Abstract
Background
People with advanced chronic kidney disease (CKD) are at risk for the development of end-stage renal disease (ESRD), but also many other adverse outcomes, including cardiovascular disease (CVD) events and death. Determination of risk factors that explain the variability in prognosis and timing of these adverse outcomes can aid patient counseling and medical decision-making.
Study Design
Prospective research cohort.
Setting & Participants
1,798 participants with eGFR <30 ml/min/1.73 m2 in the CRIC Study were followed up for a median of 5.5 years.
Predictors
Age, race, sex, eGFR, proteinuria, diabetes mellitus, body mass index, ejection fraction, systolic blood pressure, history of cardiovascular disease, and history.
Outcomes
ESRD, CVD (congestive heart failure, stroke, myocardial infarction, peripheral artery disease), and death.
Results
Baseline age of the cohort was 60 years; 46% were women, and 46% were African American. While 52.3% of participants progressed to ESRD during follow-up, the path by which this occurred was variable. For example, the predicted 1-year probabilities for a hypothetical 60-year-old white woman with eGFR 30 ml/min/1.73 m2, 1.8 g/d of proteinuria, and no diabetes or CVD (risk characteristics similar to the average participant), were 3.3%, 4.1%, and 0.3%, for first developing CVD, ESRD, and death, respectively. For a 40-year-old African-American man with similar characteristics but higher systolic blood pressure, the corresponding 1-year probabilities were 2.4%, 13.2%, and 0.1%. For all participants, the development of ESRD or CVD increased the risk of subsequent mortality, with no differences by patient race or body-mass index.
Limitations
The CRIC population was specifically recruited for kidney disease, and the vast majority had seen a nephrologist.
Conclusions
The prognosis and timing of adverse outcomes in CKD varies by patient characteristics. These results may help guide the development of personalized approaches for managing patients with advanced CKD.
Index words: Chronic kidney disease (CKD), CKD progression, disease trajectory, end-stage renal disease (ESRD), cardiovascular disease (CVD), mortality, pre-ESRD death, incident ESRD, adverse event, advanced CKD, risk factor, prognosis, kidney function decline, CRIC (Chronic Renal Insufficiency Cohort)
Patients with chronic kidney disease (CKD) are at high risk for adverse outcomes, including cardiovascular disease (CVD) events, end-stage renal disease (ESRD), and death.1–3 More advanced kidney disease, as indicated by lower glomerular filtration rate (GFR) and/or higher albuminuria, generally portends higher risk.4 However, the absolute risks of CVD, ESRD, and death in advanced CKD are ill-defined. Improved knowledge of the risk factors for and relative timing of individual adverse outcomes in patients with advanced CKD could enhance patient counseling and medical decision making. For example, arteriovenous fistula placement might be deferred in a person with a low likelihood of progression to ESRD or a high risk of pre-ESRD death.
Results of previous studies conflict regarding the absolute risk of adverse outcomes in advanced CKD: a clinical trial reported a preponderance of ESRD, whereas health system data demonstrated higher risk of pre-ESRD death.5–7 These discrepancies may relate to differences in nephrology care or differences in patient characteristics between the study populations, such as age or racial distribution or the presence of heart failure, diabetes, or the level of proteinuria. For example, a higher representation of patients with significant proteinuria might result in higher rates of ESRD. Whether individual patients can be meaningfully risk-stratified for the short-term risks of ESRD, CVD, and death on the basis of demographic or health characteristics is unknown.
Using participants in the Chronic Renal Insufficiency Cohort (CRIC) with advanced CKD (defined as eGFR <30 ml/min/1.73 m2), the aim of this study was to evaluate the frequency, timing, and risk associations of adverse outcomes by baseline patient characteristics. We hypothesized that pre-ESRD outcomes of CVD and death would be common, and that the frequency and sequence of adverse events over a 1-year time horizon would vary by baseline patient characteristics. We also hypothesized that the development of ESRD or CVD would increase the risk for subsequent mortality.
METHODS
Study Population
Participants for the present study were selected from the CRIC study, a prospective cohort of people with CKD, which has been previously described.8 In brief, eligibility for initial study enrollment into CRIC was determined by eGFR based on the best available estimating equation at the time, the MDRD (Modification of Diet in Renal Disease) Study equation: eGFR 20–70 ml/min/1.73 m2 for participants aged 21–44 years, 20–60 ml/min/1.73 m2 for participants aged 45–64 years, and 20–50 ml/min/1.73 m2 for participants aged 65–74 years.8–10 Participants were excluded for polycystic kidney disease, multiple myeloma, active glomerulonephritis, a previous dialysis requirement, recent immunotherapy for kidney disease and/or vasculitis, New York Heart Association class III or IV heart failure, and cirrhosis since these patients were expected to have very different natural history of disease than the target population.8 Enrollment occurred at seven US clinical centers (13 enrollment sites in Philadelphia, Pennsylvania; Baltimore, Maryland (2 sites); Cleveland, Ohio (3 sites); Ann Arbor and Detroit, Michigan (2 sites); Chicago, Illinois; New Orleans, Louisiana; and San Francisco and Oakland, California) during May 2003-August 2008. A total of 3,939 participants completed a baseline visit; participants have since been followed up every 6 months by telephone contact and annually with clinical visits. Laboratory tests of kidney function (creatinine and cystatin C) were performed at each clinical visit. For the present study, only those participants with eGFR <30 ml/min/1.73 m2 at baseline or observed during follow-up through the middle of 2013 were included (N=1,798). The threshold of eGFR 30 ml/min/1.73 m2 (determined using the CRIC-specific equation for creatinine and cystatin C, which most accurately reflects measured GFR in the CRIC cohort) was selected based on guidelines for the definition of “severely decreased” kidney function (CKD stage G4).11–13 The study protocol was approved by the institutional review boards of all participating centers (protocol NA_00044034 at Johns Hopkins University). All participants provided written informed consent.
Outcomes
We defined three outcomes of interest: first CVD event after eGFR <30 ml/min/1.73 m2, ESRD, and death (through mid-2013). The CVD events included congestive heart failure, ischemic or hemorrhagic stroke (probable or definite), myocardial infarction (probable or definite), and peripheral artery disease (occlusive artery disease resulting in amputation or revascularization procedures), and were analyzed as a composite variable. Events were ascertained through alternating telephone and in-person contact every six months, through which participants were questioned about intervening hospitalizations, procedures, and the development of ESRD or CVD events. In addition, diagnostic codes from all hospitalizations were obtained, and those with codes indicating cardiovascular events were flagged for physician adjudication of the medical record. Criteria for definite and probable congestive heart failure, stroke, myocardial infarction, and peripheral artery disease have been published previously.14 Incident ESRD was determined through self-report, review of medical records, and linkage to the US Renal Data System. Deaths were ascertained through contact with next of kin, review of medical records and death certificates, and search of the Social Security Death vital status and death certificate files, where possible.
Covariates
Serum creatinine was initially calibrated to the Cleveland Clinic Research Laboratory (Cleveland, OH) and then to isotope-dilution mass spectrometry-traceable standards; cystatin C was measured using a Siemens BNII Machine and internally calibrated. Both biomarkers were converted to eGFR using a CRIC-specific equation.12,13 Proteinuria was quantified as protein-creatinine ratio from 24-hour urine collections or, if that was not available, from random spot urine protein-creatinine ratio (n=166). If spot urine protein-creatinine ratio was also missing, proteinuria was imputed using the spot urine albumin-creatinine ratio (n=7).15,16 Race/ethnicity, age, current smoking, and medication use were self-reported. Diabetes was assessed as any diabetes medication use, fasting plasma glucose level ≥ 126 mg/dL, or nonfasting plasma glucose level ≥ 200 mg/dL. Systolic blood pressure was measured at the clinic visit after 5 minutes of rest; the average of three measurements was taken.17 Weight and height were measured twice and averaged before calculating body mass index (BMI). History of CVD was defined as self-report of coronary artery disease (myocardial infarction, angina, prior coronary revascularization), congestive heart failure, stroke or transient ischemic attack, or peripheral artery disease (claudication, amputation, or angioplasty). Ejection fraction was estimated using systolic and diastolic volumes of the left ventricle by the single-plane Simpson’s rule method from echocardiography performed at each center in accordance with a centralized imaging protocol. Echocardiograms were then read by a single trained sonographer at the University of Pennsylvania. Echocardiograms were performed per CRIC protocol at year 1, year 4, eGFR <20 ml/min/1.73 m2, and after ESRD.18 If an echocardiogram was not performed at the first visit in which a participant developed eGFR <30 ml/min/1.73 m2, ejection fraction from the most recent antecedent echocardiogram was used. Nephrology care was ascertained by the following question: “Have you ever seen a nephrologist or a kidney doctor?”
Statistical Methods
Participant characteristics at the first visit in which eGFR <30 ml/min/1.73 m2 was observed were presented for the overall study population and according to the first outcome of interest (CVD event, ESRD, or death). Participants who withdrew before the end of the study (n=102) and those who survived to the end of follow-up without the development of an event of interest (n=384) were described separately. The cumulative incidence of first events was determined using competing risk regression using the user-written Stata command stcompadj. Results were adjusted to eGFR 30 ml/min/1.73 m2 to account for differences in the first observed eGFR below the threshold of 30 ml/min/1.73 m2. Subsequent analyses of cumulative incidence of ESRD and death after CVD, and CVD and death after ESRD were not adjusted for eGFR and were performed using competing risk regression with the Stata command stcompet. The incidence of death after ESRD and CVD was estimated using standard Kaplan-Meier techniques since there was no competing event.
The adjusted associations between baseline characteristics and the first event of interest were assessed as sub-hazard ratios (HRs) from competing risk models, with the other two events treated as a composite competing event to the event of interest.19 For comparison purposes, we also listed cause-specific HRs from Cox regression, censoring at the incidence of competing events (Table S1, available as online supplementary material). The difference between cause-specific HRs and sub-HRs for a particular risk factor will depend both on the risk factor’s association with the competing event as well as the frequency of the competing event. Because cause-specific HRs do not directly correspond to cumulative incidence, they are less useful in the setting of an absolute risk prediction model. On the other hand, they may show stronger risk factor associations when a risk factor affects both the outcome of interest and the competing event.20–22 For this study, risk factors were selected a priori from those previously reported to be associated with CVD, ESRD, and death in the literature.3,17,23–25 Serum albumin was selected a priori for evaluation as a risk factor, but it was not significant in multivariable analysis and thus excluded from the final model. The BMI was evaluated as a categorical variable (BMI <25, 25–29.9, 30–35, and >35 kg/m2) according to the National Heart, Lung, and Blood Institute’s classification of normal weight, overweight, grade 1 obesity, and grade 2–3 obesity.26 There were very few people with BMI <18.5 kg/m2, hence they were included in the “normal weight” category. As per previous studies,4 proteinuria was log-transformed and scaled to the natural log of 2, such that the coefficient for this variable represents the increase in risk associated with a doubling of urine protein levels. For the variables included in the ESRD as a first event competing risk model (Table 2), we evaluated the association with continuous eGFR decline (in ml/min/1.73 m2 per year) using mixed models with the same covariates.
Table 2.
Subhazard ratios for first CVD, ESRD, and death event, among CRIC participants reaching eGFR <30 ml/min/1.73 m2
| CVD Event | ESRD Event | Death Event | |
|---|---|---|---|
| Age, per 10-y older | 1.18 (1.04–1.32) | 0.76 (0.70–0.83) | 1.77 (1.41–2.24) |
| Male sex | 0.99 (0.78–1.25) | 1.22 (1.01–1.49) | 1.15 (0.77–1.71) |
| Race | |||
| Black | 0.90 (0.71–1.14) | 1.16 (0.93–1.43) | 1.69 (1.09–2.62) |
| Other | 0.83 (0.54–1.28) | 1.39 (0.99–1.96) | 0.82 (0.38–1.76) |
| White | 1.00 (reference | 1.00 (reference | 1.00 (reference |
| Hispanic vs non-Hispanic ethnicity | 0.89 (0.60–1.32) | 0.80 (0.58–1.11) | 2.23 (1.20–4.15) |
| eGFR, per 5-ml/min/1.73 m2 higher | 0.96 (0.83–1.11) | 0.76 (0.66–0.86) | 0.88 (0.69–1.12) |
| Urine PCR, per 2-fold higher | 0.98 (0.92–1.03) | 1.34 (1.27–1.41) | 0.89 (0.82–0.98) |
| BMI category | |||
| 25–29.9 kg/m2 | 1.07 (0.73–1.58) | 0.98 (0.74–1.29) | 0.80 (0.43–1.50) |
| 30–35 kg/m2 | 1.31 (0.90–1.90) | 0.84 (0.62–1.14) | 0.96 (0.53–1.73) |
| >35 kg/m2 | 1.33 (0.92–1.92) | 0.76 (0.57–1.03) | 1.01 (0.56–1.82) |
| 25 kg/m2 | 1.00 (reference | 1.00 (reference | 1.00 (reference |
| Diabetes | 1.69 (1.31–2.18) | 0.82 (0.67–1.01) | 1.17 (0.77–1.78) |
| SBP, per 10–mm Hg higher | 1.08 (1.03–1.14) | 1.03 (0.99–1.08) | 0.92 (0.82–1.02) |
| Any CVD History | 2.16 (1.70–2.75) | 0.65 (0.52–0.82) | 1.04 (0.70–1.53) |
| Current smoking | 1.16 (0.85–1.58) | 0.84 (0.65–1.10) | 2.01 (1.25–3.25) |
| Ejection fraction, per 5% greater | 0.85 (0.81–0.91) | 1.06 (1.00–1.12) | 0.98 (0.88–1.10) |
Note: Associations reflect sub-hazard ratios (95% confidence intervals) for each outcome, considering all other outcomes as competing risks, and adjusted for all variables shown in the table. Due to missing values detailed in Table 1, population size is 1384. Bold font indicates statistically significant values (p<0.05).
Abbreviations: eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; PCR, protein-creatinine ratio; BMI, body mass index; SBP, systolic blood pressure; CRIC, Chronic Renal Insufficiency Cohort; CVD, cardiovascular disease
The probability (absolute risk) of having developed a first event of ESRD, CVD, and death at 1-year was estimated for several hypothetical patient scenarios using the baseline subhazard and sub-HRs estimated in the competing risk models. Because statistical software for multivariable competing risk regression can only estimate cumulative incidence in the setting of two competing events, the sum of the three probabilities was scaled to the 1-year probability of having any one of those events (i.e., a composite variable of ESRD, CVD, and death) estimated using Cox regression with the same covariates. Scenarios were chosen to reflect common profiles of patients seen in nephrology clinical practice.
The adjusted associations of patient characteristics and all mortality events (i.e., both pre-and post-ESRD and CVD) were assessed using Cox proportional hazards with the same covariates as well as two time-varying variables indicating: (1) onset of ESRD, and (2) CVD event after eGFR 30 ml/min/1.73 m2. A priori, we were interested in whether mortality risk associations between age, race, BMI and diabetes changed pre- and post-ESRD; this was assessed by including a product term for each of risk factor with time-varying ESRD. A positive finding would suggest that risk-relationships differed in the pre- and post-ESRD period (e.g., the often-cited “obesity paradox” suggests that higher BMI is a risk factor for death in CKD but protective in ESRD). We also assessed the interaction between baseline eGFR and proteinuria and ESRD, expecting the kidney function measurements to diminish in importance after the development of ESRD. All analyses were performed using Stata/MP 13.1 (College Station, TX).
RESULTS
Baseline Characteristics of Participants
There were 1,798 participants who enrolled in CRIC with eGFR <30 ml/min/1.73 m2 (N=807) or who developed eGFR <30 ml/min/1.73 m2 during the course of follow-up (N=991). At the first qualifying study visit (i.e., the first visit in which a participant had an observed eGFR <30 ml/min/1.73 m2), mean age was 59.9 years and mean time since CRIC study enrollment was 1.6 years. Of these study participants, 45.8% were women, 46.2% were black, and 16.7% identified as Hispanic ethnicity (either black or white). The vast majority (90%) were under the care of a nephrologist. The average eGFR by the CRIC formula13 was 25 ml/min/1.73 m2, and mean proteinuria was 1.8 g/day. Nearly all had hypertension (95.0%), and 61.6% had diabetes mellitus. A minority of participants (18%) had an ejection fraction <45%.
When classified by first event (CVD event, ESRD, or death), the 678 (37.7%) participants who first developed ESRD were younger, with higher blood pressure and proteinuria (Table 1). In contrast, the 455 (25.3%) participants who had a pre-ESRD CVD event were older, with higher BMI; they were also on more anti-hypertensive medications, had higher prevalence of CVD (particularly a history of myocardial infarction or coronary revascularization), and had lower ejection fraction on echocardiogram. The 162 (9.0%) participants who had death as a first event had the oldest mean age and were much more likely to be smokers. The 384 (21.4%) participants who survived to the end of follow-up event-free were also older (average age, 63.1 years), with lower systolic blood pressure (mean, 120 mmHg) and proteinuria (median, 0.2 g/day) than the other participants.
Table 1.
Baseline characteristics of CRIC participants reaching eGFR <30 ml/min/1.73 m2, stratified by first outcome
| Overall (N=1,798) |
Withdrew (n=119 [6.6%]) |
Event-Free (n=384 [21.4%]) |
CVD Event (n=455 [25.3%]) |
ESRD Event (n=678 [37.7%]) |
Death Event (n=162 [9.0%]) |
|
|---|---|---|---|---|---|---|
| Age, y | 59.9 (11.3) | 58.6 (12.5) | 63.1 (10.2) | 62.2 (9.3) | 55.8 (12.1) | 63.9 (8.3) |
| Female sex | 45.8 | 51.3 | 52.3 | 44.6 | 42.2 | 44.4 |
| Race | ||||||
| White | 41.0 | 41.2 | 50.5 | 39.6 | 37.5 | 37.7 |
| Black | 46.2 | 46.2 | 36.7 | 50.3 | 46.8 | 54.3 |
| Other | 12.8 | 12.6 | 12.8 | 10.1 | 15.8 | 8.0 |
| Hispanic ethnicity | 16.7 | 16.0 | 15.9 | 12.3 | 20.8 | 14.8 |
| High school graduate | 72.5 | 78.2 | 76.6 | 70.5 | 72.4 | 64.2 |
| Under nephrology care | 90.0 | 87.4 | 90.4 | 88.4 | 91.3 | 90.7 |
| BMI, kg/m2 | 32.5 (8.1) | 32.2 (7.2) | 32.2(7.9) | 33.9 (8.6) | 31.9 (7.9) | 32.6 (8.6) |
| BMI category | ||||||
| <18.5 kg/m2 | 0.8 | 0 | 0.8 | 0.2 | 1 | 2.5 |
| 18.5–24.99 kg/m2 | 15.2 | 16 | 15.4 | 11.4 | 17.1 | 14.2 |
| 25–29.99 kg/m2 | 26.5 | 28.6 | 28.9 | 22.4 | 27 | 24.1 |
| 30–34.99 kg/m2 | 42.3 | 38.7 | 40.6 | 47 | 39.8 | 38.9 |
| ≥35 kg/m2 | 15.2 | 13.4 | 13 | 17.6 | 13.9 | 17.9 |
| Systolic BP, mmHg | 131.0 (23.8) | 128.4 (20.4) | 120.3 (20.6) | 133.5 (23.2) | 136.0 (23.6) | 130.2 (27.0) |
| Diastolic BP, mmHg | 69.1 (13.4) | 69.1 (13.3) | 65.3 (11.4) | 67.3 (13.3) | 73.1 (13.7) | 66.9 (13.1) |
| Diabetes | 61.6 | 53.8 | 49.2 | 74.3 | 61.5 | 61.1 |
| History of CVD | 45.8 | 27.7 | 40.9 | 67.7 | 34.8 | 54.9 |
| History of CHF | 16.3 | 9.2 | 9.1 | 32.1 | 9.7 | 21.6 |
| History of MI/revasc | 30.0 | 16 | 26.6 | 47.0 | 22.1 | 33.3 |
| History of stroke | 13.2 | 11.8 | 11.7 | 19.3 | 9.6 | 16.0 |
| Current smoker | 14.8 | 10.9 | 10.4 | 15.4 | 15.5 | 23.5 |
| eGFR, mL/min/1.73 m2 | 24.9 (3.9) | 25.5 (3.4) | 26.5 (3.0) | 24.7 (3.8) | 23.9 (4.2) | 24.7 (3.9) |
| Proteinuria (g/d) | 0.7 [0.1–2.2] | 0.4 [0.1–1.5] | 0.2 [0.1–0.5] | 0.6 [0.1–2.3] | 1.6 [0.6–3.5] | 0.3 [0.1–1.3] |
| Serum albumin, g/dL | 3.8 (0.5) | 3.9 (0.5) | 4.0 (0.4) | 3.8 (0.5) | 3.7 (0.5) | 3.8 (0.5) |
| ACEi or ARB use | 71.7 | 66.4 | 77.6 | 70.5 | 69.3 | 68.5 |
| No. of BP drug classes | 3.2 (1.5) | 2.8 (1.4) | 2.9 (1.4) | 3.6 (1.3) | 3.1 (1.5) | 3.3 (1.3) |
| Statin use | 64.1 | 48.7 | 65.9 | 69.0 | 61.9 | 61.1 |
| Ejection Fraction, % | 52.2 (9.3) | 55.1 (8.5) | 53.5 (7.9) | 49.2 (11.5) | 53.2 (7.7) | 51.6 (10.9) |
| Ejection Fraction Category | ||||||
| > 50% | 71.3 | 58 | 70.6 | 53.2 | 69.5 | 56.2 |
| 46%-50% | 10.7 | 6.7 | 10.2 | 10.3 | 9.6 | 7.4 |
| 36%-45% | 12 | 5 | 10.7 | 13 | 11.1 | 7.4 |
| ≤35% | 6 | 2.5 | 2.6 | 12.3 | 2.4 | 7.4 |
Note: Participant characteristics reflect values obtained from the first CRIC study visit in which eGFR <30 mL/min/1.73 m2. Values for categorical variables are given as percentages; values for continuous variables, as mean ± standard deviation or median [interquartile range]. All p-values for comparison <0.05 with the exception of ACEi/ARB use. All variables have complete data with the exception of BMI (n=1771), systolic and diastolic blood pressure (n=1794 and 1792, respectively), proteinuria (n=1580), medication use (n=1785), and ejection fraction (n=1605). Echocardiogram parameters were obtained at exams prior to the baseline visit for the majority of the participants (90%).
Abbreviations: BMI, body-mass index; BP, blood pressure; CRIC, Chronic Renal Insufficiency Cohort; CVD, cardiovascular disease; revasc, revascularization; eGFR, estimated glomerular filtration rate calculated using the CRIC equation; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Risk of ESRD, CVD, and Death as First Events in Participants With eGFR <30 ml/min/1.73 m2
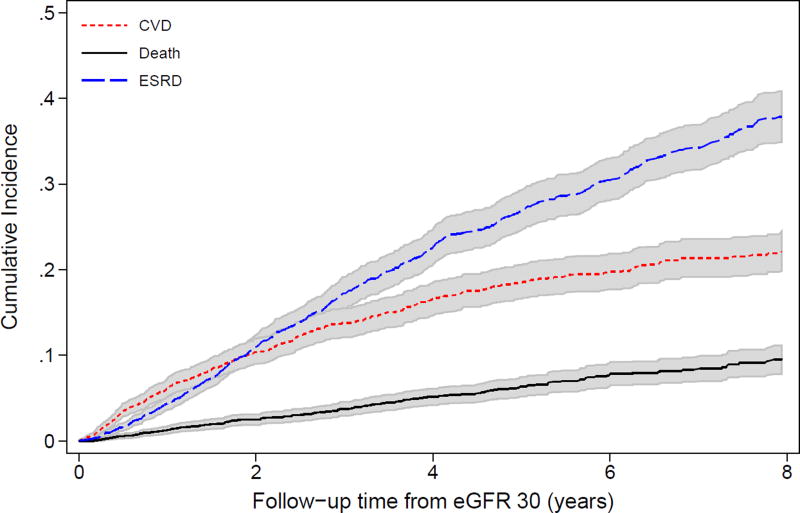
Median time from the qualifying CRIC study visit (i.e., when eGFR was first observed to be <30 ml/min/1.73 m2) to death or end of follow-up was 5.5 (interquartile range [IQR], 3.2–7.3) years. Median time from the qualifying CRIC study visit to first event (CVD event, ESRD, death, or end-of-follow-up) was 2.6 (IQR, 1.1–4.7) years. At approximately 1.8 years of follow-up, ESRD surpassed CVD events as the most common first outcome in the overall population (Figure 1). This was similar in the 807 CRIC participants who enrolled with an eGFR <30 ml/min/1.73 m2 as well as the 991 participants who developed eGFR <30 ml/min/1.73 m2 during follow-up (Figure S1).
Figure 1.
Cumulative incidence of first events (ESRD, CVD, or death) over time among CRIC study participants from eGFR 30 ml/min/1.73 m2 (N=1798).
Patient characteristics at eGFR 30 ml/min/1.73 m2 were significantly associated with first outcomes (Table 2). Risk factors for pre-ESRD CVD events included older age, higher systolic blood pressure, the presence of diabetes and pre-existing CVD, and lower ventricular ejection fraction. Risk factors for death as a first event included older age, black race, Hispanic ethnicity, and current smoking. In contrast, risk factors for first developing ESRD included younger age, male sex, lower eGFR, higher proteinuria, and the absence of prior CVD.
Further investigation of risk factors associated with eGFR decline confirmed much of the associations seen with first ESRD: there was a faster yearly decline with younger age (0.40 [95% CI, 0.21–0.58] ml/min/1.73 m2 per year faster per 10 years younger), higher proteinuria (0.66 [95% CI, 0.57–0.75] ml/min/1.73 m2 per year faster per two-fold higher), lower baseline eGFR (0.63 [95% CI, 0.37–0.88] ml/min/1.73 m2 per year faster per 5 ml/min/1.73 m2 lower), and higher systolic blood pressure (0.28 [95% CI, 0.19–0.37] ml/min/1.73 m2 per year faster per 10 mmHg higher). In contrast, male sex and prior CVD were not associated with faster eGFR decline.
1-Year Probability of Events for Various Scenarios
Combining the sub-HRs and baseline subhazard of first events, we estimated the 1-year probability of first events for hypothetical scenarios. For a patient scenario close to the study mean (60-year-old white woman with eGFR of 30 ml/min/1.73 m2, proteinuria of 1.8 g/day, systolic blood pressure of 130 mmHg, no diabetes mellitus or history of CVD, an ejection fraction of 50%, BMI of 30 kg/m2, and no current smoking), the 1-year probability of remaining CVD-, ESRD-, and death-free was 92.3% (Table S2). The predicted probability of developing a first CVD event was similar to the 1-year predicted probability of first developing ESRD (3.3% versus 4.1%), and the probability of death was 0.3%. In contrast, a 40-year-old black man with similar risk factors but a systolic blood pressure of 150 mmHg had a predicted probability of remaining event-free of 84.3% at 1-year, with a much greater risk of first developing ESRD compared to a first CVD event (13.2% versus 2.4%).
Subsequent Risks After First Events
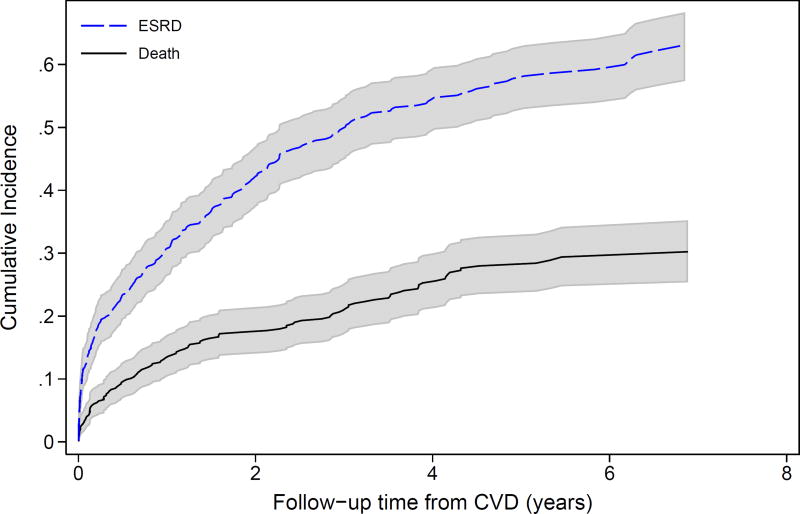
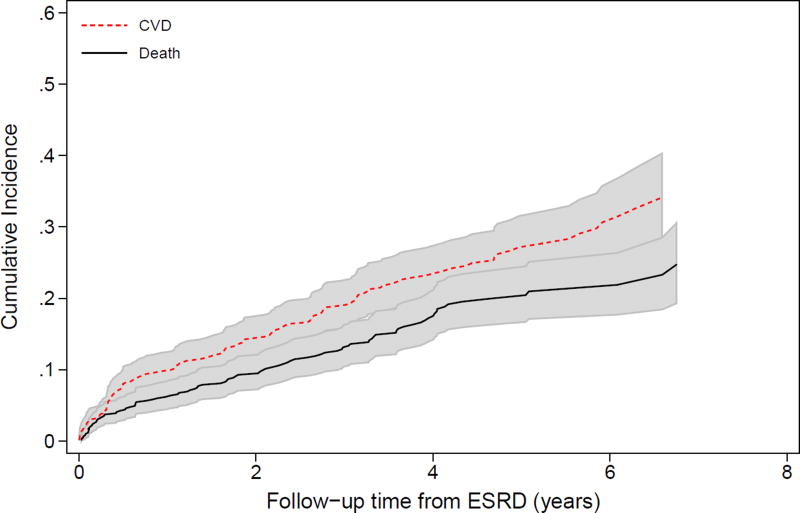
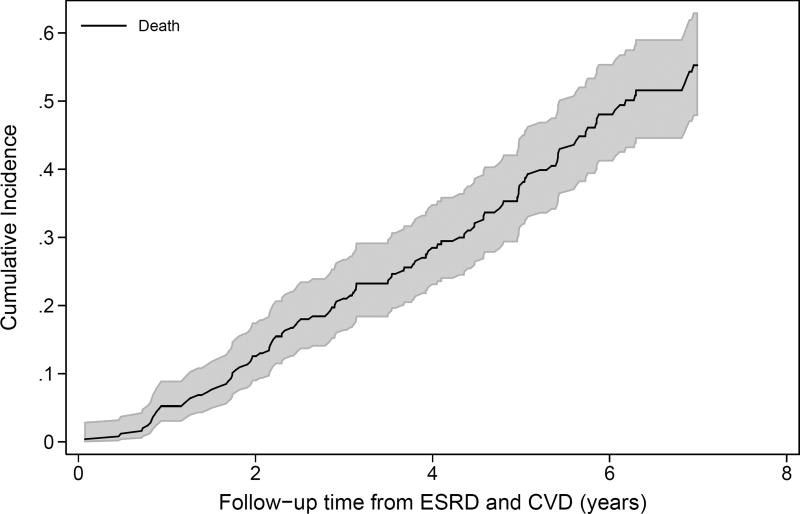
After the initial adverse event, the incidence of subsequent adverse events increased. For example, both ESRD and pre-ESRD death after a first CVD event were high (Figure 2A). Four years after a first CVD event, over 50% of the participants had developed ESRD, and 20% had died prior to ESRD. Four years after ESRD as first event, nearly 40% of participants had developed either CVD or pre-CVD death (Figure 2B). Among those who had had a CVD event and then ESRD, the risk of a subsequent CVD event was higher still (Figure S2). Death after a participant had both ESRD and a CVD event was extremely high, with 50% mortality by 6 years (Figure 2C). In analyses adjusting for baseline covariates, both the development of ESRD (HR, 3.68; 95% CI, 2.22–6.09) and CVD (HR, 2.43; 95% CI, 1.93–3.06) were significantly associated with higher risk of mortality (Table 3). Of note, there were no significant interactions of ESRD with age, sex, race, or BMI for mortality risk, suggesting that risk relationships between each characteristic and death were similar pre- and post-ESRD. There was a significant interaction between baseline eGFR and time-varying ESRD as well as diabetes and time-varying ESRD, with both measures having weaker, non-statistically significant associations with death in the post-ESRD period. The coefficient for proteinuria declined after ESRD onset, but the interaction was not statistically significant (p=0.5).
Figure 2.
Cumulative incidence of subsequent events (ESRD, CVD, or death) over time among CRIC study participants. (A) reflects the incidence of ESRD and pre-ESRD death after the occurrence of a first CVD event (N=455); (B) reflects the occurrence of CVD or pre-CVD death after a first ESRD event (N=678); and (C) reflects the incidence of death after the occurrence of ESRD then CVD or CVD then ESRD, with time at risk accruing after the latter event (N=248).
Table 3.
Multivariable associations between baseline characteristics and death, adjusted for time-varying ESRD and time-varying CVD
| Death Event | |
|---|---|
| Age, per 10-y older | 1.54 (1.36–1.75) |
| Male sex | 1.07 (0.86–1.33) |
| Race | |
| Black | 1.03 (0.81–1.30) |
| Other | 1.03 (0.69–1.54) |
| White | 1.00 (reference) |
| Hispanic vs non-Hispanic ethnicity | 1.18 (0.83–1.70) |
| eGFR, per 5-ml/min/1.73 m2 higher* | 0.79 (0.65–0.96); 1.19(1.00–1.44) |
| Urine PCR, per 2-fold higher | 1.02 (0.97–1.08) |
| BMI category | |
| 25–30 kg/m2 | 0.85 (0.61–1.17) |
| 30–35 kg/m2 | 0.91 (0.66–1.26) |
| >35 kg/m2 | 0.94 (0.68–1.30) |
| <25 kg/m2 | 1.00 (reference) |
| Diabetes* | 1.60 (1.15–2.21); 0.94 (0.68–1.31) |
| SBP, per 10-mm Hg higher | 0.99 (0.94–1.04) |
| Any CVD History | 1.60 (1.28–2.00) |
| Current smoking | 1.74 (1.33–2.27) |
| Ejection fraction, per 5% greater | 0.92 (0.87–0.97) |
| Time-varying ESRD | 3.68 (2.22–6.09) |
| Time-varying CVD | 2.43 (1.93–3.06) |
Note: Values are given as hazard ratio (95% confidence interval). Bold font indicates statistically significant relationships. There were no significant interactions between age, sex, race, proteinuria, BMI, and time-varying ESRD.
Abbreviations: eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; PCR, protein-creatinine ratio; BMI, body mass index; SBP, systolic blood pressure; CVD, cardiovascular disease
There were significant interactions between eGFR and time-varying ESRD and diabetes and time-varying ESRD. Coefficients shown reflect the pre-ESRD and post-ESRD relationship, respectively.
DISCUSSION
This study of 1,798 heterogeneous participants with advanced CKD demonstrates the variability in prognosis and timing of adverse outcomes. While the majority of participants progressed to ESRD during follow-up, the path by which this occurred differed by baseline characteristics. Older patients were more likely to develop CVD prior to ESRD; younger patients tended to have faster declines in eGFR without intervening CVD events. Once participants developed ESRD or CVD, the risk of subsequent adverse outcomes increased. For example, the risk of mortality was approximately 2-fold higher after an ESRD or CVD event. These results, and particularly their potential application to individual patients, can help guide patient counseling and therapy.
Knowledge of the range and sequence of adverse outcomes in CKD can inform patient care and counseling in several ways. Improving physician cognizance of the absolute risks not only of ESRD but also CVD and death may increase vigilance in prescribing traditionally cardiovascular-based therapies such as statins, aspirin, and smoking cessation.27 Indeed, the high degree of overlap between ESRD and CVD – the risk of CVD was extremely high after ESRD, as was the risk of ESRD after CVD – supports assertions that CKD be considered a CVD risk equivalent.28 Our study adds to the existing literature by quantifying the risk and sequence of individual adverse outcomes, as well as risk factor associations for their development.
Similar to the results of the African American Study of Kidney Disease and Hypertension (AASK),5 the absolute risks of ESRD and CVD were higher than the risk of death in CRIC participants. Corresponding rates of ESRD and death have been shown in the MDRD Study as well as the Italian Target Blood Pressure Levels in CKD (TABLE-CKD) study.29,30 Interestingly, studies in health care utilization datasets have reported opposite findings, with death being the most common first outcome in advanced CKD.6,7 Heterogeneity in absolute risk of ESRD has also been reported by the CKD Prognosis Consortium, where it appeared to track with world region.31 Our results capture adverse outcomes among volunteers in a longitudinal cohort study, which tend to represent patients who are relatively healthy, but suggest that some of these differences may relate to underlying patient characteristics, particularly the age, racial makeup, and prevalence of diabetes and CVD in the population. Others have hypothesized that referral to nephrology (which may vary widely by region) might explain differences.7 In CRIC, the vast majority of participants had seen a nephrologist at least once, but we have no information on the intensity of nephrology involvement in individual patient care. We note that CRIC participants have, on average, excellent blood pressure control with a mean systolic blood pressure of 130 mmHg; over 70% are receiving angiotensin-converting enzyme inhibitors or angiotensin receptor blockers.
Results from this study suggest that the best prognosis in advanced CKD is among participants with low levels of proteinuria and well-controlled blood pressure, with much of the risk relating to higher rates of CVD and ESRD. These findings are consistent with the long-term follow-up from historical clinical trials. For example, AASK participants with lower levels of proteinuria had slower progression of CKD. Among AASK participants with higher proteinuria, more intensive treatment of blood pressure resulted in slower progression of CKD, consistent with the MDRD Study, which showed a lower risk of kidney failure among those randomized to the lower blood pressure treatment arm.32,33 They are also consistent with recent results from the Systolic Blood Pressure Intervention Trial (SPRINT), where intensive blood pressure control reduced all-cause mortality and a composite cardiovascular outcome in those with and without CKD (note that the mean eGFRs were 81 and 48 ml/min/1.73m2 in the two groups, respectively).34 Among SPRINT participants with CKD, there was a trend toward a lesser number of ESRD events in the intensive treatment arm (6 versus 10 events), although this difference was not statistically significant, likely due to a reduced sample size. Palit and colleagues also reported a strong relationship between systolic and diastolic blood pressure and the development of ESRD among patients with eGFR ≤30 ml/min/1.73 m2, with pulse pressure being a strong predictor of cardiovascular events.35 Our data similarly suggests that high blood pressure is a risk factor, particularly for the development of CVD.
An interesting aspect of our study is the ability to follow participants from the development of eGFR <30 ml/min/1.73 m2 through death or end-of-follow-up, allowing evaluation of both pre-and post-ESRD risk associations. A traditional school of thought posits that blacks exhibit better survival on dialysis compared to whites, which has been suggested as a partial explanation for disparities in transplantation rates.36 Similarly, higher BMI has been proposed as a protective factor for patients on dialysis.37 However, these observations are limited in that they stem from a prevalent dialysis population, which by definition requires survival to dialysis. Our study adds to this literature by evaluating the risk of death in the pre- and post-dialysis periods. With the caveat that the population comprised volunteers in a research cohort, we found no difference in mortality risk between the pre- or post-ESRD period by race or BMI. The only risk factors that changed in importance after the development of ESRD were baseline eGFR and diabetes mellitus, both of which became non-significant after ESRD.
This study has strengths and limitations. Kidney function was estimated using a CRIC-specific equation that incorporates both creatinine and cystatin C, and thus likely closely corresponds to the measured GFR.38 On the other hand, cystatin C and a study-specific equation are not routinely used in clinical practice, which may limit the generalizability of the data. Creatinine, cystatin C, and proteinuria were assessed according to research protocol, and presumably reflect values when a participant was in steady state rather than acute illness. Participants were followed up closely for intervening events, and CVD and ESRD events were adjudicated by expert consensus. Few studies have echocardiogram data in advanced CKD, and ejection fraction proved to be an important risk factor.18 On the contrary, the CRIC population was specifically recruited for kidney disease, and the vast majority had previously seen a nephrologist. This differs from the “real-world” setting, where many patients with advanced CKD have severe comorbidities, such as cancer or advanced heart failure, and are not necessarily receiving nephrology care. Conversely, the CRIC population may be fairly representative of a patient population seen in a nephrology practice in the United States. Additional work and validation of outcome prediction in patients with CKD may help inform delivery of “personalized medicine”; however, we acknowledge that the precision of such models will depend on the amount of variation explained by known covariates, and that such models may be much more accurate in the short-term.15,31,39
In summary, we report the clinical outcomes among CRIC participants with eGFR <30 ml/min/1.73 m2, demonstrating that while ESRD is the most common first event over the long-term, the risks of CVD and death are interrelated, with each adverse outcome increasing the risk for subsequent events. The work represents a novel approach to outcomes assessment in CKD as it focuses on advanced CKD, considering multiple end points at once, with evaluation not only of the occurrence of events but also the sequence. In clinical practice, an integrated approach to preventing CVD, ESRD, and death—potentially through the reduction of blood pressure and proteinuria—may provide optimal care in patients with advanced CKD. Additional work to individualize outcome prediction may inform a personalized approach that could help guide patient counseling and medical decision-making.
Supplementary Material
Figure S1: Cumulative incidence of first events over time in participants from eGFR = 30, stratified by if they enrolled with or developed eGFR < 30.
Figure S2: Cumulative incidence of recurrent CVD or pre-CVD death over time among participants who developed CVD, and then ESRD after eGFR = 30.
Table S1: Cause-specific HRs for CVD, ESRD, and death, censored at first event.
Table S2: 1-y probability of remaining event-free, or first developing CVD event, ESRD, or death, for hypothetical scenarios.
Acknowledgments
The CRIC Study Investigators are Harold I. Feldman, MD, MSCE, Alan S. Go, MD, John W. Kusek, PhD, James P. Lash, MD, Akinlolu Ojo, MD PhD, Mahboob Rahman, MD, and Raymond R. Townsend, MD.
Support: Funding for the CRIC Study was obtained under a cooperative agreement from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award (CTSA; National Institutes of Health [NIH]/National Center for Advancing Translational Sciences [NCATS] UL1TR000003), Johns Hopkins University UL1 TR-000424, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the NCATS component of the NIH and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research UL1TR000433, University of Illinois at Chicago CTSA UL1RR029879, Tulane Center of Biomedical Research Excellence (COBRE) for Clinical and Translational Research in Cardiometabolic Diseases P20 GM109036, and Kaiser Permanente NIH/National Center for Research Resources University of California San Francisco—Clinical and Translational Science Institute UL1 RR-024131. In addition, Drs Grams and Hsu have received support for this project from the NIDDK (K08DK092287 and R01DK70939, respectively). None of the funders had any role in study design; collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Because no editor without conflicts of interest was available at the time of manuscript submission, the peer-review and decision-making processes were handled entirely by an Editorial Board Member (Robert N. Foley, MD) who served as Acting Editor-in-Chief. Details of the journal’s procedures for potential editor conflicts are given in the Information for Authors & Journal Policies.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Contributions: Research idea and study design: MEG, LJA, C-yH; data acquisition: WY, XW, ACP, EH, MRW, BGJ, TS, LJA, C-yH, LLH, JHS, JH; data analysis/interpretation: MEG, C-yH, CMR, LAI, XW, WY, JHS; statistical analysis: MEG, XW, WY; supervision or mentorship: C-yH, LJA, MRW, BGJ. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Peer Review: Evaluated by 3 external peer reviewers, a statistician, and an Acting Editor-in-Chief.
Note: The supplementary material accompanying this article (doi: _______ ) is available at www.ajkd.org Supplementary Material Descriptive Text for Online Delivery
References
- 1.Astor BC, Matsushita K, Gansevoort RT, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int. 2011;79(12):1331–1340. doi: 10.1038/ki.2010.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. NEJM. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 3.van der Velde M, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79(12):1341–52. doi: 10.1038/ki.2010.536. [DOI] [PubMed] [Google Scholar]
- 4.Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alves TP, Wang X, Wright JT, Jr, et al. Rate of ESRD exceeds mortality among African Americans with hypertensive nephrosclerosis. J Am Soc Nephrol. 2010;21(8):1361–1369. doi: 10.1681/ASN.2009060654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004;164(6):659–663. doi: 10.1001/archinte.164.6.659. [DOI] [PubMed] [Google Scholar]
- 7.Minutolo R, Lapi F, Chiodini P, et al. Risk of ESRD and death in patients with CKD not referred to a nephrologist: a 7-year prospective study. Clin J Am Soc Nephrol. 2014 Sep 5;9(9):1586–1593. doi: 10.2215/CJN.10481013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feldman HI, Appel LJ, Chertow GM, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. J Am Soc Nephrol. 2003;14(7 Suppl 2):S148–153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 9.Lash JP, Go AS, Appel LJ, et al. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4(8):1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer MJ, Go AS, Lora CM, et al. CKD in Hispanics: Baseline characteristics from the CRIC (Chronic Renal Insufficiency Cohort) and Hispanic-CRIC Studies. Am J Kidney Dis. Aug. 2011;58(2):214–227. doi: 10.1053/j.ajkd.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kidney Disease: Improving Global O. Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int.Suppl. 2013;3:1–150. Journal Article. [Google Scholar]
- 12.Ku E, Xie D, Shlipak M, et al. Change in Measured GFR Versus eGFR and CKD Outcomes. J Am Soc Nephrol. 2015 Nov 24; doi: 10.1681/ASN.2015040341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson AH, Yang W, Hsu CY, et al. Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. Aug. 2012;60(2):250–261. doi: 10.1053/j.ajkd.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu KD, Yang W, Go AS, et al. Urine neutrophil gelatinase-associated lipocalin and risk of cardiovascular disease and death in CKD: results from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. Feb. 2015;65(2):267–274. doi: 10.1053/j.ajkd.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tangri N, Stevens LA, Griffith J, et al. A predictive model for progression of chronic kidney disease to kidney failure. JAMA. 2011;305(15):1553–1559. doi: 10.1001/jama.2011.451. [DOI] [PubMed] [Google Scholar]
- 16.Grams ME, Li L, Greene TH, et al. Estimating Time to ESRD Using Kidney Failure Risk Equations: Results From the African American Study of Kidney Disease and Hypertension (AASK) Am J Kidney Dis. 2015;65(3):394–402. doi: 10.1053/j.ajkd.2014.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bansal N, McCulloch CE, Rahman M, et al. Blood pressure and risk of all-cause mortality in advanced chronic kidney disease and hemodialysis: the chronic renal insufficiency cohort study. Hypertension. Jan. 2015;65(1):93–100. doi: 10.1161/HYPERTENSIONAHA.114.04334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bansal N, Keane M, Delafontaine P, et al. A longitudinal study of left ventricular function and structure from CKD to ESRD: the CRIC study. Clin J Am Soc Nephrol. 2013;8(3):355–362. doi: 10.2215/CJN.06020612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 20.Dignam JJ, Zhang Q, Kocherginsky MN. The Use and Interpretion of Competing Risks Regression Models. Clinical Cancer Research. 2012;18(8):2301–2308. doi: 10.1158/1078-0432.CCR-11-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grams ME, Coresh J. Assessing risk in chronic kidney disease: a methodological review. Nat Rev Nephrol. Jan. 2013;9(1):18–25. doi: 10.1038/nrneph.2012.248. [DOI] [PubMed] [Google Scholar]
- 22.Austin PC, Lee DS, Fine JP. Introduction to the Analysis of Survival Data in the Presence of Competing Risks. Circulation. 2016;133(6):601–609. doi: 10.1161/CIRCULATIONAHA.115.017719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gansevoort RT, Matsushita K, van der Velde M, et al. Lower estimated GFR higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int. 2011;80(1):93–104. doi: 10.1038/ki.2010.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fox CS, Matsushita K, Woodward M, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012;380(9854):1662–1673. doi: 10.1016/S0140-6736(12)61350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsu CY, Iribarren C, McCulloch CE, Darbinian J, Go AS. Risk factors for end-stage renal disease: 25-year follow-up. Arch Intern Med. 2009 Feb 23;169(4):342–350. doi: 10.1001/archinternmed.2008.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary. Expert Panel on the Identification, Evaluation, and Treatment of Overweight in Adults. Am J Clin Nutr. 1998 Oct;68(4):899–917. doi: 10.1093/ajcn/68.4.899. [DOI] [PubMed] [Google Scholar]
- 27.Wanner C, Tonelli M. KDIGO Clinical Practice Guideline for Lipid Management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Int. Jun. 2014;85(6):1303–1309. doi: 10.1038/ki.2014.31. [DOI] [PubMed] [Google Scholar]
- 28.Tonelli M, Muntner P, Lloyd A, et al. Risk of coronary events in people with chronic kidney disease compared with those with diabetes: a population-level cohort study. Lancet. 2012;380(9844):807–14. doi: 10.1016/S0140-6736(12)60572-8. [DOI] [PubMed] [Google Scholar]
- 29.Menon V, Wang X, Sarnak MJ, et al. Long-term outcomes in nondiabetic chronic kidney disease. Kidney Int. Jun. 2008;73(11):1310–1315. doi: 10.1038/ki.2008.67. [DOI] [PubMed] [Google Scholar]
- 30.De Nicola L, Chiodini P, Zoccali C, et al. Prognosis of CKD patients receiving outpatient nephrology care in Italy. Clin J Am Soc Nephrol. 2011;6(10):2421–2428. doi: 10.2215/CJN.01180211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tangri N, Grams ME, Levey AS, et al. Multinational Assessment of Accuracy of Equations for Predicting Risk of Kidney Failure: A Meta-analysis. JAMA. 2016 Jan 12;315(2):164–174. doi: 10.1001/jama.2015.18202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Appel LJ, Wright JT, Jr, Greene T, et al. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med. 2010 Sep 2;363(10):918–929. doi: 10.1056/NEJMoa0910975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarnak MJ, Greene T, Wang X, et al. The effect of a lower target blood pressure on the progression of kidney disease: long-term follow-up of the modification of diet in renal disease study. Ann Intern Med. 2005 Mar 1;142(5):342–351. doi: 10.7326/0003-4819-142-5-200503010-00009. [DOI] [PubMed] [Google Scholar]
- 34.Wright JT, Jr, Williamson JD, Whelton PK, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015 Nov 26;373(22):2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palit S, Chonchol M, Cheung AK, Kaufman J, Smits G, Kendrick J. Association of BP with Death, Cardiovascular Events, and Progression to Chronic Dialysis in Patients with Advanced Kidney Disease. Clin J Am Soc Nephrol. 2015 Jun 5;10(6):934–940. doi: 10.2215/CJN.08620814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kucirka LM, Grams ME, Lessler J, et al. Association of race and age with survival among patients undergoing dialysis. JAMA. 2011;306(6):620–626. doi: 10.1001/jama.2011.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doshi M, Streja E, Rhee CM, et al. Examining the robustness of the obesity paradox in maintenance hemodialysis patients: a marginal structural model analysis. Nephrol Dial Transplant. 2016;31(8):1310–9. doi: 10.1093/ndt/gfv379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. NEJM. 2012;367(1):20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grams ME, Coresh J. Predicting Risk of RRT in Patients with CKD. Clin J Am Soc Nephrol. 2016 Dec 27; doi: 10.2215/CJN.11841116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Cumulative incidence of first events over time in participants from eGFR = 30, stratified by if they enrolled with or developed eGFR < 30.
Figure S2: Cumulative incidence of recurrent CVD or pre-CVD death over time among participants who developed CVD, and then ESRD after eGFR = 30.
Table S1: Cause-specific HRs for CVD, ESRD, and death, censored at first event.
Table S2: 1-y probability of remaining event-free, or first developing CVD event, ESRD, or death, for hypothetical scenarios.