Abstract
Insect disturbance are important agents of change in forest ecosystems around the globe, yet their spatial and temporal distribution and dynamics are not well understood. Remote sensing has gained much attention in mapping and understanding insect outbreak dynamics. Consequently, we here review the current literature on the remote sensing of insect disturbances. We suggest to group studies into three insect types: bark beetles, broadleaved defoliators, and coniferous defoliators. By so doing, we systematically compare the sensors and methods used for mapping insect disturbances within and across insect types. Results suggest that there are substantial differences between methods used for mapping bark beetles and defoliators, and between methods used for mapping broadleaved and coniferous defoliators. Following from this, we highlight approaches that are particularly suited for each insect type. Finally, we conclude by highlighting future research directions for remote sensing of insect disturbances. In particular, we suggest to: 1) Separate insect disturbances from other agents; 2) Extend the spatial and temporal domain of analysis; 3) Make use of dense time series; 4) Operationalize near-real time monitoring of insect disturbances; 5) Identify insect disturbances in the context of coupled human-natural systems; and 6) Improve reference data for assessing insect disturbances. Since the remote sensing of insect disturbances has gained much interest beyond the remote sensing community recently, the future developments identified here will help integrating remote sensing products into operational forest management. Furthermore, an improved spatiotemporal quantification of insect disturbances will support an inclusion of these processes into regional to global ecosystem models.
Keywords: Insect disturbance, Biotic disturbance, Bark beetle, Defoliation, Forest health, Insect outbreak
1. Introduction
Disturbances by insects are natural processes in forest ecosystems and an integral driver of their dynamics, helping to maintain healthy and heterogeneous forests that can provide important ecosystem services (Raffa et al., 2009). However, many forest ecosystems have experienced an increase in the rate, magnitude and frequency of insect disturbances, with recent disturbance activity considerably exceeding levels known from 20th century experience (Millar and Stephenson, 2015). This raised concerns regarding the impact of insect disturbances on biogeochemical cycles (Edburg et al., 2012), especially the carbon cycle (Kurz et al., 2008; Seidl et al., 2014), biodiversity (Beudert et al., 2015; Müller et al., 2008), and the economic value of forests (Dale et al., 2001). Despite the importance of forest insects for tree mortality globally, there is a lack of consistent data sets tracking insect disturbances systematically through space and time (Kautz et al., 2016). This data gap substantially hampers the development of process-based models for making informed prediction of potential future changes under global climate change, and thus the development of adequate management strategies (Kautz et al., 2016; Seidl et al., 2011).
Forest insects can be broadly grouped into xylophagous (e.g. bark beetles) and folivorous insects (e.g. defoliators). There exist also smaller groups of mucivores insects (fluid-feeders), though we do not focus on those here as they are less important in most forest ecosystems. Many bark beetle species of importance in the context of forest disturbance regimes reproduce in the phloem tissue of live and dead trees and – through introduction of associated fungal pathogens – disrupt the translocation of water and nutrients within the tree. A successful infestation of bark beetles can thus be mortal for trees (Raffa and Berryman, 1983). Defoliating insects feed on the needles or leaves of trees, essentially impacting the trees capacity to perform photosynthesis. This can lead to growth reduction, deformation, and – in conjunction with secondary pressures such as simultaneous bark beetle attacks or drought – tree mortality (Cooke et al., 2007). However, after the collapse of defoliator populations trees usually resprout in the following year, or – in the case of some broadleaved tree species – even in the same year.
Insect disturbances act at varying spatial and temporal scales that all need to be considered to develop a holistic understanding of their dynamics, and subsequently predict their future changes (Raffa et al., 2008). While much research has utilized laboratory and field data to understand the drivers of host colonization by insects and their reproductive success, those small-scale drivers are often not sufficient for predicting the landscape- to regional-scale infestation patterns observed in recent outbreaks (Seidl et al., 2015; Senf et al., 2017; Simard et al., 2012). Hence, besides tree- and stand-scale factors, it is also important to understand how insect populations interact with their surrounding landscape, as well as with regional-scale climate. Tackling those larger-scale processes requires data that a) are spatially explicit, b) cover large geographic extents, c) deliver a temporal resolution that fits the life-cycle of the insect of interest, and d) allows for assessing long time series to capture the long-term natural fluctuations that are inherent to insect dynamics. While dendroecology and long-term ecological monitoring allow for an extensive historical view on insect disturbances over relatively large geographic extents (Swetnam and Lynch, 1993), they do not offer the spatially explicit perspective needed for understanding the patterns and interactions of insect outbreaks at the landscape scale. Furthermore, the finest temporal resolution that can be consistently obtained from dendroecological investigations is often only in the range of decades, and an attribution to specific disturbance agents remains challenging (e.g., Janda et al., 2016). Remote sensing largely fulfills all the above-mentioned criteria and serves as a powerful approach to study insect outbreaks across large areas at fine spatial and temporal scale (McDowell et al., 2015; Trumbore et al., 2015).
The biological differences between bark beetles, defoliators of coniferous trees, and defoliators of broadleaved trees explained above suggest that there are specific advantages and disadvantages of applying particular remote sensing methods for mapping their occurrence and infestation severity. However, even though there exist reviews from a regional perspective (Hall et al., 2016), focussing on specific insect types (Rullan-Silva et al., 2013; Wulder et al., 2006a), or general forest health decline (Lausch et al., 2016), we yet lack a systematic, comprehensive, and global assessment of the methods best suited for the remote sensing of varying forest insect agents. A systematic review of the methods applied, and a better understanding of their underlying biological and ecological processes, will help to improve future studies that aim at mapping and estimating insect disturbances. Consequently, we systematically reviewed the approaches employed in the remote sensing of forest insect disturbances, specifically addressing the following three questions:
What are the insect types, species, and biomes that have been studied using remote sensing?
What are the methods best suited for mapping disturbances from bark beetles, defoliators of coniferous trees, and defoliators of broadleaved trees?
What are the challenges for current remote sensing approaches, and how can they be overcome in the future?
We first present a systematic literature review to answer research questions 1 and 2. Subsequently, using a more qualitative approach, we synthesize the results of the systematic review to address research question 3.
2. Systematic literature review
2.1. Database search
For obtaining an initial sample of the relevant literature we searched the ISI Web of Science database (http://www.webofknowledge.com/) with general search terms focussing on the mapping of forest insect disturbances by remote sensing, using the following search string: TS = (bark beetle* OR defoliator* OR insect* OR pest*) AND TS = (forest* OR tree*) AND TS = (remote sensing OR remotely sensed OR mapping OR satellite* OR earth observation*).1 This initial search led to a total of n = 868 studies. We screened the titles and abstracts of those studies to exclude studies obviously unrelated to our review (e.g., medical studies, studies using remote sensing for guiding field work, simulation studies), which decreased the total number of studies to 149. For those studies, we downloaded the full text for further screening. We subsequently analyzed each study by the following criteria set for the inclusion in our review:
A specific insect agent must be defined. Studies mapping general forest decline or change due to multiple agents were not considered.
A map was produced or could easily be produced with the methods described in the paper. Experimental studies limited to a few selected pixels or simulation studies were excluded.
Studies mapping infestations in plantations or orchards were not considered.
Approaches must be (semi-) automatic. Studies applying manual digitalisation of remote sensing data or manual mapping from aircrafts (aerial surveys or sketch maps) were not considered.
After applying these criteria, a total of 59 studies were selected for inclusion in the analysis. However, we noted that 16 studies that were initially not included in our sample were frequently cited in other studies considered in our review. After checking them against the above described criteria, we also added those studies to our literature base, yielding a final number of n = 75 studies to be included in the systematic review.
2.2. Information extraction and analysis
For each study in the sample, we extracted the same set of attributes for analysis (Table 1). In particular, we noted the insect type (i.e., bark beetle, defoliator coniferous, defoliator broadleaved), the insect species, if the species was native to the ecosystem studied, and its primary host species. Furthermore, we recorded the response variable and how reference data was collected, as well as the location of the study area. To characterize the sensor used in each study, we recorded the sensor name, the spatial, temporal, and spectral properties of the sensor, as well as the sensor. Finally, we noted the classification/regression model used for mapping/estimating infestation, if a fitting technique was applied (for temporal smoothing, etc.), if auxiliary data was used in the model, as well as the measure of accuracy/model performance and the level of accuracy/model performance obtained. For a detailed description of the attributes see Table 1.
Table 1.
Information extracted from the literature.
| Attribute | Possible values | Description |
|---|---|---|
| Insect type | BB, DC, DB, Other | Insect type: bark beetle (BB), coniferous defoliator (DC), broadleaved defoliator (DB), fluid feeders, etc. (others) |
| Insect species | – | Binomial name of the insect species. If more than one species within one genus were mentioned, we only recorded the genus. |
| Native | Yes, No | Whether the insect species is native to the forest ecosystem studied. |
| Host species | – | Binomial name of the host species. If more than one species within one genus were mentioned, we only recorded the genus. If more than one host species of varying genera were studied, we only noted the major host. |
| Response type | Occurrence, Severity | Whether the study mapped solely the occurrence of an infestation or also its severity. If severity was mapped, its unit/measure was also specified. |
| Reference data | – | How reference data used for model calibration and validation was collected (i.e., source and method) |
| Latitude/longitude | – | Location of the study area. If no location was given in the paper, we either used Google Maps to determine the approximate location, or we used the Landsat WRS2 reference system to identify the center location of the Landsat footprint used in the analysis. |
| Sensor | – | Sensor used. If no sensor was specified, the field was left empty. |
| Platform | Satellite, Air, UAV | The platform of the sensor, that is satellite-borne, air-borne, or un-maned aerial vehicle (UAV) |
| Spatial properties | CR, MR, HR, VHR | The resolution of the sensors used: coarse resolution: > 100 m; medium resolution: 10–100 m; high resolution: 1–10 m; very high resolution: < 1 m. |
| Temporal properties | SD, MD, D, VD | The temporal resolution of the data stream: SD = single date; MD = multi date (more than one image but less than annual coverage); D = Dense (annual coverage); VD = Very dense (intra-annual coverage). |
| Spectral properties | VI, Multi, Hyper, Lidar, SAR | The spectral properties used in the analysis: VI (a single vegetation index or band was used; the index name was noted), Multi (multi-spectral bands or multiple spectral indices were used), Hyper (hyper-spectral bands or multiple narrow-band indices were used), Lidar (Light detection and ranging), and SAR (Synthetic Aperture Radar). |
| Temporal fitting | – | The algorithm that was used for fitting/smoothing the spectral signal over time. If no such algorithm was used, the field was left empty. |
| Model | – | The model that was used for detecting infestations, and applied to create maps. |
| Auxiliary | Yes, No | Was auxiliary data (e.g., climate information) used in addition to remote sensing data? |
| Accuracy measure | – | The measure of accuracy reported in the study. If more than one measure was given, we always preferred overall accuracy over producer’s/user’s accuracies, and the coefficient of determination (R2 or pseudo-R2) over the root mean square error, respectively. |
| Accuracy | – | Level of accuracy, according to accuracy measure described above. If several methods/scenarios were compared, we always extracted the overall highest accuracy achieved. |
After extracting the information for each study included in our analysis, we first mapped and visualized the spatial and temporal distribution of studies and insect types. Moreover, we extracted the biome the study site was located in using the biome classification by Olson et al. (2001). We created statistical summaries of all sensor attributes and methods applied to study insect disturbances, grouped by the four insect types. Finally, we investigated the distributions of accuracies achieved when mapping/estimating disturbances. While many different measures are used in the literature for estimating accuracy/model performance, we here focused on overall accuracy as measure of map accuracy and the coefficient of determination (R2 or pseudo-R2) as measure of model performance.
2.3. Results of the systematic review
2.3.1. Insect types and biomes
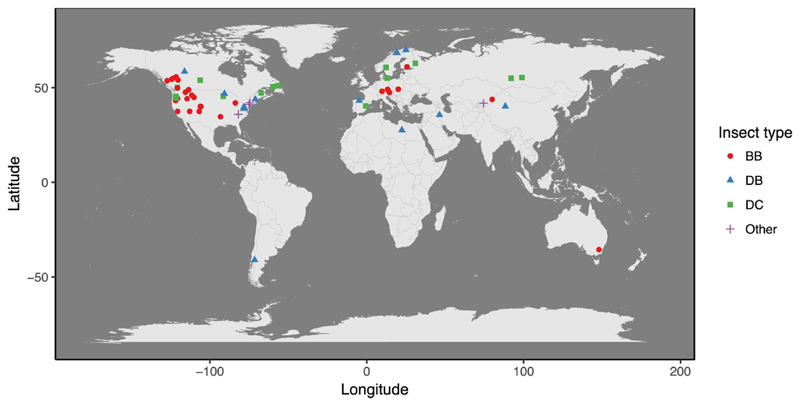
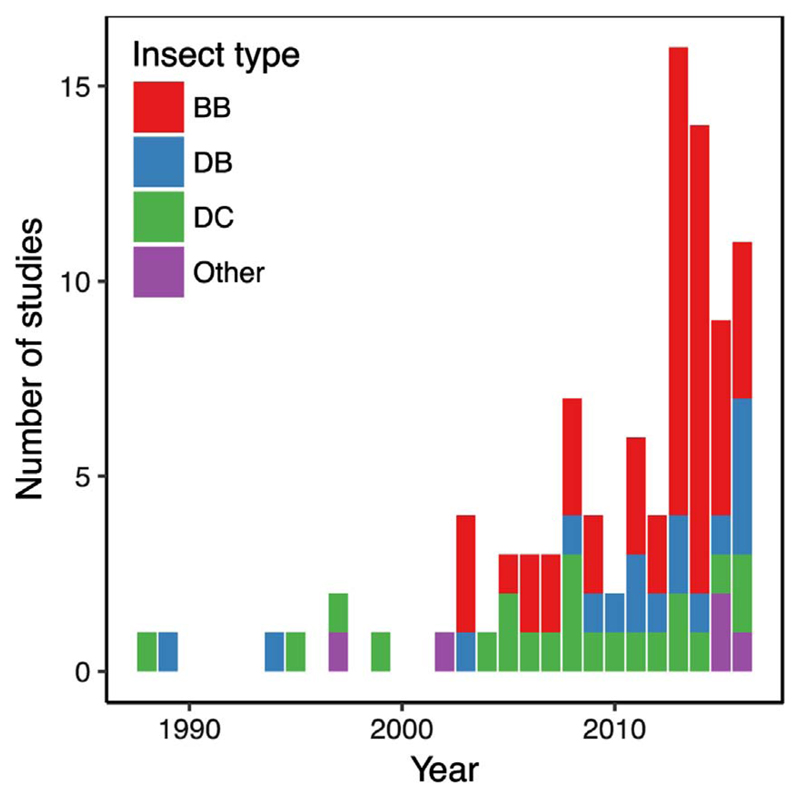
In total, there were 27 insect species studied using remote sensing, of which five were non-native (Table 2). Most studies were located in North America and Europe, whereby the European studies clustered either in the boreal biome or in selected sites in mountainous environments (Fig. 1; Table 3). There was only one study located in South America, Australia, and Africa, respectively; and only few studies were located in Asia. Bark beetles and broadleaved defoliators were mostly studied in temperate forest ecosystems, while coniferous defoliators were mostly studied in the boreal forests (Table 3). Very few studies (7%) were located in the subtropical region and no study was located in the tropics. From a historic perspective, remote sensing was mostly used from 2003 onwards for mapping insect disturbances, with a substantial increase in recent years (Fig. 2). While early studies nearly exclusively mapped defoliating insects, the recent increase in remote sensing-based studies of forest insect disturbances was largely related to studies mapping the bark beetle Dendroctonus ponderosae in North America.
Table 2.
Number of studies by insect species. See Table 1 for abbreviations of insect type.
Fig. 1.
Spatial distribution of studies and insect types according to our sample.
Table 3.
Distribution of studies across biomes.
| Biome | Number of studies | ||||
|---|---|---|---|---|---|
| BB | DB | DC | Others | Total | |
| Boreal Forests/Taiga | 1 | – | 11 | – | 12 |
| Deserts & Xeric Shrublands | 1 | 2 | 1 | – | 4 |
| Flooded Grasslands & Savannas | – | – | – | – | – |
| Mangroves | – | – | – | – | – |
| Mediterranean Forests, Woodlands & Scrub | – | – | 1 | – | 1 |
| Montane Grasslands & Shrublands | – | – | – | – | – |
| Temperate Broadleaved & Mixed Forests | 1- | 8 | 2 | 3 | 23 |
| Temperate Conifer Forests | 20 | – | 3 | – | 23 |
| Temperate Grasslands, Savannas & Shrublands | 4 | 1 | 1 | 2 | 8 |
| Tropical & Subtropical Coniferous Forests | – | – | – | – | – |
| Tropical & Subtropical Dry Broadleaved Forests | – | – | – | – | – |
| Tropical & Subtropical Grasslands, Savannas & Shrublands | – | – | – | – | – |
| Tropical & Subtropical Moist Broadleaved Forests | – | – | – | – | – |
| Tundra | – | 4 | – | – | 4 |
| Total | 36 | 15 | 19 | 5 | 75 |
Fig. 2.
Temporal distribution of studies on the remote sensing of insect disturbances by insect types. Insect types include bark beetles (BB), broadleaved defoliators (DB), coniferous defoliators (DC), and others.
2.3.2. Sensors and methods
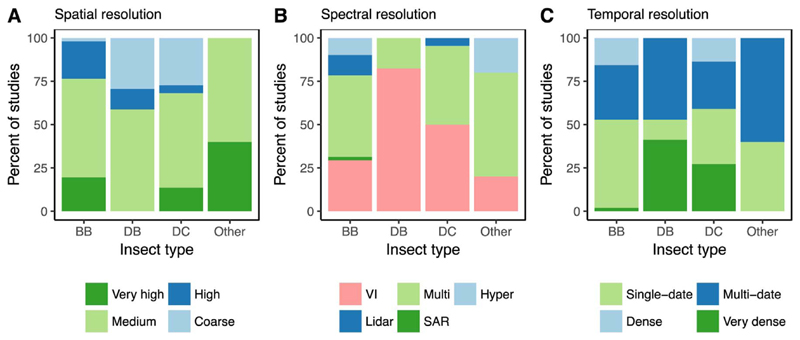
A variety of sensors were used to study forest insect disturbances (Table 4). A total of 13% of the studies used coarse resolution data (thereof 75% MODIS), 57% of the studies used medium resolution data (thereof 83% Landsat), 15% of the studies used high resolution data (most frequent: HyMap, QuickBird, RadpidEye, and WorldView-2), and another 15% of the studies used very high resolution data (including Lidar, which accounted for 7% of the total number of studies). Coarse resolution data (i.e., MODIS) was mainly used for mapping the effect of defoliators, and only a single study applied it for mapping bark beetle disturbances (Fig. 3A). Medium resolution data was used equally across all insect types, while high resolution data was particularly used for mapping the effects of bark beetles (Fig. 3A).
Table 4.
Sensors used to study forest insect disturbances.
Fig. 3.
Percent of studies using specific temporal, spectral, and spatial properties. The summary statistics are stratified by insect type. Insect types include bark beetles (BB), broadleaved defoliators (DB), coniferous defoliators (DC), and others.
Differences between insect types were also evident in the spectral properties of sensors. The majority of studies mapping defoliation of broadleaved and coniferous trees used a single spectral index (82% and 50%, respectively). The most frequently used spectral index for mapping broadleaved defoliation was the Normalized Difference Vegetation Index (NDVI; 43%), while a variety of indices was used for mapping coniferous defoliation (NDVI, Normalized Burn Ratio [NBR], Moisture Stress Index [MSI], Leave Area Index [LAI]). For mapping bark beetle disturbances, studies mostly relied on multi-spectral data (47%), though 29% of the studies also used single spectral indices. The most frequently used spectral index for mapping the effects of bark beetles was the difference in the Tasseled Cap Wetness component (Enhanced Wetness Difference Index [EWDI]), followed by the NBR and Disturbance Index (DI). Only 12% of the studies mapping bark beetle disturbances used Lidar data, and another 10% used hyperspectral imagery. Interestingly, hyperspectral data was neither used in the context of broadleaved nor coniferous defoliators, and Lidar was only used in one study on coniferous defoliators.
In terms of temporal resolution, our analysis showed that very dense time series (i.e., more than one image per year) were mainly used for mapping defoliation, especially of broadleaved trees (Fig. 3). Multi-date information was used throughout all insect types, and few studies used dense (i.e., annual) time series for mapping bark beetle disturbances and coniferous defoliation. Single-date data was used in more than 50% of the studies mapping the effect of bark beetles, though it was less commonly used for mapping defoliation. Time series fitting techniques were either used with very dense MODIS time series data (i.e., TIMESAT; Joensson and Eklundh (2004)) or annual Landsat time series (i.e., LandTrendr; Kennedy et al. (2010)). Only one study used a fusion approach (Enhanced Spatial and Temporal Adaptive Reflectance Fusion Model; Zhu et al. (2010)), combining very dense and multi-date time series.
Studies mapping disturbance occurrence and severity were nearly equally distributed in the literature (55% and 46%, respectively), with only minor differences between insect types. For studies mapping bark beetle infestation severity, the most frequently used measures were percent mortality, percent dead stems, and percent basal area mortality. For coniferous defoliators, severity was mostly measured in categories (i.e., three to five levels of defoliation intensity), while for broadleaved forests defoliation severity was mostly measured as percent canopy loss.
As for calibration and validation data, most of the studies utilized field data (55%), followed by interpretation of very high resolution remote sensing data (28%). Interestingly, VHR data interpretation was more often used in the case of bark beetles than for mapping defoliators. The remaining studies either used aerial overview survey (AOS) maps (8%), medium resolution image or time series interpretation (6%), helicopter surveys (4%), or Lidar (2%) as reference data.
The majority of studies mapping disturbance occurrence used classification models such as random forest (20%), maximum likelihood (14%), or (logistic) regression (12%). However, one quarter (25%) of the studies mapping disturbance occurrence relied on a simple rule-based approach (Table 5). Studies estimating the severity of infestation most frequently used statistical regression models (37%), followed by random forest regression (19%), and rule based approaches that classify a continuous measure into severity classes (12%).
Table 5.
Models used to map forest insect disturbances.
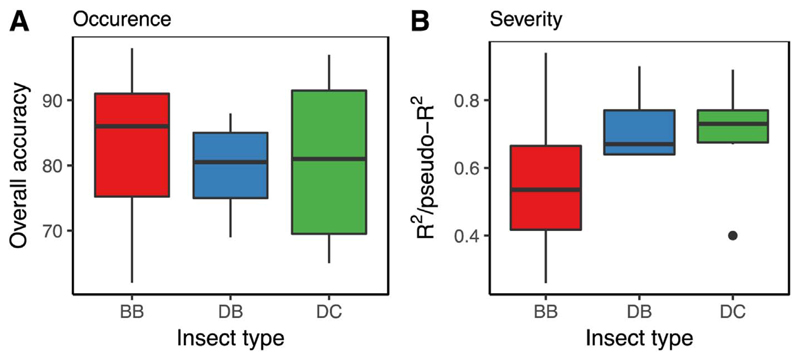
Mapping accuracies did not show any substantial differences between the three insect types, with overall accuracies ranging from 60 to > 90% (Fig. 4a). For estimating disturbance severity, however, higher modeling performance was found for defoliators than for bark beetles (Fig. 4b).
Fig. 4.
Overall accuracy (A) and coefficient of determination (B) achieved when mapping forest insect disturbance occurrence/severity.
3. Current approaches in the remote sensing of forest insect disturbances
3.1. Bark beetles
Landsat is currently the most prominent sensor for mapping bark beetle disturbances, suggesting that spatial resolution is a key feature for mapping bark beetle infestation. Indeed, the high number of studies employing sub-pixel approaches (i.e., mapping infestation severity) and using high and very-high resolution data for analyzing bark beetle disturbances further support this conclusion. Landsat also delivers continuous observations over several years, which in turn allows to monitor outbreaks of bark beetles, which often last several years (Meigs et al., 2015b).
From a spectral perspective, our results show that spectral information from the short-wave infrared range of the electromagnetic spectrum is of particular importance for detecting bark beetle infestations, likely due to the sensitivity of this spectral region to changes in needle water content. Changes in the needle water content allow for an accurate detection of trees turning red (red-attack stage) and grey (grey-attack stage) in the wake of an infestation (Skakun et al., 2003; Wulder et al., 2006a).
Studies increasingly rely on multi-date time series of medium resolution data to capture changes in the spectral signal associated with changes in the needle water content (Goodwin et al., 2008; Meddens et al., 2013). With high and very-high spatial resolution data, however, the analysis of single-date images is still the norm (e.g., Hicke and Logan, 2009; Pontius et al., 2008). Following the opening of the USGS Landsat Archive in 2008 (Wulder et al., 2012), the use of dense (i.e., annual) time series became more prominent (e.g., Meddens and Hicke, 2014; Meigs et al., 2011). Using dense time series of Landsat data allows for an annual analysis of bark beetle disturbances, which delivers detailed information on the spatial and temporal patterns of outbreaks (Meigs et al., 2015b). Very dense time series (i.e., intra-annual) are rarely used for mapping bark beetle infestations, suggesting that the intra-annual variation in the spectral signal is less important in this context, and does not counterbalance the loss of spatial information that comes with current high frequency, coarse resolution sensors (i.e., MODIS or AVHRR).
Finally, we found that field plots and image interpretation (i.e., airborne very-high resolution data) were the primary sources of reference data for mapping bark beetle disturbances. While studies utilizing field data mostly measured mortality as percent basal area killed or number of trees killed (e.g., Bright et al., 2014; Meigs et al., 2015b), very-high resolution data interpretation was mostly used for estimating a sub-pixel fraction of mortality (i.e., percent of Landsat pixel infested; e.g., Meddens and Hicke (2014)). Moreover, there were differences between studies mapping infestation in early stages (red-attack) and later stages (grey-attack). Some studies even aimed at mapping very early infestation stages (green-attack) with the aim of providing decision support for risk management actions (Fassnacht et al., 2012).
3.2. Coniferous defoliators
The majority of studies mapping disturbances of coniferous trees by defoliators used medium resolution data, with Landsat and SPOT being the most prominent sensors (e.g., Franklin et al., 2008; Olsson et al., 2012; Sangüesa-Barreda et al., 2014). While older works employed supervised classification of single-date multi-spectral data (e.g., Franklin et al., 1995; Luther et al., 1997), more recent studies almost exclusively relied on comparing one vegetation index between two or more dates in time to track vegetation changes caused by defoliation (Meigs et al., 2011; Meigs et al., 2015b; Olsson et al., 2012; Sangüesa-Barreda et al., 2014; Senf et al., 2015). A wide variety of vegetation indices was used that all made use of the near-infrared and shortwave-infrared bands. Changes in those bands can detect chlorosis and structural changes in the canopy caused by insect defoliation, but the relationship between coniferous insect defoliation and the corresponding spectral responses is poorer understood than for bark beetles (Senf et al., 2015).
Coniferous defoliation was often associated with decrease in one or several spectral indices that lasted over several years, highlighting that coniferous defoliation is a gradual process (Vogelmann et al., 2009). Indeed, there is evidence that disturbances by coniferous defoliators are on average longer in duration than disturbances by bark beetles, with the latter resulting in tree mortality within a few years (Meigs et al., 2011; Wulder et al., 2006a). Nonetheless, also very dense time series information – i.e., intra-annual variation in the spectral signal – were used to map coniferous defoliators, although with mixed results (Eklundh et al., 2009; Olsson et al., 2016a). While defoliation was indeed visible in the intra-annual changes of vegetation index time series, the spatial resolution of the underlying time series data (MODIS) limits the applicability in heterogeneous landscapes. Nonetheless, dense time series information could enhance the detectability of defoliation by delivering spectral information from peak feeding periods within the year, where differences between infested and un-infested forests are largest (Fraser and Latifovic, 2005).
A feature that was outstanding for mapping coniferous defoliation was the frequent use of severity categories (i.e., trace, low, medium, strong defoliation) instead of a measurable unit of insect impact (as with bark beetles and broadleaved defoliators). A reason for this might be the fact that measuring long-term (i.e., over several years) needle loss caused by defoliation is challenging and cost-intensive (Långström and Hellqvist, 2004), in particular compared to assessing deadwood (as with bark-beetles) or loss in foliage within one year (as with most broadleaved defoliators).
3.3. Broadleaved defoliators
Medium and coarse spatial resolution sensors were most frequently used for mapping broadleaved defoliation, and many studies employed intra-annual time series analysis for analyzing these data sets (Olsson et al., 2016b; Townsend et al., 2012). This suggests that for mapping the impact of broadleaved defoliators the intra-annual variation in the spectral signal is of particular importance. Several studies showed that a denser time series improves the detectability of defoliation in broadleaved trees, because most broadleaved trees are able to resprout in the same year of the disturbance (De Beurs and Townsend, 2008; Gartner et al., 2016; Spruce et al., 2011). Denser time series thus result in a higher chance of obtaining a cloud free observation during the short period of maximum defoliation. In the context of broadleaved defoliators, higher temporal resolution thus seems to compensate for coarser spatial resolution of the applied sensors. However, there were also studies using Landsat intra-annual spectral variability (Townsend et al., 2012) or fused products of varying sensors (Gartner et al., 2016) to overcome spatial limitations of coarser spatial resolution data.
We found that most studies using very dense time series for mapping broadleaved defoliation only used a single spectral index instead of multi-spectral data. This finding might suggest that the use of very dense time series hampers the use of multi-spectral information with current algorithms. Interestingly, most studies mapping broadleaved defoliation used near-infrared based indices, which clearly contrasts with studies mapping bark beetle infestation and coniferous defoliation. This finding suggests that changes in leaf area are more important for detecting broadleaved defoliation than changes in water content and changes in the overall canopy structure. Consequently, reference data for measuring broadleaved defoliation severity mostly included field data that measured the loss in leaf area (e.g., Hall et al., 2003; Rullan-Silva et al., 2015; Townsend et al., 2012).
4. Challenges and ways forward
4.1. Separate insect disturbances from other agents
Forest insects are often not the only disturbance agent present in a forest ecosystem. In order to map insect disturbances and estimate their severity it is thus necessary to discriminate between insect and other disturbance agents, a step that is largely neglected in the current literature. Most studies assessed here either simply assumed that all spectral anomalies found in their analysis related to one particular insect agent (e.g., based on local knowledge of the authors), or they used existing masks (i.e., harvest masks, forest type masks) to exclude areas unlikely to be affected by the insect in focus. While those approaches are common in remote sensing studies, they result in an overestimation of the true mapping accuracy, since the accuracies reported in the studies often apply only to a subpopulation of the map (e.g., all coniferous forest pixels). Several studies thus explicitly separated insect disturbances from other agents such as fire or harvest, which often have a substantially different spectral behaviour (e.g., Goodwin et al., 2008; Meigs et al., 2015b; Senf et al., 2015). However, insect disturbances are often triggered by other disturbances – bark-beetle outbreaks following blowdown (Seidl and Rammer, 2016) or defoliation following drought (Senf et al., 2016) – which can make a clear distinction between different disturbance agents challenging. In particular, clusters of small-scale disturbances might result in pixels spectrally mixed between both disturbance agents (i.e., bark beetle at the edge of blowdowns); or one disturbance might transition into another disturbance, leading to a single decrease in spectral signals (i.e., drought triggering subsequent defoliation). Hence, a potential improvement of current approaches would be to improve the discriminatory power between insect disturbances and unrelated other disturbance agents (i.e., fire, harvest, flood, avalanches), while simultaneously acknowledging that insect disturbances often interact with each other and might thus not be separable from other disturbances (i.e., blowdown, drought).
Separating insect disturbances from other disturbances is challenging, though there has been substantial improvement in recent years (Hermosilla et al., 2015; Kennedy et al., 2015). Specifically, the use of spectral-temporal information has emerged as particularly important for separating varying disturbance agents in general and insect agents in particular. Spectral characteristics include the magnitude of change (i.e., the absolute or relative change in a vegetation index or spectral band) and the spectral signature of change (i.e., how changes manifest in a multi-spectral feature space). Spectral characteristics can be derived from single- and multi-date approaches, as well as from dense and very dense time series. Temporal characteristics, however, which describe the duration of an event, can solely be quantified from dense, that is annual or intra-annual, time series. Duration was found important in a variety of studies separating insect disturbances from other disturbance agents (Kennedy et al., 2012; Meigs et al., 2015a). Finally, there is recent evidence that spatial characteristics, that is the size and shape of disturbances patches, might deliver important information for separating disturbance agents (Hermosilla et al., 2015).
A fourth information stream that yet has seldom been utilized for discriminating insect disturbances from other disturbance agents is information on the intra-annual timing of a disturbance. The intra-annual timing – i.e., information of when a disturbance occurred within a year – might contain important information on the underlying agent of change. For example, blowdown from cyclonal storm systems mostly occurs in winter in Europe’s forests, while bark beetle infestation reaches its maximum in spring and summer. Such approaches will become more feasible with an increasing available of temporally dense medium resolution image time series (Zhu et al., 2012). They could further be facilitated by using fused products between, e.g., Landsat and MODIS (Hilker et al., 2011).
4.2. Extend the spatial and temporal domain of analysis
Many studies analyzed here were limited to single watersheds, protected areas, or other geographically distinct landscape units. Only very few studies mapped insect disturbances over larger geographic gradients, covering several climatic and or biogeographic regions (e.g., Meigs et al., 2015b; Senf et al., 2015). Such large-scale assessments are, however, crucial for a better understanding of the impacts of forest disturbances at landscape and regional scales (Hicke et al., 2012; Kautz et al., 2016). Thus, while localized case studies often result in very detailed maps of insect infestation that are useful for forest management (e.g., Lausch et al., 2013; Olsson et al., 2012), large scale mapping approaches could yield important scientific insights (Kautz et al., 2016; Trumbore et al., 2015). Remote sensing has proven to be operational for mapping harvest and fire disturbances over very large geographic extents (Hermosilla et al., 2016), and computational resources are now available to conduct landscape to regional scale assessments of forest insect disturbances. Currently, a limiting factor is the transferability of approaches developed for specific case studies and/or specific agents to a wider geographic extent. Improving the generalizability and transferability of existing approaches will thus help in conducting regional to global assessments of forest insect disturbances.
Remote sensing offers a consistent insight over several decades into forest disturbances, and can thus shed light on outbreaks that would otherwise be poorly documented (Assal et al., 2014). Landsat data was most often used for historic assessments of insect disturbances ( ± 30 years of data). However, only few studies have made use of the full potential by integrating Landsat MSS to date (Assal et al., 2014; Pflugmacher et al., 2012). Integrating Landsat MSS, and thus extending the time series to 40+ years, might substantially improve our understanding of insect disturbance dynamics. In particular, a longer time series of spectral observations would allow to cover several outbreak cycles and thus increase our ability to test hypothesis on underlying drivers (Senf et al., 2016).
4.3. Make use of dense time series
Our systematic review revealed that for mapping defoliators – in particular broadleaved defoliators – a high intra-annual data availability is advantageous for reliably estimating infestation occurrence and severity. However, we also found that this requirement is currently only fulfilled by coarse-resolution sensors, such as MODIS, which have important drawbacks resulting from their spatial resolution (e.g., in the highly heterogeneous landscapes of Central Europe). Consequently, mapping defoliation might be significantly improved by increasing intra-annual data availability for medium- and high-spatial resolution sensors. An important development in this regard is the recent launch of Sentinel-2, which has a 10- to 20-m spatial resolution and a ten-day revisit time (five days when combining Sentinel-2a/b). Different resolution sensors can also be combined into one data stream to further increase data availability (Wulder et al., 2015). However, those approaches limit analyses to recent years, where dense time series of varying sensors are available, and thus preclude the historic perspective offered by, e.g., the Landsat sensor family.
Furthermore, based on our synthesis of the literature the timing of image acquisition within the year can substantially influence the detectability of defoliation. This finding likely also applies to bark beetle disturbances, which are best detected by using observations from late summer that allow for detecting most of the spring infestations and still steer clear of the regular needle loss that happens in fall. Hence, utilizing the timing of image acquisition in the context of the respective insect agent and vegetation phenology would further enhance the detectability and attribution of insect infestations. This might be particularly important for ephemeral disturbances and ecosystems with very high recovery rates. Insights on optimal dates for image acquisition can, for instance, be gained from utilizing models of insect populations. For example, De Beurs and Townsend (2008) utilized the BioSIM model (Régnière and Saint-Amant, 2014) to model annual population development of gypsy moth and thus to identify the optimal day of image acquisition. Similarly, phenological information can be utilized to identify the optimal image acquisition date in terms of maximum greenness (Frantz et al., 2017), which might increase the separability of infested and uninfested forests (Fraser and Latifovic, 2005).
4.4. Operationalize near-real time monitoring of insect disturbances
Satellite images allow for the (near) real-time detection of forest disturbances (Verbesselt et al., 2012). Remote sensing thus can be used as early-warning tool for upcoming large scale insect infestations, since regional outbreaks often emerge from localized epicenters (Seidl et al., 2015; Senf et al., 2017; Simard et al., 2012). However, to date only few studies exist that utilize remote sensing for (near) real-time monitoring of insect disturbances (Olsson et al., 2016b); as well as there are only few operational applications (e.g. https://forwarn.forestthreats.org/). Reasons might be the often complex processing steps involved in pre-processing remote sensing data (i.e., radiometric, geometric, and atmospheric corrections; cloud masking), missing ground-truth data, and the often low data availability with medium spatial resolution sensors (Wulder et al., 2009). This also explains why current studies detecting insect infestations in near real-time make exclusive use of MODIS, which is available as a ready-to-use product already shortly after acquisition. Nevertheless, with the recent increase in ready-to-use medium spatial resolution satellite products (i.e., the Landsat surface reflectance higher-level data products), the potential to combine several medium-resolution sensors into one data stream (Wulder et al., 2015), and the increase in cloud-based processing environments that have many standard disturbance detection algorithms already implemented (e.g., Google Earth Engine), it is likely that the use of medium spatial resolution remote sensing for (near) real-time monitoring of forest insect disturbances will increase significantly in the future. Also, Unmanned Aerial Vehicles (UAVs) might significantly improve rapid assessments of insect disturbances, yet there are only few studies testing the potential of UAVs for insect disturbance mapping (Nasi et al., 2015).
Moreover, remote sensing has been used to assess the susceptibility of forests to insect infestation, which in turn can guide management action to prevent or contain the eruption of large-scale outbreaks. Approaches included the use of hyper-spectral imagery to detect changes in chlorophyll absorption as indicator of tree vigor (Lausch et al., 2013), and using Landsat time series to assess the stress-level of forest stands as an indicator of their susceptibility to bark beetle attacks (Hais et al., 2016). The former approach might be substantially enhanced by the recent development of space-born hyper-spectral instruments such as the Environmental Mapping and Analysis Program (EnMAP) mission (Foerster et al., 2016).
4.5. Identify insect disturbances in coupled human-natural systems
Forests in many parts of the world are coupled human and natural systems, i.e. their dynamics is an emerging property of an integrated and interactive set of social and ecological drivers (Liu et al., 2007). Frequently, forests prone to insect infestation are under active forest management, resulting in human disturbances (i.e., sanitation and/or salvage harvest) superimposing the insect disturbance signal. Hence, it is likely that the spectral properties of insect disturbances in managed forests resemble the spectral properties of harvest or selective logging. As a consequence, studies mapping disturbances in managed forests might often underestimate the true rate of insect disturbance. Overcoming this issue is challenging since it requires separating between scheduled harvests and salvage/sanitation harvests, which might require incorporating auxiliary data that is not always available (i.e., aerial overview surveys; Schroeder et al. (2012)). Nevertheless, the increased use of dense and very dense time series will support detecting multiple disturbances such as salvage logging following an insect disturbance, and separating between different harvest types (Jarron et al., 2016).
4.6. Improve reference data for assessing insect disturbances
During our systematic review, we found that field data was the major source of reference data for calibrating and validating insect disturbance models. Field data is the ‘gold standard’ in modeling, since it represents exact measurements that depict the true condition on the ground (ground-truthing). However, field data is also costly to acquire, which limits its use over large geographic extents. Some studies therefore made use of existing inventory data that monitor relevant measurements (i.e., changes in basal area deadwood) over time (Meigs et al., 2015b). However, forest inventory data are not consistently available across countries (Gschwantner et al., 2016; Levers et al., 2014; Neumann et al., 2016) and are often difficult to combine with remote sensing data due to mismatch in plot size and spatial resolution of remote sensing sensors. To better integrate existing field and inventory data with remote sensing approaches we suggest two improvements: First, we urge that all researcher publicly archive their field data upon publication (e.g., in archives such as Dryad or Figshare), explicitly including spatial metadata to enable a linkage with remote sensing information. Second, researchers and managers planning new inventories or field campaigns could include considerations of remote sensing in their campaign designs to accrue added value from fusing terrestrial and earth observation data sources in the future.
While field data was overall the most important source of calibration and validation data, the interpretation of very-high spatial resolution imagery was especially important as reference data for mapping bark beetle disturbances. Bark beetle disturbances can be easily delineated in very-high resolution imagery, which makes it an important data source for forest management (Meddens et al., 2011). Very-high resolution imagery also allows for creating reference data over large geographic extents at relatively low costs (Olofsson et al., 2014). Finally, it also can help scaling field data to medium spatial resolution remote sensing data (Wulder et al., 2004). An increased availability and accessibility of very-high spatial resolution imagery can thus significantly improve the creation of high-quality reference data sets for calibrating remote sensing models of insect disturbances. The often large databases of many governmental agencies hold great potential in this regard (e.g., https://eros.usgs.gov/aerial-photography for the US or http://www.nrcan.gc.ca/earth-sciences/geomatics/satellite-imagery-air-photos/9265 for Canada). However, the data must be made accessible to researchers at low costs.
A relatively new method for acquiring reference data is the use of Landsat spectral trajectories, that is the interpretation of disturbance occurrence and agents using dense Landsat time series and corresponding image chips (see Cohen et al. (2010) for further information). Interpreting Landsat spectral trajectories and image chips though trained interpreters allows for an assessment of forest disturbances at the plot level over very large geographic extents, and was used successfully in various recent studies as tool for obtaining calibration and validation data (Hermosilla et al., 2015; Kennedy et al., 2012; Meigs et al., 2015b; Potapov et al., 2015; Senf et al., 2015). However, while high-severity disturbances such as harvest and fire can be easily identified in spectral trajectories, it is much more challenging to detect the subtle disturbances caused by many insects. The quality of reference data thus varies with disturbance severity and with the density of observations (Cohen et al., 2010).
5. Conclusion
We here presented a systematic review of the literature on the remote sensing of insect disturbances. We found that specific methods and sensors are particular useful for specific types of insect agents – that is bark beetles, defoliators of broadleaved trees, and defoliators of coniferous trees. While our review documents a significant increase in studies mapping insect disturbances in recent years, we also identified several challenges with current approach. To overcome those challenges, we suggest six areas of improvement:
Separate insect disturbances from other agents;
Extend the spatial and temporal domain of analysis;
Make use of dense time series;
Operationalize near-real time monitoring of insect disturbances;
Identify insect disturbances in the context of coupled human-natural systems; and
Improve reference data for assessing insect disturbances;
Addressing these issues in future studies can help integrating remote sensing-based maps into operational forest management, and support an inclusion of insect disturbances as integrated processes into regional to global ecosystem models.
Acknowledgements
The work leading to this publication was supported by the German Academic Exchange Service (DAAD) with funds from the German Federal Ministry of Education and Research (BMBF) and the People Programme (Marie Curie Actions) of the European Union’s Seventh Framework Programme (FP7/2007-2013) under REA grant agreement Nr. 605728 (P.R.I.M.E. – Postdoctoral Researchers International Mobility Experience). RS acknowledges support from a START grant of the Austrian Science Fund FWF (grant agreement Y895-B25). This work contributed to the Landsat Science Team (https://landsat.usgs.gov/landsat-science-team).
Footnotes
TS=Topic search, including title, abstract, and author keywords. Asterisks (*) are wildcards for any type and number or character.
References
- Adelabu S, Mutanga O, Adam E. Evaluating the impact of red-edge band from Rapideye image for classifying insect defoliation levels. ISPRS J Photogramm Remote Sens. 2014;95:34–41. [Google Scholar]
- Assal TJ, Sibold J, Reich R. Modeling a historical mountain pine beetle outbreak using landsat MSS and multiple lines of evidence. Remote Sens Environ. 2014;155:275–288. [Google Scholar]
- Babst F, Esper J, Parlow E. Landsat TM/ETM plus and tree-ring based assessment of spatiotemporal patterns of the autumnal moth (Epirrita autumnata) in northernmost Fennoscandia. Remote Sens Environ. 2010;114:637–646. [Google Scholar]
- Beudert B, Bassler C, Thorn S, Noss R, Schroder B, Dieffenbach-Fries H, Foullois N, Muller J. Bark beetles increase biodiversity while maintaining drinking water quality. Conser Lett. 2015;8:272–281. [Google Scholar]
- Bright BC, Hudak AT, McGaughey R, Andersen HE, Negron J. Predicting live and dead tree basal area of bark beetle affected forests from discrete-return lidar. Can J Remote Sens. 2013;39:S99–S111. [Google Scholar]
- Bright BC, Hudak AT, Kennedy RE, Meddens AJH. Landsat time series and lidar as predictors of live and dead basal area across five bark beetle-Affected forests. IEEE J Sel Top Appl Earth Obs Remote Sens. 2014;7:3440–3452. [Google Scholar]
- Cohen WB, Yang Z, Kennedy R. Detecting trends in forest disturbance and recovery using yearly Landsat time series: 2: timeSync—tools for calibration and validation. Remote Sens Environ. 2010;114:2911–2924. [Google Scholar]
- Cook S, Cherry S, Humes K, Guldin J, Williams C. Development of a satellite-based hazard rating system for Dendroctonus frontallis (Coleoptera: Scolytidae) in the Ouachita Mountains of Arkansas. J Econ Entomol. 2007;100:381–388. doi: 10.1603/0022-0493(2007)100[381:doashr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Cooke BJ, Nealis VG, Regniere J. Insect defoliators as periodic disturbances in northern forest ecosystems. In: Johnson EA, Miyanishi K, editors. Plant Disturbance Ecology: the Process and the Response. Elsevier; Burlington, MA: 2007. pp. 487–525. [Google Scholar]
- Coops NC, Wulder MA, White JC. Integrating remotely sensed and ancillary data sources to characterize a mountain pine beetle infestation. Remote Sens Environ. 2006;105:83–97. [Google Scholar]
- Dale VH, Joyce LA, McNulty S, Neilson RP, Ayres MP, Flannigan MD, Hanson PJ, Irland LC, Lugo AE, Peterson CJ, Simberloff D, et al. Climate change and forest disturbances. Bioscience. 2001;51:723–734. [Google Scholar]
- De Beurs K, Townsend P. Estimating the effect of gypsy moth defoliation using MODIS. Remote Sens Environ. 2008;112:3983–3990. [Google Scholar]
- DeRose RJ, Long JN, Ramsey RD. Combining dendrochronological data and the disturbance index to assess Engelmann spruce mortality caused by a spruce beetle outbreak in southern Utah, USA. Remote Sens Environ. 2011;115:2342–2349. [Google Scholar]
- Edburg SL, Hicke JA, Brooks PD, Pendall EG, Ewers BE, Norton U, Gochis D, Gutmann ED, Meddens AJH. Cascading impacts of bark beetle-caused tree mortality on coupled biogeophysical and biogeochemical processes. Front Ecol Environ. 2012;10:416–424. [Google Scholar]
- Eklundh L, Johansson T, Solberg S. Mapping insect defoliation in Scots pine with MODIS time-series data. Remote Sens Environ. 2009;113:1566–1573. [Google Scholar]
- Fassnacht FE, Latifi H, Koch B. An angular vegetation index for imaging spectroscopy data-Preliminary results on forest damage detection in the Bavarian National Park, Germany. Int J Appl Earth Obs Geoinf. 2012;19:308–321. [Google Scholar]
- Fassnacht FE, Latifi H, Ghosh A, Joshi PK, Koch B. Assessing the potential of hyperspectral imagery to map bark beetle-induced tree mortality. Remote Sens Environ. 2014;140:533–548. [Google Scholar]
- Foerster S, Carrère V, Rast M, Staenz K. Preface: the environmental mapping and analysis program (EnMAP) mission: prep aring for its scientific exploitation. Remote Sens. 2016;8:957. [Google Scholar]
- Franklin A, Waring RH, McGreight RW, Cohen WB, Fiorella M. Aerial and satellite sensor detection and classification of western spruce budworm defoliation in a subalpine forest. Can J Remote Sens. 1995;21:299–308. [Google Scholar]
- Franklin SE, Wulder MA, Skakun RS, Carroll AL. Mountain pine beetle red-attack forest damage classification using stratified landsat ™ data in british columbia, Canada. Photogramm Eng Remote Sens. 2003;69:283–288. [Google Scholar]
- Franklin SE, Fan H, Guo X. Relationship between Landsat TM and SPOT vegetation indices and cumulative spruce budworm defoliation. Int J Remote Sens. 2008;29:1215–1220. [Google Scholar]
- Frantz D, Röder A, Stellmes M, Hill J. Phenology-adaptive pixel-based compositing using optical earth observation imagery. Remote Sens Environ. 2017;190:331–347. [Google Scholar]
- Fraser RH, Latifovic R. Mapping insect-induced tree defoliation and mortality using coarse spatial resolution satellite imagery. Int J Remote Sens. 2005;26:193–200. [Google Scholar]
- Fraser RH, Abuelgasim A, Latifovic R. A method for detecting large-scale forest cover change using coarse spatial resolution imagery. Remote Sens Environ. 2005;95:414–427. [Google Scholar]
- Gartner P, Forster M, Kleinschmit B. The benefit of synthetically generated RapidEye and Landsat 8 data fusion time series for riparian forest disturbance monitoring. Remote Sens Environ. 2016;177:237–247. [Google Scholar]
- Gilichinsky M, Olsson H, Solberg S. Reflectance changes due to pine sawfly attack detected using multitemporal SPOT satellite data. Remote Sens Lett. 2013;4:10–18. [Google Scholar]
- Goodwin NR, Coops NC, Wulder MA, Gillanders S, Schroeder TA, Nelson T. Estimation of insect infestation dynamics using a temporal sequence of Landsat data. Remote Sens Environ. 2008;112:3680–3689. [Google Scholar]
- Gooshbor L, Pir Bavaghar M, Amanollahi J, Ghobari H. Monitoring infestations of oak forests by tortrix viridana (Lepidoptera: tortricidae) using remote sensing. Plant Prot Sci. 2016;52:270–276. [Google Scholar]
- Gschwantner T, Lanz A, Vidal C, Bosela M, Di Cosmo L, Fridman J, Gasparini P, Kuliešis A, Tomter S, Schadauer K. Comparison of methods used in European National Forest Inventories for the estimation of volume increment: towards harmonisation. Ann For Sci. 2016;73:807–821. [Google Scholar]
- Hais M, Wild J, Berec L, Brůna J, Kennedy R, Braaten J, Brož Z. Landsat imagery spectral Trajectories—important variables for spatially predicting the risks of bark beetle disturbance. Remote Sens. 2016;8:687. [Google Scholar]
- Hall RJ, Fernandes RA, Hogg EH, Brandt JP, Butson C, Case BS, Leblanc SG. Relating aspen defoliation to changes in leaf area derived from field and satellite remote sensing data. Can J Remote Sens. 2003;29:299–313. [Google Scholar]
- Hall RJ, Castilla G, White JC, Cooke BJ, Skakun RS. Remote sensing of forest pest damage: a review and lessons learned from a Canadian perspective. Can Entomol. 2016:1–61. [Google Scholar]
- Hanavan RP, Pontius J, Hallett R. A 10-Year assessment of hemlock decline in the catskill mountain region of new York state using hyperspectral remote sensing techniques. J Econ Entomol. 2015;108:339–349. doi: 10.1093/jee/tou015. [DOI] [PubMed] [Google Scholar]
- Hart SJ, Veblen TT. Detection of spruce beetle-induced tree mortality using high- and medium-resolution remotely sensed imagery. Remote Sens Environ. 2015;168:134–145. [Google Scholar]
- Havasova M, Bucha T, Ferencik J, Jakus R. Applicability of a vegetation indices-based method to map bark beetle outbreaks in the High Tatra Mountains. Ann For Res. 2015;58:295–310. [Google Scholar]
- Hermosilla T, Wulder MA, White JC, Coops NC, Hobart GW. Regional detection, characterization: and attribution of annual forest change from 1984 to 2012 using Landsat-derived time-series metrics. Remote Sens Environ. 2015;170:121–132. [Google Scholar]
- Hermosilla T, Wulder MA, White JC, Coops NC, Hobart GW, Campbell LB. Mass data processing of time series Landsat imagery: pixels to data products for forest monitoring. Int J Digital Earth. 2016;9:1035–1054. [Google Scholar]
- Hicke JA, Logan J. Mapping whitebark pine mortality caused by a mountain pine beetle outbreak with high spatial resolution satellite imagery. Int J Remote Sens. 2009;30:4427–4441. [Google Scholar]
- Hicke JA, Allen CD, Desai AR, Dietze MC, Hall RJ, Ted Hogg EH, Kashian DM, Moore D, Raffa KF, Sturrock RN, Vogelmann J. Effects of biotic disturbances on forest carbon cycling in the United States and Canada. Global Change Biol. 2012;18:7–34. [Google Scholar]
- Hilker T, Coops NC, Gaulton R, Wulder MA, Cranston J, Stenhouse G. Biweekly disturbance capture and attribution: case study in western Alberta grizzly bear habitat. J Appl Remote Sens. 2011;5:053568–053568. [Google Scholar]
- Immitzer M, Atzberger C. Early detection of bark beetle infestation in Norway spruce (Picea abies, L.) using worldView-2 data. Photogramm Fernerkundung Geoinf. 2014:351–367. [Google Scholar]
- Janda P, Trotsiuk V, Mikoláš M, Bače R, Nagel TA, Seidl R, Seedre M, Morrissey RC, Kucbel S, Jaloviar P, Jasík M, et al. The historical disturbance regime of mountain Norway spruce forests in the Western Carpathians and its influence on current forest structure and composition. For Ecol Manage. 2016;388:67–78. doi: 10.1016/j.foreco.2016.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarron L, Hermosilla T, Coops N, Wulder M, White J, Hobart G, Leckie D. Differentiation of alternate harvesting practices using annual time series of landsat data. Forests. 2016;8:15. [Google Scholar]
- Jepsen JU, Hagen SB, Hogda KA, Ims RA, Karlsen SR, Tommervik H, Yoccoz NG. Monitoring the spatio-temporal dynamics of geometrid moth outbreaks in birch forest using MODIS-NDVI data. Remote Sens Environ. 2009;113:1939–1947. [Google Scholar]
- Joensson P, Eklundh L. TIMESAT – a program for analyzing time-series of satellite sensor data. Comput Geosci. 2004;30:833–845. [Google Scholar]
- Jones C, Song CH, Moody A. Where's woolly: an integrative use of remote sensing to improve predictions of the spatial distribution of an invasive forest pest the Hemlock Woolly Adelgid. For Ecol Manage. 2015;358:222–229. [Google Scholar]
- Kantola T, Lyytikainen-Saarenmaa P, Coulson RN, Holopainen M, Tchakerian MD, Streett DA. Development of monitoring methods for Hemlock Woolly Adelgid induced tree mortality within a Southern Appalachian landscape with inhibited access. Iforest-Biogeosci For. 2016;9:178–186. [Google Scholar]
- Kautz M, Meddens AJH, Hall RJ, Arneth A. Biotic disturbances in Northern Hemisphere forests – a synthesis of recent data, uncertainties and implications for forest monitoring and modelling. Global Ecol Biogeogr. 2016 doi: 10.1111/geb.12558. [DOI] [Google Scholar]
- Kennedy RE, Yang ZG, Cohen WB. Detecting trends in forest disturbance and recovery using yearly Landsat time series: 1. LandTrendr – Temporal segmentation algorithms. Remote Sens Environ. 2010;114:2897–2910. [Google Scholar]
- Kennedy RE, Yang ZQ, Cohen WB, Pfaff E, Braaten J, Nelson P. Spatial and temporal patterns of forest disturbance and regrowth within the area of the Northwest Forest Plan. Remote Sens Environ. 2012;122:117–133. [Google Scholar]
- Kennedy RE, Yang Z, Braaten J, Copass C, Antonova N, Jordan C, Nelson P. Attribution of disturbance change agent from Landsat time-series in support of habitat monitoring in the Puget Sound region, USA. Remote Sens Environ. 2015;166:271–285. [Google Scholar]
- Kharuk VI, Ranson KJ, Kozuhovskaya AG, Kondakov YP, Pestunov IA. NOAA/AVHRR satellite detection of Siberian silkmoth outbreaks in eastern Siberia. Int J Remote Sens. 2004;25:5543–5555. [Google Scholar]
- Kharuk VI, Ranson KJ, Fedotova EV. Spatial pattern of Siberian silkmoth outbreak and taiga mortality. Scand J For Res. 2007;22:531–536. [Google Scholar]
- Kurz WA, Dymond CC, Stinson G, Rampley GJ, Neilson ET, Carroll AL, Ebata T, Safranyik L. Mountain pine beetle and forest carbon feedback to climate change. Nature. 2008;452:987–990. doi: 10.1038/nature06777. [DOI] [PubMed] [Google Scholar]
- Långström B, Hellqvist CC. Comparison of methods for estimation of needle losses in scots pine following defoliation by bupalus piniaria. Silva Fennica. 2004 Jan;38:15–27. [Google Scholar]
- Latifi H, Fassnacht FE, Schumann B, Dech S. Object-based extraction of bark beetle (Ips typographus L.) infestations using multi-date LANDSAT and SPOT satellite imagery. Prog Phys Geogr. 2014a;38:755–785. [Google Scholar]
- Latifi H, Schumann B, Kautz M, Dech S. Spatial characterization of bark beetle infestations by a multidate synergy of SPOT and Landsat imagery. Environ Monit Assess. 2014b;186:441–456. doi: 10.1007/s10661-013-3389-7. [DOI] [PubMed] [Google Scholar]
- Lausch A, Heurich M, Gordalla D, Dobner HJ, Gwillym-Margianto S, Salbach C. Forecasting potential bark beetle outbreaks based on spruce forest vitality using hyperspectral remote-sensing techniques at different scales. For Ecol Manage. 2013;308:76–89. [Google Scholar]
- Lausch A, Erasmi S, King D, Magdon P, Heurich M. Understanding forest health with remote sensing – Part I—a review of spectral traits, processes and remote-sensing characteristics. Remote Sens. 2016;8:1029. [Google Scholar]
- Lawrence R, Labus M. Early detection of Douglas-Fir beetle infestation with subcanopy resolution hyperspectral imagery. Western J Appl For. 2003;18:202–206. [Google Scholar]
- Leckie DG, Ostaff DP. Classification if airborne multispectral scanner data for mapping current defoliation by the spruce budworm. For Sci. 1988;34:259–275. [Google Scholar]
- Levers C, Verkerk PJ, Müller D, Verburg PH, Butsic V, Leitão PJ, Lindner M, Kuemmerle T. Drivers of forest harvesting intensity patterns in Europe. For Ecol Manage. 2014;315:160–172. [Google Scholar]
- Liang L, Chen Y, Hawbaker T, Zhu Z, Gong P. Mapping mountain pine beetle mortality through growth trend analysis of time-series landsat data. Remote Sens. 2014;6:5696–5716. [Google Scholar]
- Liu J, Dietz T, Carpenter SR, Alberti M, Folke C, Moran E, Pell AN, Deadman P, Kratz T, Lubchenco J, Ostrom E, et al. Complexity of coupled human and natural systems. Science. 2007;317:1513–1516. doi: 10.1126/science.1144004. [DOI] [PubMed] [Google Scholar]
- Long JA, Lawrence RL. Mapping percent tree mortality due to mountain pine beetle damage. For Sci. 2016;62:392–402. [Google Scholar]
- Luther JE, Franklin SE, Hudak J, Meades JP. Forecasting the susceptibility and vulnerability of balsam fir stands to insect defoliation with Landsat Thematic Mapper data. Remote Sens Environ. 1997;59:77–91. [Google Scholar]
- Müller J, Bußler H, Goßner M, Rettelbach T, Duelli P. The European spruce bark beetle Ips typographus in a national park: from pest to keystone species. Biodivers Conserv. 2008;17:2979–3001. [Google Scholar]
- McDowell NG, Coops NC, Beck PS, Chambers JQ, Gangodagamage C, Hicke JA, Huang CY, Kennedy R, Krofcheck DJ, Litvak M, Meddens AJ, et al. Global satellite monitoring of climate-induced vegetation disturbances. Trends Plant Sci. 2015;20:114–123. doi: 10.1016/j.tplants.2014.10.008. [DOI] [PubMed] [Google Scholar]
- Meddens AJH, Hicke JA. Spatial and temporal patterns of Landsat-based detection of tree mortality caused by a mountain pine beetle outbreak in Colorado, USA. For Ecol Manage. 2014;322:78–88. [Google Scholar]
- Meddens AJH, Hicke JA, Vierling LAI. Evaluating the potential of multispectral imagery to map multiple stages of tree mortality. Remote Sens Environ. 2011;115:1632–1642. [Google Scholar]
- Meddens AJH, Hicke JA, Vierling LA, Hudak AT. Evaluating methods to detect bark beetle-caused tree mortality using single-date and multi-date Landsat imagery. Remote Sens Environ. 2013;132:49–58. [Google Scholar]
- Meigs GW, Kennedy RE, Cohen WB. A Landsat time series approach to characterize bark beetle and defoliator impacts on tree mortality and surface fuels in conifer forests. Remote Sens Environ. 2011;115:3707–3718. [Google Scholar]
- Meigs GW, Campbell JL, Zald HSJ, Bailey JD, Shaw DC, Kennedy RE. Does wildfire likelihood increase following insect outbreaks in conifer forests? Ecosphere. 2015a;6 art118. [Google Scholar]
- Meigs GW, Kennedy RE, Gray AN, Gregory MJ. Spatiotemporal dynamics of recent mountain pine beetle and western spruce budworm outbreaks across the Pacific Northwest Region, USA. For Ecol Manage. 2015b;339:71–86. [Google Scholar]
- Millar CI, Stephenson NL. Temperate forest health in an era of emerging megadisturbances. Science. 2015;349:823–826. doi: 10.1126/science.aaa9933. [DOI] [PubMed] [Google Scholar]
- Muchoney DM, Haack BN. Change detection for monitoring forest defoliation. Photogramm Eng Remote Sens. 1994;60:1243–1251. [Google Scholar]
- Murfitt J, He YH, Yang J, Mui A, De Mille K. Ash decline assessment in emerald ash borer infested natural forests using high spatial resolution images. Remote Sensing. 2016;8 [Google Scholar]
- Nasi R, Honkavaara E, Lyytikainen-Saarenmaa P, Blomqvist M, Litkey P, Hakala T, Viljanen N, Kantola T, Tanhuanpaa T, Holopainen M. Using UAV-based photogrammetry and hyperspectral imaging for mapping bark beetle damage at tree-level. Remote Sens. 2015;7:15467–15493. [Google Scholar]
- Neumann M, Moreno A, Mues V, Härkönen S, Mura M, Bouriaud O, Lang M, Achten WMJ, Thivolle-Cazat A, Bronisz K, Merganič J, et al. Comparison of carbon estimation methods for European forests. For Ecol Manage. 2016;361:397–420. [Google Scholar]
- Nielsen MM, Heurich M, Malmberg B, Brun A. Automatic mapping of standing dead trees after an insect outbreak using the window independent context segmentation method. J For. 2014;112:564–571. [Google Scholar]
- Olofsson P, Foody GM, Herold M, Stehman SV, Woodcock CE, Wulder MA. Good practices for estimating area and assessing accuracy of land change. Remote Sens Environ. 2014;148:42–57. [Google Scholar]
- Olson DM, Dinerstein E, Wikramanayake ED, Burgess ND, Powell GVN, Underwood EC, D'amico JA, Itoua I, Strand HE, Morrison JC, Loucks CJ, et al. Terrestrial Ecoregions of the World: a New Map of Life on Earth: a new global map of terrestrial ecoregions provides an innovative tool for conserving biodiversity. Bioscience. 2001;51:933–938. [Google Scholar]
- Olsson PO, Jonsson AM, Eklundh L. A new invasive insect in Sweden – Physokermes inopinatus: tracing forest damage with satellite based remote sensing. For Ecol Manage. 2012;285:29–37. [Google Scholar]
- Olsson PO, Kantola T, Lyytikainen-Saarenmaa P, Jonsson AM, Eklundh L. Development of a method for monitoring of insect induced forest defoliation – limitation of MODIS data in Fennoscandian forest landscapes. Silva Fennica. 2016a;50 [Google Scholar]
- Olsson PO, Lindstrom J, Eldundh L. Near real-time monitoring of insect induced defoliation in subalpine birch forests with MODIS derived NDVI. Remote Sens Environ. 2016b;181:42–53. [Google Scholar]
- Ortiz SM, Breidenbach J, Kandler G. Early detection of bark beetle green attack using terraSAR-X and RapidEye data. Remote Sens. 2013;5:1912–1931. [Google Scholar]
- Paritsis J, Veblen TT, Smith JM, Holz A. Spatial prediction of caterpillar (Ormiscodes) defoliation in Patagonian Nothofagus forests. Landscape Ecol. 2011;26:791–803. [Google Scholar]
- Pflugmacher D, Cohen WB, Kennedy RE. Using Landsat-derived disturbance history (1972–2010) to predict current forest structure. Remote Sens Environ. 2012;122:146–165. [Google Scholar]
- Pontius J, Martin M, Plourde L, Hallett R. Ash decline assessment in emerald ash borer-infested regions: a test of tree-level: hyperspectral technologies. Remote Sens Environ. 2008;112:2665–2676. [Google Scholar]
- Potapov PV, Turubanova SA, Tyukavina A, Krylov AM, McCarty JL, Radeloff VC, Hansen MC. Eastern Europe's forest cover dynamics from 1985 to 2012 quantified from the full Landsat archive. Remote Sens Environ. 2015;159:28–43. [Google Scholar]
- Régnière J, Saint-Amant R. BioSIM 10 – User’s Manual. Laurentian Forestry Centre; Québec: 2014. [Google Scholar]
- Radeloff VC, Mladenoff DJ, Boyce MS. Detecting jack pine budworm defoliation using spectral mixture analysis: separating effects from determinants. Remote Sens Environ. 1999;69:156–169. [Google Scholar]
- Raffa KF, Berryman AA. The role of host plant resistance in the colonization behavior and ecology of bark beetles (Coleoptera: scolytidae) Ecol Monogr. 1983;53:27–49. [Google Scholar]
- Raffa KF, Aukema BH, Bentz BJ, Carroll AL, Hicke JA, Turner MG, Romme WH. Cross-scale drivers of natural disturbances prone to anthropogenic amplification: the dynamics of bark beetle eruptions. Bioscience. 2008;58:501. [Google Scholar]
- Raffa KF, Aukema B, Bentz BJ, Carroll A, Erbilgin N, Herms DA, Hicke JA, Hofstetter RW, Katovich S, Lindgren BS. A Literal Use of Forest Health Safeguards Against Misuse and Misapplication. 2009 [Google Scholar]
- Royle DD, Lathrop RG. Monitoring hemlock forest health in New Jersey using Landsat TM data and change detection techniques. For Sci. 1997;43:327–335. [Google Scholar]
- Royle DD, Lathrop RG. Discriminating Tsuga canadensis hemlock forest defoliation using remotely sensed change detection. J Nematol. 2002;34:213–221. [PMC free article] [PubMed] [Google Scholar]
- Rullan-Silva CD, Olthoff AE, Delgado de la Mata JA, Pajares-Alonso JA. Remote monitoring of forest insect defoliation – a review. For Syst. 2013;22:377. [Google Scholar]
- Rullan-Silva C, Olthoff AE, Pando V, Pajares JA, Delgado JA. Remote monitoring of defoliation by the beech leaf-mining weevil Rhynchaenus fagi in northern Spain. For Ecol Manage. 2015;347:200–208. [Google Scholar]
- Sangüesa-Barreda G, Camarero JJ, García-Martín A, Hernández R, de la Riva J. Remote-sensing and tree-ring based characterization of forest defoliation and growth loss due to the Mediterranean pine processionary moth. For Ecol Manage. 2014;320:171–181. [Google Scholar]
- Schroeder TA, Wulder MA, Healey SP, Moisen GG. Detecting post-fire salvage logging from Landsat change maps and national fire survey data. Remote Sens Environ. 2012;122:166–174. [Google Scholar]
- Seidl R, Rammer W. Climate change amplifies the interactions between wind and bark beetle disturbances in forest landscapes. Landscape Ecol. 2016 doi: 10.1007/s10980-016-0396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl R, Fernandes PM, Fonseca TF, Gillet F, Jönsson AM, Merganičová K, Netherer S, Arpaci A, Bontemps J-D, Bugmann H, González-Olabarria JR, et al. Modelling natural disturbances in forest ecosystems: a review. Ecol Modell. 2011;222:903–924. [Google Scholar]
- Seidl R, Schelhaas MJ, Rammer W, Verkerk PJ. Increasing forest disturbances in Europe and their impact on carbon storage. Nat Clim Change. 2014;4:806–810. doi: 10.1038/nclimate2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl R, Muller J, Hothorn T, Bassler C, Heurich M, Kautz M. Small beetle: large-scale drivers: how regional and landscape factors affect outbreaks of the European spruce bark beetle. J Appl Ecol. 2015;53:530–540. doi: 10.1111/1365-2664.12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senf C, Pflugmacher D, Wulder MA, Hostert P. Characterizing spectral–temporal patterns of defoliator and bark beetle disturbances using Landsat time series. Remote Sens Environ. 2015;170:166–177. [Google Scholar]
- Senf C, Wulder MA, Campbell EM, Hostert P. Using Landsat to assess the relationship between spatiotemporal patterns of western spruce budworm outbreaks and regional-scale weather variability. Can J Remote Sens. 2016;42:706–718. [Google Scholar]
- Senf C, Campbell EM, Pflugmacher D, Wulder MA, Hostert P. A multi-scale analysis of western spruce budworm outbreak dynamics. Landscape Ecol. 2017;32:501–514. [Google Scholar]
- Simard M, Powell EN, Raffa KF, Turner MG. What explains landscape patterns of tree mortality caused by bark beetle outbreaks in Greater Yellowstone? Global Ecol Biogeogr. 2012;21:556–567. [Google Scholar]
- Skakun RS, Wulder MA, Franklin SE. Sensitivity of the thematic mapper enhanced wetness difference index to detect mountain pine beetle red-attack damage. Remote Sens Environ. 2003;86:433–443. [Google Scholar]
- Solberg S, Næsset E, Hanssen KH, Christiansen E. Mapping defoliation during a severe insect attack on Scots pine using airborne laser scanning. Remote Sens Environ. 2006;102:364–376. [Google Scholar]
- Solberg S. Mapping gap fraction: LAI and defoliation using various ALS penetration variables. Int J Remote Sens. 2010;31:1227–1244. [Google Scholar]
- Spruce JP, Sader S, Ryan RE, Smoot J, Kuper P, Ross K, Prados D, Russell J, Gasser G, McKellip R. Assessment of MODIS NDVI time series data products for detecting forest defoliation by gypsy moth outbreaks. Remote Sens Environ. 2011;115:427–437. [Google Scholar]
- Swetnam TW, Lynch AM. Multicentury: regional-scale patterns of western spruce budworm outbreaks. Ecol Monogr. 1993;63:399–424. [Google Scholar]
- Thayn JB. Using a remotely sensed optimized Disturbance Index to detect insect defoliation in the Apostle Islands, Wisconsin, USA. Remote Sens Environ. 2013;136:210–217. [Google Scholar]
- Townsend PA, Singh A, Foster JR, Rehberg NJ, Kingdon CC, Eshleman KN, Seagle SW. A general Landsat model to predict canopy defoliation in broadleaf deciduous forests. Remote Sens Environ. 2012;119:255–265. [Google Scholar]
- Trumbore S, Brando P, Hatmann H. Forest health and global change. Science. 2015;349:814–818. doi: 10.1126/science.aac6759. [DOI] [PubMed] [Google Scholar]
- Vastaranta M, Kantola T, Lyytikäinen-Saarenmaa P, Holopainen M, Kankare V, Wulder M, Hyyppä J, Hyyppä H. Area-based mapping of defoliation of scots pine stands using airborne scanning LiDAR. Remote Sens. 2013;5:1220–1234. [Google Scholar]
- Verbesselt J, Robinson A, Stone C, Culvenor D. Forecasting tree mortality using change metrics derived from MODIS satellite data. For Ecol Manage. 2009;258:1166–1173. [Google Scholar]
- Verbesselt J, Zeileis A, Herold M. Near real-time disturbance detection using satellite image time series. Remote Sens Environ. 2012;123:98–108. [Google Scholar]
- Vogelmann JE, Rock BN. Use of thematic mapper data for the detection of forest damage caused by the pear thrips. Remote Sens Environ. 1989;30:217–225. [Google Scholar]
- Vogelmann JE, Tolk B, Zhu ZL. Monitoring forest changes in the southwestern United States using multitemporal Landsat data. Remote Sens Environ. 2009;113:1739–1748. [Google Scholar]
- Walter JA, Platt RV. Multi-temporal analysis reveals that predictors of mountain pine beetle infestation change during outbreak cycles. For Ecol Manage. 2013;302:308–318. [Google Scholar]
- White JC, Coops NC, Hilker T, Wulder MA, Carroll AL. Detecting mountain pine beetle red attack damage with EO-1 hyperion moisture indices. Int J Remote Sens. 2007;28:2111–2121. [Google Scholar]
- Wulder MA, Hall RJ, Coops NC, Franklin SE. High spatial resolution remotely sensed data for ecosystem characterization. Bioscience. 2004;54:511–521. [Google Scholar]
- Wulder MA, Skakun RS, Dymond CC, Kurz WA, White JC. Characterization of the diminishing accuracy in detecting forest insect damage over time. Can J Remote Sens. 2005;31:421–431. [Google Scholar]
- Wulder MA, Dymond CC, White JC, Leckie DG, Carroll AL. Surveying mountain pine beetle damage of forests: a review of remote sensing opportunities. For Ecol Manage. 2006a;221:27–41. [Google Scholar]
- Wulder MA, White JC, Bentz B, Alvarez MF, Coops NC. Estimating the probability of mountain pine beetle red-attack damage. Remote Sens Environ. 2006b;101:150–166. [Google Scholar]
- Wulder MA, Ortlepp SM, White JC, Coops NC, Coggins SB. Monitoring tree-level insect population dynamics with multi-scale and multi-source remote sensing. J Spat Sci. 2008;53:49–61. [Google Scholar]
- Wulder MA, White JC, Carroll AL, Coops NC. Challenges for the operational detection of mountain pine beetle green attack with remote sensing. For Chron. 2009;85:32–38. [Google Scholar]
- Wulder MA, Masek JG, Cohen WB, Loveland TR, Woodcock CE. Opening the archive: how free data has enabled the science and monitoring promise of Landsat. Remote Sens Environ. 2012;122:2–10. [Google Scholar]
- Wulder MA, Hilker T, White JC, Coops NC, Masek JG, Pflugmacher D, Crevier Y. Virtual constellations for global terrestrial monitoring. Remote Sens Environ. 2015;170:62–76. [Google Scholar]
- Zhu X, Chen J, Gao F, Chen X, Masek JG. An enhanced spatial and temporal adaptive reflectance fusion model for complex heterogeneous regions. Remote Sens Environ. 2010;114:2610–2623. [Google Scholar]
- Zhu Z, Woodcock CE, Olofsson P. Continuous monitoring of forest disturbance using all available Landsat imagery. Remote Sens Environ. 2012;122:75–91. [Google Scholar]