Abstract
Wnt10b is a signaling protein regulating skin development and homeostasis, and the expression of Wnt10b is restricted to epidermal keratinocytes in embryonic and postnatal skin. Recent studies indicate an elevated expression of Wnt10b in skin tumors. However, how Wnt10b regulates skin tumorigenesis remains largely unknown. Here we report that continuous expression of Wnt10b mediates transformation of epidermal keratinocytes through activating genes involved in EGF/MAPK signaling pathways. We first established a prolonged Wnt10b overexpression system in JB6P− cells to represent the elevated Wnt10b expression level in skin keratinocytes. Through expression assays and observations under phase-contrast microscopy, prolonged expression of Wnt10b activated Wnt/β-catenin pathway and induced morphological changes of cells showing longer protrusions and multilayer growth, indicating early-stage cell transformation. Wnt10b also increased cellular proliferation and migration according to BrdU incorporation and cell mobility assays. Furthermore, multi-doses of AdWnt10b treatment to JB6P—cells induced colony formation, stronger invasive ability in transwell system, and anchorage-independent growth in agar gel. In molecular level, AdWnt10b treatment induced increased transcriptional expressions of Egf, downstream Mapk pathway factors, and MMPs. Administration of Wnt antagonist DKK1 blocked the tumor promotion process induced by Wnt10b. Taken together, these findings clearly demonstrate that Wnt10b promotes epidermal keratinocyte transformation through induced Egf pathway.
Keywords: Wnt10b, DKK1, Epidermal keratinocyte, JB6 cell, Tumorigenesis
Introduction
Carcinogenesis consists of a multistage process, including initiation, promotion, and progression (Hennings et al. 1993). The mouse skin serves as an excellent model for multistage carcinogenesis study. The JB6 mouse epidermal cell lines, which include the tumor promotion-sensitive P+ cells (JB6P+) and tumor promotion-resistant P− cells (JB6P−), have been well established for more than 30 years to study the association of molecular events with cellular transformation during tumor promotion (Colburn et al. 1980). Previous studies show that the JB6P+ cells can be induced to malignancy by several tumor promoters such as TNF-α (Singh et al. 1995), TPA (Colburn et al. 1981), EGF (Colburn and Gindhart 1981), and other tumor promoters (Hsu et al. 2000). These factors activate AP-1 (Dong et al. 1994; Bernstein and Colburn 1989) and NF-κB (Schmidt et al. 1996), which are required for inducing JB6P+ cell transformation. During the transformation of JB6P+ cells, it has been demonstrated that many genes and pathways are involved, including Nrf2 (Paredes-Gonzalez et al. 2014), Ref-1 (Yang et al. 2007), PI3K/Akt (Ouyang et al. 2006), and PKM2 (Li et al. 2014; Wittwer et al. 2011). On the other hand, JB6P− cells resist undergoing these tumorigenic transformations due to the deficiency of AP-1 activation. Nevertheless, JB6P− cells can be restored to response to stimulation of TPA, EGF, or TNF-α and converted to tumor promotion-sensitive phenotype when transfected with stable wild-type MAPK (Erks; Huang et al. 1998) or overexpressed p65 phosphorylation (Hu et al. 2004).
Wnt family members play an important role in controlling skin development and maintaining homeostasis of the epidermis as well as its appendages (Andl et al. 2002; Widelitz 2008; Lei et al. 2013). As a member of the Wnt ligands, Wnt10b is detected in normal murine keratinocytes of epidermis and hair follicles, functioning to initiate anagen reentry (Li et al. 2013) and enhance the keratinocyte differentiation as well as hair shaft growth via activating the canonical Wnt signal pathway (Ye et al. 2013; Li et al. 2011). Wnt10b is also detected in high level in some skin tumors. In the mouse papillomas and skin squamous cell carcinomas (SCC) induced by the two-stage chemical carcinogenesis protocol, Wnt10b expression is upregulated, especially in less differentiated cells of the tumors (Bhatia and Spiegelman 2005). In the M2SMO-induced mouse skin neoplasm resembling human basal cell carcinoma (BCC), the expression of Wnt10b gene is also elevated (Yang et al. 2008). All these studies suggest a close correlation of Wnt10b expression with skin tumor promotion. However, mechanisms of how elevated expression of Wnt10b promotes tumorigenesis of skin remain unclear.
Under physiological conditions, skin epidermis has its resistance to the internal disorder to maintain its homeostasis. In the current study, by applying adenoviral infection into tumor promotion-resistant JB6P− cells rather than JB6P+ cells, we examined the effects of sustained overexpression of Wnt10b on stimulating the proliferation, migration, invasion, and cluster formation capacity of the skin keratinocytes. Accompanying with the activation of the canonical Wnt signaling pathway, we also investigated the molecules required for JB6P− cell conversion to tumor promotion-sensitive type, JB6P+ cell transformation, and tumor progression. We further studied the roles of Wnt10b in JB6P− cell transformation by applying Wnt inhibitor DKK1. Our data indicate that prolonged Wnt10b could stimulate the expressions of Egf and downstream Mapk factors to accumulate neoplasm phenotype of mouse skin keratinocytes, which could be partially rescued by DKK1 as the antagonist.
Materials and methods
Adenoviruses and plasmids
Wnt10b adenoviruses (AdWnt10b) and AdGFP (control) were kindly gifted from Dr. Tong-Chuan He at University of Chicago, USA. The AdWnt10b vector contains an entire length of murine Wnt10b cDNA compared with AdGFP vector. Dkk1 expression plasmid and pEGFP-N1 control plasmid were described as our previous studies (Lei et al. 2011, 2012, 2014). The adenoviruses were propagated in HEK293 cells according to the protocol (He et al. 1998).
Cell culture, infection of adenovirus, and transfection of plasmid in vitro
JB6 Cl 30-7b (JB6P−) mouse epithelial cell line (ATCC, Manassas, USA) was commercially available. Cells were cultured in DMEM (Hyclone, Utah, USA) containing 10 % FBS (Hyclone, Utah, USA) and incubated in a humidified atmosphere containing 5 % CO2 at 37 °C. For adenovirus infection assay, 1 × 106 cells were seeded to the 6-well dish. 1 μ1 of diluted 1 × 107 AdWnt10b or AdGFP was added to the culture dish 1 day after cell seeding. The adenovirus infection rate was analyzed by observing GFP-positive cells using fluorescence microscopy (Nikon, Tokyo, Japan). For plasmid transfection in vitro, 4 μg mouse recombinant Dkk1 expression plasmid or pEGFP-N1 control plasmid was transfected into JB6P− cells using a lipofectamine 2000 kit (Life Technologies, Grand Island, USA).
Intradermal cell injection
1 × 105 cells treated with AdWnt10b or AdGFP for up to three times (Fig. S1a) were subcutaneously injected into the axilla region of nude mice. Sample was harvested 12 days after injection (n = 4). Hematoxylin and eosin staining was applied to observe the phenotype of injected region.
Western blot
Wnt10b expression was detected by Western blot. 50 μ1 of the supernatant (culture medium of adenovirus-treated cells) was filtered through a 0.2-μm strainer, and the cell extract was loaded individually to a 12 % SDS-PAGE and then transferred onto a PVDF membrane under a voltage of 200 V for 1.5 h. The PVDF membrane adsorbed with protein was incubated with anti-Wnt10b (Goat, 1:200, Santa Cruz, USA) primary antibody followed by secondary antibody at 4 °C for overnight. Finally, the blot was developed with a developer kit (Thermo Scientific, Pittsburgh, USA).
RT-PCR
RNA extraction, reverse transcription, and PCR were performed using TRIZOL reagent (Invitrogen, Carlsbad, USA), ReverTra Ace-α (TOYOBO, Osaka, Japan), and 2× PCR master mix (Tiangen Biotech, Beijing, China), respectively. PCR primers were listed in supplementary Table 1.
Cell proliferation detection assay
Ki67 and BrdU immunostaining were used to determine cell proliferation (Figs. 3a, b, 6a and Fig. S4a) with anti-Ki67 (mouse, 1:100; Sigma-Aldrich, USA) and anti-BrdU (mouse, 1:100; Sigma-Aldrich, USA) antibodies. BrdU (Sigma-Aldrich, St. Louis, USA) or PBS as control was added into culture media with a final concentration of 10 μmol/L 1 h before the cell samples were harvested.
Fig. 3.
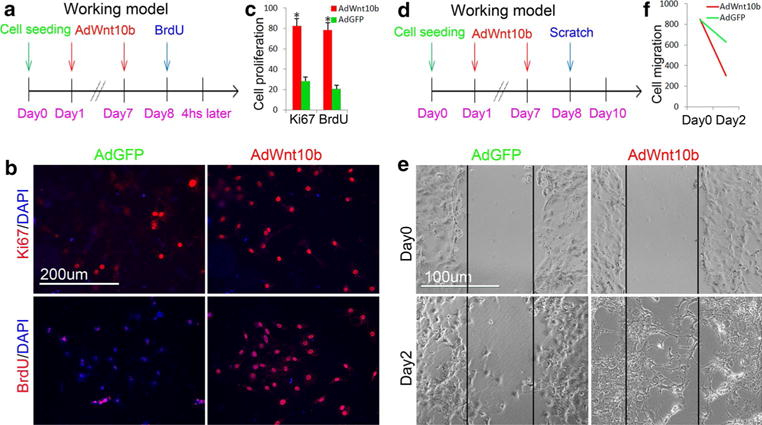
Wnt10b promoted proliferation and mobility of JB6P− cells. a Experimental design illustrated that JB6P− cells were treated with two doses of AdWnt10b and followed by BrdU incorporation assay. b, c Immunofluorescent staining and statistical analysis showed that Ki67- and BrdU-positive cells were increased after two doses of AdWnt10b treatment compared with AdGFP-treated cells. d Experimental design showed timing of two doses of AdWnt10b treatment to JB6P− cells followed by scratch assay. e, f The migration rate was increased in JB6P− cells treated with two doses of AdWnt10b. n > 5, *p < 0.05
Fig. 6.
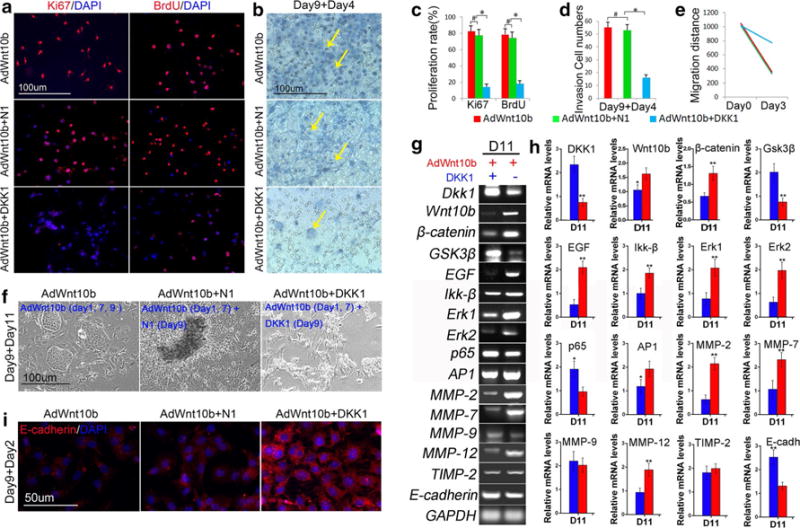
Wnt antagonist Dkk1 impaired the transformation effect triggered by Wnt10b in JB6P− cells. a, c Immunostaining and statistical analysis revealed that Ki67- and BrdU-positive cells were decreased after sequential two doses of AdWnt10b combined with one dose of Dkk1 treatments (AdWnt10b + DKK1) compared within the AdWnt10b or AdWnt10b + N1-treated cells. b, d H&E staining and statistical analysis revealed that the number of the cells invaded to the lower part of transwell system was significantly decreased in AdWnt10b + DKK1-treated group compared with the AdWnt10b or AdWnt10b + N1-treated groups. e Bar chart showed that cell migration rate was significantly decreased in AdWnt10b + DKK1-treated group compared with the AdWnt10b- or AdWnt10b + N1-treated groups. f Phase-contrast microscopy showed multilayer growth in AdWnt10b + N1-treated group and cell transformation abrogation in AdWnt10b + DKK1-treated group. g, h RT-PCR and bar charts revealed significantly decreased mRNA levels of Wnt10b, β-catenin, EGF, Ikk-β, Erk1, Erk2, MMP-2, 7, and 12, and dramatically increased mRNA levels of Dkk1, Gsk3β, p65, and E-cadherin at different time points in AdWnt10b + DKK1-treated groups when compared to those of the AdWnt10b + N1-treated groups. i E-cadherin expression was remarkably increased in AdWnt10b + DKK1-treated group compared with the AdWnt10b- or AdWnt10b + N1-treated groups. n > 5, *p < 0.05, **p < 0.01
Scratch assay
Cell migration ability was analyzed by scratch assay according to the previous description (Liang et al. 2007). AdWnt10b or AdGFP, or AdWnt10b + DKK1, or AdWnt10b + N1-treated cells were seeded in six-well dish, respectively. Cell monolayer was scratched with 200-μ1 pipette tips and photographed under a phase-contrast microscope daily after scratch. Migration rate was analyzed by measuring the average distance between the scratch.
Transwell invasion assay
To test the cell invasion ability, 1 × 106 cells were seeded onto an 8-μm-pore-size transwell dish (Millipore, MA, USA) covered with BME agar containing 10 % FBS. One microliter of diluted 1 × 107 AdWnt10b or AdGFP was added to the transwell dish (Fig. 4a, b). These cells were fixed with acetone for 10 min and stained with hematoxylin and eosin after the noninvasive cells on the upper side of the transwell membrane were gently removed thoroughly with a cotton stick. Then the stained cells were photographed and counted within 10 random fields.
Fig. 4.
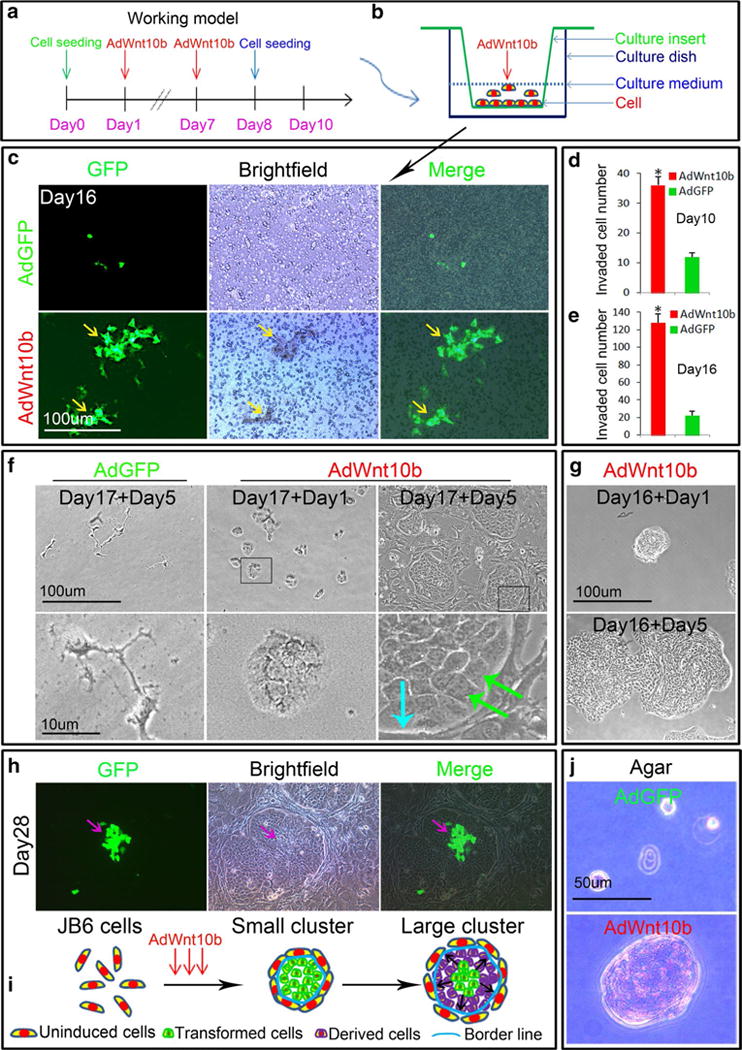
Wnt10b enhanced invasive ability of JB6P− cells. a, b Schematic drawing showed the timing of multiple AdWnt10b treatments and cell seeding into transwell culture system. c, e Fluorescent, bright-field images, and statistical analysis revealed that more cells were invaded to the lower part of transwell system at day 10 or day 16 after AdWnt10b treatments. f The cells harvested from the lower layer of the transwell system at day 17 after AdWnt10b treatments could form clusters 5 days after cultivation (baby blue arrow border line between cluster and neighbor cells, green arrows polarized cells at the border). g Small cluster picked from lower transwell system 16 days after AdWnt10b treatment could quickly expand to large cluster 5 days after culture. h, i Fluorescent, bright-field images, and schematic drawing showed that the large cluster surrounded by untransformed JB6 cells could be expanded from a small group of cells infected with AdWnt10b. j Anchorage-Independent Growth Assay determined robust cluster formation of JB6P− cells after three doses of AdWnt10b treatment, which was not occurred in AdGFP-treated group. n > 5, *p < 0.05
Immunofluorescence
Cells cultured on cover slide were washed with PBS and fixed in acetone for 10 min at 4 °C, followed by being incubated with primary antibodies: anti-Ki67 (mouse, 1:100; Sigma-Aldrich, USA), anti-BrdU (mouse, 1:100; Sigma-Aldrich, USA), and β-catenin (Rabbit, 1:100; Boster, Wuhan, China), anti-E-cadherin (Rabbit, 1:100; Boster, Wuhan, China), relevant secondary antibodies and counter-stained with DAPI (1:1000; Sigma-Aldrich, USA). Fluorescence was detected by fluorescence microscopy (Nikon, Tokyo, Japan).
Anchorage-Independent Transformation Assay
Transformation assays were performed as described previously (Lu et al. 2008). 1 × 104 cells were exposed to AdWnt10b or AdGFP in BME agar containing 10 % FBS. The cultures were maintained in a 37 °C, 5 % CO2 incubator for 2.5 weeks, and the AdWnt10b and AdGFP-induced cell colonies were scored by the methods described (Colburn and Gindhart 1981).
Statistic analysis
All experiments were repeated at least three times and expressed as mean ± SD. All statistical significance among experimental groups and control groups were evaluated by using Student’s t test. A value of p < 0.05 was accepted as significantly different.
Results
Prolonged Wnt10b induced canonical Wnt signaling pathway in JB6P− cells
We first ectopically expressed Wnt10b in JB6P− by using AdWnt10b to analyze whether those cells could be infected. Two days after infection, about 60 % GFP-expressing keratinocytes were detected by fluorescence microscopy (Fig. 1a, e). By repeating infection with AdWnt10b, the numbers of GFP-expressing keratinocytes were remarkably increased (Fig. 1b, e), compared with the AdGFP-treated group (Fig. 1c–e). RT-PCR and statistic analysis showed that the relative mRNA levels of Wnt10b and β-catenin were significantly increased 2 and 8 days after AdWnt10b treatment compared with AdGFP-treated groups (Fig. 1f–h). High levels of Wnt10b protein were detected in both of the cell lysates and culture medium supernatant of keratinocytes, which were repeatedly treated with AdWnt10b for three times at day 15 (Fig. 1i, j), whereas Wnt10b protein could hardly be detected in AdGFP-treated group by Western blot (Fig. 1i, j). After two doses of AdWnt10b treatment, β -catenin as the key molecule of canonical Wnt pathway was translocated into the nuclei, compared with AdGFP-treated control (Fig. 1k).
Fig. 1.
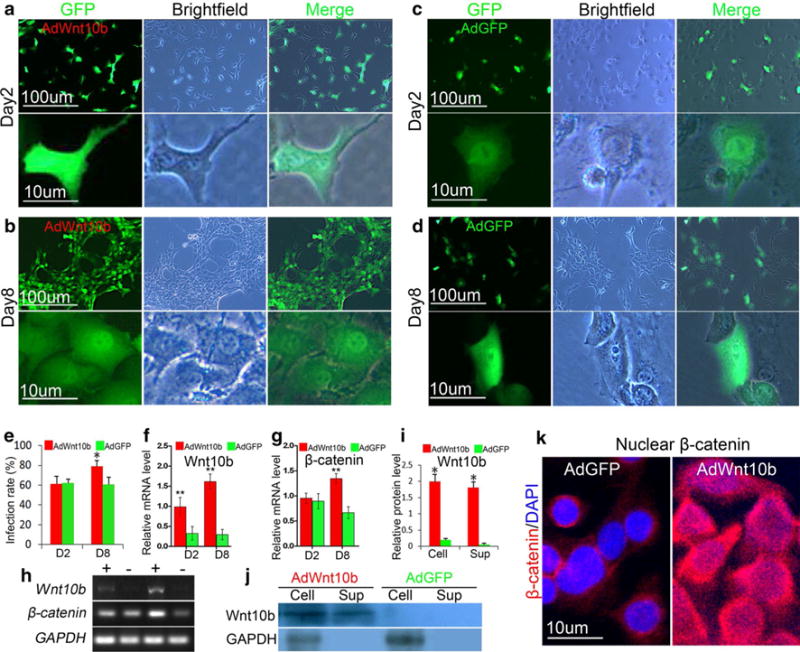
Wnt10b induced activation of canonical Wnt signaling pathway in JB6P− cells. a–d Fluorescent and bright-field images of JB6P− cells at 2 or 8 days after infection with AdWnt10b or AdGFP. e Infection rate was increased in JB6P− cells which received a second dose of AdWnt10b treatment. f–h RT-PCR and statistical analysis showed that mRNA levels of Wnt10b and β-catenin were increased in JB6-cells after AdWnt10b treatment. i, j Western blot and statistical analysis revealed that Wnt10b protein was enhanced in cells and supernatant of culture medium in AdWnt10b-treated group. k β-Catenin was translocated into the nuclei of JB6P− cells infected with a second dose of AdWnt10b. n > 5, *p < 0.05, **p < 0.01
Wnt10b triggered a stepwise morphological transformation of JB6P− cells
To test the effects of Wnt10b on skin keratinocytes, we applied AdWnt10b into the JB6P− cells once a week for successive 4 weeks (Fig. S1a). Two days after AdWnt10b treatment (3 days after cell seeding), the cells were confluent, with longer protrusions than those of the control cells. After a second AdWnt10b treatment at day 8, the cells grew densely and formed cell stack in 2–3 layers. Surprisingly, after the cells were treated with the third dose of AdWnt10b at day 16, a lot of cell clusters were formed (Fig. 2a, b and Fig. S1b). The cells surrounding the cluster were polarized, with sharp boundary insulating from the cells outside at day 22. The clusters grew larger, and the shape of the cells in the cluster was changed from quadrate to cobblestone-like structures at day 28 (Fig. 2a).
Fig. 2.
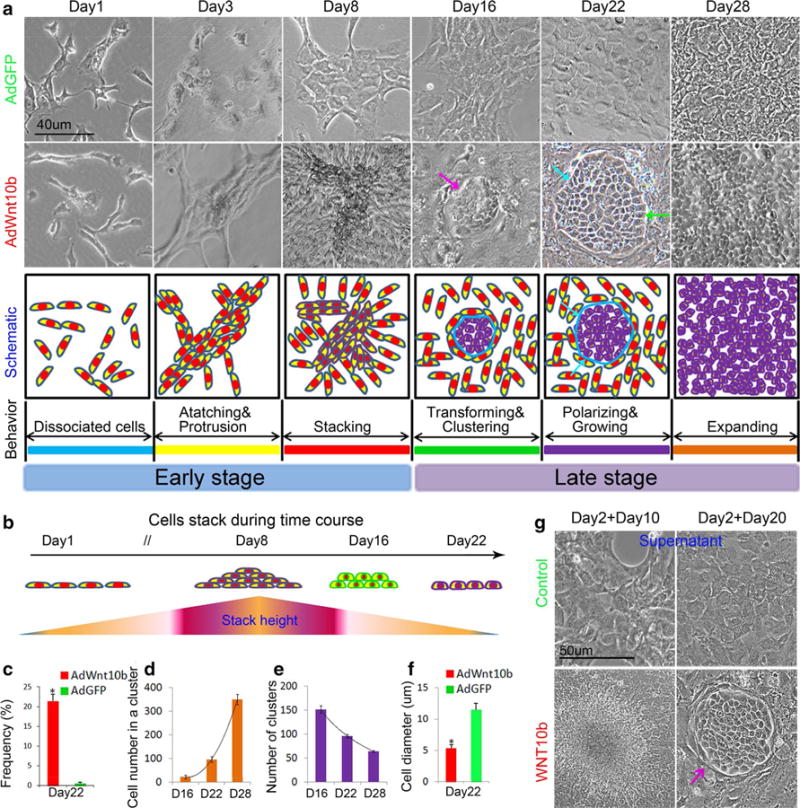
Wnt10b triggered a stepwise transformation of JB6P− cells. a Phase-contrast microscopy and schematic drawing showed that cells treated by Wnt10b gradually transformed from longer protrusions, stacking, cluster formation to expanding growth. b Schematic drawing displayed cells grew from one layer to multiple layers and then to single layer again during AdWnt10b treatments (purple arrow cluster, baby blue arrow border line between cluster and neighbor cells, green arrows polarized cells at the border). c Cell transformation incidence was about 21.5 % after AdWnt10b induction (n > 6). d Cell numbers in one cluster were gradually increased from Day 16 to Day 28 in AdWnt10b-treated group. e Number of clusters was gradually reduced from Day 16 to Day 28 in AdWnt10b-treated group. f Cell diameter was decreased at Day 28 after AdWnt10b treatment. g Cell stack and cluster formation of JB6P− cells at Day 10 or Day 20 after treatment with 2 or 3 doses of supernatant harvested from AdWnt10b-treated cells (purple arrow cluster). n > 5, *p < 0.05
To investigate the relationship between the cell stack and the prospective cell cluster, we trypsinized the cells to observe the morphology before the cluster formation at day 15 (Fig. S1c). Interestingly, we found that the cells located at the bottom layer of the cell stack branched out and changed their shape, resembling the cells in the prospective clusters (Fig. S1c). The upper layers of the cell stack could be easily washed. Thus, we proposed a two-stage process of the transformation, early and late stages, depending on the timing of cell clustering (Fig. 2a).
The incidence of cluster formation induced by prolonged AdWnt10b treatment was about 21.5 % (Fig. 2c, n > 6). The cell numbers in one cluster were gradually increased from approximately 25 cells at day 16 to around 100 cells at day 22 and over 300 cells at day 28 (Fig. 2d). The number of clusters was decreased from approximately 150 at day 16 to around 70 at day 28 due to the cell confluence (Fig. 2e). The cell diameter was significantly decreased from day 22 compared with those in AdGFP-treated group (Fig. 2f).
By adding half-fresh culture medium combined with half-filtered (0.2 μm filter) culture medium from AdWnt10b or AdGFP-treated cells to the newly seeded cells, we were able to ascertain the influence of ectopic Wnt10b treatment on the cluster induction. Cells showed multilayer growth after the second treatment with culture medium from AdWnt10b-treated cell group (Day 2, cell seeding; Day 10, observation; Fig. 2g) and were induced to form cluster after the third treatment (Day 2, cell seeding; Day 20, observation). The cluster formation incidence was also about 21.3 % (Fig. S1d). No fluorescence was observed in these cells (data not shown). These results demonstrate that the cell transformation is due to the Wnt10b induction rather than the influence of adenoviruses.
Overexpression of Wnt10b promoted proliferation and mobility of JB6P− cells at early stage
Next, we determined the early events of effects of Wnt10b overexpression on the skin keratinocytes (Fig. 3a, d). Statistic analysis and immunostaining revealed that keratinocytes infected with two doses of AdWnt10b showed significantly increased expressions of Ki67 and BrdU at day 8, compared with those in the AdGFP-treated group (Fig. 3b, c). The cell migration was then analyzed due to the observation of protrusions (Figs. 2b, 3d). It was obviously noticed that the two doses of AdWnt10b-treated cells migrated and closed the wound more quickly than those of the controls (Fig. 3e, f).
Sustained overexpression of Wnt10b enhanced invasive ability of JB6P− cells at late stage
In the analysis of potentially different cellular behavior between different subpopulations caused by continuous Wnt10b treatment at later stages, we set up a transwell system to investigate the invasive ability of the skin keratinocytes treated by AdWnt10b up to three times (Fig. 4a, b). Ten or sixteen days later, we scraped off the cells in the upper layer of the culture insert and detected cells invaded to the lower layer of the culture insert. Direct fluorescence (Fig. S2a), H&E staining (Fig. S2b), and statistic chart (Fig. 4d) showed that, compared with AdGFP-treated group, there were more cells invaded to the lower layer of the culture insert at day 10 in AdWnt10b-treated group. This was especially true at day 16 after the cells were treated with the third dose of AdWnt10b, with more cells invaded into the lower culture insert and clustered together (Fig. 4c, e and Fig. S2b).
Expansion ability of Wnt10b-induced clusters
The cells at the lower layer of the culture insert were digested with trypsin and cultured (Fig. S2c). One day after seeding, the cells clustered together (Day 17, cells were treated for 17 days; Day 1, 1 day after cell seeding) and grew into large clusters 5 days later (Day 17 + Day 5; Fig. 4f). These clusters were also well distinguished with the neighboring cells by the bright border line (Fig. 4f, baby blue arrow). The cells at outmost layer of clusters were also polarized (Fig. 4f, green arrows). To further determine whether the cluster requires the neighboring cells to expand, we picked one cluster from the lower insert and dropped it into culture plate. Five days later, the small cluster could quickly expand to a big cluster (Fig. 4g). The expanded cell cluster was originated from a small population of AdWnt10b-treated cells as seen by GFP (Fig. 4h, i), since adenovirus could not propagate in JB6 cells.
Later, to further determine the effect of Wnt10b on skin keratinocytes clustering and transformation, we performed the anchorage-independent transformation assay. Sixteen days later, the AdWnt10b repeatedly treated cells that formed big colonies and grew in an anchorage-independent manner, compared with AdGFP-treated group, which could only form very small clusters (Fig. 4j and Fig. S3a). There were about 170 cells in 1 Wnt10b-induced cluster on average (Fig. S3b).
We also subcutaneously injected the multi-doses of AdWnt10b- or AdGFP-treated cells into the nude mice. Twelve days later, H&E staining showed neoplasm formation in AdWnt10b-treated group at the dermal region, when compared to AdGFP-treated group (Fig. S3c).
Wnt10b activated genes in the Wnt, EGF, and MAPK pathways
To further probe the molecular mechanisms underlying the cell transformation after Wnt10b treatment, we used PCR to screen and compare gene expression in AdWnt10b- and AdGFP-treated cells at different time points. Compared with AdGFP-treated groups, Wnt10b and β-catenin were significantly increased, while Gsk3β was decreased in AdWnt10b-treated groups (Fig. 5a, b). Interestingly, among various factors reported to be able to induce JB6P+ cell transformation, Egf expression was significantly enhanced after two-dose treatments in AdWnt10b group. The expression of Mapk downstream molecules important for JB6P+ cell transformation such as Ikk-β, Erk1, and Erk2 was also remarkably elevated, while p65 expression was dramatically decreased in AdWnt10b-treated group compared with the AdGFP-treated group (Fig. 5a, c). Importantly, AP-1 as the effecter during JB6P+ cell transformation started to be significantly elevated after a second dose of AdWnt10b treatment (Fig. 5a, c).
Fig. 5.
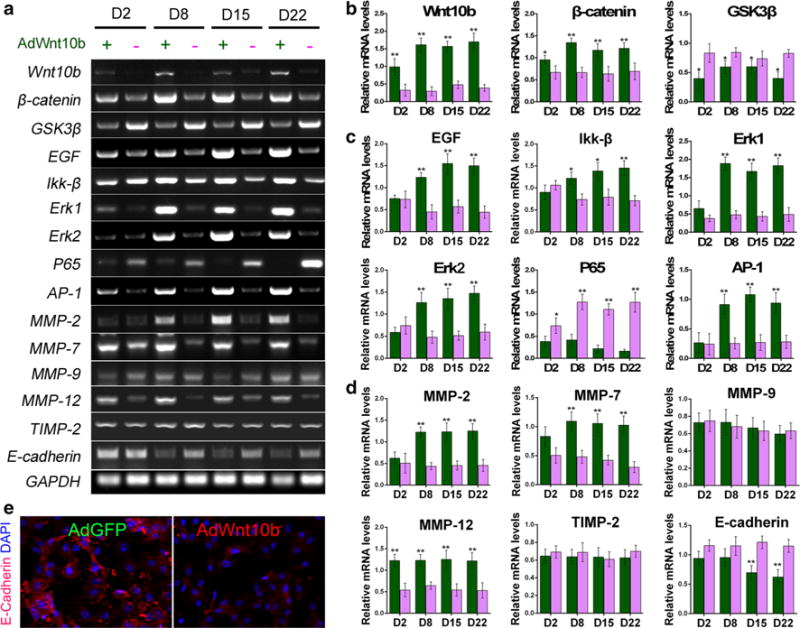
Wnt10b activated gene expressions of Wnt, EGF, and MAPK pathways. a–d RT-PCR and bar charts revealed significant increase in Wnt10b, β-catenin, EGF, Ikk-β, Erk1, Erk2, AP-1, MMP-2, 7, 12 and dramatic decrease in Gsk3β, p65, and E-cadherin at different time points in AdWnt10b-treated groups when compared to those of the AdGFP-treated groups. e Immunostaining showed E-cadherin expression was decreased in AdWnt10b-treated cells compared with the AdGFP-treated group. n > 5, *p < 0.05, **p < 0.01
In observation of strong invasive and expansion abilities of the cells after multiple Wnt10b treatments, we detected MMP expression and found that MMP-2, 7, and 12 were significantly increased in AdWnt10b-treated group when compared to the AdGFP-treated group (Fig. 5a, d). PCR and immunostaining showed E-cadherin was significantly decreased in AdWnt10b-treated group compared with AdGFP-treated group (Fig. 5d, e).
Wnt antagonist Dkkl impaired the transformation effect triggered by Wnt10b in JB6P− cells
The cells treated twice by AdWnt10b were transfected with DKK1 to examine the reversibility of AdWnt10b-treated cell transformation (Fig. S4a). Compared with AdWnt10b + N1 and AdWnt10b control groups, statistic analysis and immunostaining demonstrated that the expressions of Ki67 and BrdU were significantly decreased in the AdWnt10b + DKK1-treated cells (Fig. 6a, c). Likewise, the migration (Fig. 6e and Fig. S4b) and invasive abilities (Fig. 6b, d) of AdWnt10b + DKK1-treated cells were significantly decreased.
We next evaluated how the cell progressed without Wnt activation by transfecting DKK1 to the cells which were treated with two doses of AdWnt10b (Fig. 6f). GFP revealed successful transfections of DKK1 and N1 plasmids into JB6P− cells (Fig. S4c). Immunofluorescence staining indicated that β-catenin was only localized at cytoplasm of most of the DKK1-treated cells (Fig. S4d). Eleven days after Dkk1 treatment, no cluster was formed (Fig. 6f). In molecular level, PCR and statistic analysis showed that DKK1 expression was increased in JB6P− cells 2 days after transfection with DKK1 expression plasmid, when compared to the control group (Fig. 6g, h). Meanwhile, Wnt10b and β-catenin were dramatically decreased in AdWnt10b + DKK1-treated group when compared to those of the two control groups (Fig. 6g, h). Correspondingly, the expressions of Egf, Ikk-β, Erk1, 2, AP-1, MMP-2, 7, and 12 were also downregulated in the AdWnt10b + DKK1-treated group compared with AdWnt10b + N1 and AdWnt10b control groups (Fig. 6g, h), while Gsk3β, p65, and E-cadherin expressions were significantly increased after DKK1 treatment (Fig. 6g–i).
Discussion
Previous studies showed that Wnt10b was highly expressed in the mouse skin papillomas, BCC, and SCC (Bhatia and Spiegelman 2005; Yang et al. 2008). In the keratinocytes of the tumor placodes induced by K14-driven noggin overexpression, there was also an increase in Wnt10b expression (Sharov et al. 2009). Although the association of Wnt10b with the skin tumor was shown in recent studies, there is no direct evidence of how Wnt10b plays a role in skin tumorigenesis. In the present study, we reported that Wnt10b could transform the immortalized JB6P− mouse skin keratinocytes into a neoplastic phenotype, which was evidenced by the stepwise morphological changes, anchorage-independent growth, and the increased potential of proliferation, migration, and invasive in vitro. Interestingly, the neoplastic transformation process could be blocked by the administration of Wnt antagonist DKK1.
Why could Wnt10b induce JB6P− cell transformation? Previous studies revealed that tumorigenesis is induced by multiple gene mutations (Kopper and Hajdu 2004). While it was reported that BCC could be induced after wounding in a smoothened transgenic mouse with overexpressed smoothened in hair stem cells (Wong and Reiter 2011), this BCC did not occur in bulge region where hair stem cells reside. Under normal conditions, hair stem cells maintain quiescent. So we may infer that during wound healing, stem cells which are separated from their proliferation-inhibitory niche could be induced to form tumors under continuous proto-oncogene stimulation. This further indicates that single gene is able to induce cells to be tumorigenetic under special environment. In the present study, the JB6P− cells are proliferating under normal conditions and this proliferative ability is even strengthened after Wnt10b stimulation. Moreover, the cell migration ability is also enhanced. These phenomena quite resemble early skin tumors promotion. The features of the prolonged Wnt10b-induced cells such as anchorage-independent growth, colony formation, and increased potential of invasion also indicate that those cells are analogous to cancer cells. Our data further support the concept that single gene can promote tumorigenesis.
How does Wnt10b promote JB6P− cell transformation? It was reported that in hair follicles, Wnt10b could specifically activate the Wnt/β-catenin signaling pathway (Li et al. 2011; Ouji et al. 2008). Here we found that accompanying with overexpression of Wnt10b, the protein level of β-catenin, the key molecule of Wnt/β-catenin signaling pathway, was increased in the nuclei of skin keratinocytes, indicating the activation of Wnt/β-catenin signaling pathway. This result was not only consistent with the previous studies, but also hinted that Wnt10b-induced skin keratinocyte transformation in our study could be mediated via activation of canonical Wnt signaling pathway. This inference was further confirmed by blocking experiment using DKK1, a secreted protein, which has been shown to antagonize Wnt/β-catenin signaling by binding to LRP6 and preventing the Wnt/Fz/LRP6 complex formation (Yamaguchi et al. 2008; Sick et al. 2006).
Another interesting phenomenon observed in the present study was that the JB6P− cells seemed to become more sensitive to AdWnt10b during the second dose of adenovirus treatment, presenting by increased infection rate, suggesting that the cell property was already changed after one dose of Wnt10b treatment. Indeed, the cellular behavior was different in AdWnt10b-treated groups, which showed longer protrusions at day 3, multilayer growth at day 8, and enhanced proliferative capacity, which was consistent with our previous studies of Wnt10b on skin keratinocytes (Li et al. 2011; Lei et al. 2014).
However, what is the neoplasm exactly? At the current stage, we do not have a clear identification. The neoplasm was formed at about day 15 after prolonged Wnt10b induction, which is in similar duration to JB6P+ cell transformation under ectopic stimuli (Su et al. 2014). While different from JB6P+ cell clustering induced by TPA, EGF, or TNF-a signaling, which showed simple cell aggregation in previous studies (Saikali et al. 2012; Han et al. 2011; Su et al. 2014), the colonies observed in the present study were totally newly induced. Also, the cell colony displayed obvious boarder to the surrounding cells, which were not transformed. Even in the anchorage-independent growth assay when the cells were repeatedly treated by Wnt10b, the newly formed colony also showed a sphere, growing in the soft agar with smooth boarder. These results indicated that the cells in the colony induced by Wnt10b were completely transformed into a neoplasm phenotype. It is accepted that the tumor tissues will create their niche (Li and Xie 2005), with extracellular matrix as the major component, which plays important roles in cancer development. The bright boarder quite resembles the cancer cell niche. The ECM surrounding the niche would then make the peripheral cells of the neoplasm to be polarized.
In molecular level, AP-1 and Nf-κB activities are required for transformation of JB6P+ cells (Dong et al. 1994). These key factors during JB6P+ cell transformation could be induced by multiple pathways including EGF, TNF-a, and downstream members JNK and Erks. In addition, JB6P− cells can be transformed into tumor promotion-sensitive cells when imposed on Erk, with phosphorylation of p65 and increases in Ikk-α and Ikk-β levels (Huang et al. 1998). It was also reported that there was a threshold of AP-1 and ERK expression level for promotion of neoplastic transformation of JB6P+ cells (Suzukawa et al. 2002). In the present study, we found that after Wnt10b treatment, AP-1 expression was dramatically increased. We also found that Erk1, Erk2, and Ikk-β were enhanced, which were supposed to be beneficial for JB6P− cell transformation. It is reported that Wnt10b-overexpressed mammary gland cells could sense EGF and form tumor colonies in soft agar (Lane and Leder 1997). It is also reported that Wnt10b and EGFR were both amplified in breast cancer (Spyratos et al. 1990; Brennan and Brown 2004). The previous studies and the present study sketch a blueprint that Wnt10b induces EGF expression, and then EGF activates downstream Erks, which is able to restore JB6P− to be tumor promotion-sensitive type. Increased and sufficient EGF level also activates AP-1, which induces transformation of the sensitive type of cells (Fig. 7).
Fig. 7.
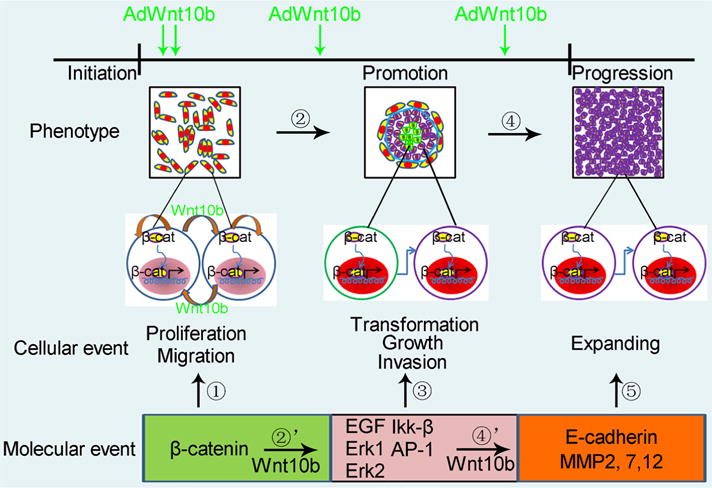
Schematic drawing of stepwise JB6P− cell transformation induced by Wnt10b. Wnt10b treatment leads to activation of Wnt/β-catenin pathway, resulting in cell proliferation and migration. Wnt pathway induces EGF and downstream Mapk factors, which transform JB6P− cells into a neoplastic phenotype. Later on, these cells expand out from the cluster, probably through decreased expression of E-cadherin and induction of MMPs
Combined cellular behavior with molecular events, it was reported that the keratinocyte transformation was closely associated with the changes of cell adhesion and ECM expression (Zhang et al. 2006). The abnormal expression and function of cell adhesion molecules and ECM components provided the molecular basis for the enhanced migration and invasion as well as the anchorage-independent growth of the transformed keratinocytes, which were often regarded as the cellular behaviors for keratinocyte transformation (Wilkins-Port and Higgins 2007). E-cadherin is a transmembrane glycoprotein and plays a key role in intercellular adhesion, ensuring the tissue integrity. Loss of E-cadherin expression directly reduces cell adhesion, and in turn, it could alter the cell morphology and enhance the cell migration and invasion, which was observed in the present study. Indeed, downregulation of E-cadherin was usually detected in the transformation of many types of epithelial cells, including skin keratinocytes, and was believed to be a molecular hallmark of tumor progression and metastasis (Soto et al. 2008; Vaughan et al. 2009; Coppe et al. 2008). Consistent with the previous studies, in this study, the decreased expression of E-cadherin both in mRNA and protein levels caused by Wnt10b overexpression suggests that E-cadherin may contribute to the morphological alteration, the enhanced migration, and invasion during Wnt10b-mediated cell transformation. In fact, the cells showing multilayer growth at day 8 after AdWnt10b treatment could be easily washed away when the culture medium was replaced, which was another evidence for decreased adhesion in Wnt10b-treated cells. Several studies showed that the activation of Wnt/β-catenin signaling downregulated E-cadherin expression by promoting the transcription of some E-cadherin suppresser, such as Twist, Snail, and Slug (Heuberger and Birchmeier 2010). However, a further study is required to validate the mechanism involved in the downregulation of E-cadherin by Wnt10b.
Later stage of carcinogenesis includes cancer cell metastasis, which is MMP dependent. It is reported that Wnt can induce MMPs secretion (Wu et al. 2007). In the present study, accompanied with cell expansion, MMP2, 7, and 12 expressions were increased. These MMPs induced by Wnt signaling may facilitate the metastatic process (Ingraham et al. 2011; Pukrop et al. 2006; Kamino et al. 2011).
In summary, our present study demonstrated that ectopically expressing Wnt10b stepwise transforms JB6P− cells into neoplastic cells through enhancing Egf and Mapk signals. Through activating the Wnt/β-catenin signaling, Wnt10b is also able to downregulate E-cadherin expression and upregulate MMPs expressions, which could facilitate the skin keratinocyte transformation and progression. These findings revealed that Wnt10b expression might exert a crucial role in promotion stage of skin tumorigenesis through activating EGF pathway.
Supplementary Material
Acknowledgments
This study was supported by Grants 81171515 from National Nature Science Foundation of China and CSTC; Innovation and Attracting Talents Program for College and University (‘111’ Project) (B06023), China; and the “BaYu” Scholars Project in Chongqing for Dr. Cheng-Ming Chuong. We thank Dr. Pin-Chi Tang (National Chung Hsing University) and Dr. Ya-Chen Liang (University of Southern California) for carefully revising the manuscript.
Abbreviations
- AdGFP
Control adenoviruses
- AdWnt10b
Wnt10b adenoviruses
- AP-1
Activator protein
- BCC
Basal cell carcinoma
- DKK1
Dickkopf 1
- ECM
Extracellular matrix
- EGF
Epidermal growth factor
- NF-KB
Nuclear factor kappa B
- PAGE
Polyacrylamide gel electrophoresis
- PBS
Phosphate-buffered saline
- PVDF
Polyvinylidene fluoride
- RT-PCR
Reverse transcription-polymerase chain reaction
- SCC
Squamous cell carcinomas
- SDS
Sodium dodecyl sulfate
- TNF-a
Tumor necrosis factor-a
- TPA
12-O-tetradecanoylphorbol-13-acetate
- Wnt10b
Wingless-type MMTV integration site family, member 10B
Footnotes
Electronic supplementary material: The online version of this article (doi:10.1007/s00418-015-1330-6) contains supplementary material, which is available to authorized users.
Conflict of interest: The authors declare no conflict of interest.
References
- Andl T, Reddy ST, Gaddapara T, Millar SE. WNT signals are required for the initiation of hair follicle development. Dev Cell. 2002;2(5):643–653. doi: 10.1016/s1534-5807(02)00167-3. [DOI] [PubMed] [Google Scholar]
- Bernstein LR, Colburn NH. AP1/jun function is differentially induced in promotion-sensitive and resistant JB6 cells. Science. 1989;244(4904):566–569. doi: 10.1126/science.2541502. [DOI] [PubMed] [Google Scholar]
- Bhatia N, Spiegelman VS. Activation of Wnt/beta-catenin/Tcf signaling in mouse skin carcinogenesis. Mol Carcinog. 2005;42(4):213–221. doi: 10.1002/mc.20077. [DOI] [PubMed] [Google Scholar]
- Brennan KR, Brown AM. Wnt proteins in mammary development and cancer. J Mammary Gland Biol Neoplasia. 2004;9(2):119–131. doi: 10.1023/B:JOMG.0000037157.94207.33. [DOI] [PubMed] [Google Scholar]
- Colburn NH, Gindhart TD. Specific binding of transforming growth factor correlates with promotion of anchorage independence in EGF receptorless mouse JB6 cells. Biochem Biophys Res Commun. 1981;102(2):799–807. doi: 10.1016/s0006-291x(81)80202-1. [DOI] [PubMed] [Google Scholar]
- Colburn NH, Koehler BA, Nelson KJ. A cell culture assay for tumor-promoter-dependent progression toward neoplastic phenotype: detection of tumor promoters and promotion inhibitors. Teratog Carcinog Mutagen. 1980;1(1):87–96. doi: 10.1002/tcm.1770010109. [DOI] [PubMed] [Google Scholar]
- Colburn NH, Wendel EJ, Abruzzo G. Dissociation of mitogenesis and late-stage promotion of tumor cell phenotype by phorbol esters: mitogen-resistant variants are sensitive to promotion. Proc Natl Acad Sci USA. 1981;78(11):6912–6916. doi: 10.1073/pnas.78.11.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppe JP, Boysen M, Sun CH, Wong BJ, Kang MK, Park NH, Desprez PY, Campisi J, Krtolica A. A role for fibroblasts in mediating the effects of tobacco-induced epithelial cell growth and invasion. Mol Cancer Res. 2008;6(7):1085–1098. doi: 10.1158/1541-7786.MCR-08-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z, Birrer MJ, Watts RG, Matrisian LM, Colburn NH. Blocking of tumor promoter-induced AP-1 activity inhibits induced transformation in JB6 mouse epidermal cells. Proc Natl Acad Sci USA. 1994;91(2):609–613. doi: 10.1073/pnas.91.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han CY, Hien TT, Lim SC, Kang KW. Role of Pin1 in UVA-induced cell proliferation and malignant transformation in epidermal cells. Biochem Biophys Res Commun. 2011;410(1):68–74. doi: 10.1016/j.bbrc.2011.05.106. [DOI] [PubMed] [Google Scholar]
- He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95(5):2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennings H, Glick AB, Greenhalgh DA, Morgan DL, Strickland JE, Tennenbaum T, Yuspa SH. Critical aspects of initiation, promotion, and progression in multistage epidermal carcinogenesis. Proc Soc Exp Biol Med. 1993;202(1):1–8. doi: 10.3181/00379727-202-43511a. [DOI] [PubMed] [Google Scholar]
- Heuberger J, Birchmeier W. Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harb Perspect Biol. 2010;2(2):a002915. doi: 10.1101/cshperspect.a002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu TC, Young MR, Cmarik J, Colburn NH. Activator protein 1 (AP-1)- and nuclear factor kappaB (NF-kappaB)-dependent transcriptional events in carcinogenesis. Free Radic Biol Med. 2000;28(9):1338–1348. doi: 10.1016/s0891-5849(00)00220-3. [DOI] [PubMed] [Google Scholar]
- Hu J, Nakano H, Sakurai H, Colburn NH. Insufficient p65 phosphorylation at S536 specifically contributes to the lack of NF-kappaB activation and transformation in resistant JB6 cells. Carcinogenesis. 2004;25(10):1991–2003. doi: 10.1093/carcin/bgh198. [DOI] [PubMed] [Google Scholar]
- Huang C, Ma WY, Young MR, Colburn N, Dong Z. Shortage of mitogen-activated protein kinase is responsible for resistance to AP-1 transactivation and transformation in mouse JB6 cells. Proc Natl Acad Sci USA. 1998;95(1):156–161. doi: 10.1073/pnas.95.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingraham CA, Park GC, Makarenkova HP, Crossin KL. Matrix metalloproteinase (MMP)-9 induced by Wnt signaling increases the proliferation and migration of embryonic neural stem cells at low O2 levels. J Biol Chem. 2011;286(20):17649–17657. doi: 10.1074/jbc.M111.229427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamino M, Kishida M, Kibe T, Ikoma K, Iijima M, Hirano H, Tokudome M, Chen L, Koriyama C, Yamada K, Arita K, Kishida S. Wnt-5a signaling is correlated with infiltrative activity in human glioma by inducing cellular migration and MMP-2. Cancer Sci. 2011;102(3):540–548. doi: 10.1111/j.1349-7006.2010.01815.x. [DOI] [PubMed] [Google Scholar]
- Kopper L, Hajdu M. Tumor stem cells. Pathol Oncol Res. 2004;10(2):69–73. doi: 10.1007/BF02893458. [DOI] [PubMed] [Google Scholar]
- Lane TF, Leder P. Wnt-10b directs hypermorphic development and transformation in mammary glands of male and female mice. Oncogene. 1997;15(18):2133–2144. doi: 10.1038/sj.onc.1201593. [DOI] [PubMed] [Google Scholar]
- Lei M, Gao X, Yang L, Yang T, Lian X. Gsdma3 gene is needed for the induction of apoptosis-driven catagen during mouse hair follicle cycle. Histochem Cell Biol. 2011;136(3):335–343. doi: 10.1007/s00418-011-0845-8. [DOI] [PubMed] [Google Scholar]
- Lei M, Bai X, Yang T, Lai X, Qiu W, Yang L, Lian X. Gsdma3 is a new factor needed for TNF-alpha-mediated apoptosis signal pathway in mouse skin keratinocytes. Histochem Cell Biol. 2012;138(3):385–396. doi: 10.1007/s00418-012-0960-1. [DOI] [PubMed] [Google Scholar]
- Lei MX, Chuong CM, Widelitz RB. Tuning Wnt signals for more or fewer hairs. J Invest Dermatol. 2013;133(1):7–9. doi: 10.1038/jid.2012.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei M, Guo H, Qiu W, Lai X, Yang T, Widelitz RB, Chuong CM, Lian X, Yang L. Modulating hair follicle size with Wnt10b/DKK1 during hair regeneration. Exp Dermatol. 2014;23(6):407–413. doi: 10.1111/exd.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Xie T. Stem cell niche: structure and function. Annu Rev Cell Dev Biol. 2005;21:605–631. doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- Li YH, Zhang K, Ye JX, Lian XH, Yang T. Wnt10b promotes growth of hair follicles via a canonical Wnt signalling pathway. Clin Exp Dermatol. 2011;36(5):534–540. doi: 10.1111/j.1365-2230.2011.04019.x. [DOI] [PubMed] [Google Scholar]
- Li YH, Zhang K, Yang K, Ye JX, Xing YZ, Guo HY, Deng F, Lian XH, Yang T. Adenovirus-mediated Wnt10b overexpression induces hair follicle regeneration. J Invest Dermatol. 2013;133(1):42–48. doi: 10.1038/jid.2012.235. [DOI] [PubMed] [Google Scholar]
- Li W, Liu J, Zhao Y. PKM2 inhibitor shikonin suppresses TPA-induced mitochondrial malfunction and proliferation of skin epidermal JB6 cells. Mol Carcinog. 2014;53(5):403–412. doi: 10.1002/mc.21988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2(2):329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- Lu Z, Liu M, Stribinskis V, Klinge CM, Ramos KS, Colburn NH, Li Y. MicroRNA-21 promotes cell transformation by targeting the programmed cell death 4 gene. Oncogene. 2008;27(31):4373–4379. doi: 10.1038/onc.2008.72. [DOI] [PubMed] [Google Scholar]
- Ouji Y, Yoshikawa M, Moriya K, Nishiofuku M, Matsuda R, Ishizaka S. Wnt-10b, uniquely among Wnts, promotes epithelial differentiation and shaft growth. Biochem Biophys Res Commun. 2008;367(2):299–304. doi: 10.1016/j.bbrc.2007.12.091. [DOI] [PubMed] [Google Scholar]
- Ouyang W, Li J, Ma Q, Huang C. Essential roles of PI-3 K/Akt/IKKbeta/NFkappaB pathway in cyclin D1 induction by arsenite in JB6 Cl41 cells. Carcinogenesis. 2006;27(4):864–873. doi: 10.1093/carcin/bgi321. [DOI] [PubMed] [Google Scholar]
- Paredes-Gonzalez X, Fuentes F, Su ZY, Kong AN. Apigenin reactivates Nrf2 anti-oxidative stress signaling in mouse skin epidermal JB6 P + cells through epigenetics modifications. AAPS J. 2014;16(4):727–735. doi: 10.1208/s12248-014-9613-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukrop T, Klemm F, Hagemann T, Gradl D, Schulz M, Siemes S, Trumper L, Binder C. Wnt 5a signaling is critical for macrophage-induced invasion of breast cancer cell lines. Proc Natl Acad Sci USA. 2006;103(14):5454–5459. doi: 10.1073/pnas.0509703103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikali M, Ghantous A, Halawi R, Talhouk SN, Saliba NA, Darwiche N. Sesquiterpene lactones isolated from indigenous Middle Eastern plants inhibit tumor promoter-induced transformation of JB6 cells. BMC Complement Altern Med. 2012;12:89. doi: 10.1186/1472-6882-12-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt KN, Amstad P, Cerutti P, Baeuerle PA. Identification of hydrogen peroxide as the relevant messenger in the activation pathway of transcription factor NF-kappaB. Adv Exp Med Biol. 1996;387:63–68. doi: 10.1007/978-1-4757-9480-9_9. [DOI] [PubMed] [Google Scholar]
- Sharov AA, Mardaryev AN, Sharova TY, Grachtchouk M, Atoyan R, Byers HR, Seykora JT, Overbeek P, Dlugosz A, Botchkarev VA. Bone morphogenetic protein antagonist noggin promotes skin tumorigenesis via stimulation of the Wnt and Shh signaling pathways. Am J Pathol. 2009;175(3):1303–1314. doi: 10.2353/ajpath.2009.090163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sick S, Reinker S, Timmer J, Schlake T. WNT and DKK determine hair follicle spacing through a reaction-diffusion mechanism. Science. 2006;314(5804):1447–1450. doi: 10.1126/science.1130088. [DOI] [PubMed] [Google Scholar]
- Singh N, Sun Y, Nakamura K, Smith MR, Colburn NH. C-JUN/AP-1 as possible mediators of tumor necrosis factor-alpha-induced apoptotic response in mouse JB6 tumor cells. Oncol Res. 1995;7(7–8):353–362. [PubMed] [Google Scholar]
- Soto E, Yanagisawa M, Marlow LA, Copland JA, Perez EA, Anastasiadis PZ. p120 catenin induces opposing effects on tumor cell growth depending on E-cadherin expression. J Cell Biol. 2008;183(4):737–749. doi: 10.1083/jcb.200805113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyratos F, Delarue JC, Andrieu C, Lidereau R, Champeme MH, Hacene K, Brunet M. Epidermal growth factor receptors and prognosis in primary breast cancer. Breast Cancer Res Treat. 1990;17(2):83–89. doi: 10.1007/BF01806288. [DOI] [PubMed] [Google Scholar]
- Su ZY, Zhang C, Lee JH, Shu L, Wu TY, Khor TO, Conney AH, Lu YP, Kong AN. Requirement and epigenetics reprogramming of Nrf2 in suppression of tumor promoter TPA-induced mouse skin cell transformation by sulforaphane. Cancer Prev Res (Phila) 2014;7(3):319–329. doi: 10.1158/1940-6207.CAPR-13-0313-T. [DOI] [PubMed] [Google Scholar]
- Suzukawa K, Weber TJ, Colburn NH. AP-1, NF-kappa-B, and ERK activation thresholds for promotion of neoplastic transformation in the mouse epidermal JB6 model. Environ Health Perspect. 2002;110(9):865–870. doi: 10.1289/ehp.02110865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan MB, Ramirez RD, Andrews CM, Wright WE, Shay JW. H-ras expression in immortalized keratinocytes produces an invasive epithelium in cultured skin equivalents. PLoS ONE. 2009;4(11):e7908. doi: 10.1371/journal.pone.0007908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widelitz RB. Wnt signaling in skin organogenesis. Organogenesis. 2008;4(2):123–133. doi: 10.4161/org.4.2.5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins-Port CE, Higgins PJ. Regulation of extracellular matrix remodeling following transforming growth factor-beta1/epidermal growth factor-stimulated epithelial-mesenchymal transition in human premalignant keratinocytes. Cells Tissues Organs. 2007;185(1–3):116–122. doi: 10.1159/000101312. [DOI] [PubMed] [Google Scholar]
- Wittwer JA, Robbins D, Wang F, Codarin S, Shen X, Kevil CG, Huang TT, Van Remmen H, Richardson A, Zhao Y. Enhancing mitochondrial respiration suppresses tumor promoter TPA-induced PKM2 expression and cell transformation in skin epidermal JB6 cells. Cancer Prev Res (Phila) 2011;4(9):1476–1484. doi: 10.1158/1940-6207.CAPR-11-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SY, Reiter JF. Wounding mobilizes hair follicle stem cells to form tumors. Proc Natl Acad Sci USA. 2011;108(10):4093–4098. doi: 10.1073/pnas.1013098108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Crampton SP, Hughes CC. Wnt signaling induces matrix metalloproteinase expression and regulates T cell transmigration. Immunity. 2007;26(2):227–239. doi: 10.1016/j.immuni.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Passeron T, Hoashi T, Watabe H, Rouzaud F, Yasumoto K, Hara T, Tohyama C, Katayama I, Miki T, Hearing VJ. Dickkopf 1 (DKK1) regulates skin pigmentation and thickness by affecting Wnt/beta-catenin signaling in keratinocytes. FASEB J. 2008;22(4):1009–1020. doi: 10.1096/fj.07-9475com. [DOI] [PubMed] [Google Scholar]
- Yang S, Misner BJ, Chiu RJ, Meyskens FL., Jr Redox effector factor-1, combined with reactive oxygen species, plays an important role in the transformation of JB6 cells. Carcinogenesis. 2007;28(11):2382–2390. doi: 10.1093/carcin/bgm128. [DOI] [PubMed] [Google Scholar]
- Yang SH, Andl T, Grachtchouk V, Wang A, Liu J, Syu LJ, Ferris J, Wang TS, Glick AB, Millar SE, Dlugosz AA. Pathological responses to oncogenic Hedgehog signaling in skin are dependent on canonical Wnt/beta3-catenin signaling. Nat Genet. 2008;40(9):1130–1135. doi: 10.1038/ng.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Yang T, Guo H, Tang Y, Deng F, Li Y, Xing Y, Yang L, Yang K. Wnt10b promotes differentiation of mouse hair follicle melanocytes. Int J Med Sci. 2013;10(6):691–698. doi: 10.7150/ijms.6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZG, Bothe I, Hirche F, Zweers M, Gullberg D, Pfitzer G, Krieg T, Eckes B, Aumailley M. Interactions of primary fibroblasts and keratinocytes with extracellular matrix proteins: contribution of alpha 2 beta 1 integrin. J Cell Sci. 2006;119(Pt 9):1886–1895. doi: 10.1242/jcs.02921. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


