Abstract
In order to gauge ongoing and future changes to disturbance regimes, it is necessary to establish a solid baseline of historic disturbance patterns against which to evaluate these changes. Further, understanding how forest structure and composition respond to variation in past disturbances may provide insight into future resilience to climate-driven alterations of disturbance regimes.
We established 184 plots (mostly 1000 m2) in 14 primary mountain Norway spruce forests in the Western Carpathians. On each plot we surveyed live and dead trees and regeneration, and cored around 25 canopy trees. Disturbance history was reconstructed by examining individual tree growth trends. The study plots were further aggregated into five groups based on disturbance history (severity and timing) to evaluate and explain its influence on forest structure.
These ecosystems are characterized by a mixed severity disturbance regime with high spatiotemporal variability in severity and frequency. However, periods of synchrony in disturbance activity were also found. Specifically, a peak of canopy disturbance was found for the mid-19th century across the region (about 60% of trees established), with the most important periods of disturbance in the 1820s and from the 1840s to the 1870s. Current stand size and age structure were strongly influenced by past disturbance activity. In contrast, past disturbances did not have a significant effect on current tree density, the amount of coarse woody debris, and regeneration. High mean densities of regeneration with height >50 cm (about 1400 individuals per ha) were observed.
Extensive high severity disturbances have recently affected Central European forests, spurring a discussion about the causes and consequences. We found some evidence that forests in the Western Carpathians were predisposed to recent severe disturbance events as a result of synchronized past disturbance activity, which partly homogenized size and age structure and made recent stands more vulnerable to bark beetle outbreak. Our data suggest that these events are still part of the range of natural variability. The finding that regeneration density and volume of coarse woody debris were not influenced by past disturbance illustrates that vastly different past disturbance histories are not likely to change the future trajectories of these forests. These ecosystems currently have high ecological resilience to disturbance. In conclusion, we suggest that management should recognize disturbances as a natural part of ecosystem dynamics in the mountain forests of Central Europe, account for their stochastic occurrence in management planning, and mimic their patterns to foster biodiversity in forest landscapes.
Keywords: Dendroecology, Forest dynamics, Landscape ecology, Disturbance synchronization, Stand structure, Spatio-temporal pattern
1. Introduction
There is widespread concern that climate change will alter natural disturbance regimes and thereby negatively impact forest ecosystems (Dale et al., 2001; Turner, 2010; Easterling et al., 2000). Recent changes in disturbance regimes in some regions, such as increased wildfire activity and large-scale insect outbreaks in North America, have already been attributed to climate change (Westerling et al., 2006; Bentz et al., 2010; Weed et al., 2013). However, in order to gauge ongoing and future changes to disturbance regimes, it is necessary to establish a solid baseline against which to evaluate these changes. This is particularly important given that disturbances are discontinuous processes that usually occur as infrequent events or episodes. Quantifying the natural range of disturbance variation over time periods of several centuries is thus important for understanding potential changes in severe large-scale events with long return intervals (Jarvis and Kulakowski, 2015).
Likewise, understanding how forest structure and composition respond to variation in past disturbances may provide insight into future resilience to climate-driven alterations of disturbance regimes (Tepley and Veblen, 2015; Kneeshaw et al., 2011; Kulakowski et al., 2017). Disturbances are by definition short events relative to the extended time frames of forest dynamics, yet they can have long-lasting effects on forest structure and composition. Disturbances can for instance, alter the age structure of forest landscapes, favor early-seral species, and change the developmental trajectories of forest ecosystems, effects that can persist for centuries after a disturbance event (Frelich, 2002; Johnstone et al., 2010; Nagel et al., 2014). Given such long-lasting impacts of disturbances on forest structure and composition, past disturbances can also strongly influence the current and future provisioning of ecosystem services to human society (Thom and Seidl, 2016). Yet, since the long-term effects of disturbances are poorly understood, the disturbance history of landscapes is currently rarely considered in forest management despite its importance for many ecosystem processes and its bearing on what constitutes “close-to-nature” management.
Disturbance change is particularly relevant for the mountain forest ecosystems of Central Europe, which are dominated by Norway spruce (Picea abies (L.) Karst.). Over the past decades, large areas of these forests have been severely damaged by windstorms (Fink et al., 2009; Holeksa et al., 2017; Schelhaas et al., 2003). Wind disturbances are commonly followed by bark beetle outbreaks (Schroeder and Lindelöw, 2002; Schelhaas et al., 2003; Wermelinger, 2004; Mezei et al., 2014), and together these two agents have resulted in widespread mortality of spruce forests throughout the region. For example, in the Tatra National Park in Slovakia, wind damaged 12,000 ha of forest in a single event in 2004 (Mezei et al., 2014). Subsequently, with the abundance of wind-felled trees serving as low-defense hosts for bark beetles, a large-scale bark beetle outbreak was triggered in the surrounding unmanaged forest reserves (Nikolov et al., 2014). Similarly, a combination of windstorms and bark beetle outbreaks resulted in widespread spruce mortality in the Bavarian Forest and Šumava National Parks (Lausch et al., 2011; Seidl et al., 2016a). Determining whether these recent disturbance events are still within the natural range of variability of the system, or whether they exceed this range as a result of drivers such as climate change or past land use, is critical for making informed decisions regarding the management of these forests.
Much recent effort has therefore been made to determine to what degree severe large-scale disturbances such as those observed recently are part of the natural disturbance regime of Norway spruce forests in Central European mountain ranges. This body of work has mainly relied on dendroecological methods to reconstruct the history of disturbance in remnant primary forest ecosystems in the region. However, the findings of these studies remain inconclusive, with some indicating a regime dominated by small-scale, low severity disturbances (Sproull et al., 2016; Szewczyk et al., 2011) and others finding evidence of larger-scale high severity events having also occurred in the past (Svoboda et al., 2012, 2014; Zielonka et al., 2010; Panayotov et al., 2015; Čada et al., 2016; Holeksa et al., 2016).
Most of these studies have been carried out within single stands or forest landscapes. The few that have addressed past disturbance patterns across larger regions (i.e. over two forest landscapes) document a complex mixed severity regime across space and time, with a predominance of intermediate severity events (Svoboda et al., 2014; Trotsiuk et al., 2014). Moreover, to date no analyses exist that link past disturbance regimes with current patterns of forest structure and composition for these forest types (but see D’Amato et al., 2008; Lecomte et al., 2006; Zenner, 2005 for analyses in other ecosystems). Such analyses would not only provide an important baseline for understanding how future changes in disturbance regimes might influence forests, but would also be valuable for informing post-disturbance management and quantifying forest resilience (Seidl et al., 2016b). In Slovakia, for example, following the recent disturbances, there was much debate among forest managers regarding the future forest development and integrity of these recently disturbed mountain forest ecosystems (Nikolov et al., 2014). The outcomes of this discussion, e.g. with regard to the question of salvage logging and re-planting, have important implications for biodiversity (Thorn et al., 2016; Fritz et al., 2008) and provisioning of ecosystem services (Thom and Seidl, 2016).
Here, we combined dendroecological approaches with an analysis of current forest structure to study primary Norway spruce forests distributed across a range of forest landscapes in the Western Carpathians of Slovakia. Specifically, we reconstructed the overall regional disturbance history and those within 14 forest stands distributed over 7 different landscapes. This extended spatial scope of the analysis was chosen to capture the potentially wide range of past disturbance activity. Subsequently, we examined the effect of past disturbance activity on contemporary forest structure and composition, including current patterns of regeneration and coarse woody debris, to assess if and how past disturbance determines current forest conditions.
2. Methods
2.1. Study area
The study was conducted in the Western Carpathian Mountains in Central Europe. This area is considered to be a biodiversity hotspot within the European temperate zone, with a large number of endemic species and large remaining populations of brown bear (Ursus arctos), Eurasian lynx (Lynx lynx), grey wolf (Canis lupus), and capercaillie (Tetrao urogallus) (Oszlányi et al., 2004; Mikoláš et al., 2015). To study landscape level disturbance dynamics, we selected stands based on the national inventory of primary forests in Slovakia, for which all forests in Slovakia were surveyed in 2009–2010 and approximately 10,000 ha of primary forests were mapped (www.pralesy.sk). Only stands in which no human activity directly affecting the tree layer were categorized as primary forest in the survey, which comprised a complex field survey, historical evidence from local experts, literature and historical military maps from the Austro-Hungarian Empire (from 1764 to 1768 and 1806 to 1869), and aerial images from 1947 to 1950. Furthermore, structural parameters (deadwood volume, natural tree species composition, etc.) were considered in the assessment in the field. All the data and further information on the methodology of their collection are available on the official primary forest inventory web site (www.pralesy.sk). We here focused on primary forest remnants of mountain spruce forests. The study plots were situated in the core areas of the primary forest polygons. Tree species composition in the study area was dominated by Norway spruce (over 90%), while other species, such as rowan (Sorbus aucuparia L.), fir (Abies alba Mill.), beech (Fagus sylvatica L.), maple (Acer pseudoplatanus L.), larch (Larix decidua Mill.), pine (Pinus spp.), and birch (Betula spp.), were admixed. More detailed information about the study area is presented in Table 1.
Table 1.
Characteristics of the study area.
| Landscapes (abbreviations) | Stands (abbreviations) | No. of plots (PSP) | Area of primary stands (hectare) | Mean elevation (m a.s.l.) | Mean annual air temperature (°C) | Mean annual precipitation (mm) | Soil Group (WRB 2014) | Bedrock |
|---|---|---|---|---|---|---|---|---|
| Eastern Tatras (A) | Bielovodská dolina (A1) | 13 | 156 | 1340 | 0–2 | 2000–2400 | Leptosols | Granitoids |
| Javorová dolina (A2) | 8 | 75 | 1439 | 0–2 | 1600–2000 | Podzols | Granitoids | |
| Zadné Med’odoly (A3) | 7 | 34 | 1499 | 0–2 | 1600–2000 | Leptosols | Limestones, dolomites | |
| Central Tatras (B) | Tichá dolina (B1) | 14 | 41 | 1414 | 0–2 | 2000–2400 | Podzols | Limestones, dolomites |
| Hlinná dolina (B2) | 10 | 63 | 1433 | 0–2 | 1200–1600 | Podzols | Metapsammites | |
| Kôprová dolina (B3) | 13 | 122 | 1434 | 0–2 | 2000–2400 | Leptosols | Granitoids | |
| Western Tatras (C) | Osobitá (C1) | 14 | 110 | 1363 | 2–4 | 1200–1600 | Leptosols | Limestones, dolomites |
| Low Tatras (D) | Bystrá dolina (D1) | 15 | 88 | 1410 | 0–2 | 1200–1600 | Podzols | Metapsammites, Granitoids |
| Ďumbier (D2) | 17 | 62 | 1495 | 0–2 | 1200–1600 | Podzols | Granitoids | |
| Great Fatra (E) | Jánošíkova kolkáreň (E1) | 20 | 239 | 1312 | 2–4 | 1200–1600 | Podzols | Granitoids |
| Smrekovica (E2) | 11 | 158 | 1386 | 2–4 | 1200–1600 | Podzols | Granitoids | |
| Orava Beskids (F) | Pilsko (F1) | 12 | 431 | 1329 | 0–2 | 1600–2000 | Podzols | Claystones, Sandstones |
| Babia hora (F2) | 10 | 249 | 1317 | 0–2 | 1200–1600 | Podzols | Claystones, Sandstones | |
| Poľana (G) | Zadná Poľana | 20 | 494 | 1378 | 2–4 | 1000–1200 | Andosols | Andesits |
2.2. Data sampling
Seven landscapes containing 14 stands (Table 1) were selected, and within the stands 184 permanent study plots (PSP) were established using stratified random sampling. A 141.4 * 141.4-m grid was overlaid on each stand. Within each grid cell, a circular sample plot was established at a restricted random position (the inner 0.49 ha core in each 2-ha cell) using GPS (see Svoboda et al., 2014 for further detail). The established PSPs were circular with an area of 1000 m2 (500 m2 in two cases), depending on the stand structure (with smaller sample sizes on plots that were recently disturbed and had a high density of regenerating trees). Elevation, aspect, slope, and slope position were recorded on each PSP. Furthermore, the diameter at breast height (DBH), species, and social status (non-suppressed – at least one half of the crown projection under open canopy condition; suppressed) of all trees with DBH ⩾ 10 cm were recorded. The crown projection in four cardinal directions of five randomly selected trees per plot was measured. The line intersect method (Harmon and Sexton, 1996) was used to measure the amount of downed deadwood with DBH ⩾ 10 cm (CWD), using a total transect length of 100 m per plot, split into five sub-transects of 20 m each. In each PSP all individuals in the regeneration layer were recorded and classified according to three height classes (0.5–1.3 m; 1.3–2.5 m; >2.5 m and <10 cm in DBH). Data on CWD and regeneration from the Babia Hora and Poǐana stands were not collected. Furthermore, 25 randomly selected, non-suppressed trees were cored at a height of 1 m above the ground (15 for 500 m2 plots). One core per tree was extracted perpendicular to the slope direction and prepared by standard dendrochronological procedures. Tree-ring widths were measured with the LintabTM sliding-stage measuring device (Rinntech, Heidelberg) with a resolution of 0.01 mm. In the event that the pith was missing from the core, the estimation of missing rings was done using Duncan’s (1989) method. The cores for which a substantial part of the sample was missing were excluded from further analyses. Finally, cores were visually cross-dated and verified using COFECHA (Holmes, 1983).
2.3. Dendrochronological analysis
Disturbance chronologies were compiled by two types of canopy accession events, as determined from radial growth patterns: (1) open canopy recruitment – rapid juvenile growth rates indicating recruitment in a former canopy gap, and (2) release – abrupt, sustained increases in tree growth indicating mortality of a former canopy (Frelich, 2002). Open canopy recruitment was defined by a threshold separating trees originating in open canopy from those found under closed canopy, based on their juvenile growth rates (Svoboda et al., 2014). Empirical data on the juvenile growth rates of sampled saplings (N = 175) growing under closed canopy and in different sizes of canopy openings were used, comparing their five-year growth rates. Thresholds were calculated using logistic regression and the intersection of specificity (an expression of the likelihood of false negatives) and sensitivity (likelihood of false positives) (Hosmer and Lemeshow, 2004). To obtain a conservative threshold estimate, we considered saplings with growth rates higher than 1.7 mm yr−1 as open canopy recruited, corresponding to gap sizes of more than 500 m2. For growth release detection, we used the boundary line criteria proposed by Black and Abrams (2003) and followed the approach also used by Svoboda et al. (2014). Specific boundary lines from all data of this study were constructed (PGC = 1664.2567 * e(−7.1423 *PG) + 684.7334 * e(−0.8271 *PG); PGC = percent growth change, PG = prior growth following Black and Abrams (2003)). A release event was defined as any growth-change value over 20% of the boundary line. Furthermore, the post-release ring widths had to exceed the pre-event 10-year running mean for at least seven years (Fraver et al., 2009) in order to signify a release event. To minimize the overestimation of disturbance detected by mature trees already in the canopy, we used a DBH threshold for separating trees in two groups, based on the probability of their presence or absence in the canopy (Lorimer and Frelich, 1989). The threshold was estimated in the same way as that for open canopy recruitment, where the DBH distribution of canopy and sub-canopy trees was separated. The DBH threshold for Norway spruce to be classified as a canopy tree in our study was found to be 23 cm without bark, and was calculated as the intersection of specificity and sensitivity (Hosmer and Lemeshow, 2004). Norway spruce is a moderately shade-tolerant species, especially in juvenile phases of development, such that more than one disturbance may be needed for it to reach the canopy (Lorimer and Frelich, 1989). Therefore, multiple canopy accessions were allowed in deriving the disturbance chronologies.
2.4. Construction of the disturbance chronologies
Canopy accession events were summed by decade to construct disturbance chronologies at the plot level. The number of growth releases and gap recruitment events were converted to total canopy area disturbed each decade, following the approach and rationale of Lorimer and Frelich (1989). Linear regression analysis was used to predict canopy area from DBH based on the present vegetation at the PSPs (crown area = 1.1038 + 0.0517 * DBH; R2 = 0.52, p < 0.001). The disturbance chronologies were truncated when the percentage of canopy available in the tree ring sample fell below 10% back through time. To analyze disturbance dynamics at different spatial scales, composite disturbance chronologies were created for the overall region, as well as for the level of landscapes and stands. All the chronologies were constructed in the same way using all events indicating disturbances. Because our focus was on spatial scales of stands and beyond, our approach aggregated disturbance evidence at the plot level without any spatial resolution of the events.
Release was detected by dating the discrete event of growth change, but regenerating trees can take several years or decades to establish and grow to a height of 1 m (coring height) (Kulakowski and Veblen, 2003). To take into account such a prolonged period of tree recruitment, we calculated a 30-year running sum of the percentage of the canopy area disturbed. This approach was used for all analyses with the exception of Fig. 1. Disturbance events were dated using the peak of the local maximum of a 30-year running sum of the percentage of the canopy area disturbed. For easier visualization of the data, we used four disturbance severity classes: Low – below 20%, Moderate – between 20% and 40%, High – 40–60% and Very high – over 60% of canopy was removed. Because we are analyzing mostly regional, landscape and stand scales, some degree of generalization was needed. Our analysis disregards potential low severity disturbance events that occurred within the 30-year periods that are counted as single events. We restricted our analysis to the period from 1790 to 1970 in each plot because in 1790 we observed a drop in the sample depth and the standard deviation (SD) of canopy area disturbed per plot with a continuously decreasing trend back through time. The upper threshold of 1970 was determined by our methodological approach (30-year running sum and missing data in recent gaps, where trees were not large enough to core). Therefore, we roughly assessed the percentage of canopy area removed in the period 1985–2010. This was done with the intersection of two GIS layers, the percentage of canopy area disturbed in the study of Griffiths et al. (2014), and the studied stand polygons delineated by the official primary forest inventory on www.pralesy.sk. This analysis likely underestimates small-scale low severity disturbance events (e.g. removal of single trees). Therefore this recent rate of canopy area removed is very conservative and serves as an illustrative example to show the difference between recent disturbances and historical disturbances.
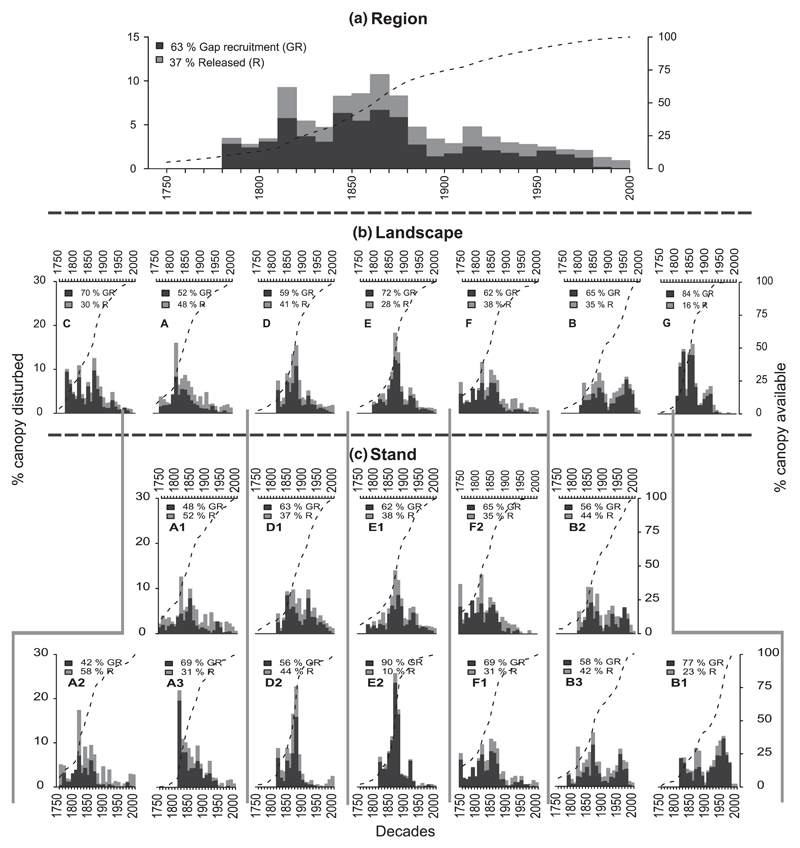
Fig. 1.
Hierarchical scheme of percent canopy disturbed for the Western Carpathian region (all plots pooled) (a), the landscape scale (b), and the stand scale (c). Disturbance chronologies were derived from analyzing two types of events indicating disturbance – gap recruitment and growth release. The percentage of these two types as well as abbreviations identifying the respective stands and landscapes (cf. Table 1) are placed in the top left corner of each panel. All disturbance events in a decade were summed, and the chronologies were truncated when the percentage of canopy available (dashed black line; secondary y axis) fell below 10% of the total number of samples. The percentage of canopy available represents the cumulative portion of canopy contributing to the chronology.
An average disturbance return interval for each plot was calculated by dividing the number of years on record by the number of disturbance events reconstructed for that period. An average rotation period for the whole area was derived as the mean time needed for each plot to be completely disturbed.
2.5. Analysis of past disturbance influence on age pattern and current forest structure
To determine the influence of past disturbance on current forest structure, we separately analyzed five groups of stands with different disturbance histories. To focus on the role of the most severe disturbance events in the recorded history of the plots, we used the maximum value of the 30-year running sum of canopy disturbance per each plot and in some cases the date of this event to delineate different disturbance history groups. Group I (“Low severity”) was characterized by events with severity below 40%, and frequently experienced events over the entire study period. Groups II and III represented “Moderate severity” disturbance regimes, with a maximum severity between 40% and 60%. They differed in the timing of the last disturbance, with Group II experiencing the last disturbance event before 1900 and Group III after 1900. Groups IV and V represent “High severity” conditions, with past disturbances affecting more than 60% of canopy trees. As with the moderate severity groups II and III, we further distinguished between those last being disturbed in the 18th and 19th century (Group IV) and those experiencing the last high severity disturbance more recently (Group V). The influence of disturbance history on stand structure was tested with linear mixed-effects models, using stands as a random effect. The variables were individually transformed (using square root, logarithmic and power transformation), and normality and homogeneity of variance were evaluated using normal probability plots and the Shapiro-Wilk test. The analysis was performed using the R language and environment for statistical computing (R Development Core Team, 2015), specifically harnessing the library “nlme” (Pinheiro et al., 2015). All tests were performed at a pre-set significance level of 0.05.
3. Results
3.1. The historical disturbance regime of the Western Carpathian region
The disturbance chronologies reconstructed at our study sites spanned the late 18th century to the end of the 20th century (Fig. 1). Most of the tree-ring evidence of disturbance consisted of open canopy recruitment events (63% on average), indicating growth in open canopy conditions for the entire life span of a current canopy tree; these patterns are also consistent with age distributions (Fig. Sup. 1). There was high temporal variability in disturbance patterns at both the level of landscapes (N = 7) and stands (N = 14) (Fig. 1b and c), but peaks in disturbance activity were evident in the periods 1810–1820 and 1840–1880 (Fig. 1a). At the stand scale, the temporal variation of disturbances is higher than at landscape and regional scales, and disturbance peaks are more pronounced (Fig. 1c). At this smallest scale of analysis, the distribution of canopy accession events over the reconstruction period varied from unimodal, to bimodal and multimodal, but temporally synchronous disturbance events were evident particularly for the 1810s and 1840s–1870s across many of the stands. In the 1810s, for instance, 56.5% of the sampled stands and 7.6% of the canopy was disturbed, whereas 93.5% of the stands (34.6% of the canopy) experienced disturbance between the 1840s and 1870s. Overall, 42.2% of the canopy was disturbed within these two time windows. Spatially less extensive disturbances were also detected through more local synchronies, e.g. between the stands in the Central Tatra landscape (B) in the 1940s and 1950s. Across the entire region, most of the canopy accession events were observed within the 19th century, except for the Central Tatra landscape (B), where a high proportion of canopy accession events was found for the 20th century.
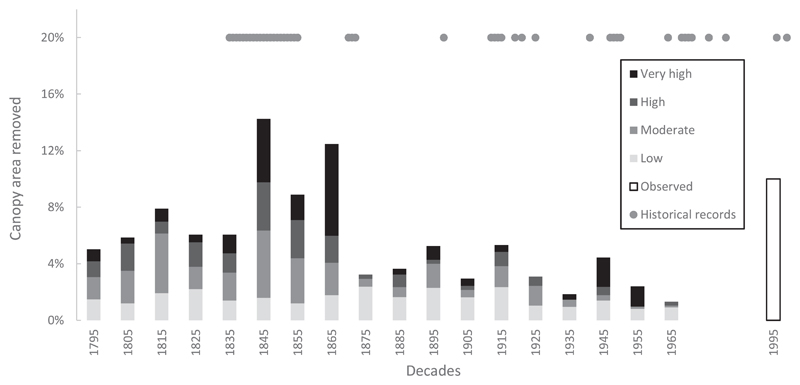
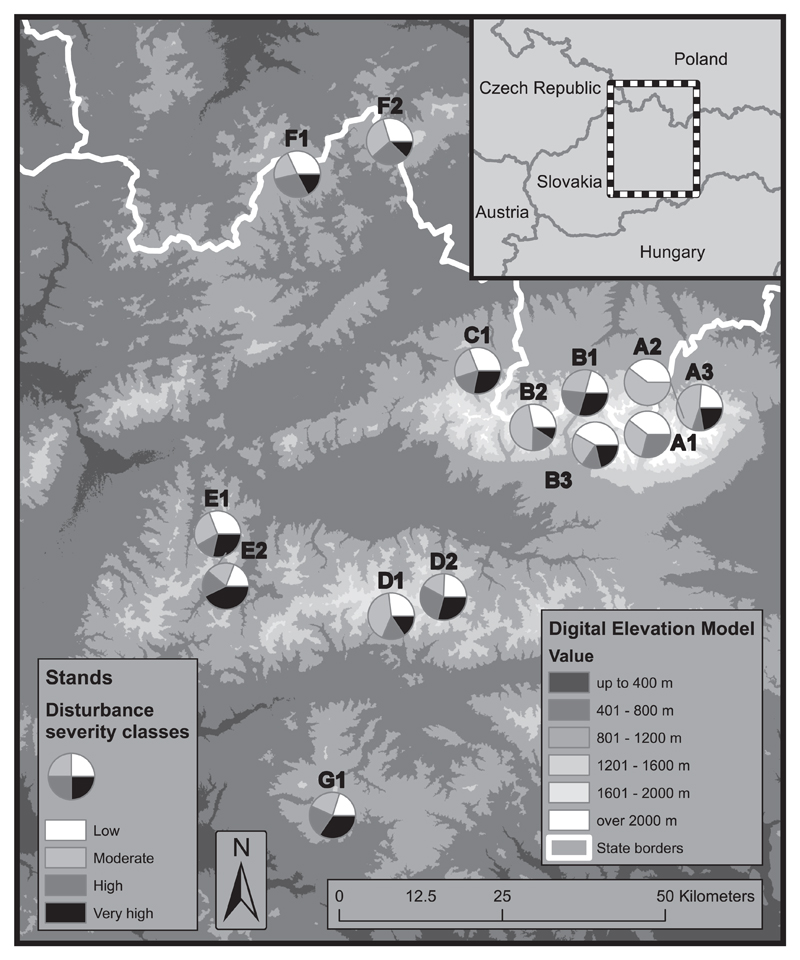
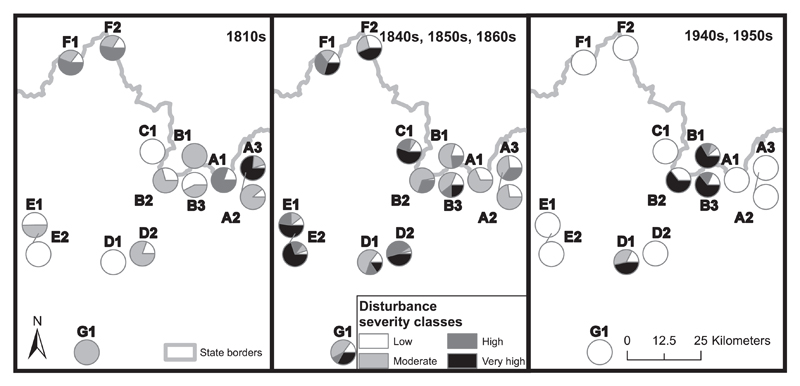
We found that most events were characterized by low disturbance severity (65.2%), which removed only 28.1% of canopy area on average. Overall, the most canopy area was removed by events with low and moderate severity. Nonetheless, severe events (High and Very high events), despite accounting for only 14.3% of the total events, were responsible for 42.3% of the total canopy area disturbed (Table 2). The average disturbance rotation period was 242 years (SD = 59.2). On average, plots were hit by disturbance every 43 years. Disturbance frequency decreased with the severity of an event: Low severity events were most frequent (return interval of 66 years), while high severity events had an order of magnitude longer return intervals (Table 2). The distribution between different severity classes remained relatively stable over time, but high severity disturbance events were particularly prevalent in the 19th century (Fig. 2). The highest severity events occurred between 1840 and 1870, but the spatial variability across the study region was high (Fig. 3). For example, a high proportion of severe disturbances was found in the stands of the Great Fatra landscape (E1 and E2), Poľana (G1), as well as some stands of the Western, Central and Low Tatras (C1, B1, D2), whereas lower severity events characterized the Eastern Tatra landscape (A1 and A2). The spatio-temporal pattern of the most important disturbance events is summarized in Fig. 4, when in the 1810s, 32.6% of the sampled stands and 7.9% of the canopy was disturbed, whereas 89.1% of the stands (35.6% of the canopy) experienced disturbance between the 1840s–1860s. During the 1940s and 1950s 34.2% of the sampled stands and 6.9% of the canopy was disturbed. Overall, 50.3% of the canopy was disturbed within these three time windows.
Table 2.
Characteristics of the disturbance regime of Norway spruce mountain forest ecosystems of the Western Carpathian region. Disturbance chronologies were constructed using a three-decade running sum window of the percentage of canopy area disturbed for all plots.
| Events by severity class | Return interval (years) | Mean severity (% canopy removed) | Proportion of canopy area disturbed (%) | Proportion of disturbance events (%) |
|---|---|---|---|---|
| All events | 43 | 20.1 | 1 | 1 |
| 0–20% Low | 66 | 8.7 | 28.1 | 65.2 |
| 20–40% Moderate | 210 | 29.0 | 29.3 | 20.4 |
| 40–60% High | 526 | 47.5 | 19.2 | 8.1 |
| Over 60% Very high | 690 | 74.9 | 23.1 | 6.2 |
Fig. 2.
The amount of canopy disturbed in the region over time, distributed over disturbance severity classes. The disturbance chronology was constructed using a three-decade running sum window of the percentage of canopy area disturbed. In the chronology we summed three decades, and the date of the events were distributed per decade (as midpoints). For easier visualization of the data, we used four disturbance severity classes: Low – below 20%, Moderate – between 20% and 40%, High – 40% to 60% and Very high – over 60% of canopy area removed. The chronology was truncated at 1790 and 1970 (see methods). Historical records of disturbance events (grey circles) are presented in the upper part of the figure (see Table Sup. 1). The observed percentage of canopy area removed was assessed by comparing the percentage of canopy area disturbed by Griffiths et al. (2014) between 1985 and 2010 with the studied stand polygons delineated by the official primary forest inventory on www.pralesy.sk.
Fig. 3.
The spatial distribution of stand level disturbance severity within the study region. The share of disturbance severities (canopy area disturbed) between 1790 and 1970 is shown at the stand level. Disturbance events were reconstructed using the same data as in Fig. 2 and Table 2. The abbreviations for different stands are described in Table 1.
Fig. 4.
The spatio-temporal distribution of stand-level disturbance severities (proportion of canopy disturbed) is shown for the three most important time periods (highest disturbance activity). Disturbance events were reconstructed using a three-decade running sum window as in Fig. 2 and Table 2.
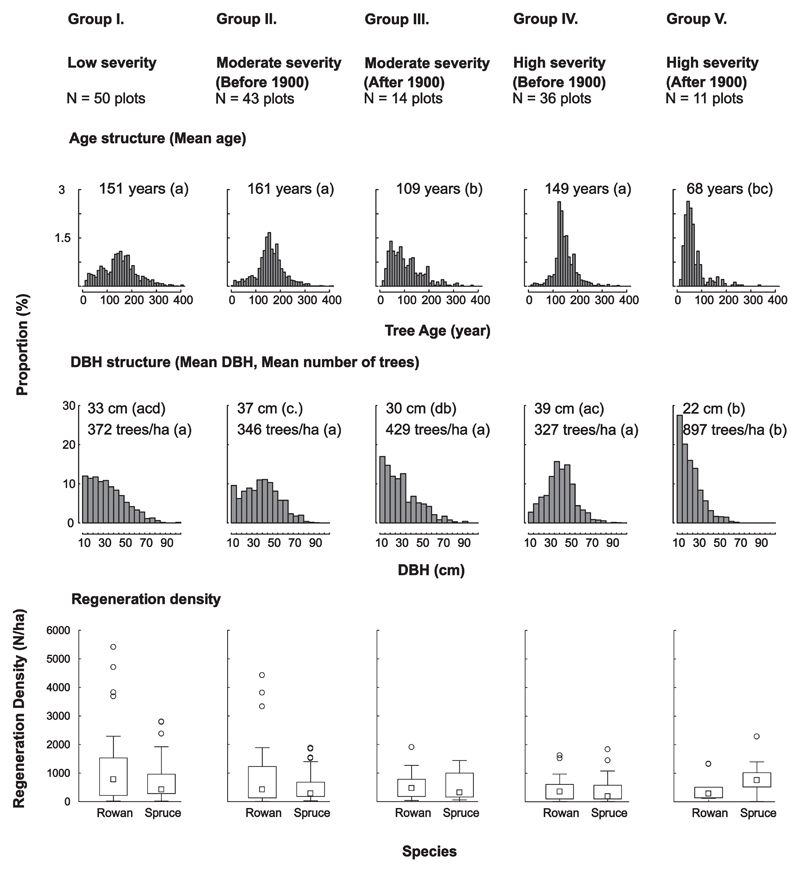
3.2. The influence of past disturbance on age pattern and current forest structure
To examine the influence of the past disturbance regime on current forest structure, the study plots were split into five groups based on the timing of the major disturbance event and the maximum disturbance severity (Fig. 5). Of all plots, approximately one third (32.4%) experienced only low severity disturbance over the study period (Group I). The majority of moderate severity (Group II) and high severity (Group IV) plots were disturbed before 1900, but a smaller percentage also experienced moderate to severe disturbance within the 20th century (9.1% and 7.1% were in Groups III and V, respectively). The latter were mostly situated on northern or western slopes, but did not differ greatly from the other groups with regard to their slope inclination (Figs. Sup. 2 and Sup. 3). As expected, mean stand ages mirrored this classification, with groups experiencing moderate and high severity disturbance after 1900 (Groups III and V) being significantly younger (p < 0.05) than the other groups. Stand age distributions showed clear peaks corresponding to the respective periods of major disturbance events. Differences in maximum ages, on the other hand, were not so clear among the groups: The oldest trees in the stands not disturbed mainly in the 20th century or disturbed with low severity only were between 431 and 495 years old (with the 90th percentile age between 199 and 249 years), while the oldest trees in stands disturbed mainly in the 20th century were 340 and 374 years old (with the 90th percentile age between 132 and 195 years).
Fig. 5.
The influence of disturbance history (characterized by past disturbance severity and the timing of the last major disturbance) on current stand structure and composition, with each column representing a different disturbance history group. Two stands (F2 and G1) were excluded from the analysis because of a lack of information on dead wood and regeneration. Statistical differences between mean values are indicated by lower case letters in parentheses and were tested with linear mixed-effects models, using stands as a random effect.
Mean DBH and age were correlated (r = 0.52, p < 0.05), and DBH distributions matched the age structures within the groups well, particularly if the sampling artifact (i.e. missing tree cores from young trees < 10 DBH) is considered. Mean DBHs differed with time since disturbance, but not between severity classes (Fig. 5). Stand density was similar among the stands with different disturbance history, except for Group V (high severity disturbance after 1900), which had a significantly higher stem density than other stands (p < 0.05). Furthermore, downed deadwood volume and regeneration density did not differ significantly between the plots, regardless of their disturbance history. The mean CWD volume per plot ranged from 83 to 157 m3 ha−1. The mean regeneration density was between 806 and 1927 trees ha−1, but there was substantial variation in regeneration density at the plot level within each disturbance history group (mean = 1427 trees/ha, standard deviation = 1717, range (min, max) = 20; 13,140 trees/ha, quantile (10%, 90%) = 237, 3078 trees/ha). The most common species in the regeneration layer were Norway spruce and rowan. However, 54% of the rowan were within the smallest height class. Other tree species were present, including fir, maple, beech, pine, and larch, but made up less than 1% of the total density.
4. Discussion
4.1. Disturbance history
We found the disturbance history of the mountain forests of the Western Carpathians to be characterized by a disturbance regime with high spatiotemporal variability in severity and frequency. Moderate and low severity disturbances dominated, but rare severe events were also evident in our disturbance reconstruction, and made a disproportional contribution to the overall canopy removed by disturbance. A similar mixed severity disturbance regime was also recently described for Norway spruce-dominated mountain forests in Central Europe by Trotsiuk et al. (2014), Svoboda et al. (2014), Holeksa et al., 2017 and Čada et al. (2016). Although the disturbance history in the region was temporally and spatially diverse, periods of synchrony in disturbance activity were also found. Specifically, a peak of canopy disturbance was found for the mid-19th century across the region, with the most important periods of disturbance in the 1820s and from the 1840s to the 1870s.
Interestingly, these periods also show strong disturbance signals in other mountain forest ecosystems of Central Europe (Trotsiuk et al., 2014; Čada et al., 2016; Svoboda et al., 2014; Janda et al., 2014; Zielonka et al., 2010), indicating that disturbance regimes might be synchronized across large spatial scales (Jarvis and Kulakowski, 2015; Seidl et al., 2016a). Historical sources (Table Sup. 1) describe events during these periods as being predominately windstorm events, often combined with bark beetle outbreaks. This suggests that the past disturbance agents in the area are identical to the most common disturbance agents in these forests today (Schelhaas et al., 2003; Müller et al., 2008). However, as these are high elevation, temperature-limited ecosystems, suboptimal temperatures and frost (Kullman and Öberg, 2009) during a cold event in the beginning of the 19th century (Büntgen et al., 2013) cannot be ruled out as a possible cause for pulses of tree mortality. Unfortunately, the methods and approaches used in this study did not allow us to distinguish between different disturbance agents (but see Zielonka et al. (2010) and Robson et al. (2015)).
The spatial patterns of the disturbance regime were highly variable, but some trends within the region could be discerned. Lower disturbance severities were, for instance, observed in the Eastern Tatras, and can be explained by the position of this particular landscape in the context of the greater Tatras mountain range, which shelters it against strong winds from predominantly westerly and northerly directions (Niedźwiedź, 1992). In contrast, landscapes exposed to these winds and without topographic sheltering were more strongly disturbed in the past, with a higher risk of severe disturbance (Čada et al., 2016). Furthermore, the region of the Central Tatras also had an elevated proportion of canopy accession events in the 20th century, suggesting an overall increased disturbance frequency. This finding is in line with the observation that this particular area is specifically influenced by strong and frequent “Bora” winds from the north (Zielonka et al., 2010; Mezei et al., 2014).
The prominent disturbance pulses of the 19th century likely also contributed to the comparatively low disturbance activity in the first part of the 20th century. The disturbance risk for the young stands originating in the mid-19th century was likely depressed for several decades, as both wind and bark beetle susceptibility increases with age in Norway spruce forests. Ips typographus attack primarily older and weakened trees, which are found in abundance after wind disturbance (Wermelinger, 2004; Økland and Bjørnstad, 2006), and also wind risk increases with tree size (and hence age) (Canham et al., 2001). Furthermore, the absence of high severity disturbance after the mid-19th century could have led to a synchronous development of regenerating forest stands as well as to spatial homogenization across the landscape through increased connectivity between susceptible patches. These factors are frequently associated with increasing risk for wind disturbance and bark beetle outbreaks (Jarvis and Kulakowski, 2015; Seidl et al., 2016a), suggesting a century-scale temporal autocorrelation between disturbance episodes in these forests.
However, it should also to be noted that the higher proportion of trees recruited in the 19th century could at least in part be explained by the availability of possible study sites, as we focused on remnants of primary forests, mostly in strictly protected reserves with specific topography (Figs. Supl. 1 and Supl. 2). Despite having a regional and landscape scale focus, our work was still carried out in a fragmented landscape, where only remnants of old-growth forests remained. Together with the fact that salvage logging has commonly been applied in the region during the last 50 years, unmanaged young post disturbance stands might be partly missing. Consequently, early-successional stages after recent high severity disturbance, such as described in Central Europe by Wild et al. (2014), Zeppenfeld et al. (2015) and Svoboda et al. (2014), were missing in our analysis. This decreases the utility of our disturbance reconstruction for recent decades, and suggests that the historical disturbance regime reconstructed from primary forests might not be fully representative of the entire region.
Nonetheless, our analysis suggests that the current disturbance pulse observed in several Western Carpathian mountain forests (Mezei et al., 2014) could at least in part result from past disturbance dynamics and the emerging synchrony in stand development. Additional drivers of the currently high disturbance activity might be climate change, resulting in an increasing probability of bark beetle outbreaks (Milad et al., 2011; Temperli et al., 2013) as well as increasing climatic extremes connected with damage to forests, such as drought and wind events (Dale et al., 2001; Seidl et al., 2014). Similar large-scale disturbance can also be observed in other parts of Central Europe (Čada et al., 2016; Lausch et al., 2011) or in North America (Berg et al., 2006). Thom et al. (2013), in their analysis of recent disturbances in neighboring Austria, derived a combined median disturbance rotation period for wind and beetles of 134 years, a value that suggests higher current disturbance activity compared to the historical values derived here (cf. Table 2). Furthermore, recent disturbances in Central Europe have largely been high severity events (Mezei et al., 2014; Nikolov et al., 2014; Kautz et al., 2011), suggesting that disturbance regimes might be changing in severity and frequency relative to their historical patterns (cf. Fig. 3). However, other evidence from Slovakia (Zielonka et al., 2010) and other parts of Central Europe (Svoboda et al., 2012, 2014; Čada et al., 2016; Holeksa et al., 2016; Brůna et al., 2013; Panayotov et al., 2015) suggests that large-scale severe disturbance events such as those observed recently might also have occurred in the past.
4.2. The influence of past disturbance on current forest structure
We found that the disturbance history of a stand had a significant influence on some aspects of its current structure, which is in line with previous investigations for other ecosystems (D’Amato et al., 2008; Zenner, 2005). Stand structural parameters such as DBH and stand age structure were strongly influenced by past disturbance activity. In contrast, the effect of past disturbances on parameters such as tree density, the amount of CWD, and regeneration was found to be only weak and not statistically significant. Maximum disturbance severity and time since the last substantial disturbance were both strong drivers of the DBH and age structure. Frequent low severity disturbances resulted in very wide DBH and age distributions, indicating high temporal variability in establishment and structures close to what can be expected for old-growth forest (D’Amato et al., 2008). In contrast, high severity disturbances created narrow DBH and age distributions or reverse J shaped distributions, indicating recent pulses of regeneration under an established overstorey cohort. Generally, time since disturbance had a stronger effect on current structures than past disturbance severity. Stands mainly influenced by disturbances occurring before 1900 as well as the low severity group had clear peaks in age structure between 100 and 200 years, while trees were considerably younger in more recently disturbed stands. Interestingly, we also found rather old trees in plots that were influenced by recent disturbance. This indicates the persistence of trees from previous generations surviving the last moderate or severe disturbance. This finding suggests that natural disturbances in the area were rarely complete and work selectively (Nagel et al., 2014; Šamonil et al., 2013), and that even in high severity disturbances, biological legacies in the form of surviving trees exist. Such legacy trees are very important for structural diversity in early successional development and contribute to the resilience of forest ecosystems to disturbance (Moning and Müller, 2009; Seidl et al., 2016b).
With regard to tree regeneration and CWD, on the other hand, we did not find significant effects of disturbance history on current conditions. In this context it should be noted that these two parameters are strongly linked in the high elevation Norway spruce forests of Central Europe, as CWD is a highly suitable microsite for spruce regeneration (Bače et al., 2012). It is reasonable to expect that the amount of CWD and subsequently the abundance of regeneration would be strongly influenced by the time since disturbance, as CWD decays over a matter of decades (Zielonka, 2006). However, no such effect was evident in our data when comparing stands that were disturbed before and after 1900. This suggests that the low severity mortality events occurring with relatively high frequency throughout all sites might play an important role in maintaining CWD levels and thus providing suitable microsites for regeneration on the landscape. Overall, our findings are in line with previous assessments suggesting that the effect of disturbances persists over different time spans depending on the ecosystem property in focus (Seidl et al., 2016b).
The amount of regeneration on all study plots was on average approximately 1400 trees per hectare with a height over 0.5 m and a DBH lower than 10 cm. Similar regeneration densities were observed in other studies of mountain spruce forests (Zeppenfeld et al., 2015; Wild et al., 2014), and these levels are assumed to be sufficient to ensure continuity of forest cover. Furthermore, there is high probability of an increasing amount of tree regeneration in the decades following large-scale dieback (Zeppenfeld et al., 2015). The overall average regeneration density was rather high across all the plots in our study, although there was considerable variation among plots. This variability contributes to high structural heterogeneity of the system at the landscape scale (Donato et al., 2012). The high densities of rowan found in the lowest height classes will probably experience high mortality, as suggested by their substantially reduced number in higher height classes. Similar trends have also recently been observed in mountain spruce forest ecosystems of the Bavarian National Park (Zeppenfeld et al., 2015).
4.3. Management implications
Extensive high severity disturbances have recently affected Central European forests, spurring a discussion among managers and policy makers as to the causes and consequences of these disturbances (Nikolov et al., 2014). An important question in this discussion has been whether such events are common, and whether these forests are adapted to these kinds of disturbances. We showed that pulses of disturbance are part of the natural disturbance regime in Norway spruce mountain forests, and that high severity disturbances have also occurred in these forest types in the past (see also Zielonka et al., 2010; Brůna et al., 2013; Svoboda et al., 2012; Čada et al., 2016). Yet, the disturbance return intervals and severity distributions estimated here suggest that recent events might be at the upper bound of the historical range of variability (HRV). This suggests that further increases in disturbance, as are expected for the region under changing climate conditions (Seidl et al., 2014), might exceed the HRV of the system. As the HRV is an important reference for understanding disturbance changes and can serve as a guideline for ecosystemoriented management (Keane et al., 2009), future research should focus on a comprehensive comparison of the regional HRV with current activities (cf. Cyr et al., 2009), as well as on estimating the future range of variability that can be expected under global climate change (see Seidl et al., 2016b).
Our results also highlight that in assessments of future trajectories of forest ecosystems, the persistent effect of past disturbances needs to be considered. It is likely, for instance, that the temporal synchronization through the heavily disturbed period in the mid-19th century has substantially contributed to the current disturbance susceptibility of the ecosystem. Furthermore, structural parameters of the forest, such as the spread and shape of the diameter distribution, indicate a lasting effect of past disturbances. The fact that these variables are important indicators of biodiversity (Paillet et al., 2010) suggests that understanding the prevailing disturbance regime (and the potential changes therein) is crucial for conservation management. The finding that regeneration density and composition was not influenced by the disturbance regime between 40 and 220 years before the present illustrates that vastly different past disturbance histories are not likely to change the future trajectories of these forests (but see Johnstone et al., 2010). In other words, these ecosystems currently exhibit high ecological resilience to disturbance (see also Zeppenfeld et al., 2015). In conclusion, we suggest that management should recognize disturbances as a natural part of ecosystem dynamics in the mountain forests of Central Europe instead of aiming to prevent them (cf. Fares et al., 2015), and should account for their stochastic occurrence in management planning (e.g. via increasing redundancies to maintain crucial ecosystem services despite disturbance, Seidl, 2014) as well as mimic their patterns (Nagel et al., 2014) to foster biodiversity in forest landscapes.
Supplementary Material
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.foreco.2016.08.014.
Acknowledgements
This study was supported by project GACR no. 15-14840S. MHN, RB, MS, PJ, VT, RCM, HM, MM received additional institutional support by project CIGA no. 20164310. VT and HM were also supported by the Faculty of Forestry and Wood Sciences, CULS, Prague (Project IGA B02/15). RS acknowledges funding from the Austrian Science Fund (FWF) through START grant Y895-B25. SK received additional institutional support by project Vega 1/0040/15, Vega 1/0057/14 and APVV-0286-10. We would like to thank all co-workers for assistance with field data collection and measurements, and two anonymous reviewers for providing valuable comments on a previous version of the manuscript. We thank to the Slovakian nature conservation authorities and forest owners for administrative support and accession of the study sites.
References
- Bače R, Svoboda M, Pouska V, Janda P, Červenka J. Natural regeneration in Central-European subalpine spruce forests: which logs are suitable for seedling recruitment? For Ecol Manage. 2012;266:254–262. [Google Scholar]
- Bentz BJ, Régnière J, Fettig CJ, Hansen EM, Hayes JL, Hicke JA, Kelsey RG, Negrón JF, Seybold SJ. Climate change and bark beetles of the Western United States and Canada: direct and indirect effects. Bioscience. 2010;60:602–613. [Google Scholar]
- Berg EE, Henry JD, Fastie CL, DeVolder AD, Matsuoka SM. Spruce beetle outbreaks on the Kenai Peninsula, Alaska, and Kluane National Park and Reserve, Yukon Territory: relationship to summer temperatures and regional differences in disturbance regimes. For Ecol Manage. 2006;227:219–232. [Google Scholar]
- Black BA, Abrams MD. Use of boundary-line growth patterns as a basis for dendroecological release criteria. Ecol Appl. 2003;13:1733–1749. [Google Scholar]
- Brůna J, Wild J, Svoboda M, Heurich M, Müllerová J. Impacts and underlying factors of landscape-scale, historical disturbance of mountain forest identified using archival documents. For Ecol Manage. 2013;305:294–306. [Google Scholar]
- Büntgen U, Kyncl T, Ginzler C, Jacks DS, Esper J, Tegel W, Heussner K-U, Kyncl J. Filling the Eastern European gap in millennium-long temperature reconstructions. Proc Natl Acad Sci. 2013;110:1773–1778. doi: 10.1073/pnas.1211485110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Čada V, Morrissey RC, Michalová Z, Bače R, Janda P, Svoboda M. Frequent severe natural disturbances and non-equilibrium landscape dynamics shaped the mountain spruce forest in central Europe. Forest Ecol Manage. 2016;363:169–178. [Google Scholar]
- Canham CD, Papaik MJ, Latty EF. Interspecific variation in susceptibility to windthrow as a function of tree size and storm severity for northern temperate tree species. Can J For Res. 2001;31:1–10. [Google Scholar]
- Cyr D, Gauthier S, Bergeron Y, Carcaillet C. Forest management is driving the eastern North American boreal forest outside its natural range of variability. Front Ecol Environ. 2009;7:519–524. [Google Scholar]
- D’Amato AW, Orwig DA, Foster DR. The influence of successional processes and disturbance on the structure of Tsuga canadensis forests. Ecol Appl. 2008;18:1182–1199. doi: 10.1890/07-0919.1. [DOI] [PubMed] [Google Scholar]
- Dale V, Joyce L, McNulty S, Neilson R, Ayres M, Flannigan M, Hanson P, Irland L, Lugo A, Peterson CJ, Simberloff D, et al. Climate change and forest disturbances. Bioscience. 2001;51:723–734. [Google Scholar]
- Donato DC, Campbell JL, Franklin JF. Multiple successional pathways and precocity in forest development: can some forests be born complex? J Veg Sci. 2012;23:576–584. [Google Scholar]
- Duncan RP. An evaluation of errors in tree age estimates based on increment cores in kahikatea (Dacrycarpus dacrydioides) N Z Nat Sci. 1989;16:1–37. [Google Scholar]
- Easterling DR, Meehl GA, Parmesan C, Changnon SA, Karl TR, Mearns LO. Climate extremes: observations, modeling, and impacts. Science. 2000;289:2068–2074. doi: 10.1126/science.289.5487.2068. [DOI] [PubMed] [Google Scholar]
- Fares S, Mugnozza GS, Corona P, Palahi M. Five steps for managing Europe’s forests. Nature. 2015;519:407–409. doi: 10.1038/519407a. [DOI] [PubMed] [Google Scholar]
- Fink AH, Brücher T, Ermert V, Krüger A, Pinto JG. The European storm Kyrill in January 2007: synoptic evolution, meteorological impacts and some considerations with respect to climate change. Nat Hazards Earth Syst Sci. 2009;9:405–423. [Google Scholar]
- Fraver S, White AS, Seymour RS. Natural disturbance in an old-growth landscape of northern Maine, USA. J Ecol. 2009;97:289–298. [Google Scholar]
- Frelich LE. Forest Dynamics and Disturbance Regimes: Studies From Temperate Evergreen-Deciduous Forests. Cambridge University Press; 2002. [Google Scholar]
- Fritz O, Gustafsson L, Larsson K. Does forest continuity matter in conservation? A study of epiphytic lichens and bryophytes in beech forests of southern Sweden. Biol Conserv. 2008;141:655–668. [Google Scholar]
- Griffiths P, Kuemmerle T, Baumann M, Radeloff VC, Abrudan IV, Lieskovsky J, Munteanu C, Ostapowicz K, Hostert P. Forest disturbances, forest recovery, and changes in forest types across the Carpathian ecoregion from 1985 to 2010 based on Landsat image composites. Remote Sens Environ. 2014;151:72–88. [Google Scholar]
- Harmon ME, Sexton J. Guidelines for measurements of woody detritus in forest ecosystems. Publication No. 20. U.S. LTER Network Office; University of Washington, Seattle, WA: 1996. [Google Scholar]
- Holeksa J, Zielonka T, Zywiec M, Fleischer P. Identifying the disturbance history over a large area of larch-spruce mountain forest in Central Europe. For Ecol Manage. 2016;361:318–327. [Google Scholar]
- Holeksa J, Jaloviar P, Kucbel S, Saniga M, Svoboda M, Szewczyk J, Szwagrzyk J, Zielonka T, Zywiec M. Models of disturbance driven dynamics in the West Carpathian spruce forests. For Ecol Manage. 2017;388:79–89. [Google Scholar]
- Holmes RL. Computer-assisted quality control in tree-ring dating and measurements. Tree-Ring Bull. 1983;44:69–75. [Google Scholar]
- Hosmer DW, Jr, Lemeshow S. Applied Logistic Regression. John Wiley & Sons: 2004. [Google Scholar]
- Janda P, Svoboda M, Bače R, Čada V, Peck JE. Three hundred years of spatio-temporal development in a primary mountain Norway spruce stand in the Bohemian Forest, central Europe. For Ecol Manage. 2014;330:304–311. [Google Scholar]
- Jarvis DS, Kulakowski D. Long-term history and synchrony of mountain pine beetle outbreaks in lodgepole pine forests. J Biogeogr. 2015;42:1029–1039. [Google Scholar]
- Johnstone JF, Hollingsworth TN, Chapin FS, Mack MC. Changes in fire regime break the legacy lock on successional trajectories in Alaskan boreal forest. Glob Change Biol. 2010;16:1281–1295. [Google Scholar]
- Kautz M, Dworschak K, Gruppe A, Schopf R. Quantifying spatio-temporal dispersion of bark beetle infestations in epidemic and non-epidemic conditions. For Ecol Manage. 2011;262:598–608. [Google Scholar]
- Keane RE, Hessburg PF, Landres PB, Swanson FJ. The use of historical range and variability (HRV) in landscape management. For Ecol Manage. 2009;258:1025–1037. [Google Scholar]
- Kneeshaw DD, Harvey BD, Reyes GP, Caron M-N, Barlow S. Spruce budworm, windthrow and partial cutting: do different partial disturbances produce different forest structures? For Ecol Manage. 2011;262:482–490. [Google Scholar]
- Kulakowski D, Veblen TT. Subalpine forest development following a blowdown in the Mount Zirkel Wilderness, Colorado. J Veg Sci. 2003;14:653–660. [Google Scholar]
- Kulakowski D, Seidl R, Holeksa J, Kuuluvainen T, Nagel TA, Panayotov M, Svoboda M, Thorn S, Vacchiano G, Whitlock C, Wohlgemuth T, et al. A walk on the wild side: Disturbance dynamics and the conservation and management of European mountain forest ecosystems. For Ecol Manage. 2017;388:120–131. doi: 10.1016/j.foreco.2016.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullman L, Öberg L. Post-little ice age tree line rise and climate warming in the Swedish Scandes: a landscape ecological perspective. J Ecol. 2009;97:415–429. [Google Scholar]
- Lausch A, Fahse L, Heurich M. Factors affecting the spatio-temporal dispersion of Ips typographus (L.) in Bavarian Forest National Park: a long-term quantitative landscape-level analysis. For Ecol Manage. 2011;261:233–245. [Google Scholar]
- Lecomte N, Simard M, Bergeron Y. Effects of fire severity and initial tree composition on stand structural development in the coniferous boreal forest of northwestern Québec, Canada. Ecoscience. 2006;13:152–163. [Google Scholar]
- Lorimer CG, Frelich LE. A methodology for estimating canopy disturbance frequency and intensity in dense temperate forests. Can J Forest Res. 1989;19:651–663. [Google Scholar]
- Mezei P, Grodzki W, Blaženec M, Jakuš R. Factors influencing the wind-bark beetles’ disturbance system in the course of an Ips typographus outbreak in the Tatra Mountains. Forest Ecol Manage. 2014;312:67–77. [Google Scholar]
- Mikoláš M, Svitok M, Tejkal M, Leitão PJ, Morrissey RC, Svoboda M, Seedre M, Fontaine JB. Evaluating forest management intensity on an umbrella species: Capercaillie persistence in central Europe. Forest Ecol Manage. 2015;354:26–34. [Google Scholar]
- Milad M, Schaich H, Bürgi M, Konold W. Climate change and nature conservation in Central European forests: a review of consequences, concepts and challenges. For Ecol Manage. 2011;261:829–843. [Google Scholar]
- Moning C, Müller J. Critical forest age thresholds for the diversity of lichens, molluscs and birds in beech (Fagus sylvatica L.) dominated forests. Ecol Indic. 2009;9:922–932. [Google Scholar]
- Müller J, Bußler H, Goßner M, Rettelbach T, Duelli P, Mueller J, Bussler H, Gossner M, Rettelbach T, Duelli P, Duelli ÆP. The European spruce bark beetle Ips typographus in a national park: from pest to keystone species. Biodivers Conserv. 2008;17:2979–3001. [Google Scholar]
- Nagel TA, Svoboda M, Kobal M. Disturbance, life-history traits, and dynamics in an old-growth forest landscape of southeastern Europe. Ecol Appl. 2014;24:663–679. doi: 10.1890/13-0632.1. [DOI] [PubMed] [Google Scholar]
- Niedźwiedź T. Climate of the Tatra Mountains. Mt Res Dev. 1992;12:131–146. [Google Scholar]
- Nikolov C, Konopka B, Kajba M, Galko J, Kunca A, Jansky L. Post-disaster forest management and bark beetle outbreak in Tatra National Park, Slovakia. Mountain Res Dev. 2014;34:326–335. [Google Scholar]
- Økland B, Bjørnstad ON. A resource-depletion model of forest insect outbreaks. Ecology. 2006;87:283–290. doi: 10.1890/05-0135. [DOI] [PubMed] [Google Scholar]
- Oszlányi J, Grodzinska K, Badea O, Shparyk Y. Nature conservation in Central and Eastern Europe with a special emphasis on the Carpathian Mountains. Environ Pollut. 2004;130:127–134. doi: 10.1016/j.envpol.2003.10.028. [DOI] [PubMed] [Google Scholar]
- Paillet Y, Bergès L, Hjälten J, Odor P, Avon C, Bernhardt-Römermann M, Bijlsma RJ, De Bruyn L, Fuhr M, Grandin U. Biodiversity differences between managed and unmanaged forests: meta-analysis of species richness in Europe. Conserv Biol. 2010;24:101–112. doi: 10.1111/j.1523-1739.2009.01399.x. [DOI] [PubMed] [Google Scholar]
- Panayotov M, Bebi P, Tsvetanov N, Alexandrov N, Laranjeiro L, Kulakowski D. The disturbance regime of Norway spruce forests in Bulgaria. Can J Forest Res. 2015;45:1143–1153. [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team Nlme: Linear and Nonlinear Mixed Effects Models. 2015 (R package version 3.1-120) [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2015. < http://www.R-project.org>. [Google Scholar]
- Robson JRM, Conciatori F, Tardif JC, Knowles K. Tree-ring response of jack pine and scots pine to budworm defoliation in central Canada. For Ecol Manage. 2015;347:83–95. [Google Scholar]
- Šamonil P, Doleželová P, Vašíčková I, Adam D, Valtera M, Král K, Janík D, Šebková B. Individual-based approach to the detection of disturbance history through spatial scales in a natural beech-dominated forest. J Veg Sci. 2013;24:1167–1184. [Google Scholar]
- Schelhaas MJ, Nabuurs GJ, Schuck A. Natural disturbances in the European forests in the 19th and 20th centuries. Glob Change Biol. 2003;9:1620–1633. [Google Scholar]
- Schroeder LM, Lindelöw Å. Attacks on living spruce trees by the bark beetle Ips typographus (Col.: Scolytidae) following a storm-felling: a comparison between stands with and without removal of wind-felled trees. Agric For Entomol. 2002;4:47–56. [Google Scholar]
- Seidl R. The shape of ecosystem management to come: anticipating risks and fostering resilience. Bioscience. 2014;64:1159–1169. doi: 10.1093/biosci/biu172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl R, Schelhaas M-J, Rammer W, Verkerk PJ. Increasing forest disturbances in Europe and their impact on carbon storage. Nat Clim Chang. 2014;4:806–810. doi: 10.1038/nclimate2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl R, Müller J, Hothorn T, Bässler C, Heurich M, Kautz M. Small beetle, large-scale drivers: how regional and landscape factors affect outbreaks of the European spruce bark beetle. J Appl Ecol. 2016a;53:530–540. doi: 10.1111/1365-2664.12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl R, Spies TA, Peterson DL, Stephens SL, Hicke JA. Searching for resilience: addressing the impacts of changing disturbance regimes on forest ecosystem services. J Appl Ecol. 2016b;53:120–129. doi: 10.1111/1365-2664.12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sproull GJ, Adamus M, Szewczyk J, Kersten G, Szwagrzyk J. Fine-scale spruce mortality dynamics driven by bark beetle disturbance in Babia Góra National Park, Poland. Eur J For Res. 2016 doi: 10.1007/s10342-016-0949-8. late spring edition. [DOI] [Google Scholar]
- Svoboda M, Janda P, Bače R, Fraver S, Nagel TA, Rejzek J, Mikoláš M, Douda J, Boublík K, Šamonil P, Čada V, et al. Landscape-level variability in historical disturbance in primary Picea abies mountain forests of the Eastern Carpathians, Romania. J Veg Sci. 2014;25:386–401. [Google Scholar]
- Svoboda M, Janda P, Nagel TA, Fraver S, Rejzek J, Bače R. Disturbance history of an old-growth sub-alpine Picea abies stand in the Bohemian Forest, Czech Republic. J Veg Sci. 2012;23:86–97. [Google Scholar]
- Szewczyk J, Szwagrzyk J, Muter E. Tree growth and disturbance dynamics in old-growth subalpine spruce forests of the Western Carpathians. Can J Forest Res-Revue Canadienne De Recherche Forestiere. 2011;41:938–944. [Google Scholar]
- Temperli Ch, Bugmann H, Elkin Ch. Cross-scale interactions among bark beetles, climate change, and wind disturbances: landscape modeling approach. Ecol Monogr. 2013;83:383–402. [Google Scholar]
- Tepley AJ, Veblen TT. Spatiotemporal fire dynamics in mixed-conifer and aspen forests in the San Juan Mountains of southwestern Colorado, USA. Ecol Monogr. 2015;85:583–603. [Google Scholar]
- Thom D, Seidl R. Natural disturbance impacts on ecosystem services and biodiversity in temperate and boreal forests. Biol Rev. 2016 doi: 10.1111/brv.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom D, Seidl R, Steyrer G, Krehan H, Formayer H. Slow and fast drivers of the natural disturbance regime in Central European forest ecosystems. For Ecol Manage. 2013;307:293–302. [Google Scholar]
- Thorn S, Bässler C, Bernhardt-Römermann M, Cadotte M, Heibl C, Schäfer H, Seibold Sa, Müller J. Changes in the dominant assembly mechanism drive species loss caused by declining resources. Ecol Lett. 2016;19:163–170. doi: 10.1111/ele.12548. [DOI] [PubMed] [Google Scholar]
- Trotsiuk V, Svoboda M, Janda P, Mikolas M, Bace R, Rejzek J, Samonil P, Chaskovskyy O, Korol M, Myklush S. A mixed severity disturbance regime in primary Picea abies (L.) Karst. Forests of the Ukrainian Carpathians. For Ecol Manage. 2014;334:144–153. [Google Scholar]
- Turner MG. Disturbance and landscape dynamics in a changing world. Ecology. 2010;91:2833–2849. doi: 10.1890/10-0097.1. [DOI] [PubMed] [Google Scholar]
- Weed AS, Ayres MP, Hicke JA. Consequences of climatic change for biotic disturbances in North American forests. Ecol Monogr. 2013;83:441–470. [Google Scholar]
- Wermelinger B. Ecology and management of the spruce bark beetle Ips typographus – a review of recent research. For Ecol Manage. 2004;202:67–82. [Google Scholar]
- Westerling AL, Hidalgo HG, Cayan DR, Swetnam TW. Warming and earlier spring increase western US forest wildfire activity. Science. 2006;313:940–943. doi: 10.1126/science.1128834. [DOI] [PubMed] [Google Scholar]
- Wild J, Kopecký M, Svoboda M, Zenáhlíková J, Edwards-Jonášová M, Herben T. Spatial patterns with memory: tree regeneration after stand-replacing disturbance in Picea abies mountain forests. J Veg Sci. 2014;25:1327–1340. [Google Scholar]
- Zenner EK. Development of tree size distributions in douglas-fir forests under differing disturbance regimes. Ecol Appl. 2005;15:701–714. [Google Scholar]
- Zeppenfeld T, Svoboda M, DeRose RJ, Heurich M, Müller J, Čížková P, Starý M, Bače R, Donato DC. Response of mountain Picea abies forests to stand-replacing bark beetle outbreaks: neighbourhood effects lead to self-replacement. J Appl Ecol. 2015;52:1402–1411. [Google Scholar]
- Zielonka T. When does dead wood turn into a substrate for spruce replacement? J Veg Sci. 2006;17:739–746. [Google Scholar]
- Zielonka T, Holeksa J, Fleischer P, Kapusta P. A tree-ring reconstruction of wind disturbances in a forest of the Slovakian Tatra Mountains, Western Carpathians. J Veg Sci. 2010;21:31–42. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.