Abstract
Purpose
We previously reported clinical outcomes and physician-reported toxicity of gemcitabine and hypofractionated stereotactic body radiation therapy (SBRT) in locally advanced pancreatic cancer (LAPC). Here we prospectively investigate the impact of gemcitabine and SBRT on patient-reported quality of life (QoL).
Methods and materials
Forty-nine LAPC patients received 33 Gy SBRT (6.6 Gy daily fractions) upfront or after ≤3 doses of gemcitabine (1000 mg/m2) followed by gemcitabine until progression. European Organization for Research and Treatment of Cancer QoL core cancer (QLQ-C30) and pancreatic cancer-specific (European Organization for Research and Treatment of Cancer QLQ-PAN26) questionnaires were administered to patients pre-SBRT and at 4 to 6 weeks (first follow-up [1FUP]) and 4 months (2FUP) post-SBRT. Changes in QoL scores were deemed clinically relevant if median changes were at least 5 points in magnitude.
Results
Forty-three (88%) patients completed pre-SBRT questionnaires. Of these, 88% and 51% completed questionnaires at 1FUP and 2FUP, respectively. There was no change in global QoL from pre-SBRT to 1FUP (P = .17) or 2FUP (P > .99). Statistical and clinical improvements in pancreatic pain (P = .001) and body image (P = .007) were observed from pre-SBRT to 1FUP. Patients with 1FUP and 2FUP questionnaires reported statistically and clinically improved body image (P = .016) by 4 months. Although pancreatic pain initially demonstrated statistical and clinical improvement (P = .020), scores returned to enrollment levels by 2FUP (P = .486). A statistical and clinical decline in role functioning (P = .002) was observed in patients at 2FUP.
Conclusions
Global QoL scores are not reduced with gemcitabine and SBRT. In this exploratory analysis, patients experience clinically relevant short-term improvements in pancreatic cancer-specific symptoms. Previously demonstrated acceptable clinical outcomes combined with these favorable QoL data indicate that SBRT can be easily integrated with other systemic therapies and may be a potential standard of care option in patients with LAPC.
Introduction
In 2015, approximately 48,960 patients received a new diagnosis of pancreatic cancer in the United States.1 Of these, nearly 40% presented with unresectable, locally advanced pancreatic cancer (LAPC).2 LAPC is defined as a pancreatic tumor with no evidence of distant metastasis and >180° superior mesenteric and/or celiac artery involvement or unreconstructable portal vein or superior mesenteric vein involvement.3 Standard of care for LAPC includes systemic chemotherapy with or without chemoradiation (CRT; gemcitabine- or 5-florouracil-based chemotherapy concurrent with 45–54 Gy radiation therapy in 1.8- to 2.5-Gy fractions)2–10; however, even with CRT, local failure occurs in approximately 25% to 35% of patients2,4 and survival rates remain poor with few surviving beyond 2 years.
Given the poor life expectancy of patients with LAPC, a major factor guiding patient and physician management decisions is treatment-related toxicity and impact on overall quality of life (QoL). Single-fraction stereotactic body radiation therapy (SBRT) for LAPC has demonstrated improved local control (80% to 100%)11–14 compared with historical reports of standard CRT; however, late gastrointestinal toxicity rates up to 47% were observed.14 Our recent multicenter phase 2 clinical trial implemented 5-fraction SBRT (33 Gy total, 6.6 Gy daily fractions) to determine if this fractionated regimen results in similar rates of local control with reduced late toxicities compared with those previously reported for single-fraction therapy. Median overall survival (OS) was 13.9 months with 78% freedom from local progression at 1 year and acceptable rates of acute and late grade ≥2 gastritis, fistula, enteritis, or ulcer toxicities of 2% and 11%, respectively.15 Although these results are promising, patient-reported measures are crucial to fully evaluate the appropriateness of SBRT, given tendencies of physician assessments to inadequately reflect treatment-related morbidity.16
Several validated metrics to assess patient-reported outcomes have emerged. Based on literature review using PubMed, the most widely accepted cancer-specific QoL questionnaire is the European Organization for Research and Treatment in Cancer QoL core cancer questionnaire (EORTC QLQ-C30).17,18 This questionnaire can be paired with an additional pancreatic cancer-specific module (EORTC QLQ-PAN26).19 These metrics have been previously used to investigate the impact of various CRT regimens for borderline resectable and LAPC patients,20–23 including 1 small study with 10 patients treated with gemcitabine and SBRT,24 and thus were selected to evaluate QoL of patients in our study.
Herein, we report the QoL outcomes of patients treated with gemcitabine and fractionated SBRT in our phase 2 trial to evaluate the patient experience of this regimen.
Methods and materials
Study participants and treatment plan
The details of the treatment regimen and patient population are reported elsewhere.15 In brief, 49 patients with histologically confirmed LAPC were treated at 3 academic institutions in the United States. Linear accelerator (Linac)-based SBRT was administered in 6.6 Gy daily fractions, 33 Gy total, upfront or after ≤3 weekly doses of gemcitabine (1000 mg/m2) within 6 weeks before SBRT followed by gemcitabine within 4 weeks of SBRT completion (median = 2.3 weeks) until progression. Institutional review boards of all 3 participating institutions approved the study protocol and patients were required to provide written informed consent before enrollment.
Quality of life assessment
QoL was measured at 3 time points for patients who were on trial without evidence of disease progression: at baseline (defined as after enrollment but before initiating SBRT, a window of 6 weeks), at first follow-up (1FUP) 4 to 6 weeks (median, 5.7 weeks; range, 3.9–7.7 weeks) following SBRT, and at second follow-up (2FUP) 4 months (median, 4 months; range, 2.9–5.5 months) following SBRT. Patients who developed disease progression or who received a second-line agent other than gemcitabine were removed from the study and did not complete subsequent QoL questionnaires.
Both the EORTC QLQ-C30, version 3.0,17,18 and QLQ-PAN2619 questionnaires were used to assess patient-reported QoL. The QLQ-C30 is a 30-question validated cancer-specific instrument that measures a global health score, our primary outcome, as well as 5 functions (physical, role, cognitive, emotional, and social) and 9 symptoms (fatigue, pain, nausea and vomiting, dyspnea, loss of appetite, insomnia, constipation, diarrhea, and financial difficulties).17 The QLQ-PAN26 is a 26-question QoL instrument for pancreatic cancer patients used to quantify pain, dietary changes, jaundice, altered bowel habit, emotional problems related to pancreatic cancer, and other symptoms including cachexia, indigestion, flatulence, dry mouth, and taste changes.19 For both the EORTC QLQ-C30 and QLQ-PAN26 questionnaires, each category is assessed by 2 to 5 questions, and responses are scored on a 4-point Likert scale. Scores are then rescaled to range from 0 to 100, with higher scores representing improved QoL on global functional categories and reduced QoL (or higher burden) on symptom categories and the QLQ-PAN26.25 The minimally clinically important difference is 5 to 10 points for the QLQ-C30 and QLQ-PAN26.26
Statistical analysis
The demographic and clinical characteristics are summarized using medians, interquartile ranges, counts, and proportions. Comparisons between those with and without 2FUP QoL scores were made using Fisher exact tests, Wilcoxon rank-sum tests, or log-rank tests for count, continuous, and survival outcomes, respectively. Because of the skewness in the QoL scores, comparisons between baseline and follow-up QoL scores were made using the Wilcoxon signed-rank test. A P value < .05 was considered statistically significant for the global health score as well as the subscales. Because of the small sample size and exploratory nature of the study, no corrections were made for multiple comparisons. With 21 subscales (14 QLQ-C30 and 7 QLQ-PAN26), we would expect just over 1 significant result to occur by chance alone at each time point at the 0.05 level.
Results
Quality of life at baseline and 1FUP
From 2010 to 2012, 49 patients with histologically confirmed LAPC were enrolled onto the clinical trial (3 at Memorial Sloan Kettering Cancer Center, 14 at Stanford Medical Center, and 32 at Johns Hopkins Hospital). Thirty-eight (78%) patients completed baseline and 1FUP questionnaires; 5 (10%) participants were missing baseline QoL, 5 (10%) participants were missing 2FUP QoL, and 1 (2%) participant was missing both baseline and 1FUP QoL. The demographic and clinical characteristics were similar for those with both QoL measurements as opposed to those missing QoL at 1 or more time points (Table 1, Table e1 available as supplementary material online only at www.practicalradonc.org). Most patients received induction gemcitabine (overall, N = 44, 90%; with paired baseline and 4- to 6-week QoL data, N = 33, 87%).
Table 1.
Baseline demographic and clinical characteristics of all patients and then comparing those with and without paired baseline and 1FUP or baseline and 2FUP QoL questionnaire data
| Characteristic | Overall (N = 49) |
Patients without baseline or 1FUP QoL data (N = 11) |
Patients with baseline and 1FUP QoL data (N = 38) |
P value | Patients without baseline or 2FUP QoL data (N = 27) |
Patients with baseline and 2FUP QoL data (N = 22) |
P value |
|---|---|---|---|---|---|---|---|
| Age ≥65 years | 35 (71%) | 10 (91%) | 25 (66%) | .14 | 22 (81%) | 13 (59%) | .12 |
| Female | 18 (37%) | 2 (18%) | 16 (42%) | .18 | 5 (19%) | 13 (59%) | .007 |
| ECOG performance status ≥ 1 | 28 (57%) | 9 (82%) | 19 (50%) | .087 | 21 (78%) | 7 (32%) | .002 |
| Tumor location: head | 41 (84%) | 8 (73%) | 33 (87%) | .36 | 22 (81%) | 19 (86%) | .72 |
| Baseline CA 19-9 ≥90 U/mL | 27 (60%) | 6 (60%) | 21 (60%) | >.99 | 16 (67%) | 11 (52%) | .37 |
| Pre-SBRT gemcitabine | 44 (90%) | 11 (100%) | 33 (87%) | .57 | 25 (93%) | 19 (86%) | .65 |
| Clinic | .44 | .71 | |||||
| Johns Hopkins | 32 (65%) | 9 (82%) | 23 (61%) | 16 (59%) | 16 (73%) | ||
| Stanford | 14 (29%) | 2 (18%) | 12 (32%) | 9 (33%) | 5 (23%) | ||
| Memorial Sloan Kettering | 3 (6%) | 0 (0%) | 3 (8%) | 2 (7%) | 1 (5%) |
CA 19–9, carbohydrate antigen 19–9; ECOG, Eastern Cooperative Oncology Group; 1FUP, first follow-up; 2FUP, second follow-up; QoL, quality of life; SBRT, stereotactic body radiation therapy.
Of the 38 individuals with paired baseline and 1FUP QoL data, 11 (29%) completed baseline questionnaires before gemcitabine therapy (only 6 of these 11 patients proceeded to receive induction gemcitabine), whereas the remaining 27 (71%) patients completed baseline questionnaires following at least 1 dose of induction gemcitabine. Of note, the distribution of role functioning scores and altered bowl habits symptoms scores were significantly higher (P = .035 and P = .046, respectively) for those who had received gemcitabine before baseline QOL assessment compared to those who did not (Table e2).
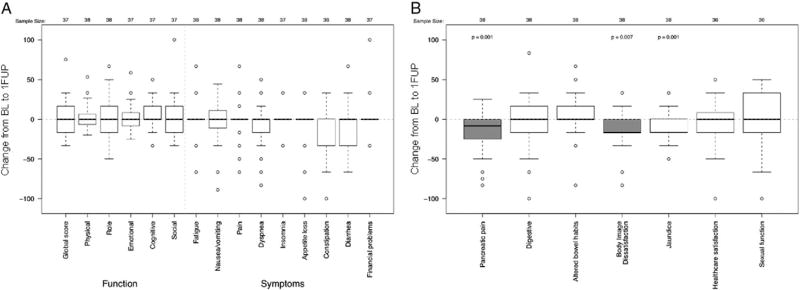
Overall, the median baseline global QLQ-C30 score for patients with paired baseline and 1FUP data was 67 (interquartile range, 50 to 84) (Table e1). The QLQ-C30 global, functional, and symptom scores did not change significantly from baseline to 1FUP after SBRT (Fig 1A). In contrast, differences were observed for components of the QLQ-PAN26 questionnaire at 1FUP (Fig 1B). Patients experienced a statistically and clinically significant decrease in pancreatic pain (median change [mΔ], −8; range, −83 to 25; P = .001) and body image dissatisfaction scores (mΔ, −17; range, 83 to 33; P = .007) following SBRT (Fig 1B). At 1FUP, there was a statistically significant decrease in jaundice scores; however, this change lacked clinical significance (mΔ, 0; range, −50 to 33; P = .001) at 1FUP. The remaining symptoms assessed by the QLQ-PAN26 questionnaire had not changed significantly at 1FUP.
Figure 1.
Changes in European Organization for Research and Treatment of Cancer QoL core cancer QLQ-C30 (A) and QLQ-PAN26 (B) quality of life scores between baseline (BL) and 1FUP for individuals completing baseline and 1FUP evaluations (N = 38). Shaded boxes highlight changes that were considered clinically significant. 1FUP, first follow-up.
QoL at baseline, 1FUP, and 2FUP in patients with longer follow-up
Of the 43 patients with pre-SBRT questionnaires, 22 (51%) completed questionnaires at 2FUP, 2 of whom were missing the 1FUP (4–6 weeks) QoL questionnaires. Patients with paired baseline and 2FUP QoL data available were more likely to be female (59% vs 19%, P = .007) and have a baseline Eastern Cooperative Oncology Group (ECOG) performance status of ≥1 (32% vs 78%, P = .002) compared with those who did not have both measurements (Table 1). Other baseline clinical characteristics were similar between these 2 groups. Individuals with paired baseline and 2FUP data had significantly more satisfaction with health care at baseline compared with those without 2FUP data (median, 83 vs 100, P = .040). No other significant differences were observed between these groups in baseline QoL values (Table 2). However, the median OS of the patients without paired baseline and 2FUP QoL data was 10.2 months (95% confidence interval, 6.2–16.7 months) compared with 18.8 months (95% confidence interval, 13.8–∞ months) for patients evaluated at both time points. Given these differences, we presented the changes in QoL from baseline to 1FUP after SBRT along with the changes from baseline to 2FUP after SBRT for the subset of patients with paired baseline and 2FUP questionnaire data (N = 20) to allow for more accurate comparisons.
Table 2.
Baseline QLQ-C30 and QLQ-PAN26 QoL scores for individuals with and without paired baseline and 2FUP QoL questionnaire data
| Patients without paired baseline and 2FUP QoL data |
Patients with paired baseline and 2FUP QoL data |
P value |
|||
|---|---|---|---|---|---|
|
|
|
||||
| N | Median (25th–75th quartiles) | N | Median (25th–75th quartiles) | ||
| QLQ-C30 | |||||
| Global QoL functioning scales | 21 | 67 (50–84) | 22 | 67 (50–84) | .98 |
| Physical functioning | 21 | 87 (80–100) | 22 | 87 (80–94) | .92 |
| Role functioning | 21 | 67 (66–84) | 22 | 75 (37–100) | .68 |
| Emotional functioning | 21 | 83 (66–100) | 22 | 79 (66–92) | .62 |
| Cognitive functioning | 21 | 83 (83–100) | 22 | 83 (66–100) | .27 |
| Social functioning | 21 | 83 (66–84) | 22 | 67 (50–84) | .16 |
| Symptom scales | |||||
| Fatigue | 21 | 33 (33–56) | 22 | 33 (22–45) | .68 |
| Nausea/vomiting | 21 | 0 (0–17) | 22 | 0 (0–17) | .37 |
| Pain | 21 | 17 (16–34) | 22 | 17 (16–34) | .56 |
| Dyspnea | 20 | 0 (0–0) | 22 | 0 (0–0) | .90 |
| Insomnia | 21 | 33 (33–34) | 22 | 33 (0–34) | .53 |
| Appetite loss | 20 | 33 (33–67) | 22 | 33 (0–67) | .91 |
| Constipation | 21 | 33 (0–34) | 22 | 33 (0–34) | .19 |
| Diarrhea | 21 | 0 (0–0) | 22 | 0 (0–25) | .81 |
| Financial problems | 21 | 0 (0–34) | 22 | 33 (0–34) | .11 |
| QLQ-PAN26 | |||||
| Pancreatic pain | 21 | 25 (16–50) | 22 | 21 (8–34) | .33 |
| Digestive | 21 | 33 (16–50) | 22 | 33 (16–46) | .97 |
| Altered bowel habits | 21 | 17 (16–34) | 22 | 17 (0–17) | .12 |
| Body image | 21 | 100 (83–100) | 22 | 83 (66–100) | .09 |
| Jaundice | 21 | 17 (0–34) | 22 | 8 (0–17) | .31 |
| Satisfaction with health care | 20 | 100 (79–100) | 22 | 83 (66–100) | .04 |
2FUP, second follow-up; QLQ-C30, European Organization for Research and Treatment of Cancer QoL core cancer; QLQ-PAN26, European Organization for Research and Treatment of Cancer QLQ-PAN26; QoL, quality of life.
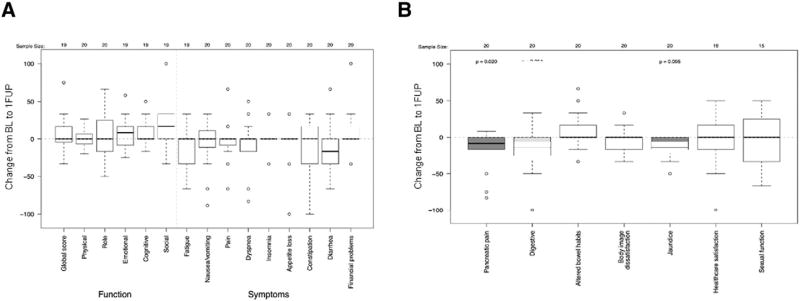
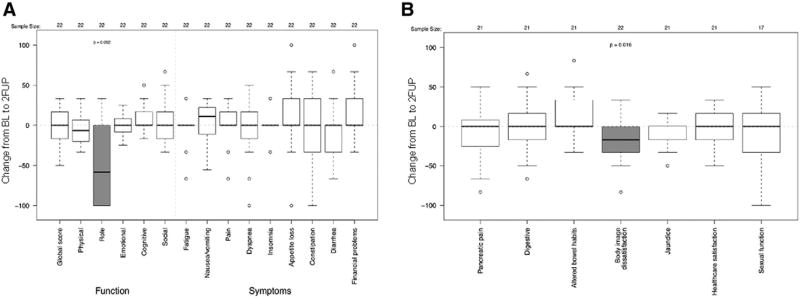
In general, the change in QLQ-C30 (Fig 2A) and QLQ-PAN26 (Fig 2B) scores from baseline to 1FUP for patients with paired baseline and 2FUP data were similar to those who only had paired baseline and 1FUP data. Patients with QoL data from all 3 time points had no significant change in body image dissatisfaction from baseline to 1FUP in this subset population (mΔ, 0; range, −33 to 33; P = .58) (Fig 2B). Yet, this subset did eventually report statistically and clinically significant decreasing body image dissatisfaction scores from baseline to 2FUP (mΔ, −17; range −83 to 33; P = .016) (Fig 3B), perhaps indicative of a delayed reaction in the subset of patients with 2FUP data given their improved baseline performance status. Role functioning measured on the QLQ-C30, which was not significantly reduced at 1FUP (Fig 2A), showed a statistically and clinically significant impairment at 2FUP (mΔ, −58; range, −100 to 33; P = .002) (Fig 3A). As with the entire cohort, pancreatic pain scores statistically and clinically significantly decreased from baseline to 1FUP (mΔ, −8; range, −83 to 25; P = .020) (Fig 2B), but returned to baseline levels at the 2FUP (mΔ, 0; range, −83 to 50; P = .49) (Fig 3B). There were insufficient events in patients with baseline and follow-up QoL questionnaires to assess the association between return of pain or functional decline with local disease. There were no differences in the median changes from baseline to 1FUP and 2FUP global QoL, pain, or pancreatic pain scores between the 10 patients returning baseline and follow-up QoL data with distant progression compared with those without distant progression.
Figure 2.
Changes in European Organization for Research and Treatment of Cancer QoL core cancer QLQ-C30 (A) and QLQ-PAN26 (B) QoL scores between baseline (BL) and 1FUP for individuals completing baseline, 1FUP, and 2FUP QoL evaluations (N = 22). Shaded boxes highlight changes that were considered clinically significant. 1FUP, first follow-up; 2FUP, second follow-up; QoL, quality of life.
Figure 3.
Changes in European Organization for Research and Treatment of Cancer QoL core cancer QLQ-C30 (A) and QLQ-PAN26 (B) QoL scores between baseline and 2FUP for individuals completing baseline and 2FUP QoL evaluations (N = 22). Shaded boxes highlight changes that were considered clinically significant. 1FUP, first follow-up; 2FUP, second follow-up; QoL, quality of life.
The distribution of QLQ-PAN26 jaundice scores also demonstrated a statistically significant decrease from baseline to 1FUP; however, this lacked clinical significance (mΔ, 0; range, −50 to 33; P = .005) (Fig 3B). Scores remained lower, although only at borderline statistical significance at 2FUP (mΔ, 0; range, −50 to 17; P = .051) (Fig 2B).
Discussion
Currently, there is no clearly defined management strategy for patients with LAPC. Patients with LAPC have historically received either chemotherapy alone or a 6-week regimen of standard CRT in sequence with chemotherapy.4 When CRT is pursued, it can be difficult for patients to receive concurrent full-dose chemotherapy and the total treatment time is extended. In addition to the long course, CRT can sometimes make it difficult for patients to receive subsequent chemotherapy and can further delay surgical evaluation. As an alternate radiation therapy approach, SBRT (33 Gy in 6.6-Gy daily fractions) allows for swift resumption of full-dose chemotherapy up to 1 week after treatment. The published clinical data from our fractionated (33 Gy, 6.6-Gy daily fractions) SBRT trial reported acceptable rates of acute and late grade ≥2 significant gastrointestinal toxicity of 2% and 11%, respectively, with 78% freedom from local progression at 1 year and median OS of 13.9 months.15 Here, we demonstrate that global QoL is not negatively impacted by SBRT. Clinically significant improvements in pancreatic pain at 1FUP, 4 to 6 weeks after SBRT, and body image at 2FUP and 4 months’ post-SBRT were observed following SBRT; however, patients demonstrated impaired role functioning at 2FUP reflecting difficulties in carrying out daily activities and hobbies. The patient-reported QoL data reported here are consistent with the physician-reported mild toxicity data of this regimen15 and support its use as an effective and well-tolerated treatment option for patients with LAPC. However, the sample size is small and the exploratory nature of the study warrants validation of the results in larger clinical trials.
By evaluating the impact of SBRT on QoL, we extended the work of others using QoL metrics to evaluate therapies for patients with borderline resectable20 and LAPC.,9,21–24, 27 In a previous study of 10 LAPC patients treated with SBRT (25 Gy, 5 fractions) delivered concurrently with full-dose gemcitabine followed by 5 additional cycles of gemcitabine,24 Gurka and colleagues previously reported no detriment in global QoL assessed by the QLQ-C30 from baseline to post-SBRT and 3 cycles of gemcitabine. No statistically significant changes in symptom scores on PAN-26 were reported in this small study. In a second report of 26 LAPC patients treated with SBRT (30 Gy, 3 fractions),27 there were no decreases in general or psychological health on SF-26, an Italian QoL metric. Here, we report the largest study of the impact of SBRT on QoL of LAPC patients.
The ECOG 4201 study9 evaluated the role of 3-dimensional conformal radiation therapy (50.4 Gy in 28 fractions over 5.5 weeks) with concurrent gemcitabine compared with gemcitabine alone. Patients in both arms reported a statistically significant decline in total Functional Assessment of Cancer Therapy-Hepatobiliary (FACT-Hep) QoL scores, hepatobiliary symptoms, and physical and functional well-being at 6 weeks following initiation of therapy; however, there were no statistically significant differences between treatment arms at baseline and beyond 6 weeks. Comparisons between ECOG 4201 and our study are limited because the investigators used the FACT-Hep questionnaire rather than the QLQ-C30 and PAN-26. No data directly comparing the EORTC QLQ-C30 or PAN-26 and FACT-Hep questionnaires exist.
Short and colleagues21 incorporated the supplemental QLQ-PAN26 with the QLQ-C30 in their trial of induction gemcitabine followed by CRT (54 Gy in 1.8-Gy fractions) with concomitant continuous-infusion 5-fluorouracil followed by 3 additional cycles of gemcitabine chemotherapy for LAPC patients. In their study, global QoL did not change from baseline to 4 weeks following CRT. Pain, jaundice, and digestive scores in their LAPC cohort remained significantly improved at 4 weeks following CRT; however, pain and digestive scores were no longer significantly improved at later follow-up. Improvement in jaundice symptoms persisted at the 4-month follow-up, but the impact of biliary stenting as a potential confounder was not assessed. Thus, similar to our study, patients receiving CRT did not experience a decline in global QoL and noted improvements in pain. Patients in our study did not observe any change in digestive scores at 1FUP or 2FUP. Although patients in our study did report statistical improvements in jaundice scores, these were not deemed clinically relevant. Importantly, patients who received standard CRT in the Short et al11 study (5–6 weeks) also experienced significant worsening of other domains including social functioning, appetite, diarrhea, and nausea and vomiting at the end of CRT. We did not include a QoL time point on day 5 of SBRT given unlikely onset of symptoms within this timeframe.
In a recent study by the University of Michigan,20 borderline resectable and LAPC patients were treated with 1 cycle of gemcitabine and oxaliplatin followed by 30 Gy in 15 fractions CRT with concurrent gemcitabine and oxaliplatin. The QLQ-C30 and QLQ-PAN26 questionnaires were administered at baseline and following chemotherapy and CRT at 6 months, limiting the ability to capture acute side effects associated with CRT. Although pancreatic pain scores were improved, similar to our patients receiving SBRT, patients receiving CRT reported a nonsignificant decrease in global QoL and a decrease in physical function (compared with a decrease in role functioning in this study).
Standard CRT, therefore, may be particularly helpful for pancreatic patients with pain; however, these patients experienced negative effects in several other QoL domains reported immediately following CRT. At 1FUP and 2FUP, our results suggest that SBRT may not negatively affect other QoL domains that are impacted by CRT while still clinically improving symptoms of pancreatic pain and body image. Still, further improvements in therapy are needed to increase durability of these improvements beyond the 1FUP or 2FUP time points. This will perhaps be achieved with improved systemic therapy, higher radiation doses, and/or radiosensitizers in the future.
There are several study limitations. First, the strength of our conclusions for the subscale items is limited because of the small sample size, which precludes adjustment for multiple comparisons. With 21 subscales, the Bonferroni correction for all subscales would be 0.002 for each time point and 0.0012 across both time points. With this number of comparisons, we would expect 1 false-positive result at each time point and observed 3 significant results for the overall population at 1FUP and 2 significant results at 1FUP and 2FUP for the subcohort of completers. Therefore, the results are exploratory and should be validated with the inclusion of QoL endpoints in larger cooperative group studies.
Second, the population completing a 2FUP QoL questionnaire was reduced (51%) as a result of death, disease progression, or receipt of second-line chemotherapy other than single-agent gemcitabine. Hence, patients completing 2FUP QoL had a greater median OS and improved performance than those who did not complete 2FUP questionnaires. This necessitated reanalysis of the baseline and 1FUP data restricted to individuals with 1FUP and 2FUP QoL data to accurately trace the pattern over time. In general, the patterns and inference were the same for this subgroup. Nevertheless, it is important to note that the 2FUP patterns cannot be generalized to those patients with a shorter survival or poor performance status. In a subsequent ongoing SBRT study, we now collect QoL data following progression to better understand QoL differences between these patients and those who have not progressed. Future research should also investigate incorporating death or severe disability into these cancer-specific QoL scales, similar to what is done with the modified Rankin score used to assess stroke,28 to avoid difficulties with missing data.
Third, patients receiving a few doses of gemcitabine before completing the baseline QoL questionnaires reported improved role functioning and worse altered bowel habits than those who had not received gemcitabine before baseline assessment indicating that receipt of chemotherapy may have impacted baseline QOL scores.
In conclusion, we demonstrate that LAPC patients prospectively evaluated during treatment with fractionated SBRT appear to experience no impairment in global QoL and have short-term improvements in symptoms commonly bothersome to this population including pancreatic pain and body image. Patients with longer follow-up demonstrate impaired role functioning several months after treatment; however, further research is necessary to elucidate if this observation is due to SBRT or natural history of pancreatic cancer, which may be curtailed in the future with improved therapies. Nevertheless, the overall physician- and patient-reported toxicity profile and short duration of SBRT is encouraging in comparison to prior investigations using CRT. Ideally, phase 3 studies would be able to better establish if SBRT improves outcomes in patients with LAPC.
Acknowledgments
Sources of support: This project was supported by the Claudio X. Gonzalez Family Foundation, Flannery Family Foundation, Alexander Family Foundation, Keeling Family Foundation, DeSanti Family Foundation, Viragh Foundation, and McKnight Family Foundation.
Footnotes
Supplementary material for this article (http://dx.doi.org/10.1016/j.prro.2016.05.005) can be found at www.practicalradonc.org.
Abstract presented at American Society of Clinical Oncology Gastrointestinal Cancers Symposium, San Francisco, California. January 16–18, 2014.
Conflicts of interest: None.
References
- 1.Siegel R, Miller KD, Jemal A, et al. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Hammel P, Huguet P, Van Laethem J-L, et al. Comparison of chemoradiotherapy (CRT) and chemotherapy (CT) in patients with a locally advanced pancreatic cancer (LAPC) controlled after 4 months of gemcitabine with or without erlotinib: Final results of the international phase III LAP 07 study. ASCO Annu Meeting Proc. 2013;31(18 Suppl) [Google Scholar]
- 3.National Comprehensive Cancer Network. Pancreatic Adenocarcinoma (version 2.2015) [Accessed March 20, 2016]; http://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf.
- 4.Moertel C, Reitemeier R, Childs D, et al. Combined 5-fluorouracil and supervoltage radiation therapy of locally unresectable gastrointestinal cancer. Lancet. 1969;2:865–869. doi: 10.1016/s0140-6736(69)92326-5. [DOI] [PubMed] [Google Scholar]
- 5.Herman JM, Wild AT, Wang H, et al. Randomized phase III multi-institutional study of TNFerade biologic with fluorouracil and radiotherapy for locally advanced pancreatic cancer: Final results. J Clin Oncol. 2013;31:886–894. doi: 10.1200/JCO.2012.44.7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ben-Josef E, Schipper M, Francis IR, et al. A phase I/II trial of intensity modulated radiation (IMRT) dose escalation with concurrent fixed-dose rate gemcitabine (FDR-G) in patients with unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2012;84:1166–1171. doi: 10.1016/j.ijrobp.2012.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Treatment of locally unresectable carcinoma of the pancreas: Comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. Gastrointestinal Tumor Study Group. J Natl Cancer Inst. 1988;80:751–755. [PubMed] [Google Scholar]
- 8.Huguet F, Andre T, Hammel P, et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol. 2007;25:326–331. doi: 10.1200/JCO.2006.07.5663. [DOI] [PubMed] [Google Scholar]
- 9.Loehrer PJ, Feng Y, Cardenes H, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: An eastern cooperative oncology group trial. J Clin Oncol. 2011;29:4105–4112. doi: 10.1200/JCO.2011.34.8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chauffert B, Mornex F, Bonnetain F, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000–01 FFCD/SFRO study. Ann Oncol. 2008;19:1592–1599. doi: 10.1093/annonc/mdn281. [DOI] [PubMed] [Google Scholar]
- 11.Koong AC, Le QT, Ho A, et al. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;58:1017–1021. doi: 10.1016/j.ijrobp.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Koong AC, Christofferson E, Le QT, et al. Phase II study to assess the efficacy of conventionally fractionated radiotherapy followed by a stereotactic radiosurgery boost in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2005;63:320–323. doi: 10.1016/j.ijrobp.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Chang DT, Schellenberg D, Shen J, et al. Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer. 2009;115:665–672. doi: 10.1002/cncr.24059. [DOI] [PubMed] [Google Scholar]
- 14.Schellenberg D, Goodman KA, Lee F, et al. Gemcitabine chemotherapy and single-fraction stereotactic body radiotherapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2008;72:678–686. doi: 10.1016/j.ijrobp.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 15.Herman JM, Chang DT, Goodman KA, et al. Phase II multi-institutional trial evaluating gemcitabine and stereotactic body radiation therapy for locally advanced unresectable pancreatic adenocarcinoma. Cancer. 2015;121:1128–1137. doi: 10.1002/cncr.29161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vistad I, Cvancarova M, Fossa SD, et al. Postradiotherapy morbidity in long-term survivors after locally advanced cervical cancer: How well do physicians’ assessments agree with those of their patients? Int J Radiat Oncol Biol Phys. 2008;71:1335–1342. doi: 10.1016/j.ijrobp.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 17.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 18.Groenvold M, Klee MC, Sprangers MA, et al. Validation of the EORTC QLQ-C30 quality of life questionnaire through combined qualitative and quantitative assessment of patient-observer agreement. J Clin Epidemiol. 1997;50:441–450. doi: 10.1016/s0895-4356(96)00428-3. [DOI] [PubMed] [Google Scholar]
- 19.Fitzsimmons D, Johnson CD, George S, et al. Development of a disease specific quality of life (QoL) questionnaire module to supplement the EORTC core cancer QoL questionnaire, the QLQ-C30 in patients with pancreatic cancer. EORTC Study Group on Quality of Life. Eur J Cancer. 1999;35:939–941. doi: 10.1016/s0959-8049(99)00047-7. [DOI] [PubMed] [Google Scholar]
- 20.Serrano PE, Herman JM, Griffith KA, et al. Quality of life in a prospective, multicenter phase 2 trial of neoadjuvant full-dose gemcitabine, oxaliplatin, and radiation in patients with resectable or borderline resectable pancreatic adenocarcinoma. Int J Radiat Oncol Biol Phys. 2014;90:270–277. doi: 10.1016/j.ijrobp.2014.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Short M, Goldstein D, Halkett G, et al. Impact of gemcitabine chemotherapy and 3-dimensional conformal radiation therapy/5-fluorouracil on quality of life of patients managed for pancreatic cancer. Int J Radiat Oncol Biol Phys. 2013;85:157–162. doi: 10.1016/j.ijrobp.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Heras P, Kritikos K, Hatzopoulos A, et al. Effect of combined treatment methods on quality of life in patients with pancreatic cancer. Am J Ther. 2009;16:316–318. doi: 10.1097/MJT.0b013e318195e33c. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein D, Spry N, Cummins MM, et al. The GOFURTGO study: AGITG phase II study of fixed dose rate gemcitabine-oxaliplatin integrated with concomitant 5FU and 3-D conformal radiotherapy for the treatment of localised pancreatic cancer. Br J Cancer. 2012;106:61–69. doi: 10.1038/bjc.2011.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gurka M, Collins SP, Slack R, et al. Stereotactic body radiation therapy with concurrent full-dose gemcitabine for locally advanced pancreatic cancer: A pilot trial demonstrating safety. Radiat Oncol. 2013;8:1. doi: 10.1186/1748-717X-8-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fayers P, Aaronson N, Bjordal K, et al., editors. The EORTC QLQ-C30 scoring manual. 3. Brussels: European Organisation for Research and Treatment of Cancer; 2001. [Google Scholar]
- 26.Osoba D, Rodrigues G, Myles J, et al. Interpreting the significance in changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 27.Polistina F, Costantin G, Casamassima F, et al. Unresectable locally advanced pancreatic cancer: A multimodal treatment using neoadjuvant chemoradiotherapy (gemcitabine plus stereotactic radiosurgery) and subsequent surgical exploration. Ann Surg Oncol. 2010;17:2092–2101. doi: 10.1245/s10434-010-1019-y. [DOI] [PubMed] [Google Scholar]
- 28.Bonita R, Beaglehole R. Modification of Rankin Scale: Recovery of motor function after stroke. Stroke. 1988;19:1497–1500. doi: 10.1161/01.str.19.12.1497. [DOI] [PubMed] [Google Scholar]