Summary
Background
Although unilateral primary aldosteronism is the most common surgically correctable cause of hypertension, no standard criteria exist to classify surgical outcomes. We aimed to create consensus criteria for clinical and biochemical outcomes and follow-up of adrenalectomy for unilateral primary aldosteronism and apply these criteria to an international cohort to analyse the frequency of remission and identify preoperative determinants of successful outcome.
Methods
The Primary Aldosteronism Surgical Outcome (PASO) study was an international project to develop consensus criteria for outcomes and follow-up of adrenalectomy for unilateral primary aldosteronism. An international panel of 31 experts from 28 centres, including six endocrine surgeons, used the Delphi method to reach consensus. We then retrospectively analysed follow-up data from prospective cohorts for outcome assessment of patients diagnosed with unilateral primary aldosteronism by adrenal venous sampling who had undergone a total adrenalectomy, consecutively included from 12 referral centres in nine countries. On the basis of standardised criteria, we determined the proportions of patients achieving complete, partial, or absent clinical and biochemical success in accordance with the consensus. We then used logistic regression analyses to identify preoperative factors associated with clinical and biochemical outcomes.
Findings
Consensus was reached for criteria for six outcomes (complete, partial, and absent success of clinical and biochemical outcomes) based on blood pressure, use of antihypertensive drugs, plasma potassium and aldosterone concentrations, and plasma renin concentrations or activities. Consensus was also reached for two recommendations for the timing of follow-up assessment. For the international cohort analysis, we analysed clinical data from 705 patients recruited between 1994 and 2015, of whom 699 also had biochemical data. Complete clinical success was achieved in 259 (37%) of 705 patients, with a wide variance (range 17–62), and partial clinical success in an additional 334 (47%, range 35–66); complete biochemical success was seen in 656 (94%, 83–100) of 699 patients. Female patients had a higher likelihood of complete clinical success (odds ratio [OR] 2·25, 95% CI 1·40–3·62; p=0·001) and clinical benefit (complete plus partial clinical success; OR 2·89, 1·49–5·59; p=0·002) than male patients. Younger patients had a higher likelihood of complete clinical success (OR 0·95 per extra year, 0·93–0·98; p<0·001) and clinical benefit (OR 0·95 per extra year, 0·92–0·98; p=0·004). Higher levels of preoperative medication were associated with lower levels of complete clinical success (OR 0·80 per unit increase, 0·70–0·90; p<0·001).
Interpretation
These standardised outcome criteria are relevant for the assessment of the success of surgical treatment in individual patients and will allow the comparison of outcome data in future studies. The variable baseline clinical characteristics of our international cohort contributed to wide variation in clinical outcomes. Most patients derive clinical benefit from adrenalectomy, with younger patients and female patients more likely to have a favourable surgical outcome. Screening for primary aldosteronism should nonetheless be done in every individual fulfilling US Endocrine Society guideline criteria because biochemical success without clinical success is by itself clinically important and older women and men can also derive post-operative clinical benefit.
Funding
European Research Council; European Union’s Horizon 2020; Else Kröner-Fresenius Stiftung; Netherlands Organisation for Health Research and Development–Medical Sciences; Japanese Ministry of Health, Labour and Welfare; Ministry of Health, Slovenia; US National Institutes of Health; and CONICYT-FONDECYT (Chile).
Introduction
Primary aldosteronism is a form of endocrine hypertension characterised by inappropriately high plasma aldosterone concentrations relative to suppressed plasma renin.1 The prevalence of primary aldosteronism is reported as 5% in the general hypertensive population,2 increasing to 10% in referred populations and 15–20% in patients with treatment-resistant hypertension,3 although estimates vary widely.4 Several studies have shown a higher prevalence of cardiovascular and cerebrovascular morbidity and mortality in patients with primary aldosteronism than in patients with primary hypertension matched for age, sex, and blood pressure,5–8 with resolution of excess risk after surgical or specific medical treatment.9–10 As such, early diagnosis and appropriate treatment of primary aldosteronism are essential to minimise the increased risk associated with this disorder.
Primary aldosteronism is classified into unilateral and bilateral forms of the disease, which must be distinguished because, although the unilateral form can respond well to adrenalectomy, the bilateral form is treated with mineralocorticoid receptor antagonists.11 Although adrenal venous sampling is the recommended procedure to distinguish primary aldosteronism subtypes,11 it is not widely available, with some centres relying on CT or MRI for determination of lateralisation.
Surgical treatment of unilateral primary aldosteronism should resolve the excessive aldosterone secretion in all patients. Persistence of primary aldosteronism after adrenalectomy suggests that the initial diagnosis was incorrect, with the patient having bilateral rather than unilateral primary aldosteronism. To define post-surgical outcomes, specific clinical and biochemical criteria are needed for persistent or recurrent disease. Reported proportions of patients achieving clinical remission vary widely between centres (16–72%); this variation is attributed to several underlying factors such as background primary hypertension, age, longstanding primary aldosteronism, advanced renal failure, or other comorbidities.12–15 However, heterogeneity might also reflect the absence of standardised criteria to classify outcomes of adrenalectomy for unilateral primary aldosteronism. As such, we hypothesised that standardised uniform outcome criteria applied across a large multicentre patient cohort might minimise the previously reported variation in outcome results. Such uniform criteria might not only improve clinical care of patients with primary aldosteronism, but could also provide a basis for comparison of outcome data from different clinical centres.
The objectives of the Primary Aldosteronism Surgical Outcome (PASO) study were to establish an international consensus for a set of standardised criteria for clinical and biochemical outcomes of adrenalectomy for unilateral primary aldosteronism; to apply these criteria to follow-up data in a large multicentre cohort of patients with primary aldosteronism from different clinical expert centres to calculate the proportion of patients achieving remission; to identify the preoperative determinants of successful outcome; and to determine the extent to which these outcome determinants might account for differing proportions of patients achieving complete success between centres.
Methods
Consensus building by the Delphi technique
For this international consensus and cohort analysis, we used the Delphi technique16 to reach an international consensus for criteria for six outcomes of adrenalectomy for unilateral primary aldosteronism—complete success (remission), partial success (improvement), and absent success (persistence), for both clinical and biochemical outcomes—and recommendations for the time and interval of follow-up. Because clinical and biochemical outcome results in primary aldosteronism are not a-priori linked and could depend on different preoperative factors, these outcome parameters were assessed and analysed separately.
The Delphi method uses a series of questionnaires sent to participants who were selected for their expertise in the treatment of primary aldosteronism, wide geographical representation, and, for this study, surgical representation. All communication was by email and replies were monitored by two core group members (TAW and MRe) who did not respond to the questionnaires. The Delphi process required four rounds of questionnaires with strict criteria to pass consensus at each round (appendix p 8).16 Participating centres are listed in the appendix (p 9); the process included 31 respondents (including six endocrine surgeons) from 28 centres, with three centres (Torino, Italy; Paris, France; and Nijmegen, Netherlands) providing individual responses from two participants each.
Application of the consensus criteria to assess outcome
We approached 15 centres of expertise to contribute patient data, of which 12 (in Australia, France, Germany, Italy, Japan, the Netherlands, Poland, Slovenia, and the USA) were able to accept the invitation. Clinical and biochemical outcomes (defined as complete, partial, or absent success) after adrenalectomy were assessed in the 12 referral centres. Data were collected within the prospective registries and analysed retrospectively. Approval from local ethics committees was obtained for the analysis of patient data in all centres. Written informed consent from patients was obtained in all centres apart from in Kyoto and Yokohama City because in Japan, according to the Ethical Guidelines for Medical and Health Research involving human subjects, this consent is not mandatory for research that does not involve the use of human biological specimens. Consecutive patients from each centre who had been adrenalectomised with follow-up data for outcome assessment were included in the analysis (panel). We included only patients diagnosed with unilateral primary aldosteronism by adrenal venous sampling and all participating centres used total adrenalectomy as the surgical procedure for the treatment of unilateral primary aldosteronism.
Primary aldosteronism was diagnosed in accordance with the US Endocrine Society guideline11 or the Japan Endocrine Society guideline.18 Measurements of biochemical and clinical parameters and adverse events after surgery are described in the appendix. Each centre applied the consensus clinical and biochemical outcome criteria to their patients and the proportions of patients in each outcome group were calculated at 6–12 months in accordance with the criteria for assessment of final outcome established by the PASO consensus (panel). Outcomes were assessed by each centre and cross-checked by members of the core group. Amendments were returned to participating centres for double-checking. In addition to the outcome categories (complete, partial, and absent success; panel), we assessed clinical and biochemical benefit, which we defined as the complete and partial success categories combined.
Statistical analyses
We used IBM SPSS Statistics version 22.0 for all statistical analyses, unless otherwise stated. The proportions of patients with different outcomes were calculated. All quantitative normally distributed variables are reported as means with SDs and quantitative non-normally distributed variables are presented as medians with IQRs. Categorical variables are presented as absolute numbers and percentages. We analysed quantitative normally distributed variables using one-way ANOVA with a post-hoc Bonferroni analysis. We analysed group differences using Kruskal-Wallis or Mann-Whitney U tests for quantitative non-normally distributed variables, and χ2 or Fisher’s exact tests for categorical variables.
We did a logistic regression analysis at the patient level to identify determinants of clinical and biochemical outcome. Odds ratios (ORs) for age were calculated per year; for potassium ion concentration, per 1 mmol/L; for antihypertensive medication, per unit of defined daily dose; for estimated glomerular filtration rate, per 1 mL/min; for systolic blood pressure, per 1 mm Hg; for BMI, per kg/m2. For sex, female patients were the reference group and for left ventricular hypertrophy, undetected was the reference. For most results, an OR greater than 1 indicates an increased likelihood of clinical or biochemical outcome and an OR less than 1 a decreased likelihood; the exception is for potassium ion concentration, which was analysed for lowest values, therefore in this case an OR greater than 1 indicates a decreased likelihood and an OR less than 1 an increased likelihood. We accounted for centre effects with a fixed-effect model using the Logistic Regression Program R and did multilevel modelling by fitting logistic regression models with centre as random effects using SPSS. SPSS gave p values to three decimal places. p values of less than 0·05 were considered significant.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Consensus was reached for a set of standardised criteria for complete, partial, and absent clinical and biochemical success based on blood pressure, use of antihypertensive drugs, plasma potassium and aldosterone concentrations, and plasma renin concentrations or activities (panel). In terms of follow-up interval, the consensus view was that the first post-surgical outcome assessment of at least blood pressure and plasma potassium concentration should be done within the first 3 months to adjust antihypertensive medication and correct hypokalaemia or hyperkalaemia if necessary. Final outcome (blood pressure, plasma potassium and aldosterone concentrations, and plasma renin concentrations or activities) should be assessed at 6–12 months after adrenalectomy and reassessed at yearly intervals indefinitely to exclude persistence or reoccurrence of the disease.
The consensus criteria were used to assess the proportions of patients with complete, partial, or absent clinical and biochemical success in 12 international expert clinical centres, each contributing 30–99 consecutively operated (unilateral laparoscopic adrenalectomy) patients with adequate follow-up data to assess outcome (705 patients in total). The period of patient inclusion and the numbers of patients diagnosed with unilateral primary aldosteronism during the same period are shown in the appendix (p 10).
Across the 12 centres, the proportions of patients achieving complete clinical success varied widely by contrast with consistently high proportions of patients with complete biochemical success (figure); biochemical data were available for 699 (99%) of the 705 patients with clinical data (six patients did not have adequate biochemical follow-up to assess outcome). Complete biochemical success was seen in 656 (94% [range 83–100]) of 699 patients, whereas only 259 (37% [range 17–62]) of 705 patients achieved complete clinical success (figure, tables 1, 2). Improvement in blood pressure control (partial clinical success) was attained in a further 334 (47% [range 35–66]) of the 705 patients, resulting in normalisation or improvement in blood pressure in more than 80% of patients.
Figure. Clinical and biochemical outcomes.
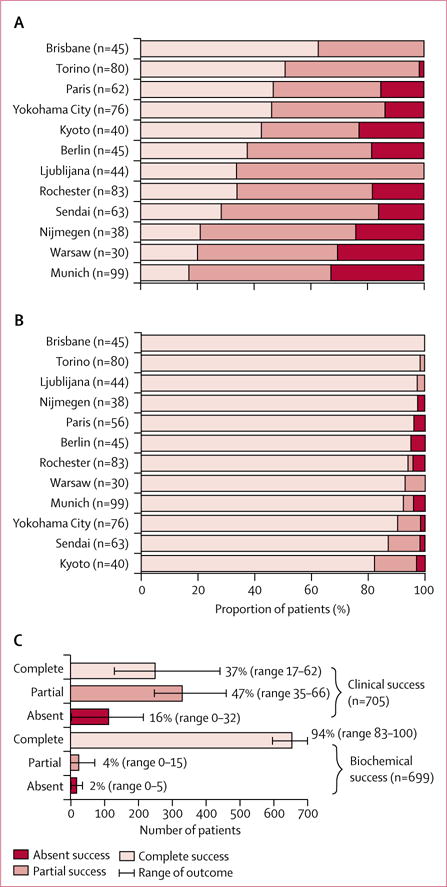
Clinical (A) and biochemical (B) outcomes were assessed in accordance with the consensus (panel). The overall clinical and biochemical outcomes of the combined cohort are shown as proportions (C).
Table 1.
Characteristics of patients with unilateral primary aldosteronism, stratified by clinical outcome
| Total cohort | Clinical success
|
Overall p value | Pairwise comparison (p values)
|
|||||
|---|---|---|---|---|---|---|---|---|
| Complete | Partial | Absent | Complete vs partial | Complete vs absent | Partial vs absent | |||
| Clinical outcome | 705 (100%) | 259 (37%) | 334(47%) | 112 (16%) | NA | NA | NA | NA |
|
| ||||||||
| Age (years) | 50.8 (10.9) | 46.4 (10.5) | 53.0 (10.3) | 54.5 (10.0) | <0001 | <0.001 | <0.001 | 0.594 |
|
| ||||||||
| Sex* | ||||||||
| Female | 311 (44%) | 172 (66%) | 113 (34%) | 26 (23%) | <0.001 | 0.035 | <0.001 | <0.001 |
| Male | 394 (56%) | 87 (34%) | 221 (66%) | 86 (77%) | NA | NA | NA | NA |
|
| ||||||||
| BMI (kg/m2; n=703) | 27.6 (5.2) | 26.1 (5.3) | 28.4 (4.7) | 29.0 (5.7) | <0.001 | <0.001 | <0.001 | 0.958 |
|
| ||||||||
| Systolic blood pressure (mm Hg; n=703) | 150.8 (21.5) | 146.3 (19.0) | 156.1 (22.6) | 145.6 (20.2) | <0.001 | <0.001 | 1.000 | <0.001 |
|
| ||||||||
| Diastolic blood pressure (mm Hg; n=703) | 91.7 (13.3) | 90.4 (11.9) | 94.1 (13.3) | 87.6 (14.7) | <0.001 | 0.002 | 0.161 | <0.001 |
|
| ||||||||
| Known duration of hypertension (months; n=612) | 96 (42 to 179) | 60 (24 to 112) | 128 (60 to 205) | 132 (60 to 228) | <0.001 | <0.001 | <0.001 | 1.000 |
|
| ||||||||
| Antihypertension medication (defined daily dose) | 3.0 (1.5 to 4.7) | 2.0 (1.0 to 3.1) | 4.0 (2.3 to 5.7) | 2.9 (1.8 to 4.0) | <0.001 | <0.001 | 0.005 | <0.001 |
|
| ||||||||
| Plasma aldosterone (pmol/L; n=703) | 848 (555 to 1382) | 877 (591 to 1437) | 868.3 (566 to 1376) | 690 (466 to 1368) | 0.077 | NA | NA | NA |
|
| ||||||||
| DRC (mU/L; n=300) | 4.0 (2.0 to 8.0) | 3.0 (1.5 to 4.9) | 4.1 (2.9 to 9.2) | 6.5 (3.5 to 14.2) | <0.001 | 0.001 | <0.001 | 0.070 |
|
| ||||||||
| ARR_DRC (n=300) | 213 (91 to 423) | 297 (165 to 586) | 224 (86 to 391) | 236 (113 to 769) | <0.001 | 0.026 | <0.001 | 0.100 |
|
| ||||||||
| PRA (pmol/L per min; n=396) | 3.8 (1.3 to 7.7) | 2.6 (1.3 to 6.4) | 3.8 (1.3 to 7.7) | 5.8 (2.6 to 7.7) | 0.026 | 0.492 | 0.013 | 0.141 |
|
| ||||||||
| ARR_PRA(n=396) | 256 (116 to 648) | 375 (126 to 807) | 257 (116 to 625) | 166 (90 to 346) | 0.006 | 0.474 | 0.004 | 0.059 |
|
| ||||||||
| Lowest serum potassium ion concentration (mmol/L; n=622) | 3.1 (2.7 to 3.4) | 3.0 (2.6 to 3.4) | 3.1 (2.8 to 3.5) | 3.2 (2.9 to 3.5) | 0.002 | 0.011 | 0.011 | 1.000 |
|
| ||||||||
| Albuminuria (mg/day; n=416) | 10.0 (2.8 to 21.5) | 10.0 (4.0 to 15.0) | 10.2 (2.8 to 33.4) | 7.1 (1.4 to 19.0) | 0.179 | NA | NA | NA |
|
| ||||||||
| Creatinine (mmol/L; n=620) | 707 (60.0 to 84.0 | 61.9 (53.0 to 74.3) | 76.9 (63.6 to 88.4) | 70.8 (61.9 to 94.0) | <0.001 | <0.001 | <0.001 | 1.000 |
|
| ||||||||
| eGFR (mL/min per 1.73 m2; n=503) | 84.8 (70.3 to 95.7) | 89.0 (78.9 to 100.0) | 79.0 (66.3 to 92.5) | 77.4 (61.6 to 93.6) | <0.001 | <0.001 | <0.001 | 1.000 |
|
| ||||||||
| Left ventricular hypertrophy (n=455) | 233/455 (51%) | 61/166 (37%) | 131/213 (62%) | 41/76 (54%) | <0.001 | <0.001 | 0.013 | 0.708 |
|
| ||||||||
| Diabetes (n=552) | 84/552 (15%) | 14/199 (7%) | 48/266 (18%) | 22/87 (25%) | <0.001 | <0.001 | <0.001 | 0.238 |
|
| ||||||||
| Lateralisation index (n=675) | 12.0 (6.3 to 26.2) | 13.9 (6.7 to 30.0) | 12.0 (6.3 to 25.7) | 8.6 (5.4 to 17.2) | 0.011 | 0.821 | 0.008 | 0.069 |
|
| ||||||||
| SI_RAV (n=604) | 20.3 (5.5 to 33.8) | 20.5 (6.8 to 33.3) | 20.3 (5.7 to 35.0) | 18.9 (4.7 to 33.5) | 0.752 | NA | NA | NA |
|
| ||||||||
| SI_LAV (n=610) | 14.0 (6.0 to 25.6) | 12.9 (6.0 to 24.3) | 14.5 (5.8 to 27.2) | 14.8 (7.8 to 25.5) | 0.386 | NA | NA | NA |
|
| ||||||||
| Contralateral ratio (n=607) | 0.3 (0.2 to 0.7) | 0.3 (0.1 to 0.6) | 0.3 (0.1 to 0.7) | 0.4 (0.2 to 0.7) | 0.056 | NA | NA | NA |
|
| ||||||||
| Presence of contralateral suppression (n=607) | 519/607 (86%) | 195/221 (88%) | 248/289 (86%) | 76/97 (78%) | 0.069 | NA | NA | NA |
|
| ||||||||
| Ipsilateral ratio (n=524) | 4.6 (2.6 to 8.7) | 4.4 (2.6 to 8.4) | 4.6 (2.7 to 8.3) | 5.5 (2.7 to 12.2) | 0.343 | NA | NA | NA |
|
| ||||||||
| Adrenal nodule (mm; n=603) | 13 (9 to 17) | 15 (10 to 18) | 12 (9 to 16) | 11 (7 to 17) | <0.001 | 0.002 | 0.002 | 0.917 |
|
| ||||||||
| Systolic blood pressure at follow-up (mm Hg; n=703) | 128.8 (13.6) | 120.9 (9.5) | 132.0 (12.6) | 137.4 (15.8) | <0.001 | <0.001 | <0.001 | <0.001 |
|
| ||||||||
| Diastolic blood pressure at follow-up (mm Hg; n=703) | 81.3 (10.0) | 76.8 (7.3) | 83.0 (10.0) | 86.5 (11.0) | <0.001 | <0.001 | <0.001 | 0.002 |
|
| ||||||||
| Defined daily dose at follow-up | 0.7 (0.0 to 2.5) | 0.0 (0.0 to 0.0) | 1.5 (0.5 to 3.0) | 3.0 (1.5 to 4.9) | <0.001 | <0.001 | <0.001 | <0.001 |
|
| ||||||||
| Pre-post Delta systolic blood pressure (mm Hg; n=703) | 22.0 (22.0) | 25.3 (19.8) | 24.1 (22.0) | 8.2 (22.1) | <0.001 | 1.000 | <0.001 | <0.001 |
|
| ||||||||
| Pre-post Delta diastolic blood pressure (mm Hg; n=703) | 10.5 (13.9) | 13.7 (11.9) | 11.1 (13.1) | 1.1 (16.5) | <0.001 | 0.067 | <0.001 | <0.001 |
|
| ||||||||
| Pre-post Delta defined daily dose | 1.7 (0.5 to 3.0) | 2.0 (1.0 to 3.1) | 2.0 (1.0 to 3.4) | −1.5 (−0.8 to 1.0) | <0.001 | 1.000 | <0.001 | <0.001 |
|
| ||||||||
| Pre-post Delta systolic blood pressure (% [SD]; n=703) | 13.3% (13.1) | 16.1% (117) | 14.1% (12.0) | 4.5% (15.1) | <0.001 | 0.172 | <0.001 | <0.001 |
|
| ||||||||
| Pre-post Delta diastolic blood pressure (% [SD]; n=703) | 10.0% (14.8) | 14.0% (11.8) | 107% (12.6) | 1.3% (20.5) | <0.001 | 0.015 | <0.001 | <0.001 |
|
| ||||||||
| Pre-post Delta defined daily dose (% [IQR]) | 767% (37.1 to 100) | 100% (100 to 100) | 53.8% (33.5 to 77.8) | −17.2% (−83.2 to 33.3) | <0.001 | <0.001 | <0.001 | <0.001 |
Data are mean (SD), n (%), n/N (%), or median (IQR), unless stated otherwise. All variables refer to baseline data unless otherwise stated. p values of less than 0.05 were considered significant. Adrenal nodule refers to diameter of largest nodule at pathology. Systolic blood pressure, diastolic blood pressure, and defined daily dose at follow-up refer to variables measured at 6–12 months. Pre-post Delta systolic blood pressure, diastolic blood pressure, or defined daily dose refer to the difference between pre-surgical and post-surgical blood pressures (systolic or diastolic blood pressure) or antihypertensive drug doses (defined daily dose). Defined daily dose is calculated according to the ATC/DDD Index 2010. Left ventricular hypertrophy was assessed with echocardiography. Lateralisation index: ([aldosterone]/[cortisol])dominant adrenal vein/([aldosterone]/[cortisol]non-dominant adrenal vein). SI_RAV: [cortisol]right adrenal vein/[cortisol]peripheral vein. SI_LAV: [cortisol]left adrenal vein/[cortisol]peripheral vein. Contralateral ratio: ([aldosterone]/[cortisol]non-dominant adrenal vein)/([aldosterone]/[cortisol]peripheral vein). Contralateral suppression defined as contralateral ratio less than 1. Ipsilateral ratio: ([aldosterone]/[cortisol]dominant adrenal vein)/([aldosterone]/[cortisol]peripheral vein). NA=not applicable. DRC=direct renin concentration. ARR=aldosterone-to-renin ratio. ARR_DRC=ARR calculated with the DRC. PRA=plasma renin activity. ARR_PRA=ARR calculated with the PRA. eGFR=estimated glomerular filtration rate. SI_RAV=selectivity index of the right adrenal vein. SI_LAV=selectivity index of the left adrenal vein.
p values are differences between sexes.
Table 2.
Characteristics of patients with unilateral primary aldosteronism, stratified by biochemical outcome
| Total cohort | Biochemical success
|
Overall p value | Pairwise comparison (p values)
|
|||||
|---|---|---|---|---|---|---|---|---|
| Complete | Partial | Absent | Complete vs partial | Complete vs absent | Partial vs absent | |||
| Biochemical outcome | 699 (100%) | 656 (94%) | 27 (4%) | 16 (2%) | NA | NA | NA | NA |
|
| ||||||||
| Age (years) | 50·8 (10·9) | 50·8 (10·9) | 51·8 (9·9) | 48·7 (12·5) | 0·718 | NA | NA | NA |
|
| ||||||||
| Sex* | ||||||||
| Female | 308 (44%) | 291 (44%) | 12 (44%) | 5 (31%) | 0·580 | NA | NA | NA |
| Male | 391 (56%) | 365 (56%) | 15 (56%) | 11 (69%) | NA | NA | NA | NA |
|
| ||||||||
| BMI (kg/m2; n=697) | 27·6 (5·2) | 27·6 (5·2) | 25·6 (5·3) | 30·0 (6·6) | 0·037 | 0·142 | 0·235 | 0·025 |
|
| ||||||||
| Systolic blood pressure (mm Hg; n=697) | 150·8 (21·5) | 151·3 (21·6) | 142·4 (20·7) | 145·8 (13·1) | 0·094 | NA | NA | NA |
|
| ||||||||
| Diastolic blood pressure (mm Hg; n=697) | 91·8 (13·3) | 92·0 (13·3) | 86·7 (14·5) | 91·5 (10·6) | 0·332 | NA | NA | NA |
|
| ||||||||
| Known duration of hypertension (months; n=606) | 96 (39 to 177) | 96 (36 to 168) | 120 (63 to 188) | 152 (42 to 241) | 0·187 | NA | NA | NA |
|
| ||||||||
| Antihypertension medication (defined daily dose) | 3·0 (1·5 to 4·7) | 3·0 (1·5 to 4·7) | 3·0 (2·0 to 3·5) | 4·0 (2·0 to 5·4) | 0·476 | NA | NA | NA |
|
| ||||||||
| Plasma aldosterone (pmol/L; n=697) | 858 (558 to 1382) | 860 (566 to 1393) | 768 (580 to 1617) | 518 (333 to 901) | 0·013 | 1·000 | 0·010 | 0·036 |
|
| ||||||||
| DRC (mU/L; n=294) | 4·0 (2·0 to 8·0) | 4·0 (2·0 to 8·0) | 11·5 (6·1 to 14·9) | 6·1 (3·2 to 14·0) | 0·185 | NA | NA | NA |
|
| ||||||||
| ARR_DRC (n=294) | 210 (89 to 414) | 216 (93 to 423) | 64 (37 to 154) | 110 (59 to 277) | 0·079 | NA | NA | NA |
|
| ||||||||
| PRA (pmol/L per min; n=396) | 3·8 (1·3 to 7·7) | 3·8 (1·3 to 7·7) | 2·6 (1·3 to 5·1) | 5·8 (3·8 to 7·7) | 0·450 | NA | NA | NA |
|
| ||||||||
| ARR_PRA (n=396) | 256 (116 to 648) | 267 (117 to 643) | 236 (112 to 876) | 79 (43 to 129) | 0·050 | NA | NA | NA |
|
| ||||||||
| Lowest serum potassium ion concentration (mmol/L; n=616) | 3·1 (2·7 to 3·5) | 3·1 (2·7 to 3·4) | 3·3 (3·0 to 3·6) | 3·0 (2·6 to 3·4) | 0·108 | NA | NA | NA |
|
| ||||||||
| Albuminuria (mg/day; n=410) | 10·0 (2·8 to 21·5) | 10·0 (2·7 to 21·1) | 10·2 (5·3 to 24·5) | 6·1 (4·5 to 14·0) | 0·745 | NA | NA | NA |
|
| ||||||||
| Creatinine (mmol/L; n=614) | 70·7 (60·0 to 84·0) | 70·7 (60·0 to 85·3) | 61·9 (53·0 to 70·7) | 70·7 (65·4 to 85·7) | 0·013 | 0·014 | 1·000 | 0·068 |
|
| ||||||||
| eGFR (mL/min per 1·73 m2; n=497) | 84·8 (70·3 to 95·7) | 84·5 (70·1 to 95·3) | 87·3 (76·8 to 95·0) | 88·8 (55·0 to 103·6) | 0·487 | NA | NA | NA |
|
| ||||||||
| Left ventricular hypertrophy (n=449) | 233/449 (52%) | 221/428 (52%) | 8/18 (44%) | 4/9 (44%) | 0·778 | NA | NA | NA |
|
| ||||||||
| Diabetes (n=546) | 84/546 (15%) | 78/516 (15%) | 3/25 (12·0%) | 3/11(27%) | 0·504 | NA | NA | NA |
|
| ||||||||
| Lateralisation index (n=669) | 11·9 (6·3 to 26·1) | 12·4 (6·5 to 27·1) | 6·5 (3·1 to 11·3) | 5·7 (4·4 to 10·0) | <0·001 | 0·001 | 0·012 | 1·000 |
|
| ||||||||
| SI_RAV (n=598) | 20·3 (5·5 to 33·8) | 20·3 (5·7 to 34·2) | 20·5 (3·6 to 28·6) | 16·6 (3·2 to 32·0) | 0·469 | NA | NA | NA |
|
| ||||||||
| SI_LAV (n=604) | 14·0 (6·0 to 25·6) | 14·0 (6·1 to 25·5) | 13·1 (5·2 to 26·8) | 14·2 (4·1 to 23·2) | 0·962 | NA | NA | NA |
|
| ||||||||
| Contralateral ratio (n=601) | 0·3 (0·2 to 0·7) | 0·3 (0·2 to 0·6) | 0·6 (0·3 to 1·2) | 0·6 (0·2 to 2·5) | 0·002 | 0·007 | 0·177 | 1·000 |
|
| ||||||||
| Presence of contralateral suppression (n=601) | 519/601 (86%) | 494/570 (87%) | 18/25 (72%) | 7/12 (58%) | 0·004 | 0·431 | 0·008 | 0·249 |
|
| ||||||||
| Ipsilateral ratio (n=518) | 4·6 (2·6 to 8·7) | 4·6 (2·6 to 8·7) | 5·3 (2·9 to 7·3) | 6·4 (2·5 to 19·9) | 0·858 | NA | NA | NA |
|
| ||||||||
| Adrenal nodule (n=597) | 13 (9 to 17) | 13 (10 to 17) | 8 (7 to 15) | 14 (7 to 19) | 0·016 | 0·012 | 1·000 | 0·232 |
|
| ||||||||
| Systolic blood pressure at follow-up (mm Hg; n=697) | 128·7 (13·7) | 128·4 (13·5) | 132·9 (16·6) | 136·2 (12·9) | 0·020 | 0·268 | 0·069 | 1·000 |
|
| ||||||||
| Diastolic blood pressure at follow-up (mm Hg; n=697) | 81·2 (10·0) | 81·0 (9·8) | 84·0 (13·1) | 86·9 (8·1) | 0·022 | 0·359 | 0·058 | 1·000 |
|
| ||||||||
| Defined daily dose at follow-up | 0·6 (0·0 to 2·5) | 0·5 (0·0 to 2·5) | 1·0 (0·3 to 2·0) | 2·2 (1·0 to 4·2) | 0·002 | 0·433 | 0·004 | 0·281 |
|
| ||||||||
| Pre-post Delta systolic blood pressure (mm Hg; n=697) | 22·1 (22·0) | 22·9 (22·0) | 9·5 (20·9) | 9·7 (17·1) | 0·001 | 0·006 | 0·050 | 1·000 |
|
| ||||||||
| Pre-post Delta diastolic blood pressure (mm Hg; n=697) | 10·5 (13·9) | 11·0 (13·8) | 2·6 (17·5) | 4·7 (9·5) | 0·002 | 0·006 | 0·219 | 1·000 |
|
| ||||||||
| Pre-post Delta defined daily dose | 1·6 (0·5 to 3·0) | 1·7 (0·5 to 3·0) | 1·5 (0·5 to 2·3) | 0·8 (–0·7 to 4·4) | 0·271 | NA | NA | NA |
|
| ||||||||
| Pre-post Delta systolic blood pressure (% [SD]; n=697) | 13·3% (13·1) | 13·8% (12·9) | 5·5% (13·6) | 6·0% (11·4) | <0·001 | 0·003 | 0·053 | 1·000 |
|
| ||||||||
| Pre-post Delta diastolic blood pressure (% [SD]; n=697) | 10·0% (14·8) | 10·6% (14·2) | 0·4% (24·3) | 4·4% (10·3) | <0·001 | <0·001 | 0·286 | 1·000 |
|
| ||||||||
| Pre-post Delta defined daily dose (% [IQR]) | 76·7% (36·7 to 100·0) | 82·2% (40·0 to 100·0) | 50·0% (33·3 to 90·0) | 27·4% (–50·1 to 70·9) | <0·001 | 0·120 | 0·002 | 0·396 |
Data are mean (SD), n (%), n/N (%), or median (IQR), unless stated otherwise. All variables refer to baseline data unless otherwise stated. p values of less than 0·05 were considered significant. Adrenal nodule refers to diameter of largest nodule at pathology. Systolic blood pressure, diastolic blood pressure, and defined daily dose at follow-up refer to variables measured at 6–12 months. Pre-post Delta systolic blood pressure, diastolic blood pressure, or defined daily dose refer to the difference between pre-surgical and post-surgical blood pressures (systolic or diastolic blood pressure) or antihypertensive drug doses (defined daily dose). Defined daily dose is calculated according to the ATC/DDD Index 2010. Left ventricular hypertrophy was assessed with echocardiography. Lateralisation index: ([aldosterone]/[cortisol])dominant adrenal vein/([aldosterone]/[cortisol]non-dominant adrenal vein). SI_RAV: [cortisol]right adrenal vein/[cortisol]peripheral vein. SI_LAV: [cortisol]left adrenal vein/[cortisol]peripheral vein. Contralateral ratio: ([aldosterone]/[cortisol]non-dominant adrenal vein)/([aldosterone]/[cortisol]peripheral vein). Contralateral suppression defined as contralateral ratio less than 1. Ipsilateral ratio: ([aldosterone]/[cortisol]dominant adrenal vein)/([aldosterone]/[cortisol]peripheral vein). NA=not applicable. DRC=direct renin concentration. ARR=aldosterone-to-renin ratio. ARR_DRC=ARR calculated with the DRC. PRA=plasma renin activity. ARR_PRA=ARR calculated with the PRA. eGFR=estimated glomerular filtration rate. SI_RAV=selectivity index of the right adrenal vein. SI_LAV=selectivity index of the left adrenal vein.
The p value is for sex in general.
Protocols for adrenal vein sampling differed between study centres (appendix pp 11–22). We therefore compared outcomes and baseline characteristics of patients after subdivision by adrenal vein sampling procedure used (unstimulated vs adrenocorticotropic hormone 1–24 stimulation) or interpretation (lateralisation index <4 vs lateralisation index ≥4). Clinical outcome was not significantly affected by the use of an unstimulated or stimulated protocol (appendix pp 23–24) or by a lateralisation index of less than 4 or of 4 or more (appendix pp 25–26).
Unadjusted baseline data for all centres combined for clinical and biochemical outcomes are shown in tables 1 and 2 and the appendix (p 27); these data show that most patients with primary aldosteronism had a history of hypertension of greater than 6 years, were hypokalaemic, and had reduced renal function. Equivalent data for each referral centre are shown in the appendix (pp 11–22). For the pooled data, we identified significant differences between patients with different outcomes in unadjusted clinical outcomes for 19 of 25 measurements (table 1). For biochemical outcomes, fewer differences were apparent; however, plasma aldosterone, creatinine, lateralisation index, contralateral suppression, contra-lateral ratio less than 1, and adrenal nodule size differed significantly between patients with different outcomes (table 2). Unadjusted analyses of patients with complete success compared with those with partial and absent clinical success combined, and of patients with complete and partial success combined compared with those with absent clinical success are shown in the appendix (pp 28–29 and 30–31, respectively).
We analysed factors associated with clinical and biochemical outcome by assessing ORs in unadjusted and adjusted analyses (table 3). We identified factors independently associated with complete success in an adjusted analysis by comparing the proportion of patients with complete success with the proportion with partial and absent success combined (table 3). Baseline systolic blood pressure was the only factor independently associated with complete biochemical success: patients with higher blood pressure had an increased likelihood of complete success (table 3). Complete clinical success was associated with four variables: female sex, lower age, fewer antihypertensive medications as measured by defined daily dose, and absence of left ventricular hypertrophy (table 3).
Table 3.
Baseline factors associated with clinical and biochemical outcomes after adrenalectomy for unilateral primary aldosteronism
| Clinical outcome
|
Biochemical outcome
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted analysis
|
Adjusted analysis
|
Unadjusted analysis
|
Adjusted analysis
|
|||||
| Odds ratio (95% CI) | p value | Odds ratio (95% CI) | p value | Odds ratio (95% CI) | p value | Odds ratio (95% CI) | p value | |
|
Complete success versus partial plus absent success
| ||||||||
| Age (per year) | 0.94 (0.92–0.95) | <0.001 | 0.95 (0.93–0.98) | <0.001 | 1.00 (0.97–1.03) | 0.911 | 0.98 (0.94–1.02) | 0.324 |
| Lowest serum potassium ion concentration (per 1 mmol/L) | 1.64 (1.22–2.21) | 0.001 | 1.43 (0.95–2.15) | 0.086 | 1.56 (0.86–2.88) | 0.147 | 1.43 (0.69–2.95) | 0.339 |
| Antihypertension medication (per 1 unit of defined daily dose) | 0.68 (0.62–0.75) | <0.001 | 0.80 (0.70–0.90) | <0.001 | 0.96 (0.85–1.09) | 0.480 | 0.87 (0.74–1.02) | 0.094 |
| eGFR (per 1 mL/min per 1.73 m2) | 1.02 (1.01–1.03) | <0.001 | 1.01 (1.00–1.02) | 0.083 | 0.99 (0.98–1.01) | 0.374 | 0.99 (0.97–1.01) | 0.169 |
| Systolic blood pressure (per 1 mm Hg) | 0.98 (0.98–0.99) | <0.001 | 0.99 (0.98–1.01) | 0.287 | 1.02 (1.00–1.04) | 0.025 | 1.03 (1.01–1.05) | 0.013 |
| BMI (per 1 kg/m2) | 0.90 (0.87–0.93) | <0.001 | 0.98 (0.94–1.03) | 0.380 | 1.02 (0.96–1.08) | 0.617 | 0.95 (0.88–1.02) | 0.149 |
| Sex (reference: female) | 4.37 (3.16–6.07) | <0.001 | 2.25 (1.40–3.62) | 0.001 | 1.22 (0.65–2.33) | 0.538 | 0.99 (0.42–2.32) | 0.977 |
| Left ventricular hypertrophy (reference: not detected) | 2.53 (1.71–3.76) | <0.001 | 1.61 (1.01–2.59) | 0.047 | 0.75 (0.34–1.64) | 0.470 | 0.70 (0.30–1.62) | 0.403 |
|
Complete plus partial success versus absent success | ||||||||
| Age (per year) | 0.96 (0.94–0.98) | <0.001 | 0.95 (0.92–0.98) | 0.004 | 1.02 (0.97–1.06) | 0.431 | 1.03 (0.96–1.11) | 0.523 |
| Lowest serum potassium ion concentration (per 1 mmol/L) | 1.53 (1.03–2.29) | 0.036 | 1.18 (0.70–2.00) | 0.540 | 0.92 (0.35–2.51) | 0.872 | 0.90 (0.26–3.12) | 0.866 |
| Antihypertension medication (per 1 unit of defined daily dose) | 1.08 (0.99–1.19) | 0.086 | 1.27 (1.08–1.50) | 0.005 | 0.88 (0.75–1.07) | 0.175 | 0.79 (0.61–1.01) | 0.062 |
| eGFR (per 1 mL/min per 1.73 m2) | 1.01 (1.00–1.03) | 0.018 | 1.01 (1.00–1.02) | 0.368 | 1.01 (0.98–1.04) | 0.553 | 1.01 (0.97–1.04) | 0.716 |
| Systolic blood pressure (per 1 mm Hg) | 1.01 (1.00–1.03) | 0.005 | 1.03 (1.01–1.04) | 0.003 | 1.01 (0.99–1.04) | 0.352 | 1.03 (0.99–1.07) | 0.140 |
| BMI (per 1 kg/m2) | 0.95 (0.91–0.98) | 0.004 | 0.90 (0.85–0.96) | 0.001 | 0.93 (0.85–1.01) | 0.071 | 0.89 (0.79–1.00) | 0.043 |
| Sex (reference: female) | 3.06 (1.94–4.97) | <0.001 | 2.89 (1.49–5.59) | 0.002 | 1.75 (0.63–5.62) | 0.302 | 1.91 (0.41–8.99) | 0.413 |
| Left ventricular hypertrophy (reference: not detected) | 1.14 (0.70–1.88) | 0.601 | 1.39 (0.76–2.54) | 0.290 | 0.76 (0.19–2.90) | 0.682 | 0.54 (0.12–2.37) | 0.416 |
|
Complete success versus absent success | ||||||||
| Age (per year) | 0.93 (0.90–0.95) | <0.001 | 0.92 (0.88–0.96) | <0.001 | 1.02 (0.97–1.06) | 0.441 | 1.02 (0.95–1.10) | 0.549 |
| Lowest serum potassium ion concentration (per 1 mmol/L) | 1.94 (1.26–3.03) | 0.003 | 1.24 (0.64–2.39) | 0.519 | 0.95 (0.36–2.58) | 0.917 | 0.98 (0.28–3.47) | 0.969 |
| Antihypertension medication (per 1 unit of defined daily dose) | 0.83 (0.74–0.94) | 0.002 | 1.05 (0.86–1.28) | 0.609 | 0.88 (0.75–1.07) | 0.177 | 0.78 (0.61–1.00) | 0.054 |
| eGFR (per 1 mL/min per 1.73 m2) | 1.03 (1.02–1.05) | <0.001 | 1.01 (0.99–1.03) | 0.177 | 1.01 (0.98–1.04) | 0.583 | 1.00 (0.97–1.04) | 0.819 |
| Systolic blood pressure (per 1 mm Hg) | 1.00 (0.99–1.01) | 0.767 | 1.02 (1.00–1.04) | 0.082 | 1.01 (0.99–1.04) | 0.319 | 1.03 (0.99–1.07) | 0.137 |
| BMI (per 1 kg/m2) | 0.91 (0.87–0.95) | <0.001 | 0.91 (0.85–0.97) | 0.004 | 0.93 (0.85–1.01) | 0.081 | 0.88 (0.79–0.99) | 0.039 |
| Sex (reference: female) | 6.54 (3.98–11.04) | <0.001 | 4.48 (2.10–9.60) | <0.001 | 1.75 (0.63–5.62) | 0.303 | 1.90 (0.40–8.97) | 0.418 |
| Left ventricular hypertrophy (reference: not detected) | 2.02 (1.17–3.51) | 0.013 | 1.89 (0.90–3.99) | 0.093 | 0.75 (0.18–2.87) | 0.670 | 0.54 (0.12–2.34) | 0.405 |
Logistic regression analyses were done at the patient level to identify factors associated with clinical and biochemical outcome. Eight covariates were entered into each model and their unadjusted odds ratios (ORs) are shown with the corresponding ORs adjusted for the confounding effects of the other covariates in the model. For most results, an OR greater than 1 indicates an increased likelihood of clinical or biochemical outcome and an OR less than 1 a decreased likelihood; the exception is for potassium ion concentration, which was analysed for lowest values, therefore in this case an OR greater than 1 indicates a decreased likelihood and an OR less than 1 an increased likelihood. The final adjusted models included data from 414 patients with complete data for all eight variables (clinical outcome: complete [n=158], partial [n=192], absent [n=64]; biochemical outcome: complete [n=387], partial [n=18], absent [n=9]). eGFR=estimated glomerular filtration rate.
Five factors were independently associated with clinical benefit when the proportion of patients with complete and partial success combined was compared with the proportion with absent success (table 3). Female sex, younger age, and lower BMI were independent determinants of clinical benefit. Patients with higher systolic blood pressure and higher antihypertensive medication use at baseline were also more likely to obtain clinical benefit from adrenalectomy (table 3).
In a comparison of complete with absent success, lower age and female sex were associated with clinical success and BMI was inversely associated with clinical and biochemical success (table 3).
Sex distribution between centres varied widely from 24% to 60% female, with nine of the 12 centres enrolling more men than women. Notwithstanding this bias, 11 of the 12 centres reported more women than men showing complete clinical remission, with 172 (55%) of 311 women versus 87 (22%) of 394 men achieving complete clinical success, whereas a greater proportion of men had partial or absent clinical success (partial success: 113 [36%] of 311 women vs 221 [56%] of 394 men; absent success: 26 [8%] of 311 women vs 86 [22%] of 394 men).
The mean age at presentation ranged from 47·7 years (SD 10·3) in Paris to 54·4 years (11·1) in Sendai, a difference of almost 7 years, which might reflect international differences in referral patterns. The mean age of patients who achieved complete clinical success was 46·4 years (10·5), which was significantly lower than the age of patients who achieved partial success (53·0 years [10·3]; p<0·001) and patients who had an absent clinical outcome (54·5 years [10·0]; p<0·001).
Adjustment for the confounding effect of centres showed a marginal effect on the associations of female sex (OR 2·26, 95% CI 1·36–3·77), younger age (OR 0·95 per extra year, 0·93–0·98), and lower antihypertensive medication use (OR 0·72 per unit increase, 0·62–0·83) with clinical remission (appendix p 32). Lower age remained a determinant of complete clinical success (OR 0·94 per extra year, 0·90–0·98) when the adjusted analysis was restricted to men (appendix p 33).
We used multilevel modelling to analyse between-centre heterogeneity; when we built the model from centre up, age and sex seemed to account for much of the heterogeneity across centres (data not shown).
Discussion
In this study, we have established a consensus on criteria to define the outcome of adrenalectomy for unilateral primary aldosteronism—a consensus built using the Delphi method of iterative questionnaires to a panel of expert participants.16 This method is particularly appropriate to reach consensus on controversial themes with divergent published evidence. Central to this method is participant anonymity within the expert panel, with communication via email to a monitoring group. This approach avoids the potential for an emotionally charged atmosphere associated with renowned experts discussing complex issues, the effect of dominant individuals, and the incentive for self-censorship. Furthermore, because communication is by email, no restrictions on participant selection exist, theoretically allowing the inclusion of unlimited numbers of experts without geographical constraints.
Researchers have reported the outcome of unilateral adrenalectomy for primary aldosteronism in previous studies, but these studies used divergent and ill-defined criteria with variable follow-up intervals to assess outcome.12,19–24 Outcome results vary widely across studies and might underestimate or overestimate the real outcome effects. Remission of blood pressure has been reported in 20–72% of patients,12,19 which is close to the results of our study (17–62%), in which we applied uniform criteria. Therefore the variability of blood pressure remission cannot be accounted for by an absence of standardised criteria or differences in post-surgical follow-up protocols. However, using standardised criteria, we report more variation of biochemical outcome (83–100%) than with previous reports (96–100%),12,19 suggesting an overestimation of the real effects of adrenalectomy in earlier studies.
The utility of the consensus lies not only in the comparison of outcome data for clinical and scientific studies, but also in its application as a quality measure of patient diagnoses. As such, complete biochemical success reflects the correct diagnosis and treatment of unilateral primary aldosteronism; the partial and absent biochemical success categories identify those patients with bilateral primary aldosteronism with asymmetrical bilateral aldosterone production rather than unilateral primary aldosteronism. Clinical outcome is not only dependent on biochemical outcome, but also on other factors such as pre-existing primary hypertension, age, duration of hypertension, and renal function.
Of the 12 international centres that submitted data for sequential patients in which the complete, partial, and absent criteria were strictly observed, nine followed the US Endocrine Society guidelines11 and three the Japan Endocrine Society guidelines18 for pathways and procedures for diagnosis. No discernible differences were seen by following one guideline rather than the other with respect to the proportion of patients classified as having complete, partial, or absent success after surgery. Analysis of individual and group data pinpointed at least three factors associated with complete clinical success or clinical benefit: age, sex, and defined daily dose of anti-hypertensive medication. Our results show that younger patients are more likely than older patients to show complete clinical success after adrenalectomy and that women, on average, have a better chance post surgery of complete clinical success than men. Young and female patients also showed a higher likelihood of clinical benefit (complete and partial success) from surgery. Higher BMI was associated with an increased likelihood of absent clinical and biochemical success. The reason for this finding is unclear, but could be related to higher aldosterone levels in patients with obesity.25
Partial clinical success occurred in almost half of patients, with an average reduction in blood pressure of 24/11 mm Hg. Such an effect also confers clinical benefit for the patient because a decrease in blood pressure, with or without a decrease in antihypertensive medication use, translates to improved long-term cardiovascular outcomes.26
The finding of a substantial difference between women and men presumably reflects the vasoprotective role of oestrogens before menopause, lessening the likelihood of irreversible vascular damage. Similarly, the older the patient (male or female), the higher the likelihood of such damage and the higher the probability of associated primary hypertension. The results of biochemical outcome of adrenalectomy are mainly determined by the correct diagnosis of unilateral aldosterone excess and a completely performed adrenalectomy. Removal of the source of aldosterone excess should therefore normalise the biochemical abnormalities. By contrast, clinical outcome is not only dependent on normalisation of aldosterone secretion, but also on the existing deleterious vascular and renal changes determined by the duration of the preceding aldosterone-induced hypertension.
Our outcome study had several important strengths: clinical and biochemical outcomes have been addressed separately; the criteria were stringently applied to a large international cohort of patients and every patient with the required outcome data could be systematically classified in accordance with the Delphi-defined criteria; and all patients were diagnosed for surgically amenable treatment by adrenal venous sampling. The main limitation of our study is that our database did not have hard endpoint outcomes and therefore we were unable to validate our surgical outcome criteria. This limitation will be addressed in a prospective multicentre validation study that is currently being planned, which will include measures of cardiovascular morbidity and mortality. Additional limitations are the absence of ambulatory blood pressure monitoring at baseline and post surgery to provide a more standardised assessment of blood pressure response to adrenalectomy and the unavoidable use of arbitrary criteria to define improvement of blood pressure control in terms of reductions in antihypertensive medication.
In conclusion, the PASO consensus offers feasible criteria for the classification of outcomes of adrenalectomy for the treatment of unilateral primary aldosteronism. The variation in baseline characteristics of the cohort contributes to the wide variation in clinical outcomes. Younger patients and female patients have a higher chance of complete clinical success and clinical benefit. Screening should still be done in every individual fulfilling the guideline criteria11 because biochemical cure is by itself clinically important and because older women and men can also derive post-operative clinical benefit. Our findings can help clinicians to inform and counsel patients about expectations after surgery based on age and sex.
Supplementary Material
Research in Context.
Evidence before this study
Adrenalectomy is the recommended treatment for unilateral primary aldosteronism in patients willing and able to undergo surgery. Proportions of patients with biochemical remission are high across various reports (96–100%); by contrast, clinical remission has been reported for a much wider range (20–72%) of patients. This variability might be real, across centres, or might reflect the absence of standardised criteria and differences in post-surgical follow-up. We noted 18 studies, published between 1998 and 2015, in which total adrenalectomy was used to treat unilateral primary aldosteronism; these studies used different outcome criteria that varied from normalisation of plasma potassium concentrations with normalisation or improvement of blood pressure to criteria that also took into account the use of antihypertensive drugs and plasma aldosterone and renin concentrations or plasma renin activities. These studies rarely separated biochemical criteria (plasma potassium, renin, and aldosterone measurements) from clinical criteria (blood pressure measurements and use of antihypertensive drugs). The assessment of biochemical success of adrenalectomy separately from clinical success is important because a patient with primary aldosteronism can be correctly biochemically diagnosed and successfully treated, but maintain background primary hypertension after successful adrenalectomy.
Added value of this study
The Primary Aldosteronism Surgery Outcome (PASO) study consisted of two parts. First, using the Delphi method, we were able to reach consensus for criteria for six outcomes (complete, partial, and absent success of clinical and biochemical outcomes) for unilateral adrenalectomy and two recommendations for the time and interval of follow-up. In an international multicentre study, we applied these criteria in a retrospective analysis of a large prospective cohort of patients who had undergone adrenalectomy. Proportions of patients with complete clinical success ranged from 17% to 62%; complete biochemical success averaged 94%. Female sex and younger age were independently associated with clinical success.
Implications of all the available evidence
This study establishes a standard set of outcome criteria for the surgical treatment of unilateral primary aldosteronism. The criteria are relevant for the assessment of the success of surgical treatment in individual patients and for the comparison of outcome data in studies. We identified that younger patients and female patients are more likely to have a favourable surgical outcome. These data will be useful for health-care professionals counselling patients with unilateral primary aldosteronism prior to adrenalectomy about the expected post-operative clinical benefit.
Panel: International consensus on surgery outcomes for unilateral primary aldosteronism.
Complete clinical success
Normal blood pressure* without the aid of antihypertensive medication
Partial clinical success
The same blood pressure as before surgery† with less antihypertensive medication‡§ or a reduction in blood pressure with either the same amount or less antihypertensive medication
Absent clinical success
Unchanged or increased blood pressure† with either the same amount or an increase in antihypertensive medication‡¶
Complete biochemical success
Correction of hypokalaemia|| (if present pre-surgery) and normalisation of the aldosterone-to-renin ratio**; in patients with a raised aldosterone-to-renin ratio post surgery, aldosterone secretion should be suppressed in a confirmatory test††‡‡
Partial biochemical success
Correction of hypokalaemia|| (if present pre-surgery) and a raised aldosterone-to-renin ratio** with one or both of the following (compared with pre-surgery): ≥50% decrease in baseline plasma aldosterone concentration; or abnormal but improved post-surgery confirmatory test result‡‡
Absent biochemical success
Persistent hypokalaemia|| (if present pre-surgery) or persistent raised aldosterone-to-renin ratio**, or both, with failure to suppress aldosterone secretion with a post-surgery confirmatory test‡‡
Outcome assessment
Outcome assessment should first be done in the 3 months post surgery, but final outcome should be assessed at 6–12 months
Annual reassessment
Outcome should be reassessed annually§§
Complete success is defined as remission, partial success as improvement, and absent success as persistence. *Normal blood pressure as defined by the European Society of Hypertension Guidelines17 for the office or outpatient setting (systolic blood pressure <140 mm Hg and diastolic blood pressure <90 mm Hg); home blood pressure measurement (systolic blood pressure <135 mm Hg and diastolic blood pressure <85 mm Hg); daytime ambulatory blood pressure monitoring (systolic blood pressure <135 mm Hg and diastolic blood pressure <85 mm Hg); and 24 h ambulatory blood pressure monitoring (systolic blood pressure <130 mm Hg and diastolic blood pressure <80 mm Hg). †Blood pressure responses based on the differences between blood pressure levels that define the blood pressure categories of hypertension,17 with unchanged blood pressure levels defined as a difference (preoperatively vs post-operatively) in systolic blood pressure of <20 mm Hg and diastolic blood pressure of <10 mm Hg; and reduction or increase in blood pressure defined as a difference in systolic blood pressure of ≥20 mm Hg or diastolic blood pressure of ≥10 mm Hg, or both; however, if a change in systolic blood pressure and an opposing change in diastolic blood pressure are reported, the blood pressure response is defined by the change in systolic blood pressure. ‡Antihypertensive medication is expressed as defined daily dose (DDD), which is the assumed average maintenance dose per day for a drug used for its main indication in adults (ATC/DDD Index 2010). Unchanged antihypertensive medication is defined as a change (decrease or increase) of less than 0·5 times the DDD between pre-surgery and post surgery; less antihypertensive medication (defined as a decrease of 0·5 or more times the DDD between pre-surgery and post surgery); and increased antihypertensive medication (defined as an increase of 0·5 or more times the DDD between pre-surgery and post surgery). §Changes in antihypertensive medication should be measured when the final outcome is assessed at 6–12 months, in accordance with the outcome assessment recommendation. ¶Reduction in blood pressureon increased antihypertensive medication is classed under the category of absent clinical success; the same applies to an increase in blood pressure on less antihypertensive medication. ||Hypokalaemia is defined as serum potassium of <3·6 mmol/L. **Normalisation of the aldosterone-to-renin ratio is defined on the basis of local laboratory reference intervals. ††If baseline plasma aldosterone is below the locally defined upper reference limit for a saline infusion or a fludrocortisone suppression confirmatory test, then these tests (the saline infusion and the fludrocortisone suppression confirmatory test) are unnecessary, even if the aldosterone-to-renin ratio is raised (this corresponds to hyporeninaemic hypoaldosteronism). ‡‡Post-surgery confirmatory test results can be defined as complete, partial, or absent biochemical success. Complete biochemical success (normal suppression) is defined as: plasma aldosterone <5 ng/dL (139 pmol/L), from a saline infusion test; urinary aldosterone <10 μg/24 h, from an oral sodium loading test at the Mayo Clinic (Rochester, MN, USA; or defined on the basis of local reference values); a decrease in plasma aldosterone after captopril by >30%, from a captopril challenge test; or a day 4 upright plasma aldosterone concentration of <166 pmol/L, from a fludrocortisone suppression test taken at 1000 h. Partial biochemical success (an abnormal but improved post-confirmatory test result) is defined as: plasma aldosterone of 5–10 ng/dL (139–277 pmol/L), from a saline infusion test; urinary aldosterone of 10–12 μg/24 h, from an oral sodium loading test at the Mayo Clinic (or defined in accordance with local reference values); or a positive day 4 upright plasma aldosterone concentration that is at least 20% lower than the pre-adrenalectomy value, from a fludrocortisone suppression test taken at 1000 h. Absent biochemical success (failure to suppress aldosterone secretion) is defined as: plasma aldosterone >10 ng/dL (>277 pmol/L), from a saline infusion test; urinary aldosterone >12 μg/24 h, from an oral sodium loading test at the Mayo Clinic (or defined in accordance with local reference values); a decrease in plasma aldosterone after captopril by ≤30%, from a captopril challenge test; or a day 4 upright plasma aldosterone concentration of >6 ng/dL (166 pmol/L), from a fludrocortisone suppression test taken at 1000 h. §§Annual reassessment includes a minimum assessment of blood pressure and potassium concentration.
Acknowledgments
This project has received funding from the European Research Council under the European Union’s Horizon 2020 research and innovation programme (grant agreement number 694913) to MRe and has been supported by grants from the Else Kröner-Fresenius Stiftung in support of the German Conn’s Registry-Else-Kröner Hyperaldosteronism Registry (2013_A182 and 2015_A171); the Deutsche Forschungsgemeinschaft (RE 752/20-1 to MRe; BE 2177/13-1 to FB; and WI 3660/1-2 [KF0252] and LE 2873/1-2 [KFO252] to Holger S Willenberg [appendix] and JWML), and the Netherlands Organisation for Health Research and Development—Medical Sciences 2010–2012 E&K (171002102) to JD. This study was also partly supported by grants from the Japanese Ministry of Health, Labour and Welfare (Grant for Research on Intractable Diseases) to FS and TN; the Ministry of Health of Slovenia (Tertiary Care Scientific grant number 20150198 of the University Medical Centre Ljubljana) to TK; the US National Institutes of Health (grant R21DK103183) to Richard J Auchus (appendix); and Chilean grants (CONICYT-FONDECYT 1160695, 1150437, and IMII P09/016- F [ICM]) to Carlos E Fardella (appendix).
Footnotes
Contributors
TAW, JWML, PM, CEG-S, JWF, and MRe designed the study.
TAW, CEG-S, JWF, and MRe interpreted the responses from the questionnaires. JWML, PM, JB, CA, FS, LA, MQ, JD, FB, KKK, UP, RM, HU, AP, TK, MN, MS, TN, and WFY Jr collected data. TAW, JWML, PM, JB, CA, FS, LA, MQ, JD, FB, KKK, UP, RM, HU, AP, TK, MN, MS, TN, WFY Jr, and MRe assessed outcome frequencies. JB and MRo did the statistical analyses. TAW, JWML, JF, CEG-S, and MRe wrote the first draft of the report. All authors made critical revisions of the report.
Declaration of interests
WFY Jr has a consulting agreement with Nihon Medi-Physics for preclinical studies related to imaging agents for adrenal glands. All other authors declare no competing interests.
For the ATC/DDD Index 2010 see http://www.whocc.no/atc_ddd_index/2010
References
- 1.Conn JW. Presidential address. I. Painting background. II. Primary aldosteronism, a new clinical syndrome. J Lab Clin Med. 1955;45:3–17. [PubMed] [Google Scholar]
- 2.Hannemann A, Wallaschofski H. Prevalence of primary aldosteronism in patient’s cohorts and in population-based studies—a review of the current literature. Horm Metab Res. 2012;44:157–62. doi: 10.1055/s-0031-1295438. [DOI] [PubMed] [Google Scholar]
- 3.Douma S, Petidis K, Doumas M, et al. Prevalence of primary hyperaldosteronism in resistant hypertension: a retrospective observational study. Lancet. 2008;371:1921–26. doi: 10.1016/S0140-6736(08)60834-X. [DOI] [PubMed] [Google Scholar]
- 4.Käyser SC, Dekkers T, Groenewoud HJ, et al. Study heterogeneity and estimation of prevalence of primary aldosteronism: a systematic review and meta-regression analysis. J Clin Endocrinol Metab. 2016;101:2826–35. doi: 10.1210/jc.2016-1472. [DOI] [PubMed] [Google Scholar]
- 5.Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45:1243–48. doi: 10.1016/j.jacc.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 6.Catena C, Colussi G, Nadalini E, et al. Cardiovascular outcomes in patients with primary aldosteronism after treatment. Arch Intern Med. 2008;168:80–85. doi: 10.1001/archinternmed.2007.33. [DOI] [PubMed] [Google Scholar]
- 7.Mulatero P, Monticone S, Bertello C, et al. Long-term cardio- and cerebrovascular events in patients with primary aldosteronism. J Clin Endocrinol Metab. 2013;98:4826–33. doi: 10.1210/jc.2013-2805. [DOI] [PubMed] [Google Scholar]
- 8.Savard S, Amar L, Plouin PF, Steichen O. Cardiovascular complications associated with primary aldosteronism: a controlled cross-sectional study. Hypertension. 2013;62:331–36. doi: 10.1161/HYPERTENSIONAHA.113.01060. [DOI] [PubMed] [Google Scholar]
- 9.Catena C, Colussi G, Lapenna R, et al. Long-term cardiac effects of adrenalectomy or mineralocorticoid antagonists in patients with primary aldosteronism. Hypertension. 2007;50:911–18. doi: 10.1161/HYPERTENSIONAHA.107.095448. [DOI] [PubMed] [Google Scholar]
- 10.Catena C, Colussi G, Di Fabio A, et al. Mineralocorticoid antagonists treatment versus surgery in primary aldosteronism. Horm Metab Res. 2010;42:440–45. doi: 10.1055/s-0029-1246185. [DOI] [PubMed] [Google Scholar]
- 11.Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:1889–916. doi: 10.1210/jc.2015-4061. [DOI] [PubMed] [Google Scholar]
- 12.Steichen O, Zinzindohoué F, Plouin PF, Amar L. Outcomes of adrenalectomy in patients with unilateral primary aldosteronism: a review. Horm Metab Res. 2012;44:221–27. doi: 10.1055/s-0031-1299681. [DOI] [PubMed] [Google Scholar]
- 13.Proye CA, Mulliez EA, Carnaille BM, et al. Essential hypertension: first reason for persistent hypertension after unilateral adrenalectomy for primary aldosteronism? Surgery. 1998;14:1128–33. doi: 10.1067/msy.1998.93108. [DOI] [PubMed] [Google Scholar]
- 14.Sechi LA, Novello M, Lapenna R, et al. Long-term renal outcomes in patients with primary aldosteronism. JAMA. 2006;295:2638–45. doi: 10.1001/jama.295.22.2638. [DOI] [PubMed] [Google Scholar]
- 15.Rossi GP, Bolognesi M, Rizzoni D, et al. Vascular remodeling and duration of hypertension predict outcome of adrenalectomy in primary aldosteronism patients. Hypertension. 2008;51:1366–71. doi: 10.1161/HYPERTENSIONAHA.108.111369. [DOI] [PubMed] [Google Scholar]
- 16.Hsu C-C, Sandford BA. The Delphi technique: making sense of consensus. Pract Assess Res Eval. 2007;12:10. [Google Scholar]
- 17.Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) 2013 ESH/ESC guidelines for the management of arterial hypertension. J Hypertens. 2013;31:1281–357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 18.Nishikawa T, Omura M, Satoh F, et al. Guidelines for the diagnosis and treatment of primary aldosteronism—the Japan Endocrine Society 2009. Endocr J. 2011;58:711–21. doi: 10.1507/endocrj.ej11-0133. [DOI] [PubMed] [Google Scholar]
- 19.Muth A, Ragnarsson O, Johannsson G, Wängberg B. Systematic review of surgery and outcomes in patients with primary aldosteronism. Br J Surg. 2015;102:307–17. doi: 10.1002/bjs.9744. [DOI] [PubMed] [Google Scholar]
- 20.Zarnegar R, Young WF, Jr, Lee J, et al. The aldosteronoma resolution score: predicting complete resolution of hypertension after adrenalectomy for aldosteronoma. Ann Surg. 2008;247:511–18. doi: 10.1097/SLA.0b013e318165c075. [DOI] [PubMed] [Google Scholar]
- 21.Waldmann J, Maurer L, Holler J, et al. Outcome of surgery for primary hyperaldosteronism. World J Surg. 2011;35:2422–27. doi: 10.1007/s00268-011-1221-5. [DOI] [PubMed] [Google Scholar]
- 22.Lim V, Guo Q, Grant CS, et al. Accuracy of adrenal imaging and adrenal venous sampling in predicting surgical cure of primary aldosteronism. J Clin Endocrinol Metab. 2014;99:2712–19. doi: 10.1210/jc.2013-4146. [DOI] [PubMed] [Google Scholar]
- 23.Aronova A, Gordon BL, Finnerty BM, Zarnegar R, Fahey TJ., 3rd Aldosteronoma resolution score predicts long-term resolution of hypertension. Surgery. 2014;156:1387–92. doi: 10.1016/j.surg.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 24.Wolley MJ, Gordon RD, Ahmed AH, Stowasser M. Does contralateral suppression at adrenal venous sampling predict outcome following unilateral adrenalectomy for primary aldosteronism? A retrospective study. J Clin Endocrinol Metab. 2015;100:1477–84. doi: 10.1210/jc.2014-3676. [DOI] [PubMed] [Google Scholar]
- 25.Rossi GP, Belfiore A, Bernini G, et al. the Primary Aldosteronism Prevalence in Hypertension Study Investigators Body mass index predicts plasma aldosterone concentrations in overweight-obese primary hypertensive patients. J Clin Endocrinol Metab. 2008;93:2566–71. doi: 10.1210/jc.2008-0251. [DOI] [PubMed] [Google Scholar]
- 26.Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957–67. doi: 10.1016/S0140-6736(15)01225-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


