Abstract
Objective
Deficits in reinforcement-based decision-making have been reported in Generalized Anxiety Disorder. However, the pathophysiology of these deficits is largely unknown, extant studies have mainly examined youth and the integrity of core functional processes underpinning decision-making remain undetermined. In particular, it is unclear whether the representation of reinforcement prediction error (PE: the difference between received and expected reinforcement) is disrupted in Generalized Anxiety Disorder. The current study addresses these issues in adults with the disorder.
Methods
Forty-six un-medicated individuals with Generalized Anxiety Disorder and 32 healthy controls group-matched on IQ, gender and age, completed a passive avoidance task while undergoing functional MRI.
Results
Behaviorally, individuals with Generalized Anxiety Disorder showed impaired reinforcement-based decision-making. Imaging results revealed that during feedback, individuals with Generalized Anxiety Disorder relative to healthy controls showed a reduced correlation between PE and activity within ventromedial prefrontal cortex, ventral striatum and other structures implicated in decision-making. In addition, individuals with Generalized Anxiety Disorder relative to healthy participants showed a reduced correlation between punishment, but not reward, PEs and activity within bilateral lentiform nucleus/putamen.
Conclusions
This is the first study to identify computational impairments during decision-making in Generalized Anxiety Disorder. PE signaling is significantly disrupted in individuals with the disorder and may underpin the decision-making deficits observed in patients with GAD.
Introduction
Generalized Anxiety Disorder is an anxiety disorder associated with pervasive worry (1). Anxiety symptoms are associated with poorer decision-making in healthy (e.g. 2, 3, 4) and clinical populations (2), particularly individuals with Generalized Anxiety Disorder (5) where reinforcement-based decision-making deficits are associated with symptom severity (5, 6). Individuals with Generalized Anxiety Disorder fail to fully show healthy optimistic bias, which has been associated with a failure to appropriately represent the value of positive events in medial prefrontal cortex (7), a region critical for reinforcement-based decision-making (8). However, the nature of the pathophysiology disrupting reinforcement-based decision-making in Generalized Anxiety Disorder remains largely unknown.
Two computational processes critical for reinforcement-based decision-making are the representation of: (i) expected value (EV)- the reinforcement expectancy associated with a stimulus/action; and (ii) prediction error (PE)- the difference between the amount of reward/punishment occurring relative to expectations (9). PE signals are thought to trigger reinforcement learning; the greater the PE, the greater the alteration in the reinforcement associated with the stimulus (9). Ventral striatum, ventromedial prefrontal cortex and posterior cingulate cortex are implicated in both the representation of EV during choice and PE signaling during feedback (10, 11). In addition, anterior insula cortex, dorsal anterior cingulate cortex/dorsomedial frontal cortex and caudate show increased BOLD response as a function of EV to sub-optimal choices (12, 13); these regions likely use EV information to guide the individual away from suboptimal choices (12, 14).
Relatively few studies have examined reinforcement-based decision-making in Generalized Anxiety Disorder and most have examined adolescents with the disorder (15–18). These studies have typically reported reduced responsiveness (other than within anterior insula cortex). Thus, adolescent individuals with Generalized Anxiety Disorder, relative to controls, showed a reduced increase in activity within caudate/putamen in anticipation of, and when receiving, reward following responding to high relative to low reward cues (15). Similarly, anxious adolescents (predominantly individuals with Generalized Anxiety Disorder) showed less ventral putamen activity relative to controls during risky relative to safe choices for monetary rewards (16). Consistent with this, though within the amygdala, adults with Generalized Anxiety Disorder showed reduced increases in amygdala activity for high uncertainty (choosing between two objects both associated with a 50% reward contingency) relative to low uncertainty choices (choosing between two objects associated with respectively a 100% and a 50% reward contingency) relative to controls (17). Moreover, recent work has revealed that anticipatory anxiety (induced by the threat of electric shocks) was associated with reduced EV signaling of reward within ventromedial prefrontal cortex and ventral striatum in healthy adults (19). However, Guyer, Choate (18) did report typical recruitment of striatal regions by adolescents with Generalized Anxiety Disorder during the anticipation of reward on the Monetary Incentive Delay task. Moreover, Benson, Guyer (15) reported that if participants with anxiety were required to chose between two responses, they showed greater increases in activity within caudate/putamen in anticipation of (and when receiving) reward following high relative to low reward cues compared to controls. Finally, Engelmann, Meyer (19) reported that anticipatory anxiety increased EV signaling of punishment within anterior insula cortex in healthy adults, while Galvan and Peris (16) reported that anxious adolescents showed greater anterior insula cortex activity relative to controls during risky relative to safe choices when responding to avoid losing money.
Given that relatively few studies have examined reinforcement-based decision-making in (particularly adult) individuals with Generalized Anxiety Disorder and the absence of patient studies utilizing model-based fMRI, we examined the computational processes involved in reinforcement-based decision-making in adults with Generalized Anxiety Disorder. Computational model-based imaging techniques allow the direct evaluation of empirically supported components of learning models of learning, such as EV and PE (20). Two main predictions were made. First, individuals with Generalized Anxiety Disorder would show impairment during reinforcement-based decision-making. Second, they would show reduced EV/PE signaling within ventromedial prefrontal cortex and ventral striatum relative to comparison individuals.
Methods
Participants
The study included 78 participants: 46 individuals meeting DSM-IV criteria for Generalized Anxiety Disorder and 32 healthy controls matched on IQ, gender and age (Table 1). Participantion was restricted to individuals aged 18 to 50 years. All were recruited from the community via advertising. Participants with Generalized Anxiety Disorder were diagnosed according to the DSM-IV (1994) criteria based on the Structural Clinical interview for DSM-IV Axis I disorders (SCID; 21) and a confirmatory clinical interview by a board-certified psychiatrist. As in our prior work, comorbidity with Social Anxiety Disorder was not exclusory. However, no subject had additional Axis-1 diagnoses other than social anxiety (N=18) and all were medication free for at least 6 months. Drug screens for benzodiazepines, cocaine metabolites, opiates, and cannabinoids (THC) were performed during the screening visit, and positive screens were exclusionary for study participation. Healthy controls with any history of psychiatric illness were excluded. All participants were in good physical health, as confirmed by a complete physical exam. All participants provided written informed consent and the National Institutes of Health Combined Neuroscience institutional review board approved all procedures. In addition, all participants completed the Inventory of Depressive Symptomatology-Self Report (IDS-SR; 22) and the individuals with Generalized Anxiety Disorder completed the Penn State Worry Questionnaire (PSWQ; 23) and the Generalized Anxiety Disorder-7 scale (24). Subjects’ level of overall social, occupational, and psychological functioning was further assessed by the Global Assessment of Functioning (GAF; Table 1). Note that no individual in the current study took part in our earlier behavioral investigation of the passive avoidance task in individuals with Generalized Anxiety Disorder, which informed the current study (6).
Table 1.
Demographics and task performance on a Passive Avoidance Task in 32 healthy comparison individuals and 46 individuals with Generalized Anxiety Disorder.
|
Healthy Comparison Individuals (N=32) |
Individuals with Generalized Anxiety Disorder (N=46) |
|||||
|---|---|---|---|---|---|---|
| Demographic Data | ||||||
| Mean | Std. Dev. | Mean | Std. Dev. | t | p | |
| age | 28.85 | 7.17 | 30.78 | 9.69 | .96 | .34 |
| IQ | 119.72 | 10.88 | 117.50 | 9.09 | .98 | .33 |
| Inventory of Depressive Symptomology | 2.28 | 2.58 | 22.70 | 8.60 | 12.61 | <.01 |
| Penn State Worry Questionnaire | 64.39 | 10.50 | ||||
| Generalized Anxiety Disorder-7 | 22.22 | 5.90 | ||||
| Global Assessment of Functioning | 61.57 | 3.88 | ||||
| N | percentage | N | percentage | χ2 | p | |
| Male participants | 11 | 34.4% | 10 | 21.7% | 1.52 | .22 |
| Task Performance Data | ||||||
|
Healthy Comparison Individuals (N=32) |
Individuals with Generalized Anxiety Disorder (N=46) |
|||||
| Overall Errors | ||||||
| Mean | Std. Dev. | Mean | Std. Dev. | t | p | |
| Run 1 | 34.93% | 12.2 | 37.15% | 10.9 | 0.84 | .40 |
| Run 2 | 27.40% | 14.4 | 36.53% | 13.6 | 2.85 | <.01 |
| Run 3 | 23.38% | 16.5 | 36.10% | 14.6 | 3.59 | <.01 |
std. dev = standard deviation
Passive Avoidance task
Participants completed a passive avoidance task that has been employed previously (25). Trials began with a 1500ms image presentation of one of four objects, where participants either responded to the object or refused to respond. The image presentation was followed by a randomly jittered fixation (0–4000ms) and by a 1500ms feedback presentation. In trials where participants chose to respond to an object, one of four outcomes was presented: win $5, win $1, lose $1 or lose $5. All objects could engender each of these outcomes. However, the feedback was probabilistic where one object resulted in a gain of $18.57 over 10 trials, one a gain of $7.14, one a loss of $18.57 and one a loss of $7.14 (participants were paid for study participation, but not on the basis of their performance). Choosing not to respond resulted in the presentation of a blank screen. Another randomly jittered fixation (0–4000ms) presentation followed feedback. Participants completed 168 trials (42 trials/object) over 3 runs.
Based on behavioral data, a learning curve was modeled establishing EVs and PEs for each object for each individual subject. PE was calculated by subtracting the EV of the current trial(t) from the feedback (F) received on the current trial(t) [coded 1 (object rewarded) or 0 (object punished)] with the formula:
EV for the first trial of each object set to 0 and updated according to the formula:
where the EV of the current trial (t) equals the EV of the previous trial (t−1) plus the PE of the previous trial multiplied by the learning rate (α). The learning rate was set at α=0.348. A learning rate was calculated for each participant via a model-fitting simulation (see Supplemental Methods) and then the average (0.348) used for the model based fMRI analyses. Average log likelihood across the sample: mean=93.00, SD=21.71).
MRI parameters
Participants were scanned using a 3-T GE Signa scanner. A total of 124 functional images per run were taken with a gradient echo planar imaging (EPI) sequence (repetition time=2900ms; echo time=27ms; 64×64 matrix; 90° flip angle; 24cm field of view). Whole-brain coverage was obtained with 46 axial slices (thickness, 2.5mm; .5mm spacing; in-plane resolution, 3.75×3.75mm). A high-resolution anatomical scan (3-dimensional spoiled gradient recalled acquisition in a steady state; repetition time=7.776ms; echo time=2.984ms; 24cm field of view; 12° flip angle; 124 axial slices; thickness, 1.2mm; 256×256 matrix) in register with the EPI data set was obtained covering the whole brain.
Imaging data preprocessing
Imaging data were preprocessed and analyzed in AFNI (26). At the individual level, functional images from the first 5 repetitions, collected prior to equilibrium magnetization, were discarded. Functional images from the 3 time series were motion corrected and spatially smoothed with a 6-mm full-width half-maximum gaussian filter. The time series were normalized by dividing the signal intensity of a voxel at each point by the mean signal intensity of that voxel for each run and multiplying the result by 100. Resultant regression coefficients represented a percentage of signal change from the mean.
Following this, four regressors were generated: objects chosen, objects refused, reward received, punishment received. All regressors were created by convolving the train of stimulus events with a gamma variate hemodynamic response function to account for the slow hemodynamic response. The participants’ anatomical scans were then individually registered to the Talairach and Tournoux atlas (27). The individuals’ functional EPI data were then registered to their Talairach anatomical scan. Linear regression modeling was performed using the 4 regressors described above plus regressors to model a first-order baseline drift function. Furthermore, the percent signal change at each voxel and time point were modulated by EV (during the choice-phase) and by PE (during the feedback-phase). This produced a modulated and un-modulated β coefficient and associated t statistic for each voxel and regressor.
fMRI data analysis
The group analysis of the BOLD data was then performed on the modulated regression coefficients from individual subject analyses. The modulated coefficients were entered into a 2(diagnosis: Generalized Anxiety Disorder, healthy controls) by 2(choice: approach, refuse) ANOVA in the choice-phase and a 2(diagnosis: Generalized Anxiety Disorder, healthy controls) by 2(feedback type: reward, punishment) in the feedback-phase. The results of the analysis using the un-modulated coefficients can be found in the Supplemental Results 1.1 and 1.2. The 3dClustSim program in AFNI was used to calculate an extent cluster threshold hold at an initial p of .005. This lead to a .05 FWE corrected extant threshold of 18 voxels (486mm3).
Results
Behavioral Results
A 2(diagnosis: Generalized Anxiety Disorder, healthy controls) by 3(task runs: first, second, third) by 2(error type: commission errors, omission errors) repeated-measures ANOVA was conducted on the error data to test for group differences in task performance over the three runs of the task (Table 1). Significant main effects of diagnosis, task run and error type were observed (F=9.24, 9.12 & 46.60 respectively p<0.05). Healthy controls made fewer errors (28.57%) than individuals with Generalized Anxiety Disorder (36.59%). Moreover, participants made significantly fewer mistakes in run 2 (32.78%) and 3 (30.88%) relative to run 1 [36.24%, t= 2.26 & 3.04 respectively, p<.027], though performance between runs 2 and 3 did not significantly differ [t=1.55, p=.124]. Participants also made more commission (21.74%) than omission errors (11.56%). Notably, a significant diagnosis-by-task run interaction was observed [F=6.37, p=.002]. In run 1, error rates did not differ between healthy controls (34.93%) and individuals with Generalized Anxiety Disorder [37.15%; t(76)=.84, p=.40]. However, by runs 2 and 3, error rates were significantly lower in the healthy controls (27.40% & 23.38%, respectively) relative to the Generalized Anxiety Disorder group [36.53% & 36.1%; t(76)=2.85 & 3.59, p<.006].
To test the validity of the learning model, we examined whether it predicted behavior (as a function of EV). In line with the model, there was a significant relationship between predicted and observed behavior [average correlation: r= .386, one-sample t-test (null: r=0), t=15.79, p<.001]. Following this, we conducted a 2(diagnosis: Generalized Anxiety Disorder, healthy controls) by 3(run: run 1, run 2, run 3) repeated-measures ANOVA on the relationship between EV and task performance data. A significant main effect of diagnosis was observed [F=6.11, p=.016] where a stronger relationship between EV and behavior was observed in healthy individuals relative to participants with Generalized Anxiety Disorder. A trend level diagnosis-by-run interaction was observed [F=2.63, p=.075], where healthy controls and participants with Generalized Anxiety Disorder did not significantly differ with respect to the correlation between EV and behavior in run 1 [t=1.04, p=.30], but did differ in runs 2 [t=2.26, p=.03] and 3 [t=2.68, p<.01].
fMRI Results
The current study sought to examine whether individuals with Generalized Anxiety Disorder would show abnormal neural recruitment during decision-making, and in particular whether they would show disrupted EV representation in ventromedial prefrontal cortex, ventral striatum and anterior insula cortex during choice and disrupted PE representation in ventromedial prefrontal cortex and ventral striatum during feedback. This was tested in the cue-phase using a 2(diagnosis: Generalized Anxiety Disorder, healthy controls) by 2(choice: approach, refuse) ANOVA conducted on the BOLD data modulated by EV and in the feedback-phase using a 2(diagnosis: Generalized Anxiety Disorder, healthy controls) by 2(feedback type: reward, punishment) conducted on the BOLD data modulated by PE.
Choice-Phase Data Modulated by Expectancies of Reinforcement
This contrast revealed no regions showing either a main effect of diagnosis or diagnosis-by-choice interaction that survived our criterion for statistical significance. However, it should be noted that in a follow-up analysis at a more lenient threshold (p=.02), a significant main effect of diagnosis within ventromedial prefrontal cortex was observed, as were significant diagnosis-by-choice interactions within ventromedial prefrontal cortex, ventral striatum and caudate (see Supplemental Results 2.0). Weaker representation of EV was seen in all three regions in individuals with Generalized Anxiety Disorder.
Regions showing a main effect of choice are detailed in the Supplemental Results 3.1.
Feedback Data Modulated by PE
Main Effect of Diagnosis
Regions showing a significant main effect of diagnosis included ventromedial prefrontal cortex, dorsal anterior cingulate cortex/dorsomedial prefrontal cortex, bilateral anterior insula cortex, posterior cingulate cortex and ventral striatum (Table 2; Figure 2). In all these regions, healthy participants showed greater modulation of activation by PE than individuals with Generalized Anxiety Disorder.
Table 2.
Brain Regions Demonstrating Differential BOLD Response Modulated by Prediction Error in 32 Healthy Comparison Individuals and 46 Individuals with Generalized Anxiety Disorder.
| Coordinates of Peak Activationb | ||||||||
|---|---|---|---|---|---|---|---|---|
| Region a | Left/Right | BA | x | y | z | F | p | Voxels |
| Diagnosis-by-Feedback Type Interaction | ||||||||
| lentiform nucleus | Right | 22.5 | 19.5 | 2.5 | 12.69 | .0006 | 37 | |
| lentiform nucleus | Left | −25.5 | −13.5 | 2.5 | 10.38 | .0019 | 26 | |
| Main Effect of Diagnosis | ||||||||
| ventromedial prefrontal cortex | Left | 10 | 7.5 | 49.5 | 2.5 | 14.43 | .0003 | 26 |
| dorsal anterior cingulate cortex/dorsomedial prefrontal cortex | Right | 6 | 31.5 | −10.5 | 56.5 | 26.42 | <.0001 | 461 |
| anterior insula cortex | Right | 13 | 55.5 | 7.5 | −0.5 | 20.26 | <.0001 | 82 |
| anterior insula cortex | Left | 13 | −46.5 | 4.5 | 2.5 | 19.01 | <.0001 | 45 |
| ventral striatum | Right | 7.5 | 19.5 | −0.5 | 12.42 | .0007 | 28 | |
| precentral gyrus | Left | 6 | −52.5 | −4.5 | 41.5 | 19.73 | <.0001 | 64 |
| precentral gyrus | Left | 6 | −16.5 | −19.5 | 62.5 | 16.12 | .0001 | 60 |
| precentral gyrus/inferior frontal gyrus | Right | 6 | 46.5 | 1.5 | 32.5 | 14.42 | .0003 | 39 |
| postcentral gyrus | Right | 6 | 25.5 | −31.5 | 65.5 | 13.31 | .0005 | 19 |
| posterior cingulate cortex | Left | 30 | −4.5 | −55.5 | 8.5 | 22.86 | <.0001 | 567 |
| fusiform gyrus | Left | 19 | −34.5 | −79.5 | −12.5 | 20.44 | <.0001 | 88 |
| fusiform gyrus | Right | 18 | 19.5 | −85.5 | −12.5 | 15.23 | .0002 | 39 |
| inferior temporal cortex | Right | 37 | 55.5 | −58.5 | −6.5 | 16.25 | .0001 | 55 |
| precuneus | Right | 19 | 28.5 | −70.5 | 32.5 | 15.67 | .0002 | 27 |
| middle occipital gyrus | Right | 18 | 28.5 | −82.5 | 8.5 | 14.01 | .0004 | 37 |
| cerebellum | Left | 18 | −10.5 | −91.5 | −18.5 | 16.90 | <.0001 | 66 |
| culmen | Left | 37 | −43.5 | −43.5 | −21.5 | 14.12 | .0003 | 65 |
According to the Talairach Daemon Atlas (http://www.nitrc.org/projects/tal-daemon/).
Based on the Tournoux & Talairach standard brain template, BA= Brodmann’s Area
Figure 2.
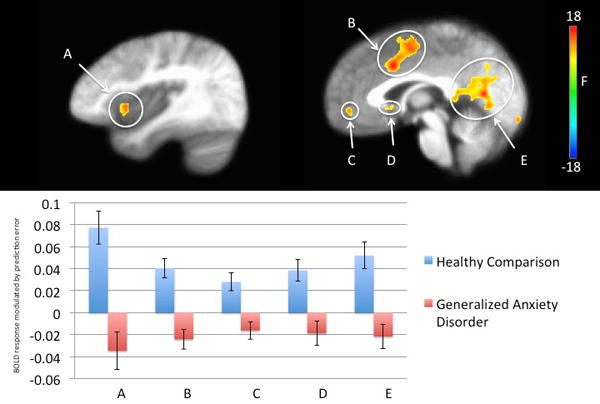
Regions showing a main effect of diagnosis in 32 healthy participants and 46 individuals with Generalized Anxiety Disorder.
Healthy participants showed greater representation of prediction error relative to patients with generalized anxiety disorder.
A. left anterior insula cortex
B. dorsal anterior cingulate cortex
C. ventromedial prefrontal cortex
D. ventral striatum
E. posterior cingulate cortex
Diagnosis-by-Feedback Type Interaction
Regions showing a significant diagnosis-by-feedback type interaction included regions of bilateral lentiform nucleus that extended into putamen and, on the right, caudate (Table 2 & Figure 3). In both regions, subjects with Generalized Anxiety Disorder showed reduced modulation by punishment prediction errors relative to healthy controls (t=3.01 & 4.24; p<0.005 * p<0.001 for left and right lentiform nucleus/putamen respectively). There were no group differences in modulation by reward prediction errors (t=1.87 & 0.99 respectively).
Figure 3.
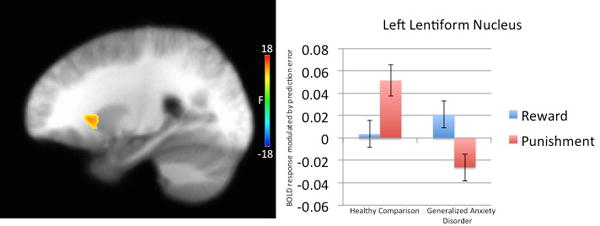
Diagnosis-by-feedback type interaction in left lentiform nucleus/putamen in 32 healthy participants and 46 subjects with Generalized Anxiety Disorder.
Individuals with generalized anxiety disorder, relative to healthy participants, showed a significantly greater difference between activation modulated by PE to rewarding relative to punishing feedback in left lentiform nucleus/putamen.
Regions showing a main effect of feedback type are detailed in the Supplemental Results 3.2.
Relationship Between Modulated Activation and Behavior
Within the sample, significant inverse correlations between modulated response in bilateral anterior insula cortex, dorsal anterior cingulate cortex/dorsomedial prefrontal cortex, ventromedial prefrontal cortex, ventral striatum and posterior cingulate cortex and omission errors were observed [r=−.362 to −.262, p<.021]. Within the subject sample, a similar pattern of results was observed. Significant inverse correlations between right anterior insula cortex and dorsal anterior cingulate cortex/dorsomedial prefrontal cortex [r=−.296 & −.299, p<.046] were observed. Inverse correlations between left anterior insula cortex, ventromedial prefrontal cortex, ventral striatum and posterior cingulate cortex were observed at trend levels [r=−.259 to −.283, p<.10].
Relationship between dysfunctional PE modulation of BOLD responses and symptom levels in individuals with Generalized Anxiety Disorder
We conducted preliminary correlational analyses focusing on the striatal regions showing group-by-feedback interactions for the PE modulated BOLD response data. Extent of atypical response, relative to healthy controls, was not related to Generalized Anxiety Disorder symptoms on the Penn State Worry Questionnaire or the Generalized Anxiety Disorder-7 scale. However, an association between atypical response to punishing feedback in left lentiform nucleus/putamen and Global Assessment of Functioning scores was observed [r= −.32, p=.03].
Discussion
The goal of the current study was to determine the extent to which individuals with GAD show dysfunction in the neural systems mediating specific computational components of decision-making. There were three main findings. First, individuals with Generalized Anxiety Disorder showed impaired reinforcement-based decision-making. Second, during feedback, they showed reduced correlation between PE and BOLD responses within ventromedial prefrontal cortex, ventral striatum, dorsal anterior cingulate cortex/dorsomedial prefrontal cortex, bilateral anterior insula cortex and posterior cingulate cortex relative to healthy controls. Third, individuals with Generalized Anxiety Disorder showed reduced correlations between punishment PEs and BOLD responses within bilateral lentiform nucleus/putamen relative to healthy controls.
Consistent with previous work (2, 5), individuals with Generalized Anxiety Disorder showed impairment in reinforcement-based learning as indexed by significantly increased errors relative to healthy controls during a passive avoidance task. The groups did not differ in performance in run 1. However, while healthy controls showed significant improvement in behavioral performance over time, individuals with Generalized Anxiety Disorder did not. These results are in line with our earlier work involving a more simplistic, behavioral version of the passive avoidance task where individuals with Generalized Anxiety Disorder similarly showed impaired performance and reduced learning as a function of time relative to the healthy comparison individuals (6).
Also consistent with predictions, individuals with Generalized Anxiety Disorder showed reduced PE representation in ventromedial prefrontal cortex and ventral striatum, as well as other regions implicated in the representation of reinforcement value (i.e., dorsal anterior cingulate cortex/dorsomedial frontal cortex, anterior insula cortex, posterior cingulate cortex; 11) relative to healthy controls. In most regions, individuals with Generalized Anxiety Disorder showed reduced modulation of BOLD responses by PEs to both rewards and punishments relative to controls. However, within lentiform nucleus/putamen patients with Generalized Anxiety Disorder showed reduced neural representation of PE during punishing, but not rewarding, feedback. Notably, previous work has reported that individuals with anxiety show both a reduced increase in activity within caudate/putamen both in anticipation of, and when receiving, reward (15) and less putamen activity relative to controls during when responding to risky relative to safe choices (16). However, previous work has not used a computational modeling approach to the decision-making deficits in Generalized Anxiety Disorder. As such, these data extend previous reports of reduced striatal activity in adolescents with the disorder when anticipating and receiving reward (15), and when making risky choices (16). Moreover, the current results are consistent with another model-based fMRI study of induced anticipatory anxiety in healthy participants, which also revealed reduced representation of decision-making computations within ventromedial prefrontal cortex and ventral striatum following anxiety induction (19).
It is probable that the reduced PE representation in regions implicated in reinforcement processing in the individuals with Generalized Anxiety Disorder contributed to their impaired task performance on the current task and in previous work (2, 5). Consistent with this, the performance of the Generalized Anxiety Disorder group on the current task was significantly inversely related to the correlation between PE and activity within right anterior insula cortex and dorsal anterior cingulate cortex/dorsomedial prefrontal cortex during feedback. Moreover, there were trends in this direction for left anterior insula cortex, ventromedial prefrontal cortex, ventral striatum and posterior cingulate cortex. All of these regions are implicated in successful decision-making (12–14).
Notably, the current data did not support our predictions with respect to representation of EV in the choice-phase. However, analysis at a more lenient threshold (p=.02) revealed that individuals with Generalized Anxiety Disorder showed a reduced correlation between EV and activity within ventromedial frontal cortex during choice. Given these findings, consistent with predictions, we suspect that our failure to support our predictions with respect to EV representation in the choice-phase is due to type II error. However, the possibility that the weak representation of PEs in individuals with Generalized Anxiety Disorder is more prominent in driving the decision-making deficits seen in this population will need to be directly tested.
The extent of reduced punishment PE representation within lentiform nucleus/putamen was related to lower Global Assessment of Functioning scores, but unrelated to more specific indices of Generalized Anxiety Disorder symptomatology. Notably, Global Assessment of Functioning scores specifically and uniquely correlated with degree of impairment on an early simplistic version of the PA task (6). In other words, individuals with Generalized Anxiety Disorder who show greater problems in reinforcement representation, particularly within regions of striatum implicated in the passive avoidance task also show greater impairment in social, occupational and psychological functioning.
A caveat should be considered: the current results might represent a general failure in attention/motivation in Generalized Anxiety Disorder rather than a specific impairment in PE representation. However, this appears unlikely for two reasons: (i) Individuals with the disorder do not show generally reduced attention/motivation. On a variety of tasks individuals with Generalized Anxiety Disorder show no impairment in reaction times or error rates relative to controls (28–30); (ii) The reduced PE representation seen here is not coupled with generally reduced responses in the subjects with Generalized Anxiety Disorder. Indeed, individuals with Generalized Anxiety Disorder showed atypically large un-modulated BOLD responses (see Supplementary Material). These data are incompatible with a general attention/motivation dysfunction account and strengthen the idea of a relatively specific neuro-computational impairment in PE representation in Generalized Anxiety Disorder.
In summary, the current study is the first to identify computational impairments in PE signaling during decision-making in Generalized Anxiety Disorder. The results suggest that the decision-making deficits observed in patients with GAD are the result of failure to appropriately represent PE. These findings have two broad implications: First, boosting the representation of PE during feedback in individuals with Generalized Anxiety Disorder might be a useful intervention goal. Second, investigating the computational processes underlying decision-making deficits in other anxiety disorders may refine diagnostic categories and differential diagnosis.
Supplementary Material
Figure 1. The Passive Avoidance Task.
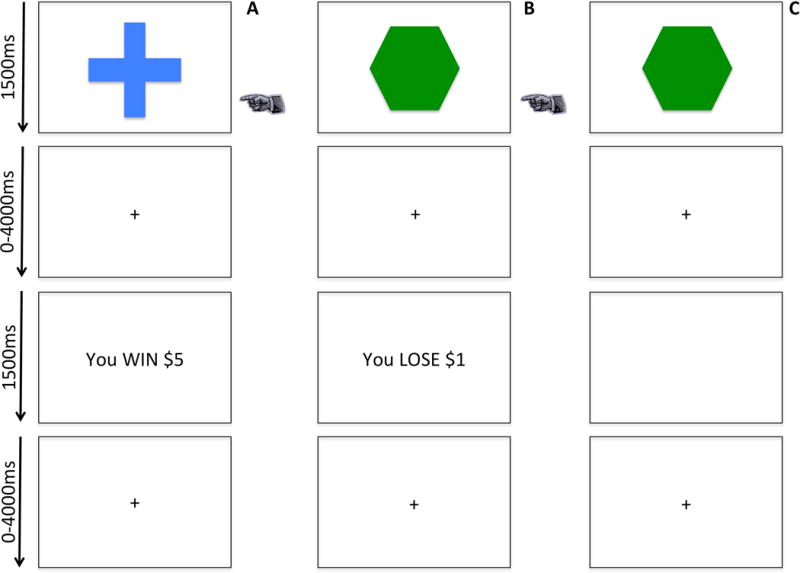
Participants chose to either respond or not to respond to four objects, presented one at a time. Reinforcement was probabilistic, such that over the course of the task, the selection of two objects would result in profit, and selection of the other two objects would result in loss.
Column A: a participant chooses to respond and receives rewarding feedback.
Column B: a participant chooses to respond and receives punishing feedback.
Column C: a participant refuses to respond and receives no feedback.
 = participant chooses object
= participant chooses object
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health [1-ZIA-MH002860, Dr. Blair principle investigator; 1-ZIA-MH002928, Dr. Averbeck principle investigator; 1-ZIA-MH002798, Dr. Grillon principle investigator]. This study was conducted under protocol number 03-M-0185, with ClinicalTrials.gov Identifier NCT00062517.
Footnotes
All authors report no financial disclosures or conflicts of interest.
References
- 1.APA: Diagnostic and Statistical Manual of Mental Disorders. 5th. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 2.Raghunathan R, Pham MT. All negative moods are not equal: Motivational influences of anxiety and sadness on decision making. Organizational Behavior and Human Decision Processes. 1999;79:56–77. doi: 10.1006/obhd.1999.2838. [DOI] [PubMed] [Google Scholar]
- 3.Maner JK, Richey JA, Cromer K, Mallott M, Lejuez CW, Joiner TE, Schmidt NB. Dispositional anxiety and risk-avoidant decision making. Personality and Individual Differences. 2007;42:665–675. [Google Scholar]
- 4.Luhman CC, Ishida K, Hajcak G. Intolerance of uncertainty and decisions about delayed, probabilistic rewards. Behavior Therapy. 2011;42:378–386. doi: 10.1016/j.beth.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 5.DeVido J, Jones M, Geraci M, Hollon N, Blair RJ, Pine DS, Blair K. Stimulus-reinforcement-based decision making and anxiety: impairment in generalized anxiety disorder (GAD) but not in generalized social phobia (GSP) Psychol Med. 2009;39:1153–1161. doi: 10.1017/S003329170800487X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teng C, Otero M, Geraci M, Blair RJ, Pine DS, Grillon C, Blair KS. Abnormal decision-making in generalized anxiety disorder: Aversison of risk or stimulus-reinforcement impairment? Psychiatry Research. doi: 10.1016/j.psychres.2015.12.031. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blair KS, Otero M, Teng CY, Geraci M, Blair RJ, Grillon C, Pine DS. Reduced optimism and a heightened neural response to everyday worries is specific to Generalized Anxiety Disorder, and not seen in Social Anxiety. American Journal of Psychiatry. doi: 10.1017/S0033291717000265. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexander WH, Brown JW. Medial prefrontal cortex as an action-outcome predictor. Nat Neurosci. 2011;14:1338–1344. doi: 10.1038/nn.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rescorla, Wagner: A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. Classical Conditioning Appleton, Century-Crofts. 1972:64–99. [Google Scholar]
- 10.O’Doherty JP. Contributions of the ventromedial prefrontal cortex to goal-directed action selection. Ann N Y Acad Sci. 2011;1239:118–129. doi: 10.1111/j.1749-6632.2011.06290.x. [DOI] [PubMed] [Google Scholar]
- 11.Clithero JA, Rangel A. Informatic parcellation of the network involved in the computation of subjective value. Soc Cogn Affect Neurosci. 2014;9:1289–1302. doi: 10.1093/scan/nst106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuhnen CM, Knutson B. The neural basis of financial risk taking. Neuron. 2005;47:763–770. doi: 10.1016/j.neuron.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Liu X, Powell DK, Wang H, Gold BT, Corbly CR, Joseph JE. Functional dissociation in frontal and striatal areas for processing of positive and negative reward information. J Neurosci. 2007;27:4587–4597. doi: 10.1523/JNEUROSCI.5227-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blair RJ, Cipolotti L. Impaired social response reversal. A case of ‘acquired sociopathy’. Brain. 2000;123(Pt 6):1122–1141. doi: 10.1093/brain/123.6.1122. [DOI] [PubMed] [Google Scholar]
- 15.Benson BE, Guyer AE, Nelson EE, Pine DS, Ernst M. Role of contingency in striatal response to incentive in adolescents with anxiety. Cogn Affect Behav Neurosci. 2015;15:155–168. doi: 10.3758/s13415-014-0307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galvan A, Peris TS. Neural correlates of risky decision making in anxious youth and healthy controls. Depress Anxiety. 2014;31:591–598. doi: 10.1002/da.22276. [DOI] [PubMed] [Google Scholar]
- 17.Yassa MA, Hazlett RL, Stark CE, Hoehn-Saric R. Functional MRI of the amygdala and bed nucleus of the stria terminalis during conditions of uncertainty in generalized anxiety disorder. J Psychiatr Res. 2012;46:1045–1052. doi: 10.1016/j.jpsychires.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guyer AE, Choate VR, Detloff A, Benson B, Nelson EE, Perez-Edgar K, Fox NA, Pine DS, Ernst M. Striatal functional alteration during incentive anticipation in pediatric anxiety disorders. Am J Psychiatry. 2012;169:205–212. doi: 10.1176/appi.ajp.2011.11010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engelmann JB, Meyer F, Fehr E, Ruff CC. Anticipatory anxiety disrupts neural valuation during risky choice. J Neurosci. 2015;35:3085–3099. doi: 10.1523/JNEUROSCI.2880-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Doherty J. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr Opin Neurobiol. 2004;14:769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 21.First MB, Spitzer RL, Gibbon M, Janet BW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. New York: Biometrics Research; New York: State Psychiatric Institute; 2002. [Google Scholar]
- 22.Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 23.Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry Questionnaire. Behav Res Ther. 1990;28:487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- 24.Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 25.White SF, Pope K, Sinclair S, Fowler KA, Brislin SJ, Williams WC, Pine DS, Blair RJ. Disrupted expected value and prediction error signaling in youths with disruptive behavior disorders during a passive avoidance task. Am J Psychiatry. 2013;170:315–323. doi: 10.1176/appi.ajp.2012.12060840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 27.Talairach, Tournoux: Co-planar stereotaxic atlas of the human brain. Stuttgart: Thieme; 1988. [Google Scholar]
- 28.Blair KS, Shaywitz J, Smith BW, Rhodes R, Geraci M, Jones M, McCaffrey D, Vythilingam M, Finger E, Mondillo K, Jacobs M, Charney DS, Blair RJR, Drevets WC, Pine DS. Response to emotional expressions in generalized social phobia and generalized anxiety disorder: evidence for separate disorders. American Journal of Psychiatry. 2008;165:1193–1202. doi: 10.1176/appi.ajp.2008.07071060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blair KS, Geraci M, Smith BW, Hollon N, Devido J, Otero M, Blair JR, Pine DS. Reduced dorsal anterior cingulate cortical activity during emotional regulation and top-down attentional control in generalized social phobia, generalized anxiety disorder, and comorbid generalized social phobia/generalized anxiety disorder. Biol Psychiatry. 2012;72:476–482. doi: 10.1016/j.biopsych.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lissek S, Kaczkurkin AN, Rabin S, Geraci M, Pine DS, Grillon C. Generalized anxiety disorder is associated with overgeneralization of classically conditioned fear. Biol Psychiatry. 2014;75:909–915. doi: 10.1016/j.biopsych.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


