Abstract
Background
Because the molecular mechanisms of morphogenesis of the hepatic cord and sinus are unclear, we investigated the involvement of bone morphogenetic protein (BMP4) in hepatic sinusoid morphogenesis.
Methods
We used embryonic chicken livers, which develop rapidly, as our model, and investigated expression of BMP-related genes. BMP4 activity was manipulated by overexpressing BMP4 and its antagonist, noggin.
Results
During hepatic cord morphogenesis, BMP4 and its receptors are expressed in both peri-sinusoidal cells and hepatoblasts as the sinusoids form, whereas noggin is expressed transiently in peri-sinusoidal cells at early stages. Suppression of BMP activity with noggin overexpression disrupted normal hepatic sinusoid structure, leading to liver congestion, failure of fibronectin deposition, and markedly reduced numbers of peri-sinusoidal cells. However, overexpression of BMP did not change sinusoidal morphology but increased endothelial cell number. Noggin overexpression resulted in disrupted cord organization, and dilated sinusoidal space, eventually leading to increased apoptosis and failed hepatocyte differentiation.
Conclusions
Our results show that proper BMP signaling mediates peri-sinusoidal cell–hepatoblast interactions during development; this is essential for hepatic cord organization among hepatoblasts, endothelium, and presumptive hepatic stellate cells.
Keywords: Epithelial morphogenesis, Epithelial–mesenchymal interaction, Tissue engineering, Liver stem cells, Fibronectin, Regeneration
Introduction
The ability of the liver to execute its vital function depends on its unique microscopic structure (i.e. hepatic cords and sinusoids). The hepatic cords are lined by a wall of sinusoids mainly composed of endothelial and hepatic stellate cells (HSC) [1]. A variety of extracellular matrix (ECM) components, including type IV collagen and fibronectin (Fn), occur along the peri-sinusoidal lining of normal liver parenchyma [2]. These different cell types and ECM components are organized into the hepatic cord configuration, which serves as the fundamental building unit of a hepatic lobule. Moreover, hepatocytes also express receptors for ECM, for example β1-integrin, which are essential for cell survival [3].
We lack a full understanding of the cellular and molecular events that govern hepatic structure morphogenesis. Previous studies have shown that endoderm-derived hepatoblasts can be regulated by molecular cues derived from mesenchymal cells [4, 5]. Our laboratory has focused on epithelial morphogenesis, especially of non-neural ectodermal organs [6]. To search for fundamental principles of epithelial morphogenesis, we investigated molecular pathways involved in chicken liver development, and found that:
The duration of active growth zone activity modulates liver size;
A shift in the position of the localized growth zone helps to shape the liver; and
Beta-catenin/Wnt signaling is involved in regulating growth zone activity during liver development [7].
Accordingly, we hypothesized that liver lobule morphogenesis requires interactions between hepatoblasts and mesenchymal cells, and proposed that members of the BMP pathway may be crucially important in the interactions underlying the morphogenetic process. BMP is known to be important in regulation of hepatoblast specification from endoderm [4, 8]. We first demonstrated that BMP and its related molecules were still expressed within the liver bud after hepatoblast specification. To further clarify the involvement of members of the BMP pathway in hepatic cord morphogenesis, we overexpressed BMP4 and its antagonist in embryonic chicken livers after hepatoblasts and mesoderm-derived peri-sinusoidal cells appeared within the liver bud. Subsequently, we assessed cell proliferation, differentiation, and overall morphological changes of the hepatic cords of livers with altered BMP activity. Our results support the proposal that BMP signaling is critical for hepatic cord morphogenesis.
Materials and Methods
RCAS Viral Production
RCAS-BMP4 or noggin was transduced into E7 chicken embryonic fibroblasts at 70 % confluence by calcium phosphate precipitation [9]. The virus was prepared as described elsewhere [7]. Increased viral titers were obtained for supernatants after centrifugation at 12,000 rpm for 30 min before injection.
In-Ovo Microinjection for Gene Misexpression
Eggs were incubated in a humidified chamber at 38°C for 3 days. The eggs of stage 20 and 21 embryos were sterilized with 70 % ethanol and a 15–20 mm diameter window was made for virus injection. The windows were closed with scotch tape and the eggs were placed back in the humidified incubator. The virus was tested for exogenous BMP activity by injection into the beak and observing changes in beak growth [9]. At E10 the liver samples were subsequently harvested for further analysis.
In-Situ Hybridization and Probe Design
Chicken embryo mRNA distribution was determined by section in-situ hybridization, by use of a procedure described elsewhere [7].
After digoxigenin labeling (Roche, Indianapolis, IN, USA), the RNA probe was detected by either the chromogenic method or by use of fluorescent dye. For the chromogenic method, the probe was first recognized by use of anti-digoxigenin antibody conjugated with alkaline phosphatase (Roche) and then detected by use of the NBT/BCIP kit (Roche), in accordance with the manufacturer’s instructions. For fluorescence staining, the probe was first recognized by use of anti-digoxigenin antibody conjugated with peroxidase (Roche). The fluorescence signal was developed by use of the tyramide signal amplification kit (Perkin–Elmer, Waltham, MA, USA). The sequences of oligonucleotides used for probe production are listed in Supplementary Table 1.
Immunochemical Staining
Embryos were sectioned to 6 μm. H&E and immunohistochemical staining were performed as described elsewhere [7]. Briefly, formalin-fixed and paraffin-embedded sections were deparaffinized and rehydrated in a graded ethanol series. Sections were treated for antigen retrieval and incubated with primary antibodies (1:200 dilution) at 4°C overnight. The color reaction was developed by use of the Envision HRP-linked polymer-detection system (Dako, Glostrup, Denmark) and sections were counterstained with hematoxylin.
The sources of the antibodies were: anti-α-SMA, A2547, Sigma-Aldrich, St. Louis, MO, USA; anti-p-smad 1/5/8, Cell Signaling Technology, Danvers, MA, USA, Cat No. 9511; anti-Fn, B3/D6, developed by Dr D.M. Fambrough, anti-LCAM, 7D6, developed by Dr W. Gallin, anti-PCNA and anti-QE, QH-1, developed by Dr F. Dieterlen, and anti-β1-integrin, V2E9, developed by Dr A.F. Horwitz, were obtained from the Developmental Studies Hybridoma Bank, Iowa City, IA, USA, developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences.
Periodic Acid Staining and TUNEL Staining
For periodic acid staining (PAS), slides were treated with 0.5 % (w/v) periodic acid (Sigma) solution for 5 min after rehydration. Thereafter, Schiff’s reagent (Sigma) was added for color development.
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining was performed by use of the TUNEL Apoptosis Detection Kit (Millipore, Billerica, MA, USA), in accordance with the manufacturer’s instructions.
Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted from liver tissues or cultured cells by use of the RNeasy Protect kit (Qiagen, Valencia, CA, USA). AMV reverse transcriptase (Roche) was used for reverse transcription, and qPCR was performed on a Stratagene Mx3000p instrument by use of the SYBR Green kit, in accordance with the manufacturer’s recommendations (Qiagen). One twentieth of the complementary DNA generated was used as template for real time qPCR analysis. The PCR reaction was: one cycle of 95°C for 10 min, 45 cycles of 95°C for 15 s, 60°C for 5 s, and 72°C for 20 s. After amplification, a final melting curve procedure was performed to determine the specificity of the PCR reaction. Primer sequences used for qPCR are listed in Supplementary Table 2.
Bead Implantation and Explant Cultures
Liver explants were prepared in a manner similar to that reported elsewhere for skin culture [10]. Briefly, the liver tissue was dissected in Hank’s buffered saline solution (Gibco/BRL) under a dissection microscope and placed on culture inserts in six-well culture dishes (Falcon). The culture medium contained DMEM and 2 % fetal calf serum and was changed every two days. The cultures were incubated at 37°C at an atmosphere of 5 % CO2 and 95 % air.
AG1-X8 ion exchange beads (Bio-rad) 100–200 mm in diameter were soaked for 2 h in either vehicle solution only or in solutions containing 0.01–5 mg/ml BMP4 or noggin protein. The beads were then washed in DMEM and placed on the surface of liver explant cultures by use of a micromanipulator (Narishigi) under a dissection microscope.
Statistics
Numeric data were normalized, and are presented as mean ± standard error (SE). Multiple comparisons among groups were performed by one-way ANOVA then the Bonferroni post-hoc test. Significance is declared when the p value is less than 0.05.
Results
Assembly of Hepatic Cords Requires Coordinated Assembly of Hepatoblasts and Peri-sinusoidal Mesenchymal Cells
At the gross level, hepatic cords are best perceived as strip-like structures emanating from the surface of the chicken liver on embryonic day 10 (E10; Fig. 1a). During development, hepatic sinusoids start to form as early as E4 (HH stages 24–25) [11]. Peri-sinusoidal cells had already appeared by this stage (Fig. 1b, arrowhead). Meanwhile, hepatoblasts congregated as interconnecting cell clusters at E4 and gradually became organized into cord-like structures by E10 (HH stages 36–37) [11].
Fig. 1.
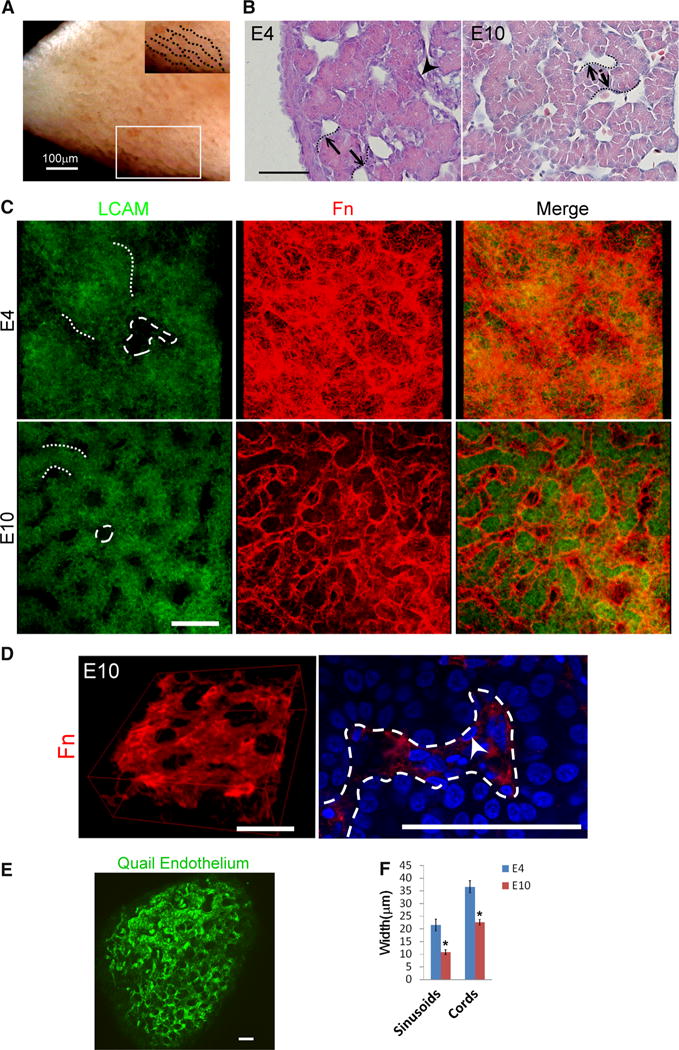
Morphogenesis of 3D hepatic cords and sinusoids. a, b Gross view and HE staining of chicken liver. Note the hepatic cords form with tube-like organization. c Two and three-dimensional staining of LCAM, and Fn staining of embryonic livers. Note that hepatic sinusoids gradually form a three-dimensional “cage-like” structure encircling peri-sinusoidal cells. d Fn immunofluorescence staining (red) of E10 liver. Left: three-dimensional reconstruction of the confocal images. Right: high-power view of Fn expression showed Fn-encircled peri-sinusoidal cells (arrow). Sinusoids are indicated by the dashed line. e Endothelial marker staining of E5 quail livers also shows the cage-like structures. f Quantification of sinusoidal and hepatic cord width. Note that the widths of hepatic cords and sinusoids decrease as the liver develops (*p < 0.05). Size bar 50 μm unless otherwise indicated, dotted lines hepatic cords, dashed lines sinusoids, arrowheads peri-sinusoidal cells. For f, 10 randomly selected sinusoids and hepatic cords from each liver sample (n = 3) were used to calculate the average width
To examine the temporal changes during this morpho-genetic process, liver microstructure was examined by use of confocal microscopy of tissues fluorescently stained with LCAM and Fn, markers of hepatoblasts [12] and hepatic sinusoids [13], respectively. Three-dimensionally, the sinusoids formed a caged structure, as indicated by Fn staining (Fig. 1c, d and Supplementary video) and the endothelial distribution (Fig. 1e). The diameters of hepatic cords and sinusoids decreased gradually as the liver developed. The widths of cords and sinusoids decreased by approximately 40 and 50 %, respectively, from E4 to E10 (Fig. 1f). Moreover, the interconnection among sinusoids became more complex.
In the chicken model, hepatoblasts were specified before E4, because they already expressed the early markers prox1 [14] and HNF4α [15] (Fig. 2a). Next, we investigated the distribution of mesenchyme-derived cells, for example endothelia and HSC, during hepatic cord morphogenesis, because they are known to be involved in liver development [16, 17]. We performed double staining for Fn (marker of sinusoidal space) and vimentin (marker of mesenchyme-derived peri-sinusoidal cells) to reveal the location of mesenchymal cells in E4 livers (Supplementary Fig. 1a). Moreover, we were able to detect SMA and flk-1 mRNA staining in these vimentin (+) cells (Supplementary Figs. 1b, c), suggesting these mesenchymal cells subsequently underwent differentiation. By E10, these endothelial cells were aligned along the hepatic cords, as indicated by in-situ hybridization staining of flk-1 and endoglin mRNA [18] (Fig. 2b). Similarly, α-SMA and p75NTR were observed in some peri-sinusoidal cells, suggesting they may be endothelial smooth muscle cells and HSC [17, 19] (Fig. 2c).
Fig. 2.
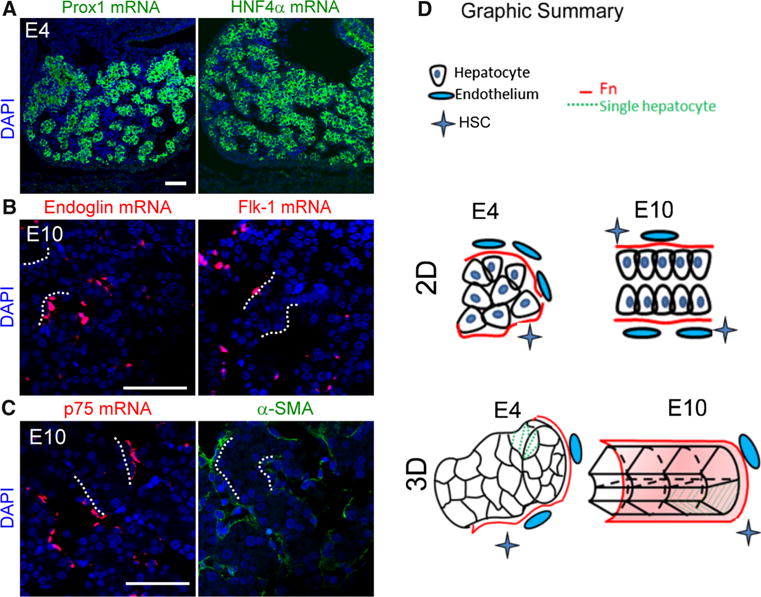
Expression of molecular markers for hepatoblasts, endothelia, and hepatic stellate cells. a In-situ hybridization shows E4 hepatoblasts express both prox1 and HNF4α mRNA, suggesting specification of endodermal cells toward hepatoblasts at this stage. b, c Distribution of endothelia (endoglin, flk-1 mRNA) and HSC (p75NTR, α-SMA) in E10 chicken livers is aligned along the hepatic cords (size bar 50 μm). d Schematic drawing of the morphogenetic process in 2D and 3D
Our results reveal coordinated morphogenesis of hepatic cords and sinusoids. This process requires:
Proper arrangement of hepatoblasts;
Remodeling of ECM components; and
Incorporation of peri-sinusoidal cells (Fig. 2d).
BMP Pathway Members Are Expressed Dynamically in Liver Progenitor Cells During Hepatic Cord Morphogenesis
We then investigated which molecules participate in the morphogenetic process. Secreted BMP4 is highly expressed within the septum transversum mesenchyme cells at early stages of endoderm specification. Delayed liver budding is observed for BMP4 knock-out mice [4], suggesting BMP4 is vital in early liver development. However, it has not been established whether BMP4 functions in hepatic cord and/or sinousoid morphogenesis after hepatoblasts are specified.
We first identified BMP4 and its main antagonist, noggin, transcripts in early liver buds after specification of hepatoblasts from endoderm. After hepatoblast specification, BMP4 was expressed throughout the liver, including hepatoblasts and peri-sinusoidal cells (Fig. 3a, left column and Supplementary Fig. 2). In contrast, peri-sinusoidal cells also express noggin, the BMP antagonist, at E4 but not at E10 (Fig. 3a, right column).
Fig. 3.
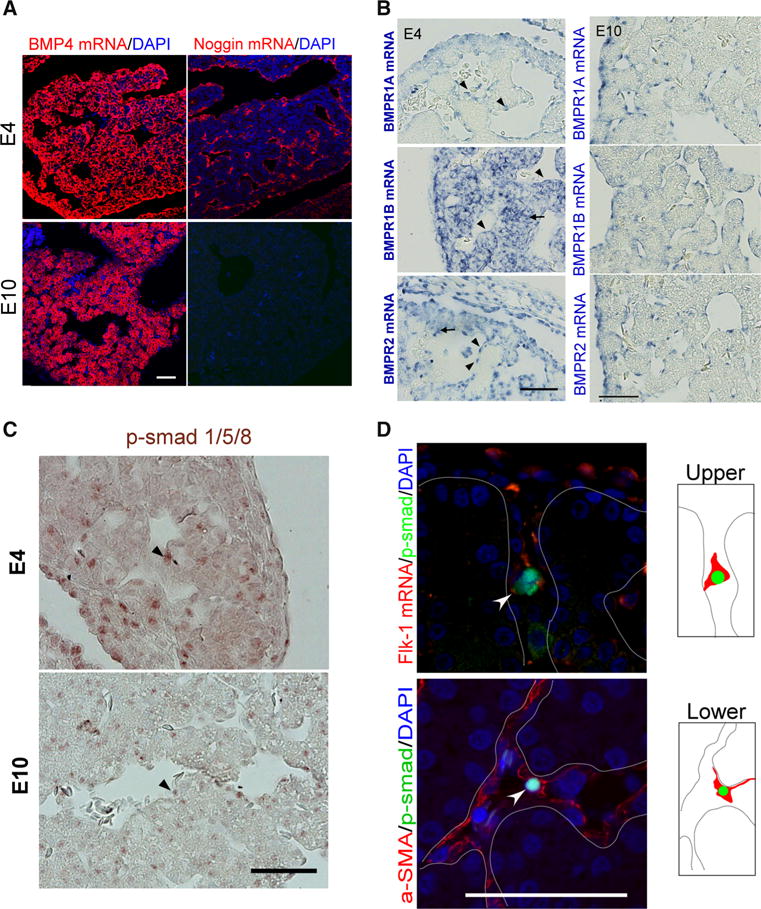
Expression of BMP pathway members in developing hepatic cords. a, b In-situ hybridization of BMP 4 and related molecules. a E4 and E10. BMP4 is expressed in hepatoblasts and peri-sinusoidal cells. Noggin is expressed in E4 peri-sinusoidal cells only. b BMPR1A, 1B, and 2 are expressed in peri-sinusoidal cells at E4 and E10. Note that at E4 (right column), the signals of BMPR1B and BMPR2 mRNA were located in the peri-sinusoidal cells (triangles) and hepatoblasts (arrows), whereas BMPR1A was mainly expressed in peri-sinusoidal cells (triangles). c Staining of p-smad 1/5/8 in E4 and E10 chicken livers. Note that nuclear staining of p-smad 1/5/8 can be observed in both peri-sinusoidal cells (triangle) and hepatoblasts. d Double staining. Nuclear p-smad 1/5/8 (arrowheads) is detected in cells expressing flk-1 mRNA and α-SMA (dotted lines hepatic cords, bar 50 μm)
BMP signaling is reported to promote endodermal specification [4] and hepatoblast differentiation into biliary epithelium [5]. At E4, we detected expression of BMP receptor transcripts, including BMPR1B and 2, in hepatoblasts and peri-sinusoidal cells (Fig. 3b, left column), whereas hepatoblasts did not express albumin mRNA until E10 (Supplementary Fig. 3). This is in accord with the important effect of BMP in regulating hepatoblast specification and differentiation. To our surprise, however, BMP receptor expression in hepatoblasts decreased as the liver developed. BMP receptor 1A, 1B, and 2 mRNAs were also detected in peri-sinusoidal cells at E10 (Fig. 3b, right column), suggesting peri-sinusoidal cells could be the targets of BMP signaling during liver lobule morphogenesis.
We then further stained the liver tissue for phosphorylated smad 1/5/8 (p-smad), the downstream BMP signaling pathway mediators, and p-smad 1/5/8 staining was identified in both hepatoblasts and peri-sinusoidal cells (Fig. 3c). At E4, p-smad staining was observed in approximately 5.17 ± 1.15 % of hepatoblasts and 6.83 ± 1.60 % of peri-sinusoidal cells. At E10, 3.83 ± 0.54 % of hepatoblasts and 4.33 ± 0.99 % of peri-sinusoidal cells expressed nuclear p-smad 1/5/8. To further clarify whether BMP can act on peri-sinusoidal cells, cells were doubly stained for p-smad 1/5/8 and a variety of peri-sinusoidal cell markers, including flk-1 mRNA and α-SMA. Nuclear p-smad was expressed by the same cells expressing the aforementioned markers (Fig. 3d, arrowheads). This suggests BMP signaling can act on peri-sinusoidal cells, including endothelia and HSC, as hepatic sinusoids form. Moreover, peri-sinusoidal cells also expressed noggin, the antagonist of BMP signaling.
BMP Signaling Affects Liver Gross Morphogenesis and Hepatoblast Differentiation
To test the function of BMP4 on liver development, we adjusted BMP activity by overexpressing BMP4 and noggin within the liver via viral transfection. RCAS viruses carrying BMP4 and noggin were injected into the liver primordia of E3 embryos and the liver samples were collected at E10. Successful viral transduction was demonstrated by staining for viral antigen (AMV-3C2) within the liver tissue. Increasing BMP activity did not result in gross or microscopic morphological changes whereas reducing BMP activity resulted in marked congestion of the livers (Fig. 4a). Moreover, histological examination revealed disrupted hepatic cord and sinusoids within noggin-overexpressing livers (Fig. 4b). However, BMP-overexpressed livers had normal sinusoids and hepatic cords. Hepatoblasts affected by noggin overexpression congregated into cell clusters (Fig. 4b, arrowhead) and the sinusoids were abnormally dilated. Some cells also seem to undergo apoptosis, because their nuclei condensed (Fig. 4b, arrow). Moreover, the cell number in noggin-overexpressed livers was markedly reduced compared with the control specimens (20.8 cells/2500 μm2 in noggin-overexpressed livers, 34.02 cells/2500 μm2 in the control samples, p = 0.002; n = 3 for each group). In contrast, in BMP-overexpressed livers the cell number was 33.7 cells/2500 μm2, which was not significantly different from that in control specimens (p = 1.0; n = 3 for each group).
Fig. 4.
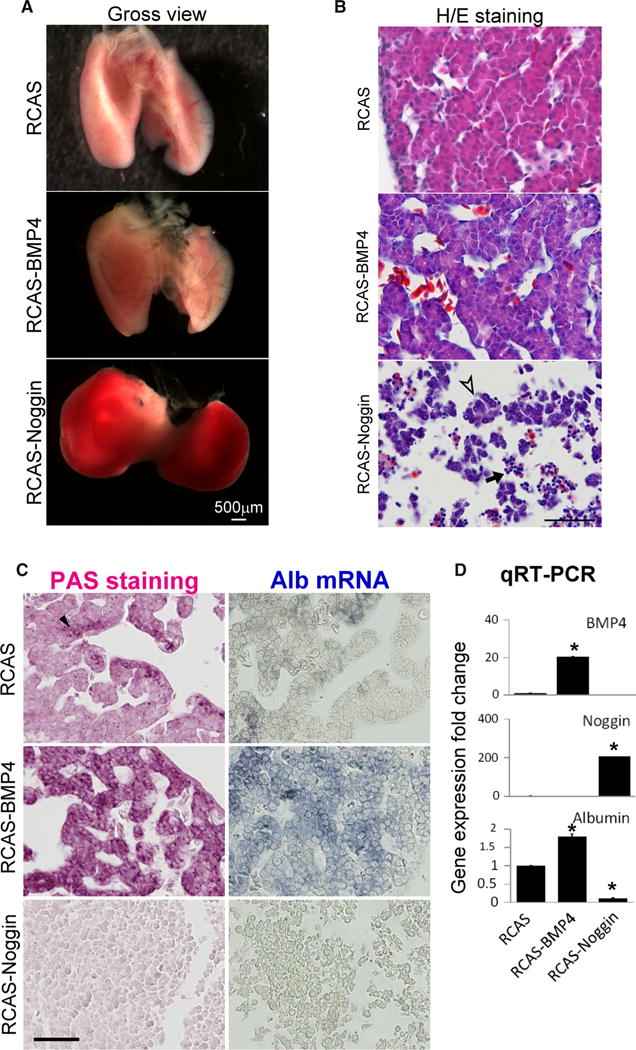
Balanced BMP activity is required for hepatic cord morphogenesis and hepatoblast differentiation. a Misexpression of noggin disrupted normal liver development and caused gross congestion, whereas livers with BMP4 overexpression appear normal. b Misexpression of noggin disrupted hepatic cord and sinusoid configurations, causing congestion and hepatoblast clusters (arrowhead). Nuclear condensation was observed for some cells (arrow apoptosis). Liver with BMP4 overexpression appears normal. c Altering BMP4 signaling affects hepatoblast differentiation, as indicated by PAS and albumin mRNA staining. d Quantitative RT-PCR revealed expression levels of BMP4, noggin, and albumin. The expression level of each target gene was normalized to the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase. (All the liver samples were collected at E10. n = 3, for each group; size bar 50 μm; *p < 0.05)
To further demonstrate that hepatoblast differentiation can be affected by BMP 4, we evaluated hepatocyte differentiation by use of periodic acid staining (PAS, specific for glycogen storage) and albumin mRNA in-situ staining. BMP4 overexpression increased staining area and intensity for both markers whereas noggin overexpression had the opposite effects (Fig. 4c). Quantitative reverse transcription PCR confirmed albumin expression changes in BMP4 and noggin overexpressing groups (Fig. 4d).
Imbalanced BMP Signaling Activity Affects ECM Deposition, Peri-sinusoidal Cell Incorporation, Cell Proliferation, and Apoptosis During Hepatic Cord Organization
As shown in Fig. 1, normal hepatic sinusoids contained Fn as a major ECM component. Therefore, we investigated whether and how sinusoidal ECM was affected by perturbing the BMP signaling pathway. In noggin-overexpressing livers, Fn expression was dramatically reduced, consistent with the altered sinusoidal morphology (Fig. 5a, 1st column). Moreover, expression of β1-integrin, the main Fn receptor, was normally located in the peri-sinusoidal cells and at the sinusoidal side of hepatoblasts. Noggin overexpression reduced β1-integrin expression in both peri-sinusoidal cells and hepatoblasts, suggesting BMP is involved in maintaining cellular matrix interactions and hepatoblast polarity (Fig. 5a, 2nd column).
Fig. 5.
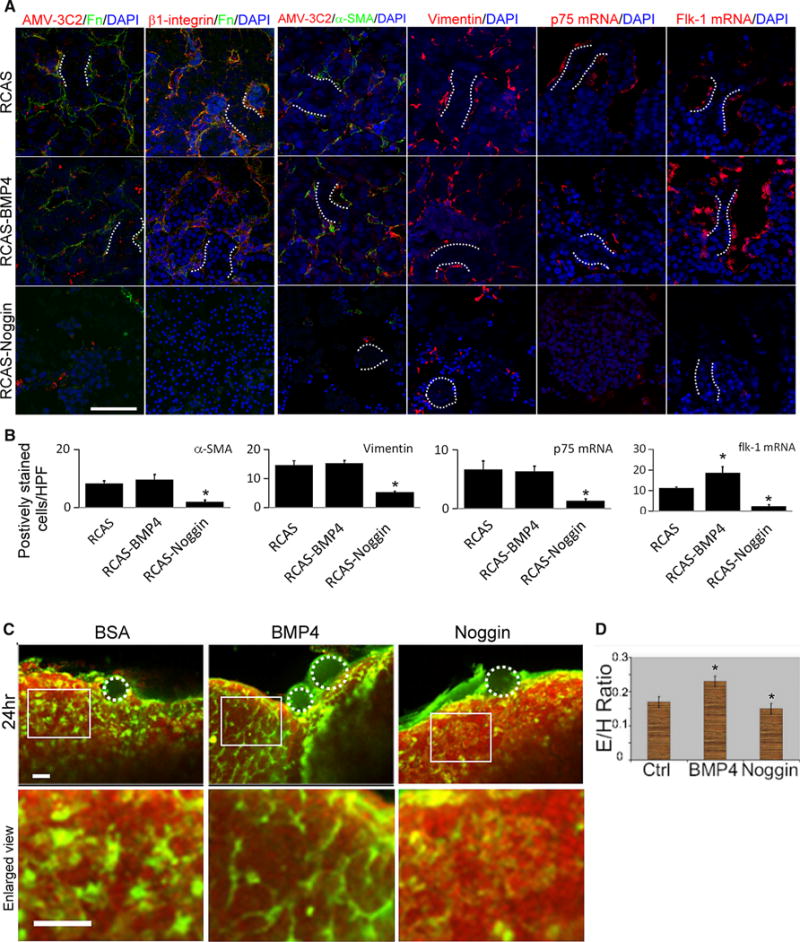
BMP signaling is required for proper assembly of ECM and for peri-sinusoidal cell incorporation. a Staining of ECM and peri-sinusoidal cell markers in BMP4-overexpressing and noggin-overexpressing livers. The left two columns reveal that noggin, but not BMP4, overexpression reduced Fn and β1-integrin staining. AMV-3C2 staining is indicative of viral infection. Ectopic noggin reduces the number of cells expressing markers of mesenchyme (vimentin), HSC (α-SMA, p75 mRNA), and endothelia (flk-1 mRNA) (right four columns). BMP4 overexpression increases the number of cells expressing flk-1 mRNA. b Quantification of peri-sinusoidal cells in BMP-overexpressing and noggin-overexpressing livers. c Several beads (white dotted circles) were placed surrounding quail liver explants. Endothelia were stained with QE antibody (green fluorescence). Nuclei are stained with PI (red). BMP4-coated beads increased the number of endothelial cells compared with controls. Images in the lower row are magnifications of those in the upper row. d Absolute number of endothelial cells per hepatocyte for control, BMP4-overexpressing, and noggin-overexpressing samples (n = 3, for each group). In b, 10 randomly selected fields from each liver sample were used for the calculations. Dotted lines, hepatic cords; size bar, 50 μm; *p < 0.05 compared with the control sample; HPF high power field
Endothelia and HSC, two major cell types of peri-sinusoidal cell, are also BMP signaling targets, as shown in Fig. 3d. Because BMP signaling is essential for sinusoidal morphogenesis, we then investigated whether BMP can regulate incorporation of peri-sinusoidal cells into developing livers. When BMP signaling was reduced, the numbers of cells expressing α-SMA, vimentin, p75NTR mRNA, and flk-1 mRNA decreased. In contrast, BMP4 overexpression increased the number of flk-1 mRNA (+) cells (Figs. 5a (3rd–6th columns), b). This finding suggested BMP signaling is required for peri-sinusoidal cell incorporation, and enhanced BMP activity increased endothelial cell number.
Antibodies to quail endothelium enable good visualization of the endothelial network (Fig. 1e). To evaluate the effects of BMP signaling on endothelia, we administered BMP4 and noggin protein-soaked beads to quail liver primordia. BMP4 and noggin-coated beads induced changes in the nearby endothelia (green fluorescence, Fig. 5c). The endothelia-to-hepatoblast ratio increased when BMP4 beads were used, whereas noggin beads reduced this ratio (Figs. 5c, d). These results were consistent with results from the RCAS misexpression experiments. In summary, we showed that BMP signaling is essential to maintain the peri-sinusoidal cells within the liver and for proper sinusoidal morphogenesis.
Because previous studies have shown that cellular interaction between hepatoblasts and endothelia is important for liver organogenesis [16], we then investigated the involvement of BMP4 in regulating cellular proliferation and differentiation. By use of PCNA and TUNEL staining, we found noggin overexpression not only reduced cell proliferation but also increased cell apoptosis, whereas BMP overexpression had no effects (Supplementary Fig. 4). In contrast, suppressing BMP activity affected not only peri-sinusoidal cells but also hepatoblasts. Proliferation and apoptosis of hepatoblasts were also affected by noggin overexpression (Supplementary Fig. 4).
Taken together, our findings suggest that balanced BMP signaling during liver development is crucial for ECM deposition, peri-sinusoidal cell incorporation, proliferation, and apoptosis.
Discussion
In this study we investigated cellular and molecular events occurring during chicken liver development. We focused on the morphogenetic process when hepatoblasts are organized into cord like structures and the sinusoids transformed into a well-organized caged structure, taking advantage of the relatively rapid transformation process which occurs within 1 week in chickens. Our results reveal that hepatoblasts are organized from multiple cell layers at E4 into double-layered cords by E10. Peri-sinusoidal cells, derived from mesoderm [17], localize to the sinusoidal side of hepatic cords at early morphogenetic stages. To form the complex sinusoidal structures, different cell types within the developing liver must communicate with each other [6, 20, 21].
Proper morphogenesis consists of many tightly regulated cellular events, including properly controlled cell proliferation, cell death, differentiation, cellular arrangement and ECM deposition [6]. We found that imbalanced BMP activity produced alterations in every component of morphogenesis examined. However, noggin-overexpressing livers still developed with a bilobular shape, with enlargement likely to be caused by congestion (Fig. 4a). Vimentin(+) cells could form cell clusters in noggin-overexpressing livers (Fig. 5a), suggesting that arrangement and incorporation of these cells were also affected. Therefore, BMP-related cell apoptosis and proliferation are more likely to be the result, rather than the cause, of altered sinusoid morphogenesis. In the following text we discuss the possible mechanism.
Effects of BMP on Non-parenchymal Cells During Liver Morphogenesis
During liver development, BMP signaling has been reported to promote differentiation of the hepatic endoderm into hepatoblasts [4, 8] and to work simultaneously with FGF signaling and ECM components to guide biliary epithelial differentiation [5]. As sinusoids form, both hepatoblasts and peri-sinusoidal cells secrete BMP4, which targets peri-sinusoidal cells and maintains their proliferative ability. In contrast, noggin was synthesized by peri-sinusoidal cells at early stages only. These different cell types use this pathway to construct the sinusoids which in turn affect hepatic cord conformation and hepatoblast differentiation.
On the basis of these results, we propose a role for BMP signaling, targeting the peri-sinusoidal cells, to coordinate the morphogenetic process which affects both hepatoblasts and peri-sinusoidal cells (Fig. 6). When BMP was over-expressed, liver structure was not affected. However, we observed that BMP bead implantation in ex-vivo embryonic liver culture increased the number of peri-sinusoidal cells (Fig. 5c, d). Moreover, BMP overexpression seemed to promote hepatoblast differentiation, as indicated by the positive staining for albumin mRNA and glycogen (Fig. 6, upper row). BMP overexpression is likely to promote infiltration or proliferation of peri-sinusoidal cells in the embryonic liver and affects hepatoblasts differentiation, which is consistent with previous studies [4, 8]. The lack of morphological defects in BMP-overexpressed livers, however, is unclear. One possible explanation is that proper interaction between hepatoblasts and peri-sinusoidal cells can still occur while BMP is over-expressed. Therefore, we observed no morphogenetic results other than an increased number of peri-sinusoidal cells and increased speed of hepatoblast differentiation.
Fig. 6.
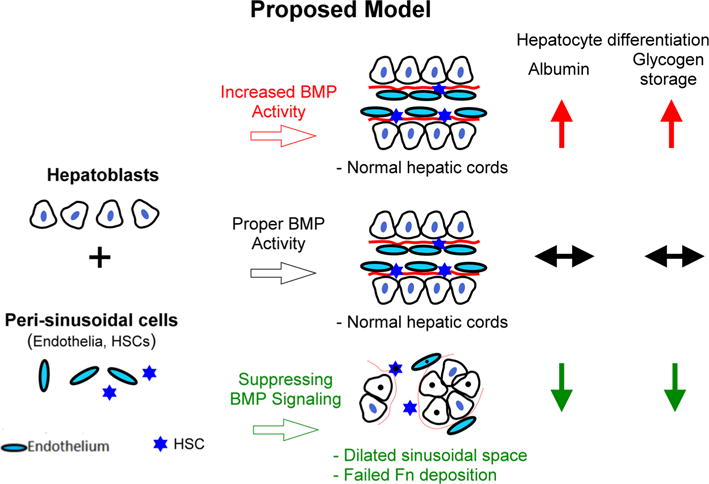
Schematic drawing showing the effects of BMP signaling on the morphogenesis of hepatic cords. Assembly of hepatic cords requires the coordinated assembly of hepatoblasts and peri-sinusoidal mesenchymal cells. Proper BMP activity levels are important for coordinated assembly of the complex epithelial structure (middle row). When BMP was overexpressed, liver structure was not affected. Over-expression of BMP promoted hepatoblast differentiation (upper row). When BMP signaling was suppressed, liver structure was disrupted and cells formed clusters, leading to abnormal fibronectin deposition and dilated sinusoidal space (lower row). Thus BMP signaling is important for regulating interactions between hepatoblast and peri-sinusoidal cells for organizing the hepatic cord and its 3D ECM
Liver structure was, however, severely disrupted when BMP signaling was suppressed by overexpression of noggin (Fig. 6, lower row). This study also showed that BMP activity is required for proper hepatoblast differentiation and maturation. However, we could not distinguish whether this effect on hepatoblasts was directly dependent on BMP signaling or indirectly affected via cell–cell or cell–matrix interactions. More studies will be required to address the mechanism.
It is worthy of note that the percentage of p-smad 1/5/8(+) cells was relatively low in the developing liver. This finding could be simply because of the time-dependent effects on expression of p-smad 1/5/8 after BMP signaling. In an in-vitro study using lung cancer cells, p-smad expression returned to baseline level (4.5 %) 2 h after BMP was added to the culture medium [22]. The fact that relatively few cells were positive for p-smad 1/5/8 did not, therefore, exclude the possibility of an important effect of BMP signaling.
BMP Signaling Affects ECM Organization
In this work we demonstrated that balanced BMP signaling levels, neither too high nor too low, are critical to regulation of the dynamics of ECM organization and successful formation of liver with the correct structure. In noggin-overexpressing livers, in which Fn and peri-sinusoidal cells disappear almost completely, hepatoblasts congregated into clusters rather than cord structures. Therefore, the phenotype we observed in noggin-overexpressing livers was likely to be because of lack of proper cell–matrix interactions. This is consistent with a report showing BMP signaling regulates Fn deposition in the foregut [23].
Three-dimensional organization of ECM has been shown to be involved in morphogenesis [24]. The importance of dynamic ECM synthesis during self-assembly of tissue structures was recently demonstrated [25]. The liver ECM is clinically significant because it can serve as the scaffold for an artificial organ. Dysregulation of liver ECM production can lead to a variety of pathological states, for example liver fibrosis or cirrhosis [26]. Furthermore, it has been proposed that proper interactions between hepatocytes and Fn may be involved in restoring the polarized hepatocyte organization during the liver regeneration required for normal hepatic cord morphogenesis [27]. Our results are consistent with this concept, because loss of proper Fn conformation accompanied the loss of polarized hepatic cord structures and hindered hepatoblast proliferation and differentiation. This is consistent with previous reports showing that ablation of integrin-linked kinase distorted liver histology and affected cell proliferation [28].
In addition, Fn is essential for hepatocyte proliferation after acute liver injury, although mice with a conditional Fn knock-out do not change their hepatic cord conformation [29]. These results suggest that factors other than Fn may contribute to maintaining hepatic cord conformation in adult livers.
Some limitations of the study must be addressed, however. First, the chicken model used in this study may not completely reflect the developmental process in mammals. Second, we were unable to quantify the protein expression of some markers and transduced genes, for example albumin, BMP, and noggin, by western blotting because of a lack of specific antibodies for chicken.
Our study reveals that hepatocyte and non-parenchymal BMP signaling are crucially important in regulating peri-sinusoidal cells and ECM organization during hepatic cord morphogenesis. BMP activity–dependent assembly of liver microstructure is critical for the establishment of hepatic circulation and subsequent hepatocyte differentiation.
Supplementary Material
Key Messages.
Both hepatoblasts and peri-sinusoidal cells contribute to the formation of hepatic cords.
BMP regulates hepatoblasts and mesenchymal cells during liver lobule morphogenesis.
3D fibronectin organization is essential for cell interactions during assembly.
Suppressing BMP signaling disrupts liver lobule structure and ECM.
Acknowledgments
This work was supported by NIH grants to CMC (NIAMS AR 47364, and AR 60306). MST was funded by a Research Fellowship Grant from E-Da hospital, Kaohsiung, Taiwan (EDAHP-10004). SS was supported by a Royal Thai Government Scholarship from Thailand when he was at USC. The work was also supported by the USC Research Center for Liver Diseases, NIH grants 5P30DK048522-06 and 5P30DK048522-07 (PI: Dr Neil Kaplowitz). We acknowledge Drs Neil Kaplowitz, Hide Tsukamato, and James Ou for discussion. We thank Michele McVeigh and the Microscopy Sub Core at the USC Center for Liver Diseases (NIH 1 P03 DK48522) for help with confocal microscopy.
Abbreviations
- HSC
Hepatic stellate cells
- ECM
Extracellular matrix
- Fn
Fibronectin
- BMP
Bone morphogenetic protein
- RCAS
Vector of replication-competent ALV LTR with a splice acceptor
- E
Embryonic day
- LCAM
Liver cell adhesion molecule
- Flk-1
Fetal liver kinase-1
- α-SMA
Alpha-smooth muscle actin
- p75NTR
p75 Neurotrophin receptor
- MMP
Matrix metalloproteinase
- PCNA
Proliferative cell nuclear antigen
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10620-015-3798-2) contains supplementary material which is available to authorized users.
Conflict of interest The authors declare no conflict of interest.
References
- 1.DeRuiter MC, Poelmann RE, Mentink MM, Vaniperen L, Gittenberger-De Groot AC. Early formation of the vascular system in quail embryos. Anat Rec. 1993;235:261–2742. doi: 10.1002/ar.1092350210. [DOI] [PubMed] [Google Scholar]
- 2.Hahn E, Wick G, Pencev D, Timpl R. Distribution of basement membrane proteins in normal and fibrotic human liver: collagen type IV, laminin, and fibronectin. Gut. 1980;21:63–71. doi: 10.1136/gut.21.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinkse GG, Voorhoeve MP, Noteborn M, Terpstra OT, Bruijn JA, De Heer E. Hepatocyte survival depends on beta1-integrin-mediated attachment of hepatocytes to hepatic extracellular matrix. Liver Int. 2004;24:218–226. doi: 10.1111/j.1478-3231.2004.0914.x. [DOI] [PubMed] [Google Scholar]
- 4.Rossi JM, Dunn NR, Hogan BL, Zaret KS. Distinct mesodermal signals, including BMPs from the septum transversum mesenchyme, are required in combination for hepatogenesis from the endoderm. Genes Dev. 2001;15:1998–2009. doi: 10.1101/gad.904601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yanai M, Tatsumi N, Hasunuma N, Katsu K, Endo F, Yokouchi Y. FGF signaling segregates biliary cell-lineage from chick hepatoblasts cooperatively with BMP4 and ECM components in vitro. Dev Dyn. 2008;237:1268–1283. doi: 10.1002/dvdy.21520. [DOI] [PubMed] [Google Scholar]
- 6.Chuong CM, Wu P, Plikus M, Jiang TX, Bruce Widelitz R. Engineering stem cells into organs: topobiological transformations demonstrated by beak, feather, and other ectodermal organ morphogenesis. Curr Top Dev Biol. 2006;72:237–274. doi: 10.1016/S0070-2153(05)72005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suksaweang S, Lin CM, Jiang TX, Hughes MW, Widelitz RB, Chuong CM. Morphogenesis of chicken liver: identification of localized growth zones and the role of beta-catenin/Wnt in size regulation. Dev Biol. 2004;266:109–122. doi: 10.1016/j.ydbio.2003.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang W, Yatskievych TA, Baker RK, Antin PB. Regulation of Hex gene expression and initial stages of avian hepatogenesis by Bmp and Fgf signaling. Dev Biol. 2004;268:312–3269. doi: 10.1016/j.ydbio.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 9.Wu P, Jiang TX, Suksaweang S, Widelitz RB, Chuong CM. Molecular shaping of the beak. Science. 2004;305:1465–1466. doi: 10.1126/science.1098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chuong C-M, Ting S, Widelitz R, Lee Y. Mechanism of skin morphogenesis. II. Retinoic acid modulates axis orientation and phenotypes of skin appendages. Development. 1992;115:839–852. doi: 10.1242/dev.115.3.839. [DOI] [PubMed] [Google Scholar]
- 11.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- 12.Gallin WJ, Chuong CM, Finkel LH, Edelman GM. Antibodies to liver cell adhesion molecule perturb inductive interactions and alter feather pattern and structure. Proc Natl Acad Sci USA. 1986;83:8235–823913. doi: 10.1073/pnas.83.21.8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desmouliere A, Darby I, Costa AM, et al. Extracellular matrix deposition, lysyl oxidase expression, and myofibroblastic differentiation during the initial stages of cholestatic fibrosis in the rat. Lab Invest. 1997;76:765–778. [PubMed] [Google Scholar]
- 14.Papoutsi M, Dudas J, Becker J, et al. Gene regulation by homeobox transcription factor Prox1 in murine hepatoblasts. Cell Tissue Res. 2007;330:209–220. doi: 10.1007/s00441-007-0477-4. [DOI] [PubMed] [Google Scholar]
- 15.Watt AJ, Garrison WD, Duncan SA. HNF4: a central regulator of hepatocyte differentiation and function. Hepatology. 2003;37:1249–1253. doi: 10.1053/jhep.2003.50273. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto K, Yoshitomi H, Rossant J, Zaret KS. Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 2001;294:559–563. doi: 10.1126/science.1063889. [DOI] [PubMed] [Google Scholar]
- 17.Asahina K, Tsai SY, Li P, et al. Mesenchymal origin of hepatic stellate cells, submesothelial cells, and perivascular mesenchymal cells during mouse liver development. Hepatology. 2009;49:998–1011. doi: 10.1002/hep.22721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheifetz S, Bellon T, Cales C, et al. Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J Biol Chem. 1992;267:19027–19030. [PubMed] [Google Scholar]
- 19.Suzuki K, Tanaka M, Watanabe N, Saito S, Nonaka H, Miyajima A. p75 Neurotrophin receptor is a marker for precursors of stellate cells and portal fibroblasts in mouse fetal liver. Gastroenterology. 2008;135:270–281. doi: 10.1053/j.gastro.2008.03.075. [DOI] [PubMed] [Google Scholar]
- 20.Zaret KS, Grompe M. Generation and regeneration of cells of the liver and pancreas. Science. 2008;322:1490–1494. doi: 10.1126/science.1161431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lemaigre F, Zaret KS. Liver development update: new embryo models, cell lineage control, and morphogenesis. Curr Opin Genet Dev. 2004;14:582–590. doi: 10.1016/j.gde.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Langenfeld E, Kong Y, Langenfeld J. Bone morphogenetic protein 2 stimulation of tumor growth involves the activation of Smad-1/5. Oncogene. 2005;25:685–692. doi: 10.1038/sj.onc.1209110. [DOI] [PubMed] [Google Scholar]
- 23.Kenny AP, Rankin SA, Allbee AW, et al. Sizzled-tolloid interactions maintain foregut progenitors by regulating fibronectin-dependent BMP signaling. Dev Cell. 2012;23:292–304. doi: 10.1016/j.devcel.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rozario T, DeSimone DW. The extracellular matrix in development and morphogenesis: a dynamic view. Dev Biol. 2010;341:126–140. doi: 10.1016/j.ydbio.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo CL, Ouyang M, Yu JY, Maslov J, Price A, Shen CY. Long-range mechanical force enables self-assembly of epithelial tubular patterns. Proc Natl Acad Sci USA. 2012;109:5576–5582. doi: 10.1073/pnas.1114781109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jaeschke H. Cellular adhesion molecules: regulation and functional significance in the pathogenesis of liver diseases. Am J Physiol. 1997;273:G602–61126. doi: 10.1152/ajpgi.1997.273.3.G602. [DOI] [PubMed] [Google Scholar]
- 27.Pujades C, Forsberg E, Enrich C, Johansson S. Changes in cell surface expression of fibronectin and fibronectin receptor during liver regeneration. J Cell Sci. 1992;102:815–820. doi: 10.1242/jcs.102.4.815. [DOI] [PubMed] [Google Scholar]
- 28.Gkretsi V, Apte U, Mars WM, et al. Liver-specific ablation of integrin-linked kinase in mice results in abnormal histology, enhanced cell proliferation, and hepatomegaly. Hepatology. 2008;48:1932–1941. doi: 10.1002/hep.22537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moriya K, Sakai K, Yan MH, Sakai T. Fibronectin is essential for survival but is dispensable for proliferation of hepatocytes in acute liver injury in mice. Hepatology. 2012;56:311–321. doi: 10.1002/hep.25624. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


