P2−/− animals employed in grafting. It would also be interesting to evaluate homing of HSPCs in mice that have SPK1 or SPK2 deficiency (double SPK1 and SPK2 deficiency are embryonic lethal).66,67,69,70 On the other hand, it might also be possible to increase the intra-BM level of S1P by blocking S1P lyase with 4-deoxypyridoxine (DOP) or with the small molecule tetrahydroxybutylimidazole (THI),9,59,77 and this could be another strategy for increasing the S1P homing gradient in BM (Figure 4).
Figure 4. Potential strategies to improve homing of HSPCs by targeting the S1P–S1P receptors axes.
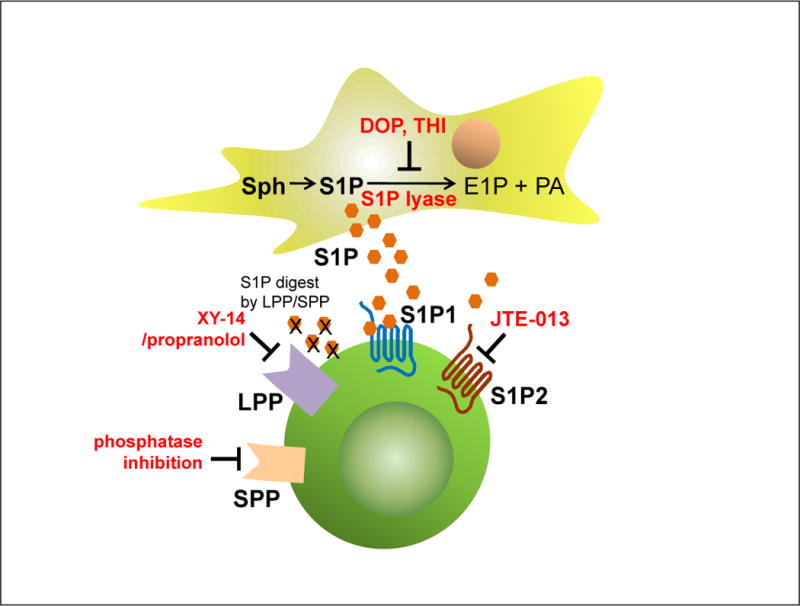
S1P-mediated homing of HSPCs could be improved by upregulating the S1P level in BM of the transplant recipients with DOP or THI, by blockade of S1P2 on transplanted donor cells (with JTE-013), or by inhibiting S1P-degrading enzymes (i.e., LPP and SPP receptors with, e.g., XY-14 analogues) on donor HSPCs. All these strategies await experimental confirmation.
Bioactive lipids in stem cell mobilization
Enhanced egress of HSPCs from the BM into PB is a part of the stress response observed in infections, strenuous exercise, and tissue and organ damage (e.g., in heart infarct or stroke).27–30,78 It can also be achieved after administration of certain drugs. Pharmacological mobilization has been exploited in hematological transplantation field as a means to obtain HSPCs for hematopoietic reconstitution.40,79,80 HSPCs circulating in PB are currently a preferred source of stem cells for transplantation, because they are easily accessible and—what is important from a clinical point of view—they also engraft faster after transplantation than HSPCs harvested from the BM under steady-state conditions.23 The most important mobilizing drugs currently employed in the clinic and in experimental animals models are (a) cytokines (e.g., G-CSF), (b) cytostatics (e.g., cyclophosphamide), (c) CXCR4- or VLA-4-blocking molecules (AMD3100 or BIO4860, respectively), and (d) certain chemokines (e.g., the growth-related oncogene protein-beta [Gro-β]).6,16,40,81
It is accepted that the mobilization process is related to i) a decrease in the SDF-1–CXCR4 axis and VLA-4 integrin–VCAM-1 interactions in BM (e.g., due to release of proteolytic enzymes or after molecular blockage after administration of small molecule antagonists against CXCR4 or VLA-4), ii) release of neurotransmitters that stimulate dopamine and β2-adrenergic receptors at the synapses of the nerves that innervate BM, iii) activation of the coagulation cascade (e.g., by release of uPAR), and finally, iv) activation of the CC.15,31,82–85 Two questions remain: which of the mechanisms is most important, and are they specific to the mobilizing agent employed? Regardless of the specific, the finding that activation of the CC occurs in all mobilization protocols investigated so far15 supports the concept that mobilization of HSPCs is a part of the innate immune response to different stress situations.15
As mentioned above, for many years a tug of war has been postulated, in which changes in SDF-1 level between BM and PB compartments orchestrate HSPC mobilization (Figure 3). However, ELISA measurements of the SDF-1 level in PB have revealed that it does not change significantly during mobilization, and there are no significant differences in plasma SDF-1 concentrations between good and poor mobilizers.13
In light of recent evidence, this process seems to be more complex and involves activation of distal parts of the CC and generation of soluble C5b-C9 (MAC), which releases free S1P, a major chemottractant for BM-residing HSPCs,9,13 from erythrocytes into plasma. As shown in Table I, activation of the CC has pleiotropic effects on retention and egress of HSPCs. Cleavage fragments from the proximal part of the CC, such as the cationic peptides C3a and desArgC3a, increase the responsiveness of HSPCs to an SDF-1 gradient73,74 and positively modulate the chemotactic effects of SDF-1 on both sides of the BM–blood barrier. In addition, a similar effect is observed in the presence of other cationic peptides, such as cathelicidin (LL-37) or β2-defensin, which are activated by CC and released from BM stroma and granulocytes.19
Our data in C5-deficient mice indicate the crucial involvement of a distal part of the CC cascade in egress of HSPCs from BM. In support of this finding, C5-deficient mice, which do not generate C5a and desArgC5a anaphylatoxins, do not activate the distal part of the CC, and lack C5b-C9 (soluble MAC), display a profound defect in HSPC mobilization.19 This effect of the distal part of CC activation can be explained (Table I) by the fact that C5a and desArgC5a released in the BM microenvironment (upstream from the BM–PB barrier) induce release of proteolytic enzymes from BM stroma and myeloid cells that are involved in detachment of HSPCs from their niches.19 In addition, activation of the CC in BM sinusoids (downstream from the BM–PB barrier) generates C5a and desArgC5a, which direct egress of granulocytes and monocytes. These latter cells are highly enriched in proteolytic enzymes (up to 100 times more than in HSPCs) and thus pave the way across the endothelial barrier to facilitate the egress of HSPCs from BM into PB.19 However, in addition to C5 cleavage fragments, some chemokines that modulate migration of granulocytes and monocytes are also involved in this process.40
Nevertheless, C5a and desArgC5a anaphylatoxins are well known and the most potent chemoattractants and activators of granulocytes and monocytes.19 The involvement of the C5a–C5a receptor (C5aR) axis in mobilization is further supported by the fact that C5aR−/− mice exhibit significant defects in mobilization of HSPCs.19 This could be explained by less effective activation and egress of granulocytes and monocytes from BM in C5aR−/− animals, which supports an important involvement of C5aR+ granulocytes and monocytes in mobilization.19 However, a direct role of the C5a–C5aR axis in egress of HSPCs must be confirmed in wild type (wt) mice reconstituted with C5aR−/− HSPCs. Poor mobilization in these animals would support a crucial role of C5 cleavage fragments in egress of granulocytes and monocytes.
On other hand, the fact that C5−/− mice display a more profound defect in mobilization than C5aR−/− animals86 points to an important involvement of the distal part of CC activation and generation of soluble C5b-C9 (MAC) in this process. It has been demonstrated that this distal product of CC activation in BM sinusoids is responsible for release of S1P from erythrocytes (the main reservoir for this bioactive lipid), and is a major chemoattractant of HSPCs in mobilized PB.7,9
The release of S1P occurs during activation of CC in BM sinusoids, despite the fact that erythrocytes are protected from MAC influence by CD55 (also known as decay accelerating factor [DAF]) and CD59 (also known as membrane inhibitor of reactive lysis [MIRL]) proteins, which are both expressed on the surface of erythrocytes and involved in the regulation of complement activation.87–89 A major role played here is by CD59 (DAF), which inhibits formation of MAC by preventing C9 binding to C5b, C6, C7, and C8.87 On the other hand, CD55 increases the removal of the complement protein C3 convertase, thereby reducing the amount of C3 that is cleaved for subsequent activation of the distal part of the CC and generation of MAC.88 This S1P-mediated mechanism regulating egress of HSPCs from BM may also play a leading role in chronic mobilization of HSPCs in patients suffering from sickle cell anemia or paroxysmal nocturnal hemoglobinuria (PNH). We envision that S1P released from erythrocytes during hemolytic crises is a major factor that mobilizes HSPCs in these patients.7,9
Based on these findings, it is attractive to propose that S1P is a major chemottractant for HSPCs in BM sinusoids and its release from erythrocytes is regulated by CC activation in a soluble MAC-dependent manner.9 S1P could also be released from activated platelets and endothelial cells. In support of this possibility, it is well known that the mobilization process also activates, in parallel to CC, the coagulation cascade.85 On the other hand, C5a anaphylatoxin released during CC activation stimulates and increases the permeability of BM endothelium.19,71,86,90 Thus, both thrombin-activated platelets and endothelial cells in response to CC activation may, in addition to erythrocytes, release S1P into BM sinusoids.91 However, the contribution of these other sources of S1P to mobilization require further study. Finally, we observed that S1P receptors are internalized after exposure to S1P (Figure 1), which may explain why, in contrast to “virgin” HSPCs isolated from BM, HSPCs isolated from mPB or from UCB display very poor chemotactic responsiveness to S1P and C1P (Figure 2). This, however can be reversed if the cells are washed of their surrounding plasma, which is enriched in bioactive lipids.
The priming effect may turn low SDF-1 levels into potent chemotactic gradients
Chemotaxis of cells can be easily evaluated in the trans-well migration assay where two chambers (an upper chamber containing tested cells and a lower chamber containing chemoattractant) are separated by a porous membrane that allows transmigration of cells in response to the chemotactic gradient. Cells that respond to this gradient migrate and accumulate in the lower chamber (Figure 5). Interestingly, as mentioned above, most of the studies on chemotactic responsiveness of HSPCs to SDF-1 were performed when SDF-1 was employed at supra-physiological (~100-fold higher than physiological) concentrations.13
Figure 5. A priming effect increases the responsiveness of HSPCs to low SDF-1 gradients.
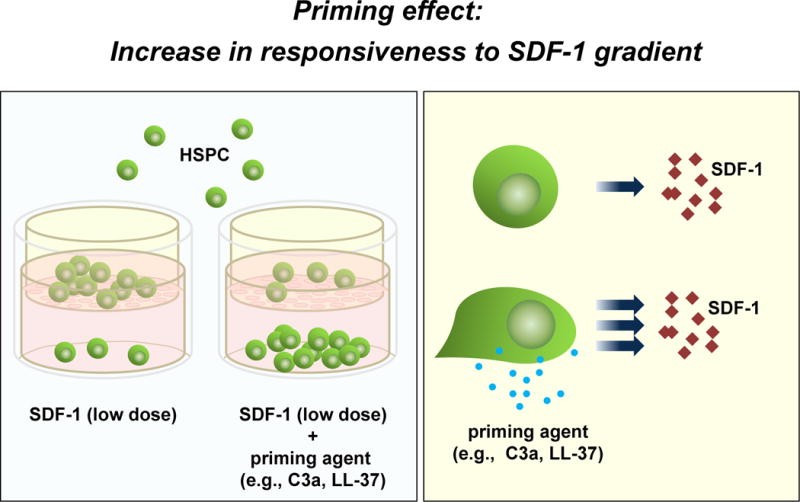
Left panel: The overall scheme of chemotactic assays performed in the Transwell system to evaluate the HSPC priming phenomenon. Right panel: In the presence of a priming agent (e.g., C3a or cathelicidin), HSPCs respond more robustly to low doses of SDF-1. This phenomenon is currently being tested in the clinic, where UCB are exposed ex vivo to a priming agent (C3a cationic peptide) before transplantation.
As discussed above, our recent findings indicate that the SDF-1 level does not significantly change in PB during HSPC mobilization13 and decreases due to the proteolytic microenvironment in BM after conditioning for transplantation by lethal irradiation.13 Moreover, since a few amino acids located at the N-terminus of this peptide are crucial for the biological activity of SDF-1,11,92 we observed that removal of this peptide fragment, for example, by metalloproteinase-2 (MMP-2) or MMP-9, does not affect detection of the SDF-1 protein in tissues by antibodies (e.g., in an ELISA assay or histochemistry) targeted to other fragments of the SDF-1 peptide. Thus, antibody-based SDF-1 detection does not correlate with the chemotactic activity of SDF-1,13 unless antibodies are specifically directed to its N-terminus and do not interact with inactive forms of SDF-1.13
On the other hand, as mentioned above, the responsiveness of HSPCs to SDF-1 could be enhanced (Figure 5) by some cationic peptides,19 such as C3 cleavage fragments (C3a and desArgC3a), as well as by cathelicidin and β2-defensin released by C5a and sMAC- activated stroma and granulocytes.19,22,73 This priming phenomenon depends on promoting the incorporation of CXCR4 into membrane lipid rafts.19 Since, membrane lipid rafts are enriched for several signaling molecules, incorporation of CXCR4 into lipid raft facilitates signaling,22 and thus CXCR4 is activated more efficiently in the presence of low doses of SDF-1.
Further studies are needed to see whether, in addition to CXCR4, S1P receptors are also lipid raft-regulated. It has been reported that stimulation of the S1P1 receptor by its agonist, FY720, may also increase the responsiveness of HSPCs to an SDF-1 gradient.93 However, this probably occurs due to intercellular crosstalk between CXCR4 and S1P receptors. Since a receptor for another bioactive lipid, C1P, has not yet been identified, it is not clear whether C1P signaling is also lipid raft-regulated. Our data indicate that this receptor is expressed on HSPCs and is sensitive to pertussis toxin, which suggests that, like S1P, it is a Gαiprotein-coupled receptor.13
In addition to cationic peptides, some other small molecules (e.g., prostaglandin E2 [PGE2] or hylauronic acid) have also been purported to increase responsiveness of HSPCs to an SDF-1 gradient.94,95 Of note, PGE2 is also a bioactive lipid derivative and, as previously reported, plays an important role in homing of HSPCs by upregulating expression of CXCR4 on HSPCs.93 This mechanism responsible for increasing chemotaxis in response to an SDF-1 gradient after pretreatment of HSPCs by PGE2.94 Interestingly, it has been reported that C5a and S1P modulate the activity of coxygenase-2 (COX2) and thus affect synthesis of PGE2 in BM,96 which explains why both S1P and C1P increase PGE2 activity in BM stromal cells9 and why an elevated PGE2 level is detectable in conditioned media harvested from irradiated BM cells.19 Thus, some of the effects of PGE2 in homing94 may be due to activation of CC and release of S1P and C1P. This, however, requires further study.
Translational aspects
All of this new knowledge about regulation of HSPC trafficking has important clinical implications for, on the one hand, how to modulate homing and engraftment of HSPCs and, on the other hand, how to promote more efficient mobilization of HSPCs to harvest enough cells for successful transplantation.
Firstly, the priming strategy of short ex vivo exposure of HSPCs to C3a or cathelicidin (LL-37) before transplantation may accelerate homing and engraftment of HSPCs.19 This strategy is currently under clinical evaluation by hematopoietic transplantation centers in Charlottesville, Virginia, USA and Minneapolis, Minnesota, USA. Accordingly, umbilical cord blood (UCB)-derived cells are primed for 30 minutes with recombinant C3a before infusion of the patients.
In optimizing mobilization protocols, it has been demonstrated that blockage of C3aR by a small molecular antagonist (SB 290157) could enhance mobilization in mice.76 This is explained by the fact that C3aR expressed on HSPCs increases adhesion and thus retention of HSPCs in BM.76 Based on this finding, further studies are needed to see whether poor mobilizers have defective or delayed activation of the CC that may affect egress of HSPCs from BM into PB.
Other potential translational strategies are related to modifying bioactive lipid gradients in BM and/or responsiveness of HSPCs to S1P (Figure 4). As mentioned above, the S1P homing level in BM of the transplant recipient might be increased by inhibiting S1P lyase using DOP or THI. This strategy, however, awaits experimental confirmation. Furthermore, since S1P2, in contrast to S1P1 and S1P3 receptors, has an inhibitory effect on the migration of stem cells,46 blockage of this receptor on HSPCs by the small molecular inhibitor JTE-013 could improve their engraftment after transplantation. There are also several inhibitors of S1P-degrading lipid phosphate phosphatases and S1P-specific phosphatases (SPP) under development.63,97 Since these enzymes are also expressed on the surface of HSPCs, their inhibitors, such as XY-14/propranolol analogues, could improve responsiveness of HSPCs to an S1P gradient. Finally, high concentrations of bioactive lipids in leucopheresis products or UCB plasma may desensitize the responsiveness of HSPCs to BM S1P and C1P homing gradients. Therefore, removal of these bioactive lipids from mPB and UCB-based grafts would increase the homing responsiveness of transplanted HSPCs to bioactive lipids.
We also propose that the in vitro chemotactic test based on responsiveness of HSPCs to S1P and C1P gradients could be introduced as a novel parameter to predict engraftment. A similar assay has been proposed in the past for SDF-1.13
Conclusions
Identification of new mechanisms that govern stem cell trafficking may have important implications, not only for hematopoietic transplants but also for cellular therapies in regenerative medicine (e.g., heart after infarct, spinal cord injuries, and stroke).
Overall, recent data provide more evidence that innate immunity and the CC regulate trafficking of HSPCs. In particular, C3a and sMAC i) enhance S1P and C1P levels in BM, ii) increase responsiveness of HSPCs to an SDF-1 gradient, and iii) promote S1P- and C1P-mediated adhesion of HSPCs in the BM microenvironment. Based on these findings, we propose modulation of CC and innate immunity components as a novel strategy for controlling both mobilization and homing of HSPCs. This could be achieved, for example, by (i) exposure of HSPCs before transplantation to some cationic peptides (e.g., C3a or cathelicidin) that enhance responsiveness of these cells to homing factors, (ii) modulating bioactive lipid levels in BM, or (iii) modulating the responsiveness of HSPCs to S1P and C1P gradients.
A new paradigm is emerging in which CC priming molecules and bioactive lipids play an important role in homing and mobilization of HSPCs. We also propose that, in addition to SDF-1, S1P and C1P play a widespread role in regulating migration of other types of stem cells, such as circulating mesenchymal stem cells, epithelial progenitor cells, and very small embryonic like stem cells (VSELs).13,98,99 Similar mechanisms of homing as those proposed for BM probably direct recruitment of non-hematopoietic stem cells in other types of organ injury, for example, in heart infarct or stroke.
Acknowledgments
This work was supported by NIH R01 DK074720, EU structural funds, KBN grant (N N401 024536), Innovative Economy Operational Program POIG.01.01.02-00-109/09-01, and the Henry M. and Stella M. Hoenig Endowment (to MZR)
Footnotes
Conflict of interest statement
The author declares no conflict of interests.
References
- 1.Levesque JP, Takamatsu Y, Nilsson SK, Haylock DN, Simmons PJ. Vascular cell adhesion molecule-1 (CD106) is cleaved by neutrophil proteases in the bone marrow following hematopoietic progenitor cell mobilization by granulocyte colony-stimulating factor. Blood. 2001;98:1289–1297. doi: 10.1182/blood.v98.5.1289. [DOI] [PubMed] [Google Scholar]
- 2.Peled A, Grabovsky V, Habler L, Sandbank J, Arenzana-Seisdedos F, Petit I, et al. The chemokine SDF-1 stimulates integrin-mediated arrest of CD34+ cells on vascular endothelium under shear flow. J Clin Invest. 1999;104:1199–1211. doi: 10.1172/JCI7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levesque JP, Hendy J, Winkler IG, Takamatsu Y, Simmons PJ. Granulocyte colony-stimulating factor induces the release in the bone marrow of proteases that cleave c-KIT receptor (CD117) from the surface of hematopoietic progenitor cells. Exp Hematol. 2003;31:109–117. doi: 10.1016/s0301-472x(02)01028-7. [DOI] [PubMed] [Google Scholar]
- 4.Levesque JP, Hendy J, Takamatsu Y, Williams B, Winkler IG, Simmons PJ. Mobilization by either cyclophosphamide or granulocyte colony-stimulating factor transforms the bone marrow into a highly proteolytic environment. Exp Hematol. 2002;30:440–449. doi: 10.1016/s0301-472x(02)00788-9. [DOI] [PubMed] [Google Scholar]
- 5.Levesque JP, Hendy J, Takamatsu Y, Simmons PJ, Bendall LJ. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J Clin Invest. 2003;111:187–196. doi: 10.1172/JCI15994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pelus LM, Bian H, King AG, Fukuda S. Neutrophil-derived MMP-9 mediates synergistic mobilization of hematopoietic stem and progenitor cells by the combination of G-CSF and the chemokines GRObeta/CXCL2 and GRObetaT /CXCL2delta4. Blood. 2004;103:110–119. doi: 10.1182/blood-2003-04-1115. [DOI] [PubMed] [Google Scholar]
- 7.Hanel P, Andre´ani P, Graler MH. Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB J. 2007;21:1202–1209. doi: 10.1096/fj.06-7433com. [DOI] [PubMed] [Google Scholar]
- 8.Seitz G, Boehmle AM, Kanz L, Möhle R. The role of sphingosine 1-phosphate receptors in the trafficking of hematopoietic progenitor cells. Ann N Y Acad Sci. 2005;1044:84–89. doi: 10.1196/annals.1349.011. [DOI] [PubMed] [Google Scholar]
- 9.Ratajczak MZ, Lee H, Wysoczynski M, Wan W, Marlicz W, Laughlin MJ, et al. Novel insight into stem cell mobilization-plasma sphingosine-1-phosphate is a major chemoattractant that directs the egress of hematopoietic stem progenitor cells from the bone marrow and its level in peripheral blood increases during mobilization due to activation of complement cascade/membrane attack complex. Leukemia. 2010;24:976–985. doi: 10.1038/leu.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma Q, Jones D, Springer TA. The Chemokine Receptor CXCR4 Is Required for the Retention of B Lineage and Granulocytic Precursors within the Bone Marrow Microenvironment. Immunity. 1999;10:463–471. doi: 10.1016/s1074-7613(00)80046-1. [DOI] [PubMed] [Google Scholar]
- 11.Christopherson IIKW, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of Hematopoietic Stem Cell Homing and Engraftment by CD26. Science. 2004;305:1000–1003. doi: 10.1126/science.1097071. [DOI] [PubMed] [Google Scholar]
- 12.Onai N, Zhang YY, Yoneyama H, Kitamura T, Ishikawa S, Matsushima K. Impairment of lymphopoiesis and myelopoiesis in mice reconstituted with bone marrow-hematopoietic progenitor cells expressing SDF-1-intrakine. Blood. 2000;96:2074–2080. [PubMed] [Google Scholar]
- 13.Kim CH, Wu W, Wysoczynski M, Abdel-Latif A, Sunkara M, Morris A, et al. Conditioning for hematopoietic transplantation activates the complement cascade and induces a proteolytic environment in bone marrow: a novel role for bioactive lipids and soluble C5b-C9 as homing factors. Leukemia. 2011 doi: 10.1038/leu.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Massberg S, Schaerli P, Knezevic-Maramica I, Köllnberger M, Tubo N, Moseman EA, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ratajczak MZ, Kim CH, Wojakowski W, Janowska-Wieczorek A, Kucia M, Ratajczak J. Innate immunity as orchestrator of stem cell mobilization. Leukemia. 2010;24:1667–1675. doi: 10.1038/leu.2010.162. [DOI] [PubMed] [Google Scholar]
- 16.Dar A, Schajnovitz A, Lapid K, Kalinkovich A, Itkin T, Ludin A, et al. Rapid mobilization of hematopoietic progenitors by AMD3100 and catecholamines is mediated by CXCR4-dependent SDF-1 release from bone marrow stromal cells. Leukemia. 2011 doi: 10.1038/leu.2011.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Granado MH, Gangoiti P, Ouro A, Arana L, González M, Trueba M, et al. Ceramide 1-phosphate (C1P) promotes cell migration Involvement of a specific C1P receptor. Cell Signal. 2009;21:405–412. doi: 10.1016/j.cellsig.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Arana L, Gangoiti P, Ouro A, Trueba M, Gómez-Muñoz A. Ceramide and ceramide 1-phosphate in health and disease. Lipids Health Dis. 2010;9:15–26. doi: 10.1186/1476-511X-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee HM, Wu W, Wysoczynski M, Liu R, Zuba-Surma EK, Kucia M, et al. Impaired mobilization of hematopoietic stem/progenitor cells in C5-deficient mice supports the pivotal involvement of innate immunity in this process and reveals novel promobilization effects of granulocytes. Leukemia. 2009;23:2052–2062. doi: 10.1038/leu.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukuoka Y, Hugli TE. Anaphylatoxin binding and degradation by rat peritoneal mast cells. Mechanisms of degranulation and control. J Immunol. 1990;145:1851–1858. [PubMed] [Google Scholar]
- 21.Mousli M, Hugli TE, Landry Y, Bronner C. A mechanism of action for anaphylatoxin C3a stimulation of mast cells. J Immunol. 1992;148:2456–2461. [PubMed] [Google Scholar]
- 22.Ratajczak MZ, Reca R, Wysoczynski M, Kucia M, Baran JT, Allendorf DJ, et al. Transplantation studies in C3-deficient animals reveal a novel role of the third complement component (C3) in engraftment of bone marrow cells. Leukemia. 2004;18:1482–1490. doi: 10.1038/sj.leu.2403446. [DOI] [PubMed] [Google Scholar]
- 23.Bonig H, Priestley GV, Oehler V, Papayannopoulou T. Hematopoietic progenitor cells (HPC) from mobilized peripheral blood display enhanced migration and marrow homing compared to steady-state bone marrow HPC. Exp Hematol. 2007;35:326–334. doi: 10.1016/j.exphem.2006.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simo´n MF, Andrew C, Miriam M, Paul SF. Circadian rhythms influence hematopoietic stem cells. Curr Opin Hematol. 2009;16:235–242. doi: 10.1097/MOH.0b013e32832bd0f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Möbius-Winkler S, Hilberg T, Menzel K, Golla E, Burman A, Schuler G, et al. Time-dependent mobilization of circulating progenitor cells during strenuous exercise in healthy individuals. J Appl Physiol. 2009;107:1943–1950. doi: 10.1152/japplphysiol.00532.2009. [DOI] [PubMed] [Google Scholar]
- 26.Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: Present and future therapeutic targets. Nat Immunol. 2005;6:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 27.Kucia M, Zhang YP, Reca R, Wysoczynski M, Machalinski B, Majka M, et al. Cells enriched in markers of neural tissuecommitted stem cells reside in the bone marrow and are mobilized into the peripheral blood following stroke. Leukemia. 2006;20:18–28. doi: 10.1038/sj.leu.2404011. [DOI] [PubMed] [Google Scholar]
- 28.Wojakowski W, Tendera M, Kucia M, Zuba-Surma E, Paczkowska E, Ciosek J, et al. Mobilization of bone marrow-derived Oct-4+ SSEA-4+ very small embryonic-like stem cells in patients with acute myocardial infarction. J Am Coll Cardiol. 2009;53:1–9. doi: 10.1016/j.jacc.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wojakowski W, Landmesser U, Bachowski R, Jadczyk T, Tendera M. Mobilization of stem and progenitor cells in cardiovascular diseases. Leukemia. 2011 doi: 10.1038/leu.2011.184. [DOI] [PubMed] [Google Scholar]
- 30.Paczkowska E, Kucia M, Koziarska D, Halasa M, Safranow K, Masiuk M, et al. Clinical evidence that very small embryonic-like stem cells are mobilized into peripheral blood in patients after stroke. Stroke. 2009;40:1237–1244. doi: 10.1161/STROKEAHA.108.535062. [DOI] [PubMed] [Google Scholar]
- 31.Lee HM, Wysoczynski M, Liu R, Shin DM, Kucia M, Botto M, et al. Mobilization studies in complement-deficient mice reveal that optimal AMD3100 mobilization of hematopoietic stem cells depends on complement cascade activation by AMD3100-stimulated granulocytes. Leukemia. 2010;24:573–582. doi: 10.1038/leu.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright DE, Bowman EP, Wagers AJ, Butcher EC, Weissman IL. Hematopoietic stem cells are uniquely selective in their migratory response to chemokines. J Exp Med. 2002;195:1145–1154. doi: 10.1084/jem.20011284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106:1901–1910. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- 34.Vagima Y, Lapid K, Kollet O, Goichberg P, Alon R, Lapidot T. Pathways implicated in stem cell migration: the SDF-1/CXCR4 axis. Methods Mol Biol. 2011;750:277–289. doi: 10.1007/978-1-61779-145-1_19. [DOI] [PubMed] [Google Scholar]
- 34.Tarnowski M, Liu R, Wysoczynski M, Ratajczak J, Kucia M, Ratajczak MZ. CXCR7: a new SDF-1-binding receptor in contrast to normal CD34(+) progenitors is functional and is expressed at higher level in human malignant hematopoietic cells. Eur J Haematol. 2010;85:472–483. doi: 10.1111/j.1600-0609.2010.01531.x. [DOI] [PubMed] [Google Scholar]
- 36.Tarnowski M, Grymula K, Liu R, Tarnowska J, Drukala J, Ratajczak J, et al. Macrophage migration inhibitory factor is secreted by rhabdomyosarcoma cells, modulates tumor metastasis by binding to CXCR4 and CXCR7 receptors and inhibits recruitment of cancer-associated fibroblasts. Mol Cancer Res. 2010;8:1328–1343. doi: 10.1158/1541-7786.MCR-10-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Basu S, Ray NT, Atkinson SJ, Broxmeyer HE. Protein phosphatase 2A plays an important role in stromal cell-derived factor-1/CXC chemokine ligand 12-mediated migration and adhesion of CD34+ cells. J Immunol. 2007;179:3075–3085. doi: 10.4049/jimmunol.179.5.3075. [DOI] [PubMed] [Google Scholar]
- 38.Gazitt Y, Liu Q. Plasma levels of SDF-1 and expression of SDF-1 receptor on CD34+ cells in mobilized peripheral blood of non-Hodgkin’s lymphoma patients. Stem Cells. 2001;19:37–45. doi: 10.1634/stemcells.19-1-37. [DOI] [PubMed] [Google Scholar]
- 39.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 40.Greenbaum AM, Link DC. Mechanisms of G-CSF-mediated hematopoietic stem and progenitor mobilization. Leukemia. 2011;25:211–217. doi: 10.1038/leu.2010.248. [DOI] [PubMed] [Google Scholar]
- 41.Lynch KR. Lysophospholipid receptor nomenclature. Biochim Biophys Acta. 2002;1582:70–71. doi: 10.1016/s1388-1981(02)00138-5. [DOI] [PubMed] [Google Scholar]
- 42.Sanchez T, Hla T. Structural and functional characteristics of S1P receptors. J Cell Biochem. 2004;92:913–922. doi: 10.1002/jcb.20127. [DOI] [PubMed] [Google Scholar]
- 43.Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol. 2008;8:753–763. doi: 10.1038/nri2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jo E, Sanna MG, Gonzalez-Cabrera PJ, Thangada S, Tigyi G, Osborne DA, et al. S1P1-selective in vivo-active agonists from high-throughput screening: off-theshelf chemical probes of receptor interactions, signaling, and fate. Chem Biol. 2005;12:703–715. doi: 10.1016/j.chembiol.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 45.Walter DH, Rochwalsky U, Reinhold J, Seeger F, Aicher A, Urbich C, et al. Sphingosine-1-phosphate stimulates the functional capacity of progenitor cells by activation of the CXCR4-dependent signaling pathway via the S1P3 receptor. Arterioscler Thromb Vasc Biol. 2007;27:275–282. doi: 10.1161/01.ATV.0000254669.12675.70. [DOI] [PubMed] [Google Scholar]
- 46.Michaud J, Im DS, Hla T. Inhibitory role of sphingosine 1-phosphate receptor 2 in macrophage recruitment during inflammation. J Immunol. 2010;184:1475–1483. doi: 10.4049/jimmunol.0901586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Graler MH, Goetzl EJ. The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G-protein-coupled receptors. FASEB J. 2004;18:551–553. doi: 10.1096/fj.03-0910fje. [DOI] [PubMed] [Google Scholar]
- 48.Boath A, Graf C, Lidome E, Ullrich T, Nussbaumer P, Bornancin F. Regulation and traffic of ceramide 1-phosphate produced by ceramide kinase: comparative analysis to glucosylceramide and sphingomyelin. J Biol Chem. 2008;283:8517–8526. doi: 10.1074/jbc.M707107200. [DOI] [PubMed] [Google Scholar]
- 49.Hannun YA, Obeid LM. The Ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J Biol Chem. 2002;277:25847–25850. doi: 10.1074/jbc.R200008200. [DOI] [PubMed] [Google Scholar]
- 50.Zheng W, Kollmeyer J, Symolon H, Momin A, Munter E, Wang E, et al. Ceramides and other bioactive sphingolipid backbones in health and disease: lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochim Biophys Acta. 2006;1758:1864–1884. doi: 10.1016/j.bbamem.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 51.Gomez-Munoz A, Kong JY, Salh B, Steinbrecher UP. Ceramide-1-phosphate blocks apoptosis through inhibition of acidsphingomyelinase in macrophages. J Lipid Res. 2004;45:99–105. doi: 10.1194/jlr.M300158-JLR200. [DOI] [PubMed] [Google Scholar]
- 52.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 53.Allende ML, Dreier JL, Mandala S, Proia RL. Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J Biol Chem. 2004;279:15396–15401. doi: 10.1074/jbc.M314291200. [DOI] [PubMed] [Google Scholar]
- 54.Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol. 2007;8:1295–1301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- 55.Pereira JP, Xu Y, Cyster JG. A role for S1P and S1P1 in immature-B cell egress from mouse bone marrow. PLoS One. 2010;5:e9277. doi: 10.1371/journal.pone.0009277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Donovan EE, Pelanda R, Torres RM. S1P3 confers differential S1P-induced migration by autoreactive and non-autoreactive immature B cells and is required for normal B-cell development. Eur J Immunol. 2010;40:688–698. doi: 10.1002/eji.200939858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brindley DN, English D, Pilquil C, Buri K, Ling ZC. Lipid phosphate phosphatases regulate signal transduction through glycerolipids and sphingolipids. Biochim Biophys Acta. 2002;1582:33–44. doi: 10.1016/s1388-1981(02)00135-x. [DOI] [PubMed] [Google Scholar]
- 58.Sciorra VA, Morris AJ. Roles for lipid phosphate phosphatases in regulation of cellular signaling. Biochim Biophys Acta. 2002;1582:45–51. doi: 10.1016/s1388-1981(02)00136-1. [DOI] [PubMed] [Google Scholar]
- 59.Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- 60.Long J, Darroch P, Wan KF, Kong KC, Ktistakis N, Pyne NJ, et al. Regulation of cell survival by lipid phosphate phosphatases involves the modulation of intracellular phosphatidic acid and sphingosine 1-phosphate pools. Biochem J. 2005;391:25–32. doi: 10.1042/BJ20050342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mechtcheriakova D, Wlachos A, Sobanov J, Kopp T, Reuschel R, Bornancin F, et al. Sphingosine 1-phosphate phosphatase 2 is induced during inflammatory responses. Cell Signal. 2007;19:748–760. doi: 10.1016/j.cellsig.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 62.Zhao Y, Kalari SK, Usatyuk PV, Gorshkova I, He D, Watkins T, et al. Intracellular generation of sphingosine 1-phosphate in human lung endothelial cells: role of lipid phosphate phosphatase-1 and sphingosine kinase 1. J Biol Chem. 2007;282:14165–14177. doi: 10.1074/jbc.M701279200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 64.Hannun YA, Obeid LM. Principles of bioactive lipid signaling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 65.Peest U, Sensken SC, Andréani P, Hänel P, Van Veldhoven PP, Gräler MH. S1P-lyase independent clearance of extracellular sphingosine 1-phosphate after dephosphorylation and cellular uptake. J Cell Biochem. 2008;104:756–772. doi: 10.1002/jcb.21665. [DOI] [PubMed] [Google Scholar]
- 66.Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest. 2000;106:951–961. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lorenz JN, Arend LJ, Robitz R, Paul RJ, MacLennan AJ. Vascular dysfunction in S1P2 sphingosine 1-phosphate receptor knockout mice. Am J Physiol Regul Integr Comp Physiol. 2007;292:R440–446. doi: 10.1152/ajpregu.00085.2006. [DOI] [PubMed] [Google Scholar]
- 68.Ishii I, Friedman B, Ye X, Kawamura S, McGiffert C, Contos JJ, et al. Selective loss of sphingosine 1-phosphate signaling with no obvious phenotypic abnormality in mice lacking its G protein-coupled receptor, LP(B3)/EDG-3. J Biol Chem. 2001;276:33697–33704. doi: 10.1074/jbc.M104441200. [DOI] [PubMed] [Google Scholar]
- 69.Escalante-Alcalde D, Hernandez L, Le Stunff H, Maeda R, Lee HS, Jr-Gang-Cheng, et al. The lipid phosphatase LPP3 regulates extra-embryonic vasculogenesis and axis patterning. Development. 2003;130:4623–4637. doi: 10.1242/dev.00635. [DOI] [PubMed] [Google Scholar]
- 70.Allende ML, Sasaki T, Kawai H, Olivera A, Mi Y, van Echten-Deckert G, et al. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J Biol Chem. 2004;279:52487–52492. doi: 10.1074/jbc.M406512200. [DOI] [PubMed] [Google Scholar]
- 71.Lee H, Ratajczak MZ. Innate immunity: a key player in the mobilization of hematopoietic stem/progenitor cells. Arch Immunol Ther Exp. 2009;57:269–278. doi: 10.1007/s00005-009-0037-6. [DOI] [PubMed] [Google Scholar]
- 72.Lévesque JP, Helwani FM, Winkler IG. The endosteal ‘osteoblastic’ niche and its role in hematopoietic stem cell homing and mobilization. Leukemia. 2010;24:1979–1992. doi: 10.1038/leu.2010.214. [DOI] [PubMed] [Google Scholar]
- 73.Ratajczak MZ, Reca R, Wysoczynski M, Yan J, Ratajczak J. Modulation of the SDF-1-CXCR4 axis by the third complement component (C3)–implications for trafficking of CXCR4+ stem cells. Exp Hematol. 2006;34:986–995. doi: 10.1016/j.exphem.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 74.Wysoczynski M, Reca R, Lee H, Wu W, Ratajczak J, Ratajczak MZ. Defective engraftment of C3aR−/− hematopoietic stem progenitor cells shows a novel role of the C3a-C3aR axis in bone marrow homing. Leukemia. 2009;23:1455–1461. doi: 10.1038/leu.2009.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bengtsson NE, Kim S, Lin L, Walter GA, Scott EW. Ultra-high-field MRI real-time imaging of HSC engraftment of the bone marrow niche. Leukemia. 2011 doi: 10.1038/leu.2011.72. [DOI] [PubMed] [Google Scholar]
- 76.Ratajczak J, Reca R, Kucia M, Majka M, Allendorf DJ, Baran JT, et al. Mobilization studies in mice deficient in either C3 or C3a receptor (C3aR) reveal a novel role for complement in retention of hematopoietic stem/progenitor cells in bone marrow. Blood. 2004;103:2071–2078. doi: 10.1182/blood-2003-06-2099. [DOI] [PubMed] [Google Scholar]
- 77.Bandhuvula P, Honbo N, Wang GY, Jin ZQ, Fyrst H, Zhang M, et al. S1P lyase: a novel therapeutic target for ischemia-reperfusion injury of the heart. Am J Physiol Heart Circ Physiol. 2011;300:H1753–1761. doi: 10.1152/ajpheart.00946.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Borlongan CV. Bone marrow stem cell mobilization in stroke: a ‘bonehead’ may be good after all! Leukemia. 2011 Jul;:5. doi: 10.1038/leu.2011.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pelus LM, Fukuda S. Chemokine-mobilized adult stem cells; defining a better hematopoietic graft. Leukemia. 2008;22:466–473. doi: 10.1038/sj.leu.2405021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Taichman RS. Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood. 2005;105:2631–2639. doi: 10.1182/blood-2004-06-2480. [DOI] [PubMed] [Google Scholar]
- 81.King AG, Horowitz D, Dillon SB, Levin R, Farese AM, MacVittie TJ, et al. Rapid mobilization of murine hematopoietic stem cells with enhanced engraftment properties and evaluation of hematopoietic progenitor cell mobilization in rhesus monkeys by a single injection of SB-251353, a specific truncated form of the human CXC chemokine GRObeta. Blood. 2001;97:1534–1542. doi: 10.1182/blood.v97.6.1534. [DOI] [PubMed] [Google Scholar]
- 82.Ramirez P, Rettig MP, Uy GL, Deych E, Holt MS, Ritchey JK, et al. BIO5192, a small molecule inhibitor of VLA-4, mobilizes hematopoietic stem and progenitor cells. Blood. 2009;114:1340–1343. doi: 10.1182/blood-2008-10-184721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 84.Spiegel A, Shivtiel S, Kalinkovich A, Ludin A, Netzer N, Goichberg P, et al. Catecholaminergic neurotransmitters regulate migration and repopulation of immature human CD34+ cells through Wnt signaling. Nat Immunol. 2007;8:1123–1131. doi: 10.1038/ni1509. [DOI] [PubMed] [Google Scholar]
- 85.Topcuoglu P, Arat M, Dalva K, Ozcan M. Administration of granulocyte-colony-stimulating factor for allogeneic hematopoietic cell collection may induce the tissue factor-dependent pathway in healthy donors. Bone Marrow Transplant. 2004;33:171–176. doi: 10.1038/sj.bmt.1704341. [DOI] [PubMed] [Google Scholar]
- 86.Reca R, Cramer D, Yan J, Laughlin MJ, Janowska-Wieczorek A, Ratajczak J, et al. A novel role of complement in mobilization: immunodeficient mice are poor granulocyte-colony stimulating factor mobilizers because they lack complement-activating immunoglobulins. Stem Cells. 2007;25:3093–3100. doi: 10.1634/stemcells.2007-0525. [DOI] [PubMed] [Google Scholar]
- 87.Farkas I, Baranyi L, Ishikawa Y, Okada N, Bohata C, Budai D, et al. CD59 blocks not only the insertion of C9 into MAC but inhibits ion channel formation by homologous C5b-8 as well as C5b-9. J Physiol. 2002;539:537–545. doi: 10.1113/jphysiol.2001.013381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ruiz-Argüelles A, Llorente L. The role of complement regulatory proteins (CD55 and CD59) in the pathogenesis of autoimmune hemocytopenias. Autoimmun Rev. 2007;6:155–161. doi: 10.1016/j.autrev.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 89.Kimberley FC, Sivasankar B, Paul Morgan B. Alternative roles for CD59. Mol Immunol. 2007;44:73–81. doi: 10.1016/j.molimm.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 90.Jalili A, Shirvaikar N, Marquez-Curtis L, Qui Y, Korol C, Lee H, et al. Fifth complement cascade protein (C5) cleavage fragments disrupt the SDF-1/CXCR4 axis: further evidence that innate immunity orchestrates the mobilization of hematopoietic stem/progenitor cells. Exp Hematol. 2010;38:321–332. doi: 10.1016/j.exphem.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yatomi Y, Ruan F, Hakomori S, Igarashi Y. Sphingosine-1-phosphate: a platelet-activating sphingolipid released from agonist-stimulated human platelets. Blood. 1995;86:193–202. [PubMed] [Google Scholar]
- 92.Sadir R, Imberty A, Baleux F, Lortat-Jacob H. Heparan sulfate/heparin oligosaccharides protect stromal cell-derived factor-1 (SDF-1)/CXCL12 against proteolysis induced by CD26/dipeptidyl peptidase IV. J Biol Chem. 2004;279:43854–43860. doi: 10.1074/jbc.M405392200. [DOI] [PubMed] [Google Scholar]
- 93.Ou WC, Liu SM, Xiong LG, Li GQ, Tan MQ. Role of sphingosine 1-phosphate receptor signaling in hematopoietic stem/progenitor cell transmigration. Nan Fang Yi Ke Da Xue Xue Bao. 2009;29:1862–1865. [PubMed] [Google Scholar]
- 94.Hoggatt J, Singh P, Sampath J, Pelus LM. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood. 2009;113:5444–5455. doi: 10.1182/blood-2009-01-201335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hoggatt J, Pelus LM. Eicosanoid regulation of hematopoiesis and hematopoietic stem and progenitor trafficking. Leukemia. 2010;24:1993–2002. doi: 10.1038/leu.2010.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pettus BJ, Kitatani K, Chalfant CE, Taha TA, Kawamori T, Bielawski J, et al. The coordination of prostaglandin E2 production by sphingosine-1-phosphate and ceramide-1-phosphate. Mol Pharmacol. 2005;68:330–335. doi: 10.1124/mol.104.008722. [DOI] [PubMed] [Google Scholar]
- 97.Brizuela L, Rábano M, Peña A, Gangoiti P, Macarulla JM, Trueba M, et al. Sphingosine 1-phosphate: a novel stimulator of aldosterone secretion. J Lipid Res. 2006;47:1238–1249. doi: 10.1194/jlr.M500510-JLR200. [DOI] [PubMed] [Google Scholar]
- 98.Ratajczak MZ. Spotlight series on stem cell mobilization: many hands on the ball, but who is the quarterback? Leukemia. 2010;24:1665–1666. doi: 10.1038/leu.2010.181. [DOI] [PubMed] [Google Scholar]
- 99.Ratajczak MZ, Kim CH, Wu W, Shin DM, Bryndza E, Kucia M, et al. The Role of Innate Immunity in Trafficking of Hematopoietic Stem Cells—An Emerging Link Between Activation of Complement Cascade and Chemotactic Gradients of Bioactive Sphingolipids. Current Topics in Innate Immunity II. doi: 10.1007/978-1-4614-0106-3_3. [DOI] [PubMed] [Google Scholar]


