Abstract
The role of prolyl hydroxylase (PHD)-3 as a hypoxia inducible factor (HIF)-1α cofactor is controversial and remains unknown in skeletal tissues. We investigated whether PHD3 controls HIF-1 transcriptional activity in nucleus pulposus (NP) cells through the pyruvate kinase muscle (PKM)-2-Jumonji domain–containing protein (JMJD5) axis. PHD3−/− mice (12.5 mo old) showed increased incidence of intervertebral disc degeneration with a concomitant decrease in expression of the HIF-1α targets VEGF-A, glucose transporter-1, and lactate dehydrogenase A. PHD3 silencing decreased hypoxic activation of HIF-1α C-terminal transactivation domain (C-TAD), but not HIF-1α-N-terminal-(N)-TAD or HIF-2α-TAD. Moreover, PHD3 suppression in NP cells resulted in decreased HIF-1α enrichment on target promoters and lower expression of select HIF-1 targets. Contrary to other cell types, manipulation of PKM2 and JMJD5 levels had no effect on HIF-1 activity in NP cells. Likewise, stabilization of tetrameric PKM2 by a chemical approach had no effect on PHD3-dependent HIF-1 activity. Coimmunoprecipitation assays showed lack of association between HIF-1α and PKM2 in NP cells. Results support the role of the PHD3 as a cofactor for HIF-1, independent of PKM2-JMJD5.—Schoepflin, Z. R., Silagi, E. S., Shapiro, I. M., Risbud, M. V. PHD3 is a transcriptional coactivator of HIF-1α in nucleus pulposus cells independent of the PKM2-JMJD5 axis.
Keywords: intervertebral disc, hypoxia, skeletal tissue, transcription factor
The intervertebral disc is the largest avascular tissue in mammals and, as a consequence, is physiologically hypoxic (1). Intervertebral disc degeneration is closely linked to low back pain, which is the leading cause of disability-adjusted life years in the United States (2). The healthy intervertebral disc tissue contains an outer annulus fibrosus (AF) derived from the embryonic sclerotome and a central nucleus pulposus (NP) derived from the embryonic notochord, which are bound by cranial and caudal cartilaginous endplates (3). The cells of the NP are finely attuned to survive in the physiologically hypoxic niche by robust expression of the basic helix–loop–helix Per-Arnt-Sim transcription factor hypoxia inducible factor (HIF)-1α (4–6). Maintenance of proper HIF-1 signaling is essential for NP cell homeostasis and survival within the intervertebral disc. Recent studies have shown that conditional deletion of HIF-1α in the NP results in cell death and replacement of the NP by a biomechanically inferior fibrocartilaginous tissue (7).
In most cell types, HIF-1α stability and activity are controlled by non–heme Fe2+-dependent molecular dioxygenases (8). Prolyl hydroxylases (PHDs) use molecular oxygen to hydroxylate 2 specific proline residues located within the oxygen-dependent degradation domain (ODD) of HIF-1α, resulting in von Hippel-Lindau (VHL)–mediated ubiquitination and proteasomal degradation (9). However, recent studies in NP cells have shown that the relationship between HIF-1α and PHDs, as well as HIF-1α and factor-inhibiting HIF-1, is unique, and that PHDs retain some enzymatic activity during hypoxia (HX) (10–13). Of particular interest is the observation that PHD3 does not have any discernible role in regulating HIF-1/2α stability, but may have an independent function in regulating HIF-1 activity in NP cells (11). The role of PHD3 in controlling HIF-1 transcriptional activity, independent of its hydroxylase function, has been debated. One detailed study has shown that PHD3 promotes HIF-1α transcriptional activity, but a more recent study suggests that PHD3 suppresses HIF-1 activity in a small ubiquitin–like modifier-dependent fashion (14, 15). However, the exact nature of this relationship and mechanism of action remain unknown in skeletal tissues, including NP.
Tetrameric PK catalyzes the final step in glycolysis, converting phosphoenolpyruvate into pyruvate. Transcripts from the PKM locus are alternatively spliced into 2 major isoforms, M1 and M2, differing by 1 exon (16). The M2 isoform has recently received much attention for its noncanonical roles in tumorigenesis, functioning as a dimer, promoting Warburg-like metabolism and enhancing transcriptional activity of Oct-4, β-catenin, and HIF-1α (17). Studies suggest that translocation of PKM2 dimers into the nucleus is controlled by another molecular dioxygenase, Jumonji domain-containing protein (JMJD)-5, which primarily serves as a histone demethylase (18). Recent evidence suggests that these noncanonical functions of PKM2 do not require protein kinase activity (19). PHD3 has been reported to control HIF-1 activity through a PKM2-p300 axis; the major objective of this study was to investigate the role of PHD3 as a cofactor for HIF-1α in NP cells, and the role of the PKM2-JMJD5 axis in this HIF-PHD3 circuit. Our study shows for the first time, to the best of our knowledge, that PHD3 in NP cells promotes hypoxic expression of a select subset of HIF-1 target genes in a C-terminal (C)-TAD-dependent manner. We demonstrate that the PKM2-JMJD5 axis plays no role in regulation of HIF-1α activity in NP cells, indicating that the HIF-PHD3 circuit in NP is novel and cell-type specific. PHD3−/− mice, at 12.5 mo of age, showed increased incidence of intervertebral disc degeneration with a concomitant decrease in expression of the C-TAD-dependent HIF-1α targets VEGF-A, glucose transporter (GLUT)-1, and lactate dehydrogenase (LDH)-A. Our findings suggest that maintenance of the HIF-PHD3 axis is critical for proper maintenance of HIF-1α signaling in the NP and for intervertebral disc homeostasis.
MATERIALS AND METHODS
Plasmids and reagents
For transactivation studies of HIF-1α and -2α the binary Gal4 reporter plasmids (HIF-1α aa 530–778; HIF-1α aa 740–826; HIF-1α aa 786–826; and HIF-2α aa 819–870) were provided by Nianli Sang (Drexel University, Philadelphia, PA, USA). The pFR-Luc (Stratagene, La Jolla, CA, USA) reporter contains a yeast Gal4-binding site upstream of a minimal promoter and the firefly luciferase gene. HIF-1α aa 530–778 P564A mutant was generated with Q5 site-directed mutagenesis kit (New England Biolabs, Ipswich, MA, USA) and verified by Sanger sequencing. Enolase (ENO)-1-wild-type (WT) promoter was provided by Gregg Semenza (Johns Hopkins University, Baltimore, MD, USA). Mission short hairpin RNA (shRNA) clones targeted against human PKM (TRCN291062 and TRCN296841) and rat HIF-1α (TRCN232222 and TRCN54450) were purchased from Sigma-Aldrich (St. Louis, MO, USA). LVshPHD3 construct was provided by Kenneth Thirstrup (H. Lundbeck A/S, Valby, Denmark) (20). PKM2-WT, PKM2-K367M, PKM2-R399E, JMJD5-WT, JMJD5-H321A, and JMJD5-ΔN80 were kindly provided by Wen-Ching Wang (National Tsing Hua University, Hsinchu City, Taiwan) (18). Hypoxia response element (HRE)-Luc (26731) by Navdeep Chandel; PHD3-WT (18960) and PHD3-H196A (22717) by William Kaelin (Dana-Farber Cancer Institute, Harvard University, Boston, MA, USA); and psPAX2 (12260) and pMD2.G (12259) by Didier Trono (École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland), were obtained from Addgene (Cambridge, MA, USA). pRLTK (Promega, Madison, WI, USA) containing the Renilla reniformis luciferase gene was used as an internal transfection control.
Generation of PHD3−/− mice
PHD3+/+ and PHD3−/− mice were kindly provided by Peter Ratcliffe (University of Oxford, Oxford, United Kingdom) (21). The mice were maintained on a mixed Swiss/129SvEv genetic background. Mice from the same litter were used for comparisons.
Immunohistological analysis
PHD3+/+ and PHD3−/− mouse spines (5 and 12.5 mo old) were harvested and fixed in 4% paraformaldehyde for 24 h and decalcified in 12.5% EDTA for 6 wk before they were embedded in paraffin. Sagittal sections, 7 μm in thickness, were deparaffinized and rehydrated in graded alcohols, and the antigens were retrieved with citrate buffer (pH 6) for 20 min. Slides were blocked in 5% normal goat serum in 1× PBS, 0.4% Triton-X for 1 h at room temperature. Sections were then sequentially incubated with antibodies [VEGF, ab46154; LDHA, ab52488; and GLUT1, ab40084 (all from Abcam), or ENO1, NB100-65252 (Novus Biologics, Littleton CO, USA), all at a concentration of 1:100) with an NP marker anti-keratin (Krt)19 antibody (1:3, TROMA-III; Developmental Studies Hybridoma Bank, Iowa City, IA, USA) in 5% normal donkey serum in 1× PBS, 0.4% Triton-X at 4°C overnight (22). After the sections were thoroughly washed, the bound primary antibodies were incubated with Alexa Fluor-594 AffiniPure F(ab′)2 conjugated anti-rabbit, anti-mouse, or anti-rat secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) for 1 h at room temperature. Vector Laboratories Mouse on Mouse (M.O.M.) Detection Kit was used for anti-mouse primary antibodies. Sections were visualized with a fluorescence microscope (AxioImager A2; Zeiss, Thornwood, NY, USA) fitted with a monochromatic camera (AxioCam MRm; Zeiss). Histology of the intervertebral discs was evaluated using standard Safranin-O, Fast green, and hematoxylin staining. To evaluate structural integrity in 12.5-mo-old mice, 4 disc levels per mouse (n = 3 animals/genotype) were scored with a modified Thompson grading system (scale of grade I–IV), evaluated by 2 independent scorers (23, 24). Lower score represents a healthier disc.
Isolation of NP and AF cells, cell treatments, and hypoxic culture
Rat and human NP and rat AF cells were isolated and characterized according to a published method (5). T/C-28, a human chondrocyte line, was kindly provided by Mary Goldring (Hospital for Special Surgery, New York, NY, USA) (25). T/C-28 cells have been extensively characterized and closely replicate important physiologic responses of primary human chondrocytes (22). Cells were maintained in DMEM and 10% fetal bovine serum (FBS) supplemented with antibiotics. To investigate the effects of stabilization of PKM2 tetramers, cells were treated with N,N′-diarylsulfonamide (DASA)-10 (20 μM) or thieno[3,2-b]pyrrole[3,2-d]pyridazinone (TEPP)-46 (50 μM) for 24 h (EMD Millipore, Billerica, MA, USA). Cells were cultured in an HX work station (Invivo2 300; Ruskinn, Bridgend, United Kingdom) with a mixture of 1% O2, 5% CO2, and 94% N2 or 5% O2, 5% CO2, and 90% N2 for 4–72 h.
Lentiviral production and transduction
HEK293T cells were seeded in 10-cm plates (6 × 106 cells/plate) in Opti-MEM with 2% heat-inactivated FBS 1 d before transfection. The cells were transfected with Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA) with 9 μg shPHD3 or shControl plasmid along with 6 μg of psPAX2 and 3 μg of pMD2.G. After 6 h, transfection medium was removed and replaced with DMEM with 10% heat-inactivated FBS and antibiotics. Lentiviral particles were harvested at 48 and 60 h after transfection and precipitated overnight with 35% PEG6000 solution. NP cells were plated in DMEM with 10% FBS 1 d before transduction. Cells were transduced with DMEM containing lentivirus along with 8 μg/ml polybrene. After 24 h, medium was removed and replaced with DMEM with 10% heat-inactivated FBS. Cells were harvested for mRNA or protein analysis 7 d after transduction.
Real-time RT-PCR analysis
Total RNA was extracted from NP cells using RNeasy minicolumns and treated with RNase-free DNase I before elution (Qiagen, Germantown, MD, USA). Purified, DNA-free RNA was converted to cDNA using EcoDry Premix (Clontech Laboratories, Mountain View, CA, USA). Template cDNA and gene-specific primers were added to SYBR Green master mix (Thermo Fisher Scientific), and mRNA expression was quantified with the Step One Plus Real-time PCR System (Thermo Fisher Scientific). Hypoxanthine-guanine phosphoribosyltransferase (HPRT) and β-actin were used to normalize gene expression. Melting curves were analyzed to verify the specificity of the RT-PCR and the absence of primer dimer formation. Each sample was analyzed in duplicate and included a template-free control. All primers used were synthesized by Integrated DNA Technologies (Coralville, IA, USA).
Protein extraction, immunoprecipitation, and Western blot analysis
Cells were placed on ice immediately after treatment and washed with ice-cold PBS. Wash buffer and lysis buffer contained 1× protease inhibitor cocktail (Roche, Basel, Switzerland), NaF (4 mM), Na3VO4 (20 mM), NaCl (150 mM), β-glycerophosphate (50 mM), and DTT (0.2 mM). Nuclear and cytosolic proteins were prepared with the CellLytic NuCLEAR extraction kit (Sigma-Aldrich). Immunoprecipitation was performed with Protein A/G Plus Agarose beads (Thermo Fisher Scientific), according to the manufacturer’s protocol, with anti-HIF-1α (Abcam Inc., Cambridge, MA, USA) or anti-PKM2 (Cell Signaling Technology, Danvers, MA, USA). Equal amounts of total or fractionated cell proteins were resolved on 8–10% SDS-polyacrylamide gels and transferred to PVDF membranes (Thermo Fisher Scientific). Membranes were blocked with 5% nonfat dry milk in 50 mM Tris (pH 7.6), 150 mM NaCl, 0.1% Tween 20 and incubated overnight at 4°C in 5% nonfat dry milk in Tris-buffered saline–Tween with anti-HIF-1α (1:500; R&D Systems, Minneapolis, MN, USA, or 1:1000, Abcam); anti-PKM2 (1:5000), anti-PKM1 (1:3000), anti-LDHA (1:1000), anti-PHD2 (1:1000), anti-GLUT1 (1:1000), anti-ENO1 (1:1000), and anti-lamin A/C (1:1000), all from Cell Signaling Technology; anti-PHD3 (1:1000; Novus Biologicals); or anti-β-tubulin (1:5000; Developmental Studies Hybridoma Bank). Immunolabeling was detected with ECL reagent (LAS4000; GE Life Sciences, Pittsburgh, PA, USA). Densitometric analysis was performed with ImageQuant TL (GE Life Sciences). The band density of the protein of interest was normalized to the band density of the housekeeping protein (β-tubulin) to control for variability in protein loading.
Chromatin immunoprecipitation
A chromatin immunoprecipitation (ChIP) assay was performed using ChIP-IT high-sensitivity kit (Active Motif, Carlsbad, CA, USA), according to the manufacturer’s recommendations. In brief, chromatin was sheared by sonication, and input DNA was generated by treating aliquots with RNase, proteinase K, and heat, followed by ethanol precipitation. DNA complexes were immunoprecipitated by incubation with anti-HIF-1α antibody (Abcam) overnight at 4°C followed by binding to protein G-agarose beads for 3 h at 4°C. Cross-links were reversed by treatment with proteinase K and heat for 2.5 h, and DNA was purified with DNA purification elution buffer (Active Motif). Real-time PCR analysis was performed with ChIP-IT quantitative PCR analysis kit (Active Motif) using the following primer pairs for validated HRE sites (from literature) as shown in Supplemental Table 1. Negative control primers and standard curve primers used were provided with the kit. Real-time PCR was performed with Power SYBR Green PCR Master Mix (Thermo Fisher Scientific). The Ct values were recorded, and the data were normalized based on primer efficiency, input DNA Ct values, amount of chromatin, and resuspension volume, based on the manufacturer’s recommendations.
Transfections and dual luciferase assay
Cells were transferred to 48-well plates at a density of 2 × 104 cells/well 1 d before transfection. For each transfection, plasmids were premixed with Lipofectamine 2000. For measuring the effect of HX or PKM2 stabilizers on HRE reporter and HIF-TAD activity, the cells in some wells were treated with activator or moved to the HX work station 24 h after transfection. Cells were harvested 48 h after transfection. The Dual-Luciferase Reporter Assay System (Promega) was used for sequential measurements of firefly and Renilla luciferase activities. Quantification of luciferase activities and calculation of relative ratios were performed with an Infinite 200 PRO plate reader (Tecan, Männedorf, Switzerland).
Statistical analysis
All measurements were performed at least in biologic triplicate. Data are presented as means ± sem. Differences between groups were analyzed by Student’s t test or 1-way ANOVA with post hoc Tukey’s test, or Dunnett’s test for multiple comparisons to a single control. A value of P < 0.05 indicated significant results.
RESULTS
PHD3−/− mice show increased incidence of disc degeneration with a concomitant decrease in expression of select HIF-1 target genes in NP tissue
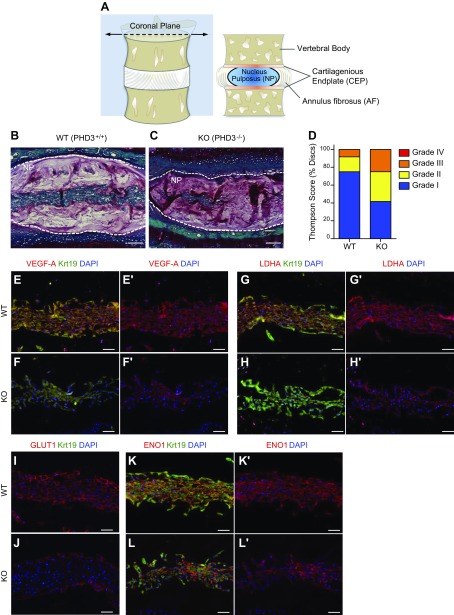
It has been shown that HIF-1α expression is critical for proper maintenance of NP tissue homeostasis in vivo (7). In vitro studies have also suggested that PHD3 promotes HIF-1α activity in cancer cells (14), as well as in NP cells (11). We therefore investigated the in vivo role of PHD3 in maintaining integrity of the intervertebral discs. We characterized the disc phenotype of 12.5-mo-old WT (Fig. 1B) and PHD3-knockout mice (Fig. 1C) and assessed the histologic incidence of degeneration of Safranin-O-stained sections using the Thompson grading scale (23, 24) (Fig. 1D). The discs of PHD3-knockout mice evidenced higher incidence of degenerative changes in tissue structure and morphology as compared to WT littermate controls (Fig. 1B–D). We then evaluated expression of known HIF-1 target proteins in the NP tissue of WT and PHD3-knockout animals. Immunohistological staining of coronal sections (Fig. 1A) of the disc showed that the expression of VEGF-A (Fig. 1E–F'), LDHA (Fig. 1G–H'), and GLUT1 (Fig. 1I, J) were markedly decreased in the NP tissue of PHD3−/− mice when compared to PHD3+/+ littermates. Expression of ENO1, another HIF-1 target, was not affected in the NP of PHD3−/− mice (Fig. 1K–L'); NP tissue identity was confirmed by costaining with a known NP phenotypic marker, Krt19 (22) (Fig. 1E–H, K, L). A similar decrease in levels of these HIF-1 targets was also observed in 5-mo-old knockout animals, without overt changes in the gross morphology of disc tissue (Supplemental Fig. 1). These results clearly show that PHD3 is critical for disc health in vivo, possibly through maintaining expression of select HIF-1 targets in the NP tissue.
Figure 1.
PHD3−/− mice show increased incidence of disc degeneration and decreased expression of select HIF-1 target genes in NP tissue. A) Schematic drawing of spinal motion segment and coronal cross section showing vertebral bodies and the intervertebral disc with its central NP, circumferential AF, and superior and inferior cartilaginous endplates (CEP). B, C) Representative Safranin-O/Fast Green/hematoxylin–stained sections of 12.5-mo-old WT (B) and PHD3−/− (C) mice. The PHD3−/− mouse showed a decreased number of NP cells and some changes in cell morphology. D) PHD3−/− mice showed increased incidence of discs with a higher grade of degeneration, as assessed by the Thompson grading scale. Four discs/animal were scored from 3 pairs of littermate animals. E–L') Representative immunofluorescence images from 12.5-mo-old WT (PHD3+/+) and knockout (PHD3−/−) mice showing NP tissue areas with a comparable number of cells. There was decreased staining of HIF-1 targets VEGF-A (E–F'), LDHA (G–H'), and GLUT1 (I, J) in knockout mice compared to WT mice. In contrast, another HIF-1 target, ENO1, shows comparable expression in PHD3+/+ (K, K') and PHD3−/− (L, L') mice. Krt19 was used as a marker to label the NP tissue compartment with VEGF-A, LDHA, and ENO1 (independent channel not shown). Scale bars, 100 μm (B, C) and 50 μm (E–L'). Images are representative from 3 independent littermate groups.
PHD3 promotes hypoxic HIF-1α activity in disc cells through a C-TAD-dependent mechanism
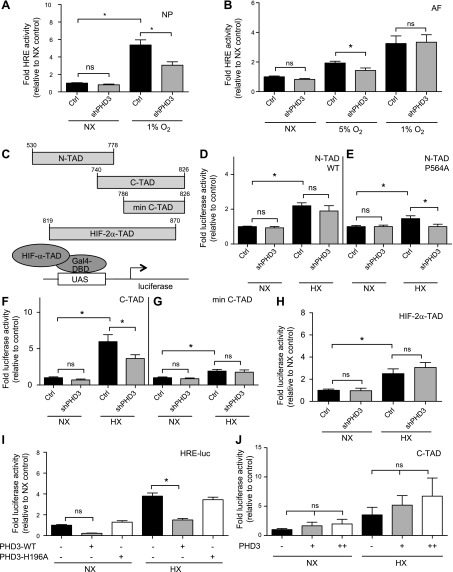
Although PHD3 controls HIF-1α activity in NP cells, the mechanism is yet unknown. To address this question, we first transiently suppressed PHD3 expression in NP cells with specific shRNA and measured HIF activity using an HRE-luciferase reporter. Knockdown of PHD3 resulted in a significant decrease in the hypoxic induction of HRE reporter activity (Fig. 2A); no effect on HRE activity was seen in normoxia (NX). As a control for these experiments, we measured AF cell response to knockdown of PHD3; there was no effect on HRE activity in AF cells in NX, but a significant decrease in HIF activity was seen at 5% O2 (Fig. 2B). The effect of shPHD3 in AF cells disappeared at 1% oxygen, suggesting that the effect of PHD3 on HIF activity may be dependent on hydroxylase function (11).
Figure 2.
PHD3 promotes hypoxic HIF-1α activity in disc cells through a C-TAD-dependent mechanism. A, B) Measurement of HIF activity in rat NP (A) (n = 7) and AF (B) (n = 3) cells using an HRE-luciferase reporter in NX and HX after PHD3 silencing. C) Gal4-HIF-α-TAD constructs used in panels D–H and the assay principle. D, E) Measurement of WTe (D) and P564A mutant (E) HIF-1α N-TAD (aa 530–778) activity in NP cells in NX and HX after PHD3 silencing (n = 3). F, G) Measurement of HIF-1α C-TAD (aa740–826) (F) (n = 7) and HIF-1α minimal C-TAD (aa 786–826) (G) (n = 3) activity in NP cells after PHD3 silencing. PHD3 silencing in HX resulted in decreased activity of HIF-1α C-TAD (aa 740–826) but not of minimal C-TAD (aa 786–826). H) Measurement of HIF-2α-TAD activity in NP cells after PHD3 silencing (n = 4). I) Measurement of HRE reporter activity in NP cells after overexpression of WT (PHD3-WT) or hydroxylase-deficient (PHD3-H196A) PHD3 (n = 3). J) PHD3 overexpression did not affect HIF-1α C-TAD activity (n = 3). Hypoxic culture for all experiments was 24 h. Data are means ± sem. Independent biologic experiments were performed with 3 technical replicates per experiment. *P < 0.05.
To further investigate the mechanism by which PHD3 promotes hypoxic activity of HIF-1 in NP cells, we used a Gal4 reporter system containing different fragments of HIF TADs fused to minimal Gal4-DNA binding domain to drive an upstream activation sequence-luciferase reporter (Fig. 2C). Knockdown of PHD3 had no effect on the activity of the N-terminal (N-TAD) of HIF-1α in both NX and HX (Fig. 2D). Because there is an overlap between the ODD and the N-TAD, we used an N-TAD construct where proline 564 was mutated to alanine (P564A), to avoid confounding effects of hydroxylation-dependent ODD stabilization. The N-TAD P564A mutant still showed a small increase in activity in HX, which was blocked by PHD3 knockdown (Fig. 2E). This finding suggests that PHD3 has a very limited effect on N-TAD activity in NP cells. However, because this effect is not seen with WT N-TAD, it is likely that it is not a primary mechanism by which PHD3 regulates HIF-1α activity in NP cells. We then investigated the effects of PHD3 knockdown on activity of HIF-1α C-TAD. The longer C-TAD construct (aa 740–826) showed a robust increase in activity in HX, which was significantly decreased upon knockdown of PHD3 (Fig. 2F). The minimal C-TAD (aa 786–826) (26) was not affected by PHD3 knockdown (Fig. 2G). On the other hand, the activity of HIF-2α-TAD was unaffected by PHD3 silencing (Fig. 2H), suggesting that PHD3 specifically activates the HIF-1α isoform, and changes in HRE reporter after PHD3 silencing are likely related to changes in HIF-1, but not HIF-2, activity.
Next, we overexpressed both WT and hydroxylase-deficient PHD3 (H196A) and examined its effect on HRE reporter activity. Overexpression of only WT PHD3 significantly decreased HIF activity in NP cells in both NX and HX (Fig. 2I). This result adds credence to the notion that although endogenous PHD3 does not play a major role in regulating HIF-1α stability in NP cells, at nonphysiologically high levels, it serves as a traditional HIF hydroxylase and results in decreased HIF-1α protein and activity (10). To circumvent the confounding effect of PHD3 overexpression on the stability of HIF-1α, we examined its ability to activate HIF-1α C-TAD that lacks the ODD. Although there was a trend of increasing C-TAD activity after PHD3 overexpression, the values unexpectedly failed to reach statistical significance (Fig. 2J). It is likely that endogenous PHD3 expression in HX was high enough that its effect on HIF activity was saturated. However, during NX, when endogenous PHD3 levels were relatively low, PHD3 overexpression had little effect on C-TAD activity, suggesting that in NP cells, PHD3 functions as an HIF-1α coactivator only in HX.
PHD3 controls expression of a select set of HIF-1 targets in NP cells by enabling HIF-1α enrichment at promoters
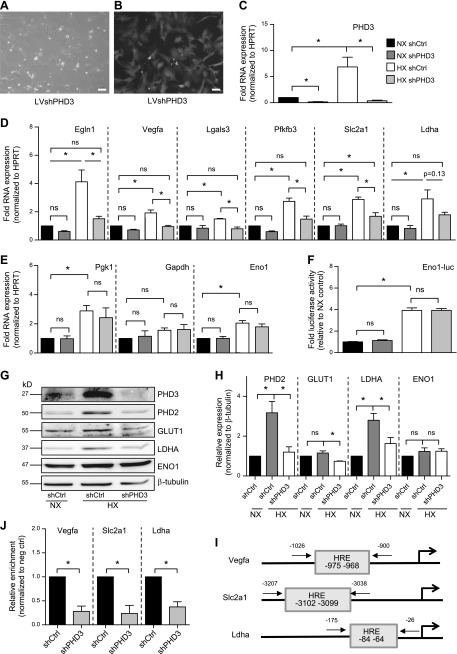
To investigate the effects of PHD3 on HIF-1 target gene expression in NP cells, we stably knocked down PHD3 using lentivirally delivered shRNA. Efficiency of transduction was monitored by yellow fluorescent protein coexpressed with the shRNA (Fig. 3A, B). Knockdown of shPHD3 in both NX and HX resulted in a robust decrease in PHD3 mRNA expression (Fig. 3C). Hypoxic induction of HIF-1 targets Egln1 (Phd2), Vegfa, Lgals3, and Pfkfb3 was completely abolished with knockdown of PHD3, and there was partial suppression of Slc2a1 (Glut1) (Fig. 3D). In contrast, there was no change in expression of any of these targets in PHD3-silenced cells in NX. Expression of Ldha also showed a trend of decreased expression after PHD3 knockdown in HX. Knockdown of PHD3 had no effect on expression of Pgk1, Gapdh, or Eno1 (Fig. 3E). We also measured the activity of an Eno1 promoter containing 2 well-characterized HIF-1 binding sites (27). Knockdown of PHD3 had no effect on Eno1 promoter activity (Fig. 3F). This result is in contrast to what has been reported in other cell types (14), suggesting that PHD3 in NP cells acts a cofactor for only a select group of HIF-1 targets and therefore is not required for global hypoxic HIF-1 transactivation. We examined expression of some of these HIF-1 targets by Western blot. Hypoxic expression of PHD2, GLUT1, and LDHA decreased after PHD3 knockdown, whereas expression of ENO1 was unaffected (Fig. 3G, H). In contrast to mRNA expression, GLUT1 and ENO1 protein levels at 72 h HX showed a trend of increased expression, most likely because elevated levels of these proteins are required for long-term hypoxic adaptation (4). PHD3 does not promote HIF-1α degradation in NP cells, and any effect on expression of HIF-1 targets is therefore independent of changes in HIF-1α protein level (10, 11).
Figure 3.
PHD3 controls expression of a select set of HIF-1 targets in NP cells through a p300-dependent mechanism. A, B) Bright-field (A) and fluorescent (B) images of rat NP cells after transduction with lentivirus coexpressing shPHD3 and enhanced green fluorescent protein. Scale bars, 10 μm. C) Measurement of PHD3 mRNA expression after transduction of NP cells with shRNA targeting PHD3 (n = 7). D) Measurement of mRNA expression of HIF-1 target genes in PHD3-silenced NP cells cultured in NX or HX for 72 h (n = 7). E) Hypoxic expression of Pgk1, Gapdh, and Eno1 in PHD3-silenced cells did not change (n = 3). F) Measurement of activity of Eno1 promoter containing 2 well-characterized HIF-1 binding sites after PHD3 knockdown (n = 7). G, H) Western blot (G) and corresponding densitometric analysis (H) of select HIF-1 targets in NP cells after stable knockdown of PHD3 (n = 4). I) Location of HRE sites within promoters of Vegfa, Slc2a1, and Ldha and location of ChIP primers used in J. J) HIF-1α enrichment at HRE sites within Vegfa, Slc2a1, and Ldha promoters decreases after knockdown of PHD3 during 72 h HX (n = 3). Luciferase assays were performed with 3 technical replicates per experiment; quantitative RT-PCR assays were performed with 2 technical replicates per experiment. Data are means ± sem. *P < 0.05.
Activation of HIF-1α target genes requires binding of HIF-1α to HRE sites in promoters. We investigated the effects of PHD3 knockdown on HIF-1α enrichment using ChIP. PHD3 silencing resulted in decreased HIF-1α enrichment at HREs within Vegfa, Slc2a1, and Ldha promoters (Fig. 3I, J). This finding suggests that PHD3 is critical for stabilizing binding of HIF-1α to the promoters of a select set of target genes and activates their transcription through increasing HIF-1α C-TAD activity. To elucidate whether PHD3 is recruited to these HIF-1 targets, we attempted to perform ChIP using the only available ChIP-grade anti-PHD3 antibody; however, it failed to meet the stringent quality controls of the assay recommended by the kit’s manufacturer (ChIP-IT high-sensitivity kit; Active Motif). As a result, we cannot conclude with certainty whether PHD3 is co-recruited with HIF-1 to the select target gene promoters.
Expression of the M2, but not M1, isoform of PK is induced by HIF-1 in NP cells
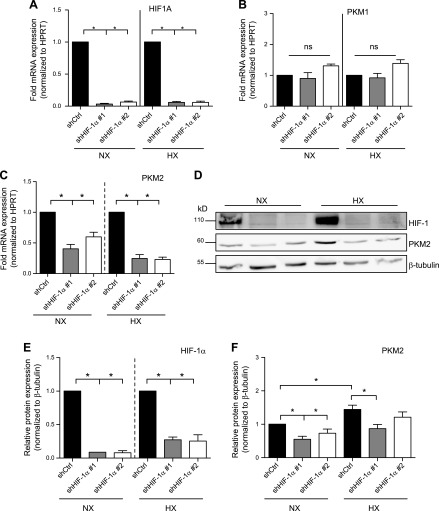
A recent report in cancer cell lines suggested that PKM2, in concert with PHD3, serves as a cofactor for HIF-1α through enhanced recruitment of p300 (14). However, the role of PKM2 in regulating HIF-1 activity and its relationship to HX in NP cells is unknown. We investigated the expression of Pkm1 and Pkm2 mRNA in NP cells. Expression of both isoforms increased during HX (Supplemental Fig. 2A, B). Expression of PKM2 at the protein level also increased at 24 h of HX (Supplemental Fig. 2C, D). We then investigated whether hypoxic induction of PKM isoforms is HIF-1 dependent. We performed stable knockdowns of HIF-1α in rat NP cells using lentiviral delivery of shRNA. HIF-1α mRNA showed a more than 90% reduction after shRNA transduction (Fig. 4A). Unlike Pkm1 (Fig. 4B), Pkm2 mRNA expression decreased after HIF-1α knockdown in both NX and HX (Fig. 4C). This observation contrasts with what has been reported in other cell types, where transcription of both isoforms is controlled by HIF-1α (14). Using Western blot analysis, we confirmed at the protein level a decrease in PKM2 expression after knockdown of HIF-1α in NP cells (Fig. 4D–F).
Figure 4.
Expression of the M2, but not M1, isoform of PK is induced by HIF-1 in NP cells. A) Measurement of HIF-1α mRNA after stable transduction of rat NP cells with 2 different lentivirally delivered shRNA sequences targeting HIF-1α (n = 4). B, C) mRNA expression of Pkm1 (B) and Pkm2 (C) in NP cells transduced with HIF-1α shRNAs (n = 3). D–F) Western blot (D) and corresponding densitometric analysis of HIF-1α (E) and PKM2 (F) after stable knockdown of HIF-1α (n = 4). Data are means ± sem. *P < 0.05.
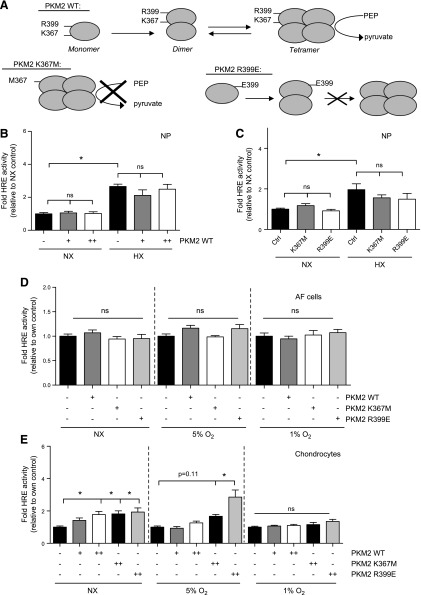
Overexpression of PKM2 does not affect HIF-1 activity in intervertebral disc cells, but does so in chondrocytes
Because HIF-1α controlled expression of PKM2 in NP cells, we wanted to investigate whether PKM2 in turn regulates HIF-1α activity, as reported recently in certain tumor types (14, 18, 28–31) and macrophages (Mϕs) (32, 33). We overexpressed WT, kinase-dead, or dimeric mutant of PKM2 (Fig. 5A) and measured HIF-1 activity using HRE reporter. Overexpression of WT (Fig. 5B) or mutant (Fig. 5C) PKM2 in NP cells had no effect on HIF activity in either NX or HX. Similar to NP cells, overexpression of any of these PKM2 constructs in AF cells did not affect HIF activity (Fig. 5D). On the other hand, in chondrocytes, overexpression of WT PKM2 resulted in increased HIF activity in NX (Fig. 5E). The magnitude of this effect was reduced by 5% O2, and reduced even further in 1% O2. The kinase-dead K367M mutant increased HIF activity in chondrocytes in NX to the same extent as WT PKM2. In addition, the R399E mutant, which fails to tetramerize and exists only in monomeric and dimeric forms, increased HIF activity in chondrocytes in NX and in 5% O2. This suggests that, unlike in NP cells, dimeric PKM2, independent of its protein kinase activity, can control HIF activity in chondrocytes.
Figure 5.
Overexpression of PKM2 does not affect HIF-1 activity in intervertebral disc cells, but does so in chondrocytes. A) WT, kinase-dead (K367M), and dimeric mutant (R399E) PKM2 constructs used in panels B–E. B, C) HIF-1 activity in rat NP cells after overexpression of WT (B) or mutant (C) PKM2 did not change. D) HIF-1 activity in AF cells remained unaffected after overexpression of WT or mutant PKM2. E) HIF-1 activity in chondrocytes after overexpression of WT or mutant PKM2 shows increase in NX and at 5% O2, only with the R399E mutant. Hypoxic culture for all experiments was 24 h. Data are means ± sem of 3 independent experiments, performed with 3 technical replicates per experiment. *P < 0.05.
PKM2 at physiologic levels does not act as an HIF-1 cofactor in NP cells and chondrocytes
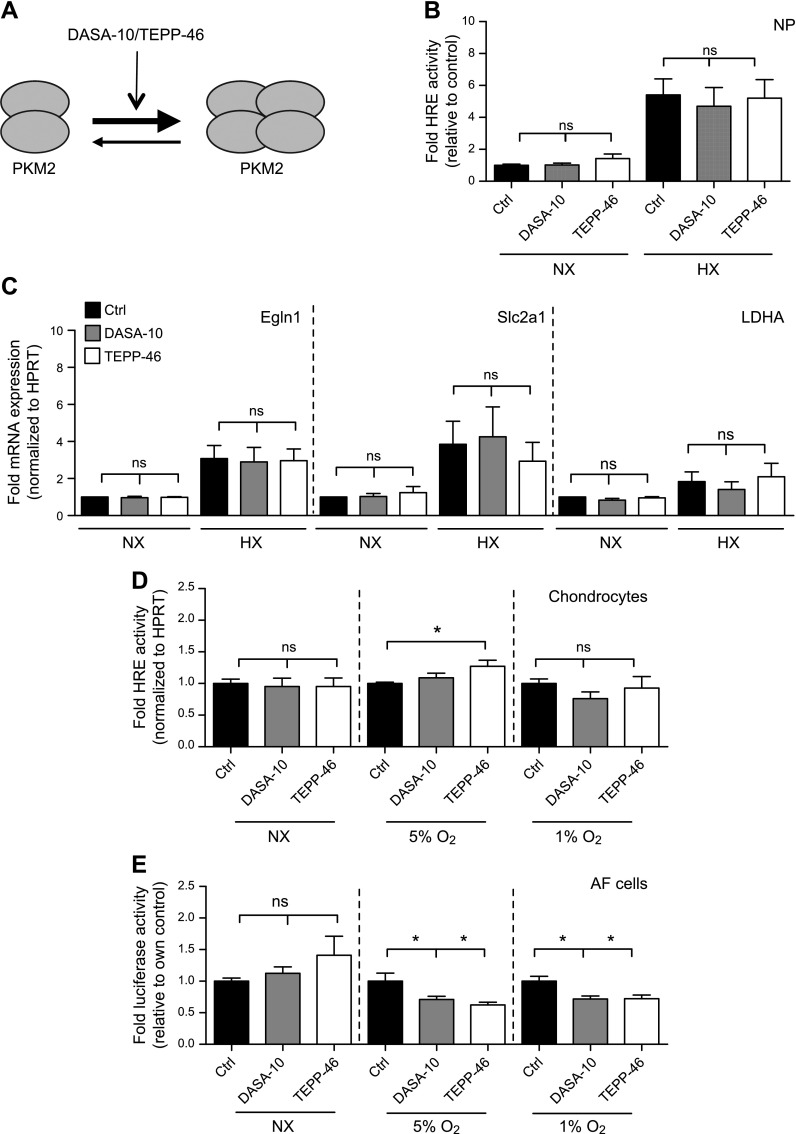
Although overexpression of PKM2 had no effect on HIF-1 activity in NP cells (Fig. 5B, C), we wanted to rule out the possibility of a saturation effect, so we sought to modulate endogenous PKM2 activity without changing its levels. To this end, we used 2 well-characterized and widely used small molecules, DASA-10 and TEPP-46, that stabilize the PKM2 tetramer and thus promote its PK activity (32, 34). Consequently, in other cell types this stabilization has been shown to decrease HIF-1 activity, in that PKM2 functions as a cofactor for HIF-1 in its dimeric and monomeric forms (Fig. 6A). Irrespective of oxygen tension, treatment of NP cells with either DASA-10 or TEPP-46 had no effect on HRE reporter activity (Fig. 6B), and on expression of Egln1, Slc2a1, or Ldha mRNA (Fig. 6C), even though expression of these transcripts had shown significant decrease upon PHD3 knockdown (Fig. 3D). These findings suggest that PHD3 regulation of HIF-1 activity in NP cells does not require PKM2. We then assessed the effect of DASA-10 and TEPP-46 on HIF-1 activity in chondrocytes and AF cells. Although overexpression of PKM2 resulted in an increase in HRE activity in chondrocytes (Fig. 5E), stabilization of the endogenous PKM2 tetramers resulted in no measurable decrease in HIF-1 activity in any of the oxygen tensions tested (Fig. 6D). This finding suggests that, similar to NP cells, PKM2 at its physiologic levels does not act as a cofactor for HIF-1 in chondrocytes. Furthermore, treatment of AF cells with DASA-10 and TEPP-46 had no effect on HIF activity in NX, but resulted in a significant decrease in HRE reporter activity in 5 and 1% oxygen (Fig. 6E). These results indicate that the potential of PKM2 to act as a cofactor for HIF-1 is very cell-type specific.
Figure 6.
Stabilization of PKM2 tetramer using small molecular activators has no effect on HIF-1 activity in NP cells or chondrocytes. A) Mechanism of action of the small molecular activators DASA-10 and TEPP-46 used in panels B–E. B) HIF activity in rat NP cells after treatment with DASA-10 (20 μM) or TEPP-46 (50 μM) for 24 h (n = 3). C) mRNA expression of HIF-1 targets in NP cells showed no change after treatment with DASA-10 (20 μM) or TEPP-46 (50 μM) during NX and HX (n = 3). D) HIF-1 activity in chondrocytes did not decrease after stabilization of PKM2 tetramer by DASA-10 (20 μM) or TEPP-46 (50 μM) treatment for 24 h in 5 and 1% Po2 (n = 4). E) HIF-1 activity in rat AF cells after treatment with DASA-10 (20 μM) or TEPP-46 (50 μM) for 24 h decreases in 5 and 1% Po2 (n = 3). Luciferase assays were performed with 3 technical replicates per experiment. Data are means ± sem. *P < 0.05.
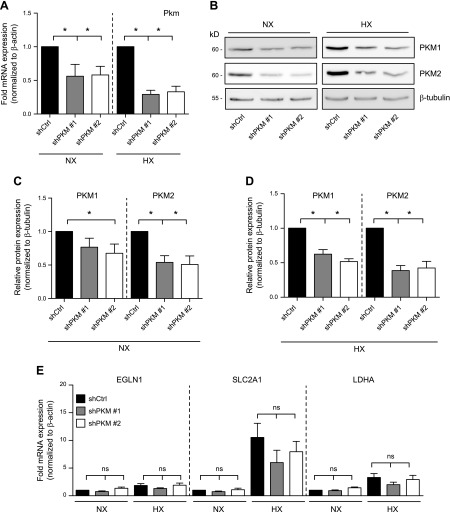
To complement PKM2 activator studies and to further support the idea that PKM2 does not control HIF-1α activity in NP cells, we silenced PKM in human NP cells using lentivirally delivered shRNA. Knockdown of PKM resulted in a significant decrease in mRNA transcribed from the PKM locus (PKM1 and PKM2) in both NX and HX (Fig. 7A). We confirmed the knockdown of PKM1 and -2 at the protein level by Western blot (Fig. 7B–D). Hypoxic induction of HIF-1 targets EGLN1, SLC2A1, and LDHA was unaffected after silencing of PKM2 (Fig. 7E). These genetic loss-of-function experiments, along with DASA-10 and TEPP-46 results, strongly support that PKM2 does not function as a regulator of HIF-1 activity in NP cells.
Figure 7.
PKM2 silencing in NP cells does not control hypoxic expression of HIF-1 targets. A) Targeting of the Pkm locus in human NP cells by 2 independent lentivirally delivered shRNA sequences resulted in decreased Pkm mRNA expression (n = 5). B–D) Western blot (B) and corresponding densitometric analysis (C, D) shows that both PKM1 and -2 levels are suppressed by either shRNA in NX (C) and HX (D) (n = 5). E) mRNA expression of HIF-1 target genes after PKM knockdown in NP cells (n = 5). Quantitative RT-PCR assays were performed with 2 technical replicates per experiment. Data are means ± sem. *P < 0.05.
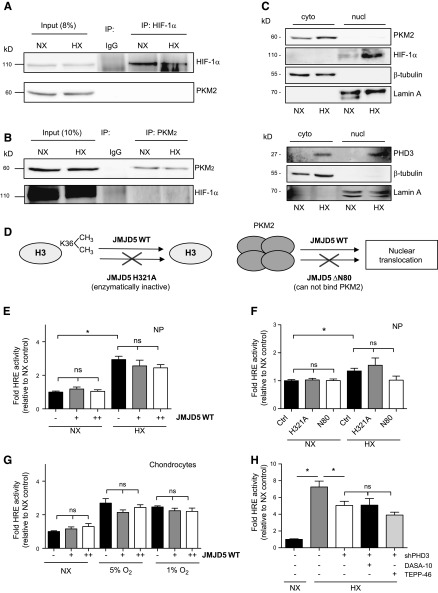
To gain further insights into the cell type specificity of this pathway, we investigated whether PKM2 and HIF-1α interact in NP cells. We immunoprecipitated HIF-1α in NP cells in both NX and HX, but were unable to detect PKM2 in the pull-down assay (Fig. 8A). Similarly, we pulled down PKM2 in NP cells and did not find association with HIF-1α (Fig. 8B). This observation is unique to NP cells, given that association between these 2 proteins has been reported in several cell types (14, 29). Because the association between HIF-1 and PKM2 is expected to occur in the nuclear compartment, we also examined the subcellular localization of PKM2 in NP cells. In both NX and HX, whereas HIF-1α is predominantly found in the nucleus, PKM2 is mostly localized within the cytosolic compartment (Fig. 8C), indicating a more traditional role as a glycolytic enzyme. PHD3 was localized to both compartments in NP cells (Fig. 8C), in agreement with a previous report (11). The histone demethylase JMJD5 has recently been proposed to promote nuclear translocation of PKM2 and subsequently promote its association with HIF-1α (18). To determine whether JMJD5 was a limiting factor and, if overexpressed, it could promote the PKM2-HIF-1α axis in NP cells and chondrocytes, we transfected cells with a WT, a demethylase-deficient mutant (H321A), or a truncated form of JMJD5 that does not associate with PKM2 (ΔN80) (Fig. 8D) (18). Irrespective of oxygen tension, overexpression of WT (Fig. 8E) or mutant (Fig. 8F) JMJD5 in NP cells did not increase HIF activity. Similarly, overexpression of WT JMJD5 in chondrocytes resulted in no significant increase in HIF-1 activity (Fig. 8G).
Figure 8.
PKM2 and HIF-1 do not interact in NP cells. A) Western blot analysis after immunoprecipitation of HIF-1α shows that HIF-1α does not interact with PKM2 in rat NP cells. Image is representative of 3 independent experiments. B) Western blot analysis after immunoprecipitation of PKM2 shows no evidence of PKM2-HIF-1α interaction in NP cells. Image is representative of 3 independent experiments. C) Western blot analysis of HIF-1α, PKM2, and PHD3 using cytoplasmic and nuclear fractions of NP cells. Images are representative of 3 independent experiments. D) Schematic of WT, demethylase deficient (H321A), and truncated (ΔN80, lacks PKM2 binding capability) JMJD5 constructs used in panels E–G. E, F) HIF activity in NP cells after overexpression of WT (E) or mutant (F) JMJD5 constructs (n = 3). G) HIF activity in chondrocytes was also unaffected after overexpression of WT or mutant JMJD5 (n = 4). H) Measurement of HRE reporter activity in PHD3-silenced NP cells after pretreatment with PKM2 stabilizers (n = 3). Luciferase assays were performed with 3 technical replicates per experiment. Data are means ± sem. *P < 0.05.
To confirm that PHD3 functions as a transcriptional coactivator for HIF-1α in NP cells independent of PKM2, we silenced PHD3 in NP cells after pretreatment with PKM2 stabilizers and measured HIF activity. Even in the presence of PKM2 stabilizers, knockdown of PHD3 resulted in decreased HIF-1 activity in HX (Fig. 8H). This result suggests that PHD3 does not require monomeric or dimeric PKM2 for it to serve as a cofactor for HIF-1.
DISCUSSION
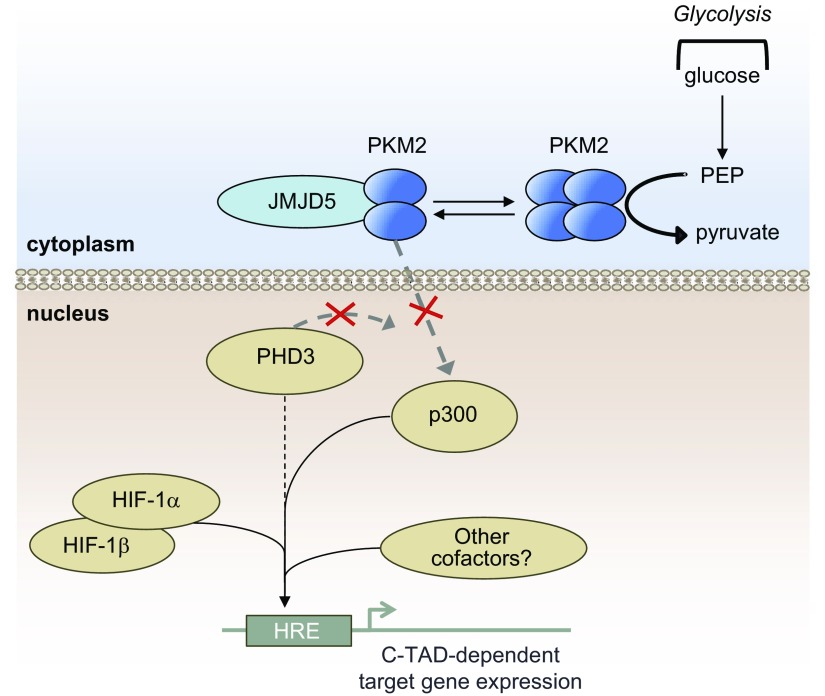
It has been shown that the regulation of HIF-1α in the hypoxic NP niche of the intervertebral disc is rather unique. In NP cells, although PHDs retain some enzymatic activity in physiologically relevant oxygen tensions (1% O2), PHD2 regulates HIF-1α stability only to a certain extent. On the other hand, although PHD3 has little effect on HIF-1α stability, there is some indication that it may contribute to maintenance of HIF-1α activity (11). However, to this point, it is not known how PHD3 controls HIF-1α target gene expression and transcriptional activity in NP cells. There have been several reports investigating noncanonical roles of the PHDs both dependent on (35–39) and independent of (40–42) their enzymatic function. Other work has shown nontraditional roles of PHDs in NP cells as cofactors of RelA in the NF-κB signaling pathway (43, 44). In other tissues, it has also been reported that PHD3 acts as either a transcriptional activator (in a PKM2-dependent manner) (14), or a transcriptional repressor (in a small ubiquitin–like modifier-dependent manner) (15) for HIF-1. Based on these reports, and considering the unequivocal importance of maintaining proper HIF-1 signaling for NP cell function and survival (7), we investigated the role of PHD3 in regulation of HIF-1 activity in these cells. We show here for the first time that in NP cells PHD3 acts as a cofactor for HIF-1, and this cofactor function is independent of the PKM2-JMJD5 axis (14, 18) (Fig. 9).
Figure 9.
PHD3 serves as a hypoxic transcriptional coactivator of HIF-1α C-TAD in NP cells in a p300-dependent and PKM2/JMJD5-independent manner. Proposed model of regulation of HIF-1 activity by PHD3 in NP cells. PHD3 serves as a hypoxic coactivator of HIF-1α in NP cells, directly or indirectly promoting HIF-1 enrichment at target loci and activating C-TAD-dependent targets, independent of PKM2/JMJD5. PKM2 in NP cells does not have cofactor function for HIF-1.
Using a loss-of-function approach, we clearly show that PHD3 in NP cells promotes HIF-1α recruitment to target loci and controls hypoxic activity of primarily the HIF-1 C-TAD. Knockdown of PHD3 was unable to affect activity of the minimal C-TAD (aa 786–826), but of a construct that also expressed a portion of the inhibitory domain (ID) located between N- and C-TAD with the minimal C-TAD. This finding suggested that PHD3 controls C-TAD activity through targeting the ID. Moreover, it clearly suggests that the role of PHD3 as a cofactor for HIF-1α is independent of the ODD, where it is traditionally reported to interact.
Although HIF-1α contains 2 transactivation domains, surprisingly little is known regarding the difference between the 2 domains and the target gene specificity they confer. There is evidence that certain HIF-1 targets are more dependent on the C-TAD (based on their relative susceptibility to regulation by FIH-1), whereas other HIF-1 targets are more dependent on the N-TAD (based on their relative resistance to regulation by FIH-1) (45). This evidence helps explain the specificity of target genes affected by PHD3 knockdown in NP cells: genes previously reported by Dayan et al. (45) as more C-TAD dependent (e.g., Vegfa, Slc2a1, and Ldha) are decreased upon knockdown of PHD3 in HX, whereas genes previously reported as more N-TAD dependent (e.g., Pgk1, Eno1, and Gapdh) are unaffected by PHD3 knockdown. This observation is peculiar that a previous report in cancer cells suggests that loss of PHD3 results in a global reduction in HIF-1 activity (14), our data show that in NP cells, PHD3 controls hypoxic expression of select HIF-1 targets likely driven by C-TAD activity. These results show that PHD3 is necessary for complete hypoxic inducibility of C-TAD-driven HIF-1 targets in NP cells. It is important to note that at its physiologic levels in NP cells, PHD3 does not promote HIF-1α degradation irrespective of oxygen tension (10, 11). Therefore, any effect on expression of HIF-1 targets is independent of changes in HIF-1α protein level.
Our data show that PHD3 promotes HIF-1 transcription in NP cells during HX, whereas knockdown of PHD3 in NX has little effect on HIF-1 activity, probably because, in NP cells, baseline levels of PHD3 in NX are lower, but show a robust increase in HX (11). However, in NX, overexpression of PHD3 does not increase HIF-1α C-TAD activity, suggesting that the function of PHD3 as HIF-1 cofactor in NP cells is partially dependent on oxygen tension. Because in NP cells PHD3 maintains enzymatic activity regardless of Po2, it is unlikely that its cofactor function is dependent only on hydroxylase activity. Rather, it is not unreasonable to assume that PHD3 mediates its effects through another cofactor present primarily in HX, or is subject to NX-specific inhibition. We could not confirm in this study whether PHD3 was directly recruited to select HIF-1 target promoters or indirectly modulated HIF-1 activity; future studies will be designed to investigate this possibility.
The M2 isoform of PK has received much attention lately for its apparent role in promoting the Warburg effect in tumors (46). In addition, it is reported that PKM2 acts as a cofactor for HIF-1 in cancer cells after being hydroxylated in a PHD3-dependent fashion (14). Given that NP cells are obligate glycolytic (4), and our finding that PHD3 promotes hypoxic expression of HIF-1 targets in NP cells, we asked the question of whether PKM2 was involved in this regulation. Using both gain- and loss-of-function assays, we show that the HIF-PKM2 relationship is cell-type specific. In NP cells, PKM2 did not have an effect on HIF-1 transcriptional activity. Whereas in chondrocytes, although forced expression of PKM2 increased HIF reporter activity in a manner dependent on oxygen tension, pharmacologically stabilizing tetrameric PKM2 (which serves as a traditional PK and fails to associate with HIF-1α), did not decrease HIF activity. Furthermore, in NP cells, there was no detectable association between HIF-1α and PKM2, and -2 was mostly excluded from the nuclear compartment, where its presence is required for cofactor function. The data support the argument that it plays no role in controlling HIF-1 activity in NP cells. It is important to note that most of the previous studies investigated the role of PKM2 in regulation of HIF-1 in cancer cell lines (14, 18, 28–31, 47). In contrast, the NP and cartilage niches are physiologically hypoxic and robustly express HIF-1α (6, 48, 49). Moreover, HIF-1α shows normoxic stability in NP cells and, to a certain extent, in articular chondrocytes (4, 5, 50), a finding that clearly suggests that in nontransformed cells from physiologically hypoxic niches, there is a fundamentally different and unique regulation of HIF-1α activity that does not involve PKM2. This hypothesis is further supported by our observation that JMJD5 has no effect on HIF-1 activity in NP cells, as well as in chondrocytes, contrasting what has been previously reported in tumor cell lines (18).
In contrast to NP, the AF niche is relatively less hypoxic, and tissue levels of HIF-1α levels are lower (6). It is therefore not surprising that normoxic HIF-1α activity in AF cells remained unaffected by PHD3 knockdown, as well as by PKM2 overexpression and tetrameric stabilization. However, at 5% oxygen, when both partial HIF-1α stabilization and PHD3 enzymatic activity are expected, suppression of PHD3 decreased HIF activity in AF cells, but not at 1%, when PHD3 was probably inactive, suggesting that the effect of PHD3 on HIF-1α activity requires hydroxylase function. On the other hand, treatment with PKM2 stabilizers decreased HIF-1 activity in both 5 and 1% oxygen, suggesting that the effects of PHD3 and PKM2 on HIF-1 activity in AF cells could be independent of one another. Further support to this premise comes from recent observations that, while stabilization of PKM2 tetramers as well as knockdown of PKM2 resulted in decreased HIF-1 activity in Mϕs (32, 33), PHD3−/− Mϕs show no change in HIF-1 target gene expression (51). This shows that effects of PKM2 on HIF-1 activity in primary Mϕs are independent of PHD3. It is also important to note that although several studies investigated the role of PKM2 as a cofactor for HIF-1α (18, 28–33), only a single study to date showed requirement of PKM2 for PHD3-mediated control of HIF-1 activity (14), something which we did not observe in NP cells. It is therefore not unreasonable to assume that in both NP and AF cells, regulation of HIF-1α activity by PHD3 is independent of the PKM2-JMJD5 axis.
The novel observation that PHD3 serves to control HIF-1 target gene expression in vivo in the NP niche is expected to have important physiologic consequences. Analysis of PHD3−/− mice strongly supported in vitro findings concerning role of PHD3 in HIF-1 target gene specificity. Previous studies have shown that PHD3 expression in NP is responsive to HIF-1α, and expression is induced in prolonged HX (11). Because targeted deletion of HIF-1α in NP compartment results in increased cell death and subsequent degeneration (7), it is hypothesized that in vivo loss of PHD3 in NP will also contribute over time to degenerative changes of the disc through compromised HIF-1 signaling. This hypothesis was supported by the observation that there is increased incidence of disc degeneration in 12.5-mo-old null mice. It is not too surprising that minimal histologic change in NP tissue architecture is seen at a 5-mo-old time point, because PHD3 is one of many coactivators for HIF-1α and loss of PHD3 is not expected to phenocopy loss of HIF-1α from the NP compartment, especially at a young age (7). Furthermore, degeneration of the intervertebral disc is a progressive and chronic disease, and ageing is one of the important risk factors for disc degeneration (52). It has been shown that loss of critical matrix protein connective tissue growth factor from the NP compartment in mice accelerates degenerative changes in the disc, but the gross morphologic changes are not evident until after 1 yr of age (24). This notion is further supported by a recent study that has shown correlation between NP degeneration and decreased HIF-1α levels (53), and another study that demonstrates a positive correlation between NP degeneration and decreased PHD3 expression (11). It is evident that HIF-1-PHD3 forms a positive feedback loop in NP cells, and that the functioning of this circuit is central to controlling NP cell function. We are currently investigating whether targeting this axis can be exploited therapeutically for treating disc disease.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the U.S. National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases Grants R01AR05565, R01AR064733, F30AR066506 (to Z.R.S.), and T32AR052273 (to E.S.S.). The authors declare no conflicts of interest.
Glossary
- AF
annulus fibrosus
- C-TAD
C-terminal TAD
- ChIP
chromatin immunoprecipitation
- DASA
N,N′-diarylsulfonamide
- ENO1
enolase 1
- FIH
factor inhibiting HIF
- FBS
fetal bovine serum
- GLUT
glucose transporter
- HIF
hypoxia inducible factor
- HRE
hypoxia response element
- HX
hypoxia
- JMJD
Jumonji domain-containing protein
- Krt19
keratin 19
- LDHA
lactate dehydrogenase A
- Mϕ
macrophage
- NP
nucleus pulposus
- N-TAD
N-terminal TAD
- NX
normoxia
- ODD
oxygen-dependent degradation domain
- PHD
prolyl hydroxylase
- PKM
pyruvate kinase muscle
- shRNA
short hairpin RNA
- TAD
transactivation domain
- TEPP
thieno[3,2-b]pyrrole[3,2-d]pyridazinone
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
Z. R. Schoepflin and E. S. Silagi performed and analyzed all the experiments and wrote the manuscript; and E. S. Silagi, I. M. Shapiro, and M. V. Risbud designed the experiments, helped in data interpretation, wrote the manuscript, and secured funding.
REFERENCES
- 1.Risbud M. V., Schipani E., Shapiro I. M. (2010) Hypoxic regulation of nucleus pulposus cell survival: from niche to notch. Am. J. Pathol. 176, 1577–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.U.S. Burden of Disease Collaborators (2013) The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA 310, 591–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scaal M. (2016) Early development of the vertebral column. Semin. Cell Dev. Biol. 49, 83–91 [DOI] [PubMed] [Google Scholar]
- 4.Agrawal A., Guttapalli A., Narayan S., Albert T. J., Shapiro I. M., Risbud M. V. (2007) Normoxic stabilization of HIF-1alpha drives glycolytic metabolism and regulates aggrecan gene expression in nucleus pulposus cells of the rat intervertebral disk. Am. J. Physiol. Cell Physiol. 293, C621–C631 [DOI] [PubMed] [Google Scholar]
- 5.Risbud M. V., Guttapalli A., Stokes D. G., Hawkins D., Danielson K. G., Schaer T. P., Albert T. J., Shapiro I. M. (2006) Nucleus pulposus cells express HIF-1 alpha under normoxic culture conditions: a metabolic adaptation to the intervertebral disc microenvironment. J. Cell. Biochem. 98, 152–159 [DOI] [PubMed] [Google Scholar]
- 6.Rajpurohit R., Risbud M. V., Ducheyne P., Vresilovic E. J., Shapiro I. M. (2002) Phenotypic characteristics of the nucleus pulposus: expression of hypoxia inducing factor-1, glucose transporter-1 and MMP-2. Cell Tissue Res. 308, 401–407 [DOI] [PubMed] [Google Scholar]
- 7.Merceron C., Mangiavini L., Robling A., Wilson T. L., Giaccia A. J., Shapiro I. M., Schipani E., Risbud M. V. (2014) Loss of HIF-1α in the notochord results in cell death and complete disappearance of the nucleus pulposus. PLoS One 9, e110768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greer S. N., Metcalf J. L., Wang Y., Ohh M. (2012) The updated biology of hypoxia-inducible factor. EMBO J. 31, 2448–2460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaakkola P., Mole D. R., Tian Y. M., Wilson M. I., Gielbert J., Gaskell S. J., von Kriegsheim A., Hebestreit H. F., Mukherji M., Schofield C. J., Maxwell P. H., Pugh C. W., Ratcliffe P. J. (2001) Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292, 468–472 [DOI] [PubMed] [Google Scholar]
- 10.Fujita N., Chiba K., Shapiro I. M., Risbud M. V. (2012) HIF-1α and HIF-2α degradation is differentially regulated in nucleus pulposus cells of the intervertebral disc. J. Bone Miner. Res. 27, 401–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujita N., Markova D., Anderson D. G., Chiba K., Toyama Y., Shapiro I. M., Risbud M. V. (2012) Expression of prolyl hydroxylases (PHDs) is selectively controlled by HIF-1 and HIF-2 proteins in nucleus pulposus cells of the intervertebral disc: distinct roles of PHD2 and PHD3 proteins in controlling HIF-1α activity in hypoxia. J. Biol. Chem. 287, 16975–16986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schoepflin Z. R., Shapiro I. M., Risbud M. V. (2016) Class I and IIa HDACs mediate HIF-1α stability through PHD2-dependent mechanism, while HDAC6, a class IIb member, promotes HIF-1α transcriptional activity in nucleus pulposus cells of the intervertebral disc. J. Bone Miner. Res. 31, 1287–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirose Y., Johnson Z. I., Schoepflin Z. R., Markova D. Z., Chiba K., Toyama Y., Shapiro I. M., Risbud M. V. (2014) FIH-1-Mint3 axis does not control HIF-1 transcriptional activity in nucleus pulposus cells. J. Biol. Chem. 289, 20594–20605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo W., Hu H., Chang R., Zhong J., Knabel M., O’Meally R., Cole R. N., Pandey A., Semenza G. L. (2011) Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell 145, 732–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Núñez-O’Mara A., Gerpe-Pita A., Pozo S., Carlevaris O., Urzelai B., Lopitz-Otsoa F., Rodríguez M. S., Berra E. (2015) PHD3-SUMO conjugation represses HIF1 transcriptional activity independently of PHD3 catalytic activity. J. Cell Sci. 128, 40–49 [DOI] [PubMed] [Google Scholar]
- 16.Israelsen W. J., Vander Heiden M. G. (2015) Pyruvate kinase: function, regulation and role in cancer. Semin. Cell Dev. Biol. 43, 43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lincet H., Icard P. (2015) How do glycolytic enzymes favour cancer cell proliferation by nonmetabolic functions? Oncogene 34, 3751–3759 [DOI] [PubMed] [Google Scholar]
- 18.Wang H.-J., Hsieh Y. J., Cheng W. C., Lin C. P., Lin Y. S., Yang S. F., Chen C. C., Izumiya Y., Yu J. S., Kung H. J., Wang W. C. (2014) JMJD5 regulates PKM2 nuclear translocation and reprograms HIF-1α-mediated glucose metabolism. Proc. Natl. Acad. Sci. USA 111, 279–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hosios A. M., Fiske B. P., Gui D. Y., Vander Heiden M. G. (2015) Lack of evidence for PKM2 protein kinase activity. Mol. Cell 59, 850–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johansen J. L., Sager T. N., Lotharius J., Witten L., Mørk A., Egebjerg J., Thirstrup K. (2010) HIF prolyl hydroxylase inhibition increases cell viability and potentiates dopamine release in dopaminergic cells. J. Neurochem. 115, 209–219 [DOI] [PubMed] [Google Scholar]
- 21.Bishop T., Gallagher D., Pascual A., Lygate C. A., de Bono J. P., Nicholls L. G., Ortega-Saenz P., Oster H., Wijeyekoon B., Sutherland A. I., Grosfeld A., Aragones J., Schneider M., van Geyte K., Teixeira D., Diez-Juan A., Lopez-Barneo J., Channon K. M., Maxwell P. H., Pugh C. W., Davies A. M., Carmeliet P., Ratcliffe P. J. (2008) Abnormal sympathoadrenal development and systemic hypotension in PHD3-/- mice. Mol. Cell. Biol. 28, 3386–3400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Risbud M. V., Schoepflin Z. R., Mwale F., Kandel R. A., Grad S., Iatridis J. C., Sakai D., Hoyland J. A. (2015) Defining the phenotype of young healthy nucleus pulposus cells: recommendations of the Spine Research Interest Group at the 2014 annual ORS meeting. J. Orthop. Res. 33, 283–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson J. P., Pearce R. H., Schechter M. T., Adams M. E., Tsang I. K., Bishop P. B. (1990) Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine 15, 411–415 [DOI] [PubMed] [Google Scholar]
- 24.Bedore J., Sha W., McCann M. R., Liu S., Leask A., Séguin C. A. (2013) Impaired intervertebral disc development and premature disc degeneration in mice with notochord-specific deletion of CCN2. Arthritis Rheum. 65, 2634–2644 [DOI] [PubMed] [Google Scholar]
- 25.Goldring M. B., Birkhead J. R., Suen L. F., Yamin R., Mizuno S., Glowacki J., Arbiser J. L., Apperley J. F. (1994) Interleukin-1 beta-modulated gene expression in immortalized human chondrocytes. J. Clin. Invest. 94, 2307–2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang B. H., Zheng J. Z., Leung S. W., Roe R., Semenza G. L. (1997) Transactivation and inhibitory domains of hypoxia-inducible factor 1alpha. Modulation of transcriptional activity by oxygen tension. J. Biol. Chem. 272, 19253–19260 [DOI] [PubMed] [Google Scholar]
- 27.Semenza G. L., Jiang B. H., Leung S. W., Passantino R., Concordet J. P., Maire P., Giallongo A. (1996) Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J. Biol. Chem. 271, 32529–32537 [DOI] [PubMed] [Google Scholar]
- 28.Azoitei N., Becher A., Steinestel K., Rouhi A., Diepold K., Genze F., Simmet T., Seufferlein T. (2016) PKM2 promotes tumor angiogenesis by regulating HIF-1α through NF-κB activation. Mol. Cancer 15, 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giannoni E., Taddei M. L., Morandi A., Comito G., Calvani M., Bianchini F., Richichi B., Raugei G., Wong N., Tang D., Chiarugi P. (2015) Targeting stromal-induced pyruvate kinase M2 nuclear translocation impairs oxphos and prostate cancer metastatic spread. Oncotarget 6, 24061–24074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim D. J., Park Y. S., Kim N. D., Min S. H., You Y. M., Jung Y., Koo H., Noh H., Kim J. A., Park K. C., Yeom Y. I. (2015) A novel pyruvate kinase M2 activator compound that suppresses lung cancer cell viability under hypoxia. Mol. Cells 38, 373–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu Z., Zhao X., Huang L., Zhang T., Yang F., Xie L., Song S., Miao P., Zhao L., Sun X., Liu J., Huang G. (2013) Proviral insertion in murine lymphomas 2 (PIM2) oncogene phosphorylates pyruvate kinase M2 (PKM2) and promotes glycolysis in cancer cells. J. Biol. Chem. 288, 35406–35416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palsson-McDermott E. M., Curtis A. M., Goel G., Lauterbach M. A., Sheedy F. J., Gleeson L. E., van den Bosch M. W., Quinn S. R., Domingo-Fernandez R., Johnston D. G., Jiang J.-K., Israelsen W. J., Keane J., Thomas C., Clish C., Vander Heiden M., Xavier R. J., O’Neill L. A. (2015) Pyruvate kinase M2 regulates Hif-1α activity and IL-1β induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell Metab. 21, 65–80 [Erratum] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang L., Xie M., Yang M., Yu Y., Zhu S., Hou W., Kang R., Lotze M. T., Billiar T. R., Wang H., Cao L., Tang D. (2014) PKM2 regulates the Warburg effect and promotes HMGB1 release in sepsis. Nat. Commun. 5, 4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anastasiou D., Yu Y., Israelsen W. J., Jiang J. K., Boxer M. B., Hong B. S., Tempel W., Dimov S., Shen M., Jha A., Yang H., Mattaini K. R., Metallo C. M., Fiske B. P., Courtney K. D., Malstrom S., Khan T. M., Kung C., Skoumbourdis A. P., Veith H., Southall N., Walsh M. J., Brimacombe K. R., Leister W., Lunt S. Y., Johnson Z. R., Yen K. E., Kunii K., Davidson S. M., Christofk H. R., Austin C. P., Inglese J., Harris M. H., Asara J. M., Stephanopoulos G., Salituro F. G., Jin S., Dang L., Auld D. S., Park H. W., Cantley L. C., Thomas C. J., Vander Heiden M. G. (2012) Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis. Nat. Chem. Biol. 8, 839–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie L., Pi X., Mishra A., Fong G., Peng J., Patterson C. (2012) PHD3-dependent hydroxylation of HCLK2 promotes the DNA damage response. J. Clin. Invest. 122, 2827–2836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie L., Pi X., Townley-Tilson W. H., Li N., Wehrens X. H., Entman M. L., Taffet G. E., Mishra A., Peng J., Schisler J. C., Meissner G., Patterson C. (2015) PHD2/3-dependent hydroxylation tunes cardiac response to β-adrenergic stress via phospholamban. J. Clin. Invest. 125, 2759–2771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng X., Zhai B., Koivunen P., Shin S. J., Lu G., Liu J., Geisen C., Chakraborty A. A., Moslehi J. J., Smalley D. M., Wei X., Chen X., Chen Z., Beres J. M., Zhang J., Tsao J. L., Brenner M. C., Zhang Y., Fan C., DePinho R. A., Paik J., Gygi S. P., Kaelin W. G. Jr., Zhang Q. (2014) Prolyl hydroxylation by EglN2 destabilizes FOXO3a by blocking its interaction with the USP9x deubiquitinase. Genes Dev. 28, 1429–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moser S. C., Bensaddek D., Ortmann B., Maure J. F., Mudie S., Blow J. J., Lamond A. I., Swedlow J. R., Rocha S. (2013) PHD1 links cell-cycle progression to oxygen sensing through hydroxylation of the centrosomal protein Cep192. Dev. Cell 26, 381–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodriguez J., Pilkington R., Garcia Munoz A., Nguyen L. K., Rauch N., Kennedy S., Monsefi N., Herrero A., Taylor C. T., von Kriegsheim A. (2016) Substrate-trapped interactors of PHD3 and FIH cluster in distinct signaling pathways. Cell Rep. 14, 2745–2760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henze A.-T., Garvalov B. K., Seidel S., Cuesta A. M., Ritter M., Filatova A., Foss F., Dopeso H., Essmann C. L., Maxwell P. H., Reifenberger G., Carmeliet P., Acker-Palmer A., Acker T. (2014) Loss of PHD3 allows tumours to overcome hypoxic growth inhibition and sustain proliferation through EGFR. Nat. Commun. 5, 5582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fu J., Taubman M. B. (2013) EGLN3 inhibition of NF-κB is mediated by prolyl hydroxylase-independent inhibition of IκB kinase γ ubiquitination. Mol. Cell. Biol. 33, 3050–3061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Y., Zhang H. S., Fong G. H., Xi Q. L., Wu G. H., Bai C. G., Ling Z. Q., Fan L., Xu Y. M., Qin Y. Q., Yuan T. L., Sun H., Fang J. (2015) PHD3 stabilizes the tight junction protein occludin and protects intestinal epithelial barrier function. J. Biol. Chem. 290, 20580–20589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fujita N., Gogate S. S., Chiba K., Toyama Y., Shapiro I. M., Risbud M. V. (2012) Prolyl hydroxylase 3 (PHD3) modulates catabolic effects of tumor necrosis factor-α (TNF-α) on cells of the nucleus pulposus through co-activation of nuclear factor κB (NF-κB)/p65 signaling. J. Biol. Chem. 287, 39942–39953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li J., Yuan W., Jiang S., Ye W., Yang H., Shapiro I. M., Risbud M. V. (2015) Prolyl-4-hydroxylase domain protein 2 controls NF-κB/p65 transactivation and enhances the catabolic effects of inflammatory cytokines on cells of the nucleus pulposus. J. Biol. Chem. 290, 7195–7207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dayan F., Roux D., Brahimi-Horn M. C., Pouyssegur J., Mazure N. M. (2006) The oxygen sensor factor-inhibiting hypoxia-inducible factor-1 controls expression of distinct genes through the bifunctional transcriptional character of hypoxia-inducible factor-1α. Cancer Res. 66, 3688–3698 [DOI] [PubMed] [Google Scholar]
- 46.Iqbal M. A., Gupta V., Gopinath P., Mazurek S., Bamezai R. N. K. (2014) Pyruvate kinase M2 and cancer: an updated assessment. FEBS Lett. 588, 2685–2692 [DOI] [PubMed] [Google Scholar]
- 47.De Wit R. H., Mujić-Delić A., van Senten J. R., Fraile-Ramos A., Siderius M., Smit M. J. (2016) Human cytomegalovirus encoded chemokine receptor US28 activates the HIF-1α/PKM2 axis in glioblastoma cells. Oncotarget 7, 67966–67985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Provot S., Zinyk D., Gunes Y., Kathri R., Le Q., Kronenberg H. M., Johnson R. S., Longaker M. T., Giaccia A. J., Schipani E. (2007) Hif-1alpha regulates differentiation of limb bud mesenchyme and joint development. J. Cell Biol. 177, 451–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schipani E., Ryan H. E., Didrickson S., Kobayashi T., Knight M., Johnson R. S. (2001) Hypoxia in cartilage: HIF-1alpha is essential for chondrocyte growth arrest and survival. Genes Dev. 15, 2865–2876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coimbra I. B., Jimenez S. A., Hawkins D. F., Piera-Velazquez S., Stokes D. G. (2004) Hypoxia inducible factor-1 alpha expression in human normal and osteoarthritic chondrocytes. Osteoarthritis Cartilage 12, 336–345 [DOI] [PubMed] [Google Scholar]
- 51.Swain L., Wottawa M., Hillemann A., Beneke A., Odagiri H., Terada K., Endo M., Oike Y., Farhat K., Katschinski D. M. (2014) Prolyl-4-hydroxylase domain 3 (PHD3) is a critical terminator for cell survival of macrophages under stress conditions. J. Leukoc. Biol. 96, 365–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hassett G., Hart D. J., Manek N. J., Doyle D. V., Spector T. D. (2003) Risk factors for progression of lumbar spine disc degeneration: the Chingford Study. Arthritis Rheum. 48, 3112–3117 [DOI] [PubMed] [Google Scholar]
- 53.Chen S., Fang X. Q., Wang Q., Wang S. W., Hu Z. J., Zhou Z. J., Xu W. B., Wang J. Y., Qin A., Fan S. W. (2016) PHD/HIF-1 upregulates CA12 to protect against degenerative disc disease: a human sample, in vitro and ex vivo study. Lab. Invest. 96, 561–569 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.