Abstract
Unlike other agonists that cause transient endothelial cell (EC) response, the products of 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine (PAPC) oxidation that contain cyclopenthenone groups, which recapitulate prostaglandin-like structure, cause sustained enhancement of the pulmonary EC barrier. The mechanisms that drive the sustained effects by oxidized PAPC (OxPAPC) remain unexplored. On the basis of the structural similarity of isoprostanoid moieties that are present in full-length oxygenated PAPC species, we used an inhibitory approach to perform the screening of prostanoid receptors as potential candidates that mediate OxPAPC effects. Results show that only prostaglandin E receptor-4 (EP4) was involved and mediated the sustained phase of the barrier-enhancing effects of OxPAPC that are associated with the activation of Rac GTPase and its cytoskeletal targets. EC incubation with OxPAPC also induced EP4 mRNA expression in pulmonary ECs and lung tissue. EP4 knockdown using gene-specific small interfering RNA did not affect the rapid phase of OxPAPC-induced EC barrier enhancement or the protective effects against thrombin-induced EC permeability, but abolished the advanced barrier enhancement phase and suppressed the protective effects of OxPAPC against more sustained EC barrier dysfunction and cell inflammatory response caused by TNF-α. Endothelial-specific knockout of the EP4 receptor in mice attenuated the protective effect of intravenous OxPAPC administration in the model of acute lung injury caused by intratracheal injection of LPS. Taken together, these results demonstrate a novel role for prostaglandin receptor EP4 in the mediation of barrier-enhancing and anti-inflammatory effects caused by oxidized phospholipids.—Oskolkova, O., Gawlak, G., Tian, Y., Ke, Y., Sarich, N., Son, S., Andreasson, K., Bochkov, V. N., Birukova, A. A., Birukov, K. G. Prostaglandin E receptor-4 receptor mediates endothelial barrier–enhancing and anti-inflammatory effects of oxidized phospholipids.
Keywords: acute lung injury, vascular permeability, cytoskeleton
Acute lung injury (ALI) and the more severe acute respiratory distress syndrome develop in response to a variety of infectious and noninfectious insults. Oxidative stress that accompanies inflammatory or mechanical tissue insult triggers the formation of bioactive oxidized phospholipid species from cell membrane phospholipids, which may modulate vascular permeability and trigger both pro- and anti-inflammatory cascades (1). Nonenzymatic oxidation of one of the major plasma membrane phospholipids, 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (PAPC), generates a group of compounds that contain various modified residues at the sn-2 position of the phospholipid. Whereas oxidatively truncated products of PAPC oxidation promote chronic vascular inflammatory processes that are involved in atherogenesis (2–5) and endothelial cell (EC) barrier dysfunction (6–8), other oxidized PAPC (OxPAPC) species have been demonstrated to inhibit the inflammatory response to bacterial agonists and blunt NF-κB–mediated expression of inflammatory genes via antagonistic interaction with the TLRs, TLR-2, -4, and -9 (9–11). OxPAPC also exhibits pronounced and sustained barrier protective effects on pulmonary vascular ECs, which were described in culture and in animal models of ALI caused by high tidal volume mechanical ventilation or intratracheal injection of viral antigens, gram-positive and -negative bacterial pathogens (10, 12–14). These effects were attributed to full-length oxygenated OxPAPC products (8).
Prostaglandins (PGs) are produced by the COX-mediated enzymatic conversion of arachidonic acid. In turn, nonenzymatic oxidation of arachidonic acid leads to the formation of their stereoisomers, isoprostaglandins (15). Similarly, oxidation of cell membrane phospholipids during acute tissue injury leads to the formation of phospholipids that contain the isoprostanoid chain in their structure (1). Receptors that are activated by PGs represent a group of GPCRs with specific affinity to different PGs (16). Among them, PGE2 and PGI2 mediate their effects in target cells by binding to specific prostanoid GPCRs PGE2 receptor (EP)1–4 and prostacyclin (PGI2) receptor (IP), respectively. PGF2α acts via PGF2 receptor (FP). GP and DP serve as PGG2 and PGD2 receptor, respectively. All of these receptors are expressed in the endothelium (17), and both EPs and IPs are expressed in lung tissues (18).
Activation of different PG receptors triggers receptor-specific signaling cascades. Certain PGs, such as PGE2 and PGI2, and its stable synthetic analogs, beraprost and iloprost, exhibit barrier protective and anti-inflammatory effects on pulmonary ECs. These effects are mediated by the stimulation of cAMP production and activation of cAMP-dependent PKA and cAMP-dependent Rap1 GTPase-specific guanine nucleotide exchange factor, Epac-1 (19–21). Both cascades lead to the activation of Rac1 GTPase. Together, activated Rap1 and Rac1 stimulate peripheral cytoskeletal remodeling and enhance cell junctions and EC barrier (22, 23). In addition, activated Rap1 and Rac1 down-regulate the agonist-induced activation of barrier-disruptive RhoA GTPase signaling via negative crosstalk mechanisms (23, 24) and attenuate the augmentation of endothelial inflammation that is caused by bacterial compounds and inflammatory cytokines (25).
We have previously found that the barrier-enhancing effects of OxPAPC are mediated by full-length oxygenated PAPC products and may be reproduced by single compounds that are contained in OxPAPC, such as 1-palmitoyl-2-(5,6-epoxyisoprostane E2)-sn-glycero-3-phosphatidyl choline, the molecule that contains the isoprostanoid group with cyclopenthenone ring (25). Whether PG receptors mediate the barrier-enhancing and anti-inflammatory effects of OxPAPC remains unknown. By using complementary molecular and pharmacologic approaches, combined with animal genetic models of endothelial-specific receptor knockout, this study tested the involvement of PG receptors in the barrier-enhancing and anti-inflammatory effects of OxPAPC. We also characterized the signaling and morphologic events that contribute to receptor-dependent EC enhancement or protection of pulmonary EC barrier against vasoactive and inflammatory insults in vitro and in the animal model of LPS-induced ALI.
MATERIALS AND METHODS
Cell culture and reagents
Human pulmonary artery endothelial cells were obtained from Lonza (East Rutherford, NJ, USA) and were used for experiments at passages 5–7. All reagents for immunofluorescence studies were purchased from Molecular Probes (Eugene, OR, USA). PAPC was obtained from Avanti Polar Lipids (Alabaster, AL, USA). PAPC was oxidized by exposure to air for 72 h. The extent of oxidation was measured by positive ion electrospray mass spectrometry, as previously described (26). After the completion of oxidation, phospholipids were stored at −70°C dissolved in chloroform and were used within 2 wk of mass spectrometry analysis. TNF-α was obtained from R&D Systems (Ann Arbor, MI, USA). The following PG receptor family inhibitors, obtained from Cayman Chemicals (Ann Arbor, MI, USA), were used: thromboxane receptor (TP) inhibitor SQ29548, DP inhibitor BWA868C, IP inhibitor CAY10449, EP4 inhibitor L161982, FP (PGI2) receptor AL8810, and EP1-3 inhibitor AH6809. Rac1, p120-catenin, and Wiskott-Aldrich syndrome protein-family verprolin-homologous protein (WAVE) Abs were purchased from BD Transduction Laboratories (San Diego, CA, USA); RhoA, EP4, IQ motif containing GTPase-activating protein 1 (IQGAP1), vascular endothelial cadherin (VE-cadherin), zonula occludens protein 1 (ZO-1), intercellular cell adhesion molecule type 1 (ICAM1), and VCAM1 Abs were from Santa Cruz Biotechnology (Santa Cruz, CA, USA); cortactin and phospho-Y421 cortactin Abs were from EMD Millipore (Billerica, MA, USA); phospho–myosin light chain, phospho-PAK1, phospho–NF-κB, and IκBα Abs were obtained from Cell Signaling Technology (Beverly, MA, USA). Unless otherwise specified, all biochemical reagents, including β-tubulin Ab, were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Small interfering RNA transfection
Human pulmonary ECs were treated with gene-specific small interfering RNA (siRNA) duplexes. Predesigned standard purity siRNA sets (Homo sapiens) were purchased from Dharmacon (Lafayette, CO, USA), and transfection of ECs with siRNA was performed as previously described (13). After 48 h of transfection, cells were used for experiments or were harvested for Western blot verification of specific protein depletion. Nonspecific, nontargeting siRNA (Dharmacon) was used as a control treatment. siRNA transfection efficiency according to our protocol exceeded 90% (27).
Quantitative RT-PCR
Analysis of EP1–4 mRNA expression by human pulmonary ECs was performed by real-time quantitative RT-PCR. Total RNA from lung tissue was isolated using Qiagen RNeasy kit, and DNA contamination was removed by using on-column DNase I from Qiagen. One microgram of RNA was reverse transcribed to cDNA using an iScript (Bio-Rad, Hercules, CA, USA) cDNA synthesis kit per the manufacturer’s instructions. Quantitative RT-PCR reactions were performed in duplicate for each sample with a final reaction volume of 10 µl in 96-well plates using SYBR green reaction mix (Quantabio) on a Bio-Rad CFX96. Gene expression fold changes were calculated according to the ΔΔCt method (28). The following primers were used: EP1 (forward) ACCTTCTTTGGCGGCTCTC, (reverse) GCACGACACCACCATGATAC; EP2 (forward) CAGTCTCCCTGCTCTTCTGC, (reverse) GCACCGAGACAATGAGAAGC; EP3 (forward) TCATCGTCGTGTACCTGTCC; (reverse) CGATGAACAACGAGGAGAGC; EP4 (29) (forward) 5′-ACCATTCCTAGATCGAACCGT-3′, (reverse) 5′-CACCACCCCGAAGATGAACAT-3′; GAPDH: (forward) 5′-GACATCAAGAAGGTGGTGAAGCAG-3′, (reverse) 5′-ATACCAGGAAATGAGCTTGACAAA-3′.
Coimmunoprecipitation, differential protein fractionation, and immunoblotting
Coimmunoprecipitation studies were performed as described elsewhere (30). In brief, after agonist stimulation, plated EC cultures were washed in ice-cold PBS and lysed on ice with cold Tris-buffered saline NP-40 lysis buffer (20 mM Tris, pH 7.4, 150 mM NaCl, 1% NP-40) that was supplemented with protease and phosphatase inhibitor cocktails (Roche, Indianapolis, IN, USA). Clarified lysates were then incubated with Abs to p120-catenin or IQGAP1 overnight at 4°C and washed 3–4 times with Tris-buffered saline NP-40 lysis buffer; complexes were then analyzed by Western blotting using appropriate Abs. In fractionation studies, membrane fractions were isolated by using a subcellular protein fractionation kit (Thermo Fisher Scientific, Waltham, MA, USA) according to manufacturer protocol. For the analysis of protein phosphorylation profile, cells were stimulated and lysed; protein extracts were then separated by SDS-PAGE, transferred to PVDF membrane, and probed with specific Abs. Equal protein loading was verified by probing membranes with the Ab to β-tubulin or the specific protein of interest. The relative intensities of immunoreactive protein bands (relative density units) were analyzed and quantified by scanning densitometry using Image Quant software (Molecular Dynamics, Sunnyvale, CA, USA).
In situ proximal ligation assay
Cells were fixed with methanol/acetone and incubated for 30 min with the mouse anti-p120-catenin monoclonal antibody (BD Transduction Labs) and rabbit polyclonal anti-VE-cadherin antibody (Cayman). Ligation and amplification were performed as described in the manufacturer’s protocol [Duolink In Situ proximal ligation assay (PLA); Sigma-Aldrich] using the following reagents: PLA probe anti-rabbit plus, PLA probe anti-mouse minus, and detection reagent Red. After PLA, samples were incubated for an additional 30 min with FITC-labeled donkey Ab against mouse IgG.
GTPase activation assays
Rac and Rho activation was evaluated in pull-down assays by using agarose beads with immobilized PAK1-PBD or rhotekin, respectively, as described in Tian et al. (31). The levels of activated small GTPases that bound to beads were evaluated by Western blot analysis and normalized to total Rac, Rap, and Rho levels.
Immunofluorescence staining
After agonist treatment, ECs that were grown on glass coverslips were fixed in PBS that contained 3.7% formaldehyde, and F-actin was visualized by immunofluorescence staining of cell monolayers with Texas Red–conjugated phalloidin. Adherens junctions were labeled with Abs against VE-cadherin, as previously described (32, 33).
Cytokine analysis
Concentrations of TNF-α or soluble ICAM-1 in control and treated cell conditioned medium samples were measured by using ELISA kits available from R&D Systems (Minneapolis, MN, USA) according to manufacturer instructions.
Permeability measurements
Measurements of transendothelial electrical resistance (TER) across confluent human pulmonary artery EC monolayers were performed by using the electrical cell–substrate impedance sensing system (Applied Biophysics, Troy, NY, USA) as previously described in Birukova et al. and Birukov et al. (34, 35). Endothelial permeability to macromolecules was monitored by express permeability testing assay that was available from Millipore (Vascular Permeability Imaging Assay). For permeability assays in the 96-well plates, pulmonary ECs were seeded on biotinylated gelatin-coated 96-well plates (3 × 104 cells/well) and grown for 48–72 h before testing. At the end of agonist stimulation time, FITC-avidin solution at a final concentration of 25 µg/ml was added directly to the culture medium for 3 min before the termination of the experiment. Unbound FITC-avidin was washed out with 200 µl PBS (pH 7.4, 37°C) for 2 cycles, 10 s each. Finally, 100 µl PBS was added to each well, and the fluorescence of matrix-bound FITC-avidin was measured on a Victor X5 Multilabel Plate Reader (PerkinElmer, Waltham, MA, USA) using an excitation wavelength of 485 nm and an emission wavelength of 535 nm.
Animal studies
All experimental protocols that involved the use of animals were approved by the University of Chicago Institutional Animal Care and Use Committee. EC-specific EP4-knockout mice were generated by cross-breeding of the endothelial VECad-Cre-ERT2 line (36) and EP4flox/flox mice in the C57B6 background, as previously described in Liang et al. (37). EP4-knockout mice and wild-type controls were anesthetized with an intraperitonal injection of ketamine (75 mg/kg) and acepromazine (1.5 mg/kg). Bacterial LPS(0.63 mg/kg body weight; Escherichia coli O55:B5) or sterile water was injected intratracheally in a small volume (20–30 µl). OxPAPC (1.5 mg/kg) or sterile saline solution was administrated 5 h after LPS instillation by i.v. injection in the external jugular vein. Control and treated groups did not significantly differ in body weight (wild-type control: 24.2 ± 2.3 g; wild-type LPS: 23.3 ± 3.8 g; wild-type LPS + OxPAPC: 22.6 ± 3.6; EP4−/− control: 25.8 ± 4.6 g; EP4−/− + LPS: 23.1 ± 5.9 g; and EP4−/− +LPS + OxPAPC 23.3 ± 5.1 g.) Animals were humanely killed by exsanguination under anesthesia 24 h after LPS challenge and were used for the evaluation of lung injury parameters.
Evaluation of lung injury parameters
After the experiment, the collection of bronchoalveolar lavage (BAL) fluid was performed using 1 ml of sterile HBSS. BAL protein concentration was determined by BCATM Protein Assay kit (Thermo Fisher Scientific). BAL inflammatory cell counting was performed by using a standard hemacytometer technique (38). As an additional parameter that reflected increased lung vascular leakiness, Evans blue accumulation in the lung tissue was evaluated as previously described in Fu et al. (38). At the end of the experiment, thoracotomy was performed and the lungs were perfused in situ via the left atrium with PBS that contained 5 mM EDTA to flush the blood off the lungs. Left lungs and right lungs were excised and imaged by a Kodak digital camera (Kodak, Rochester, NY, USA).
Statistical analysis
Results are expressed as means ± sd. Experimental samples were compared with controls by unpaired Student’s t test. For multiple-group comparisons, a 1-way ANOVA and post hoc multiple comparison tests were used. A value of P < 0.05 was considered statistically significant.
RESULTS
Electrical cell–substrate impedance sensing system and express permeability testing assay screening of prostanoid receptors that mediate the sustained phase of EC barrier-enhancing response to OxPAPC
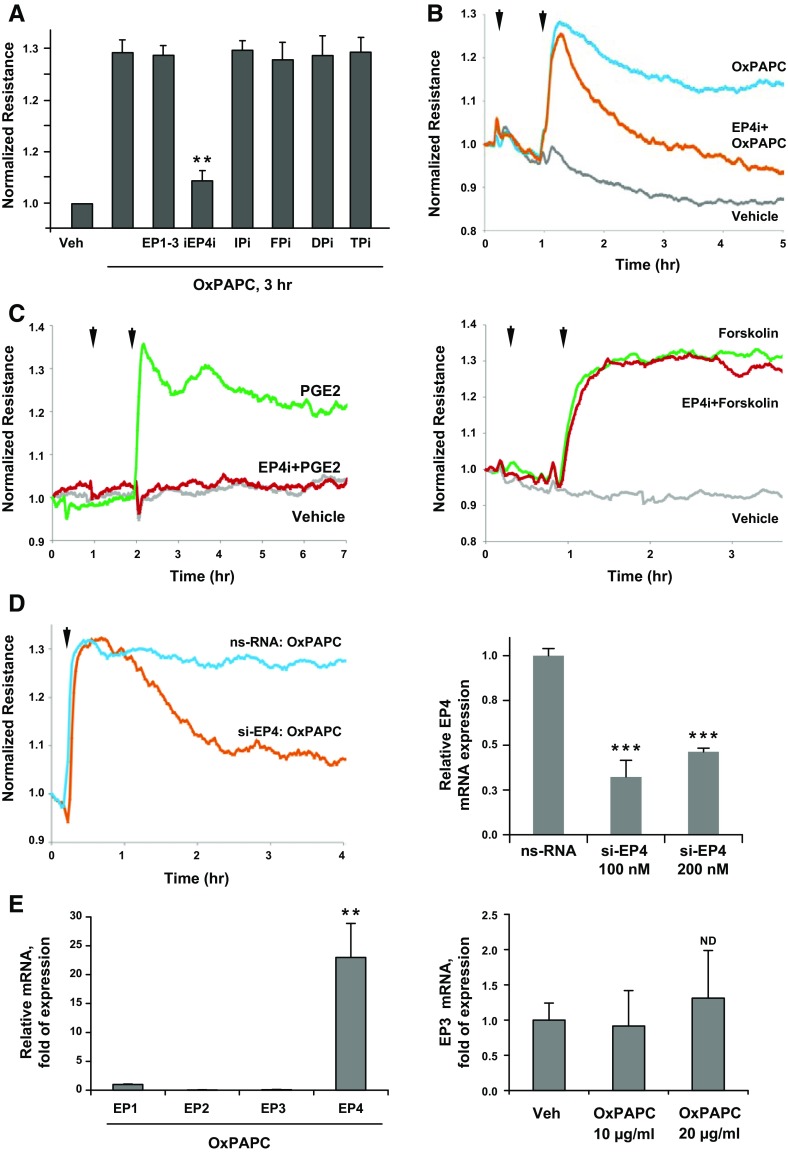
OxPAPC induces sustained EC barrier enhancement, which can be monitored by an increase in the TER of human pulmonary EC monolayers (34). We performed screening of potential receptors that mediate OxPAPC-induced EC barrier enhancement by using pharmacologic inhibitors of prostanoid receptors. Pulmonary ECs that were pretreated with pharmacologic antagonists of prostanoid receptors were stimulated with OxPAPC, and TER measurements after 3 h of OxPAPC treatment were compared. Analysis of the time-dependent development of EC barrier enhancement in response to OxPAPC showed that, although none of PG receptor inhibitors affected the immediate phase of the OxPAPC-induced TER increase in the first 15 min of treatment (data not shown), the sustained phase of OxPAPC-induced EC barrier enhancement response (after 2 h of OxPAPC addition) was dependent on EP4 (Fig. 1A). Results show that the OxPAPC-induced TER increase was not affected by specific inhibitors of EP1-3 (AH6809), IP (AH6809), FP (AH6809), DP (AH6809), and TP (AH6809) prostanoid receptors. In turn, pretreatment with the EP4 inhibitor, L161982, abolished the OxPAPC-induced EC barrier enhancement (Fig. 1B). Cell treatment with another EP4 inhibitor, GW627368, caused the same effect (data not shown).
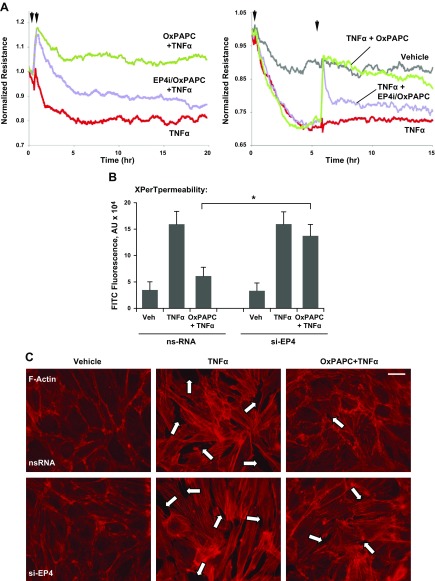
Figure 1.
Analysis of PG receptors involved in the sustained barrier-enhancing effect of OxPAPC. A) ECs were pretreated for 1 h with antagonists of EP1–3 (AH6809, 25 µM), EP4 (L161982, 3 µM), IP (CAY10449, 50 nM), FP (AL8810, 3 µM), DP (BWA868C, 50 nM), and TP (SQ29548, 0.5 µM), followed by stimulation with OxPAPC (15 µg/ml, 3 h). Bar graphs depict results of TER measurements shown as means ± sd of 4 independent experiments. B, C) Time-dependent analysis of TER changes was performed in ECs that were pretreated with EP4 inhibitor (first arrow) and stimulated with OxPAPC (B), PGE2 (C, left) or forskolin (C, right; second arrow). D) Analysis of TER in ECs that were treated with nonspecific or EP4-specific siRNA after stimulation with OxPAPC. Bar graph: RT-PCR analysis of EP4 mRNA levels in ECs that were treated with nonspecific and EP4-specific siRNA at 100 and 200 nM. E) EP1–4 mRNA levels in OxPAPC-stimulated human pulmonary ECs were measured by RT-PCR as described in Materials and Methods. Left bar graph depicts OxPAPC-induced changes in mRNA expression for each receptor expressed as fold of EP mRNA after 4 h of OxPAPC treatment normalized to GAPDH. Right graph represents the fold induction of EP3 mRNA normalized to GAPDH after treatment with 2 OxPAPC concentrations. ND, no difference; ns, nonspecific; veh, vehicle. Results are presented as means ± sd (n = 6). **P < 0.01, ***P < 0.001.
In control experiments, EP4 inhibitor completely blocked the EC barrier enhancement that was caused by canonical EP4 ligand, PGE2, and did not affect EC barrier enhancement caused by intracellular cAMP elevation upon addition of nonreceptor activator of adenylate cyclase, forskolin (Fig. 1C). These data support the notion that OxPAPC-induced EC barrier enhancement is a receptor-mediated event that involves EP4. Of importance, TER studies depicted in Fig. 1B, C show that EP4 antagonist completely blunted the rapid TER increase caused by PGE2, but did not affect the initial phase of the TER increase caused by OxPAPC. Instead, EP4 antagonist abolished the sustained phase of the OxPAPC barrier-enhancing response in pulmonary ECs. In addition to pharmacologic EP4 inhibition, abolishment of sustained phase of the OxPAPC-induced barrier-enhancing response was also observed in pulmonary ECs with siRNA-induced EP4 knockdown (Fig. 1D). The bar graph depicts results of RT-PCR analysis of EP4 mRNA levels in ECs that were treated with nonspecific and EP4-specific siRNA. Of interest, RT-PCR analysis showed that treatment with OxPAPC dramatically up-regulated EP4 expression without significantly affecting the mRNA expression levels of EP1–3 (Fig. 1E).
Effects of EP4 inhibition on OxPAPC-induced Rac1 signaling and the reorganization of actin cytoskeleton and adherens junctions
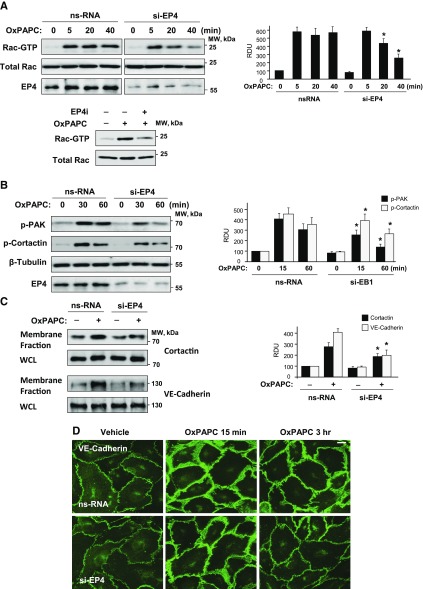
Previous studies have demonstrated a key role for Rac1 GTPase signaling in OxPAPC-induced EC barrier enhancement (25, 34). We next examined effects of EP4 inhibition on the OxPAPC-induced activation of the Rac1 signaling pathway. Inhibition of EP4 by siRNA-induced knockdown or pharmacologic inhibitor did not considerably affect the peak of OxPAPC-induced rapid Rac activation at 5 min, but significantly reduced Rac activity at later time points (Fig. 2A). Rac1-induced activation of its effector serine/threonine protein kinase, PAK1, is manifested by PAK1 autophosphorylation at Thr423 (39). Tyrosine phosphorylation of another regulator of cortical actin polymerization, cortactin, is also promoted by activated Rac1 (40, 41). Consistent with Rac1 activation patterns, OxPAPC caused sustained phosphorylation of PAK1 and cortactin, which was significantly reduced by EP4 knockdown (Fig. 2B).
Figure 2.
Role of EP4 in OxPAPC-induced activation of Rac signaling and cell junction remodeling. ECs were treated with nonspecific or EP4-specific siRNA (100 nM, 48 h). A) EP4 knockdown suppresses prolonged OxPAPC-induced Rac activation (upper). Efficiency of EP4 knockdown was verified by Western blot. This effect is also recapitulated by EC treatment with EP4 inhibitor (lower). B) Effect of EP4 knockdown on prolonged OxPAPC-induced phosphorylation of PAK and cortactin. C) Effect of EP4 knockdown on OxPAPC-induced cortactin and VE-cadherin translocation to the cell membrane detected by subcellular fractionation assay. Bar graphs depict the quantitative densitometry analysis of Western blot data. D) EP4 knockdown suppresses the prolonged enhancement of adherens junctions caused by OxPAPC (15 µg/ml, 3 h) monitored by immunofluorescence staining of transmembrane adherence junction structural protein, VE-cadherin. ns, nonspecific; RDU, relative density unit; WCL, whole-cell lysate. Scale bar, 5 µm. *P < 0.05 vs. nonspecific (ns)RNA.
Activation of cortical actin polymerization driven by regulatory actin-binding proteins, such as cortactin, and assembly of VE-cadherin–positive adherens junctions are key mechanisms of EC barrier enhancement and barrier restoration (42–44). We evaluated the effects of EP4 inhibition on OxPAPC-induced cortical actin cytoskeletal dynamics and remodeling of adherens junctions by using biochemical and imaging approaches. Subcellular fractionation studies showed increased accumulation of cortactin and VE-cadherin in the cell membrane fraction that was observed after 3 h of OxPAPC treatment, which was markedly suppressed in ECs with EP4 knockdown (Fig. 2C). Involvement of EP4 signaling in the sustained barrier-enhancing effects of OxPAPC was further evaluated in experiments with siRNA-induced EP4 protein depletion. Immunofluorescence staining of OxPAPC-stimulated EC monolayers with and without EP4 knockdown showed enhancement of adherens junctions in both OxPAPC-stimulated control and EP4-depleted ECs after 15 min of stimulation as reflected by the increased intensity of VE-cadherin–positive immunofluorescence staining (Fig. 2D). However, sustained VE-cadherin accumulation at cell junctions after 3 h of OxPAPC stimulation was not observed in ECs with depleted EP4 (Fig. 2D, bottom).
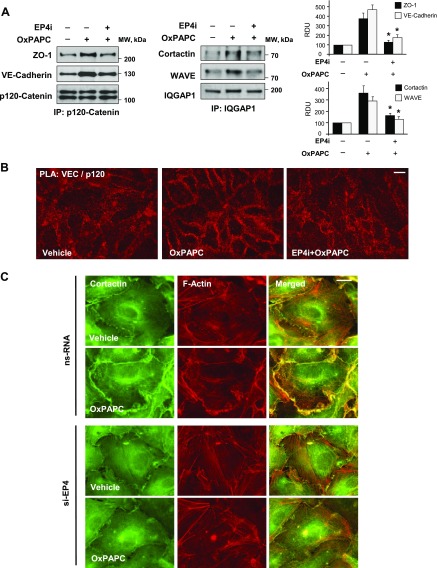
Coimmunoprecipitation assays with an Ab to adherens junction protein, p120-catenin, showed that the OxPAPC-induced association of p120-catenin with VE-cadherin and tight junction protein ZO-1—shown to be critical for OxPAPC-induced EC barrier enhancement (45)—was significantly reduced in ECs with inhibited EP4 signaling (Fig. 3A, left). Similarly, OxPAPC-stimulated formation of function complex that contained actin cytoskeletal regulators, cortactin, WAVE, and IQGAP1, that are involved in the control of cortical actin dynamics and cytoskeleton–cell junction interactions was not observed after 3 h of OxPAPC treatment in ECs with inhibited EP4 (Fig. 3A, right).
Figure 3.
Inhibition of EP4 attenuates OxPAPC-induced functional interactions of cell junction and cytoskeletal proteins. ECs were treated with vehicle or EP4 inhibitor, L161982 (3 µM, 1 h), before OxPAPC stimulation (15 µg/ml, 3 h). A) Coimmunoprecipitation assays: EP4 inhibitor attenuates OxPAPC-induced association of ZO-1, VE-cadherin, and p120-catenin (left), and association of cortactin, WAVE, and IQGAP1 (right). Membrane reprobing with p120-catenin and IQGAP1 was used as a protein load normalization control. Bar graph depicts quantitative analysis of immunoprecipitated protein complexes. B) PLA (red) with OxPAPC-stimulated ECs with or without pretreatment with EP4 inhibitor by using mouse onoclonal anti–p120-catenin and rabbit polyclonal anti–VE-cadherin Abs. OxPAPC-increased PLA signal at the area of adherens junctions was suppressed in ECs that were pretreated with EP4 inhibitor. Scale bar, 10 µm. C) EP4 knockdown suppresses OxPAPC-induced peripheral accumulation of cortactin and cortical F-actin remodeling. Immunofluorescence staining of cortactin (green), F-actin (red), and merged images (right) of OxPAPC-stimulated EC monolayers with and without EP4 knockdown. IP, immunoprecipitation; ns, nonspecific; RDU, relative density unit. Scale bar, 5 µm. *P < 0.05 vs. OxPAPC alone.
Adherens junction enhancement is associated with increased interactions of adherens junction proteins. Visualization of intracellular colocalization and protein interaction of VE-cadherin and p120-catenin was performed by using PLA. Increased accumulation of both proteins at the cell junction areas of OxPAPC-stimulated ECs, and their interaction was suppressed by EC pretreatment with EP4 inhibitor (Fig. 3B). Immunofluorescence detection of actin cytoskeleton arrangement and cortactin intracellular localization in the OxPAPC-stimulated EC monolayers showed enhancement of peripheral F-actin staining coinciding with the accumulation of cortactin at the cortical cytoskeletal compartment after 3 h of stimulation in EC without EP4 knockdown. However, enhanced cortical F-actin rim and peripheral increased cortactin immunoreactivity was not observed in ECs with EP4 knockdown (Fig. 3C).
OxPAPC-induced EC barrier protection against thrombin-induced permeability is not mediated by EP4
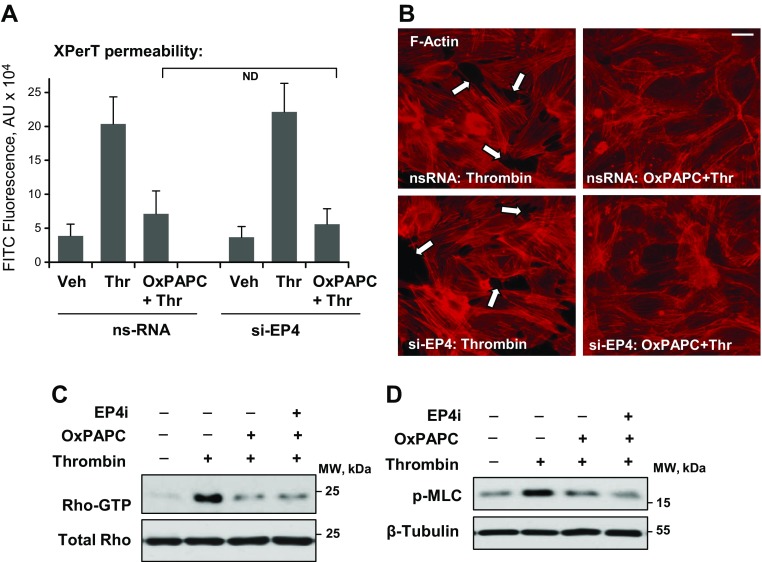
We next tested the effect of EP4 knockdown on OxPAPC-induced protection against thrombin-induced permeability by using a fluorimetry-based permeability assay for macromolecules in 96-well plate format as described in Materials and Methods. Thrombin induced a rapid increase in EC permeability, which started developing in the first minute after thrombin addition and reached maximal levels by 15 min, and was significantly attenuated by pretreatment of pulmonary EC monolayers with OxPAPC (Fig. 4A). Knockdown of EP4 did not affect the protective effect of OxPAPC against this rapid thrombin-induced EC permeability response.
Figure 4.
EP4 is not involved in the OxPAPC protective effects against thrombin-induced EC permeability. Pulmonary EC monolayers that were pretreated with control or EP4-specific siRNA were pretreated with OxPAPC (15 µg/ml, 30 min) or vehicle followed by thrombin (0.5 U/ml) stimulation. A) Analysis of EC permeability for macromolecules by using FITC-labeled avidin tracer. Accumulation of FITC-avidin on the substrate that underlies EC monolayers reflecting EC barrier dysfunction was assessed by fluorimetric analysis as described in Materials and Methods (n = 6). B) Effect of EP4 knockdown on the OxPAPC-induced attenuation of thrombin-induced formation of actin stress fibers and paracellular gaps. F-actin was visualized by cell staining with Texas Red phalloidin. Paracellular gaps are marked by arrows. Results are representative of 3 independent experiments. Scale bar, 10 µm. C, D) Thrombin-induced activation of RhoA GTPase measured in pulldown assays (C) and Rho kinase–mediated phosphorylation of myosin light chains (MLCs) (D) detected by Western blot was evaluated in ECs after thrombin challenge with or without OxPAPC or EP4 inhibitor L161982 (3 µM, 1 h) pretreatment. Membrane probing with Ab to β-tubulin was used as a normalization control. ND, no difference; ns, nonspecific; thr, thrombin; veh, vehicle.
In agreement with the results of permeability assays, the F-actin stress fiber formation that was observed 15 min after thrombin stimulation was dramatically attenuated by OxPAPC pretreatment, and this OxPAPC effect was not influenced by the molecular inhibition of EP4 (Fig. 4B). Thrombin-induced EC permeability was associated with the rapid activation of small GTPase Rho and an increase in myosin light chain phosphorylation. This EC response to thrombin was abolished by OxPAPC pretreatment, but was independent of EP4 inhibition (Fig. 4C, D).
EP4 mediates OxPAPC-induced protection against sustained EC barrier dysfunction and inflammatory activation caused by TNF-α
TNF-α is a potent activator of the EC inflammatory response and sustained barrier dysfunction (46–48). Analysis of the EC monolayer barrier function using the measurement of TER demonstrated that EC pretreatment with OxPAPC markedly attenuated TNF-α–induced barrier dysfunction. OxPAPC induced a sustained EC barrier protective effect, and TER in OxPAPC-pretreated EC monolayers did not decline even after 15–20 h of TNF-α challenge. In contrast, the protective effect of OxPAPC in pulmonary ECs that were treated with EP4 inhibitor became negligible after 20 h of treatment (Fig. 5A, left).
Figure 5.
EP4 mediates the protective effect of OxPAPC against TNF-α–induced EC barrier dysfunction. A) Pulmonary ECs with or without L161982 (3 µM, 1 h) pretreatment were stimulated with OxPAPC (15 µg/ml, first arrow) and further incubated with TNF-α (20 ng/ml, second arrow; left). ECs with or without EP4 inhibitor pretreatment were challenged with TNF-α for 6 h followed by OxPAPC post-treatment (right). TER measurements were performed over 20- or 15-h time periods. B) Effect of siRNA-induced EP4 knockdown on the OxPAPC protective effect against TNF-α–induced EC permeability for macromolecules was evaluated by using FITC-labeled avidin tracer as described above (n = 6). C) ECs with or without siRNA-induced EP4 knockdown pretreated with OxPAPC or vehicle (30 min) were challenged with TNF-α (6 h) followed by immunofluorescence analysis of actin stress fiber and paracellular gap foramtion. F-actin was visualized by cell staining with Texas Red phalloidin. Paracellular gaps are marked by arrows. Ns, nonspecific; veh, vehicle. Scale bar, 20 µm. *P < 0.05.
In more clinically relevant settings of OxPAPC post-treatment, the addition of OxPAPC 6 h after TNF-α challenge caused rapid and sustained increase in TER, which reached basal levels observed in nonstimulated cells (Fig. 5A, right). Of interest, OxPAPC post-treatment of TNF-α–challenged ECs that were preincubated with EP4 inhibitor caused rapid elevation of TER, reaching similar levels observed in ECs without EP4 inhibitor; however, this elevation was transient and declined within the next 2 h.
The role of EP4 in the OxPAPC long-term barrier protective effects against TNF-α was further verified by permeability assays for macromolecules. The increased permeability registered in TNF-α–treated EC monolayers 6 h after the addition of TNF-α was significantly attenuated by OxPAPC. This OxPAPC effect was abolished in ECs with EP4 knockdown (Fig. 5B). TNF-α–induced EC barrier dysfunction was associated with the disruption of EC monolayer integrity and increased F-actin stress fiber formation observed after 6 h of TNF-α treatment (Fig. 5C). This barrier-disruptive TNF-α effect was dramatically attenuated by OxPAPC. siRNA-induced EP4 knockdown suppressed the barrier-protective effect of OxPAPC (Fig. 5C).
Activation of the NF-κB–mediated inflammatory signaling cascade by bacterial and nonbacterial pathogens is reflected by the phosphorylation of the NF-κB subunit and degradation of the inhibitory IκBα subunit (49, 50). As a canonical activator of the NF-κB pathway, TNF-α induced phosphorylation of NF-κB and degradation of IκBα, which was attenuated in OxPAPC-pretreated ECs. This anti-inflammatory effect of OxPAPC was attenuated by cell pretreatment with a pharmacologic antagonist of EP4 (Fig. 6A).
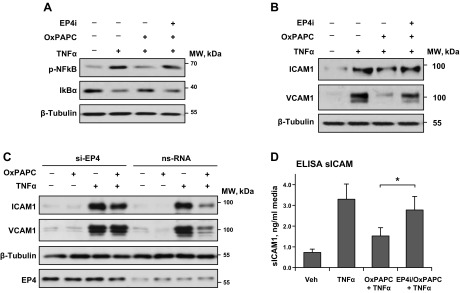
Figure 6.
EP4 mediates the protective effect of OxPAPC against TNF-α–induced EC inflammatory activation. A, B) ECs that were preincubated with vehicle or EP4 inhibitor L161982 (3 µM, 1 h) were treated with OxPAPC (15 µg/ml, 30 min) followed by TNF-α (20 ng/ml) challenge. NF-κB phosphorylation and IκBα degaradation was evaluated 3 h after TNF-α addition (A); ICAM-1 and VCAM-1 expression were evaluated 6 h after TNF-α addition (B). C) Effect of EP4 knockdown on OxPAPC-induced inhibition of ICAM-1 and VCAM-1 expression caused by TNF-α (6 h). ECs were transfected with EP4-specific siRNA or nonspecific RNA 48 h before agonist stimulation. Probing with β-tubulin Ab was used as normalization control. Efficiency of EP4 knockdown was verified by Western blot. D) Inhibition of EP4 attenuates the suppressing effect of OxPAPC on TNF-α–induced production of soluble ICAM-1 by pulmonary ECs (n = 4). Ns, nonspecific; veh, vehicle. *P < 0.05.
Inflammatory activation of vascular endothelium by TNF-α stimulates the NF-κB–dependent expression of adhesion molecules ICAM-1 and VCAM-1, which promotes increased neutrophil adhesion to the vascular EC and neutrophil transmigration via the EC monolayer and development of lung inflammation. Treatment with OxPAPC inhibited TNF-α–induced expression of ICAM-1 and VCAM-1 that was observed 6 h after TNF-α treatment (Fig. 6B). This effect of OxPAPC was also attenuated by EC treatment with EP4 inhibitor. As a complimentary approach, we performed EP4 molecular inhibition by using siRNA-induced EP4 knockdown. EP4 knockdown did not affect the TNF-α–induced increase in ICAM-1 and VCAM-1 protein expression by pulmonary ECs, but attenuated the protective effect of OxPAPC (Fig. 6C). Measurements of the soluble ICAM-1 that was released by TNF-α–activated ECs into culture medium after 6 h of agonist treatment showed an inhibitory effect of OxPAPC, which was abolished in ECs that were pretreated with EP4 antagonist (Fig. 6C). Taken together, these results strongly suggest a role for EP4 in the mediation of the sustained phase of OxPAPC-induced EC barrier protection and the inhibition of TNF-α–induced endothelial inflammation.
EP4 mediates the barrier-protective and anti-inflammatory effects of OxPAPC in vivo
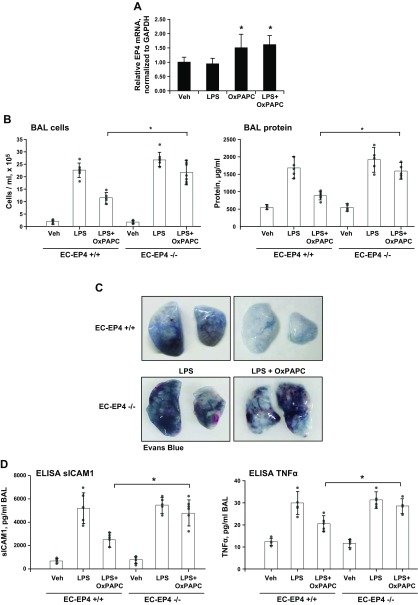
The role of EP4 in mediating the barrier-protective and anti-inflammatory effects of OxPAPC was further tested by using a genetic animal model of endothelial-specific EP4-knockout mice. Generation and characterization of EC-specific EP4-knockout mice (EC-EP4−/−) was described in a previous study (37). Age-matched EP4flox/flox mice were used as controls. Intratracheal LPS treatment did not affect EP4 mRNA expression in the lungs, whereas OxPAPC alone or in combination with LPS modestly increased EP4 mRNA levels (Fig. 7A). These changes are also consistent with the EP4 induction that was observed in the OxPAPC-stimulated pulmonary EC cultures (Fig. 1E). Next, parameters of lung injury in EC-EP4−/− mice and matching controls were analyzed 24 h after LPS administration. The protective effects of OxPAPC post-treatment against LPS-induced lung injury were significantly attenuated in EC-EP4−/− mice (Fig. 7A). Evaluation of OxPAPC protective effects against LPS-induced lung vascular leak was further performed by analysis of Evans blue accumulation in the lung parenchyma. Results showed that the pronounced OxPAPC protective effect against Evans blue accumulation in the lungs of LPS-challenged mice was abolished in animals with EP4 knockout (Fig. 7B). Further analysis of BAL samples showed induction of soluble ICAM-1 and TNF-α levels caused by LPS. Expression of these markers of lung inflammation was significantly attenuated by single OxPAPC i.v. injection (Fig. 7C). This inhibitory effect of OxPAPC on LPS-induced TNF-α and soluble ICAM-1 production was suppressed in EC-EP4−/− mice.
Figure 7.
Endothelial-specific EP4 knockout abolishes the protective effects of OxPAPC against LPS-induced lung injury. Injection of OxPAPC (1.5 mg/kg, i.v.) in EC-specific EP4−/− mice and matching controls was performed 5 h after LPS administration (0.7 mg/kg i.t.). The experiment was terminated after 24 h of LPS challenge. A) Effects of LPS and OxPAPC treatment on EP4 mRNA levels in lung tissue of wild-type mice were evaluated by real-time RT-PCR (n = 7). B) Measurements of total cell counts (left) and protein concentration (right) in BAL fluid (n = 4). C) Analysis of Evans blue–labeled albumin extravasation into the lung parenchyma, reflecting vascular leak. Shown are representative images of lungs that were excised from the chest and perfused with PBS. D) ELISA assay of soluble ICAM-1 (sICAM-1) and TNF-α levels in BAL samples (n = 3). Veh, vehicle. *P < 0.05.
DISCUSSION
Oxidized phospholipids exhibit a broad spectrum of biologic activities, which are mediated by specific receptors [reviewed previously in Bochkov et al. (1)]. The barrier-enhancing effects of OxPAPC on pulmonary ECs that were first discovered by our group are stimulated by full-length oxygenated phospholipid products that contain the isoprostanoid group (8). This study describes for the first time, to our knowledge, the activation of EP4 by OxPAPC and demonstrates the EP4 role in the sustained phase of OxPAPC barrier-protective and anti-inflammatory effects in pulmonary EC culture and the animal model of LPS-induced lung injury.
Oxidized phospholipid products that contain esterified isoprostanoids, such as 1-palmitoyl-2-(5,6-epoxyisoprostane E2)-sn-glycero-3-phosphatidyl choline, activate EP2 and DP, which recognize PGE2 and PGD2 (51). EP2 and DP have been implicated as potential receptors that are responsible for OxPAPC-induced cAMP elevation, and up-regulation of CREB-dependent gene expression and monocyte activation in atherosclerosis models has been previously described (51). However, our new data indicate that, despite the well-recognized ability of EP2 to elevate cAMP levels (52, 53) implicated in EC barrier-protective effects (54), the inhibition of EP2 (Fig. 1), or EC treatment with butaprost (data not shown), the PGE2 analog that causes the selective activation of EP2 (55) did not affect pulmonary EC barrier properties. Instead, this study shows the involvement of EP4 in OxPAPC barrier-enhancing and anti-inflammatory effects.
Our previous studies have described the initiating mechanistic events that are triggered by OxPAPC to increase vascular integrity (56). We found that OxPAPC directly binds the cell membrane localized chaperone protein, GRP78, which leads to GRP78 translocation to the caveolin-enriched signaling microdomains and transactivation of sphingosine 1-phosphate receptor 1 by Src- and Fyn-tyrosine kinase–dependent mechanism. As a result, the sphingosine 1-phosphate receptor 1–induced activation of Rac1 pathway drives the assembly of adherens junctions and the remodeling of peripheral actin cytoskeleton, which leads to EC barrier enhancement (56). GRP78-mediated rapid activation of Rac1 also causes the activation of p190RhoGAP, which is a negative regulator of RhoA activity (57, 58) that is also involved in the inhibition of the rapid EC permeability response to thrombin (59). GRP78 knockdown blocks the initial phase of the OxPAPC-induced EC barrier enhancement response and inhibition of thrombin-induced permeability (56); thus, the GRP78-mediated acute mechanism of OxPAPC-induced signaling explains the rapid barrier protective effects of OxPAPC against thrombin-induced, RhoA-dependent EC permeability.
Our new data demonstrate that inhibition of EP4 did not affect the initial phase, but abolished the sustained phase of the EC barrier-enhancing response to OxPAPC. EP4 inhibition also suppressed the sustained attenuation of barrier-disruptive and proinflammatory signaling by OxPAPC. The protective effects of OxPAPC against LPS-induced lung injury were also significantly attenuated in conditional EC-specific EP4-knockout mice. The different routes of intratracheal LPS and intravenous OxPAPC administration in in vivo experiments further support the endothelial-specific mechanism of OxPAPC-induced, EP4-dependent modulation of lung barrier and inflammation by OxPAPC shown in this study. The EC-specific EP4 knockout that was tested in this study does not impose any major phenotypic alterations in healthy animals. Similarly, global EP4 knockout does not cause noticeable phenotypic effects in young and adult mice, with the exception of lower bone content, which may result in delayed fracture healing (60). EP4 ablation may have effects on aging, as it was associated with increased fibrosis and dilated cardiomyopathy in aged cardiac-specific EP4-knockout mice (61); however, the global EP4 knockout and the loss of EP4 in other cell types may have an additional effect on host defense during infection or inflammation. EP4-mediated cAMP elevation is crucial for the maturation and cytokine-activated chemotaxis of resident and peripheral blood myeloid dendritic cells (62, 63). Activation of EP2- and EP4-mediated signaling and the cAMP pathway in T cells up-regulates IL-23 and IL-1 receptor expression, differentially regulates IFN-γ production, and inhibits the production of the anti-inflammatory cytokine IL-10 in Th17 cells (64).
How EP4 controls a prolonged phase of EC barrier enhancement and protection remains to be elucidated. In addition to Gs-mediated elevation of cAMP, activation of EP4 stimulates PI3K-dependent signaling (65). In vascular ECs, this pathway causes additional stimulation of Rac1 signaling and Rac1-dependent barrier-protective cytoskeletal mechanisms by activating Rac1-specific nucleotide exchange factor Tiam1 (66). The PI3K–Rac1 pathway is a common mechanism that is involved in the sustained barrier-enhancing effects of several agonists, including hepatocyte growth factor, sphingosine 1-phosphate, high-MW hyaluronans, OxPAPC, and others (13, 67, 68). Taking into consideration the role of EP4 signaling in OxPAPC sustained barrier-enhancing effects, the prominent induction of EP4 expression by OxPAPC that was observed in this study can make an additional contribution to OxPAPC-induced barrier enhancement. Alternatively, EP4 recruitment to caveolin-enriched signaling microdomains, where EP4 binds OxPAPC and becomes fully activated, may occur with a delay. Precise analysis of this mechanism was outside the scope of this study and warrants further investigation. It is also important to acknowledge the potential impact of EP4 activation by OxPAPC in other cells, such as alveolar macrophages, which may stimulate additional anti-inflammatory effects. EP4 activation suppressed the cytokine release by human alveolar macrophages (69). LPS-induced activation of NF-κB signaling was also suppressed by EP4-associated EPRAP, an inhibitor of the p105 subunit of the NF-κB inflammatory complex (70).
In addition to EP4 induction in pulmonary ECs caused by OxPAPC described in this study, another study by our group showed a marked induction of EP4 levels in the brain vasculature after brain ischemia and reperfusion in a model of cerebral ischemia (37). In that model, conditional EP4 knockout in brain neurons or brain microvascular ECs worsened stroke injury and decreased cerebral reperfusion. Thus, the published and new findings may reflect a new paradigm of tissue recovery and restoration of normal lung homeostasis. Oxidized phospholipids that were generated during lung injury trigger EP4 induction in vascular endothelium and stimulate barrier-recovery and anti-inflammatory responses. We speculate that the mechanisms of EP4-dependent EC barrier recovery by OxPAPC may involve 2 signaling pathways: 1) activation of cAMP-dependent PKA and Epac-Rap1 cascades of EC barrier repair and suppression of NFκB inflammatory cascade; and 2) activation of PI3K signaling. In support of this mechanism, the EP4 activation by pharmacologic EP4 activator suppressed endotoxin-induced neutrophil infiltration in airways in vivo (71).
Potent anti-inflammatory effects of OxPAPC against LPS-induced inflammation in the cotreatment model that has been described in previous studies have been associated with competitive antagonism of OxPAPC against LPS ligation of the TLR4 receptor (9). The current study shows that OxPAPC may inhibit TLR-independent EC inflammation and barrier dysfunction that are caused by other inflammatory mediators (TNF-α) via suppression of NF-κB signaling. This effect may be associated with sustained OxPAPC inhibitory effects on RhoA signaling, which, in turn, leads to the suppression of RhoA-assisted activation of p38-MAPK and NF-κB inflammatory cascades (72–74). Therefore, EP4-mediated stimulation of Rac1 signaling during the late phase of OxPAPC treatment may contribute to this mechanism by promoting Rac1–RhoA negative crosstalk and suppressing the innate inflammatory response.
The model of LPS-induced inflammation that was tested in this study has certain limitations. Although LPS or endotoxin are products of bacterial wall breakdown detected in the lungs and plasma of patients who are septic and shown to cause serious lung dysfunction, these do not represent the full spectrum of pathologic effects caused by live bacteria, such as the release of bacterial toxins and bacterial invasion, effects on immune cells, bacterial clearance, and phagocytosis. It is important to note in this regard that the suppression of the early host response to live bacteria by inhibition of chemokine production and neutrophil infiltration may have adverse effects and lead to decreased bacterial clearance and the worsening of ALI. For example, down-regulation of plasminogen activator inhibitor-1 function showed an early protective effect in a murine model of Pseudomonas aeruginosa pneumonia by improving the alveolar capillary barrier and reducing pulmonary edema, but later suppressing neutrophil accumulation and bacterial clearance from the airspace, which leads to increased mortality (75). Thus, understanding the delicate balance between early inflammatory response caused by bacterial pathogens, modulation of cytokine storm at the peak of inflammation, and control of lung vascular permeability to prevent pulmonary edema is a critical point for the development of mechanism-based therapeutic interventions in the course of bacterial ALI. Additional studies are underway to evaluate the effects of the OxPAPC-EP4 axis in such clinically relevant models of lung injury caused by live bacterial infection and, specifically, to investigate the involvement of the OxPAPC-EP4 mechanism in improved survival and accelerated ALI recovery.
In summary, this study demonstrates for the first time the involvement of EP4 in the barrier-protective and anti-inflammatory effects of OxPAPC in models of EC barrier dysfunction caused by vasoactive and proinflammatory agonists. We characterized EP4 as a PG receptor that mediates the prolonged phase of OxPAPC-induced EC barrier enhancement, which plays an essential role in the suppression of lung endothelial inflammation and hyperpermeability in the injured lung. This mechanism may reflect normal homeostatic control of lung injury and recovery.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health (NIH) National Heart, Lung, and Blood Institute Grants HL076259, HL087823, HL107920, and HL130431 and NIH National Institute of General Medical Sciences Grant GM114171. The authors thank Mohan Tulapurkar (University of Maryland, Baltimore, MD, USA), for superior technical assistance and analysis of EP4 expression in lung tissue samples.
Glossary
- ALI
acute lung injury
- BAL
bronchoalveolar lavage
- DP
prostaglandin D2 receptor
- EC
endothelial cell
- EP
prostaglandin E2 receptor
- EP4
prostaglandin E receptor-4
- FP
prostaglandin F2 receptor
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- ICAM1
intercellular cell adhesion molecule type 1
- IP
prostaglandin I2 receptor
- IQGAP1
IQ motif containing GTPase-activating protein 1
- OxPAPC
oxidized PAPC; PAPC, 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine
- PG
prostaglandin
- PLA
proximal ligation assay
- siRNA
small interfering RNA
- TER
transendothelial electrical resistance
- TP
thromboxane receptor
- VE-cadherin
vascular endothelial cadherin
- WAVE
Wiskott-Aldrich syndrome protein-family verprolin-homologous protein
- ZO-1
zonula occludens protein 1
AUTHOR CONTRIBUTIONS
O. Oskolkova, V. N. Bochkov, A. A. Birukova, and K. G. Birukov designed the research; G. Gawlak, Y. Tian, Y. Ke, N. Sarich, and S. Son performed the research; O. Oskolkova, G. Gawlak, Y. Tian, V. N. Bochkov, A. A. Birukova, and K. G. Birukov analyzed the data; K. Andreasson provided EC-specific EP4-knockout mice; and O. Oskolkova, A. A. Birukova, and K. G. Birukov wrote the paper.
REFERENCES
- 1.Bochkov V. N., Oskolkova O. V., Birukov K. G., Levonen A. L., Binder C. J., Stöckl J. (2010) Generation and biological activities of oxidized phospholipids. Antioxid. Redox Signal. 12, 1009–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bochkov V. N., Mechtcheriakova D., Lucerna M., Huber J., Malli R., Graier W. F., Hofer E., Binder B. R., Leitinger N. (2002) Oxidized phospholipids stimulate tissue factor expression in human endothelial cells via activation of ERK/EGR-1 and Ca++/NFAT. Blood 99, 199–206 [DOI] [PubMed] [Google Scholar]
- 3.Lusis A. J. (2000) Atherosclerosis. Nature 407, 233–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leitinger N., Tyner T. R., Oslund L., Rizza C., Subbanagounder G., Lee H., Shih P. T., Mackman N., Tigyi G., Territo M. C., Berliner J. A., Vora D. K. (1999) Structurally similar oxidized phospholipids differentially regulate endothelial binding of monocytes and neutrophils. Proc. Natl. Acad. Sci. USA 96, 12010–12015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cole A. L., Subbanagounder G., Mukhopadhyay S., Berliner J. A., Vora D. K. (2003) Oxidized phospholipid-induced endothelial cell/monocyte interaction is mediated by a cAMP-dependent R-Ras/PI3-kinase pathway. Arterioscler. Thromb. Vasc. Biol. 23, 1384–1390 [DOI] [PubMed] [Google Scholar]
- 6.Muñoz N. M., Desai A., Meliton L. N., Meliton A. Y., Zhou T., Leff A. R., Dudek S. M. (2012) Group V phospholipase A2 increases pulmonary endothelial permeability through direct hydrolysis of the cell membrane. Pulm. Circ. 2, 182–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butler B. D., Davies I., Drake R. E. (1989) Effect of lysophosphatidylcholine on the filtration coefficient in intact dog lungs. Am. J. Physiol. 257, H1466–H1470 [DOI] [PubMed] [Google Scholar]
- 8.Birukova A. A., Starosta V., Tian X., Higginbotham K., Koroniak L., Berliner J. A., Birukov K. G. (2013) Fragmented oxidation products define barrier disruptive endothelial cell response to OxPAPC. Transl. Res. 161, 495–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bochkov V. N., Kadl A., Huber J., Gruber F., Binder B. R., Leitinger N. (2002) Protective role of phospholipid oxidation products in endotoxin-induced tissue damage. Nature 419, 77–81 [DOI] [PubMed] [Google Scholar]
- 10.Ma Z., Li J., Yang L., Mu Y., Xie W., Pitt B., Li S. (2004) Inhibition of LPS- and CpG DNA-induced TNF-alpha response by oxidized phospholipids. Am. J. Physiol. Lung Cell. Mol. Physiol. 286, L808–L816 [DOI] [PubMed] [Google Scholar]
- 11.Erridge C., Kennedy S., Spickett C. M., Webb D. J. (2008) Oxidized phospholipid inhibition of Toll-like receptor (TLR) signaling is restricted to TLR2 and TLR4: roles for CD14, LPS-binding protein, and MD2 as targets for specificity of inhibition. J. Biol. Chem. 283, 24748–24759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nonas S., Miller I., Kawkitinarong K., Chatchavalvanich S., Gorshkova I., Bochkov V. N., Leitinger N., Natarajan V., Garcia J. G., Birukov K. G. (2006) Oxidized phospholipids reduce vascular leak and inflammation in rat model of acute lung injury. Am. J. Respir. Crit. Care Med. 173, 1130–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singleton P. A., Chatchavalvanich S., Fu P., Xing J., Birukova A. A., Fortune J. A., Klibanov A. M., Garcia J. G., Birukov K. G. (2009) Akt-mediated transactivation of the S1P1 receptor in caveolin-enriched microdomains regulates endothelial barrier enhancement by oxidized phospholipids. Circ. Res. 104, 978–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meliton A., Meng F., Tian Y., Sarich N., Mutlu G. M., Birukova A. A., Birukov K. G. (2015) Oxidized phospholipids protect against lung injury and endothelial barrier dysfunction caused by heat-inactivated Staphylococcus aureus. Am. J. Physiol. Lung Cell Mol. Physiol., 308, L550–L562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauer J., Ripperger A., Frantz S., Ergün S., Schwedhelm E., Benndorf R. A. (2014) Pathophysiology of isoprostanes in the cardiovascular system: implications of isoprostane-mediated thromboxane A2 receptor activation. Br. J. Pharmacol. 171, 3115–3131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hata A. N., Breyer R. M. (2004) Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol. Ther. 103, 147–166 [DOI] [PubMed] [Google Scholar]
- 17.Alfranca A., Iñiguez M. A., Fresno M., Redondo J. M. (2006) Prostanoid signal transduction and gene expression in the endothelium: role in cardiovascular diseases. Cardiovasc. Res. 70, 446–456 [DOI] [PubMed] [Google Scholar]
- 18.Breyer R. M., Bagdassarian C. K., Myers S. A., Breyer M. D. (2001) Prostanoid receptors: subtypes and signaling. Annu. Rev. Pharmacol. Toxicol. 41, 661–690 [DOI] [PubMed] [Google Scholar]
- 19.Birukova A. A., Zagranichnaya T., Fu P., Alekseeva E., Chen W., Jacobson J. R., Birukov K. G. (2007) Prostaglandins PGE2 and PGI2 promote endothelial barrier enhancement via PKA- and Epac1/Rap1-dependent Rac activation. Exp. Cell Res. 313, 2504–2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Métrich M., Berthouze M., Morel E., Crozatier B., Gomez A. M., Lezoualc’h F. (2010) Role of the cAMP-binding protein Epac in cardiovascular physiology and pathophysiology. Pflugers Arch. 459, 535–546 [DOI] [PubMed] [Google Scholar]
- 21.Lorenowicz M. J., Fernandez-Borja M., Kooistra M. R., Bos J. L., Hordijk P. L. (2008) PKA and Epac1 regulate endothelial integrity and migration through parallel and independent pathways. Eur. J. Cell Biol. 87, 779–792 [DOI] [PubMed] [Google Scholar]
- 22.Dejana E., Tournier-Lasserve E., Weinstein B. M. (2009) The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev. Cell 16, 209–221 [DOI] [PubMed] [Google Scholar]
- 23.Spindler V., Schlegel N., Waschke J. (2010) Role of GTPases in control of microvascular permeability. Cardiovasc. Res. 87, 243–253 [DOI] [PubMed] [Google Scholar]
- 24.Birukova A. A., Tian X., Tian Y., Higginbotham K., Birukov K. G. (2013) Rap-afadin axis in control of Rho signaling and endothelial barrier recovery. Mol. Biol. Cell 24, 2678–2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Birukova A. A., Meng F., Tian Y., Meliton A., Sarich N., Quilliam L. A., Birukov K. G. (2015) Prostacyclin post-treatment improves LPS-induced acute lung injury and endothelial barrier recovery via Rap1. Biochim. Biophys. Acta 1852, 778–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watson A. D., Leitinger N., Navab M., Faull K. F., Hörkkö S., Witztum J. L., Palinski W., Schwenke D., Salomon R. G., Sha W., Subbanagounder G., Fogelman A. M., Berliner J. A. (1997) Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. J. Biol. Chem. 272, 13597–13607 [DOI] [PubMed] [Google Scholar]
- 27.Birukova A. A., Alekseeva E., Mikaelyan A., Birukov K. G. (2007) HGF attenuates thrombin-induced permeability in the human pulmonary endothelial cells by Tiam1-mediated activation of the Rac pathway and by Tiam1/Rac-dependent inhibition of the Rho pathway. FASEB J. 21, 2776–2786 [DOI] [PubMed] [Google Scholar]
- 28.Pfaffl M. W. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vallerie S. N., Kramer F., Barnhart S., Kanter J. E., Breyer R. M., Andreasson K. I., Bornfeldt K. E. (2016) Myeloid cell prostaglandin E2 receptor EP4 modulates cytokine production but not atherogenesis in a mouse model of type 1 diabetes. PLoS One 11, e0158316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Birukova A. A., Malyukova I., Poroyko V., Birukov K. G. (2007) Paxillin-beta-catenin interactions are involved in Rac/Cdc42-mediated endothelial barrier-protective response to oxidized phospholipids. Am. J. Physiol. Lung Cell. Mol. Physiol. 293, L199–L211 [DOI] [PubMed] [Google Scholar]
- 31.Tian Y., Gawlak G., Shah A. S., Higginbotham K., Tian X., Kawasaki Y., Akiyama T., Sacks D. B., Birukova A. A. (2015) Hepatocyte growth factor-induced Asef-IQGAP1 complex controls cytoskeletal remodeling and endothelial barrier. J. Biol. Chem. 290, 4097–4109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Birukova A. A., Chatchavalvanich S., Rios A., Kawkitinarong K., Garcia J. G., Birukov K. G. (2006) Differential regulation of pulmonary endothelial monolayer integrity by varying degrees of cyclic stretch. Am. J. Pathol. 168, 1749–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Birukova A. A., Cokic I., Moldobaeva N., Birukov K. G. (2009) Paxillin is involved in the differential regulation of endothelial barrier by HGF and VEGF. Am. J. Respir. Cell Mol. Biol. 40, 99–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Birukov K. G., Bochkov V. N., Birukova A. A., Kawkitinarong K., Rios A., Leitner A., Verin A. D., Bokoch G. M., Leitinger N., Garcia J. G. (2004) Epoxycyclopentenone-containing oxidized phospholipids restore endothelial barrier function via Cdc42 and Rac. Circ. Res. 95, 892–901 [DOI] [PubMed] [Google Scholar]
- 35.Birukova A. A., Birukov K. G., Smurova K., Adyshev D., Kaibuchi K., Alieva I., Garcia J. G., Verin A. D. (2004) Novel role of microtubules in thrombin-induced endothelial barrier dysfunction. FASEB J. 18, 1879–1890 [DOI] [PubMed] [Google Scholar]
- 36.Monvoisin A., Alva J. A., Hofmann J. J., Zovein A. C., Lane T. F., Iruela-Arispe M. L. (2006) VE-cadherin-CreERT2 transgenic mouse: a model for inducible recombination in the endothelium. Dev. Dyn. 235, 3413–3422 [DOI] [PubMed] [Google Scholar]
- 37.Liang X., Lin L., Woodling N. S., Wang Q., Anacker C., Pan T., Merchant M., Andreasson K. (2011) Signaling via the prostaglandin E2 receptor EP4 exerts neuronal and vascular protection in a mouse model of cerebral ischemia. J. Clin. Invest. 121, 4362–4371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fu P., Birukova A. A., Xing J., Sammani S., Murley J. S., Garcia J. G., Grdina D. J., Birukov K. G. (2009) Amifostine reduces lung vascular permeability via suppression of inflammatory signalling. Eur. Respir. J. 33, 612–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bokoch G. M. (2003) Biology of the p21-activated kinases. Annu. Rev. Biochem. 72, 743–781 [DOI] [PubMed] [Google Scholar]
- 40.Jacobson J. R., Dudek S. M., Singleton P. A., Kolosova I. A., Verin A. D., Garcia J. G. (2006) Endothelial cell barrier enhancement by ATP is mediated by the small GTPase Rac and cortactin. Am. J. Physiol. Lung Cell. Mol. Physiol. 291, L289–L295 [DOI] [PubMed] [Google Scholar]
- 41.Head J. A., Jiang D., Li M., Zorn L. J., Schaefer E. M., Parsons J. T., Weed S. A. (2003) Cortactin tyrosine phosphorylation requires Rac1 activity and association with the cortical actin cytoskeleton. Mol. Biol. Cell 14, 3216–3229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dudek S. M., Jacobson J. R., Chiang E. T., Birukov K. G., Wang P., Zhan X., Garcia J. G. (2004) Pulmonary endothelial cell barrier enhancement by sphingosine 1-phosphate: roles for cortactin and myosin light chain kinase. J. Biol. Chem. 279, 24692–24700 [DOI] [PubMed] [Google Scholar]
- 43.Dejana E., Orsenigo F., Molendini C., Baluk P., McDonald D. M. (2009) Organization and signaling of endothelial cell-to-cell junctions in various regions of the blood and lymphatic vascular trees. Cell Tissue Res. 335, 17–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harris E. S., Nelson W. J. (2010) VE-cadherin: at the front, center, and sides of endothelial cell organization and function. Curr. Opin. Cell Biol. 22, 651–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Birukova A. A., Zebda N., Fu P., Poroyko V., Cokic I., Birukov K. G. (2011) Association between adherens junctions and tight junctions via Rap1 promotes barrier protective effects of oxidized phospholipids. J. Cell. Physiol. 226, 2052–2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kiemer A. K., Weber N. C., Fürst R., Bildner N., Kulhanek-Heinze S., Vollmar A. M. (2002) Inhibition of p38 MAPK activation via induction of MKP-1: atrial natriuretic peptide reduces TNF-alpha-induced actin polymerization and endothelial permeability. Circ. Res. 90, 874–881 [DOI] [PubMed] [Google Scholar]
- 47.Hocking D. C., Phillips P. G., Ferro T. J., Johnson A. (1990) Mechanisms of pulmonary edema induced by tumor necrosis factor-alpha. Circ. Res. 67, 68–77 [DOI] [PubMed] [Google Scholar]
- 48.Kakiashvili E., Speight P., Waheed F., Seth R., Lodyga M., Tanimura S., Kohno M., Rotstein O. D., Kapus A., Szászi K. (2009) GEF-H1 mediates tumor necrosis factor-alpha-induced Rho activation and myosin phosphorylation: role in the regulation of tubular paracellular permeability. J. Biol. Chem. 284, 11454–11466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu Y. C., Yeh W. C., Ohashi P. S. (2008) LPS/TLR4 signal transduction pathway. Cytokine 42, 145–151 [DOI] [PubMed] [Google Scholar]
- 50.Khakpour S., Wilhelmsen K., Hellman J. (2015) Vascular endothelial cell Toll-like receptor pathways in sepsis. Innate Immun. 21, 827–846 [DOI] [PubMed] [Google Scholar]
- 51.Li R., Mouillesseaux K. P., Montoya D., Cruz D., Gharavi N., Dun M., Koroniak L., Berliner J. A. (2006) Identification of prostaglandin E2 receptor subtype 2 as a receptor activated by OxPAPC. Circ. Res. 98, 642–650 [DOI] [PubMed] [Google Scholar]
- 52.Yagami T., Koma H., Yamamoto Y. (2016) Pathophysiological roles of cyclooxygenases and prostaglandins in the central nervous system. Mol. Neurobiol. 53, 4754–4771 [DOI] [PubMed] [Google Scholar]
- 53.Yokoyama U., Iwatsubo K., Umemura M., Fujita T., Ishikawa Y. (2013) The prostanoid EP4 receptor and its signaling pathway. Pharmacol. Rev. 65, 1010–1052 [DOI] [PubMed] [Google Scholar]
- 54.Fukuhara S., Sakurai A., Sano H., Yamagishi A., Somekawa S., Takakura N., Saito Y., Kangawa K., Mochizuki N. (2005) Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol. Cell. Biol. 25, 136–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Regan J. W., Bailey T. J., Pepperl D. J., Pierce K. L., Bogardus A. M., Donello J. E., Fairbairn C. E., Kedzie K. M., Woodward D. F., Gil D. W. (1994) Cloning of a novel human prostaglandin receptor with characteristics of the pharmacologically defined EP2 subtype. Mol. Pharmacol. 46, 213–220 [PubMed] [Google Scholar]
- 56.Birukova A. A., Singleton P. A., Gawlak G., Tian X., Mirzapoiazova T., Mambetsariev B., Dubrovskyi O., Oskolkova O. V., Bochkov V. N., Birukov K. G. (2014) GRP78 is a novel receptor initiating a vascular barrier protective response to oxidized phospholipids. Mol. Biol. Cell 25, 2006–2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Noren N. K., Arthur W. T., Burridge K. (2003) Cadherin engagement inhibits RhoA via p190RhoGAP. J. Biol. Chem. 278, 13615–13618 [DOI] [PubMed] [Google Scholar]
- 58.Bustos R. I., Forget M. A., Settleman J. E., Hansen S. H. (2008) Coordination of Rho and Rac GTPase function via p190B RhoGAP. Curr. Biol. 18, 1606–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Birukova A. A., Zebda N., Cokic I., Fu P., Wu T., Dubrovskyi O., Birukov K. G. (2011) p190RhoGAP mediates protective effects of oxidized phospholipids in the models of ventilator-induced lung injury. Exp. Cell Res. 317, 859–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li M., Healy D. R., Li Y., Simmons H. A., Crawford D. T., Ke H. Z., Pan L. C., Brown T. A., Thompson D. D. (2005) Osteopenia and impaired fracture healing in aged EP4 receptor knockout mice. Bone 37, 46–54 [DOI] [PubMed] [Google Scholar]
- 61.Harding P., Yang X. P., Yang J., Shesely E., He Q., LaPointe M. C. (2010) Gene expression profiling of dilated cardiomyopathy in older male EP4 knockout mice. Am. J. Physiol. Heart Circ. Physiol. 298, H623–H632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Legler D. F., Krause P., Scandella E., Singer E., Groettrup M. (2006) Prostaglandin E2 is generally required for human dendritic cell migration and exerts its effect via EP2 and EP4 receptors. J. Immunol. 176, 966–973 [DOI] [PubMed] [Google Scholar]
- 63.Scandella E., Men Y., Gillessen S., Förster R., Groettrup M. (2002) Prostaglandin E2 is a key factor for CCR7 surface expression and migration of monocyte-derived dendritic cells. Blood 100, 1354–1361 [DOI] [PubMed] [Google Scholar]
- 64.Boniface K., Bak-Jensen K. S., Li Y., Blumenschein W. M., McGeachy M. J., McClanahan T. K., McKenzie B. S., Kastelein R. A., Cua D. J., de Waal Malefyt R. (2009) Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling. J. Exp. Med. 206, 535–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Konya V., Marsche G., Schuligoi R., Heinemann A. (2013) E-type prostanoid receptor 4 (EP4) in disease and therapy. Pharmacol. Ther. 138, 485–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Singleton P. A., Dudek S. M., Chiang E. T., Garcia J. G. (2005) Regulation of sphingosine 1-phosphate-induced endothelial cytoskeletal rearrangement and barrier enhancement by S1P1 receptor, PI3 kinase, Tiam1/Rac1, and alpha-actinin. FASEB J. 19, 1646–1656 [DOI] [PubMed] [Google Scholar]
- 67.Singleton P. A., Salgia R., Moreno-Vinasco L., Moitra J., Sammani S., Mirzapoiazova T., Garcia J. G. (2007) CD44 regulates hepatocyte growth factor-mediated vascular integrity. Role of c-Met, Tiam1/Rac1, dynamin 2, and cortactin. J. Biol. Chem. 282, 30643–30657 [DOI] [PubMed] [Google Scholar]
- 68.Tauseef M., Kini V., Knezevic N., Brannan M., Ramchandaran R., Fyrst H., Saba J., Vogel S. M., Malik A. B., Mehta D. (2008) Activation of sphingosine kinase-1 reverses the increase in lung vascular permeability through sphingosine-1-phosphate receptor signaling in endothelial cells. Circ. Res. 103, 1164–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ratcliffe M. J., Walding A., Shelton P. A., Flaherty A., Dougall I. G. (2007) Activation of E-prostanoid4 and E-prostanoid2 receptors inhibits TNF-alpha release from human alveolar macrophages. Eur. Respir. J. 29, 986–994 [DOI] [PubMed] [Google Scholar]
- 70.Minami M., Shimizu K., Okamoto Y., Folco E., Ilasaca M. L., Feinberg M. W., Aikawa M., Libby P. (2008) Prostaglandin E receptor type 4-associated protein interacts directly with NF-kappaB1 and attenuates macrophage activation. J. Biol. Chem. 283, 9692–9703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Konya V., Maric J., Jandl K., Luschnig P., Aringer I., Lanz I., Platzer W., Theiler A., Bärnthaler T., Frei R., Marsche G., Marsh L. M., Olschewski A., Lippe I. T., Heinemann A., Schuligoi R. (2015) Activation of EP4 receptors prevents endotoxin-induced neutrophil infiltration into the airways and enhances microvascular barrier function. Br. J. Pharmacol. 172, 4454–4468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mammoto T., Parikh S. M., Mammoto A., Gallagher D., Chan B., Mostoslavsky G., Ingber D. E., Sukhatme V. P. (2007) Angiopoietin-1 requires p190 RhoGAP to protect against vascular leakage in vivo. J. Biol. Chem. 282, 23910–23918 [DOI] [PubMed] [Google Scholar]
- 73.Guo F., Tang J., Zhou Z., Dou Y., Van Lonkhuyzen D., Gao C., Huan J. (2012) GEF-H1-RhoA signaling pathway mediates LPS-induced NF-κB transactivation and IL-8 synthesis in endothelial cells. Mol. Immunol. 50, 98–107 [DOI] [PubMed] [Google Scholar]
- 74.Wu T., Xing J., Birukova A. A. (2013) Cell-type-specific crosstalk between p38 MAPK and Rho signaling in lung micro- and macrovascular barrier dysfunction induced by Staphylococcus aureus-derived pathogens. Transl. Res. 162, 45–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goolaerts A., Lafargue M., Song Y., Miyazawa B., Arjomandi M., Carlès M., Roux J., Howard M., Parks D. A., Iles K. E., Pittet J. F. (2011) PAI-1 is an essential component of the pulmonary host response during Pseudomonas aeruginosa pneumonia in mice. Thorax 66, 788–796 [DOI] [PMC free article] [PubMed] [Google Scholar]