Abstract
Background
Chronic kidney disease (CKD) is common and associated with cardiovascular disease, cerebrovascular disease and cognitive function, although the nature of this relationship remains uncertain.
Study Design
Cross-sectional cohort using baseline data from the Systolic Blood Pressure Intervention Trial (SPRINT)
Setting and Participants
Participants in SPRINT, a randomized clinical trial of blood pressure targets in older community-dwelling adults with cardiovascular disease, CKD or high cardiovascular disease risk and without diabetes or known stroke, who underwent detailed neurocognitive testing in the cognition substudy, SPRINT-Memory and Cognition in Decreased Hypertension (SPRINT-MIND)
Predictors
Urine albumin-creatinine ratio (ACR) and estimated glomerular filtration rate (eGFR)
Outcomes
Cognitive function, a priori defined as 5 cognitive domains based on 11 cognitive tests using zscores, and abnormal white matter volume quantified by brain magnetic resonance imaging
Results
Among 9361 SPRINT participants, 2800 participated in SPRINT-MIND and 2707 had complete data; 637 had brain imaging. Mean age was 68 years, 37% were women, 30% were black, and 20% had known cardiovascular disease. Mean eGFR was 70.8 ± 20.9 ml/min/1.73 m2 and median urine ACR was 9.7 (IQR, 5.7–22.5) mg/g. In adjusted analyses, higher ACR was associated with worse global cognitive function, executive function, memory and attention, such that each doubling of urine ACR had the same association with cognitive performance as being 7 months, 10 months, 6 months, and 14 months older, respectively. Lower eGFR was independently associated with worse global cognitive function and memory. In adjusted models, higher ACR but not eGFR was associated with larger abnormal white matter volume.
Limitations
Cross-sectional only, no patients with diabetes were included.
Conclusions
In older adults, higher urine ACR and lower eGFR have independent associations with global cognitive performance with different affected domains. Albuminuria concurrently identifies a higher burden of abnormal brain white matter disease, suggesting vascular disease may mediate these relationships.
Keywords: Kidney disease, cognition, dementia, albuminuria, brain, cardiovascular disease (CVD), cerebrovascular disease, brain imaging, white matter volume, neurocognitive test battery, urinary albumin-creatinine ratio (UACR), estimated glomerular filtration rate (eGFR), kidney function, global cognitive function, executive function, memory
Chronic kidney disease (CKD) in older adults is an important public health problem affecting up to 26 million individuals in the United States.1 The prevalence of CKD worldwide continues to rise,1 likely reflecting an aging population and increasingly common cardiovascular disease risk factors, including hypertension, diabetes, and obesity. It is particularly common in older adults, with moderate or severe (stage 3 or 4) CKD affecting nearly 11% of individuals aged 60–69 years and more than 1 in 3 persons aged 70 years and older.1
Chronic kidney disease is defined by either reduced kidney function, most often identified through serum creatinine-based estimates of the glomerular filtration rate (GFR), or by evidence of kidney damage, most often identified by the presence of albumin in the urine.2 It is associated with a marked increase in cardiovascular disease risk.3 This risk extends to all vascular beds, including the cerebrovascular circulation, and the frequency of cerebrovascular disease, including acute and subclinical stroke, is also substantially increased in CKD.4,5 Given this cerebrovascular disease burden, it is not surprising that cognitive impairment is common in individuals with CKD, particularly in older adults.6–10 Many of the studies exploring the association between CKD and cognitive function relied on omnibus cognitive screening instruments rather than more detailed neurocognitive testing, lacked brain imaging, or did not have concurrent ascertainment of cognitive functioning and both kidney disease markers.11–14 One study using a cognitive screening test noted that, when eGFR was preserved, albuminuria was associated independently with incident cognitive impairment, while, when albuminuria was minimal, low eGFR was associated independently with cognitive impairment.7 Another study in older adults found an association between albuminuria and performance on cognitive tests assessing executive function but not on tests assessing memory along with an association between albuminuria and abnormal brain white matter volume.10 A third more recent study evaluated data from participants in the Maastricht Study, a population-based cohort in the Netherlands with a low prevalence of CKD, and showed an association between albuminuria and lower information processing speed, particularly among older participants, but no significant association between GFR and cognitive performance.15 All studies enrolled participants with diabetes.
Viewed in sum, findings from these and other studies suggest that cerebrovascular disease may link CKD and cognitive impairment.16 It remains unclear whether albuminuria and reduced GFR each are associated with different cognitive domain profiles. To explore the relationships among kidney markers, cognitive function, and cerebrovascular disease in a high cardiovascular disease risk population without diabetes or known stroke, we evaluated participants in the Systolic Blood Pressure Intervention Trial (SPRINT) cognition substudy, SPRINT-Memory and Cognition in Decreased Hypertension (SPRINT-MIND), with concurrent assessment of kidney function, detailed cognitive testing, and, in a subset, brain imaging measures of cerebrovascular disease. We hypothesized that albuminuria and reduced eGFR are independently correlated with poorer cognitive function and a larger burden of abnormal white matter volume.
Methods
Study Population
A multicenter randomized trial, SPRINT (ClinicalTrials.gov study number NCT01206062) compared two strategies for treating systolic blood pressure: one targeted the standard goal of <140 mm Hg and the other a more intensive target of <120 mm Hg. Enrollment focused on volunteers age 50 years or older with an average baseline systolic blood pressure ≥130 mm Hg and evidence of cardiovascular disease, CKD, 10-year Framingham cardiovascular disease risk score ≥15%, or age ≥75 years. Major exclusion criteria included history of known stroke, diabetes mellitus (current glycosylated hemoglobin ≥6.5% or current use of medications to treat hyperglycemia at screening), proteinuria exceeding 1 g/d, and eGFR below 20 mL/min/1.73 m2.17 Recruitment of a high number of participants with CKD stage 3 was a study goal.17 SPRINT recruited 9361 people at 102 clinics in the United States from November 2010 through March 2013, including 2648 with CKD, 1877 with a history of cardiovascular disease, and 2636 age 75 years or older. Recruitment for SPRINT-MIND targeted 2800 participants at baseline, enriched for older SPRINT participants, while a subset of SPRINT-MIND, SPRINT-MIND Magnetic Resonance Imaging (MRI), targeted approximately 640 MIND participants for brain imaging. All participants provided written informed consent. The study was approved by the institutional review board at each participating study site.
Study Variables
Study laboratory measurements were drawn at the baseline SPRINT visit. Serum creatinine concentration was measured by enzymatic procedure on a Roche analyzer, and is IDMS-traceable for calibration. Urine albumin was measured by an immunoturbidometric method on a Roche analyzer, while cholesterol was measured enzymatically on a Roche analyzer using a cholesterol esterase/cholesterol oxidase method. Urine albumin was quantified along with urine creatinine in random spot urine specimens, with the urine albumin-creatinine ratio (ACR, in mg/g) used to account for urine concentration. For this report, the CKD-EPI (CKD Epidemiology Collaboration) creatinine equation was used to estimate GFR. Blood pressure was the average of three automated measurements assessed while participants were seated in isolation following 5 minutes of rest. Although diabetes was an exclusion criterion, individuals not on medications for diabetes with a screening hemoglobin A1c below 6.5% were eligible.
Cognitive Testing
Cognitive tests were administered in a private room by SPRINT cognitive test administrators, all of whom had undergone detailed training on the cognitive test battery through an iterative process of test administration and feedback. Cognitive test administrators were certified to a criterion level of performance prior to testing with annual review of test materials and recertification. All SPRINT participants underwent a cognitive screen at baseline, consisting of the Montreal Cognitive Assessment (MoCA), the Digit Symbol Substitution Test, and the Logical Memory Subtest of the Weschler Memory Scale (4th edition, Story A). The expanded MIND cognitive battery targeted a subset of 2800 SPRINT participants, with tests selected to provide detailed information on multiple cognitive domains. Cognitive tests were a priori assigned to specific cognitive domains (Table S1, available as online supplementary material). Trail Making Test scores were converted to speed by taking the reciprocal, and summary scores were generated for each of these 5 domains. For participants missing data on up to 2 individual tests, results for these missing tests were imputed using k nearest neighbors (kNN), while, for participants missing 3 or more test results, these were set to missing. Individual test results were standardized using the SPRINT-MIND cohort as [(X-median)]/[interquartile Range (IQR)]. These scores, similar to z scores, were then summed to create domain scores. Higher domain scores represent better performance.
Magnetic Resonance Imaging
Brain MRI in SPRINT was performed using 3.0-T scanners.18 The University of Pennsylvania managed MRI quality control, with each field center performing quarterly phantom scans for the evaluation of scanner stability and image distortion. The MRI scanner performance across the clinical centers was stable over the duration of the study. After preprocessing T1 scans to correct intensity in-homogeneities, the brain was partitioned into 148 anatomical regions of interest and then into 10 larger regions by applying a multi-atlas consensus-based label fusion method.19 White matter lesions were segmented to further characterize brain tissue as normal or abnormal using a supervised learning-based multimodal segmentation technique.20 Abnormal white matter volume, which has been associated with cardiovascular disease risk factors and presence of cerebrovascular disease,21,22 was operationally defined as a non-mass lesion having fast spin-echo fluid attenuated inversion recovery (FLAIR) signal intensity greater than that of normal gray matter in a vascular distribution.
Statistical Analyses
Models were sequentially fit regardless of univariate associations. Unadjusted linear regression models evaluate the association between either eGFR or natural log-transformed urine ACR and cognitive domains. Model 2 further adjusts for age, race, education and study network, while model 3 adjusts for model 2 variables as well as sex; history of diabetes; cardiovascular disease; use of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers; systolic blood pressure; diastolic blood pressure; total, LDL, and HDL cholesterol; and either eGFR or log-transformed albuminuria. Because eGFR often has a non-linear association with cross-sectional and longitudinal outcomes, reflecting the association of serum creatinine concentration with muscle mass, we further examined clinically relevant eGFR strata.3 We used quantile regression, with abnormal white matter volume as the dependent variable, to explore the association of both urine ACR and eGFR with abnormal white matter volume, reflecting the highly skewed distribution of abnormal white matter volume. These models adjust for similar variables as models evaluating the association between kidney markers and cognitive performance.
Results
Participant Characteristics
Among 2921 SPRINT participants receiving the extended cognitive battery, 214 were missing key data, including urine ACR (n=152), eGFR (n=22) or sufficient cognitive testing (n=64) with 26 of these individuals missing more than one of these items, resulting in 2707 participants with complete essential data. Participants with missing data were similar to those with complete data except for slightly lower educational achievement. Mean baseline eGFR was 70.8 ± 20.9 ml/min/1.73 m2 and median baseline urine ACR was 9.7 (IQR, 5.7–22.5) mg/g (Tables 1 and 2). Mean age was 68.4 ± 8.6 years, 36.7% were women, 30.4% were black, and 20.1% had a history of cardiovascular disease. A majority of participants had normal albuminuria levels (51.2%), while 28.7% had high normal albuminuria (10–29 mg/g), 17.2% had moderate albuminuria (30–299 mg/g), and 2.9% had severe albuminuria (≥300 mg/g). There were 493 (18.2%) participants with an eGFR of 45 to <60 ml/min/1.73 m2 and 306 (11.3%) with eGFR below 45 ml/min/1.73 m2. Higher urine ACR was associated with older age, prevalent cardiovascular disease, higher systolic blood pressure and lower eGFR, while lower eGFR was associated with older age, white race, prevalent cardiovascular disease, lower diastolic blood pressure and higher urine ACR. Other characteristics stratified by ACR and eGFR categories are shown in Tables 1 and Table 2.
Table 1.
Baseline characteristics stratified by urine ACR
| Variable | Total (N=2707) |
ACR <10 mg/g (n=1386) |
ACR 10−29 mg/g (n=776) |
ACR 30−299 mg/g (n=466) |
ACR ≥300 mg/g (n=79) |
P for Trend |
|---|---|---|---|---|---|---|
| Demographic Characteristics | ||||||
| Age (years) | 68.4 (8.6) | 67.1 (8.1) | 69.5 (8.6) | 69.9 (9.3) | 70.9 (8.9) | <0.001 |
| Female sex | 994 (36.7%) | 469 (33.8%) | 342 (44.1) | 153 (32.8%) | 30 (38%) | <0.001 |
| Race/Ethnicity | 0.8 | |||||
| White | 1600 (59.1%) | 803 (57.9%) | 475 (61.2%) | 281 (60.3%) | 41 (52%) | |
| Black | 824 (30.4%) | 451 (32.5%) | 205 (26.4%) | 138 (29.6%) | 30 (38%) | |
| Hispanic | 225 (8.3%) | 104 (7.5%) | 75 (9.7%) | 40 (8.6%) | 6 (8%) | |
| Other | 58 (2.1%) | 28 (2.0%) | 21 (2.7%) | 7 (1.5%) | 2 (3%) | |
| Education | 0.09 | |||||
| Below HS Graduate | 227 (8.4%) | 111 (8.0%) | 71 (9.2%) | 34 (7.3%) | 11 (14%) | |
| HS Graduate | 456 (16.9%) | 220 (15.9%) | 136 (17.5%) | 84 (18.0%) | 16 (20%) | |
| Beyond HS | 2024 (74.8%) | 1055 (76.2%) | 569 (73.3%) | 348 (74.7%) | 52 (66%) | |
| Medical History | ||||||
| Cardiovascular Disease | 543 (20.1%) | 247 (17.8%) | 163 (21.0%) | 109 (23.4%) | 24 (30%) | 0.004 |
| Diabetes History | 38 (1.4%) | 16 (1.2%) | 13 (1.7%) | 5 (1.1%) | 4 (5%) | 0.03 |
| Hypertension | 2538 (93.8%) | 1284 (92.6%) | 727 (93.7%) | 448 (96.1%) | 79 (100%) | 0.005 |
| Smoking | 0.3 | |||||
| Never | 1181 (43.7%) | 618 (44.7%) | 347 (44.8%) | 185 (39.7%) | 31 (39%) | |
| Former | 1184 (43.8%) | 604 (43.6%) | 324 (41.8%) | 218 (46.8%) | 38 (48%) | |
| Current | 339 (12.5%) | 162 (11.7%) | 104 (13.4%) | 63 (13.5%) | 10 (13%) | |
| Hypertension Medications | ||||||
| ACE inhibitor | 1004 (37.1%) | 495 (35.7%) | 298 (38.4%) | 174 (37.3%) | 37 (47%) | 0.2 |
| ARB | 584 (21.6%) | 315 (22.7%) | 148 (19.1%) | 104 (22.3%) | 17 (22%) | 0.3 |
| Calcium channel blocker | 966 (35.7%) | 422 (30.5%) | 292 (37.6%) | 214 (45.9%) | 38 (48%) | <0.001 |
| B-blocker | 845 (31.2%) | 379 (27.3%) | 257 (33.1%) | 171 (36.7%) | 38 (48%) | <0.001 |
| Diuretic | 1208 (44.6%) | 635 (45.8%) | 346 (44.6%) | 187 (40.1%) | 40 (51%) | 0.1 |
| Clinical Results | ||||||
| Systolic BP (mm Hg) | 138.8 (16.0) | 136.24 (14.7) | 140.7 (16.3) | 142.1 (17.0) | 145.9 (20.6) | <0.001 |
| Diastolic BP (mm Hg) | 77.3 (11.8) | 77.27 (10.9) | 76.9 (12.1) | 78.1 (13.1) | 78.7 (14.5) | 0.3 |
| BMI (kg/m2) | 29.8 (5.7) | 29.89 (5.4) | 29.6 (5.8) | 30.2 (6.1) | 29.4 (5.8) | 0.3 |
| Laboratory Results | ||||||
| Urine ACR (mg/g) | 9.7 (5.7, 22.5) | 5.8 (4.4, 7.6) | 15.1 (12.2, 20.0) | 59.5 (40.0, 105.0) | 475.0 (382.4, 961.9) | <0.001 |
| eGFR (ml/min/1.73m2) | 70.8 (20.9) | 73.4 (18.3) | 72.0 (21.5) | 63.7 (24.0) | 53.8 (20.7) | <0.001 |
| Cholesterol | ||||||
| Total (mg/dL) | 189.9 (41.1) | 191.6 (39.9) | 191.3 (42.6) | 183.2 (41.5) | 186.1 (42.9) | 0.001 |
| HDL (mg/dL) | 53.4 (14.5) | 53.1 (13.7) | 54.7 (15.4) | 51.5 (15.0) | 55.1 (15.8) | 0.001 |
| LDL (mg/dL) | 112.3 (35.4) | 115.0 (34.3) | 111.6 (36.6) | 106.6 (35.7) | 104.9 (35.7) | <0.001 |
| Triglycerides (mg/dL) | 122.4 (71.1) | 118.2 (62.4) | 125.8 (80.8) | 128.0 (76.4) | 128.8 (76.3) | 0.02 |
Note: Values for categorical variables are given as number (percentage); values for continuous variables, as mean ± standard deviation or median [interquartile range]. Conversion factors for units: cholesterol in mg/dL to mmol/L, ×0.02586; triglycerides in mg/dL to mmol/L, ×0.01129; ACR in mg/g to mg/mmol, ×0.113).
HS, high school; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BP, blood pressure; BMI, body mass index; ACR, albumin-creatinine ratio; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Table 2.
Baseline Characteristics by eGFR Category
| Variable | Total (N=2707) |
eGFR >90 (n=530) |
eGFR 75–90 (n=700) |
eGFR 60–74 (n=678) |
eGFR 45–59 (n=493) |
eGFR <45 (n=306) |
P for trend |
|---|---|---|---|---|---|---|---|
| Demographic Characteristics | |||||||
| Age (years) | 68.4 (8.6) | 62.7 (5.8) | 67.4 (7.6) | 69.1 (8.5) | 72.0 (8.6) | 72.8 (9.1) | <0.001 |
| Female sex | 994 (36.7%) | 188 (36.0%) | 232 (33.2%) | 248 (36.2%) | 196 (39.6%) | 130 (42.5%) | 0.04 |
| Race/Ethnicity | <0.001 | ||||||
| White | 1600 (59.1%) | 216 (41.3%) | 425 (60.9%) | 438 (63.9%) | 320 (64.7%) | 201 (65.7%) | |
| Black | 824 (30.4%) | 241 (46.1%) | 190 (27.2%) | 186 (27.2%) | 130 (26.3) | 77 (25.2%) | |
| Hispanic | 225 (8.3%) | 57 (10.9%) | 65 (9.3%) | 50 (7.3%) | 31 (6.3%) | 22 (7.2%) | |
| Other | 58 (2.1%) | 9 (1.7%) | 18 (2.6%) | 11 (1.6%) | 14 (2.8%) | 6 (2.0%) | |
| Education | 0.5 | ||||||
| Below HS Graduate | 227 (8.4%) | 44 (8.4%) | 52 (7.5%) | 63 (9.2%) | 37 (7.5%) | 31 (10.1%) | |
| HS Graduate | 456 (16.9%) | 95 (18.2%) | 115 (16.5%) | 102 (14.9%) | 87 (17.6%) | 57 (18.6%) | |
| Beyond HS | 2024 (74.8%) | 384 (73.4%) | 531 (76.1%) | 520 (75.9%) | 371 (75.0%) | 218 (71.2%) | |
| Medical History | |||||||
| Cardiovascular Disease | 543 (20.1%) | 78 (14.9%) | 118 (16.9%) | 141 (20.6%) | 119 (24.0%) | 87 (28.4%) | <0.001 |
| Diabetes History | 38 (1.4%) | 3 (0.6%) | 7 (1.0%) | 10 (1.5%) | 10 (2.0%) | 8 (2.6%) | 0.09 |
| Hypertension | 2538 (93.8%) | 489 (93.5%) | 648 (92.8%) | 634 (92.6%) | 469 (94.8%) | 298 (97.4%) | 0.03 |
| Smoking | <0.001 | ||||||
| Never | 1181 (43.7%) | 187 (35.8%) | 290 (41.6%) | 326 (47.7%) | 236 (47.7%) | 142 (46.4%) | |
| Former | 1184 (43.8%) | 215 (41.2%) | 308 (44.1%) | 290 (42.5%) | 226 (45.7%) | 145 (47.4%) | |
| Current | 339 (12.5%) | 120 (23.0%) | 100 (14.3%) | 67 (9.8%) | 33 (6.7%) | 19 (6.2%) | |
| Hypertension Medications | |||||||
| ACE Inhibitor | 1004 (37.1%) | 173 (33.1%) | 254 (36.4%) | 256 (37.4%) | 200 (40.4%) | 121 (39.5%) | 0.1 |
| ARB | 584 (21.6%) | 102 (19.5%) | 130 (18.6%) | 156 (22.8%) | 107 (21.6%) | 89 (29.1%) | 0.003 |
| Calcium channel blocker | 966 (35.7%) | 178 (34.0%) | 238 (34.1%) | 235 (34.3%) | 184 (37.2%) | 131 (42.8%) | 0.06 |
| Β-blocker | 845 (31.2%) | 122 (23.3%) | 206 (29.5%) | 226 (33.0%) | 166 (33.5%) | 125 (40.9%) | <0.001 |
| Diuretic | 1208 (44.6%) | 239 (45.7%) | 285 (40.8%) | 279 (40.7%) | 241 (48.7%) | 164 (53.6%) | <0.001 |
| Clinical Results | |||||||
| Systolic BP (mm Hg) | 138.8 (16.0) | 140.1 (16.7) | 138.5 (15.6) | 138.6 (15.21) | 138.4 (16.5) | 138.2 (16.7) | 0.3 |
| Diastolic BP (mm Hg) | 77.3 (11.8) | 81.1 (11.1) | 77.8 (11.0) | 77.7 (11.4) | 75.0 (12.4) | 72.7 (12.2) | <0.001 |
| BMI (kg/m2) | 29.8 (5.7) | 30.4 (5.9) | 29.8 (5.9) | 29.7 (5.3) | 29.8 (5.6) | 29.3 (5.7) | 0.06 |
| Laboratory Results | |||||||
| Urine ACR (mg/g) | 9.68 (5.7, 22.5) | 8.80 (5.7, 16.7) | 9.1 (5.8, 18.6) | 8.3 (5.1, 16.5) | 10.5 (5.7, 29.5) | 25.1 (10.8, 102.3) | <0.001 |
| eGFR (ml/min/1.73m2) | 70.8 (20.9) | 99.6 (13.1) | 80.5 (6.3) | 66.8 (4.9) | 53.1 (4.3) | 36.6 (6.4) | <0.001 |
| Cholesterol | |||||||
| Total (mg/dL) | 189.9 (41.1) | 193.5 (44.0) | 190.8 (40.8) | 191.0 (39.4) | 185.9 (40.2) | 186.0 (41.7) | 0.02 |
| HDL (mg/dL) | 53.4 (14.5) | 53.8 (15.0) | 53.7 (14.4) | 52.9 (14.5) | 53.9 (14.8) | 52.1 (13.7) | 0.3 |
| LDL (mg/dL) | 112.3 (35.4) | 115.7 (37.9) | 113.1 (34.6) | 113.9 (33.9) | 108.0 (34.3) | 107.8 (36.4) | <0.001 |
| Triglycerides (mg/dL) | 122.4 (71.1) | 121.0 (74.1) | 121.1 (69.5) | 121.7 (66.9) | 121.7 (76.2) | 130.1 (69.9) | 0.4 |
Note: eGFRs expressed in mL/min/1.73 m2. Values for categorical variables are given as number (percentage); values for continuous variables, as mean ± standard deviation or median [interquartile range]. Conversion factors for units: cholesterol in mg/dL to mmol/L, ×0.02586; triglycerides in mg/dL to mmol/L, ×0.01129; ACR in mg/g to mg/mmol, ×0.113).
HS, high school; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BP, blood pressure; BMI, body mass index; ACR, albumin-creatinine ratio; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Kidney Markers and Cognitive Function
Raw scores on cognitive tests stratified by urine ACR and by eGFR are presented in tables a and b, respectively, of Item S1. In unadjusted analyses, higher urine ACR levels were associated with significantly worse performance on all cognitive domains. The association between urine ACR and cognition remained robust to partial and full adjustment for all cognitive domains except language and a borderline statistically significant association with memory (Table 3, Figure 1a, Figure S1). To place these into context, each doubling of urine ACR had the same association with cognitive performance as being 7 months older for global cognitive function, 10 months for executive function, 6 months for memory, and 14 months for attention/concentration. In unadjusted analyses, lower eGFR was associated with worse performance of tests of global cognitive function, executive function, attention/concentration and memory. These associations were attenuated after multivariable adjustment but remained significant for global cognition and memory (Table 3, Figure 1b). While the relationship between log transformed ACR and cognitive performance appeared continuous, there appeared to be a threshold eGFR effect, such that only at an eGFR below 60 ml/min/1.73m2 was performance significantly poorer on tests of global cognitive function, executive function, and memory, compared to individuals with an eGFR 75–90 ml/min/1.73m2, while individuals with eGFR levels >90 ml/min per 1.73m2 also had slightly poorer performance on global cognitive function and executive function domains (Figure 1b, Table S2).
Table 3.
Association between kidney disease markers and cognitive domains.
| Cognitive Domain | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| Urine ACR | Estimate ± SE | P-value | Estimate ± SE | P-value | Estimate ±SE | P-value |
| Global Cognitive Function | −0.061 (0.011) | <0.001 | −0.029 (0.009) | <0.001 | −0.024 (0.009) | 0.008 |
| Executive Function | −0.068 (0.011) | <0.001 | −0.038 (0.009) | <0.001 | −0.032 (0.010) | <0.001 |
| Memory | −0.051 (0.011) | <0.001 | −0.023 (0.010) | 0.02 | −0.017 (0.010) | 0.09 |
| Attention/Concentration | −0.054 (0.011) | <0.001 | −0.033 (0.010) | <0.001 | −0.030 (0.010) | 0.003 |
| Language | −0.015 (0.011) | 0.2 | 0.002 (0.010) | 0.8 | 0.001 (0.010) | 0.9 |
| eGFR | ||||||
| Global Cognitive Function | 0.052 (0.010) | <0.001 | 0.024 (0.008) | 0.003 | 0.020 (0.008) | 0.02 |
| Executive Function | 0.050 (0.010) | <0.001 | 0.021 (0.008) | 0.02 | 0.014 (0.009) | 0.1 |
| Memory | 0.054 (0.010) | <0.001 | 0.027 (0.009) | 0.002 | 0.027 (0.009) | 0.002 |
| Attention/Concentration | 0.026 (0.010) | 0.006 | 0.014 (0.009) | 0.1 | 0.010 (0.009) | 0.3 |
| Language | 0.007 (0.010) | 0.5 | 0.001 (0.009) | 0.9 | 0.0001 (0.009) | 0.9 |
Note: Estimates for urine ACR are reported per one log base 2 increase (doubling) while eGFR is per each 10−ml/min/1.73 m2 increase Model 1: Univariate. Model 2: Model 1 plus age, race, education and study network. Model 3: Model 2 plus sex; diabetes history; body mass index; cardiovascular disease; use of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers; systolic blood pressure; diastolic blood pressure; total, high-density lipoprotein and low-density lipoprotein cholesterol; and either eGFR or log-transformed urine ACR.
ACR, albumin-creatinine ratio; eGFR, estimated glomerular filtration rate; SE, standard error
Figure 1.
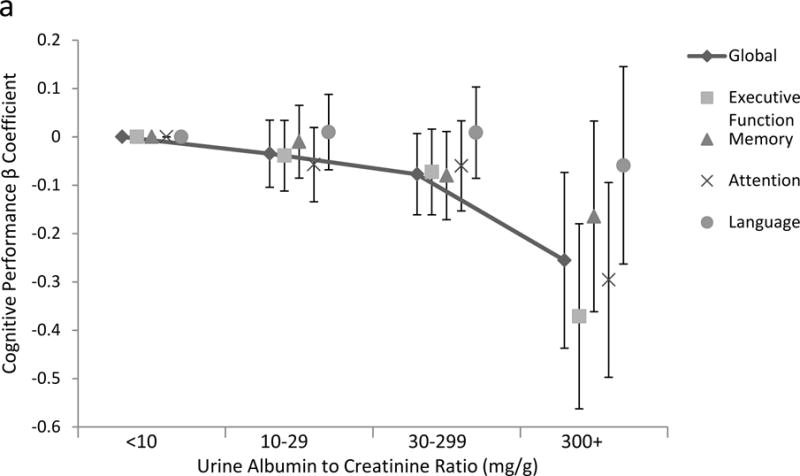
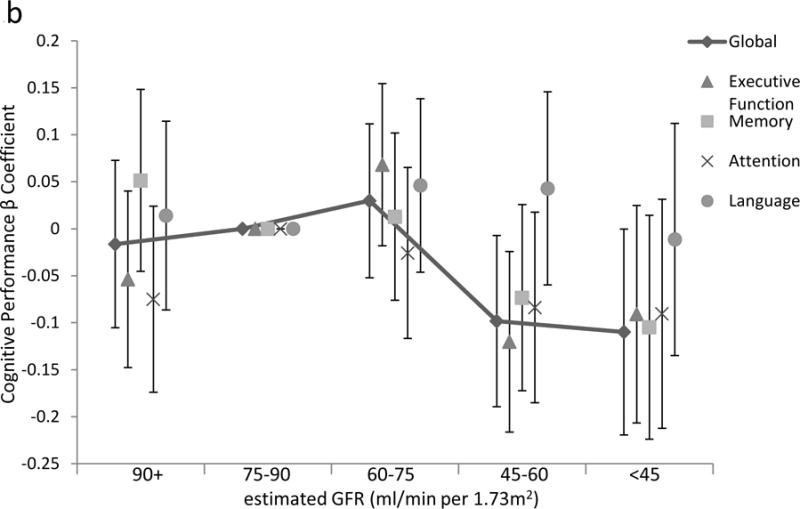
Association of (a) urine albumin-creatinine ratio and (b) estimated glomerular filtration rate (GFR) with cognitive domains, showing non-linear associations between either urine ACR or estimated GFR equations and cognitive performance, shown on the y-axis as the parameter estimate for the ACR or GFR subgroup for each domain. Reference is ACR below 10 mg/g and eGFR of 75 to less than 90 ml/min/1.73m2, respectively. In multivariable analyses adjusting for model 3 variables including estimated GFR for ACR models and ACR for estimated GFR models, p for trend for the association with global cognitive function is 0.03 and 0.02, for executive function 0.002 and <0.001, for memory 0.18 and 0.08, for attention/concentration 0.04 and 0.3, and for language 0.9 and 0.3, for ACR and GFR models, respectively. Lines are presented for only global cognitive function.
Kidney Markers and Brain Abnormal White Matter Volume
A subset of 637 participants in SPRINT-MIND had concurrent brain MRI. SPRINT-MIND MRI participants were younger, had less cardiovascular disease and higher eGFR than those who did not undergo imaging (Table S3). In multivariable models, higher urine ACR was associated with significantly larger abnormal white matter volume (Table 4). There was no association between eGFR and abnormal white matter volume after adjustment for age, intracranial volume, sex, race, education, and scanner.
Table 4.
Association of eGFR and ACR with abnormal white matter volume based on quantile regression
| Urine ACR | eGFR | |||
|---|---|---|---|---|
| Model and Comparison | Estimate ± SE | p-value | Estimate ±SE | p-value |
| Model 1 | ||||
| Continuous | 0.25 (0.06) | <0.001 | 0.08 (0.03) | 0.01 |
| 30 vs 5 mg/g (calculated) | 0.44 (0.11) | – | – | |
| 300 vs 30 mg/g (calculated) | 0.57 (0.14) | – | – | |
| Model 2 | ||||
| Continuous | 0.20 (0.05) | <0.001 | 0.04 (0.03) | 0.2 |
| 30 vs 5 mg/g (calculated) | 0.36 (0.09) | – | – | |
| 300 vs 30 mg/g (calculated) | 0.46 (0.12) | – | – | |
| Model 3 | ||||
| Continuous | 0.17 (0.05) | 0.001 | 0.03 (0.03) | 0.3 |
| 30 vs 5 mg/g (calculated) | 0.30 (0.09) | – | – | |
| 300 vs 30 mg/g (calculated) | 0.38 (0.12) | – | – | |
Note: Data derived from the SPRINT-MIND MRI subcohort of 637 participants. The estimates denote median change. White matter volume is in cm3. For urine ACR, estimates are for each 1−U change in natural log transformed ACR. For eGFR, estimates are per each 10−ml/min/1.73m2 decrease in eGFR. Model 1 is univariate. Model 2 adjusts for intracranial volume, age, MRI scanner type, education, and race. Model 3 adjusts for model 2 components as well as diabetes history, body mass index, cardiovascular disease, use of angiotensin-cnoverting enzyme inhibitors or angiotensin receptor blockers, sex, systolic blood pressure, diastolic blood pressure and either eGFR or natural log transformed urine ACR. The comparisons at clinically relevant urine ACR strata are calculated from the continuous model as: (estimate*[log(30)−log(5)]) and (estimate*[log(300)−log(5)]) for each model and can be interpreted as the estimate associated with an ACR of 30 mg/g or 300 mg/g as compared to a reference value of 5 mg/g.
ACR, albumin-creatinine ratio; eGFR, estimated glomerular filtration rate; MRI, magnetic resonance imaging; SE, standard error; SPRINT-MIND, Systolic Blood Pressure Intervention Trial-Memory and Cognition in Decreased Hypertension
Discussion
Among SPRINT-MIND participants, higher urine albumin concentration was independently associated with worse cognitive functioning in multiple domains, including global cognitive function, executive function and attention, while lower eGFR was independently associated with worse global function and memory. The magnitude of association between urine ACR and cognitive performance was notable, with each doubling of the urine ACR akin to the effect of being 6 to 14 months older for most cognitive domains. In the subset of SPRINT-MIND participants who had brain MRI, higher urine ACR was associated with a greater burden of abnormal white matter volume. Albuminuria and reduced eGFR had independent associations with global cognition with different patterns of affected domains, such that both were statistically significant in multiple models that adjusted for both CKD markers. The association of urine ACR with executive function and attention in conjunction with the association between urine ACR and abnormal white matter volume suggests that reductions in these cognitive domains could be mediated at least in part by cerebrovascular disease and altered brain perfusion. In contrast, the pattern of cognitive impairment associated with reduced eGFR and the absence of an association with abnormal white matter volume suggests that reduced eGFR may reflect a partially distinct pathophysiologic process of cognitive dysfunction.
Cerebrovascular disease often manifests with neurocognitive changes in processing speed, concentration and executive functioning, skills necessary for complex attention, shifting between mental tasks, and initiating and stopping actions.23 This pattern of cognitive impairment has been referred to as ‘subcortical’, reflecting a pattern of brain white matter changes associated with deficits in these cognitive domains.24 Executive functions have particular significance for an individual’s ability to engage in medical decision-making and medication management and may affect health behaviors and outcomes.25,26 These results from SPRINT-MIND are consistent with those of prior studies showing an association between CKD and poorer cognitive function, particularly executive function and attention/concentration domains,7,10,27–30 and expand on those findings by demonstrating an independent and potentially differential association of urine ACR and reduced eGFR with cognitive function and brain vascular disease in a large cohort of community-dwelling adults with hypertension with detailed assessment of cognitive function.
Albuminuria is both an indicator of glomerular disease and a robust marker of cardiovascular disease risk.3,31–33 Reduced eGFR is also a powerful predictor of cardiovascular disease risk,3 with the caveat that serum creatinine concentration is not only a marker of glomerular filtration but also is associated with muscle mass. Accordingly, a high eGFR (consistent with low serum creatinine concentration) may reflect cachexia rather than kidney function and, therefore, may also be associated with worse clinical outcomes, explaining the absence of an association within higher eGFR levels.34–36 While it is possible that kidney disease itself contributes to worse cognitive function, it is more likely that the presence of CKD is a marker of cardiovascular disease risk, reflecting overall vascular disease burden. Endothelial dysfunction and other sequelae of vascular disease lead to a loss of vascular integrity in the glomerulus and subsequent albuminuria;37 in the setting of similar systemic factors, it is likely that the brain microvasculature has processes like those in the kidney, resulting in increased levels of abnormal brain white matter. These hypothesized mechanisms are consistent with our findings in SPRINT-MIND and SPRINT MRI, where, in addition to the aforementioned associations between abnormal white matter and urine albumin concentration, lower eGFR was associated with higher cerebral blood flow, a potential marker of impaired cerebrovascular autoregulation.18
Much of the literature evaluating the association between cognition and kidney disease markers relies on cognitive screening tests,11–14 while few studies have employed more detailed neurocognitive testing. In addition, few studies have concurrent measurements of albuminuria and eGFR. Separate analyses from the Cardiovascular Health Study (CHS) demonstrated associations between albuminuria and incident dementia, in a pattern most consistent with vascular dementia,9 and between reduced eGFR and incident dementia, again in a pattern most consistent with vascular dementia;27 however these analyses did not account for both kidney markers simultaneously. In both the cross-sectional Maastricht Study and the longitudinal Rancho Bernardo Study, albuminuria but not GFR was associated with worse cognitive performance, with both of these population-based studies having relatively low prevalence of CKD and including participants with diabetes.15,28 Several smaller cohorts of CKD stages 3–5 also show poorer performance on tests of executive function.38,39 Supporting the relationship between CKD markers and worse cognitive functioning, one study of 335 elderly individuals with substantial comorbidity described an independent association of albuminuria with larger white matter disease burden and with worse cognitive performance, particularly in executive function.10 In a longitudinal analysis of 19,399 adults without cognitive impairment at baseline participating in the REGARDS (Reasons for Geographic and Racial Disparities in Stroke) Study, Kurella Tamura and colleagues noted that, when eGFR was preserved, albuminuria was associated independently with incident cognitive impairment defined using the 6-item screener; however, when albuminuria was minimal (<10 mg/g), low eGFR was associated independently with cognitive impairment, suggesting that albuminuria and low eGFR identify different risk states for incident cognitive impairment. SPRINT consolidates many of these findings in a non-diabetic, non-stroke cohort with a high prevalence of CKD by demonstrating that higher urine ACR level is associated with worse cognitive function and larger abnormal white matter volume and suggesting that reduced eGFR is also associated with cognitive function as eGFR declines below 60 ml/min/1.73m2.
These analyses from SPRINT-MIND stress the need to be aware of the heightened risk of worse cognitive performance among individuals with CKD and suggest that cognitive screening in some patients with CKD may have a role in interdisciplinary patient management. Knowledge of cognitive impairment in these individuals is important for optimizing multiple aspects of patient care. First, patients with cognitive impairment are susceptible to delirium, which can lead to premature death, functional decline, falls, and institutionalization.40 Second, patients with chronic conditions, including CKD, often receive complex medication regimens that require intact cognitive functioning to manage. In fact, among SPRINT participants, those randomized to the intensive blood pressure target required 3 different blood pressure medications, on average, in addition to medications they were taking for other purposes.41 Third, the finding that markers of CKD are associated with cognitive function suggests that targeting CKD and vascular disease prevention could reduce the risk of subsequent cognitive impairment. In this regard, longitudinal analyses from SPRINT-MIND are eagerly awaited.
This study has several limitations. First, it is a cross-sectional report and conclusions regarding causality or reverse causation cannot be drawn. Longitudinal data from SPRINT-MIND, likely available in 2018, will better be able to address this question. Second, although we adjusted for education, other social factors that were not available may confound the association between kidney disease markers and cognitive performance. Third, the associations, although statistically significant, are of uncertain clinical significance. However, the finding that each doubling of the urine ACR has a similar association with many cognitive domains as being 6 to 14 months older suggests that the presence of CKD is an important marker of cognitive performance. Differences in associations by domain should be interpreted with caution, as tests within a specific domain may be less precise than those within a different domain. Additionally, some of the cognitive tests have some intrinsic limitations; for example, the recorded Trail-Making Test, Part B, time is a function of both time and the number of errors, such that participants making five errors were automatically scored as having a maximum time score of 300 seconds. Critically, both components relate to underlying executive function capabilities. Fourth, serum creatinine-based GFR estimates may be confounded by muscle mass, particularly in older individuals. Finally, many cognitive tests do not precisely map to specific cognitive domains, making categorization of cognitive tests into specific categories somewhat arbitrary. This is partially addressed through use of a global cognition score.
This study has multiple strengths as well. It encompasses a large, community dwelling population which should be generalizable to clinical populations cared for in many outpatient settings. SPRINT recruited individuals without known stroke, reinforcing the concept that microvascular complications affect brain function. SPRINT also targeted older individuals and individuals with CKD, providing adequate numbers to examine relationships across the spectrum of kidney function. Finally, SPRINT-MIND utilized a comprehensive battery of cognitive tests and obtained brain imaging in nearly a quarter of these participants, providing sufficient data to support the hypothesized links among kidney disease markers, cognitive functioning and brain structure.
In conclusion, in a large population of non-diabetic community-dwelling adults with risk factors for cardiovascular disease, CKD markers are associated with worse cognitive performance. Most notably, the presence of albuminuria, even at very low levels, is associated with worse global cognitive function and worse performance on tests evaluating executive function and attention/cognition domains while concurrently identifying a higher burden of abnormal brain white matter disease. These findings suggest that vascular disease may mediate these interrelationships and identify patients with kidney disease as a high risk population for cognitive impairment.
Supplementary Material
Table S1: Tasks included in the SPRINT-MIND cognitive battery.
Table S2: Association between eGFR subdivided into clinically relevant categories and cognitive function.
Table S3: Baseline characteristics of the SPRINT-MIND MRI subcohort.
Figure S1: Association of urine ACR with cognitive domains, highlighting only ACR < 300 mg/g.
Item S1: Cognitive function summary score components by ACR and eGFR groups.
Acknowledgments
Support: SPRINT is funded with federal funds from the National Institutes of Health (NIH), including the National Heart, Lung, and Blood Institute, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the National Institute on Aging, and the National Institute of Neurological Disorders and Stroke, under contract numbers HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, HHSN268200900049C, and Inter-Agency Agreement Number A-HL-13-002-001. It was also supported in part with resources and use of facilities through the US Department of Veterans Affairs. The SPRINT investigators acknowledge the contribution of study medications (azilsartan and azilsartan combined with chlorthalidone) from Takeda Pharmaceuticals International, Inc. All components of the SPRINT study protocol were designed and implemented by the investigators. The investigative team collected, analyzed, and interpreted the data. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, the US Department of Veterans Affairs, or the US Government. The SPRINT investigators also acknowledge the support from the following clinical and translational science awards funded by the National Center for Advancing Translational Sciences: Case Western Reserve University: UL1TR000439; Ohio State University: UL1RR025755; University of Pennsylvania: UL1RR024134 and UL1TR000003; Boston University: UL1RR025771; Stanford University: UL1TR000093; Tufts University: UL1RR025752, UL1TR000073 and UL1TR001064; University of Illinois: UL1TR000050; University of Pittsburgh: UL1TR000005; University of Texas Southwestern: 9U54TR000017-06; University of Utah: UL1TR000105-05; Vanderbilt University: UL1 TR000445; George Washington University: UL1TR000075; University of California, Davis: UL1 TR000002; University of Florida: UL1 TR000064; University of Michigan: UL1TR000433; Tulane University: P30GM103337; Centers of Biomedical Research Excellence Award, National Institute of General Medical Sciences. Mind the Kidneys, an ancillary study that contributed data to this report, is funded by R01 DK092241 from the NIDDK to Dr Kurella Tamura.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
In line with AJKD’s procedures for potential conflicts of interest for editors, described in the Information for Authors & Journal policies, Editorial Board Morgan E. Grams, MD, PhD, MHS, served as Acting Editor-in-Chief and handled the peer-review and decision-making processes.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Contributions: Research idea and study design: DEW, GJC, LC, PLK, SO, JDW, MKT; data acquisition: JN, APA, GJC, MC, LC, WEH, AAK, AJL, SO, MGS, YMS, CBW, JDW, MKT; data analysis/interpretation: DEW, SAG, MKT; statistical analysis: SAG. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Peer Review: Evaluated by 3 external peer reviewers, a statistician, and an Acting Editor-in-Chief.
Supplementary Material
Note: The supplementary material accompanying this article (doi: _______) is available at www.ajkd.org
References
- 1.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007 Nov 7;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Stevens PE, Levin A, Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group M Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013 Jun 4;158(11):825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 3.Chronic Kidney Disease Prognosis Consortium. Matsushita K, van der Velde M, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010 Jun 12;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiner DE, Tighiouart H, Levey AS, et al. Lowest systolic blood pressure is associated with stroke in stages 3 to 4 chronic kidney disease. J Am Soc Nephrol. 2007 Mar;18(3):960–966. doi: 10.1681/ASN.2006080858. [DOI] [PubMed] [Google Scholar]
- 5.Seliger SL, Gillen DL, Longstreth WT, Jr, Kestenbaum B, Stehman-Breen CO. Elevated risk of stroke among patients with end-stage renal disease. Kidney Int. 2003 Aug;64(2):603–609. doi: 10.1046/j.1523-1755.2003.00101.x. [DOI] [PubMed] [Google Scholar]
- 6.Yaffe K, Kurella-Tamura M, Ackerson L, et al. Higher levels of cystatin C are associated with worse cognitive function in older adults with chronic kidney disease: the chronic renal insufficiency cohort cognitive study. J Am Geriatr Soc. 2014 Sep;62(9):1623–1629. doi: 10.1111/jgs.12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurella Tamura M, Muntner P, Wadley V, et al. Albuminuria, kidney function, and the incidence of cognitive impairment among adults in the United States. Am J Kidney Dis. 2011 Nov;58(5):756–763. doi: 10.1053/j.ajkd.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurella Tamura M, Wadley V, Yaffe K, et al. Kidney function and cognitive impairment in US adults: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Am J Kidney Dis. 2008 Aug;52(2):227–234. doi: 10.1053/j.ajkd.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seliger SL, Siscovick DS, Stehman-Breen CO, et al. Moderate Renal Impairment and Risk of Dementia among Older Adults: The Cardiovascular Health Cognition Study. J Am Soc Nephrol. 2004 Jul;15(7):1904–1911. doi: 10.1097/01.asn.0000131529.60019.fa. [DOI] [PubMed] [Google Scholar]
- 10.Weiner DE, Bartolomei K, Scott T, et al. Albuminuria, cognitive functioning, and white matter hyperintensities in homebound elders. Am J Kidney Dis. 2009 Mar;53(3):438–447. doi: 10.1053/j.ajkd.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurella M, Chertow GM, Fried LF, et al. Chronic kidney disease and cognitive impairment in the elderly: the health, aging, and body composition study. J Am Soc Nephrol. 2005 Jul;16(7):2127–2133. doi: 10.1681/ASN.2005010005. [DOI] [PubMed] [Google Scholar]
- 12.Kurella M, Yaffe K, Shlipak MG, Wenger NK, Chertow GM. Chronic kidney disease and cognitive impairment in menopausal women. Am J Kidney Dis. 2005 Jan;45(1):66–76. doi: 10.1053/j.ajkd.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 13.Slinin Y, Paudel ML, Ishani A, et al. Kidney function and cognitive performance and decline in older men. J Am Geriatr Soc. 2008 Nov;56(11):2082–2088. doi: 10.1111/j.1532-5415.2008.01936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vupputuri S, Shoham DA, Hogan SL, Kshirsagar AV. Microalbuminuria, peripheral artery disease, and cognitive function. Kidney Int. 2008 Feb;73(3):341–346. doi: 10.1038/sj.ki.5002672. [DOI] [PubMed] [Google Scholar]
- 15.Martens RJ, Kooman JP, Stehouwer CD, et al. Estimated GFR, Albuminuria, and Cognitive Performance: The Maastricht Study. Am J Kidney Dis. 2017 Feb;69(2):179–191. doi: 10.1053/j.ajkd.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 16.Seliger SL, Weiner DE. Cognitive impairment in dialysis patients: focus on the blood vessels? Am J Kidney Dis. 2013 Feb;61(2):187–190. doi: 10.1053/j.ajkd.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ambrosius WT, Sink KM, Foy CG, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT) Clinical trials. 2014 Oct;11(5):532–546. doi: 10.1177/1740774514537404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamura MK, Pajewski NM, Bryan RN, et al. Chronic kidney disease, cerebral blood flow, and white matter volume in hypertensive adults. Neurology. 2016 Mar 29;86(13):1208–1216. doi: 10.1212/WNL.0000000000002527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lao Z, Shen D, Liu D, et al. Computer-assisted segmentation of white matter lesions in 3D MR images using support vector machine. Academic radiology. 2008 Mar;15(3):300–313. doi: 10.1016/j.acra.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zacharaki EI, Kanterakis S, Bryan RN, Davatzikos C. Measuring brain lesion progression with a supervised tissue classification system. Medical image computing and computer-assisted intervention: MICCAI … International Conference on Medical Image Computing and Computer-Assisted Intervention. 2008;11(Pt 1):620–627. doi: 10.1007/978-3-540-85988-8_74. [DOI] [PubMed] [Google Scholar]
- 21.Launer LJ, Lewis CE, Schreiner PJ, et al. Vascular factors and multiple measures of early brain health: CARDIA brain MRI study. PLoS One. 2015;10(3):e0122138. doi: 10.1371/journal.pone.0122138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brickman AM, Zahra A, Muraskin J, et al. Reduction in cerebral blood flow in areas appearing as white matter hyperintensities on magnetic resonance imaging. Psychiatry Res. 2009 May 15;172(2):117–120. doi: 10.1016/j.pscychresns.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Brien JT, Erkinjuntti T, Reisberg B, et al. Vascular cognitive impairment. Lancet Neurol. 2003 Feb;2(2):89–98. doi: 10.1016/s1474-4422(03)00305-3. [DOI] [PubMed] [Google Scholar]
- 24.Graham NL, Emery T, Hodges JR. Distinctive cognitive profiles in Alzheimer’s disease and subcortical vascular dementia. Journal of neurology, neurosurgery, and psychiatry. 2004 Jan;75(1):61–71. [PMC free article] [PubMed] [Google Scholar]
- 25.Waldstein SR, Wendell CR. Neurocognitive function and cardiovascular disease. J Alzheimers Dis. 2010;20(3):833–842. doi: 10.3233/JAD-2010-091591. [DOI] [PubMed] [Google Scholar]
- 26.Amirian E, Baxter J, Grigsby J, Curran-Everett D, Hokanson JE, Bryant LL. Executive function (capacity for behavioral self-regulation) and decline predicted mortality in a longitudinal study in Southern Colorado. J Clin Epidemiol. 2010 Mar;63(3):307–314. doi: 10.1016/j.jclinepi.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barzilay JI, Fitzpatrick AL, Luchsinger J, et al. Albuminuria and dementia in the elderly: a community study. Am J Kidney Dis. 2008 Aug;52(2):216–226. doi: 10.1053/j.ajkd.2007.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jassal SK, Kritz-Silverstein D, Barrett-Connor E. A prospective study of albuminuria and cognitive function in older adults: the Rancho Bernardo study. Am J Epidemiol. 2010 Feb 1;171(3):277–286. doi: 10.1093/aje/kwp426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarnak MJ, Tighiouart H, Scott TM, et al. Frequency of and risk factors for poor cognitive performance in hemodialysis patients. Neurology. 2013 Jan 29;80(5):471–480. doi: 10.1212/WNL.0b013e31827f0f7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murray AM, Bell EJ, Tupper DE, et al. The Brain in Kidney Disease (BRINK) Cohort Study: Design and Baseline Cognitive Function. Am J Kidney Dis. 2016 Apr;67(4):593–600. doi: 10.1053/j.ajkd.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gutierrez OM, Khodneva YA, Muntner P, et al. Association between urinary albumin excretion and coronary heart disease in black vs white adults. JAMA. 2013 Aug 21;310(7):706–714. doi: 10.1001/jama.2013.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fox CS, Matsushita K, Woodward M, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012 Nov 10;380(9854):1662–1673. doi: 10.1016/S0140-6736(12)61350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Velagaleti RS, Gona P, Larson MG, et al. Multimarker approach for the prediction of heart failure incidence in the community. Circulation. 2010 Oct 26;122(17):1700–1706. doi: 10.1161/CIRCULATIONAHA.109.929661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shlipak MG, Matsushita K, Arnlov J, et al. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013 Sep 5;369(10):932–943. doi: 10.1056/NEJMoa1214234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levey AS, Inker LA, Coresh J. GFR estimation: from physiology to public health. Am J Kidney Dis. 2014 May;63(5):820–834. doi: 10.1053/j.ajkd.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005 May 19;352(20):2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 37.Wolf G, Chen S, Ziyadeh FN. From the periphery of the glomerular capillary wall toward the center of disease: podocyte injury comes of age in diabetic nephropathy. Diabetes. 2005 Jun;54(6):1626–1634. doi: 10.2337/diabetes.54.6.1626. [DOI] [PubMed] [Google Scholar]
- 38.Kurella M, Chertow GM, Luan J, Yaffe K. Cognitive impairment in chronic kidney disease. J Am Geriatr Soc. 2004 Nov;52(11):1863–1869. doi: 10.1111/j.1532-5415.2004.52508.x. [DOI] [PubMed] [Google Scholar]
- 39.Thornton WL, Shapiro RJ, Deria S, Gelb S, Hill A. Differential impact of age on verbal memory and executive functioning in chronic kidney disease. J Int Neuropsychol Soc. 2007 Mar;13(2):344–353. doi: 10.1017/S1355617707070361. [DOI] [PubMed] [Google Scholar]
- 40.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014 Mar 8;383(9920):911–922. doi: 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.SPRINT Research Group. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015 Nov 26;373(22):2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Tasks included in the SPRINT-MIND cognitive battery.
Table S2: Association between eGFR subdivided into clinically relevant categories and cognitive function.
Table S3: Baseline characteristics of the SPRINT-MIND MRI subcohort.
Figure S1: Association of urine ACR with cognitive domains, highlighting only ACR < 300 mg/g.
Item S1: Cognitive function summary score components by ACR and eGFR groups.


