Abstract
Background
Approximately 40% of HIV infected patients have chronic meningitis at various stages during the infection, 59% are asymptomatic. This is a diagnosis of exclusion and a confounding factor in cerebrospinal fluid (CSF) analysis, any other causes of chronic meningitis by opportunistic or co-infection must be ruled out. The aim of this study was to analyze CSF lactic acid (LA) as an adjuvant biomarker in chronic meningitis due to HIV.
Methods
CSF LA was quantified in 223 CSF samples by the Dimension AR (Dade Behring, Deerfield, IL, USA), distributed into nine groups: 1) HIV positive with an increase in CSF WBCs (n = 17); 2) HIV positive with normal CSF (n = 20); 3) enterovirus meningitis (n = 33); 4) Herpesviridae meningoencephalitis (n = 30); 5) fungal meningitis (n = 25); 6) tuberculosis (TB) meningitis (n = 17); 7) toxoplasmosis (n = 18); 8) neurosyphilis (n = 6); 9) control group (n = 57).
Results
CSF LA (median; IQR) was higher in samples with TB meningitis (5.5; 2.9–7.5 mmol/L) and Cryptococcus neoformans meningitis (3.9; 2.7–5.8 mmol/L) compated with samples with HIV chronic meningitis (1.7; 1.4–1.9 mmol/L) and other groups (p≤0.0001). For the diagnosis of HIV chronic meningitis, using a cut-off of 3.5 mmol/L, CSF LA showed high sensitivity and negative predictive value, although low specificity.
Conclusions
CSF LA helps to discriminate between C. neoformans or TB meningitis and HIV chronic meningitis: CSF LA can be included with the methods currently used to identify these specific pathogens, though it does not replace them. It is rapid, inexpensive and easy to perform, and can be used in developing countries.
Keywords: Cryptococcus neoformans, central nervous system, cerebrospinal fluid, chronic meningitis, HIV, lactic acid, lymphocytic meningitis, tuberculosis, viral meningitis
Introduction
Approximately 40% of HIV infected patients have chronic meningitis during various stages during the infection, with 59% being asymptomatic (1–3). This is a diagnosis of exclusion and a confounding factor in cerebrospinal fluid (CSF) analysis. Any other causes of chronic meningitis by opportunistic or co-infections as Cryptococcus neoformans; tuberculosis, syphilis and toxoplamosis need to be ruled out (2).
The differential diagnosis between opportunistic or co-infections and HIV related chronic viral meningitis is crucial both for choosing the most appropriate therapeutic option and prognosis. This is a difficult issue to tackle in clinical practice.
The present study was aimed to analyze CSF lactic acid (LA) in chronic meningitis (by HIV, C. neoforman tuberculosis, syphilis and toxoplamosis) and acute viral meningitis (by enterovirus and Herpesvidae) in order to identify a CSF adjuvant biomarker that could help in the differential diagnosis between an increase in CSF cells due to HIV and opportunistic infections. In order to calculate the operational characteristics of CSF LA, it is essential to discriminate between HIV related chronic meningitis and opportunistic infections.
Materials and methods
A prospective longitudinal study was conducted at the clinical pathology laboratory of Hospital de Clínicas, Universidade Federal do Paraná (HC-UFPR). This study was approved by the HC-UFPR IRB.
All CSF samples were obtained by lumbar puncture (LP). CSF LA was determined using the Dimension AR machine (Dade Behring, Deerfield, IL, USA).
CSF total protein (TP) was quantified using a turbidimetric pyrogallol red method and CSF glucose was quantified using an enzymatic method. CSF total cell count (WBCs) was performed using a Fuchs Rosenthal chamber. A WBC count >4 cells/mm3 was considered increased. For the differential cell count, CSF samples were concentrated by Shandon Cytospin (Pittsburgh, PA, USA) and the slides were stained using the May-Grünwald-Giemsa technique. The predominance of cells was only considered when it was higher than 50%.
A total 223 CSF samples were studied. Demographic, CSF biochemistry and cell characteristics and HIV status of all the studied groups are shown in Table 1. The samples were distributed into the following groups:
Group 1. HIV positive with increased CSF WBCs (n = 17); CSF samples with increased number of WBCs with predominance of lymphocytes (≥50%) and glucose ≥2.78 mmol/L. CSF VDRL; direct bacterioscopic and mycologic examination, fungal and bacterial cultures were negative.
Group 2. HIV positive with normal CSF biochemistry and cell characteristics (n = 20).
Group 3. enterovirus meningitis (n = 33). CSF samples with increased WBCs with a predominance of lymphocytes (≥50%) and glucose ≥2.78 mmol/L. Polymerase chain reaction (PCR) for enterovirus (4) positive in all samples. No case was HIV positive.
Group 4. Herpesviridae meningoencephalitis (n = 30). CSF samples with increased WBCs with a predominance of lymphocytes (≥50%) and glucose ≥2.78 mmol/L. PCR for HSV-1, HSV-2, CMV, EBV, VZV, HHV-8, HHV-6A, HHV-6B or HHV-7 (4) positive. HIV was positive in 8 (27%) cases.
Group 5. Fungal meningitis (n = 25). Diagnosed by direct mycological examination, C. neoformans antigen latex agglutination or culture; 22 samples were positive for C. neoformans and three samples had other species of fungus (Candida guilliermondii; Histoplasma capsulatum; Fusarium sp.). Seventeen (68%) cases were HIV positive.
Group 6. Tuberculosis (TB) meningitis (n = 17). CSF samples with increased WBCs and with a predominance of lymphocytes and glucose ≤2.78 mmol/L. Mycobacterium tuberculosis identified by positive BAAR culture, positive BAAR direct observation on CSF smear by Ziehl Nielsen method or positive PCR (confirmed TB meningitis, n = 6); altered CSF with Mycobacterium tuberculosis identified in sputum (probable TB meningitis, n = 5); altered CSF, no Mycobacterium tuberculosis identified, although with response to empiric TB treatment (possible TB meningitis, n = 6). HIV was positive in 13 (76%) cases.
Group 7. Neurotoxoplasmosis (n = 18), CT or MRI compatible with neurotoxoplasmosis, clinical response to specific therapy. Ten cases (56%) with increased CSF WBCs. HIV was positive in all cases.
Group 8. Neurosyphilis (n = 6), CSF showing an increase in cells with a predominance of lymphocytes, increase in protein, CSF VDRL positive. Four cases (67%) with increased CSF WBCs. HIV positive in two (33%) cases.
Group 9. Control group, HIV negative, no meningitis (n = 57). Control group; CSF samples from patients with clinical suspicion of acute meningitis. WBCs, total protein and glucose were normal. Bacteria were not isolated by culture or on a Gram stain.
Table 1.
CSF biochemistry and cell characteristics of all groups studied (median; IQR).
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| n | 17 | 20 | 33 | 30 | 25 | 17 | 18 | 6 | 57 |
| Male, n (%) | 10 (59%) | 7 (35%) | 21 (64%) | 20 (67%) | 14 (56%) | 15 (88%) | 13 (72%) | 4 (67%) | 21 (37%) |
| Age years | 43; 32–49 | 44; 35–56 | 7; 4–9 | 30; 10–47 | 41; 26–54 | 33; 30–50 | 42; 36–49 | 40; 30–55 | 10; 4–40 |
| HIV +, n (%) | 17 (100%) | 20 (100%) | 0 | 8 (27%) | 17 (68%) | 13 (76%) | 18 (100%) | 2 (33%) | 0 |
| RBC, cells/mm3 | 1.5; 0–13 | 0.3; 0–2 | 6; 1.5–39 | 5.6; 1.3–40 | 5.0; 1.0–25 | 2.4; 0.3–225 | 6.2; 1.5–453 | 6.5; 1.5–121 | 0.3; 0–1.3 |
| ↑ CSF WBC, n (%) | 17 (100%) | 0 | 33 (100%) | 30 (100%) | 23 (92%) | 17 (100%) | 10 (56%) | 4 (67%) | 0 |
| WBC, cells/mm3 | 10; 8–22 | 1.6; 0.6–2.7 | 55; 15–118 | 79; 15–193 | 27; 12–71 | 130; 11–314 | 6.3; 2.8–23 | 11; 2.3–132 | 0.9; 0.5–1.9 |
| Lymphocytes, % | 96; 95–98 | – | 70; 24–85 | 86; 63–98 | 94; 85–98 | 85; 73–95 | 95; 90–98 | 98 | – |
| Glucose, mmol/L | 2.94; 2.39–3.39 | 3; 2.83–3.61 | 3.72; 3.16–4.16 | 2.72; 1.94–3.55 | 1.78; 1.05–2.5 | 1.55; 0.94–2.44 | 2.61; 2.22–3.44 | 3.44; 1.83–4.27 | 3.55; 3.16–4 |
| TP, g/L | 0.47; 0.35–0.78 | 0.38; 0.32–0.45 | 0.41; 0.27–0.58 | 0.74; 0.45–2.12 | 0.94; 0.56–1.73 | 2.65; 0.69–4.36 | 0.97; 0.48–1.41 | 0.62; 0.30–1.01 | 0.25; 0.16–0.35 |
Statistical analysis
The results are shown as median±interquartile range (IQR). Continuous variables were compared using the non-parametric testing to compare all five groups with the Kruskal-Wallis (KW) non-parametric test. A two-by-two comparison of groups was performed using the Mann-Whitney (MW) test. A p-value ≤0.05 was considered significant. The confidence interval was 95%.
In order to evaluate the operational characteristics (5) of CSF LA quantification for the diagnosis of HIV related chronic meningitis and chronic meningitis caused by opportunistic infections or co-infections, group 1 was compared to groups 5, 6, 7 and 8. Groups 7 and 8 included only cases with an increase in CSF WBCs. The CSF LA cut-off used was 3.5 mmol/L.
The following formulas were used: sensitivity: [TrP/(Tr-P + FN)] × 100; specificity: [TN/(TN + FP)] × 100; positive predictive value: [TrP/(TrP + FP)] × 100; negative predictive value (NPV): [TN/(TN + FN)] × 100; detection rate: TrP/total tested; Youden index: [(sensitivity + specificity) − 1] ; error ratio: (FP + FN)/TrP; combined error: (FP + FN)/total tested.
(TrP, true positive; TN, true negative; FP, false positive; FN, false negative); likelihood ratio for a positive test (LR +) = sensitivity/(1 − specificity).
Results
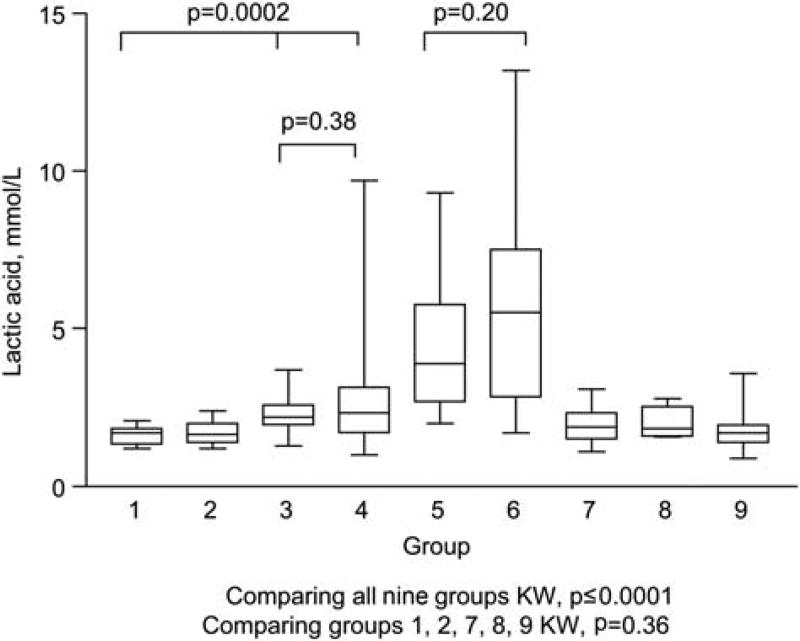
The CSF LA (median; IQR) was higher in samples with TB meningitis (5.5; 2.9–7.5 mmol/L) and fungal meningitis (3.9; 2.7–5.8 mmol/L) compared to samples with HIV related chronic meningitis (1.7; 1.4–1.9 mmol/L) and the other groups. A comparison of all nine groups (KW, p≤0.0001) (Figure 1 and Table 2) showed no difference between the fungal meningitis and TB meningitis groups (MW, p = 0.20).
Figure 1.
CSF lactic acid concentration in the groups studied.
Table 2.
CSF lactic acid (mmol/L) concentrations in all groups studied.
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| n | 17 | 20 | 33 | 30 | 25 | 17 | 18 | 6 | 57 |
| Median | 1.7 | 1.7 | 2.2 | 2.4 | 3.9 | 5.5 | 1.9 | 1.9 | 1.7 |
| IQR | 1.4–1.9 | 1.4–2.0 | 1.9–2.6 | 1.7–3.2 | 2.7–5.8 | 2.9–7.5 | 1.5–2.4 | 1.6–2.6 | 1.4–2.0 |
| Mean | 1.6 | 1.7 | 2.3 | 2.8 | 4.3 | 5.8 | 1.9 | 2.0 | 1.7 |
| SD | 0.3 | 0.4 | 0.5 | 1.9 | 2.1 | 3.4 | 0.5 | 0.5 | 0.5 |
KW, p≤0.0001.
There was no difference between the group with HIV chronic meningitis and groups 2, 7, 8 and 9 (p = 0.36), even when only the cases of increased CSF WBCs in groups 7 and 8 were analyzed. There was a difference between the group with HIV chronic meningitis and the groups with acute meningitis due to enterovirus (group 3) and the group with herpes virus (group 4) (KW, p = 0.0002). No difference (MW, p = 0.38) was found in the groups with acute meningitis (3 and 4).
In the TB group (group 6) median; IQR in cases with confirmed TB (6 cases) 6.7; 5.6–10 mmol/L; in the cases with probable TB (5 cases) was 2.9; 1.7–9 mmol/L, and in cases with possible TB (6 cases) 4.5; 2.4–9.8 mmol/L (KW, p = 0.13). A comparison confirmed those cases where differences were likely to occur (MW, p = 0.08).
CSF LA <3.5 mmol/L was found in group 1 in 17 (100%) samples; group 2 in 19 (100%); group 3 in 32 (97%); group 4 in 26 (87%); group 5 in 10 (42%); group 6 in 5 (29%); group 7 in 18 (100%); group 8 in 6 (100%) and group 9 in 56 samples (98%).
The operational characteristics of CSF LA, using a cut-off of 3.5 mmol/L, for the differential diagnosis between HIV related chronic meningitis and other causes of chronic meningitis are shown in Table 3.
Table 3.
The operational characteristics of CSF LA usig a cut-off of 3.5 mmol/L to diagnose chronic meningitis by HIV and chronic meningitis due to fungus, tuberculosis, toxoplasmosis and syphilis.
| Operational characteristics | Group 1 compared with | |
|---|---|---|
|
|
||
| Groups 5, 6 | Groups 5, 6, 7a, 8a | |
| Sensitivity, % | 100 | 100 |
| Specificity, % | 62 | 46 |
| PPV, % | 52 | 36 |
| NPV, % | 100 | 100 |
| Detection rate, % | 29 | 23 |
| Youden index | 0.62 | 0.46 |
| Error ratio, % | 90 | 180 |
| Combined error, % | 27 | 41 |
| Presumptive positive | 0.56 | 0.64 |
| Eficiency, % | 73 | 59 |
| LR+ | 2.6 | 1.9 |
| LR− | 0 | 0 |
Cases with increase of CSF WBC.
Discussion
The normal range for lumbar CSF LA in most reports ranges from 1.1 to 2.2 mmol/L (6, 7). LA concentrations in the control group were (median; IQR) 1.7; 1.4–2.0 mmol/L. In this study, CSF LA was higher in chronic meningitis by TB and fungus, caused primarily by C. neoformans than HIV chronic meningitis. In tuberculosis meningitis, CSF LA was higher in the cases that were culture positive (6.7, 5.6–10 mmol/L), being three-fold higher than the control group, which is in accordance with other studies that also showed that neither the clinical stage of tuberculosis meningitis nor prognosis was related to CSF LA concentrations (8). Repeated CSF studies persistently showed high LA concentrations in the early stages of illness despite adequate antituberculous therapy (8). The CSF LA concentration helps differentiate between partially treated pyogenic meningitis and tuberculous meningitis. The increase in CSF LA is not specific for meningitis and interpretation must take into consideration the patient’s clinical status (9). Studies investigating the role of CSF LA in helping differentiate between tuberculous meningitis and aseptic meningitis used an upper limit of normal for CSF LA of 2.75 mmol/L which allowed detection of 24 out of 26 cases of tuberculous meningitis (sensitivity of 92%). However, if a concentration of 3.85 mmol/L was used as the upper limit of normal, then 18 out of 26 cases were detected (sensitivity of 69%) (10).
There was a difference among the groups with HIV chronic meningitis and the group with acute viral meningitis by enterovirus and herpesviridae meningoencephalitis. However, the CSF LA values were lower than 3.5 mmol/L in all groups and thus there was no clinical relevance.
The sensitivity of CSF LA in our study was 100%. However, the specificity of LA for differentiating HIV related chronic meningitis from fungal meningitis or tuberculosis was low. Including toxoplasmosis and syphilis, two conditions that could also cause chronic meningitis, the specificity is even lower (46%). In this study, all cases with toxoplasmosis or syphilis showed LA to be lower than 3.5. Thus, LA does not help differente between these opportunistic infections and co-infections. The NPV is high, therefore a LA higher than 3.5 rules out chronic meningitis by HIV. However, when values are lower than 3.5, no diagnosis can be made because the PPV is low due to the previous considerations regarding toxoplasmosis and syphilis. Both the NPV and PPV depend on the prevalence of the disease on the population. Previous studies show that CSF LA exhibited the highest sensitivity (89%–100%) and specificity (96%–100%) at values ≥3.5 mmol/L to discriminate between acute untreated bacterial meningitis and viral meningitis (11, 12). This limit proved to be also relevant for tuberculosis (8), Listeria monocytogenes (13) and fungal meningitis (14). The CSF LA test fulfills the criteria of an optimum test; higher discrimination limits at >5.0 mmol/L reduce diagnostic sensitivity, and a lower limit, such as 2.8 mmol/L decreases diagnostic specificity and does not allow differentiation of viral meningitis from controls (15).
CSF LA is considered a good biomarker to differentiate acute bacterial from viral meningitis at a cut-off of 3.5 mmol/L (6, 15–22). In the current study we show that CSF LA can also help different between HIV related chronic meningitis, and tuberculosis and cryptococcus meningitis, although it does not help in differentiating between HIV related chronic meningitis and toxoplasmosis or neurosyphilis; two frequent causes of chronic meningitis in HIV patients. CSF LA is also not helpful for differentiating between HIV meningitis and other causes of viral meningitis, such as caused by enterovirus and the herpesviridae family. According to some authors, the test is unlikely to be of much help in differentiating between partly treated bacterial meningitis from viral meningitis, but it is valuable for differentiating between tuberculous meningitis and viral meningitis, modified bacterial meningitis and parameningeal septic states (23).
CSF LA is increased in fungal meningitis. CSF LA concentrations in fungal meningitis ranged from 3.2 to 13.3 mmol/L. Due to the poor sensitivity of stained smear or wet preparations and cultures when <5 mL of CSF are used for culture, an increased LA value in a patient showing or not showing signs of meningitis should raise the suspicion of fungal infection (14). Some investigators describe overlapping CSF LA concentrations in viral, partially treated bacterial meningitis and tuberculosis meningitis. This limits the value of the assay as a diagnostic test for the differential diagnosis of these disorders (24).
Bacteria can produce both D- and L-lactate, while mammalian cells produce only L-lactate. Increased CSF lactate in meningitis consists mostly of L-lactate and originates predominantly from host cells. CSF D-lactate is of limited diagnostic value (25). Since the LA concentration in bacterial meningitis increases linearly with the number of LA-producing cells, it can be concluded that the increased LA concentration results from CSF pleocytosis. If LA concentrations are higher than the normal distribution, additional sources of LA production, such as cerebral hypoxia must be assumed (26). The CSF concentration of LA is directly dependent upon its production rate by the brain. There is substantial evidence in patients and in experimental animals that blood and CSF LA concentrations are largely independent of each other (7, 27). Thus, intravenous infusion of LA in dogs was sufficient to raise the blood concentration six-fold, yet failed to increase CSF concentrations (7). This is important in clinical practice because it eliminates the need for collecting matched serum. CSF LA concentrations are also useful in the diagnosis of post surgical acute bacterial meningitis when there is not a specific increase in cells and proteins (28, 29). There is no correlation between CSF and blood. In the cases of low CSF LA, values are similar to those in serum (7, 27). This is a very important characteristic of LA determinations and an advantage over CSF glucose determinations which are dependent on blood glucose concentrations.
Elevated CSF LA is associated with low CSF glucose concentrations. While a moderate elevation in LA is often observed with a normal CSF glucose concentration, the occurrence of a very low CSF glucose concentration has been invariably associated with a substantial increase in LA concentrations. This is indicative of the occurrence of increased anaerobic glycolysis by the adjacent cerebral tissue or by cellular infiltrates in the leptomeninges in the pathogenesis of reduction in glucose content and increases in LA (30).
The measurement of CSF LA has been considered by some investigators to be clinically useful in the differential diagnosis of disorders associated with low CSF glucose and as guidance on response to therapy (31).
There is substantial evidence that whenever cerebral glycolysis is increased, whether this is due to anaerobic glycolysis, hypoxia, ischemia, seizures or meningitis (32), the LA and pyruvate concentrations in brain and CSF, and the lactate-to-pyruvate ratio are typically increased. However, measurement of CSF LA concentrations showed that these were greater than the ratio of CSF to blood glucose for the diagnosis of bacterial meningitis in postoperative neurological patients (17, 33).
The blood brain barrier (BBB) shows low permeability to the diffusion of LA; in the normal pH range the LA molecules are mostly dissociated. The penetration of acid radicals of metabolic origin through the BBB depends on the characteristics of the exchange mechanisms (34).
The central nervous system (CNS) and immune system are the main targets of HIV infection (35). The frequency of neurological manifestations as AIDS defining diseases varies according to the geographic area. At autopsy, neurological findings are found in 75%–90% of the patients. Intracranial mass lesions account for 50% of neurologic disorders and are among the most common neurologic complications of HIV infection, due to opportunistic infections or CNS primary neoplasm. These occur in patients with CD4 counts of <200 cells/mm3 (36).
It is possible that HIV penetrates the CNS during the initial stages of infection. During this period, the viral load in the peripheral blood is as high as it is in the terminal stage of the illness that can drive CNS penetration (37, 38).
Chronic meningitis can be caused by HIV in 13 (1) to 40% (2, 3) of HIV positive individuals, at any stage of HIV infection. CSF characteristics are similar to those of other viral meningitis. CSF cytological and biochemical findings in AIDS patients limit the interpretation of CSF. CSF abnormalities are quite frequent in these patients, are non-specific and difficult to interpret. In these circumstances, a systematic search to identify the etiologic agent using microbiological and/or immunological assays or molecular biology, when available, should be routinely performed (39).
C. neoformans is the most common life-threatening fungal pathogen that infects patients with AIDS. It is a very common CNS opportunistic infection, occurring in about 10% of AIDS patients (40). In AIDS patients and those with cryptococcosis, abnormal CSF is found in 69%, and in the group with no identified neurological dysfunction, abnormal CSF is found in 61%. Lymphocytes were the most frequent cells in both groups (39).
In order to identify a biomarker that could help differentiating between HIV related chronic meningitis and other opportunistic chronic meningitis, such as those caused by M. tuberculosis, C. neoformans or syphilis could be of great clinical importance. LA has proved to be helpful in these cases.
In conclusion, CSF LA can be used routinely in laboratories. It can be measured rapidly on automated instruments and is easy to perform. In addition, it is a powerful test to discriminate between HIV-related chronic meningitis and opportunistic chronic meningitis, with high sensitivity and NPVs, although low specificity. CSF LA is a biomarker that helps discriminate between C. neoformans or suspicion of TB meningitis and HIV related chronic meningitis. Although it is important to stress that it does not replace the current methods used to identify these specific pathogens, it can be associated with them.
Footnotes
Conflict of interest statement
Authors’ conflict of interest disclosure: The authors stated that there are no conflicts of interest regarding the publication of this article.
Research funding: None declared.
Employment or leadership: None declared.
Honorarium: None declared.
References
- 1.Hollander H, McGuire D, Burack JH. Diagnostic lumbar puncture in HIV-infected patients: analysis of 138 cases. Am J Med. 1994;96:223–8. doi: 10.1016/0002-9343(94)90146-5. [DOI] [PubMed] [Google Scholar]
- 2.Livramento JA, Machado LR, Spina-Franca A. Cerebrospinal fluid abnormalities in 170 cases of AIDS. Arq Neuropsiquiatr. 1989;47:326–31. doi: 10.1590/s0004-282x1989000300013. [DOI] [PubMed] [Google Scholar]
- 3.Marshall DW, Brey RL, Cahill WT, Houk RW, Zajac RA, Boswell RN. Spectrum of cerebrospinal fluid findings in various stages of human immunodeficiency virus infection. Arch Neurol. 1988;45:954–8. doi: 10.1001/archneur.1988.00520330032007. [DOI] [PubMed] [Google Scholar]
- 4.Pozo F, Tenório A. Detection and typing of lympgotropic herpesviruses by multiplex polymerase chain reaction. J Virol Meth. 1999;79:9–19. doi: 10.1016/s0166-0934(98)00164-5. [DOI] [PubMed] [Google Scholar]
- 5.Galen RS, Gambino SR. Beyond normality: the predictive value and efficiency of medical diagnoses. New York: John Wiley & Sons; 1975. p. 237. [Google Scholar]
- 6.Negrini B, Kelleher KJ, Wald ER. Cerebrospinal Fluid findings in aseptic versus bacterial meningitis. Pediatrics. 2000;105:316–19. doi: 10.1542/peds.105.2.316. [DOI] [PubMed] [Google Scholar]
- 7.Posner JB, Plum F. Independence of blood and cerebrospinal fluid lactate. Arch.Neurol. 1967;16:492–6. doi: 10.1001/archneur.1967.00470230044005. [DOI] [PubMed] [Google Scholar]
- 8.Tang LM. Serial lactate determinations in tuberculous meningitis. Scand J Infect Dis. 1988;20:81–3. doi: 10.3109/00365548809117221. [DOI] [PubMed] [Google Scholar]
- 9.el Mdaghri N, Benbachir M, Tazi-Lakhsassi L, Himmich H. Significance of the determination of lactic acid in the cerebrospinal fluid for the differential diagnosis of meningitis. Pathol Biol. 1985;33:227–31. [PubMed] [Google Scholar]
- 10.Donald PR, Malan C. Cerebrospinal fluid lactate and lactate dehydrogenase levels as diagnostic aids in tuberculous meningitis. S Afr Med J. 1985;67:19–20. [PubMed] [Google Scholar]
- 11.Briem H. Comparison between cerebrospinal fluid concentrations of glucose, total protein, chloride, lactate, and total amino acids for differential diagnosis of patients with meningitis, Scand. J Infect Dis. 1983;15:277–84. doi: 10.3109/inf.1983.15.issue-3.08. [DOI] [PubMed] [Google Scholar]
- 12.Lindquist L, Linn T, Hansson LO, Kalin M, Axelsson G. Value of cerebrospinal fluid analysis in the diferential diagnosisd of meningitis: a study of 710 patients with suspected central system infection. Eur J Clin Microbiol Infect Dis. 1988;7:374–80. doi: 10.1007/BF01962340. [DOI] [PubMed] [Google Scholar]
- 13.Cunha AB, Fatehpuria R, Eisenstein LE. Listeria monocytogenes encephalitis mimicking Herpes simples encephalitis: the differential diagnostic importance of cerebrospinal fluid lactic acid levels. Heart Lung. 2007;36:226–31. doi: 10.1016/j.hrtlng.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Body BA, Oneson RH, Herold DA. Use of cerebrospinal fluid lactic acid concentration in diagnosis of fungal meningitis. Ann Clin Lab Sci. 1987;17:429–34. [PubMed] [Google Scholar]
- 15.Kleine TO, Zwerenz P, Zofel P, Zöfel P, Shiratori K. New and old diagnostic markers of meningitis in cerebrospinal fluid (CSF) Brain Research Bull. 2003;61:287–97. doi: 10.1016/s0361-9230(03)00092-3. [DOI] [PubMed] [Google Scholar]
- 16.Deisenhammer F, Bartos A, Egg R, Gilhus NE, Giovannoni G, Rauer S, et al. EFNS Task Force. Guidelines on routine cerebrospinal fluid analysis. Report from an EFNS task force. Eur J Neurol. 2006;13:913–22. doi: 10.1111/j.1468-1331.2006.01493.x. [DOI] [PubMed] [Google Scholar]
- 17.Leib SL, Boscacci R, Gratzl O, Zimmerli W. Predictive value of cerebrospinal fluid (CSF) Lactate versus CSF/Blood glucose ratio for the diagnosis of bacterial meningitis following neurosurgery. Clin Infec Dis. 1999;29:69–74. doi: 10.1086/520184. [DOI] [PubMed] [Google Scholar]
- 18.Cabeça HL, Gomes HR, Machado LR, Livramento JA. Dosage of lactate in the cerebrospinal fluid in infectious diseases of the central nervous system. Arq Neuropsiquiatr. 2001;59:843–8. doi: 10.1590/s0004-282x2001000600002. [DOI] [PubMed] [Google Scholar]
- 19.de Almeida SM, Faria FL, Fontes KG, Buczenko GM, Berto DB, Raboni SM, et al. Quantification of cerebrospinal fluid lactic acid in infectious and noninfectious neurological diseases. Clin Chem Lab Med. 2009;47:755–61. doi: 10.1515/CCLM.2009.160. [DOI] [PubMed] [Google Scholar]
- 20.Donald PR, Malan C. Cerebrospinal fluid lactate and lactate dehydrogenase activity in the rapid diagnosis of bacterial meningitis. S Afr Med J. 1986;69:39–42. [PubMed] [Google Scholar]
- 21.Knight JA, Dudek SM, Haymond RE. Early (chemical) diagnosis of bacterial meningitis-cerebrospinal fluid glucose, lactate, and lactate dehydrogenase compared. Clin Chem. 1981;27:1431–4. [PubMed] [Google Scholar]
- 22.Jordan GW, Statland B, Halsted C. CSF lactate in diseases of the CNS. Arch Intern Med. 1984;143:85–7. [PubMed] [Google Scholar]
- 23.Mandal BK, Dunbar EM, Hooper J, Parker L. How useful is cerebrospinal fluid lactate estimation in differential diagnosis of meningitis? J Infect. 1983;6:231–7. doi: 10.1016/s0163-4453(83)93597-1. [DOI] [PubMed] [Google Scholar]
- 24.D’Souza E, Mandal BK, Hooper J, Parker L. Lactic acid concentration in cerebrospinal fluid and differential diagnosis of meningitis. Lancet. 1978;2:579–80. doi: 10.1016/s0140-6736(78)92918-5. [DOI] [PubMed] [Google Scholar]
- 25.Wellmer A, Prange J, Gerber J, Zysk G, Lange P, Michel U, et al. D- and L-lactate in rabbit and human bacterial meningitis. Scand J Infect Dis. 2001;33:909–13. doi: 10.1080/00365540110076732. [DOI] [PubMed] [Google Scholar]
- 26.Kölmel HW, von Maravic M. Correlation of lactic acid level, cell count and cytology in cerebrospinal fluid of patients with bacterial and non-bacterial meningitis. Acta Neurol Scand. 1988;78:6–9. doi: 10.1111/j.1600-0404.1988.tb03610.x. [DOI] [PubMed] [Google Scholar]
- 27.Gladden LB. Lactate metabolism: a new paradigm for the third millennium. J Physiol. 2004;558:5–30. doi: 10.1113/jphysiol.2003.058701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ponka A, Ojala K, Teppo AM, Weber TH. The differential diagnosis of bacterial and aseptic meningitis using cerebrospinal fluid laboratory tests. Infection. 1983;11:129–31. doi: 10.1007/BF01641290. [DOI] [PubMed] [Google Scholar]
- 29.Cunha BA. The usefulness of CSF lactic acid levels in central Nervous System infections with decreased CSF glucose. Clin Infect Dis. 2004;38:1260–1. doi: 10.1086/424751. [DOI] [PubMed] [Google Scholar]
- 30.Fishman RA. Cerebrospinal Fluid in Diseases of the Nervous System. Philadelphia: Saunders; 1992. p. 431. [Google Scholar]
- 31.Brook I. The importance of lactic acid in body fluids in the detection of of bacterial infections. Rev Infect Dis. 1981;3:470–8. doi: 10.1093/clinids/3.3.470. [DOI] [PubMed] [Google Scholar]
- 32.Tunkel AR, Hartman BJ, Kaplan SL, Kaufman BA, Roos KL, Scheld WM, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;39:1267–84. doi: 10.1086/425368. [DOI] [PubMed] [Google Scholar]
- 33.Wong GK, Poon WS, Ip M. Use of ventricular cerebrospinal fluid lactate measurement to diagnose cerebrospinal fluid infection in patients with intraventricular haemorrhage. J Clin Neurosc. 2008;15:654–5. doi: 10.1016/j.jocn.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 34.Weyne J, van Leuven F. Lactic acid in the brain and cerebrospinal fluid in different conditions of hyperlactacidemia. Arch Int Physiol Biochim. 1973;81:925–30. doi: 10.3109/13813457309074494. [DOI] [PubMed] [Google Scholar]
- 35.Berger JR, Moskowitz L, Fischl M, Kelley RE. Neurologic disease as the presenting manifestation of acquired immunodeficiency syndrome. Aids and Neurologic Disorders. 1987;80:683–5. doi: 10.1097/00007611-198706000-00004. [DOI] [PubMed] [Google Scholar]
- 36.American academy of neurology. Evaluation and management of intracranial mass lesions in AIDS. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 1998;50:21–6. doi: 10.1212/wnl.50.1.21. [DOI] [PubMed] [Google Scholar]
- 37.Resnick L, DiMarzo-Veronese F, Schupbach, Tourtellotte WW, HO DD, Müller F, et al. Intra-blood-brain-barrier synthesis of HTLV-III specific IgG in patients with neurologic symptons associated with AIDS or AIDS-related complex. N Engl J Med. 1985;313:1498–504. doi: 10.1056/NEJM198512123132402. [DOI] [PubMed] [Google Scholar]
- 38.Resnick L, Berger JR, Shapshak P, Tourtellotte WW. Early penetration of the blood brain barrier by HIV. Neurology. 1988;38:9–14. doi: 10.1212/wnl.38.1.9. [DOI] [PubMed] [Google Scholar]
- 39.Garlipp CR, Rossi CL, Bottini PV. Cerebrospinal fluid profiles in acquired immunodeficiency syndrome with and without neurocryptococcosis. Rev Inst Med Trop Sao Paulo. 1997;39:323–5. doi: 10.1590/s0036-46651997000600003. [DOI] [PubMed] [Google Scholar]
- 40.Dal Pan GJ, McArthur JC. Neuroepidemiology of HIV infection. Neuroepidemiology. 1996;14:359–82. doi: 10.1016/s0733-8619(05)70262-0. [DOI] [PubMed] [Google Scholar]