Abstract
The extracellular domain of influenza A ion channel membrane matrix protein 2 (M2e) is considered to be a potential candidate to develop a universal influenza A vaccine. However poor immunogenicity of M2e presents a significant roadblock. We have developed a vaccine formulation comprising of the consensus M2e peptide conjugated to gold nanoparticles (AuNPs) with CpG as a soluble adjuvant (AuNP-M2e+sCpG). We demonstrate that intranasal delivery of AuNP-M2e+sCpG in mice induces lung B cell activation and robust serum anti-M2e immunoglobulin G (IgG) response, with stimulation of both IgG1 and IgG2a subtypes. Using Madin-Darby canine kidney (MDCK) cells infected with A/California/04/2009 (H1N1pdm) pandemic strain, or A/Victoria/3/75 (H3N2), or the highly pathogenic avian influenza virus A/Vietnam/1203/2004 (H5N1) as immunosorbants we further show that the antibodies generated are also capable of binding to the homotetrameric form of M2 expressed on infected cells. Lethal challenge of vaccinated mice with A/California/04/2009 (H1N1pdm) pandemic strain, A/Victoria/3/75 (H3N2), and the highly pathogenic avian influenza virus A/Vietnam/1203/2004 (H5N1) led to 100%, 92%, and 100% protection, respectively. Overall, this study helps to lay the foundation of a potential universal influenza A vaccine.
Keywords: adjuvants, CpG, gold nanoparticles, influenza vaccine, intranasal vaccination, M2e, universal influenza vaccine
1. INTRODUCTION
Since 1918, four influenza pandemics have struck the globe. Following the 1957 and 1968 pandemics, the world witnessed its fourth pandemic in 2009, which resulted in an estimated 284,500 respiratory and cardiovascular deaths, worldwide (Dawood et al., 2012). The danger of yet another influenza pandemic continues to loom threateningly, and it is often quoted that it is not a question of “if, but when” (Allen, 2006), the next pandemic will strike. Furthermore, as a seasonal event, influenza virus strikes each year, and typically results in up to 0.5 million deaths worldwide (WHO, 2014). To prevent these devastating events there is an urgent need to develop a universal influenza vaccine, which can provide broad cross protection against different influenza A subtypes.
Influenza virus is an enveloped virus containing two major membrane glycoproteins, hemagglutinin (HA) and neuraminidase (NA). HA allows the virus to infect cells through interaction with sialic-acid residues on receptors, and NA is a receptor-destroying enzyme that enables the virus to escape from infected cells to spread infection (Gamblin and Skehel, 2010). Current influenza vaccines rely on HA and NA as antigens to induce neutralizing antibodies, which inhibit virus infection and replication in humans. However, these antibodies are stimulated mostly against the immunodominant epitopes of HA, and these epitopes are highly variable between different influenza strains (Krammer et al., 2015). As a result existing vaccines are only protective against influenza strains included in the vaccine and offer poor to no protection against other strains. The situation gets more complicated because new variants of influenza virus emerge each year due to antigenic shift and drift, which forces reformulation of the influenza vaccine every year (Subbarao et al., 2006).
To develop a universal influenza vaccine, conserved sequences that are shared by different influenza viruses must be used as vaccine antigens. In addition to HA and NA, the influenza virus surface contains a third membrane protein called the ion channel membrane matrix protein 2 (M2) (Holsinger and Alams, 1991; Lamb et al., 1985; Schnell and Chou, 2008). The 23 amino acid extracellular domain of M2 (M2e) has remained fairly conserved since the 1918 influenza outbreak (Reid et al., 2002), and thus it is an attractive target to develop a universal influenza A vaccine. However, a major challenge in developing a vaccine based on M2e is that M2 naturally occurs in very small numbers on the virus surface (about 16–20 molecules per virion) (Holsinger and Alams, 1991; Lamb et al., 1985) and is poorly immunogenic. To enhance the immunogenicity of M2e various approaches have been employed including fusion of M2e to different carriers such as hepatitis B virus core protein (Neirynck et al., 1999), bacterially-derived outer membrane vesicles (Rappazzo et al., 2016), virus-like particles (Kim et al., 2014; Wang et al., 2012), through attachment to flagellin domains (Wang et al., 2014) or elastin-like polypeptides (Ingrole et al., 2014), and use of nanoparticles with soluble antigens (Seth et al., 2015; Wibowo et al., 2014).
We recently demonstrated that attachment of the consensus M2e peptide to gold nanoparticles (AuNPs) can significantly enhance M2e immunogenicity, and that intranasal delivery of M2e-conjugated AuNPs (AuNP-M2e) with soluble CpG (sCpG) as an adjuvant (AuNP-M2e+sCpG) can completely protect mice from a lethal challenge with influenza A/PR/8/34 (H1N1) (Tao and Gill, 2015; Tao et al., 2014). In the present study we sought to further investigate whether this vaccine formulation can offer protection against a broader spectrum of influenza A subtypes, including the A/California/04/2009 (H1N1) pandemic strain, A/Victoria/3/75 (H3N2), and the highly pathogenic avian influenza virus A/Vietnam/1203/2004 (H5N1), and to characterize the mucosal immune response generated by the vaccine.
2. MATERIALS AND METHODS
2.1 Chemicals
Gold (III) chloride trihydrate (520918-5G), trisodium citrate dihydrate (S1804-500G), phosphate-citrate buffer tablet (P4809-50TAB) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Tween20 (BP337-100) was purchased from Fisher Scientific (Waltham, MA, USA). O-Phenylenediamine (OPD) (00-2003) was obtained from Life Technologies (Carlsbad, CA, USA). Milli-Q water with resistance of 18.2 MΩ cm was used in all the experiments.
2.2 Peptides, antibodies and oligonucleotides
Consensus M2e peptide (acetylated-SLLTEVETPIRNEWGSRSNDSSDC-amidated, MW: 2736 Da) was chemically synthesized by AAPPTec (Louisville, KY, USA). Underlined serines (S) are intentional substitutions of native cysteine to serine. CpG oligodeoxynucleotide (ODN) 1826 VacciGrade™ (5′-TCCATGACGTTCCTGACGTT-3′) was purchased from InvivoGen (San Diego, CA, USA). Alexa-Fluor 488-labeledanti-mouse IgG antibody was obtained from Life Technologies (Carlsbad, CA, USA). All other secondary antibodies were purchased from Southern Biotech (Birmingham, AL, USA).
2.3 Animals
Six to eight week old female BALB/c mice were obtained from Charles River Laboratories. All animal treatments were performed according to Utah State University and Texas Tech University Animal Care and Use Committee (IACUC) approved procedures.
2.4 Viruses
Pandemic influenza A/California/04/2009 (H1N1pdm), strain designation 175190, was received from Dr. Elena Govorkova, Department of Infectious Diseases, St. Jude Children’s Research Hospital, Memphis TN. The virus was adapted for replication in the lungs of BALB/c mice by nine sequential passages in mice. Virus was plaque purified in Madin-Darby canine kidney (MDCK) cells and a virus stock was prepared by growth in embryonated chicken eggs and then MDCK cells. Influenza A/Victoria/3/75 (H3N2) virus was obtained from the American Type Culture Collection (Manassas, VA) and mouse adapted by serial passages in the lungs of BALB/c mice. Following mouse-adaptation a virus stock was prepared by growth in MDCK cells. Influenza A/Vietnam/1203/2004 (H5N1) was obtained from the Centers for Disease Control (Atlanta, GA, USA). Viral propagation was done in MDCK cells (American Type Culture Collection, Manassas, VA, USA). Parent virus was passaged once to prepare a challenge pool. The challenge pool was then titrated in MDCK cells before use. Influenza A/California/04/2009 (H1N1pdm) and A/Victoria/3/75 (H3N2) are biosafety level (BSL)-2 viruses while A/Vietnam/1203/2004 (H5N1) is a BSL-3+ select agent. Appropriate safety and operational procedures were followed for both BSLs.
2.5 Vaccine formulation and immunization schedule
AuNPs with a diameter of 12 nm were synthesized as described (Tao et al., 2014) by the Turkevich method (Kimling et al., 2006; Tkachenko et al., 2005) of reduction of gold III) chloride trihydrate (HAuCl4·3H2O) with trisodium citrate dihydrate (Na3C6H5O7.2H2O). Tween 20 (0.1%) was added into AuNP suspension to improve the stability of AuNPs. Vaccine dose containing M2e and sCpG was prepared as described previously (Tao et al., 2014). Briefly, tween (0.1%) was added to synthesized AuNPs, they were centrifuged to remove excess supernatant, and M2e was added dropwise. After overnight equilibration, CpG was added.
All vaccinated mice received 8.2 μg M2e, 60 μg AuNPs and 20 μg sCpG per animal. As shown in our previous study (Tao et al., 2014), out of the 8.2 μg M2e approximately 1.25 μg of M2e is bound to AuNPs while the remaining M2e is present in soluble form. CpG is present in soluble form (sCpG) and is not attached to AuNPs.
All formulations were administered in a volume of 25 μl given drop wise to the nares at day 1 and was repeated (boosted) at day 21.
2.6 Influenza virus challenge experiment
The virus challenge study for each influenza A strain contained two groups of mice (vaccinated and placebo, n=12 or 13 per group). Vaccinated mice received the vaccine as described above, while the placebo group received sterile saline solution. Blood was collected through cheek bleed at days 0, 21, and 42. The collected sera was processed and stored at −20 °C until analysis.
Mice were anesthetized by intraperitoneal (i.p.) injection of ketamine/xylazine (50 mg/kg//5 mg/kg) prior to challenge by the intranasal route with 3xLD50 of influenza A/California/04/2009 (H1N1pdm) virus per mouse; or 3xLD50 of influenza A/Victoria/3/75 (H3N2) virus per mouse; or approximately 1xLD90 of influenza A/Vietnam/1203/2004 (H5N1) virus per mouse. All mice were administered virus for challenge on day 42 post first immunization. Mice were weighed prior to virus challenge and then every other day thereafter to assess the effects of vaccination on ameliorating weight loss due to virus infection. All mice were also observed for morbidity and mortality through day 19 post-challenge. Death was used as the experimental end point.
2.7 ELISAs
M2e-specific antibodies generated by immunized mice were measured by ELISA as described before (Tao and Gill, 2015). Briefly, ninety-six well plates (Maxisorp, Nunc) were coated overnight with M2e peptide in phosphate buffered saline (PBS). After blocking with bovine serum albumin, serum from individual mice at 1:1600 dilution was added to the wells. Horseradish peroxidase (HRP)-labeled goat anti-IgG antibody was used to detect mouse IgG antibody. Color was developed with OPD as a substrate, the reaction was stopped with phosphoric acid, and absorbance at 492 nm was recorded with SpectraMax Plus384 microplate reader (Molecular Devices LLC., CA, USA). Serum dilution of 1:1600 was selected for total IgG analysis because in two previous studies we have shown that this dilution falls in the linear range of serum dilution curves of mice vaccinated with the same vaccine formulation at the same schedule (Tao and Gill, 2015; Tao et al., 2014).
For measurement of IgG1 and IgG2a subtypes, the above procedure was repeated by using serum of mice diluted to 1:1600, and by using HRP-labeled anti-IgG1 and anti-IgG2a secondary antibodies. We chose 1:1600 serum dilution for IgG1 and IgG2a analysis based on our previous study (Tao et al., 2014).
2.8 Measurement of local mucosal immune responses
To measure local mucosal and cellular immune responses, a cohort of mice with two groups (vaccinated and naive, n=5 per group) was vaccinated on days 0 and 21, and on day 42, nasal and lung washes were collected to measure local IgG and IgA antibodies. A dilution of 512 and 64 was selected to determine anti-M2e IgG and IgA antibodies, respectively, because these dilutions lie in the linear range of both nasal and lung wash dilution curves (supplementary data, Fig S1). HRP-conjugated anti-IgG and anti-IgA secondary antibodies were used (Southern Biotech, Birmingham, AL, USA). Spleen, lung and bone marrow of these mice were used to prepare single cell suspensions, which were used to analyze cytokine and chemokine production after in vitro stimulation of the cells with the vaccine formulation.
2.9 Measurement of B cells and cytokines/chemokines
In another experiment, mice (n=5 per group) were vaccinated on day 0 and day 21, and 10 days later (day 31), single cell suspensions from their lung, spleen and bone marrow were used to examine B cell populations using flow cytometry to determine if any differences existed between the local (lung) and distal (spleen and bone marrow) organs. Naïve mice (n=5 per group) were used as controls.
Single cell suspensions from lung, spleen, and bone marrow were obtained by passing tissue pieces through a 40-μm mesh filter for mechanical cell dissociation. Lymphocytes from lung were enriched by density-gradient centrifugation with Nycodenz™ 1.077 (Cosmo Bio USA, Inc., CA, USA).
Isolated cells were cultured in triplicates at a concentration of 1×106 cells per well in a 96 well plate for 72 h at 37°C with 5% CO2 with either 3.25 μg/ml of M2e, 24 μg/ml of AuNPs, or 5μg/ml of concanavalin A (positive control), all in RPMI media supplemented with 10% heat-inactivated fetal calf serum and penicillin-streptomycin antibiotics. The RPMI culture media was used as the negative control. Supernatant of cultured cells were collected after 72 h of culture for cytokine and chemokine analysis using a chemiluminescent ELISA-based assay according to the manufacturer’s instructions (Quansys Biosciences Q-Plex™ Array, Logan, UT). Each supernatant was tested for the following: interleukin-1α (IL-1α), IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-10, IL-12p70, IL-17, monocyte chemoattractant protein-1 (MCP-1), gamma interferon (IFN-γ), tumor necrosis factor alpha (TNFα), macrophage inflammatory protein-1α (MIP-1α), granulocyte macrophage colony stimulating factor(GM-CSF), and regulated upon activation, normal T cell expressed and secreted (RANTES). Cytokine and chemokine titers are reported in pg/ml.
Cell surface staining of single cell suspensions was analyzed with flow cytometry using the following antibodies: PE-CF594 Anti-mouse CD45 (30-F11; BD), Brilliant Violet 711 anti-mouse CD3 (17A2, Biolegend), Alexa Fluor 488 anti-mouse CD19 (6D5; Biolegend), Brilliant Violet 605 anti-mouse CD86 (GL-1, Biolegend) and Alexa Fluor 700 anti-mouseB220 (RA3-6B2, Biolegend). Samples were acquired using LSRFortessa (BD) and analyzed with FlowJo software.
2.10 Acetone fixation of MDCK cell monolayers
MDCK cells were seeded into 96-well tissue culture plates at a density of 5×106 cells per plate in Minimal Essential Media (MEM) (Hyclone, Logan, UT, USA) containing 5% fetal bovine serum (Hyclone, Logan, UT, USA) and incubated at 37°C in a CO2 incubator. After incubation for 24 h, the MDCK cells were washed twice with MEM and then infected with approximately 50 cell culture infectious doses (CCID50) of influenza A/California/04/2009 (H1N1pdm), A/Victoria/3/75 (H3N2), or A/Vietnam/1203/2004 (H5N1) virus. Following incubation for 24 h, the infected MDCK cells were washed twice with PBS, and then fixed by the addition of cold 70% acetone. The monolayers were fixed to the plates by incubation with acetone at room temperature for 20 min. After fixation, the plates were rinsed once with PBS. After acetone fixation and washing, the 96-well plates were sealed with parafilm and stored at 4 °C until use. Likewise, uninfected MDCK cells were also acetone fixed. Plates were used within 72 h of fixation.
2.11 Immunofluorescence antibody assay (IFA)
Acetone-fixed, infected and un-infected MDCK cells were blocked with 100 μl 1% BSA-PBS for 2 h at room temperature. After blocking, 50 μl pooled sera (1:50 dilution in 0.1% BSA-PBST) was added to wells, plates were incubated for 1 h at room temperature, washed with PBST, 50 μl Alexa-Fluor 488-labeled secondary antibody (10 μg/ml) was added into wells and incubated for 1 h at room temperature. The plates were then washed with PBST three times and fluorescence was visualized using Nikon TI-FL microscope (Nikon, Melville, NY, USA).
2.12 ELISA using infected MDCK cells
Acetone-fixed, infected and un-infected MDCK cells were blocked with 100 μl 1% BSA-PBS for 2 h at room temperature. After blocking, 50 μl pooled sera (1:200 dilution in 0.1% BSA-PBST) was added to wells and plates were incubated for 1 h. Then plates were incubated with 50 μl of 1:4000 dilution of horseradish peroxidase (HRP)-labeled anti-IgG antibody for 1 h. Plates were washed three times with PBST between each step using the microplate washer. Color was developed by adding OPD substrate for 10 min. Then reaction was terminated by adding 50 μl of 3M phosphoric acid. Absorbance at 492 nm was recorded using a microplate reader.
2.13 Statistical analysis
All statistical analyses were performed using GraphPad Prism for windows, version 6.0 (GraphPad Software, Inc., La Jolla, CA). Comparison of antibody and cytokine titers between groups of mice was performed with two-way analysis of variance (ANOVA) and a Bonferroni test at a value of p<0.05 for statistical significance. Kaplan-Meier survival curves were generated and compared by the Log-rank (Mantel-Cox) test followed by pairwise comparison using the Gehan-Breslow-Wilcoxon test. The mean body weights were analyzed by analysis of variance (ANOVA) followed by Tukey’s multiple comparison test.
3. RESULTS
3.1 Design strategy for AuNP-M2e+sCpG vaccine formulation
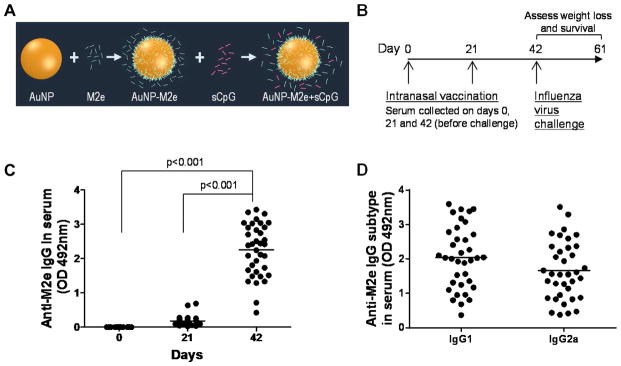
It has been shown in several studies that M2e by itself is a poor immunogen (De Filette et al., 2008; Li et al., 2011). Thus, we sought to enhance the immunogenicity of M2e by creating a particle comprising of a AuNP core with M2e attached to its surface. Gold enables use of a simple one-step mixing process to attach M2e to its surface by exploiting the ‘gold-thiol’ chemistry, eliminating the need for multi-step conjugation and purification procedures. To exploit the ‘gold-thiol’ chemistry for attachment of M2e to the AuNP surface, an extra cysteine residue was added to the M2e sequence at it’s ‘C’ terminus (acetylated-SLLTEVETPIRNEWGSRSNDSSDC*-amidated; C*: cysteine added to help attach M2e to AuNPs). The ‘thiol’ in the side-chain of cysteine can allow M2e to bind to the AuNP surface upon simple mixing of M2e in an aqueous AuNP solution. We also changed the two internal cysteines to serines (underlined) to prevent aggregation of gold nanoparticles during vaccine formulation. This substitution has previously been shown to not impair the ability of monoclonal antibodies raised against M2e with cysteines to bind to it (Mozdzanowska et al., 2003). We also included sCpG as a soluble adjuvant to further stimulate the innate immune system. sCpG is a short synthetic DNA molecule rich in CG motifs that can stimulate toll-like receptor 9 (TLR 9) (Zabel et al., 2013). The schematic of this nanoparticle-based vaccine is shown in Fig 1A.
Fig. 1.
(A) Scheme of vaccine design. M2e is conjugated to AuNPs. By keeping M2e in excess in the solution, complete surface-coverage of AuNPs with M2e is ensured at all times. Soluble CpG (sCpG) is an unmethylated CG-rich oligonucleotide found in viral and bacterial genome. sCpG, is a known TLR-9 agonist, which enhances the immune response and is used as an adjuvant in the formulation. We used CpG 1826: 5′-TCCATGACGTTCCTGACGTT-3′ with phosphorothioate linkages. sCpG stays in solution and does not attach to AuNPs. (B) Vaccination schedule. Mice (n=37) were vaccinated with AuNP-M2e+sCpG on days 0 and 21, and serum was collected on days 0, 21 and 42 for analysis. (C) M2e-specific IgG antibody response in mouse serum. M2e-specific IgG antibody in 1:1600 diluted serum of individual mice at days 0, 21 and 42. Each circle* represents an individual animal and the horizontal bar represents the mean. (D) M2e-specific IgG1 and IgG2a response in mouse serum. M2e-specific IgG1 and IgG2a antibodies in 1:1600 diluted serum of individual mice at day 42. Each circle* represents an individual animal and the horizontal bar represents the mean. Optical density (OD) of the ELISA reaction was measured at 492 nm wavelength. *: Serum of one vaccinated mouse was not available in sufficient quantity and it was thus not included in the ELISAs.
3.2 Stimulation of M2e-specific antibodies in mouse serum by AuNP-M2e+sCpG
In our previous study we had demonstrated that intranasal vaccination with AuNP-M2e+sCpG stimulates strong M2e-specific IgG response in serum after two doses (Tao et al., 2014). In the present study mice were immunized with AuNP-M2e+sCpG following the same vaccination schedule (shown in Fig 1B). It can be seen from Fig 1C that after two doses there is a significant increase in anti-M2e serum IgG antibodies (p<0.001), and all mice responded to the vaccine (mean optical density (OD) of 2.25±0.76), indicating good reproducibility. It has previously been shown that upon vaccination with M2e, protection against influenza challenge correlates with levels of IgG2a subtype (El Bakkouri et al., 2011). Thus, we evaluated the relative proportion of IgG1 and IgG2a, and found that AuNP-M2e+sCpG vaccine stimulates both IgG1 and IgG2a response (Fig 1D). The ratio of the mean OD values of IgG1 to IgG2a was 1.2 at a serum dilution of 1:1600, which was in the linear range for both antibodies. To validate that OD values can be used to compare the relative amounts of IgG1 and IgG2a we performed ELISAs with plates coated with purified mouse IgG1 and IgG2a. Fig S2 (supplementary data) shows that when plates are coated with purified mouse IgG1 or IgG2a, and then detected with secondary anti-IgG1 or anti-IgG2a Abs, similar OD values are obtained. Further, insignificant cross-reactivity between anti-IgG1 and IgG2a, or anti-IgG2a and IgG1 antibodies was observed.
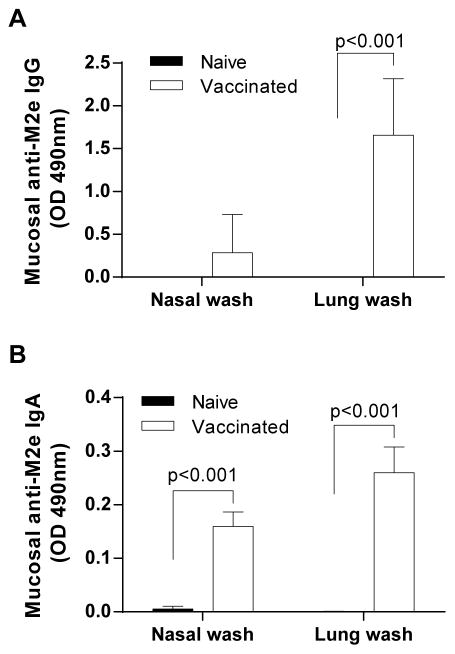
3.3 Stimulation of M2e-specific antibodies in nasal and lung washes by AuNP-M2e+sCpG
Mucosal immunity is important for antiviral immunity against influenza virus. In order to evaluate whether i.n. immunization with AuNP-M2e+sCpG vaccine could induce mucosal antibody response, M2e-specfic IgG and IgA antibodies from nasal and lung washes before challenge were measured using ELISA. As shown in Fig 2A, significantly higher levels of anti-M2e IgG were observed in lung washes compared to naïve mice, while in nasal washes anti-M2e IgG was not significantly enhanced. Importantly though, significant enhancement in anti-M2e IgA was seen in both nasal and lung washes in the vaccinated group compared to the naïve mice (p<0.001) (Fig 2B).
Fig. 2. M2e-specific IgG and IgA antibody response in nasal wash and lung wash.
(A) M2e-specific IgG antibody in 1:512 diluted nasal and lung wash of individual mice. (B) M2e-specific IgA antibodies in 1:64 diluted nasal and lung wash of individual mice. The vertical columns represent the mean and the error bars represent standard deviation. Optical density (OD) of the ELISA reaction was measured at 492 nm wavelength.
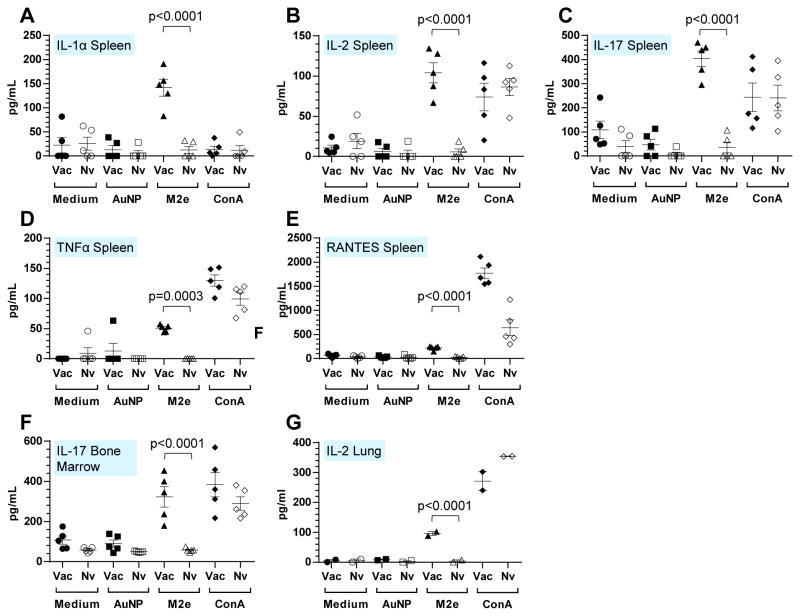
3.4 Determination of M2e specific cellular responses
The immune response generated in mice upon i.n. immunization with the AuNP-M2e+sCpG vaccine was further characterized by measuring ex vivo cytokine production from splenocytes, bone marrow cells, and lung cells after stimulation with the M2e peptide. As described in the method section, a total of 16 cytokine were tested. The splenocytes (Fig 3A–E) of vaccinated mice as compared to naïve mice exhibited a significant increase in cytokine levels for IL-1α, IL-2, IL-17, TNFα and RANTES after ex vivo stimulation with the M2e peptide but not after AuNP or media-alone stimulation. Similarly, increase in IL-17 secretion was seen for bone marrow cells, and IL-2 secretion for lung cells of vaccinated mice after stimulation with M2e (Fig 3F and G). Other cytokines did not exhibit a statistically significant change between vaccinated and naïve mice.
Fig. 3. M2e specific T cell response in spleen, bone marrow and lung.
Cytokine production after in vitro re-stimulation by using medium, AuNP, M2e peptide and ConA was analyzed by cytokine multiplex assay. Study included two groups (n=5 per group, vaccinated and naïve). (A) IL-1α, (B) IL-2, (C) IL-17, (D) TNFα and (E) RANTES in spleen; (F) IL-17 in bone marrow. Each symbol represents an animal and the horizontal bar represents the mean. (G) IL-2 in lung. Samples were prepared as duplicates.
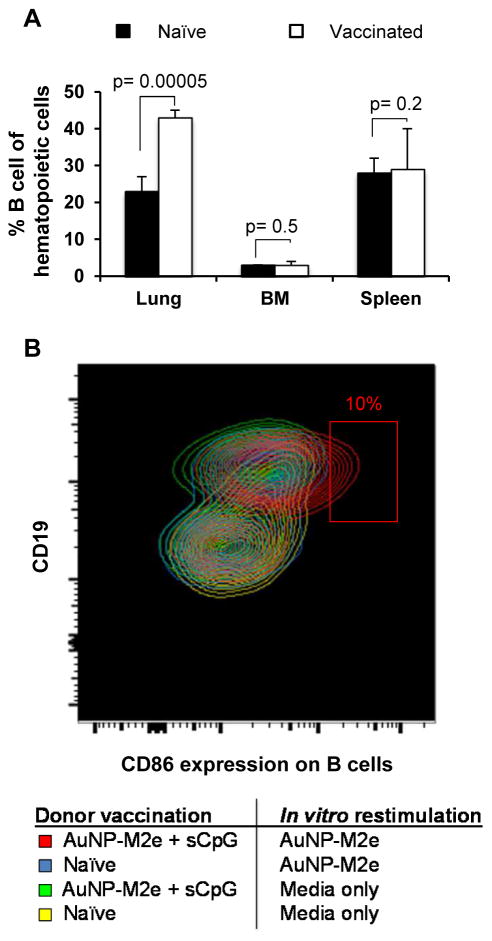
3.5 Assessment of B cells in the lung, spleen, and bone marrow of vaccinated mice
To examine the effect of AuNP-M2e+sCpG vaccination on lung B cells in comparison to B cells in organs not directly exposed to the vaccine, single cell suspensions of the lung, spleen, and bone marrow were generated ten days after the last vaccination, and then incubated in vitro with media alone or AuNP-M2e for 72 hours. The study included two groups of mice (vaccinated and naive, n=5 per group). In each sample, the B cell frequency, and the B cell activation status was assessed using flow cytometry. The B cell activation status was determined by measurement of CD86 expression, which is a potent costimulatory molecule that is induced on antigen presenting cells upon their activation (Sharpe, 2009). As expected, after re-stimulation with M2e, B cell numbers increased in lung cells of mice vaccinated with AuNP-M2e+sCpG, compared to naïve controls (Fig 4A), however, no differences in B cell frequency were observed in spleen or bone marrow derived cells of vaccinated vs. naïve control mice. In addition, a subset (about 10%) of AuNP-M2e re-stimulated lung B cells upregulated the costimulatory molecule CD86 (B7.2), while B cells stimulated with media-only did not. This upregulation was seen only if the cells were isolated from the lungs of previously AuNP-M2e+sCpG vaccinated animals but not naïve mice (Fig 4B). Altogether, this data demonstrates that the frequency of lung B cells increases in Balb/c mice upon intranasal vaccination with AuNP-M2e+sCpG, and that an enhanced percentage of M2e-specific B cells from the lungs of AuNP-M2e+sCpG vaccinated donor mice respond rapidly to in vitro AuNP-M2e re-stimulation. As BALB/c mice are polyclonal, the expected frequency of M2e-responsive B cells is low, but should increase slightly after vaccination, which is what we observe.
Fig. 4. B cells are increased in lungs of AuNP-M2e+sCpG vaccinated mice, and respond rapidly to in vitro restimulation with AuNP-M2e.
B cell frequencies and CD86 expression was determined by flow cytometry. Hematopoietic cells were identified as CD45+ cells, and B cells as CD45+CD19+B220+CD3–cells. Study included two groups (n=5 per group, vaccinated and naïve). (A) Average frequency and standard deviation of B cells in the lungs, spleens and bone marrow (BM) of naïve vs. AuNP-M2e+sCpG vaccinated donor mice, regardless of their in vitro re-stimulation. (B) CD86 expression was determined on B cells isolated from naïve or AuNP-M2e+sCpG vaccinated donor lungs after 96 hours of media control or AuNP-M2e restimulation in vitro.
3.6 Serum IgG induced by AuNP-M2e+sCpG can bind M2 on influenza-infected MDCK cells
Homotetrameric M2 protein is incorporated only in small numbers in influenza virions (Holsinger and Alams, 1991; Lamb et al., 1985), but is expressed in larger numbers on cells infected with the influenza virus (Lamb et al., 1985). Anti-M2e antibodies have been shown to not prevent infection in vitro, but to only reduce plaque formation for some but not all influenza strains (Zebedee and Lamb, 1988). It has been suggested that the binding of anti-M2e antibodies to M2 on infected cells and not mature influenza virions potentiates the protective effect from M2e-based vaccination. Accordingly, we examined the ability of anti-M2e antibodies to bind M2 expressed on virus-infected cells using IFA and ELISA.
3.6.1 IFA using MDCK-infected cells
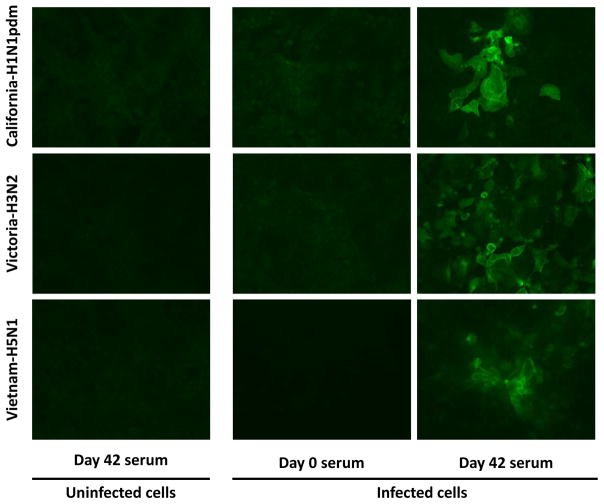
Fig 5 shows that serum from vaccinated mice collected 3 weeks after the second vaccine dose, i.e. at day 42, shows strong fluorescence staining of MDCK cells infected with A/California/04/2009 (H1N1pdm), A/Victoria/3/75(H3N2), and A/Vietnam/1203/2004 (H5N1), but not with uninfected MDCK cells. On the other hand, the pre-immune serum from these mice did not exhibit any fluorescence staining upon incubation with infected MDCK cells. Altogether, this result shows that anti-M2e antibodies stimulated by AuNP-M2e+sCpG can bind to M2 expressed on the surface of virus-infected cells.
Fig. 5. Immunofluorescence Assay (IFA) on influenza-infected MDCK cells.
Acetone-fixed-influenza virus-infected MDCK cells were used to evaluate the ability of antibodies generated by AuNP-M2e+sCpG to bind M2 expressed on cells. Immunofluorescence assays were performed using day 0 and day 42 mouse serum at 1:50 dilution after pooling sera of vaccinated mice. Uninfected MDCK cells were used as a negative control. California-H1N1pdm: A/California/04/2009 (H1N1pdm); Victoria-H3N1: A/Victoria/3/75 (H3N2); Vietnam-H5N1: A/Vietnam/1203/2004 (H5N1).
3.6.2 ELISA using MDCK-infected cells
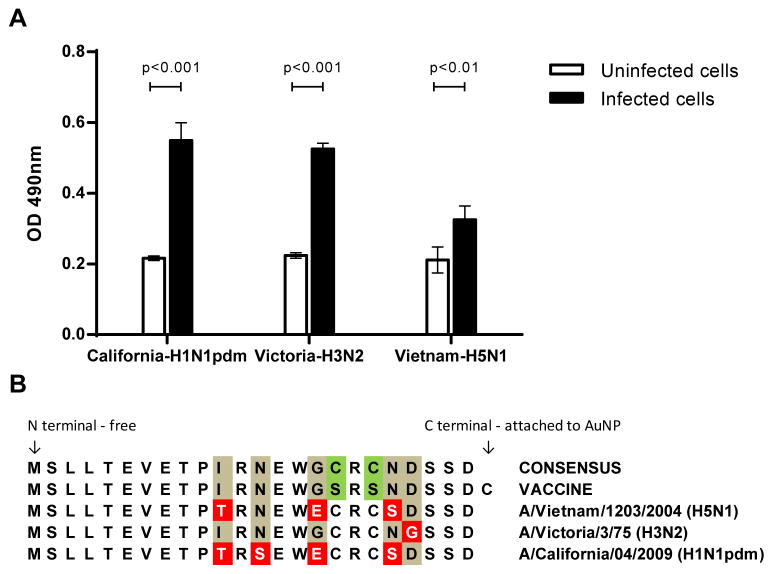
Binding of serum anti-M2e IgG to M2 expressed on the surface of virus-infected cells was further confirmed by performing ELISA using MDCK cells infected with A/California/04/2009 (H1N1pdm), A/Victoria/3/75(H3N2), or A/Vietnam/1203/2004 (H5N1) as an immune adsorbant. This ELISA shows that serum IgG antibodies from mice vaccinated with AuNP-M2e+sCpG have a significantly higher binding to infected MDCK cells as compared to their non-specific binding to uninfected MDCK cells (Fig 6A).
Fig. 6. Cell-based ELISA using influenza-infected MDCK cells.
MDCK cells that were acetone fixed before infection or after infection with A/California/04/2009 (H1N1pdm) (California-H1N1pdm), A/Victoria/3/75 (H3N2) (Victoria-H3N2), or A/Vietnam/1203/2004(H5N1) (Vietnam-H5N1) were used as immunoabsorbants to evaluate the ability of antibodies induced by the AuNP-M2e+sCpG vaccine to bind M2 in its homotetrameric form expressed on infected cells. (A) Day 42 mouse serum was used at 1:50 dilution after pooling sera of vaccinated mice. The vertical columns represent the mean and the error bars represent standard deviation. Optical density (OD) of the ELISA reaction was measured at 492 nm wavelength. (B) Amino acid sequences of: consensus M2e, M2e used for immunization, and M2e for the three viruses used to infect MDCK cells.
A comparison between the amino acid sequences of M2e used as a vaccine, the consensus M2e sequence, and M2e found on the three different influenza strains is given in Fig 6B. The M2e sequences of A/California/04/2009 (H1N1pdm), A/Victoria/3/75(H3N2), and A/Vietnam/1203/2004 (H5N1) differ from our M2e vaccine sequence by four, one, and three residues, respectively, in addition to the purposeful modifications we made to consensus M2e (cysteine to serine changes and inclusion of C-terminal cysteine). Despite these differences, the antibodies stimulated by the AuNP-M2e+sCpG vaccine could recognize M2 of these three viruses expressed on infected MDCK cells.
3.7 AuNP-M2e+sCpG protects mice against lethal challenges with influenza A H1N1, H3N2 and H5N1 subtypes
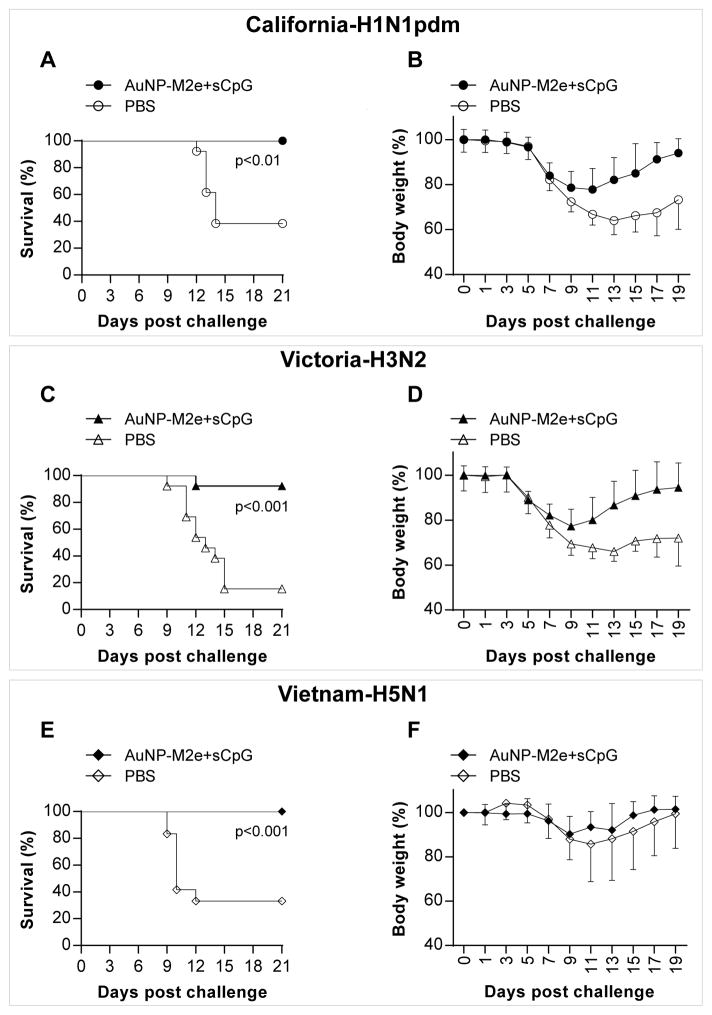
Next we assessed the ability of AuNP-M2e+sCpG vaccine to protect mice against lethal infection with influenza A/California/04/2009 (H1N1pdm), A/Victoria/3/75 (H3N2), and A/Vietnam/1203/2004 (H5N1) viruses. Vaccinated (n=12) and placebo (n=13) groups of mice were challenged with 3xLD50 of influenza A/California/04/2009 (H1N1pdm) virus per mouse, 3xLD50 of influenza A/Victoria/3/75 (H3N2) virus per mouse (n=13), or 1xLD90 of influenza A/Vietnam/1203/2004 (H5N1) virus per mouse (n=12). The following survival rates were observed (Fig 7): 100% (12/12 vaccinated mice) vs 38% (5/13 placebo mice, p<0.01) for A/California/04/2009 (H1N1pdm) challenge; 92 % (12/13 vaccinated mice) vs 15 % (2/13 placebo mice, p<0.01) for A/Victoria/3/75 (H3N2) challenge; and 100 % (12/12 vaccinated mice) vs 33 % (4/12 placebo mice, p<0.001) for A/Vietnam/1203/2004 (H5N1) challenge. Both vaccinated and placebo mice exhibited weight loss after virus challenge. However, while the vaccinated mice recovered their body weight, the placebo mice that had survived the challenge continued to have significantly lower body weight 19 days post challenge (Fig 7). Thus, the AuNP-M2e+sCpG vaccine administered intranasally could protect mice against three different influenza A subtypes.
Fig. 7. Effectiveness of AuNP-M2e+sCpG vaccine in mice against H1N1, H3N2 and H5N1 influenza A subtypes.
Mice were vaccinated with AuNP-M2e+sCpG or saline (placebo) on days 0 and 21, and challenged on day 42 with 3xLD50 of California-H1N1pdm: A/California/04/2009 (H1N1pdm); or 3xLD50 of Victoria-H3N1: A/Victoria/3/75 (H3N2); or 1xLD90 of Vietnam-H5N1: A/Vietnam/1203/2004 (H5N1). (A) Survival rate and (B) body weight after pdm-CA2009-H1N1 virus infection. n=12 in AuNP-M2e+sCpG group, n=13 in placebo group. (C)Survival rate and (D) body weight after Victoria-H3N2 virus infection. n=13 in both groups. (E)Survival rate and (F) body weight after Vietnam-H5N1 virus infection. n=12 in both groups.
4. DISCUSSION
We have previously seen that the survival rate of mice vaccinated with AuNP-M2e+sCpG upon challenge with approximately 5xLD50 of influenza A/PR/8/34 (H1N1) was 100% (Tao et al., 2014). Encouraged with these findings, we performed the current study to investigate whether AuNP-M2e+sCpG could provide protection against other influenza virus subtypes. To provide a stringent test for the effectiveness of AuNP-M2e+sCpG as a universal influenza A vaccine, we decided to include the 2009 pandemic strain A/California/04/2009 (H1N1pdm), the A/Victoria/3/75 (H3N2) strain, and the highly pathogenic avian influenza strain A/Vietnam/1203/2004 (H5N1), which has been implicated as a potential pandemic threat (Peiris et al., 2007).
In our previous publication we had demonstrated that M2e alone or M2e mixed with sCpG elicited a poor anti-M2e IgG response, while M2e attached to AuNPs (but without sCpG) induced a moderate immune response with partial protection (Tao et al., 2014). We also observed that only when M2e was attached to AuNPs, and sCpG was included as an adjuvant did a robust and protective immune response get generated. Similarly, in the current study, we found that intranasal immunization with AuNP-M2e+sCpG induced robust systemic M2e specific IgG antibody response. The IgG1 to IgG2a ratio was found to be 1.2, which is within the range of 0.33 to 2 observed in other studies. These studies have used different carriers for M2e and deliver the vaccines via different routes (intramuscular, intranasal, subcutaneous or microneedle patch) (Kim et al., 2013; Rappazzo et al., 2016; Wang et al., 2014; Wang et al., 2013). The intranasal vaccination also promoted local production of IgG in lungs and IgA antibodies in nasal and lung washes of mice. Similar results have also been previously reported wherein mucosal immunity was stimulated when M2e-based vaccines were administered intranasally (Chowdhury et al., 2014; Wang et al., 2013; Zhang et al., 2009). It should be noted that mucosal IgA antibody in the upper respiratory tract plays a major role in preventing the initial viral infection, whereas the IgG antibody can offer further protection against infection of lungs (Clements et al., 1986; Renegar et al., 2004). As demonstrated by IFA and MDCK-based ELISA, serum IgG antibodies were found to bind to the homotetrameric form of M2 from influenza strains A/California/04/2009 (H1N1pdm), A/Victoria/3/75 (H3N2), and A/Vietnam/1203/2004 (H5N1). Using ELISA we have previously also shown that serum from mice vaccinated with AuNP-M2e+sCpG can bind to M2 expressed on virions of A/Philippines/2/82 (H3N2), A/PR/8/34 (H1N1), and A/WSN/1933(H1N1)(Tao et al., 2014). Together, these results demonstrate that the anti-M2e antibodies stimulated by AuNP-M2e+sCpG can bind to the homotetrameric form of M2 from different influenza A subtypes. As expected, we were also able to demonstrate that B cells in the lungs of mice that had previously been intranasally vaccinated with AuNP-M2e-sCpG exhibited immunological memory to AuNP-M2e re-challenge in vitro, as visualized by the upregulation of the potent co-stimulatory molecule CD86 on the surface of 10% of lung B cells. Interestingly, while B cells in the lung expanded upon inhalation of the vaccine containing the cognate antigen M2e (without the adjuvant), the B cells in the bone marrow, the site where B cells develop, and in the spleen, an organ rich in B cells that filters blood borne pathogens, did not, suggesting that intranasal vaccination with AuNP-M2e-sCpG elicits influenza A M2e-specific humoral immunity specifically in the lungs, which could be beneficial because lungs are a potential site for future influenza virus encounter.
Vaccination of mice with AuNP-M2e+sCpG not only induced strong humoral responses but also enhanced cellular responses. Significantly higher level of IL-2 and TNFα were detected in response to stimulation with M2e peptide in vaccinated mice compared to naïve mice. This result is consistent with a study by Wang et al. (Wang et al., 2008) who used an M2e vaccine incorporating flagellin, a TLR-5 agonist as an adjuvant, and other studies that have used CpG-ODN as an adjuvant (Decker et al., 2000; Knuschke et al., 2013). It is known that IL-2 plays a central role in activation of T cells and Natural Killer cells while TNFα is produced by activation of macrophages and is effective in defense against intracellular pathogens (Elkon et al., 1997; Granucci et al., 2004; Lan et al., 2008). Although, the IgG1 to IgG2a ratio of 1.2 suggests a Th2 response, higher levels of IL-2 also indicate a Th1 response. Furthermore, the induction of chemokine RANTES in spleen is reported to be very important for allowing immune cells to migrate towards the sites of infection (Kaufmann et al., 2001). A high amount of IL-17 was observed in both spleen and bone marrow. IL-17 is known to help recruit neutrophils (Miyamoto et al., 2003), which could enhance protection through phagocytosis. Indeed, IL-17 has been detected in the lungs of mice after influenza infection. It was observed that depletion of IL-17 with anti-IL-17 antibody led to higher mortality from influenza A infection (Hamada et al., 2009). We also observed an increase in IL-1α after in vitro splenocyte restimulation. IL-1α is a pro-inflammatory cytokine, which is released from necrotizing cells. IL-1α helps to recruit neutrophils (Rider et al., 2011) and it has been shown to help clear influenza infection (Schmitz et al., 2005). Altogether, a combination of enhanced M2e specific IgG and IgA antibody titers and an increased production of cytokine levels coincided with protection against lethal challenge with influenza A/California/04/2009 (H1N1pdm), A/Victoria/3/75 (H3N2), and the highly pathogenic avian influenza virus A/Vietnam/1203/2004 (H5N1) in mice vaccinated with AuNP-M2e+sCpG. This result is significant considering that the M2e sequence that we used as the vaccine differed by up to four amino acids from the M2 sequence of the challenge strains. This suggests that the vaccine AuNP-M2e+sCpG induces broadly binding antibodies against M2 of different influenza subtypes. To study this aspect further, in future experiments it will be important to assess the ability of serum from vaccinated mice to cross react with M2e peptides from other influenza subtypes that are considerably more different in amino acid sequence than the M2e peptide used as the vaccine. It will also be important to test the AuNP-M2e+sCpG formulation against other influenza A subtypes, in mice, and in other animal models including ferrets.
An important aspect of our vaccine formulation is that it is completely synthetic and easily scalable for mass production. AuNPs, M2e-peptide, and CpG can be readily synthesized in large quantities using chemistry-based processes, and the vaccine can then be prepared by simply mixing the different components. Furthermore, because the formulation is delivered as intranasal drops, it is also painless, needle-free, and has potential for self-administration. In conclusion, the data presented here supports further evaluation of AuNP-M2e+sCpG as a candidate universal influenza A vaccine.
Supplementary Material
Highlights.
M2e-conjugated gold nanoparticles with CpG as an adjuvant generated robust anti-M2e serum IgG antibodies in mice.
Anti-M2e IgG and IgA antibodies were also stimulated in nasal and lung mucosal secretions.
Anti-M2e IgG antibody could recognize homotetrameric M2 protein of H1N1, H3N2, and H5N1 subtypes.
Generated anti-M2e immunity could protect mice from challenge with influenza 2009 pandemic H1N1, H3N2, and H5N1 subtypes.
Acknowledgments
Research reported in this publication was supported in part by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Number R21AI099575. Preclinical Services offered by the NIAID were utilized for this study (contract HHSN272201000039I from the Respiratory Diseases Branch, NIAID, NIH to Utah State University). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
GLOSSARY
- AuNPs
gold nanoparticles
- M2e
extracellular domain of matrix protein 2
- CpG
oligonucleotides that contain unmethylated CpG dinucleotides motifs (C:cytosine; G:guanine)
- sCpG
soluble form of CpG that is not attached to gold nanoparticles
- CCID50
Cell culture infective dose 50%
- IgG
Immunoglobulin G
- IgG1
Immunoglobulin G1
- IgG2a
Immunoglobulin G2a
- LD50
Lethal dose 50%
- MDCK
Madin-Darby canine kidney
- OD
Optical density
- pdm
pandemic
- pdm-CA2009-H1N1
pandemic A/California/04/2009 (H1N1)
- Victoria-H3N1
A/Victoria/3/75 (H3N2)
- Vietnam-H5N1
A/Vietnam/1203/2004 (H5N1)
Footnotes
FINANCIAL DISCLOSURE/CONFLICT OF INTEREST
The authors have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen PJ. Avian influenza pandemic: not if, but when. Pediatr Nurs. 2006;32:76–81. [PubMed] [Google Scholar]
- Chowdhury MY, Li R, Kim J-H, Park M-E, Kim T-H, Pathinayake P, Weeratunga P, Song MK, Son H-Y, Hong S-P. Mucosal Vaccination with Recombinant Lactobacillus casei-Displayed CTA1-Conjugated Consensus Matrix Protein-2 (sM2) Induces Broad Protection against Divergent Influenza Subtypes in BALB/c Mice. PLoS One. 2014:9. doi: 10.1371/journal.pone.0094051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements M, Betts R, Tierney E, Murphy B. Serum and nasal wash antibodies associated with resistance to experimental challenge with influenza A wild-type virus. J Clin Microbiol. 1986;24:157–160. doi: 10.1128/jcm.24.1.157-160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawood FS, Iuliano AD, Reed C, Meltzer MI, Shay DK, Cheng PY, Bandaranayake D, Breiman RF, Brooks WA, Buchy P, Feikin DR, Fowler KB, Gordon A, Hien NT, Horby P, Huang QS, Katz MA, Krishnan A, Lal R, Montgomery JM, Molbak K, Pebody R, Presanis AM, Razuri H, Steens A, Tinoco YO, Wallinga J, Yu H, Vong S, Bresee J, Widdowson MA. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect Dis. 2012;12:687–695. doi: 10.1016/S1473-3099(12)70121-4. [DOI] [PubMed] [Google Scholar]
- De Filette M, Martens W, Smet A, Schotsaert M, Birkett A, Londoño-Arcila P, Fiers W, Saelens X. Universal influenza A M2e-HBc vaccine protects against disease even in the presence of pre-existing anti-HBc antibodies. Vaccine. 2008;26:6503–6507. doi: 10.1016/j.vaccine.2008.09.038. [DOI] [PubMed] [Google Scholar]
- Decker T, Schneller F, Kronschnabl M, Dechow T, Lipford GB, Wagner H, Peschel C. Immunostimulatory CpG-oligonucleotides induce functional high affinity IL-2 receptors on B-CLL cells: costimulation with IL-2 results in a highly immunogenic phenotype. Exp Hematol. 2000;28:558–568. doi: 10.1016/s0301-472x(00)00144-2. [DOI] [PubMed] [Google Scholar]
- El Bakkouri K, Descamps F, De Filette M, Smet A, Festjens E, Birkett A, Van Rooijen N, Verbeek S, Fiers W, Saelens X. Universal Vaccine Based on Ectodomain of Matrix Protein 2 of Influenza A: Fc Receptors and Alveolar Macrophages Mediate Protection. J Immunol. 2011;186:1022–1031. doi: 10.4049/jimmunol.0902147. [DOI] [PubMed] [Google Scholar]
- Elkon KB, Liu CC, Gall JG, Trevejo J, Marino MW, Abrahamsen KA, Song X, Zhou JL, Old LJ, Crystal RG, Falck-Pedersen E. Tumor necrosis factor alpha plays a central role in immune-mediated clearance of adenoviral vectors. Proc Natl Acad Sci U S A. 1997;94:9814–9819. doi: 10.1073/pnas.94.18.9814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamblin SJ, Skehel JJ. Influenza hemagglutinin and neuraminidase membrane glycoproteins. J Biol Chem. 2010;285:28403–28409. doi: 10.1074/jbc.R110.129809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granucci F, Zanoni I, Pavelka N, van Dommelen SL, Andoniou CE, Belardelli F, Degli Esposti MA, Ricciardi-Castagnoli P. A contribution of mouse dendritic cell–derived IL-2 for NK cell activation. J Exp Med. 2004;200:287–295. doi: 10.1084/jem.20040370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H, Garcia-Hernandez MdlL, Reome JB, Misra SK, Strutt TM, McKinstry KK, Cooper AM, Swain SL, Dutton RW. Tc17, a Unique Subset of CD8 T Cells That Can Protect against Lethal Influenza Challenge. The Journal of Immunology. 2009;182:3469–3481. doi: 10.4049/jimmunol.0801814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsinger LJ, Alams R. Influenza virus M2 integral membrane protein is a homotetramer stabilized by formation of disulfide bonds. Virology. 1991;183:32–43. doi: 10.1016/0042-6822(91)90115-r. [DOI] [PubMed] [Google Scholar]
- Ingrole RS, Tao W, Tripathy JN, Gill HS. Synthesis and Immunogenicity Assessment of Elastin-Like Polypeptide-M2e Construct as an Influenza Antigen. Nano Life. 2014;4:1450004. doi: 10.1142/s1793984414500044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann A, Salentin R, Meyer RG, Bussfeld D, Pauligk C, Fesq H, Hofmann P, Nain M, Gemsa D, Sprenger H. Defense against influenza A virus infection: essential role of the chemokine system. Immunobiology. 2001;204:603–613. doi: 10.1078/0171-2985-00099. [DOI] [PubMed] [Google Scholar]
- Kim MC, Lee YN, Hwang HS, Lee YT, Ko EJ, Jung YJ, Cho MK, Kim YJ, Lee JS, Ha SH, Kang SM. Influenza M2 virus-like particles confer a broader range of cross protection to the strain-specific pre-existing immunity. Vaccine. 2014;32:5824–5831. doi: 10.1016/j.vaccine.2014.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MC, Song JM, OE, Kwon YM, Lee YJ, Compans RW, Kang SM. Virus-like particles containing multiple M2 extracellular domains confer improved cross-protection against various subtypes of influenza virus. Mol Ther. 2013;21:485–492. doi: 10.1038/mt.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimling J, Maier M, Okenve B, Kotaidis V, Ballot H, Plech A. Turkevich method for gold nanoparticle synthesis revisited. J Phys Chem B. 2006;110:15700–15707. doi: 10.1021/jp061667w. [DOI] [PubMed] [Google Scholar]
- Knuschke T, Sokolova V, Rotan O, Wadwa M, Tenbusch M, Hansen W, Staeheli P, Epple M, Buer J, Westendorf AM. Immunization with biodegradable nanoparticles efficiently induces cellular immunity and protects against influenza virus infection. J Immunol. 2013;190:6221–6229. doi: 10.4049/jimmunol.1202654. [DOI] [PubMed] [Google Scholar]
- Krammer F, Palese P, Steel J. Advances in universal influenza virus vaccine design and antibody mediated therapies based on conserved regions of the hemagglutinin. Curr Top Microbiol Immunol. 2015;386:301–321. doi: 10.1007/82_2014_408. [DOI] [PubMed] [Google Scholar]
- Lamb RA, Zebedee SL, Richardson CD. Influenza virus M2 protein is an integral membrane protein expressed on the infected-cell surface. Cell. 1985;40:627. doi: 10.1016/0092-8674(85)90211-9. [DOI] [PubMed] [Google Scholar]
- Lan RY, Selmi C, Gershwin ME. The regulatory, inflammatory, and T cell programming roles of interleukin-2 (IL-2) J Autoimmun. 2008;31:7–12. doi: 10.1016/j.jaut.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Li K, Luo J, Wang C, He H. α-Galactosylceramide potently augments M2e-induced protective immunity against highly pathogenic H5N1 avian influenza virus infection in mice. Vaccine. 2011;29:7711–7717. doi: 10.1016/j.vaccine.2011.07.136. [DOI] [PubMed] [Google Scholar]
- Miyamoto M, Prause O, Sjöstrand M, Laan M, Lötvall J, Lindén A. Endogenous IL-17 as a Mediator of Neutrophil Recruitment Caused by Endotoxin Exposure in Mouse Airways. The Journal of Immunology. 2003;170:4665–4672. doi: 10.4049/jimmunol.170.9.4665. [DOI] [PubMed] [Google Scholar]
- Mozdzanowska K, Feng J, Eid M, Kragol G, Cudic M, Otvos L, Jr, Gerhard W. Induction of influenza type A virus-specific resistance by immunization of mice with a synthetic multiple antigenic peptide vaccine that contains ectodomains of matrix protein 2. Vaccine. 2003;21:2616–2626. doi: 10.1016/s0264-410x(03)00040-9. [DOI] [PubMed] [Google Scholar]
- Neirynck S, Deroo T, Saelens X, Vanlandschoot P, Jou WM, Fiers W. A universal influenza A vaccine based on the extracellular domain of the M2 protein. Nat Med. 1999;5:1157–1163. doi: 10.1038/13484. [DOI] [PubMed] [Google Scholar]
- Peiris JM, De Jong MD, Guan Y. Avian influenza virus (H5N1): a threat to human health. Clin Microbiol Rev. 2007;20:243–267. doi: 10.1128/CMR.00037-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappazzo CG, Watkins HC, Guarino CM, Chau A, Lopez JL, DeLisa MP, Leifer CA, Whittaker GR, Putnam D. Recombinant M2e outer membrane vesicle vaccines protect against lethal influenza A challenge in BALB/c mice. Vaccine. 2016;34:1252–1258. doi: 10.1016/j.vaccine.2016.01.028. [DOI] [PubMed] [Google Scholar]
- Reid AH, Fanning TG, Janczewski TA, McCall S, Taubenberger JK. Characterization of the 1918 “Spanish” influenza virus matrix gene segment. J Virol. 2002;76:10717–10723. doi: 10.1128/JVI.76.21.10717-10723.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renegar KB, Small PA, Boykins LG, Wright PF. Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. J Immunol. 2004;173:1978–1986. doi: 10.4049/jimmunol.173.3.1978. [DOI] [PubMed] [Google Scholar]
- Rider P, Carmi Y, Guttman O, Braiman A, Cohen I, Voronov E, White MR, Dinarello CA, Apte RN. IL-1α and IL-1β Recruit Different Myeloid Cells and Promote Different Stages of Sterile Inflammation. The Journal of Immunology. 2011;187:4835–4843. doi: 10.4049/jimmunol.1102048. [DOI] [PubMed] [Google Scholar]
- Schmitz N, Kurrer M, Bachmann MF, Kopf M. Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. J Virol. 2005;79:6441–6448. doi: 10.1128/JVI.79.10.6441-6448.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell JR, Chou JJ. Structure and mechanism of the M2 proton channel of influenza A virus. Nature. 2008;451:591–595. doi: 10.1038/nature06531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth A, Ritchie FK, Wibowo N, Lua LH, Middelberg AP. Non-carrier nanoparticles adjuvant modular protein vaccine in a particle-dependent manner. PLoS One. 2015;10:e0117203. doi: 10.1371/journal.pone.0117203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe AH. Mechanisms of costimulation. Immunol Rev. 2009;229:5–11. doi: 10.1111/j.1600-065X.2009.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbarao K, Murphy BR, Fauci AS. Development of effective vaccines against pandemic influenza. Immunity. 2006;24:5–9. doi: 10.1016/j.immuni.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Tao W, Gill HS. M2e-immobilized gold nanoparticles as influenza A vaccine: Role of soluble M2e and longevity of protection. Vaccine. 2015;33:2307–2315. doi: 10.1016/j.vaccine.2015.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao W, Ziemer KS, Gill HS. Gold nanoparticle-M2e conjugate coformulated with CpG induces protective immunity against influenza A virus. Nanomedicine. 2014;9:237–251. doi: 10.2217/nnm.13.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkachenko A, Xie H, Franzen S, Feldheim DL. In: Assembly and Characterization of Biomolecule-Gold Nanoparticle Conjugates and Their Use in Intracellular Imaging NanoBiotechnology Protocols. Rosenthal SJ, Wright DW, editors. Humana Press; 2005. pp. 85–99. [DOI] [PubMed] [Google Scholar]
- Wang BZ, Quan FS, Kang SM, Bozja J, Skountzou I, Compans RW. Incorporation of membrane-anchored flagellin into influenza virus-like particles enhances the breadth of immune responses. J Virol. 2008;82:11813–11823. doi: 10.1128/JVI.01076-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang BZ, Gill HS, He C, Ou C, Wang L, Wang YC, Feng H, Zhang H, Prausnitz MR, Compans RW. Microneedle delivery of an M2e-TLR5 ligand fusion protein to skin confers broadly cross-protective influenza immunity. J Control Release. 2014;178:1–7. doi: 10.1016/j.jconrel.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang BZ, Gill HS, Kang SM, Wang L, Wang YC, Vassilieva EV, Compans RW. Enhanced influenza virus-like particle vaccines containing the extracellular domain of matrix protein 2 and a Toll-like receptor ligand. Clin Vaccine Immunol. 2012;19:1119–1125. doi: 10.1128/CVI.00153-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Wang Y-C, Feng H, Ahmed T, Compans RW, Wang B-Z. Virus-like particles containing the tetrameric ectodomain of influenza matrix protein 2 and flagellin induce heterosubtypic protection in mice. Biomed Res Int. 2013;2013 doi: 10.1155/2013/686549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Influenza (Seasonal) 2014 Website: http://www.who.int/mediacentre/factsheets/fs211/en/
- Wibowo N, Hughes FK, Fairmaid EJ, Lua LH, Brown LE, Middelberg AP. Protective efficacy of a bacterially produced modular capsomere presenting M2e from influenza: extending the potential of broadly cross-protecting epitopes. Vaccine. 2014;32:3651–3655. doi: 10.1016/j.vaccine.2014.04.062. [DOI] [PubMed] [Google Scholar]
- Zabel F, Kundig TM, Bachmann MF. Virus-induced humoral immunity: on how B cell responses are initiated. Curr Opin Virol. 2013;3:357–362. doi: 10.1016/j.coviro.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Zebedee SL, Lamb RA. Influenza A virus M2 protein: monoclonal antibody restriction of virus growth and detection of M2 in virions. J Virol. 1988;62:2762–2772. doi: 10.1128/jvi.62.8.2762-2772.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G-g, Li D-x, Zhang H-h, Zeng Y-m, Chen L. Enhancement of mucosal immune response against the M2eHBc+ antigen in mice with the fusion expression products of LTB and M2eHBc+ through mucosal immunization route. Vet Res Commun. 2009;33:735–747. doi: 10.1007/s11259-009-9222-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.