Abstract
Preterm birth (PTB) is the leading cause of neonatal mortality and morbidity, with few prevention and treatment options. Uterine contraction is a central feature of PTB, so gaining new insights into the mechanisms of this contraction and consequently identifying novel targets for tocolytics are essential for more successful management of PTB. Here we report that myometrial cells from human and mouse express bitter taste receptors (TAS2Rs) and their canonical signaling components (i.e., G-protein gustducin and phospholipase C β2). Bitter tastants can completely relax myometrium precontracted by different uterotonics. In isolated single mouse myometrial cells, a phenotypical bitter tastant (chloroquine, ChQ) reverses the rise in intracellular Ca2+ concentration ([Ca2+]i) and cell shortening induced by uterotonics, and this reversal effect is inhibited by pertussis toxin and by genetic deletion of α-gustducin. In human myometrial cells, knockdown of TAS2R14 but not TAS2R10 inhibits ChQ’s reversal effect on an oxytocin-induced rise in [Ca2+]i. Finally, ChQ prevents mouse PTBs induced by bacterial endotoxin LPS or progesterone receptor antagonist mifepristone more often than current commonly used tocolytics, and this prevention is largely lost in α-gustducin-knockout mice. Collectively, our results reveal that activation of the canonical TAS2R signaling system in myometrial cells produces profound relaxation of myometrium precontracted by a broad spectrum of contractile agonists, and that targeting TAS2Rs is an attractive approach to developing effective tocolytics for PTB management.—Zheng, K., Lu, P., Delpapa, E., Bellve, K., Deng, R., Condon, J. C., Fogarty, K., Lifshitz, L. M., Simas, T. A. M., Shi, F., ZhuGe, R. Bitter taste receptors as targets for tocolytics in preterm labor therapy.
Keywords: uterine smooth muscle, relaxation, chloroquine, G-protein coupled receptor
Preterm birth (PTB), defined as birth occurring before 37 wk of gestation, is the leading cause of infant morbidity and mortality. Worldwide, nearly 15 million PTBs and 1 million neonatal deaths resulting from PTBs occur annually. In the United States, 1 in 10 pregnancies ends before term, affecting over 500,000 newborns each year and accounting for over 70% of fetal morbidity (1). Moreover, PTB is a major risk factor for many lifelong disabilities and diseases in the progeny, such as diabetes and developmental disorders (2–4). The human and financial impact of PTB on the family and the health care system is staggering. Despite the magnitude, impact, and costs of PTB, relatively few advances have been made in treatment and prevention, particularly for early PTBs occurring before 34 wk of gestation. A major reason for this may be the complexity in the etiology of PTB. So far, it is known that this disorder can be linked to conditions as diverse as infection, inflammation, stress, and genetic mutations (5, 6). Moreover, these conditions are often not diagnosed before preterm labor begins.
Yet regardless of the initiating factor, PTB can ultimately be linked to increased myometrial contractility (7–9). Therefore, unless high risk factors to the fetus or the mother are clearly identified, alleviating myometrial contractions with tocolytics has been one of the basic mechanisms used to delay or even prevent PTB. With tocolytics, pregnant women may be able to be transferred to the most appropriate hospital and treated with corticosteroids prenatally. So far several classes of tocolytics have been used clinically to delay preterm labor. Among them, nifedipine targets voltage-dependent Ca2+ channels to stop Ca2+ influx, leading to myometrial relaxation; β-adrenergic receptor agonists increase cAMP, which in turn decreases cytosolic Ca2+ and inhibits the contractile apparatus, hence relaxing myometrium; and indomethacin, a cyclooxygenase inhibitor, prevents the conversion of arachidonic acid to prostaglandins, which is a critical hormone for parturition. Magnesium sulfate, despite having long been used to delay preterm labor, has unclear molecular targets. Although these tocolytics are in clinical use, they have many adverse effects or safety concerns for mother, fetus, or both, and none of them are recommended by the U.S. Food and Drug Administration (FDA) for PTB prevention. Moreover, they are often not effective in extending pregnancy for more than 48 h, and they have not been able to reduce the incidence of PTBs (7, 9, 10). Hence, finding new molecular targets that mediate myometrial relaxation and developing new classes of effective and safe tocolytics are necessary for better PTB management.
Recently, bitter taste receptors (TAS2Rs, or T2R family) have become an attractive target for developing smooth muscle relaxants (11, 12). Traditionally, bitter taste, 1 of 5 basic taste qualities, is thought to guide organisms to avoid harmful toxins and noxious substances, and is critical to animal and human survival. It has long been known that specialized epithelial cells in the taste buds of the tongue detect bitter tastants via TAS2Rs and initiate the sensation of bitterness (13–15). However, emerging evidence has gradually brought attention to cells in extraoral tissues, where bitter tastants can generate different biologic responses tailored to their location (16). In airway smooth muscle, bitter tastants appear to activate these receptors to fully relax precontracted airways (11, 12). It remains little explored whether bitter tastants can exert such potent relaxation in other types of smooth muscle. Given the central role of myometrial contraction in PTB, and lack of any major advancement in tocolytics for PTB in the last 3 decades (17), we were prompted to determine whether TAS2R activation can relax myometrium and whether these receptors can serve as targets for tocolytics for preterm labor. In this study, we used genetic approaches to firmly establish that activation of bitter taste receptors can potently relax contracted myometrium by inhibiting uterotonic-induced Ca2+ signaling. Furthermore, we demonstrated that this form of relaxation is more effective than currently used tocolytics in preventing PTBs in 2 clinically relevant mouse models of PTB. These findings raise the possibility that a new class of tocolytics targeting TAS2Rs could be developed for better PTB treatment or prevention.
MATERIALS AND METHODS
Animal preparation
Female C57BL/6 or α-gustducin-knockout (Gnat3−/−) mice (a gift from R. F. Margolskee, Monell Chemical Senses Center, Philadelphia, PA, USA) were bred with C57/BL6 male mice, and the day of vaginal plugging was designated as d 0 of pregnancy. Experimental protocols for animal research were approved by the Institutional Animal Care and Use Committees at the University of Massachusetts Medical School.
Preparation of myometrial tissues
Female C57BL/6 and Gnat3−/− mice were humanely killed by CO2 and cervical dislocation at d 18 of pregnancy. Uteri were quickly removed and transferred to ice-cold and oxygenated Krebs physiologic buffer (KPS), which was composed of (mM): 118.07 NaCl, 4.69 KCl, 2.52 CaCl2, 1.16 MgSO4, 1.01 NaH2PO4, 25 NaHCO3, and 11.10 glucose. Uterine tissue from the antimesometrial border (i.e., the side opposite the implantation site) was selected for the experiments below. Human uterine specimens from peripartum hysterectomies were collected under a human subject exemption protocol (No. 13590 to R.Z.G.) approved by the Committee for Protection of Human Subjects in Research at the University of Massachusetts Medical School, given the use of deidentified uterine tissues to be discarded after the procedure. Hysterectomies were performed on multiparous reproductive-age women at the time of indicated deliveries for medical reasons (i.e., placenta accreta, postpartum hemorrhage resulting from uterine atony) at the University of Massachusetts Memorial Medical Center. Patients with blood-borne infection, including hepatitis C and HIV, were excluded from this study. Once the specimens were transferred to the laboratory, the endometrium and connective tissues were removed, and the remaining myometrium was placed in ice-cold and oxygenated KPS until they were processed for the experiments.
Measurement of myometrial contractility
Uterine specimens were cut into longitudinal strips (5 × 1.5 mm), which were then transferred to 5-ml muscle baths containing ice-cold oxygenated KPS at 37°C. The strips were mounted on a wire myograph chamber (610-M; Danish Myo Technology, Aarhus, Denmark), and tension was measured by a PowerLab (ADInstruments, Colorado Springs, CO, USA) recording device. Each smooth-muscle strip was equilibrated for 60 min and then applied a 0.1-g load. To test the contractile response, each strip was stimulated twice with KCl (60 mM), separated by 10 min, before proceeding to other treatments. The measurement of human myometrium contraction was performed in the same way. The order and treatment time of agonists and antagonists are indicated in the figures and figure captions. Percentage relaxation for a given tocolytic was calculated as [100 × (FU − FT)/(FU − FB)], where F is the area under the force curve at a fixed concentration of a testing compound divided by time at that concentration, FU is the F generated by uterotonic (e.g., oxytocin, OT) before tocolytics (e.g., bitter tastant chloroquine [ChQ]) application, FT is the F at a given concentration of tocolytics, and FB is the basal value before uterotonic application. OT at 100 nM can generate sustained and highly reproducible contraction in mouse uteri. After OT’s response reaching a plateau, OT-induced contraction decreased by 13 ± 3% (n = 5) over 40 min, potentially as a result of some desensitization of OT receptors. All the data from mouse uteri were corrected for this time-dependent effect using an assumption that the decay is a linear process over time. When, after application of a uterotonic, tissue could not maintain a sustained contraction, the mean force at 15 to 30 min after the uterotonic application was calculated as the time control in the experiments with human uterine strips.
Isolation of mouse and human uterine smooth muscle cells
Uteri from d 18 pregnant mice were quickly removed and placed in a prechilled dissociation solution consisting of (mM): 135 NaCl, 6 KCl, 5 MgCl2, 0.1 CaCl2, 0.2 EDTA, 10 HEPES, and 10 glucose (pH 7.3). After gently removing the endometrium, the uteri were cut into longitudinal strips (5 × 1.5 mm). The tissue strips were first incubated in a dissociation medium containing 30 U/ml papain, 1 mM DTT, and 0.5 mg/ml bovine serum albumin (BSA) at 35°C for 30 min, then transferred to a dissociation medium containing 3 U/ml collagenase F and 0.5 mg/ml BSA at 35°C for another 5 min. Finally, the strips were agitated with a fire-polished wide-bore glass pipette to release the cells. Human myometrial cells were isolated in the same way.
Measurement of cell shortening
Myometrial cells in cell suspension were added to a recording chamber superfused with the bath solution at room temperature consisting of (mM): 130 NaCl, 5.5 KCl, 2.2 CaCl2, 1 MgCl2, and 10 HEPES, pH adjusted to 7.4 with NaOH. Once the cells were attached to the bottom of the chamber, they were imaged using a custom-built wide-field digital imaging system (18). The measurements were performed within 20 min to avoid the situation where the cells become attached to the bottom too strongly. The lengths of cells were determined using custom software to manually trace down the center of the cell, as previously described (17).
Immunohistochemical analyses
Isolated mouse and human myometrial cells were fixed in 4% paraformaldehyde for 2 min and washed with PBS. The nonspecific binding of primary antibodies was blocked by incubation with PBS containing 5% BSA and 0.2% Triton X-100 for 1 h. Incubation was carried out overnight at 4°C with a rabbit polyclonal antibody to GNAT3 (dilution 1:250; Santa Cruz Biotechnology, Santa Cruz, CA, USA). The specificity of the GNAT3 antibody has been established (19–21). After washing in PBS, cells were incubated with an Alexa Fluor 555–conjugated goat anti-rabbit antibody (dilution 1:500; Cell Signaling Technology, Danvers, MA, USA) for 1 h. Negative controls were performed by omitting the primary antibody. Immunoreactivity was evaluated with a FV1000 confocal laser scanning microscope system (Olympus, Tokyo, Japan).
Measurement of global intracellular Ca2+ concentration
Fluorescence images using Fluo-3 as a calcium indicator were obtained using a custom-built wide-field digital imaging system. The camera was interfaced to a custom-made inverted microscope, and the cells were imaged using a ×20 1.3 NA objective (Nikon, Tokyo, Japan) for global intracellular Ca2+ concentration ([Ca2+]) measurement. The 488 nm line of an argon ion laser provided fluorescence excitation, with a shutter to control exposure duration; emission of the Ca2+ indicator was monitored at wavelengths >500 nm. The images were acquired at the speed of 1 Hz for global [Ca2+] measurement. Subsequent image processing and analysis was performed off-line using a custom-designed software package running on a Linux/PC workstation. [Ca2+]i was represented as ΔF/F0 × 100, with F calculated by integrating fluo-3 over entire cells for global [Ca2+].
Mouse preterm labor model and toxicity studies
In separate experiments, d 16 pregnant mice were treated either subcutaneously with 150 µg mifepristone (RU-486; Sigma-Aldrich, St. Louis, MO, USA) or intraperitoneally with 10 µg LPS (Sigma-Aldrich). In both experimental series, pregnant mice were subsequently randomly assigned to 1 of 4 experimental groups and administered, via intrauterine injections, one of the following: vehicle (saline, 400 µl), ChQ (2 mg/dam), MgSO4 (10 mg/dam), or albuterol (100 ng/dam). The number of molecules of ChQ and tocolytics used was set to 10 times the molecule number in 0.4 ml of solution at the concentration that causes maximal myometrial relaxation in vitro. Mice were checked every 24 h for delivery. In another experiment, d 16 pregnant Gnat3−/− mice were treated with 150 µg RU-486 or 10 µg LPS subcutaneously, followed by an intrauterine injection of 2 mg ChQ.
Vector construction and lentivirus production
The short hairpin RNA (shRNA) sequences for TAS2R10 and TAS2R14 were annealed and inserted into pLKO.1, a third-generation self-inactivating lentivirus vector containing a blasticidin-resistant gene for selection. Meanwhile, a scrambled sequence was utilized as the control to discount any gene silencing that may have resulted from the delivery method. The constructed pLKO.1 vector and another 3 package vectors were cotransfected into 293FT cells (Thermo Fisher Scientific, Waltham, MA, USA) with lipofectamine according to the manufacturer’s instructions. The medium was replaced 16 h after transfection. Two days later, the virus-containing medium was pooled and passed through a 0.45-μm filter to remove cell debris, and was then ready to infect target cells or be frozen at −80°C.
Cell culture and lentivirus infection
hTERT-infected human myometrial (hTERT-HM) cells, a immortalized cell line derived from human uterine smooth muscle cells, were cultured in DMEM/F-12 (Thermo Fisher Scientific) containing 10% (v/v) FBS (Thermo Fisher Scientific) at 37°C in a humidified atmosphere of 5% CO2 and 95% air. The medium was changed every other day. When the cells reached 90% of confluence, they were detached by treatment with 0.25% (w/v) trypsin and reseeded at a density of 1 × 104 cells/cm2.
hTERT-HM cells were infected with lentivirus when the cells grew to 30–50% confluence at a multiplicity of infection of 200. The medium was changed 12 h after infection, when more than 95% of the cells were still viable. Three days later, all infected cells were selected with 4 μg/ml blasticidin (Thermo Fisher Scientific) in the medium for 10 d. Transduced cells after selection were utilized for functional studies.
RT-PCR and real-time quantitative PCR
The uterine smooth muscles from humans and mice were carefully isolated and quickly cleaned by removing connective tissues. The samples were then frozen and ground to homogeneity in liquid nitrogen. Trizol (Thermo Fisher Scientific) was used directly to lyse the cells in culture dishes. Total cellular RNA was isolated by using Trizol as described in the manufacturer’s instructions. Baseline-Zero DNase (Epicentre, Madison, WI, USA) was deployed to digest the residual genome (following the manuals). Then, 2 µg of isolated RNA from each sample was reverse transcribed into cDNA using SuperScript III reverse transcriptase (Thermo Fisher Scientific). A negative control was prepared with the omission of SuperScript III reverse transcriptase. Both the cDNA synthesis and negative control products were diluted to 200 µl, of which 1 µl was used as template for amplification of genes in the TAS2R signaling pathway. The housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) or β-actin was used as a positive control.
Quantitative PCR (qPCR) was carried out to determine the mRNA levels of TAS2Rs with iTaq Universal SYBR Green Supermix (Bio-Rad, Hercules, CA, USA) in accordance with the manufacturer’s protocols. Because the expression of TAS2Rs is relatively low, the input RNA (cDNA) for each well was 10 ng (the upper limit for use), and the PCR cycling consisted of 50 cycles of amplification of the template cDNA with primer annealing at 60°C. Then, the relative level of expression of each target gene was calculated using the 2−ΔΔCt method. Those with Ct values > 35 were considered as having poor precision and excluded, because it is generally accepted that 35 cycles of a PCR reaction should be sufficient to amplify even a single transcript up to measurable levels (22). Target genes were normalized against the housekeeping gene β-actin before further analysis. All the primers for mouse and human TAS2Rs and gustatory signal components are listed in Tables 1 and 2.
TABLE 1.
Primer sequences for mouse TAS2Rs and gustatory signal components
| Sequence, 5′–3′ |
||
|---|---|---|
| Name | Forward | Reverse |
| Tas2R102 | AGAGAGGTTCTATCAATATGGAAGG | CAAGAGGAAGGACACCATAATTC |
| Tas2R103 | AGGCCAACCGTGAAAAGAT | GAGTGTGTTTACTTGAAGTCTGTC |
| Tas2R104 | CTTAATTATAACGTGGCTAGCTTCC | ACTTTCATGGCTTTCATGTGAG |
| Tas2R105 | ATCGGCTTAGCAACTTCCAGG | AGAAGATGCTTAGGCTGGTGG |
| Tas2R106 | TTCTCACTACATCTTCTTCTGCTTG | AGGCATACAACTGCATCTTTCT |
| Tas2R107 | TTCCAACTCTGTATTTCTCTGGC | TAATTTTTCCGCTGGTGGA |
| Tas2R108 | CTAATTTTCAACACCCAGTG | CCCAATTATGTGTTCAGGA |
| Tas2R109 | TCAGTTACATTACTGGTGTCTCTGG | TTGACAGGGACAAAACAACG |
| Tas2R110 | GGATTCAACCCCATTTCCCA | TTAATGTGGGCCATGGTGCT |
| Tas2R113 | GCATAGACTGGGTCCAAAGAAG | CAGTTTCGTGTAATGCTGGGTA |
| Tas2R114 | CCCAGTTGTTGCGAAGATGGTT | CTGCCTGCGATGTCTCCAAAGT |
| Tas2R115 | CCTTCAGCAGATCATTTCCTCAC | TAAAAGATGCTGAGGCTGGTAG |
| Tas2R116 | GATTGCCTCCCTCCTCCTGTAT | CCTTTCTCATCTTAGCATTGCCC |
| Tas2R117 | ACACTGTTGGTGTCATTGCTCCTC | ATGCTGCTGCATCCTCTTGTG |
| Tas2R118 | ACAACTTTGGTACTTATTCTAGGCC | AGGAATACCAAAGCCAATCA |
| Tas2R119 | CCATCCCTCACCCACTCTTTCT | GCTGGTCTGACCCGAGTTGTAT |
| Tas2R120 | GTTGCATATCTTGGGATGGTGA | CAGGGAATAGATTAAGAGCAGAAC |
| Tas2R121 | TGATCTTCTTGATGCTCAAC | GGAGAAGATTAACAGGATGA |
| Tas2R122 | GTCCTAGGCACACTCATGTATTTC | TGGTATGTATGCCCTGTAGTTTC |
| Tas2R123 | ATGATCAGTTCTCAATTGCTGC | GTACTGACATCTCTAGGTTGTTTGG |
| Tas2R124 | CATGCTTCTGGGAAGCTTGG | CGTAGCACTAGGGTCTCTATCTCCT |
| Tas2R125 | GCACTCAGTTTCCCAGACTTGT | GTCTTCGGAGCCTTTAGCATAG |
| Tas2R126 | CTCCATCTGGTTGAGTACTCC | GATAGAGCCCAACAAGAACC |
| Tas2R129 | CACATCTATACCCTTTACACTTTCC | CTCCTCAAAATTTGCATGGA |
| Tas2R130 | GTGCAGTATCCAGACACCTACAA | TGACTGGGAGGCTAAAACACA |
| Tas2R131 | ACTGCTTGAAACACTTCCTATTTGG | AAGAGAATGAGGAAAGACACCAG |
| Tas2R134 | GAATAGCCGTCCTAAACAAC | TGACAGTCCACCAATGATCA |
| Tas2R135 | TTGGAATGTCACTGGGAATAGC | CCAAAGATACGAGGAGCAAACT |
| Tas2R136 | AAGCAAATGGACACCAGGGCAACT | GTGGTGAAGGGAACTAAACTGC |
| Tas2R137 | AAACCACTTCAGTATTTGGC | TGAGGGCAGAATAGATCTTA |
| Tas2R138 | TTCCAGCAGATGAAAGACCCAC | GAAGCGGACAATCTTGGAGCAG |
| Tas2R139 | GTTGATAGAAATTACCTCCTCC | GGAGCTGAAGGAAATAAACA |
| Tas2R140 | GTCACTTCTCCTCTTGTTTGTAAAC | TGTGTTGCATGTTCTTCAGATG |
| Tas2R143 | CGAACCTTATTGGCATCCTCTG | ACCAGCAGCCTGGGAACTAACT |
| Tas2R144 | ATCACCTCACTCAAGAGGCACAC | TCCAGACACTGTAAGCACCAAG |
| mGnat3 | GAAAGGGCATCTGAATACCAGC | GAAAGGGCATCTGAATACCAGC |
| mGnb3 | CAGGACAGCAGAAGACAGTG | GTCATCTGAGCCAGTGCAG |
| mGng13 | CCCAGCCTCACTCCACAGAT | CCTCTTGAAGGCCAGTTGG |
| mpLCβ2 | ATGCAGCAGAACATGGCACT | CCAGCTCAGGCATCAAGAT |
| mActin | AGGCCAACCGTGAAAAGAT | AGAGCATAGCCCTCGTAGA |
TABLE 2.
Primer sequences for human TAS2Rs and gustatory signal components
| Sequence, 5′–3′ |
||
|---|---|---|
| Name | Forward | Reverse |
| TAS2R1 | CCATGGATGATCCTGGGGTCTC | GATAAGCAATGGCACTGAGAAC |
| TAS2R3 | ATGGTATGGATGCTGTTGGGTG | GAGAAGATGAGCAAAGAGTAGGAG |
| TAS2R4 | TCTGCTTCCTTGCTAATACACTCC | TTTGGTCCCCATATCCATCCCT |
| TAS2R5 | GCAGCATTCGGTATCCCTTTG | GCAGCATTCGGTATCCCTTTG |
| TAS2R7 | AGCACAGAAGCCCATGTGAGA | TGACTTGAGGGGTAGATTAGAGC |
| TAS2R8 | CTTCTGGACATTTGCCAACTAC | CTTCTGGACATTTGCCAACTAC |
| TAS2R9 | GATGGTTCCCTTTATCCTTTGC | CCCTCATGTGGGCCTCTGTA |
| TAS2R10 | AGTGAGTCAGTGTTTGGGGTTT | CAATTAGGTTACCGGAGGCATA |
| TAS2R13 | ACTGGCTGGACCGATATGAAAG | AGGCCACAGTAAATGGTGTTA |
| TAS2R14 | CATACCCTTTACTTTGTCCCTG | AGGATAAGCCATTCCCATCACC |
| TAS2R16 | CTTGCTTACCGTGTTCTACTGC | ATGAGCCTGGAACTGATACTGA |
| TAS2R19 | TCTCACTGCTCTGGTGGTCTC | GCAAGCCACATGCTGAAATGGT |
| TAS2R20 | GATAGTGTTGGGGTCTTTGTTCT | ATCAGGGTCAGAGTGAATGGTA |
| TAS2R30 | GACCTCCTTTCTTCTGTTATGTGC | AATCAGGATGAATGGGTGGGTT |
| TAS2R31 | GTCATTCTGGTGATGCTGTTGG | TTTTATGTGGACCTTGGTGCTG |
| TAS2R38 | TTTTGGGATGTAGTGAAGAGGC | CTGGAAGTGGGTAAGCTGGATA |
| TAS2R39 | CATCAGCTCAACCTCCCTAAG | CAATCCAGTAATTCTCCACCTC |
| TAS2R40 | TCCATTCCTATCCCCTCCTCC | TTTGATGGCCCCTATGTGAGC |
| TAS2R41 | TGCTCTTTAGCCTGCTGAGTC | AGTTCAGGAAGTGCCAGTGTAG |
| TAS2R42 | GACTGGTAAACTGCTCTGAAGGGAT | GCAAGCCAGGTTGTCAAGTGATT |
| TAS2R43 | AGTGTCATTCTGGTGGTGCTG | TGTGGACCTTGGTACTGGGAT |
| TAS2R45 | TGTCATTCTGGTGGTGCTGTTG | TTTATGTGGACCTTGGTACTGG |
| TAS2R46 | TGACCTCCTTCCTCTTGTTATG | TCAGGATGAATGGGTGGGTTG |
| TAS2R50 | CTGGTGATACTGTTGGGGACT | GCTTTTATGTGGACCTTGGTG |
| TAS2R60 | GGAGTGGGTGCTACGGAGAAT | GAAGACAGGGTGGGTGAAGGT |
| hGNAT3 | GATAGAATAACAGCATCTGGGTATG | TTGAGGAACAGGACAATGGAGG |
| hGNB3 | CCTACTATTCGCTGGCTACGACG | TGTTCACTGCCTTCCACTTCC |
| hpLCβ2 | GCAGGACCCACTCATAGCA | GGAGGGCCCTCAGCCAGG |
| hGAPDH | TGACGCTGGGGCTGGCATTG | GGCTGGTGGTCCAGGGGTCT |
Statistical analysis
Unless stated otherwise, data are reported as means ± sem, and n represents the number of myometrial cells, uterine strips, or mice. Statistical analyses of differences were carried with Student’s t test when data was from independent groups. Dose–response curves underwent ANOVA followed by post hoc pairwise Student’s t tests at each dosing level. PTB rate was examined by the χ2 test. The significance level was set at P < 0.05.
RESULTS
Myometrial cells express TAS2Rs and their signaling elements
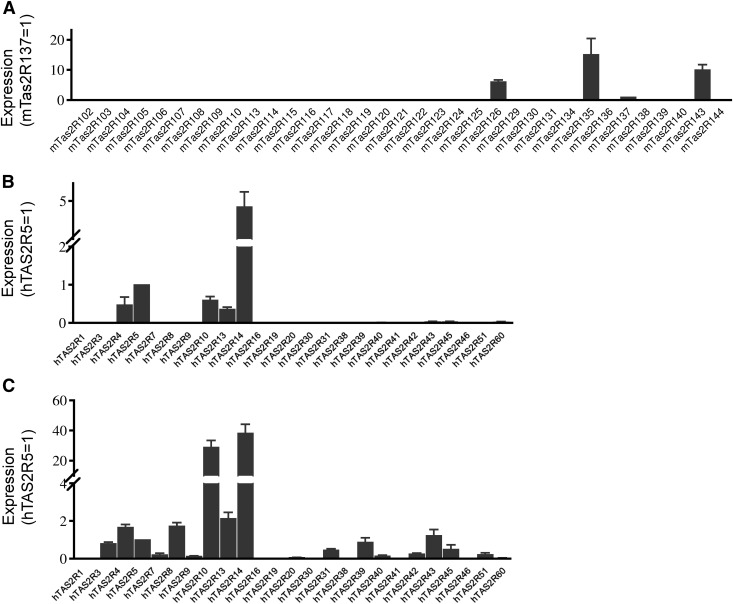
To probe the potential roles of TAS2Rs in uterine contractility and in preventing preterm labor, we first examined whether TAS2Rs are present in myometrial cells from pregnant mice and humans. Of 35 mouse Tas2Rs, we detected mRNA transcripts for TAS2R126, -135, -137, and -143 in myometrium from pregnant mice at d 18 (Fig. 1A and Table 1). Out of a total of 25 human TAS2Rs, we detected the transcripts for TAS2R4, -5, -10, -13, and -14 in freshly collected human myometrial tissues (Fig. 1B) and for TAS2R3, -4, -5, -7, -8, -10, -13, -14, -31, -39, -42, -43, -45, and -50 in cultured hTERT-HM cells (Fig. 1C and Table 2). Several mouse Tas2Rs (e.g., Tas2R115 and -108) had inconsistent Ct values ∼35, a cutoff for our quantification, and were therefore not included in the results. However, we cannot rule out the existence of these Tas2rs in myometrial cells, as these values may merely reflect their extremely low expression level or a qPCR efficiency <100%.
Figure 1.
Mouse and human myometrium express TAS2Rs. A) Tas2R expression pattern in myometrium from d 18 pregnant mice. B) TAS2R expression in myometrium from women in third trimester of pregnancy. C) TAS2R expression in hTERT-HM cells. Quantitative PCR primers for TAS2Rs are listed in Tables 1 and 2. To visualize differences among TAS2Rs, Ct values for each TAS2R was normalized against β-actin and then against Tas2R137 in mice and TAS2R5 in humans.
In taste cells, bitter tastants bind to TAS2Rs to activate the pertussis toxin (PTX)-sensitive G protein gustducin, which in turn induces a phospholipase C β2 (PLCβ2) and inositol trisphosphate (IP3) signaling cascade (21, 23). We therefore examined mRNAs for these elements of the TAS2R signaling pathway. As shown in Supplemental Fig. 1A, B, we detected mRNAs for the elements in the TAS2R signaling pathway in myometrial cells from mouse and human. Using a specific antibody for gustducin (19–21), we detected gustducin in both human and mouse myometrial cells (Supplemental Fig. 1C–E and Tables 1 and 2). These results indicate that myometrial cells from both mouse and human express the canonical TAS2R signaling cascade, raising the possibility that bitter tastants act on this pathway to generate their responses in these cells.
Bitter tastants relax myometrium completely
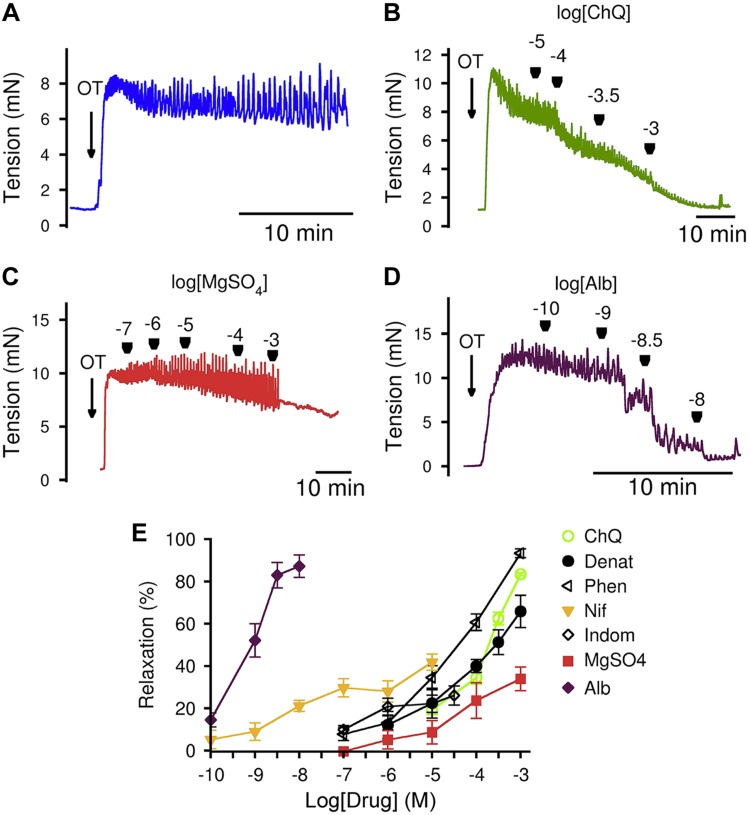
To assess the effects of bitter tastants on myometrial contractility, we used freshly isolated mouse uterine strips and measured their force generation under isometric conditions. OT, a hormone critical for parturition, was used as a contractile agent. Figure 2 shows bitter tastants ChQ, denatonium, and 1,10-phenanthroline dose-dependently suppressed the OT-induced contraction of mouse myometrium from d 18 pregnant mice. As a comparison, we also examined the effects of 4 currently used tocolytics: MgSO4, indomethacin, nifedipine, and albuterol (Fig. 2). Although the concentration required to initiate the relaxation was greater for bitter tastants than for nifedipine and albuterol, the former caused stronger relaxation than nifedipine (∼100% by bitter tastants vs. ∼60% by nifedipine at the concentration without effect of solvent on myometrium). Compared to MgSO4 and indomethacin, bitter tastants relax myometrium both more completely and at a lower dose.
Figure 2.
Bitter tastants completely relax OT-induced contraction of uterine strips from d 18 pregnant mice. A) OT (100 nM)-induced sustained contraction of uterine strips from d 18 pregnant mice. B–D) Dose-dependent effects of ChQ (B), MgSO4 (C), and albuterol (D) on 100 nM OT-induced mouse uterine strip contraction. E) Summarized results of experiments A–D, another 2 bitter tastants, denatonium (Denat) and 1, 10-phenanthroline (Phen), nifedipine (Nif), and indomethacin (Indom). Data are means ± sem; n = 4–6 mice. Strips were placed in KPS containing 1.16 mM MgSO4 (i.e., final concentration of MgSO4 was 2.16 mM in C, and also in experiments in Fig. 4); and that to avoid potential relaxing effects of solvent (i.e., ethanol for nifedipine and DMSO for indomethacin), higher levels of nifedipine and indomethacin were not examined. Relaxation calculation is described in Materials and Methods.
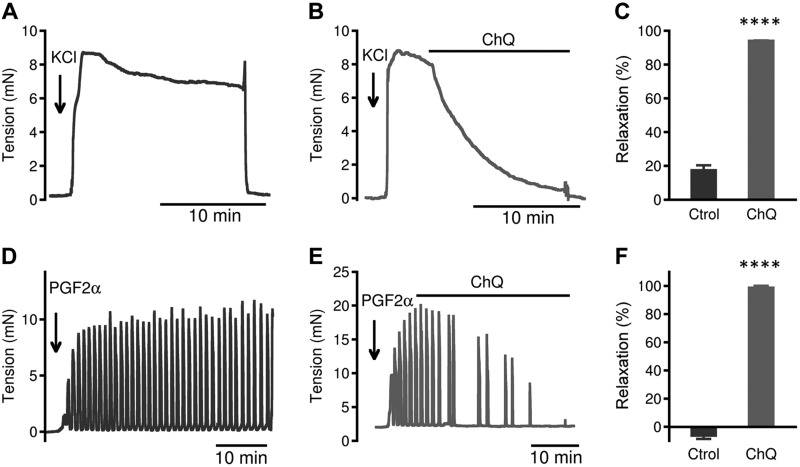
To test the generality of bitter tastants in reversing uterotonic-induced contraction, we also examined the effect of ChQ on uterine contraction caused by KCl and prostaglandin F2α (PGF2α, another critical hormone for parturition). As shown in Fig. 3, ChQ at 1 mM can fully reverse both KCl- and PGF2α-induced contraction of the uterus from d 18 pregnant mice.
Figure 3.
ChQ relaxes KCl- and PGF2α-induced contraction of myometrial strips from d 18 pregnant mice. A–C) Force response to 40 mM KCl alone (A), relaxation caused by 1 mM ChQ (B), and summarized results (C) (means ± sem; n = 5 for each group; KCl alone vs. KCl + ChQ, unpaired Student’s t test). D–F) Force response to 5 µM PGF2α alone (D), relaxation caused by 1 mM ChQ (E), and summarized results (F) (means ± sem; n = 5 for each group; PGF2α alone vs. PGF2α + ChQ, unpaired Student’s t test). ****P < 0.0001.
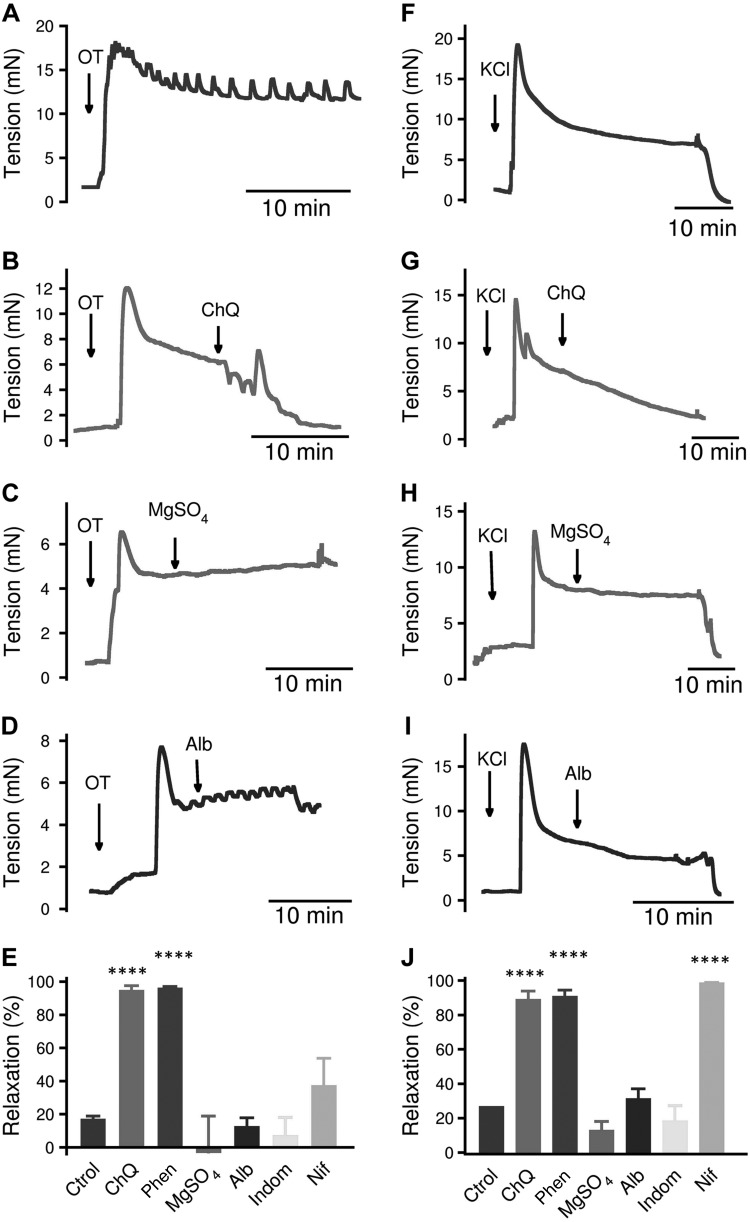
We also studied the effect of bitter tastants on contractions in human pregnant myometrium. We found that ChQ and 1,10-phenanthroline almost fully reverses OT- and KCl-induced contraction of human myometrium (Fig. 4B, E, G,J). This reversal effect is much greater than all 4 clinically used tocolytics—MgSO4, indomethacin, nifedipine, and albuterol—in OT-induced contraction (Fig. 4C–E), and greater than MgSO4, indomethacin, and albuterol in KCl-induced contraction (Fig. 4H–J). These contraction bioassays indicate that bitter tastants can relax human pregnant uterus contracted by uterotonics potentially better than currently used tocolytics, which have several different types of targets (e.g., albuterol targets β2-adrenergic receptors, indomethacin targets cyclooxygenase 1 and 2, and nifedipine targets Ca2+ channels) in myometrial cells.
Figure 4.
Bitter tastants relax human uterine strips more completely than currently used tocolytics. A) Representative tension response to 100 nM OT in uterine strips from women in late pregnancy. B–D) Tension responses caused by 1 mM ChQ (B), 1 mM MgSO4 (C), and 10 nM albuterol (D) on 100 nM OT-precontracted uterine strips from women in late pregnancy. E) Summarized results of experiments A–D. Data are means ± sem; n = 5–6 strips. F) Representative tension response to 60 mM KCl in uterine strips from women in late pregnancy. G–I) Tension responses caused by 1 mM ChQ (G), 1 mM MgSO4 (H), and 10 nM albuterol (I) on 60 mM KCl-precontracted uterine strips from women in late pregnancy. J) Summarized results of experiments F–I. Data are means ± sem; n = 4–7 strips. ****P < 0.0001, uterotonic alone vs. uterotonic + tocolytic, unpaired Student’s t test.
Bitter tastants activate canonical Tas2R signaling to modestly raise [Ca2+]i in uncontracted myometrial cells
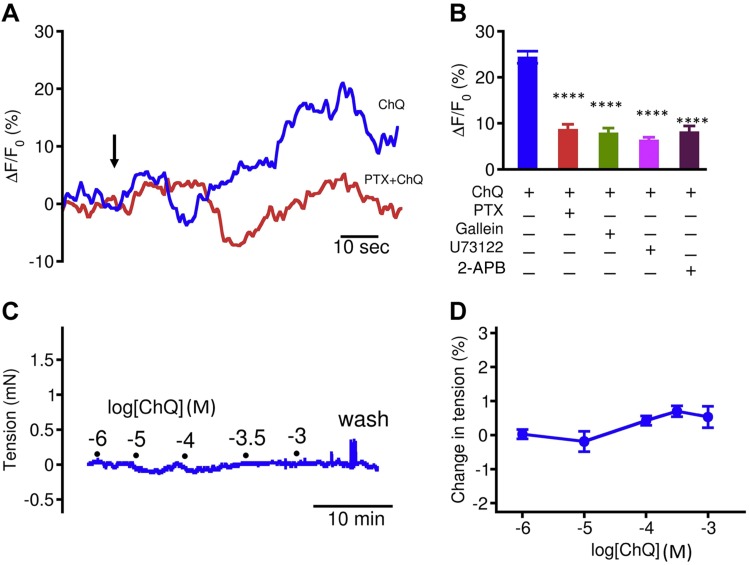
In light of our findings that myometrial cells express TAS2Rs and their signaling components, and that bitter tastants can fully relax uterotonic-induced myometrial contractions, we hypothesized that bitter tastants activate TAS2R signaling to mediate the myometrial relaxation. To test this hypothesis, we chose ChQ in the following series of experiments because it is a widely used antimalarial drug and a commonly used bitter tastant for studying bitter taste responses. Because [Ca2+]i is a key signal mediating myometrial contractility (24–26), we decided to examine whether ChQ affects [Ca2+]i, and, if so, to study the underlying mechanism.
We examined the effect of ChQ on Ca2+ signaling in myometrial cells at rest. In freshly isolated myometrial cells from d 18 pregnant mice, ChQ alone modestly increased global [Ca2+]i (Fig. 5). This rise in [Ca2+]i is not sufficient to contract myometrium (Fig. 5). We next examined the cause of the modest global [Ca2+]i rise produced by ChQ by blocking the components in the canonical TAS2R signaling cascade (Fig. 5). In pregnant mouse myometrial cells, PTX (1 µg/ml and 6–8 h pretreatment) reduced the ChQ-induced increase in global [Ca2+]i to 36.7 ± 4.8% of the rise seen in the control cells (n = 16). We further found that gallein (20 µM and 30 min pretreatment), a blocker of the Gβγ dimer of PTX sensitive G proteins (27, 28), reduced the ChQ-mediated increase in [Ca2+]i to 33.2 ± 5.0% (n = 14) of the controls. Finally, U73122 (3 µM and 20 min pretreatment), a blocker of PLCβ, and 2-aminoethoxydiphenyl borate (50 µM and 20 min pretreatment), an IP3 receptor antagonist, inhibited the ChQ-induced increases in [Ca2+]i to 26.7 ± 2.9% (n = 15) and 33.7 ± 5.7% (n = 14) of controls, respectively. To assess whether the same signaling pathway is operative in human myometrial cells, we carried out the same series of experiments using cultured human myometrial cells hTERT-HM. We found that ChQ can increase [Ca2+]i to a higher level than in primary mouse myometrial cells (Supplemental Fig. 2). The blockers of TAS2R signaling exerted similar inhibitory effects on the ChQ-induced rise in [Ca2+]i in human myometrial cells (Supplemental Fig. 2) as they did in native pregnant mouse myometrial cells (Fig. 5). These results indicate that bitter tastants activate the TAS2R signaling transduction pathway to modestly release Ca2+ from internal stores.
Figure 5.
ChQ activates TAS2R signaling to modestly raise [Ca2+]i without changes in force generation in myometrium from d 18 pregnant mice. A) Representative traces showing [Ca2+]i after 1 mM ChQ, or 1 mM ChQ + 1 µg/ml PTX pretreatment. Arrow indicates time when ChQ was applied to cells. PTX and other inhibitors were applied as pretreatment as described in text. B) Effects of inhibitors of TAS2R signaling pathway on ChQ-induced rise in [Ca2+]i. ChQ causes oscillations in [Ca2+]i; peak [Ca2+]i in A was calculated for comparison; n = 9 cells for ChQ (1 mM) alone, n = 16 for PTX (1 µg/ml), n = 14 for galleon (20 µM), n = 15 for U73122 (3 µM), and n = 14 for 2-aminoethoxydiphenyl borate (2-APB) (50 µM). ChQ alone vs. ChQ + inhibitor, unpaired Student’s t test. C) Representative trace showing that ChQ alone does not affect myometrial contraction. D) Summarized results of experiments in C; n = 5. ****P < 0.0001.
Gustducin contributes to bitter tastant-mediated change in [Ca2+]i and cell shortening in myometrial cells
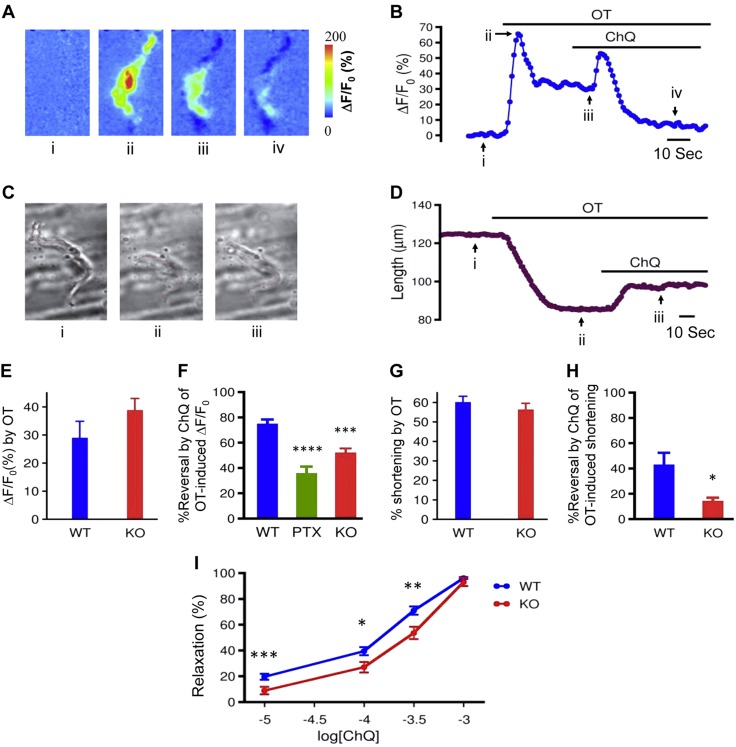
Having found that the modest increase in [Ca2+]i produced by bitter tastants acting on resting cells did not change mouse uterine contractility, we went on to study the hypothesis that bitter tastants reverse the uterotonic-induced rise in [Ca2+]i, resulting in myometrial relaxation. As shown in Fig. 6, OT (600 nM) rapidly increased [Ca2+]i. Notably, ChQ (1 mM) largely reversed this increase in [Ca2+]i (Fig. 6A, B, F). As noted in the fluorescence imaging, OT at 600 nM also shortened myometrial cells. To more precisely quantify the length change caused by OT, we stimulated the myometrial cells without loading them with Ca2+ indicators. We found that OT can markedly shorten the cells (Fig. 6C, D), and ChQ at 1 mM can robustly reverse the shortening by 42.7 ± 9.7% (Fig. 6C, D, H). These results demonstrated that bitter tastants can reverse the contractile agonist-induced rise in [Ca2+]i, which in turn could cause myometrial relaxation.
Figure 6.
ChQ activates TAS2R-coupled G protein gustducin in myometrial cells. A, B) Effects of 1 mM ChQ on 600 nM OT-induced Ca2+ rise in d 18 pregnant mouse myometrial cells. Fluo-3 fluorescent images (A) show changes in [Ca2+]i displayed as ΔF/F0 and were taken at time indicated by black arrows and roman letters on time course (B) of [Ca2+]i (represented as ΔF/F0 integrated over entire cell). Transient rise in [Ca2+]i after ChQ was applied is likely due to activation of TAS2R signaling pathway, resulting in IP3 generation (Supplemental Fig. 3). C, D) Effects of 1 mM ChQ on 600 nM OT-induced cell shortening in d 18 pregnant mouse myometrial cells. Changes in cell length (C, red lines) taken at time indicated on time course (D) of length change. E) Comparison of OT-induced change in [Ca2+]i in myometrial cells from WT mice and Gnat3−/− mice. Experiments were performed as in A; values were calculated on basis of ΔF/F0 at time points marked as i and ii in A and B. F) Summarized results showing percentage reversal by ChQ of OT-induced Ca2+ rise in WT cells with and without PTX (1 μg/ml) treatment, and in Gnat3−/− cells. Note that percentage reversal is [100 × (iii − iv)/(iii − i)], where i, ii, and iii are marked in B. G) Comparison of OT-induced change in cell length of myometrial cells between WT mice and Gnat3−/− mice. Experiments were performed as in C; values were calculated on basis of lengths at time points marked i and ii in C and D. H) Summarized results showing percentage reversal by ChQ of OT-induced shortening of myometrial cells from WT and Gnat3−/− mice. Percentage reversal is [100 × (ii − iii)/(ii − i)], where i, ii, and iii are marked in D. Means ± sem; n = 10–19 cells for (A–H). I) Relaxation by ChQ of 600 nM OT-induced contraction of myometrium from Gnat3−/− mice is suppressed compared to that in strips from WT mice; n = 13 for WT and 9 for Gnat3−/− mice. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; unpaired Student’s t test (E–H), and ANOVA and post hoc pairwise Student’s t tests (I).
To examine whether ChQ reverses the OT-induced [Ca2+]i rise through the activation of TAS2Rs, we first used strategies to interfere with gustducin, because all TAS2Rs are coupled with this G protein. One strategy used was to disrupt the interaction between TAS2Rs and gustducin with PTX (which catalyzes the ADP-ribosylation of the gustducin α subunit, preventing the gustducin from interacting with TAS2Rs). We found that PTX (1 µg/ml and 6–8 h pretreatment) decreased bitter tastant’s reversal of the rise in [Ca2+]i caused by OT (Fig. 6F). Another strategy used Gnat3−/− mice. We found that the OT-induced increase in [Ca2+]i and that the cell shortening were comparable between wild-type (WT) cells and Gnat3−/− cells (Fig. 6E, G). However, ChQ’s reversal effects on the OT-induced rise in [Ca2+]i and cell shortening were significantly reduced in Gnat3−/− cells compared to the WT cells (Fig. 6F, H and Supplemental Fig. 3). Moreover, ChQ’s reversal effects on OT-induced myometrial contractions were reduced when gustducin was knocked out; this effect was more prominent in the lower ChQ levels (Fig. 6I). These results strongly suggest that gustducin plays an important role in ChQ-induced responses in myometrial cells.
Bitter tastant ChQ activates human TAS2R14 to alter Ca2+ responses in human myometrial cells
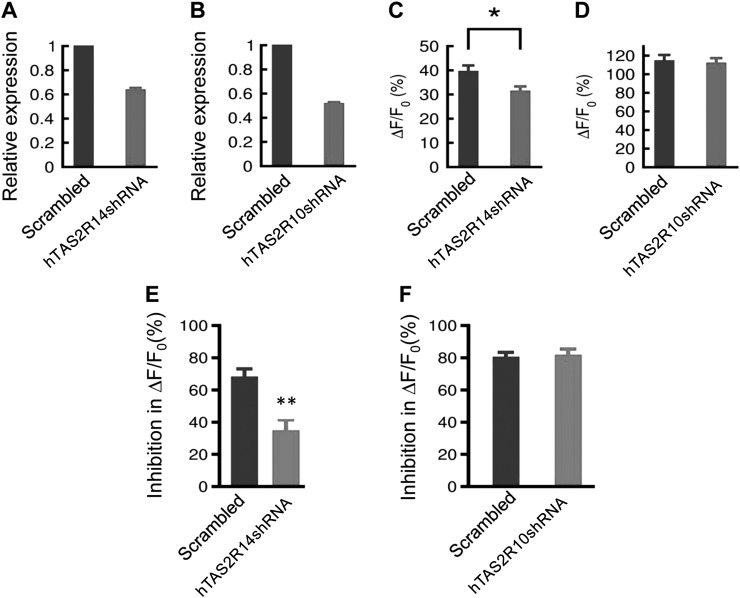
Given the lack of TAS2R-knockout mice and the difficulties in knocking down genes in freshly isolated myometrial cells, we used shRNA applied to human cultured myometrial cells to test whether ChQ activates TAS2Rs to generate myometrial responses. It is known that ChQ activates TAS2R3, -7, -10, -14, and -39 with different affinities and effective concentrations of ChQ for TAS2R10 and -14 are 10 mM and 10 µM, respectively (29). Considering that TAS2R10 and -14 are expressed most abundantly in human cultured myometrial cells (Fig. 1C) and that only 1 mM ChQ is required to produce maximal effects in relaxing myometrium and in decreasing the OT-induced rise in [Ca2+]i, we hypothesized that TAS2R14 but not TAS2R10 is required for the ChQ-induced Ca2+ changes in human myometrial cells. Using shRNA, we knocked down TAS2R14 and TAS2R10 separately in cultured human myometrial cells (Fig. 7A, B and Table 3). As shown in Fig. 7C, D, the shRNA for TAS2R10 exerted no effect on the ChQ-induced rise in [Ca2+]i in resting cells, while the shRNA for TAS2R14 produced an inhibition of the ChQ-induced rise in [Ca2+]i. We then tested the effects of these shRNAs on ChQ’s ability to reverse any OT-induced rise in [Ca2+]i. In the control cells with scrambled shRNAs, ChQ markedly inhibited the OT-induced increase in [Ca2+]i (Fig. 7E, F). The shRNA for TAS2R10 exerted no effect on ChQ’s effect on the OT-induced rise in [Ca2+]i (Fig. 7F), but the shRNA for TAS2R14 significantly inhibited ChQ’s effect on the OT-induced rise in [Ca2+]i (Fig. 7E). These results indicate that TAS2R14 mediates the ChQ-induced effect on [Ca2+]i in human myometrial cells.
Figure 7.
ChQ activates TAS2Rs in cultured human myometrial cells. A, B) Efficiency of shRNA knockdown targeting human TAS2R14 (A) and TAS2R10 (B) as assessed by qPCR. C, D) [Ca2+]i rise induced by 10 mM ChQ is significantly suppressed by shRNA knockdown targeting human TAS2R14 (C), but not TAS2R10 (P = 0.75) (D). E, F) shRNA knockdown targeting human TAS2R14 (E), but not TAS2R10 (F), inhibits reversal by 1 mM ChQ of 600 nM OT-induced Ca2+ rise. Data are means ± sem; n = 30–73 cells. *P < 0.05, **P < 0.01; scrambled vs. shRNAi, unpaired Student’s t test.
TABLE 3.
Sequences of Tas2R shRNAs
| Sequence, 5′–3′ |
||
|---|---|---|
| Name | Top strand | Bottom strand |
| Tas2R10-shRNA | CCGGGCATTGACTGTGCCAAGAATACTCGAGTATTCTTGGCACAGTCAATGCTTTTTG | AATTCAAAAAGCATTGACTGTGCCAAGAATACTCGAGTATTCTTGGCACAGTCAATGC |
| Tas2R14-shRNA | CCGGGCCTGGTTTGGTTAATATTCGCTCGAGCGAATATTAACCAAACCAGGCTTTTTG | AATTCAAAAAGCCTGGTTTGGTTAATATTCGCTCGAGCGAATATTAACCAAACCAGGC |
| Scramble | CCGGGCAAGGGCAACATCCTGTTCGCTCGAGCGAACAGGATGTTGCCCTTGCTTTTTG | AATTCAAAAAGCAAGGGCAACATCCTGTTCGCTCGAGCGAACAGGATGTTGCCCTTGC |
Bitter tastant ChQ protects against mouse PTBs better than currently used tocolytics
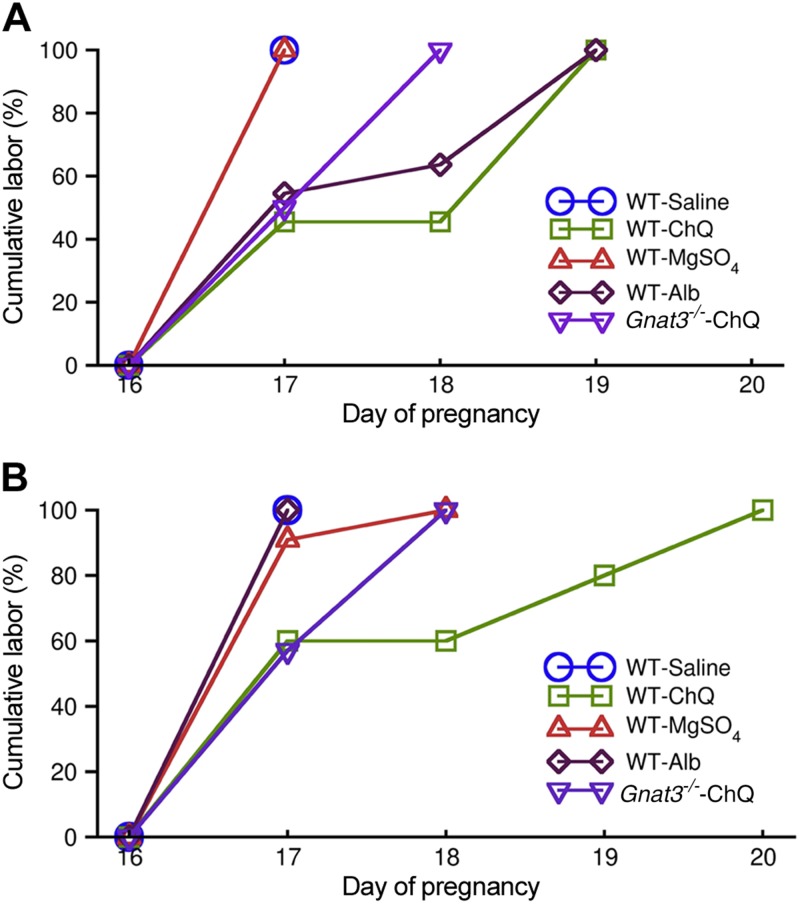
The strong relaxation produced by bitter tastants implies that these compounds may be able to prevent or treat PTB. To assess this, we first established a mouse model of preterm labor with bacterial endotoxin LPS by making only minor modifications of a protocol developed by Kaga et al. (30) (i.e., i.p. injection of LPS at 10 µg/mouse on d 16 of pregnancy). LPS was used because inflammation is one of the major risk factors for preterm labor (31). Consistent with the results from Kaga et al. (30), all mice delivered dead litters 1 d after LPS injection (i.e., d 17) (Fig. 8A and Supplemental Fig. 4). When administering ChQ (0.4 ml of 5 mg/ml) to the uteri 3 h after the LPS injection, more than 50% mice delivered their litters on d 19 (i.e., full-term birth) (Fig. 8A). More importantly, all the litters were delivered alive (Supplemental Fig. 4). As a comparison, when MgSO4 (0.4 ml of 25 mg/ml) was administered to the uteri 3 h after LPS, all mice still delivered their litters, all dead, on d 17 (Fig. 8A) (i.e., it exerted no protection from LPS-induced PTB); when albuterol (0.4 ml of 0.25 µg/ml) was applied to the uteri 3 h after LPS, 50% of mice gave birth on d 17 and the remaining 50% on d 19, and more than 90% of the pups were alive (Supplemental Fig. 4).
Figure 8.
ChQ, via gustducin, rescues LPS- or RU-486-induced preterm labor better than MgSO4 and albuterol. A) Mean cumulative labor induced by LPS followed by treatment with saline, ChQ, MgSO4, or albuterol in WT mice, or followed by treatment with ChQ in Gnat3−/− mice. LPS (10 µg, i.p.) was administered on d 16. Lines for WT-saline and WT-MgSO4 are overlapped. P < 0.05 saline vs. ChQ; P < 0.05 saline vs. albuterol; P < 0.01 WT ChQ vs. Gnat3−/− ChQ; n = 11 for each group and P value from χ2 test. B) Mean cumulative labor induced by RU-486 followed by intrauterine administration of saline, ChQ, MgSO4, or albuterol in WT mice, or followed by treatment with ChQ in Gnat3−/− mice. RU-486 (150 mg, s.c.) was administered on d 16. Lines for WT-saline and WT-albuterol are overlapped. P < 0.05 saline vs. ChQ, P < 0.05 WT ChQ vs. Gnat3−/− ChQ; n = 10–11 for each group, χ2 test.
Progesterone is a key hormonal regulator of the female reproductive system and plays a major role in preparing the uterus for implantation and in the establishment and maintenance of pregnancy (32, 33). PTB in women is associated with changes in circulating hormones such as progesterone deficits (34–38) or with inadequate responses to progesterone (32, 39). To further demonstrate the effectiveness of bitter tastants in preventing PTB, we established another mouse model of preterm labor with the progesterone receptor antagonist RU-486. Similar to the protocol developed by Dudley et al. (40), we applied a subcutaneous injection of RU-486 to d 16 pregnant mice. With this experimental protocol, we found that RU-486 induced labor in 100% of the mice on d 17 (Fig. 8B). When administering ChQ (0.4 ml of 5 mg/ml) to the uteri 3 h after the RU-486 injection, of 10 mice, 6 gave birth on d 17 (i.e., no protection from RU-486), 2 delivered on d 19 (i.e., full term), and 2 delivered on d 20 (i.e., delayed labor). The litters in all RU-486-treated groups were dead. As a comparison, both MgSO4 and albuterol gave essentially no protection from RU-486-induced preterm labor (Fig. 8B). These results suggest that the bitter tastant ChQ can provide better protection in preventing LPS- or RU-486-induced PTB in mice than currently used tocolytics.
ChQ protects mice from PTBs via gustducin
To test whether gustducin is involved in ChQ-induced protection from PTB, we injected LPS or RU-486 followed 3 h later by ChQ into Gnat3−/− mice. As shown in Fig. 8A, in the Gnat3−/− mice administered LPS, the ChQ treatment resulted in 60% of the mice delivering on d 17 and another 40% on d 18; all the litters were dead (Supplemental Fig. 4). Hence, the protection from PTBs by ChQ in the Gnat3−/− mice was significantly weaker than that in the WT mice. In the Gnat3−/− mice administered RU-486, the ChQ treatment resulted in 50% of the mice delivering on d 17 and another 50% on d 18, and all the pups were dead when delivered. Therefore, the protection provided by ChQ from RU-486 induced PTB in the Gnat3−/− mice was also significantly weaker than that in the WT mice. These results indicate that gustducin is a critical molecule mediating ChQ-induced protection from PTBs in mice.
DISCUSSION
In this study, we have found that both human and mouse myometrial cells express TAS2Rs and their signaling components. Bitter tastants activate these receptors and can fully relax myometrial contraction even when this contraction is induced by different uterotonics that target different receptors and ion channels. These results highlight an attractive characteristic of bitter tastants as tocolytics because the cause of PTB is multifactorial and often unknown, and therefore a tocolytic with a broad spectrum of applicability could provide better protection from PTB than those with a narrow spectrum effect. Currently, progesterone is the only pharmacologic reagent approved by FDA for preventing PTB (41, 42). According to the U.S. Institutes of Medicine Committee on Understanding Premature Birth and Assuring Healthy Outcomes (26), 3 possibilities may account for the effectiveness of progesterone: 1) progesterone inhibits a pathway shared by diverse causes of PTB, 2) progesterone exerts diverse effects that act on several different pathways, or 3) progesterone is very effective against one highly prevalent pathway or cause. Our results suggest that bitter tastants may possess similar characteristics to those of progesterone, and hence they are potentially useful for human preterm labor management.
Another attractive characteristic of bitter tastants as revealed in this study is that their relaxing effects are more complete, particularly in human myometrium (from patients with placenta accreta or postpartum hemorrhage resulting from uterine atony), than those seen with clinically used tocolytics. Moreover, in vivo, bitter tastants can protect against PTB in a gustducin-dependent manner in mice in 2 models that mimic human PTB clinically, and this protection is superior to currently used tocolytics. Although mice may not be an ideal model for human PTB, and parturition is a complex process involving not only uterine contraction but also cervix remodeling (43), our findings suggest that TAS2R-mediated uterine relaxation is an attractive approach for preterm labor therapeutics. Because bitter tastants and current tocolytics act on different molecular targets, our study further suggests that bitter tastants may complement current medicine to better treat PTB. The potential of using bitter tastants as tocolytics is of importance, given that for the past 3 decades there has been only one medicine—progesterone—approved by the FDA for preventing PTB. Significantly, many thousands of bitter tastants are available in nature and in laboratories worldwide; it is likely that specific, safe, and effective bitter tastant tocolytics could be discovered and developed. It is anticipated that bitter tastants may benefit patients sooner than one would usually expect, because many FDA-approved drugs have a strong, bitter taste, so one may be able to find new uses for old drugs. ChQ, one bitter tastant tested in this study, is “the best drug and the least harmful for the pregnant women against all types of malaria provided drug resistance is not the problem” (44). Malaria is likely a major contributor to PTB in regions in which it is endemic (5). There is evidence that ChQ not only treats malaria but also prevents the PTBs associated with it (45–47). The current view is that elimination of the Plasmodium parasitic infection, which causes malaria, is the proximate reason PTBs are prevented. However, our new finding in this study raises a possibility that ChQ could additionally be activating TAS2Rs in myometrial cells, resulting in PTB prevention.
The molecular mechanisms underlying bitter tastant–induced smooth muscle relaxation remain controversial. In this study, we found that suppressing [Ca2+]i raised by uterotonics is likely a causal signal in bitter tastant–induced myometrial relaxation, as it is in airway smooth muscle (12). It is beyond the scope of this study to pinpoint the molecular targets of bitter tastants that led to the decrease in this [Ca2+]i, although the potential targets include (but are not limited to) big-conductance Ca2+-activated K+ channels (11), nonselective cation channels (48), and IP3 receptor (49) and l-type voltage-dependent Ca2+ channels (12). Because KCl-induced myometrial contraction can be fully reversed by bitter tastants, l-type Ca2+ channels may be a major target of bitter tastants in uterine smooth muscle. It would be important to further investigate this possibility because l-type Ca2+ channels are the key channels underlying action potentials in uterine smooth muscle cells, and they play essential roles in hormone-induced contraction (25, 50, 51).
TAS2Rs have been increasingly detected in the cells and tissues outside the oral cavity, and they mediate diverse biologic functions dependent their location. Bitter tastants relax airway, vascular, bladder, and gastrointestinal smooth muscle, and they also contract pulmonary arteries and gastrointestinal smooth muscle (11, 12, 52–55). The wide expression of TAS2Rs on the one hand reveals their important biologic roles, and on the other hand poses a challenge to find bitter tastants that act on a specific cell. An attractive advantage for the uterus is that bitter tastants can be applied locally (e.g., via the vagina), which can substantially reduce its systemic effects and at the same time allow their desired effects.
To date, the functional role of bitter tastants has been inferred from studying pharmacologic interventions. It is thus essential to establish genetically that bitter tastants activate TAS2R signaling to generate their biologic function. This study is novel in that we used a genetic approach to demonstrate that bitter tastants act on the TAS2R signaling pathway to alter [Ca2+]i, relax myometrial tissues, and, more importantly, protect from PTBs in two different mouse models. However, a direct demonstration of TAS2R involvement in bitter tastant–induced relaxation awaits the generation of TAS2R-knockout mice. Interestingly, gustducin knockout partially inhibits ChQ’s ability to reverse an OT-induced myometrial contraction at lower ChQ concentrations but fails to do so at the highest ChQ concentration (i.e., 1 mM). These observations are consistent with others’ reports that gustducin knockdown can only partially prevent bitter tastants from inducing biologic responses in both oral and extraoral tissues (14, 20, 54, 56, 57). This raises a possibility that at high concentrations, ChQ may activate targets other than TAS2R or TAS2Rs to produce uterine relaxation. Alternatively, this suggests that TAS2Rs in uterine smooth muscle may also couple with other G proteins. This is highly likely because biochemical evidence indicates that TAS2Rs can also couple with transducin and other G proteins (58, 59). These results highlight the need to delete TAS2Rs directly to understand the molecular mechanisms of bitter tastant–induced relaxation and other biologic responses. An interesting finding in this study is that although mice have 35 TAS2Rs, mouse myometrial cells express only a small number of them (i.e., TAS2R126, -135, -137, and -143), and moreover, 3 of these 4 TAS2Rs (i.e., TAS2R126, -137, and -143) cluster (i.e., have no other genes between them) in the genome. This genomic pattern raises the possibility that there exists a shared transcription regulation factor controlling the expression of these 3 TAS2Rs in myometrial cells. Finding this mechanism could provide insight into the roles of TAS2Rs in uterine function, pregnancy, and parturition.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank R. F. Margolskee (Monell Chemical Senses Center, Philadelphia, PA, USA) for providing Gnat3−/− mice. This study was supported, in part, by the National Natural Science Foundation of China (31172206 and 31572403 to F.S.), and by the U.S. National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases Grant NIDDK098586, and NIH National Heart, Lung, and Blood Institute Grant HL117104 (both to R.Z.G.). The authors declare no conflicts of interest.
Glossary
- [Ca2+]i
intracellular Ca2+ concentration
- BSA
bovine serum albumin
- ChQ
chloroquine
- FDA
U.S. Food and Drug Administration
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- Gnat3
α-gustducin
- hTERT-HM
hTERT-infected human myometrial
- IP3
inositol trisphosphate
- KPS
Krebs physiologic buffer
- OT
oxytocin
- PGF2α
prostaglandin F2α
- PLCβ2
phospholipase C β2
- PTB
preterm birth
- PTX
pertussis toxin
- qPCR
quantitative PCR
- RU-486
mifepristone
- shRNA
short hairpin RNA
- T2R/TAS2R
bitter taste receptor
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
F. Shi and R. ZhuGe initiated and directed research; K. Zheng, and P. Lu designed and performed experiments and analyzed data; K. Bellve, K. Fogarty, R. Deng, J. Condon, E. Delpapa, L. Lifshitz, and T. Simas contributed new reagents and analytic tools; and R. ZhuGe designed experiments, analyzed data, and wrote the article with input from all other authors.
REFERENCES
- 1.Blencowe H., Cousens S., Chou D., Oestergaard M., Say L., Moller A. B., Kinney M., Lawn J.; Born Too Soon Preterm Birth Action Group (2013) Born too soon: the global epidemiology of 15 million preterm births. Reprod. Health 10(Suppl 1), S2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erdei C., Dammann O. (2014) The perfect storm: preterm birth, neurodevelopmental mechanisms, and autism causation. Perspect. Biol. Med. 57, 470–481 [DOI] [PubMed] [Google Scholar]
- 3.Ortinau C., Neil J. (2015) The neuroanatomy of prematurity: normal brain development and the impact of preterm birth. Clin. Anat. 28, 168–183 [DOI] [PubMed] [Google Scholar]
- 4.Simmons L. E., Rubens C. E., Darmstadt G. L., Gravett M. G. (2010) Preventing preterm birth and neonatal mortality: exploring the epidemiology, causes, and interventions. Semin. Perinatol. 34, 408–415 [DOI] [PubMed] [Google Scholar]
- 5.Romero R., Dey S. K., Fisher S. J. (2014) Preterm labor: one syndrome, many causes. Science 345, 760–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swaggart K. A., Pavlicev M., Muglia L. J. (2015) Genomics of preterm birth. Cold Spring Harb. Perspect. Med. 5, a023127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Illanes S. E., Pérez-Sepúlveda A., Rice G. E., Mitchell M. D. (2014) Preterm labour: association between labour physiology, tocolysis and prevention. Expert Opin. Investig. Drugs 23, 759–771 [DOI] [PubMed] [Google Scholar]
- 8.Smith R. (2007) Parturition. N. Engl. J. Med. 356, 271–283 [DOI] [PubMed] [Google Scholar]
- 9.Van Vliet E. O., Boormans E. M., de Lange T. S., Mol B. W., Oudijk M. A. (2014) Preterm labor: current pharmacotherapy options for tocolysis. Expert Opin. Pharmacother. 15, 787–797 [DOI] [PubMed] [Google Scholar]
- 10.Mitchell B. F., Aguilar H. N., Mosher A., Wood S., Slater D. M. (2013) The uterine myocyte as a target for prevention of preterm birth. Facts Views Vis. Obgyn 5, 72–81 [PMC free article] [PubMed] [Google Scholar]
- 11.Deshpande D. A., Wang W. C., McIlmoyle E. L., Robinett K. S., Schillinger R. M., An S. S., Sham J. S., Liggett S. B. (2010) Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat. Med. 16, 1299–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang C.-H., Lifshitz L. M., Uy K. F., Ikebe M., Fogarty K. E., ZhuGe R. (2013) The cellular and molecular basis of bitter tastant–induced bronchodilation. PLoS Biol. 11, e1001501 Erratum: PLoS Biol. 2013;11(3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chandrashekar J., Mueller K. L., Hoon M. A., Adler E., Feng L., Guo W., Zuker C. S., Ryba N. J. (2000) T2Rs function as bitter taste receptors. Cell 100, 703–711 [DOI] [PubMed] [Google Scholar]
- 14.Wong G. T., Gannon K. S., Margolskee R. F. (1996) Transduction of bitter and sweet taste by gustducin. Nature 381, 796–800 [DOI] [PubMed] [Google Scholar]
- 15.Chaudhari N., Roper S. D. (2010) The cell biology of taste. J. Cell Biol. 190, 285–296; erratum (2010) 191, 429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Behrens M., Meyerhof W. (2011) Gustatory and extragustatory functions of mammalian taste receptors. Physiol. Behav. 105, 4–13 [DOI] [PubMed] [Google Scholar]
- 17.Zhuge R., Bao R., Fogarty K. E., Lifshitz L. M. (2010) Ca2+ sparks act as potent regulators of excitation–contraction coupling in airway smooth muscle. J. Biol. Chem. 285, 2203–2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ZhuGe R., Tuft R. A., Fogarty K. E., Bellve K., Fay F. S., Walsh J. V. Jr. (1999) The influence of sarcoplasmic reticulum Ca2+ concentration on Ca2+ sparks and spontaneous transient outward currents in single smooth muscle cells. J. Gen. Physiol. 113, 215–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rozengurt N., Wu S. V., Chen M. C., Huang C., Sternini C., Rozengurt E. (2006) Colocalization of the alpha-subunit of gustducin with PYY and GLP-1 in L cells of human colon. Am. J. Physiol. Gastrointest. Liver Physiol. 291, G792–G802 [DOI] [PubMed] [Google Scholar]
- 20.Janssen S., Laermans J., Verhulst P. J., Thijs T., Tack J., Depoortere I. (2011) Bitter taste receptors and α-gustducin regulate the secretion of ghrelin with functional effects on food intake and gastric emptying. Proc. Natl. Acad. Sci. USA 108, 2094–2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang L., Shanker Y. G., Dubauskaite J., Zheng J. Z., Yan W., Rosenzweig S., Spielman A. I., Max M., Margolskee R. F. (1999) Ggamma13 colocalizes with gustducin in taste receptor cells and mediates IP3 responses to bitter denatonium. Nat. Neurosci. 2, 1055–1062 [DOI] [PubMed] [Google Scholar]
- 22.Mestdagh P., Feys T., Bernard N., Guenther S., Chen C., Speleman F., Vandesompele J. (2008) High-throughput stem-loop RT-qPCR miRNA expression profiling using minute amounts of input RNA. Nucleic Acids Res. 36, e143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y., Hoon M. A., Chandrashekar J., Mueller K. L., Cook B., Wu D., Zuker C. S., Ryba N. J. (2003) Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell 112, 293–301 [DOI] [PubMed] [Google Scholar]
- 24.Aguilar H. N., Mitchell B. F. (2010) Physiological pathways and molecular mechanisms regulating uterine contractility. Hum. Reprod. Update 16, 725–744 [DOI] [PubMed] [Google Scholar]
- 25.Sanborn B. M., Ku C. Y., Shlykov S., Babich L. (2005) Molecular signaling through G-protein-coupled receptors and the control of intracellular calcium in myometrium. J. Soc. Gynecol. Investig. 12, 479–487 [DOI] [PubMed] [Google Scholar]
- 26.Committee on Understanding Premature Birth and Assuring Healthy Outcomes. (2007) Diagnosis and treatment of preterm labor. In Preterm Birth: Causes, Consequences, and Prevention (Behrman R. E., Butler A. S., eds.), pp. 259–310, National Academies Press, Washington, DC: [PubMed] [Google Scholar]
- 27.Morrey C., Estephan R., Abbott G. W., Levi R. (2008) Cardioprotective effect of histamine H3-receptor activation: pivotal role of G beta gamma–dependent inhibition of voltage-operated Ca2+ channels. J. Pharmacol. Exp. Ther. 326, 871–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orr A. W., Pallero M. A., Murphy-Ullrich J. E. (2002) Thrombospondin stimulates focal adhesion disassembly through Gi- and phosphoinositide 3-kinase-dependent ERK activation. J. Biol. Chem. 277, 20453–20460 [DOI] [PubMed] [Google Scholar]
- 29.Meyerhof W., Batram C., Kuhn C., Brockhoff A., Chudoba E., Bufe B., Appendino G., Behrens M. (2010) The molecular receptive ranges of human TAS2R bitter taste receptors. Chem. Senses 35, 157–170 [DOI] [PubMed] [Google Scholar]
- 30.Kaga N., Katsuki Y., Obata M., Shibutani Y. (1996) Repeated administration of low-dose lipopolysaccharide induces preterm delivery in mice: a model for human preterm parturition and for assessment of the therapeutic ability of drugs against preterm delivery. Am. J. Obstet. Gynecol. 174, 754–759 [DOI] [PubMed] [Google Scholar]
- 31.Elovitz M. A., Mrinalini C. (2004) Animal models of preterm birth. Trends Endocrinol. Metab. 15, 479–487 [DOI] [PubMed] [Google Scholar]
- 32.Ehn N. L., Cooper M. E., Orr K., Shi M., Johnson M. K., Caprau D., Dagle J., Steffen K., Johnson K., Marazita M. L., Merrill D., Murray J. C. (2007) Evaluation of fetal and maternal genetic variation in the progesterone receptor gene for contributions to preterm birth. Pediatr. Res. 62, 630–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romero R., Yeo L., Chaemsaithong P., Chaiworapongsa T., Hassan S. S. (2014) Progesterone to prevent spontaneous preterm birth. Semin. Fetal Neonatal Med. 19, 15–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stamatelou F., Deligeoroglou E., Farmakides G., Creatsas G. (2009) Abnormal progesterone and corticotropin releasing hormone levels are associated with preterm labour. Ann. Acad. Med. Singapore 38, 1011–1016 [PubMed] [Google Scholar]
- 35.Darne J., McGarrigle H. H., Lachelin G. C. (1987) Increased saliva oestriol to progesterone ratio before preterm delivery: a possible predictor for preterm labor? Br. Med. J. (Clin. Res. Ed.) 294, 270–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lachelin G. C., McGarrigle H. H., Seed P. T., Briley A., Shennan A. H., Poston L. (2009) Low saliva progesterone concentrations are associated with spontaneous early preterm labour (before 34 weeks of gestation) in women at increased risk of preterm delivery. BJOG 116, 1515–1519 [DOI] [PubMed] [Google Scholar]
- 37.Priya B., Mustafa M. D., Guleria K., Vaid N. B., Banerjee B. D., Ahmed R. S. (2013) Salivary progesterone as a biochemical marker to predict early preterm birth in asymptomatic high-risk women. BJOG 120, 1003–1011 [DOI] [PubMed] [Google Scholar]
- 38.Maged A. M., Mohesen M., Elhalwagy A., Abdelhafiz A. (2015) Salivary progesterone and cervical length measurement as predictors of spontaneous preterm birth. J. Matern. Fetal Neonatal Med. 28, 1147–1151 [DOI] [PubMed] [Google Scholar]
- 39.Manuck T. A., Lai Y., Meis P. J., Dombrowski M. P., Sibai B., Spong C. Y., Rouse D. J., Durnwald C. P., Caritis S. N., Wapner R. J., Mercer B. M., Ramin S. M. (2011) Progesterone receptor polymorphisms and clinical response to 17-alpha-hydroxyprogesterone caproate. Am. J. Obstet. Gynecol. 205, 135.e131–135.e139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dudley D. J., Branch D. W., Edwin S. S., Mitchell M. D. (1996) Induction of preterm birth in mice by RU486. Biol. Reprod. 55, 992–995 [DOI] [PubMed] [Google Scholar]
- 41.Gupta S., Roman A. S. (2012) 17-α Hydroxyprogesterone caproate for the prevention of preterm birth. Womens Health (Lond) 8, 21–30 [DOI] [PubMed] [Google Scholar]
- 42.Jarde A., Lutsiv O., Park C. K., Beyene J., Dodd J. M., Barrett J., Shah P. S., Cook J. L., Saito S., Biringer A. B., Sabatino L., Giglia L., Han Z., Staub K., Mundle W., Chamberlain J., McDonald S. D. (2017) Effectiveness of progesterone, cerclage and pessary for preventing preterm birth in singleton pregnancies: a systematic review and network meta-analysis. [E-pub ahead of print] BJOG doi: 10.1111/1471-0528.14624 [DOI] [PubMed] [Google Scholar]
- 43.Mitchell B. F., Taggart M. J. (2009) Are animal models relevant to key aspects of human parturition? Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R525–R545 [DOI] [PubMed] [Google Scholar]
- 44.Krishna U. T. D., Daftary S. (2001) Pregnancy at Risk Current Concepts, 4th ed., Jaypee Brothers Medical Publishers, New Delhi, India: [Google Scholar]
- 45.Radeva-Petrova D., Kayentao K., ter Kuile F. O., Sinclair D., Garner P. (2014) Drugs for preventing malaria in pregnant women in endemic areas: any drug regimen versus placebo or no treatment. Cochrane Database Syst. Rev. (10):CD000169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garner P., Gülmezoglu A. M. (2006) Drugs for preventing malaria in pregnant women. Cochrane Database Syst. Rev. (4):CD000169 [DOI] [PubMed] [Google Scholar]
- 47.Ngassa P. C. (2000) Malaria parasitaemia and the risk of preterm labour: a re-evaluation of the evidence. Afr. J. Reprod. Health 4, 53–61 [Google Scholar]
- 48.Zhang T., Luo X. J., Sai W. B., Yu M. F., Li W. E., Ma Y. F., Chen W., Zhai K., Qin G., Guo D., Zheng Y. M., Wang Y. X., Shen J. H., Ji G., Liu Q. H. (2014) Non-selective cation channels mediate chloroquine-induced relaxation in precontracted mouse airway smooth muscle. PLoS One 9, e101578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tan X., Sanderson M. J. (2014) Bitter tasting compounds dilate airways by inhibiting airway smooth muscle calcium oscillations and calcium sensitivity. Br. J. Pharmacol. 171, 646–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moynihan A. T., Smith T. J., Morrison J. J. (2008) The relaxant effect of nifedipine in human uterine smooth muscle and the BK(Ca) channel. Am. J. Obstet. Gynecol. 198, 237.e1–237.e8 [DOI] [PubMed] [Google Scholar]
- 51.Shmygol A., Blanks A. M., Bru-Mercier G., Gullam J. E., Thornton S. (2007) Control of uterine Ca2+ by membrane voltage: toward understanding the excitation–contraction coupling in human myometrium. Ann. N. Y. Acad. Sci. 1101, 97–109 [DOI] [PubMed] [Google Scholar]
- 52.Sai W. B., Yu M. F., Wei M. Y., Lu Z., Zheng Y. M., Wang Y. X., Qin G., Guo D., Ji G., Shen J., Liu Q. H. (2014) Bitter tastants induce relaxation of rat thoracic aorta precontracted with high K(+). Clin. Exp. Pharmacol. Physiol. 41, 301–308 [DOI] [PubMed] [Google Scholar]
- 53.Upadhyaya J. D., Singh N., Sikarwar A. S., Chakraborty R., Pydi S. P., Bhullar R. P., Dakshinamurti S., Chelikani P. (2014) Dextromethorphan mediated bitter taste receptor activation in the pulmonary circuit causes vasoconstriction. PLoS One 9, e110373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Avau B., Rotondo A., Thijs T., Andrews C. N., Janssen P., Tack J., Depoortere I. (2015) Targeting extra-oral bitter taste receptors modulates gastrointestinal motility with effects on satiation. Sci. Rep. 5, 15985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sakai H., Sato K., Kai Y., Chiba Y., Narita M. (2016) Denatonium and 6-n-propyl-2-thiouracil, agonists of bitter taste receptor, inhibit contraction of various types of smooth muscles in the rat and mouse. Biol. Pharm. Bull. 39, 33–41 [DOI] [PubMed] [Google Scholar]
- 56.Clapp T. R., Trubey K. R., Vandenbeuch A., Stone L. M., Margolskee R. F., Chaudhari N., Kinnamon S. C. (2008) Tonic activity of Galpha-gustducin regulates taste cell responsivity. FEBS Lett. 582, 3783–3787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Caicedo A., Pereira E., Margolskee R. F., Roper S. D. (2003) Role of the G-protein subunit alpha-gustducin in taste cell responses to bitter stimuli. J. Neurosci. 23, 9947–9952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ueda T., Ugawa S., Yamamura H., Imaizumi Y., Shimada S. (2003) Functional interaction between T2R taste receptors and G-protein alpha subunits expressed in taste receptor cells. J. Neurosci. 23, 7376–7380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ruiz-Avila L., McLaughlin S. K., Wildman D., McKinnon P. J., Robichon A., Spickofsky N., Margolskee R. F. (1995) Coupling of bitter receptor to phosphodiesterase through transducin in taste receptor cells. Nature 376, 80–85 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.