Abstract
Forest disturbances are sensitive to climate. However, our understanding of disturbance dynamics in response to climatic changes remains incomplete, particularly regarding large-scale patterns, interaction effects and dampening feedbacks. Here we provide a global synthesis of climate change effects on important abiotic (fire, drought, wind, snow and ice) and biotic (insects and pathogens) disturbance agents. Warmer and drier conditions particularly facilitate fire, drought and insect disturbances, while warmer and wetter conditions increase disturbances from wind and pathogens. Widespread interactions between agents are likely to amplify disturbances, while indirect climate effects such as vegetation changes can dampen long-term disturbance sensitivities to climate. Future changes in disturbance are likely to be most pronounced in coniferous forests and the boreal biome. We conclude that both ecosystems and society should be prepared for an increasingly disturbed future of forests.
Natural disturbances, such as fires, insect outbreaks and windthrows, are an integral part of ecosystem dynamics in forests around the globe. They occur as relatively discrete events, and form characteristic regimes of typical disturbance frequencies, sizes and severities over extended spatial and temporal scales1,2. Disturbances disrupt the structure, composition and function of an ecosystem, community or population, and change resource availability or the physical environment3. In doing so, they create heterogeneity on the landscape4, foster diversity across a wide range of guilds and species5,6 and initiate ecosystem renewal or reorganization7,8.
Disturbance regimes have changed profoundly in many forest ecosystems in recent years, with climate being a prominent driver of disturbance change9. An increase in disturbance occurrence and severity has been documented over large parts of the globe, for example, for fire10,11, insect outbreaks12,13 and drought14,15. Such alterations of disturbance regimes have the potential to strongly impact the ability of forests to provide ecosystem services to society6. Moreover, a climate-mediated increase in disturbances could exceed the ecological resilience of forests, resulting in lastingly altered ecosystems or shifts to non-forest ecosystems as tipping points are crossed16–18. Consequently, disturbance change is expected to be among the most profound impacts that climate change will have on forest ecosystems in the coming decades19.
The ongoing changes in disturbance regimes in combination with their strong and lasting impacts on ecosystems have led to an intensification of disturbance research in recent years. There is a long tradition of disturbance research in ecology3,20,21, with an increasing focus on understanding the links between disturbance and climate in recent decades1,22,23. Syntheses on the effects of climate change on important disturbance agents such as fire24, bark beetles25, pathogens26 and drought15 summarize recent advances of a highly prolific field of study. Considerably less synthetic knowledge is available on interactions among disturbance agents27–29. Furthermore, to date, no global synthesis exists that integrates insights on changing disturbance regimes across agents and regions. Yet, the main drivers of disturbance change are global in scale (for example, climate warming), rendering such a global synthesis highly relevant30,31.
Specifically, a comprehensive analysis of the multiple pathways via which climate might influence forest disturbances is still lacking. Interactions between different disturbance agents can, for instance, result in strong and nonlinear effects of climate change on disturbance activity32. In contrast, climate-mediated vegetation changes can dampen the climate sensitivity of disturbances33. Many assessments of disturbance responses to climate change are currently neglecting such complex effect pathways34,35. More commonly still, the effects of changing disturbance regimes are disregarded entirely in analyses of future forest development36,37 and studies quantifying the climate change mitigation potential of forest ecosystems38, potentially inducing significant bias39,40.
Here we review the current understanding of forest disturbances under climate change, focusing on naturally occurring agents of disturbance. Specifically, we synthesize the existing knowledge of how climate change may affect disturbance regimes via direct, indirect and interaction effects. We reviewed the disturbance literature published from 1990 onwards, applying a consistent analysis framework over a diverse set of major forest disturbance agents, including four abiotic (fire, drought, wind, as well as snow and ice) and two biotic agents (insects and pathogens). We compiled evidence for climate effects from all biomes and continents, and analysed it in a qualitative modelling framework. We tested the hypothesis that climate change will considerably increase forest disturbance activity at the global scale, and specifically that positive, amplifying effects of climate change on disturbances dominate negative, dampening effects.
Literature review and analysis
We screened the literature for peer-reviewed English-language papers addressing the climate sensitivity of forest disturbances (that is, a change in disturbance in response to a change in climate). Due to conceptual advances in disturbance ecology in the 1980s3,21 and the increasing availability of climate scenario data and remotely sensed information, we chose to focus our analysis on research emerging from 1990 onwards. Material was selected by searching for our six focal disturbance agents (fire, drought, wind, snow and ice, insects, and pathogens) or applicable aliases (for example, bark beetles or defoliators for the insects category), in combination with the terms climate and/or climatic change in the title, abstract and/or key words of published papers. In the context of drought, it is important to note that here we applied an ecological definition rather than a meteorological one, that is, we focused on events of severe water limitation that affect ecosystem structure and functioning, and thus fall under the definition of ecological disturbance. After initially screening the abstracts of several thousands of papers, studies not directly addressing climatic controls of disturbances (for example, work describing disturbance patterns but not their climatic drivers) and those unrelated to the subject matter (for example, work on insect species that are reproducing in dead trees and are thus not acting as a disturbance agent) were excluded, and 674 papers were selected for detailed review. As individual papers frequently contained evidence for more than one climatic effect on disturbances, 1,669 observations were extracted from the selected papers (see Supplementary Text as well as Supplementary Table 1 and Supplementary Figs 1 and 2). We conducted an in-depth uncertainty analysis of the information synthesized from the literature, assessing how well the data corresponded with the variable of interest in our analysis (that is, disturbance activity and changes therein) and evaluating the methodological rigour applied in its generation (see Supplementary Text and Supplementary Figs 3–5). We subsequently omitted information that we deemed to be a poor proxy for disturbance change or of limited methodological rigour, resulting in 1,621 observations available for analysis (Supplementary Dataset 1).
We applied a common analysis scheme to all reviewed papers. For each paper we recorded meta-data on study location, methodological approach (empirical, experimental or simulation-based) and the disturbance agent(s) studied. We distinguished direct, indirect and interaction effects of climate change41–43 on disturbances in our analysis of the literature. Direct effects were defined as the unmediated impacts of climate variables on disturbance processes. Examples included changes in the frequency or severity of wind events and drought periods, changes in lightning activity or climate-mediated changes in the metabolic rates of pests and pathogens. Indirect effects were defined as changes in the disturbance regime through climate effects on vegetation and other ecosystem processes not directly related to disturbances. Prominent processes considered here are climate-mediated changes in the tree population and community composition, and include an alteration of the disturbance susceptibility through a change in tree species composition, size, density (for example, fuel available for burning) and distribution, as well as changes in tree-level vulnerability (for example, changes in soil anchorage of trees against wind due to variation in soil frost). Interaction effects were defined as linked or compounding relationships between disturbance agents27, such as an increased risk of bark beetle outbreaks resulting from wind disturbance (creating large amounts of effectively defenceless breeding material supporting the build-up of beetle populations) or drought (weakening tree defences against beetles). Only interactions between the six agents investigated here were considered explicitly.
To characterize the climate sensitivity of disturbances, we first collated the evidence for direct, indirect and interaction effects of climate change for each of the six disturbance agents studied. We screened the information for key climatic drivers of disturbances, and analysed their variation over biomes. As an auxiliary variable, we determined the response time of the ecosystem (that is, the time needed to respond to a respective change in a climate driver) on an ordinal scale. Subsequently, we synthesized the literature regarding potential future changes in the disturbance regime. This analysis was conducted at two levels. First, the sign of the climate effect (positive, more disturbance; negative, less disturbance) in response to changes in the respective climate variable(s) was assessed. Interaction effects were grouped by directionality (links between individual agents) and also analysed for the sign of the interaction. This information was synthesized qualitatively, scrutinizing whether amplifying or dampening climate change impacts prevail for each disturbance agent (Supplementary Fig. 6). We conducted this analysis separately for two broad trajectories of change: (1) warmer and wetter conditions, which assume an increase in both indicators of the thermal environment and water availability (for example, warmer temperatures, higher levels of precipitation and soil moisture, or lower levels of water deficit and drought indices); and (2) warmer and drier conditions, with an opposite direction of change for indicators of water availability under warming temperatures (see Supplementary Text for details). Second, we calculated a relative effect size (disturbance change in response to future climate change relative to baseline climate conditions, with a value of one indicating no change) across all the potential future climate conditions studied in the literature. Relative effect sizes were tested against the null hypothesis of no change in disturbance as a result of climate change using Wilcoxon signed rank sum tests. All analyses were conducted using the R language and environment for statistical computing44, specifically employing the packages ‘circlize’45 and ‘fsmb’46.
Pathways of climate influence
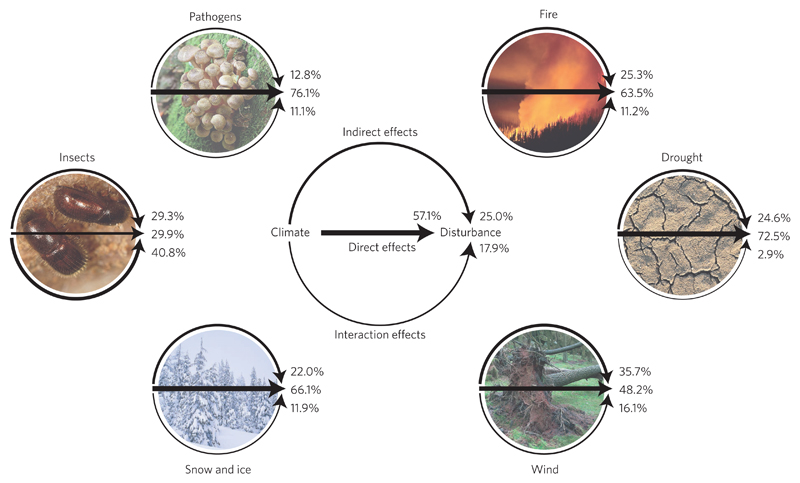
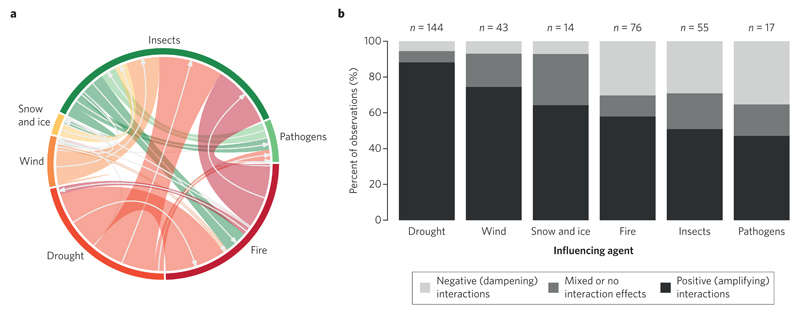
We found evidence for a substantial influence of climate on disturbances via all three scrutinized pathways, that is, direct, indirect and interaction effects. More than half of the observations reported in the literature related to direct climate effects (57.1%), which were the most prominent pathway of climate influence for all analysed agents except insects (Fig. 1). Direct effects were found to be particularly pronounced for abiotic agents: abiotic disturbances are often the direct consequence of climatic extremes, and are thus highly sensitive to changes in their occurrence, intensity and duration (Table 1). Furthermore, 25.0% of the analysed observations reported indirect effects of climate change on disturbances. Climate-mediated changes in forest structure and composition were particularly relevant in the context of wind disturbance. Also interactions between disturbance agents are well documented in the analysed literature (17.9% of the overall observations). For insects, for instance, 40.8% of the reported effects were associated with disturbance interactions. Links between abiotic (influencing agent) and biotic (influenced agent) disturbances were found to be particularly strong (Fig. 2a). The large majority of the recorded interaction effects were positive or predominately positive (71.0%), indicating an amplification of disturbance as a result of the interaction between agents. In particular, disturbances by drought and wind strongly facilitate the activity of other disturbance agents, such as insects and fire (Fig. 2b and Supplementary Table 2). Overall, only 16.2% of the studies on disturbance interactions reported a negative or predominately negative (that is, dampening) effect between interacting disturbance agents.
Figure 1. Distribution of evidence for direct, indirect and interaction effects of climate change on forest disturbance agents in the reviewed literature.
For every agent, arrow widths and percentages indicate the relative prominence of the respective effect as expressed by the number of observations extracted from the analysed literature supporting it. The central panel displays the aggregate result over all disturbance agents. Direct effects are unmediated impacts of climate on disturbance processes, while indirect effects describe a climate influence on disturbances through effects on vegetation and other ecosystem processes. Interaction effects refer to the focal agent being influenced by other disturbance agents. Image credits: David R. Frazier Photolibrary/Alamy Stock Photo (fire); PhotoDisc/Getty Images/Don Farrell (drought); Chris Warham/Alamy Stock Photo (wind); Royalty-Free/Corbis (snow and ice); Nigel Cattlin/Alamy Stock Photo (insects); and Naturepix/Alamy Stock Photo (pathogens).
Table 1. Important processes through which climate influences forest disturbances.
| Disturbance agent | Direct effects: climate impact through changes in… | Indirect effects: climate impact through changes in… | Interaction effects: climate impact through changes in… |
|---|---|---|---|
| Fire | Fuel moisture24 Ignition (for example, lightning activity) Fire spread (for example, wind speed66) |
Fuel availability (for example, vegetation productivity67) Flammability (for example, vegetation composition) Fuel continuity (for example, vegetation structure68) |
Fuel availability (for example, via wind or insect disturbance) Fuel continuity (for example, avalanche paths as fuel breaks69) |
| Drought | Occurrence of water limitation Duration of water limitation70 Intensity of water deficit70 |
Water use and water-use efficiency (for example, tree density and competition) Susceptibility to water deficit (for example, tree species composition71) |
Water use and water-use efficiency (for example, insect-related density changes) Susceptibility to water deficit (for example, fire-mediated changes in forest structure72) |
| Wind | Occurrence of strong winds73 Duration of wind events74 Intensity of wind events (for example, peak wind speeds)75 |
Tree anchorage (for example, soil frost75) Wind exposure (for example, tree growth76) Wind resistance (for example, tree species composition54) |
Wind exposure (for example, insect disturbances increases canopy roughness) Soil anchorage (for example, pathogens decrease rooting stability77) Resistance to stem breakage (for example, pathogens decrease stability) |
| Snow and ice | Snow occurrence78 Snow duration79 Occurrence of freezing rain80 |
Exposure of forest to snow81 Avalanche risk82 |
Avalanche risk (for example, through gap formation by bark beetles83) |
| Insects | Agent metabolic rate (for example, reproduction35) Agent behaviour (for example, consumption84) Agent survival85 |
Host distribution and range86 Agent–host synchronization (for example, budburst87) Host defence (for example, carbohydrate reserves) |
Host presence and abundance33 Host resistance and defence (for example, through changes in drought88) |
| Pathogens | Agent metabolic rate (for example, respiration52) Agent abundance89 |
Host abundance and diversity90 Host defence91 |
Agent interaction and asynchrony92 Agent dispersal (for example, through vector insects93) |
Figure 2. Interactions between forest disturbance agents.
a, The sector size in the outer circle indicates the distribution of interactions over agents, while the flows through the centre of the circle illustrate the relative importance of interactions between individual agents (as measured by the number of observations reporting on the respective interaction). Arrows point from the influencing agent to the agent being influenced by the interaction. b, Sign of the interaction effect induced by the influencing agent on the influenced agent. n, number of observations.
Climate drivers and response times
The climatic drivers of disturbances varied strongly with agent and region. However, temperature-related variables were the most prominent climatic drivers reported in the forest disturbance literature (42.0%). Water availability was a second important climatic influence on disturbance regimes (37.9%). The importance of temperature-related variables on the disturbance regime increased with latitude and was highest in the boreal biome (Supplementary Fig. 9). Conversely, the importance of water availability decreased with latitude and was highest in the tropics. In addition to temperature and water availability, a wide range of other climate-related variables were associated with disturbance change, ranging from wind speed and atmospheric moisture content to snow pack and atmospheric CO2 concentration.
The response times of the disturbance regime to changes in the climate system varied widely, ranging from annual to centennial scales. Response times were clearly related to the type of climate effect, with disturbance interactions constituting the fastest responding pathway and indirect effects being the slowest (Supplementary Fig. 10). For interaction effects, the analysed literature reports a response time of <6 years in 81.0% of the reviewed cases, and only 9.0% of the studied interaction effects have a response time of >25 years. For indirect effects, only 38.6% of the systems responded within the first five years of the respective climatic forcing, while 44.6% of the responses took >25 years.
Potential future disturbance change
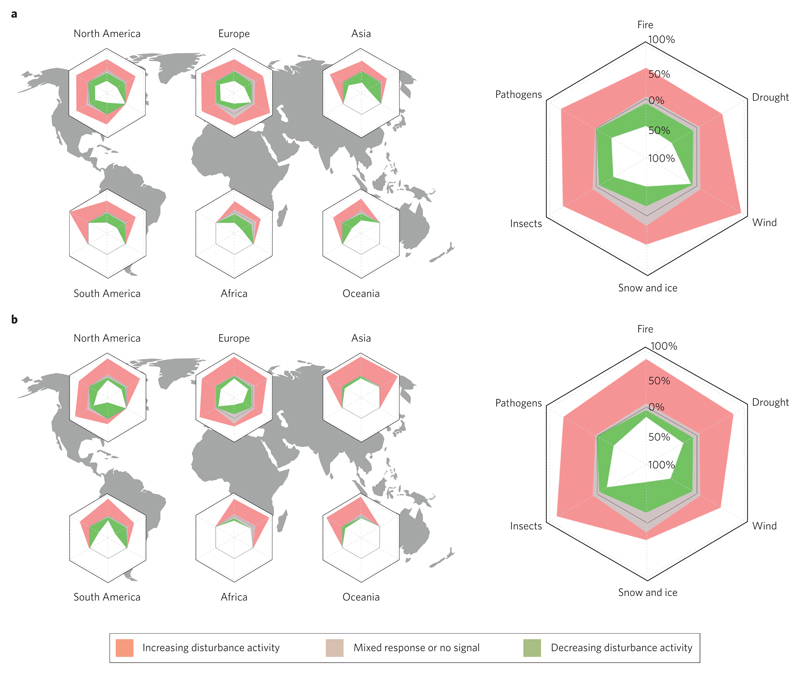
At the global scale, our analysis suggests that disturbances from five out of the six analysed agents are likely to increase in a warming world. The exception was disturbances from snow and ice, which are likely to decrease in the future, especially under warmer and drier conditions (Supplementary Figs 7 and 11). For warmer and drier future conditions, the large majority of studies suggested an increase in fires (82.4% of the observations), drought (74.2%) and insect activity (78.4%) (Fig. 3). Under warmer and wetter conditions, the evidence for increased activity from these disturbance agents was significantly reduced (55.0%, 51.2% and 65.3%, respectively). Wetter conditions were found to particularly foster wind disturbance (expected to increase in 89.1% of the cases) and pathogen activity (69.0%). Indirect climate effects were dampening the overall climate sensitivity of the system more often than direct climate effects (Supplementary Table 2 and Supplementary Figs 7 and 8), although no significant differences in effect sizes were found (Supplementary Fig. 13). Interaction effects were largely amplifying climate sensitivity (Fig. 2).
Figure 3. Global disturbance response to changing temperature and water availability.
a,b, Radar surfaces indicate the distribution of evidence (% of observations) for increasing or decreasing disturbance activity under warmer and wetter (a) as well as warmer and drier (b) climate conditions. The large radar plots to the right summarize the responses over all continents. Disturbance agents with less than four observations were omitted in the analysis. Only direct and indirect climate effects are considered here. More details on the qualitative modelling applied can be found in the Supplementary Information.
Across all scenarios considered in the analysed literature, the ratio between disturbances under future climate to disturbances under baseline conditions was significantly positive (P < 0.05). The exception was disturbances from snow and ice, which decreased significantly (median effect size of 0.345 over all studies and climate change scenarios; see Supplementary Fig. 11). Disturbances from all other agents increased under future climate change, with median effect sizes of between 1.34 and 1.51. Climate-related disturbance effects were positive across all biomes (P < 0.001) and moderately increased with latitude (Supplementary Fig. 12), with the highest values reported for the boreal zone (1.71). Furthermore, coniferous forests had a significantly higher future disturbance effect size than broadleaved and mixed forest types (Supplementary Fig. 14). Also, longer response times of disturbances to climate change were associated with increased effect sizes (Supplementary Fig. 15).
Discussion and conclusion
We found strong support for the hypothesis that climate change could markedly modify future forest disturbance regimes at the global scale. Our analysis of the global forest disturbance literature suggests that disturbances from fire, insects and pathogens in particular are likely to increase in a warming world (regardless of changes in water availability). These agents and their interactions currently dominate disturbance regimes in many forests of the world, and will probably gain further importance globally in the coming decades. Future changes of disturbances caused by other agents, such as drought, wind and snow, will be strongly contingent on changes in water availability, which can be expected to vary more strongly locally and intra-annually than temperature changes. Wind disturbance, for instance, which is currently the most important disturbance agent in Europe40, is expected to respond more strongly to changes in precipitation (and the corresponding changes in tree soil anchorage and tree growth) than to warming temperatures (compare Fig. 3). Yet the most influential climate variable determining wind disturbance remains the frequency and intensity of strong winds, for which current and future trends remain inconclusive47,48. In general, our global summary of the climate sensitivity of forest disturbance regimes suggests that the recently observed increases in disturbance activity10,40,49 are likely to continue in the coming decades as climate warms further50,51.
Our synthesis of effect pathways showed that direct climate effects were by far the most prominently reported impact in the analysed literature. This underlines the importance of climatic drivers as inciting factors of tree mortality, and highlights the strong dependence of developmental rates of biotic disturbance agents on climatic conditions26,35. However, the prominence of direct effects in the literature may at least partially result from the fact that they are easier to study and isolate (for example, in laboratory experiments52) than indirect and interaction effects. Publication bias might thus result in an overestimation of the importance of direct effects relative to indirect and interaction effects in our analysis.
Indirect effects, mediated by climate-related changes in vegetation structure and composition, were most frequently reported for wind disturbance, but were documented in the literature for all six studied disturbance agents. They are slower than climate effects via direct and interaction pathways, with response times frequently in the range of several decades. Also, indirect effects are often dampening disturbance increases (Supplementary Table 2 and Supplementary Figs 7 and 8), for example, when trees susceptible to an increasingly aggressive insect pest are outcompeted by individuals or species better adapted to warmer climates, ultimately resulting in a system less vulnerable to disturbances33,53. A second important class of dampening indirect effects occur when a previous disturbance event lowers the probability for subsequent disturbances by the same agent, for example, through a disturbance-induced alteration of forest structure or the depletion of the resource a disturbance agent depends on54–56. The temporal mismatch observed between direct and indirect effects (Supplementary Fig. 10) suggests that disturbances will probably increase further in the coming decades, as dampening effects of changes in forest structure and composition take effect only with considerable delay. Here it has to be noted that our estimate of response times to climatic changes is necessarily truncated by the observation periods of the underlying studies. It might thus be biased against longterm effects8 and underestimate the full temporal extent of climate effects on disturbances.
Evidence for potential changes in disturbance interactions was found for all six investigated agents. In this context, it is noteworthy that the large majority of the interaction effects reported in the literature are positive, that is, they amplify disturbance activity. We showed that interactions are especially important for the dynamics of biotic disturbance agents. As an increasing disturbance activity under climate change also means an increasing propensity for disturbance interactions, biotic agents could be particularly prone to further intensification via the influence of other disturbance agents29,57. This is of growing concern, as amplification of disturbances through interactions could also increase the potential for the exceedance of ecological thresholds and tipping points27,58.
In particular, the indirect and interaction effects of climate change on disturbance regimes need to be better understood to comprehensively assess future trajectories of disturbance in a changing world. The complexity of disturbance interactions complicates predictions of future forest change, and highlights the need for further research comprising multiple interacting disturbance agents and larger spatiotemporal scales. Dynamic vegetation models are prime tools for this domain of inquiry59. Simulation models are able to consistently track vegetation–disturbance feedbacks over time frames of decades to centuries33,60 and allow controlled experiments to isolate the effects of interactions between different agents32,60. However, many current disturbance models either do not explicitly consider vegetation processes, or disturbance agents are simulated in isolation, neglecting potential interaction effects. Future work should thus focus on integrating disturbance and vegetation dynamics in models, to address the complex interrelations between climate, vegetation and disturbance61,62. Furthermore, long-term ecological observations and dedicated experimentation are needed to improve our understanding of changing disturbance regimes, and provide the data needed for parameterizing and evaluating the above-mentioned simulation models59.
Our analysis revealed a strong bias of the literature towards agents such as fire, drought, insects and pathogens, as well as ecosystems located in North America and Europe (Supplementary Table 1 and Supplementary Fig. 1). However, climate change is a global phenomenon, affecting forests in all regions of the world. To obtain a more comprehensive understanding of the global patterns of disturbance change, considerable knowledge gaps on the climate sensitivity of disturbance regimes need to be filled. It remains unclear, for instance, whether the increasing effect of future climate change with latitude reported here (Supplementary Fig. 9) is the result of an increased exposure of boreal forests to climate change in combination with naturally lower tree species diversity, or whether it is simply the effect of a publication bias towards these ecosystems. Furthermore, the fact that disturbance research is currently focused on a limited number of agents could be increasingly problematic in the future, as agents that were of little regional relevance in the past could gain importance under changing climatic conditions. In this regard, it should be noted that invasive alien pests63,64 were not in the focus of our analysis, but are likely to contribute considerably to future changes in disturbance regimes.
Climate-induced changes in disturbance regimes are a major challenge for the sustainable provisioning of ecosystem services to society6,14. Our finding of prominent indirect effects suggests that forest management can actively modulate the climate sensitivity of disturbance regimes via modifying forest structure and composition. However, mitigating the direct effects of a changing climate through management will be rarely possible, which suggests that future management will need to find ways of coping with disturbance change. A promising approach in this regard is to foster the resilience of forests to changing disturbance regimes, enabling their recovery from and adaptation to disturbances17,65, to ensure a continuous provisioning of ecosystem services18 and, ultimately, prepare both ecosystems and society for an increasingly disturbed future of forests.
Supplementary Material
Supplementary information is available in the online version of the paper.
Acknowledgements
This work is the result of a working group within the European Union (EU) COST Action PROFOUND (FP1304) and the IUFRO Task Force on Climate Change and Forest Health. R.S. acknowledges funding from a START grant of the Austrian Science Fund FWF (Y 895-B25). M.K. acknowledges funding from the EU FP7 project LUC4C, grant 603542. M.Peltoniemi was funded by EU Life+ (LIFE12 ENV/FI/000409). C.P.O.R. acknowledges funding from the German Federal Ministry of Education and Research (BMBF, grant no. 01LS1201A1). D.M.-B. was funded by a Marie-Curie IEF grant (EU grant 329935). J.W. was funded by the long-term research and development project RVO 67985939 (The Czech Academy of Sciences). M.S., V.T. and J.W. acknowledge support from a project of the Ministry of Education, Youth and Sports no. LD15158. M.Petr acknowledges funding support from Forestry Commission (UK) funded research on climate change impacts. J.H. acknowledges funding from the Foundation for Research of Natural Resources in Finland, grant no. 2015090. M.S. and V.T. acknowledge funding from the project GAČR 15-14840S “EXTEMIT - K”, no. CZ.02.1.01/0.0/0.0/15_003/0000433 financed by OP RDE.
Footnotes
Author contributions
R.S. and C.P.O.R. initiated the research. R.S. and D.T. designed the study, with feedback from all authors during workshops in Vienna, Austria (April 2015) and Novi Sad, Serbia (November 2015). G.V., D.A., P.M., C.P.O.R. and R.S. reviewed the fire literature. D.M.-B., M.Petr and V.T. reviewed the drought literature. J.W., M.J.L., M.F. and T.N. reviewed the wind literature. D.T. and T.N. reviewed the snow and ice literature. M.K., D.T., M.J.L., M.S. and J.W. reviewed the literature on insects. M.Peltoniemi, J.H. and M.Petr reviewed the literature on pathogens. R.S. conducted the analyses. All authors contributed to writing and revising the manuscript.
Additional information
Reprints and permissions information is available online at www.nature.com/reprints. Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Turner MG. Disturbance and landscape dynamics in a changing world. Ecology. 2010;91:2833–2849. doi: 10.1890/10-0097.1. [DOI] [PubMed] [Google Scholar]
- 2.Frelich LE. Forest Dynamics and Disturbance Regimes: Studies from Temperate Evergreen–Deciduous Forests. Cambridge Univ. Press; 2002. [Google Scholar]
- 3.White PS, Pickett STA. In: The Ecology of Natural Disturbance and Patch Dynamics. Pickett STA, White PS, editors. Academic Press; 1985. pp. 3–13. [Google Scholar]
- 4.Turner MG, Tinker DB, Romme WH, Kashian DM, Litton CM. Landscape patterns of sapling density, leaf area, and aboveground net primary production in postfire lodgepole pine forests, Yellowstone National Park (USA) Ecosystems. 2004;7:751–775. [Google Scholar]
- 5.Beudert B, et al. Bark beetles increase biodiversity while maintaining drinking water quality. Conserv Lett. 2015;8:272–281. [Google Scholar]
- 6.Thom D, Seidl R. Natural disturbance impacts on ecosystem services and biodiversity in temperate and boreal forests. Biol Rev. 2016;91:760–781. doi: 10.1111/brv.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holling CS, Gunderson LH. In: Panarchy Understanding the transformations in human and natural systems. Gunderson LH, Holling CS, editors. Island Press; 2002. pp. 25–62. [Google Scholar]
- 8.Thom D, Rammer W, Seidl R. Disturbances catalyze the adaptation of forest ecosystems to changing climate conditions. Global Change Biol. 2017;23:269–282. doi: 10.1111/gcb.13506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seidl R, Schelhaas M-J, Lexer MJ. Unraveling the drivers of intensifying forest disturbance regimes in Europe. Global Change Biol. 2011;17:2842–2852. [Google Scholar]
- 10.Westerling AL. Increasing western US forest wildfire activity: sensitivity to changes in the timing of spring. Philos Trans R Soc Lond B. 2016;371 doi: 10.1098/rstb.2015.0178. 20150178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pechony O, Shindell DT. Driving forces of global wildfires over the past millennium and the forthcoming century. Proc Natl Acad Sci USA. 2010;107:19167–19170. doi: 10.1073/pnas.1003669107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paritsis J, Veblen TT. Dendroecological analysis of defoliator outbreaks on Nothofagus pumilio and their relation to climate variability in the Patagonian Andes. Global Change Biol. 2011;17:239–253. [Google Scholar]
- 13.Kautz M, Meddens AJH, Hall RJ, Arneth A. Biotic disturbances in Northern Hemisphere forests — a synthesis of recent data, uncertainties and implications for forest monitoring and modelling. Global Ecol Biogeogr. 2017;26:533–552. [Google Scholar]
- 14.Millar CI, Stephenson NL. Temperate forest health in an era of emerging megadisturbance. Science. 2015;349:823–826. doi: 10.1126/science.aaa9933. [DOI] [PubMed] [Google Scholar]
- 15.Allen CD, Breshears DD, McDowell NG. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere. 2015;6:1–55. [Google Scholar]
- 16.Reyer CPO, et al. Forest resilience and tipping points at different spatio-temporal scales: approaches and challenges. J Ecol. 2015;103:5–15. [Google Scholar]
- 17.Johnstone JF, et al. Changing disturbance regimes, climate warming and forest resilience. Front Ecol Environ. 2016;14:369–378. [Google Scholar]
- 18.Seidl R, Spies TA, Peterson DL, Stephens SL, Hicke JA. Searching for resilience: addressing the impacts of changing disturbance regimes on forest ecosystem services. J Appl Ecol. 2016;53:120–129. doi: 10.1111/1365-2664.12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindner M, et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For Ecol Manage. 2010;259:698–709. [Google Scholar]
- 20.White PS. Pattern, process, and natural disturbance in vegetation. Bot Rev. 1979;45:229–299. [Google Scholar]
- 21.Sousa WP. The role of disturbance in natural communities. Annu Rev Ecol Syst. 1984;15:353–391. [Google Scholar]
- 22.Dale VH, et al. Climate change and forest disturbances. Bioscience. 2001;51:723–734. [Google Scholar]
- 23.Overpeck JT, Rind D, Goldberg R. Climate induced changes in forest disturbance and vegetation. Nature. 1990;343:51–53. [Google Scholar]
- 24.Williams AP, Abatzoglou JT. Recent advances and remaining uncertainties in resolving past and future climate effects on global fire activity. Curr Clim Change Rep. 2016;2:1–14. [Google Scholar]
- 25.Raffa KF, et al. In: Climate Change and Insect Pests. Björkman C, Niemelä P, editors. CABI; 2015. pp. 173–201. [Google Scholar]
- 26.Sturrock RN, et al. Climate change and forest diseases. Plant Pathol. 2011;60:133–149. [Google Scholar]
- 27.Buma B. Disturbance interactions: characterization, prediction, and the potential for cascading effects. Ecosphere. 2015;6 art70. [Google Scholar]
- 28.Foster CN, Sato CF, Lindenmayer DB, Barton PS. Integrating theory into disturbance interaction experiments to better inform ecosystem management. Global Change Biol. 2016;22:1325–1335. doi: 10.1111/gcb.13155. [DOI] [PubMed] [Google Scholar]
- 29.Jactel H, et al. Drought effects on damage by forest insects and pathogens: a meta-analysis. Global Change Biol. 2012;18:267–276. [Google Scholar]
- 30.Peters DPC, et al. Cross-system comparisons elucidate disturbance complexities and generalities. Ecosphere. 2011;2 art81. [Google Scholar]
- 31.Berrang-Ford L, Pearce T, Ford JD. Systematic review approaches for climate change adaptation research. Reg Environ Change. 2015;15:755–769. [Google Scholar]
- 32.Seidl R, Rammer W. Climate change amplifies the interactions between wind and bark beetle disturbance in forest landscapes. Landscape Ecol. 2017 doi: 10.1007/s10980-016-0396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Temperli C, Bugmann H, Elkin C. Cross-scale interactions among bark beetles, climate change, and wind disturbances: a landscape modeling approach. Ecol Monogr. 2013;83:383–402. [Google Scholar]
- 34.Flannigan M, et al. Global wildland fire season severity in the 21st century. For Ecol Manage. 2013;294:54–61. [Google Scholar]
- 35.Jönsson AM, et al. Modelling the potential impact of global warming on Ips typographus voltinism and reproductive diapause. Clim Change. 2011;109:695–718. [Google Scholar]
- 36.Hanewinkel M, Cullmann DA, Schelhaas M-J, Nabuurs G-J, Zimmermann NE. Climate change may cause severe loss in the economic value of European forest land. Nat Clim Change. 2013;3:203–207. [Google Scholar]
- 37.Schröter D, et al. Ecosystem service supply and vulnerability to global change in Europe. Science. 2005;310:1333–1337. doi: 10.1126/science.1115233. [DOI] [PubMed] [Google Scholar]
- 38.Naudts K, et al. Europe’s forest management did not mitigate climate warming. Science. 2016;351:597–601. doi: 10.1126/science.aad7270. [DOI] [PubMed] [Google Scholar]
- 39.Running SW. Ecosystem disturbance, carbon, and climate. Science. 2008;321:652–653. doi: 10.1126/science.1159607. [DOI] [PubMed] [Google Scholar]
- 40.Seidl R, Schelhaas M-J, Rammer W, Verkerk PJ. Increasing forest disturbances in Europe and their impact on carbon storage. Nat Clim Change. 2014;4:806–810. doi: 10.1038/nclimate2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clark JS, Bell DM, Kwit MC, Zhu K. Competition-interaction landscapes for the joint response of forests to climate change. Global Change Biol. 2014;20:1979–1991. doi: 10.1111/gcb.12425. [DOI] [PubMed] [Google Scholar]
- 42.Bentz BJ, et al. Climate change and bark beetles of the western United States and Canada: direct and indirect effects. Bioscience. 2010;60:602–613. [Google Scholar]
- 43.Liu Z, Wimberly MC. Direct and indirect effects of climate change on projected future fire regimes in the western United States. Sci Total Environ. 2016;542:65–75. doi: 10.1016/j.scitotenv.2015.10.093. [DOI] [PubMed] [Google Scholar]
- 44.R Development Core Team R. A Language and Environment for Statistical Computing (R Foundation for Statistical Computing) 2016 [Google Scholar]
- 45.Gu Z, Gu L, Eils R, Schlesner M, Brors B. circlize implements and enhances circular visualization in R. Bioinformatics. 2014;30:2811–2812. doi: 10.1093/bioinformatics/btu393. [DOI] [PubMed] [Google Scholar]
- 46.Nakazawa M. fmsb: Functions for Medical Statistics Book with some Demographic Data R package v.0.5.1. 2014 http://CRAN.R-project.org/package=fmsb.
- 47.Knutson TR, et al. Tropical cyclones and climate change. Nat Geosci. 2010;3:157–163. [Google Scholar]
- 48.Bender MA, et al. Modeled impact of anthropogenic warming on the frequency of intense Atlantic hurricanes. Science. 2010;327:454–458. doi: 10.1126/science.1180568. [DOI] [PubMed] [Google Scholar]
- 49.Meddens AJH, Hicke JA, Ferguson CA. Spatiotemporal patterns of observed bark beetle-caused tree mortality in British Columbia and the western United States. Ecol Appl. 2012;22:1876–1891. doi: 10.1890/11-1785.1. [DOI] [PubMed] [Google Scholar]
- 50.Stocker TF, et al., editors. IPCC. Climate Change 2013: The Physical Science Basis. Cambridge Univ. Press; 2013. [Google Scholar]
- 51.Solomon S, Plattner G-K, Knutti R, Friedlingstein P. Irreversible climate change due to carbon dioxide emissions. Proc Natl Acad Sci USA. 2009;106:1704–1709. doi: 10.1073/pnas.0812721106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Müller MM, et al. Predicting the activity of Heterobasidion parviporum on Norway spruce in warming climate from its respiration rate at different temperatures. For Pathol. 2014;44:325–336. [Google Scholar]
- 53.Cruickshank MG, Jaquish B, Nemec AFL. Resistance of half-sib interior Douglas-fir families to Armillaria ostoyae in British Columbia following artificial inoculation. Can J For Res. 2010;40:155–166. [Google Scholar]
- 54.Panferov O, Doering C, Rauch E, Sogachev A, Ahrends B. Feedbacks of windthrow for Norway spruce and Scots pine stands under changing climate. Environ Res Lett. 2009;4:045019. [Google Scholar]
- 55.Cowden MM, Hart JL, Schweitzer CJ, Dey DC. Effects of intermediate-scale wind disturbance on composition, structure, and succession in Quercus stands: implications for natural disturbance-based silviculture. For Ecol Manage. 2014;330:240–251. [Google Scholar]
- 56.Seidl R, Donato DC, Raffa KF, Turner MG. Spatial variability in tree regeneration after wildfire delays and dampens future bark beetle outbreaks. Proc Natl Acad Sci USA. 2016;113:13075–13080. doi: 10.1073/pnas.1615263113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stadelmann G, Bugmann H, Wermelinger B, Bigler C. Spatial interactions between storm damage and subsequent infestations by the European spruce bark beetle. For Ecol Manage. 2014;318:167–174. [Google Scholar]
- 58.Paine RT, Tegner MJ, Johnson EA. Compounded perturbations yield ecological surprises. Ecosystems. 1998;1:535–545. [Google Scholar]
- 59.Becknell JM, et al. Assessing interactions among changing climate, management, and disturbance in forests: a macrosystems approach. Bioscience. 2015;65:263–274. [Google Scholar]
- 60.Temperli C, Veblen TT, Hart SJ, Kulakowski D, Tepley AJ. Interactions among spruce beetle disturbance, climate change and forest dynamics captured by a forest landscape model. Ecosphere. 2015;6:art231. [Google Scholar]
- 61.Seidl R, et al. Modelling natural disturbances in forest ecosystems: a review. Ecol Modell. 2011;222:903–924. [Google Scholar]
- 62.Keane RE, et al. Representing climate, disturbance, and vegetation interactions in landscape models. Ecol Model. 2015;309–310:33–47. [Google Scholar]
- 63.Valenta V, Moser D, Kuttner M, Peterseil J, Essl F. A high-resolution map of emerald ash borer invasion risk for southern central Europe. Forests. 2015;6:3075–3086. [Google Scholar]
- 64.Gandhi KJK, Herms DA. Direct and indirect effects of alien insect herbivores on ecological processes and interactions in forests of eastern North America. Biol Invasions. 2010;12:389–405. [Google Scholar]
- 65.Seidl R. The shape of ecosystem management to come: anticipating risks and fostering resilience. Bioscience. 2014;64:1159–1169. doi: 10.1093/biosci/biu172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Billmire M, French NHF, Loboda T, Owen RC, Tyner M. Santa Ana winds and predictors of wildfire progression in southern California. Int J Wildland Fire. 2014;23:1119–1129. doi: 10.1071/wf13046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pausas JG, Ribeiro E. The global fire-productivity relationship. Global Ecol Biogeogr. 2013;22:728–736. [Google Scholar]
- 68.Bowman DM, Murphy BP, Williamson GJ, Cochrane MA. Pyrogeographic models, feedbacks and the future of global fire regimes. Global Ecol. 2014;32:821–824. [Google Scholar]
- 69.Ryan KC. Dynamic interactions between forest structure and fire behavior in boreal ecosystems. Silva Fenn. 2002;36:13–39. [Google Scholar]
- 70.Cook BI, Smerdon JE, Seager R, Coats S. Global warming and 21st century drying. Clim Dyn. 2014;43:2607–2627. [Google Scholar]
- 71.Suarez ML, Kitzberger T. Recruitment patterns following a severe drought: long-term compositional shifts in Patagonian forests. Can J For Res. 2008;38:3002–3010. [Google Scholar]
- 72.Harvey BJ, Donato DC, Turner High and dry: postfire drought and large stand-replacing burn patches reduce postfire tree regeneration in subalpine forests. Global Ecol Biogeogr. 2016;25:655–669. [Google Scholar]
- 73.Donat MG, Leckebusch GC, Wild S, Ulbrich U. Future changes in European winter storm losses and extreme wind speeds inferred from GCM and RCM multi-model simulations. Nat Hazards Earth Syst Sci. 2011;11:1351–1370. [Google Scholar]
- 74.Peltola H, et al. Impacts of climate change on timber production and regional risks of wind-induced damage to forests in Finland. For Ecol Manage. 2010;260:833–845. [Google Scholar]
- 75.Usbeck T, et al. Increasing storm damage to forests in Switzerland from 1858 to 2007. Agric For Meteorol. 2010;150:47–55. [Google Scholar]
- 76.Moore JR, Watt MS. Modelling the influence of predicted future climate change on the risk of wind damage within New Zealand’s planted forests. Global Change Biol Biol. 2015;21:3021–3035. doi: 10.1111/gcb.12900. [DOI] [PubMed] [Google Scholar]
- 77.Whitney RD, Fleming RL, Zhou K, Mossa DS. Relationship of root rot to black spruce windfall and mortality following strip clear-cutting. Can J For Res. 2002;32:283–294. [Google Scholar]
- 78.Teich M, Marty C, Gollut C, Grêt-Regamey A, Bebi P. Snow and weather conditions associated with avalanche releases in forests: rare situations with decreasing trends during the last 41years. Cold Regions Sci Technol. 2012;83:77–88. [Google Scholar]
- 79.Gregow H, Peltola H, Laapas M, Saku S, Venäläinen A. Combined occurrence of wind, snow loading and soil frost with implications for risks to forestry in Finland under the current and changing climatic conditions. Silva Fenn. 2011;45:35–54. [Google Scholar]
- 80.Cheng CS, Auld H, Li G, Klaassen J, Li Q. Possible impacts of climate change on freezing rain in south-central Canada using downscaled future climate scenarios. Nat Hazards Earth Syst Sci. 2007;7:71–87. [Google Scholar]
- 81.Kilpeläinen A, et al. Impacts of climate change on the risk of snow-induced forest damage in Finland. Clim Change. 2010;99:193–209. [Google Scholar]
- 82.Bebi P, Kulakowski D, Rixen C. Snow avalanche disturbances in forest ecosystems—state of research and implications for management. For Ecol Manage. 2009;257:1883–1892. [Google Scholar]
- 83.Maroschek M, Rammer W, Lexer MJ. Using a novel assessment framework to evaluate protective functions and timber production in Austrian mountain forests under climate change. Reg Environ Change. 2015;15:1543–1555. [Google Scholar]
- 84.Lemoine NP, Burkepile DE, Parker JD. Variable effects of temperature on insect herbivory. PeerJ. 2014;2:e376. doi: 10.7717/peerj.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Battisti A, et al. Expansion of geographic range in the pine processionary moth caused by increased winter temperatures. Ecol Appl. 2005;15:2084–2096. [Google Scholar]
- 86.Evangelista PH, Kumar S, Stohlgren TJ, Young NE. Assessing forest vulnerability and the potential distribution of pine beetles under current and future climate scenarios in the Interior West of the US. For Ecol Manage. 2011;262:307–316. [Google Scholar]
- 87.Schwartzberg EG, et al. Simulated climate warming alters phenological synchrony between an outbreak insect herbivore and host trees. Oecologia. 2014;175:1041–1049. doi: 10.1007/s00442-014-2960-4. [DOI] [PubMed] [Google Scholar]
- 88.Gaylord ML, et al. Drought predisposes piñon-juniper woodlands to insect attacks and mortality. New Phytol. 2013;198:567–578. doi: 10.1111/nph.12174. [DOI] [PubMed] [Google Scholar]
- 89.Aguayo J, Elegbede F, Husson C, Saintonge F-X, Marçais B. Modeling climate impact on an emerging disease, the Phytophthora alni-induced alder decline. Global Change Biol. 2014;20:3209–3221. doi: 10.1111/gcb.12601. [DOI] [PubMed] [Google Scholar]
- 90.Vacher C, Vile D, Helion E, Piou D, Desprez-Loustau M-L. Distribution of parasitic fungal species richness: influence of climate versus host species diversity. Divers Distrib. 2008;14:786–798. [Google Scholar]
- 91.Karnosky DF, et al. Interacting elevated CO2 and tropospheric O3 predisposes aspen (Populus tremuloides Michx.) to infection by rust (Melampsora medusae f. sp. tremuloidae) Global Change Biol. 2002;8:329–338. [Google Scholar]
- 92.Garnas JR, Houston DR, Ayres MP, Evans C. Disease ontogeny overshadows effects of climate and species interactions on population dynamics in a nonnative forest disease complex. Ecography. 2012;35:412–421. [Google Scholar]
- 93.Tsui CKM, et al. Population structure and migration pattern of a conifer pathogen, Grosmannia clavigera, as influenced by its symbiont, the mountain pine beetle. Mol Ecol. 2012;21:71–86. doi: 10.1111/j.1365-294X.2011.05366.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.