Abstract
Pulmonary neuroendocrine cells (PNECs) are the only innervated airway epithelial cells. To what extent neural innervation regulates PNEC secretion and function is unknown. Here, we discover that neurotrophin 4 (NT4) plays an essential role in mucus overproduction after early life allergen exposure by orchestrating PNEC innervation and secretion of GABA. We found that PNECs were the only cellular source of GABA in airways. In addition, PNECs expressed NT4 as a target-derived mechanism underlying PNEC innervation during development. Early life allergen exposure elevated the level of NT4 and caused PNEC hyperinnervation and nodose neuron hyperactivity. Associated with aberrant PNEC innervation, the authors discovered that GABA hypersecretion was required for the induction of mucin Muc5ac expression. In contrast, NT4−/− mice were protected from allergen-induced mucus overproduction and changes along the nerve–PNEC axis without any defects in inflammation. Last, GABA installation restored mucus overproduction in NT4−/− mice after early life allergen exposure. Together, our findings provide the first evidence for NT4-dependent neural regulation of PNEC secretion of GABA in a neonatal disease model. Targeting the nerve–PNEC axis may be a valid treatment strategy for mucus overproduction in airway diseases, such as childhood asthma.—Barrios, J., Patel, K. R., Aven, L., Achey, R., Minns, M. S., Lee, Y., Trinkaus-Randall, V. E., Ai, X. Early life allergen-induced mucus overproduction requires augmented neural stimulation of pulmonary neuroendocrine cell secretion.
Keywords: NT4, innervation, GABA, childhood asthma
Mucus overproduction is a salient symptom of patients with chronic airway diseases, such as asthma. Excessive mucus blocks airway flow and is a major contributor to morbidity and mortality in people with asthma. The production of excessive mucus in people with asthma involves an increase in the number of mucin-producing secretory cells (1). However, unlike human airways, mouse airways have few mucin-producing cells under normal conditions. Allergen exposure induces mucus overproduction by up-regulating mucin expression in secretory cells (2, 3). While the origin of cells that produce mucin differs between humans and mice, mechanisms underlying mucus overproduction in allergic inflammation are evolutionally conserved. These include master transcriptional factors, such as SPDEF and STAT6, as well as key cytokines, such as IL-13 (4–6). In addition, an airway GABAergic system was found to be aberrantly activated to induce mucus overproduction in adult mouse models and human airway epithelium cell lines (7, 8). These previous studies of GABA signaling exclusively relied on pharmaceutical approaches (7, 8). As a result, the precise functional source of GABA in airways and mechanisms of GABA secretion remain unclear.
Asthma often progresses from wheezing in early childhood into a chronic disease (9, 10). Risk factors for asthma include exposure to allergens, respiratory viruses, ozone, and pollutants in infancy and early childhood. As the lung continues to grow after birth, detrimental environmental exposure may disrupt the developmental process and ultimately cause long-lasting changes in airway structure and function. Notably, clinical data indicate that commonly used treatment strategies for asthma, such as early life introduction of inhaled corticosteroid that temporally dampens inflammation, have little beneficial impact on disease progression (11). These findings raise the possibility that the pathogenesis of persistent asthma may involve developmental factors.
Changes in neural innervation may be a developmental factor that connects early episodes of exposure to prolonged airway dysfunction. For example, respiratory syncytial viral infection in neonatal guinea pigs altered sensory neuropeptide expression in airways (12). Exposure to ozone, cigarette smoke, or house dust mite allergen also increased airway innervation in neonatal rodents and infant rhesus monkeys (13–16). In a previous study, we showed that neonatal allergen exposure in mice aberrantly increased the level of airway innervation (17). In contrast, allergen exposure in adult mice has no effect on innervation (17). Compared to adult mouse models, allergen exposure during the first 3 wk after birth induced similar but modest eosinophilic inflammation (17). However, the neonatal mouse model exhibited persistent airway hyperreactivity that was functionally linked to airway smooth muscle (ASM) hyperinnervation (17). Mechanistically, we showed that neurotrophin 4 (NT4) was expressed by developing ASM, while nerves selectively expressed the specific NT4 receptor tropomyosin receptor kinase B (TrkB), thereby establishing innervation during postnatal development (17). Early life allergen exposure in mice activated mast cells as a de novo source of NT4, which in turn aberrantly increased ASM innervation (18). Genetic disruption of NT4 and TrkB signaling prevented allergen-induced increases in airway innervation and diminished airway hyperreactivity without any effect on allergic inflammation (17). Last, consistent with abnormal neural development in animal models of neonatal exposure, the levels of neurotrophins were positively associated with respiratory viral infection in infants and with severity of asthma in children (19, 20). These findings highlight unique changes in airway innervation after early life exposures and emphasize the disease relevance of the animal models of childhood asthma.
In addition to ASM, the other innervated cell type in airways is pulmonary neuroendocrine cells (PNECs). PNECs exist as solitary cells or in clusters called neuroendocrine bodies (NEBs). PNECs express a variety of bioactive amines and neuropeptides including calcitonin gene-related peptide (CGRP) and are innervated by a neurocircuitry that consists of sensory afferents mostly from nodose ganglion neurons and vagal efferents (21–23). PNECs are sensors for oxygen and inflammation during the transition to air breathing and are mechanotransducers in adult lungs via secretion of bioactive mediators (22, 24). Compared to other neuroendocrine organs, such as the gut, pancreas, pituitary gland, and the reproductive system, where endocrine secretion is regulated by neural activities to accommodate for fundamental bodily functions, how PNECs become innervated and whether nerves regulate PNEC secretion and function in airway physiology and diseases are unknown.
In this study, we investigated the mechanism of PNEC innervation during postnatal development and assessed the role of the nerve–PNEC axis in a neonatal mouse model of allergic inflammation. We identified NT4 as an essential factor for PNEC innervation and PNECs as the only cellular source of GABA in airways. Notably, GABA is converted from glutamate by glutamate decarboxylase 67 (Gad67) and Gad65 that are encoded by Gad1 and Gad2 genes, respectively. In addition, a specific vesicular GABA transporter, Slc32A1, is responsible for packaging GABA into secretory vesicles before secretion (25). To identify the functional relationship between NT4 and PNEC-derived GABA, our study used genetic approaches to disrupt NT4 and GABA biosynthesis. These assays, in combination with GABA rescue in NT4−/− mice, revealed a critical role of NT4-dependent neural regulation of PNEC secretion of GABA in mucus overproduction after early life allergen exposure.
MATERIALS AND METHODS
Mice
C57BL6, NT4−/−, ShhCre+, TrkBCreERT2/+, Slc32a1f/f, RosatmRed/tmRed, and Gad1-GFP were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Mice were of the C57BL/6 genetic background except Slc32a1flox/flox mice, which were of a mixed C57/129 background. All mouse experiments were approved by the Institutional Animal Care and Use Committee at Brigham and Women’s Hospital.
OVA exposure in neonatal mice
For ovalbumin (OVA) exposure, pups were sensitized by intraperitoneal injections of 10 μg OVA (Sigma-Aldrich, St. Louis, MO, USA) in Imject alum (Thermo Fisher Scientific, Waltham, MA, USA) on postnatal day (P)5 and 10, followed by 3 consecutive challenges with 3% aerosolized OVA solution on P18, 19, and 20 (17). Control pups were challenged with PBS. Mice were humanely killed at P21 for bodily fluid and tissue collection. Serum levels of OVA-specific IgE and GABA were measured by ELISA M036005 (MD Biosciences, St. Paul, MN, USA) and NC0436948 (Rocky Mountain Diagnostics, Colorado Springs, CO, USA). For GABA rescue assay, NT4−/− mice were subjected to intratracheal delivery of GABA (10 µg/g body weight; Sigma-Aldrich) daily between P14 and 20. For methacholine (Mch, a stable mimic of acetylcholine) rescue assay, NT4−/− mice were nebulized for 10 min daily between P15 and 20 with Mch (30 mg/ml; Sigma-Aldrich).
Bronchoalveolar lavage counts
Mouse lungs were flushed with 0.5 ml cold PBS before bronchoalveolar lavage (BAL) was collected. Cells were pelleted, resuspended in 200 μl PBS, and spun onto a histology slide by using Cytospin (Thermo Fisher Scientific). The slides stained with Hema3 stain. Two hundred cells were counted to determine relative abundance of different immune cell types.
TrkB lineage tracing
TrkBCreERT2/+ mice were crossed with a Rosa(tmRed) reporter line. TrkBCreERT2/+;Rosa(tmRed) mice received 6 tamoxifen injections (0.2 mg per injection) between P5–7 and P15–17. Lungs were collected at P21 and processed for histology.
Lung slice preparation and culture
Lung lobes were infused with 1.5% low-melting agarose (Thermo Fisher Scientific). After cooling on ice for 15 min, the lobes were sliced 150-µm thick with a VF-300 microtome (Precisionary Instruments, Greenville, NC, USA). Lung slices were recovered overnight in DMEM/F-12 medium (1:1; Thermo Fisher Scientific) before treatment with GABA (10 mg/ml), CGRP (10 µM), substance P (10 µM), dopamine (10 µM), and Mch (10 μM). After 2 d, slices were collected for gene expression assays.
Histology and immunohistochemistry
Lungs were fixed in 4% paraformaldehyde/PBS. Paraffin-embedded sections (5 μm) of right cranial lobes were collected for periodic acid–Schiff (PAS) staining of mucus (Sigma-Aldrich) and for antibody staining. For staining of nodose ganglion neurons, frozen sections of dissected nodose ganglions were used. For double staining of PNECs and nerves, lung slices were used. Primary antibodies included the following: mouse anti-NT4 (1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-Uchl1 (1:100; Thermo Fisher Scientific), guinea pig anti-P2X3 (1:100; EMD Millipore, Billerica, MA, USA), mouse anti–neural class III β-tubulin antibody (TuJ1) (1:50; R&D Systems, Minneapolis, MN, USA), rabbit anti-CGRP (1:1000, Sigma-Aldrich), goat anti-Foxa3 (1:50; Santa Cruz Biotechnology), goat anti-Scgb1a1 (1:1000; Santa Cruz Biotechnology), mouse anti-Gad67 (1:500; EMD Millipore), and mouse anti-Foxj1 (1:300, eBioscience, San Diego, CA, USA). For mouse primary antibodies, tissue sections were blocked using the Mouse on Mouse Kit (M.O.M.; Vector Laboratories, Burlingame, CA, USA) before incubation with secondary antibodies. All secondary antibodies were purchased from Thermo Fisher Scientific. Nuclei were stained using Hoechst dye (1:1000). Fluorescent images were collected by a digital camera (Leica, Wetzlar, Germany) for tissue sections and by confocal microscopy (Carl Zeiss GmbH, Jena, Germany) for lung slices. Compressed z stack images were quantified by ImageJ software (Image Processing and Analysis in Java; National Institutes of Health, Bethesda, MD, USA; http://imagej.nih.gov/) to determine the NEB innervation density by normalizing TuJ1 immunoreactive area to the number of cells within the cluster.
Real-time quantitative PCR
RNA was extracted from lung samples with an RNeasy kit (Qiagen, Germantown, MD, USA), followed by reverse transcription using Supercript III Reverse Transcriptase (Thermo Fisher Scientific). TaqMan primers for Muc5ac (Mm01276704_m1), Gad1 (Mm04207430_g1), and 18S (Mm03928990_g1) were purchased from Thermo Fisher Scientific. The relative gene expression was measured by normalizing to 18S rRNA using ΔΔCt.
Lung epithelial cell sorting
Lungs were digested with 0.1% collagenase A (Roche, Basel, Switzerland), 2.4 U/ml dispase (Roche), and 6 U/ml DNase I (Qiagen). Single-cell suspensions were stained with rat anti-mouse CD45 and rat anti-mouse CD31 (BD Pharmingen, Franklin Lakes, NJ, USA) and rat anti-mouse CD326 (eBioscience). Cell isolation was performed using FACSAria III (BD Biosciences, San Jose, CA, USA). Isotype antibodies were used as controls. The CD31−CD45−CD326+ populations were deemed lung epithelium cells (18).
Primary nodose ganglion neuron culture and Ca2+ imaging
Nodose ganglions were dissected from P18 mice, followed by enzymatic dissociation using 2.4 U/ml dispase (Roche). A total of 1000 cells were plated in each glass-bottomed dish (0.5 mm in diameter) precoated with polylysine. Cells were cultured in neurobasal medium supplemented with B-27 (Thermo Fisher Scientific). Recombinant human NT4 (N1780; Sigma-Aldrich) was added at a concentration of 100 ng/ml, while control cultures had bovine serum albumin (BSA). After 3 d, cells were washed with neurobasal medium before cells were loaded with 5 µM Fluo-3 AM fluorescent dye (Thermo Fisher Scientific) for 20 min at 37°C and 5% CO2. Images were collected every 100 ms on a Zeiss LSM 880 confocal microscope. Approximately 50 frames were captured to establish baseline fluorescence (F0). Cells were stimulated with ATP (100 µM; Sigma-Aldrich), and images were continuously captured. Analysis was performed by Fiji (https://fiji.sc/)/ImageJ software. Relative fluorescence was calculated by normalizing Fluo-3 AM fluorescence after ATP stimulation to the baseline fluorescence (Ft/F0) (26). To block the P2X3 receptor, cultures were treated for 20 min before imaging and during the course of imaging with a selective inhibitor, A-317491 (1 µM, A2979; Sigma-Aldrich) (27). After imaging, primary nodose ganglion neuron cultures were fixed, followed by P2X3 staining.
Statistical analysis
Data are presented as means ± sem from at least 3 independent experiments. Statistical significance was determined by paired 2-tailed Student’s t test or 2-way ANOVA with appropriate post hoc tests using GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA). Values of P < 0.05 were deemed significant.
RESULTS
NT4 was required for mucus overproduction after early life allergen exposure in mice
We utilized the disease-mimicking early life exposure model to study the mechanism of mucus overproduction. Repetitive exposure to allergens such as OVA (Fig. 1A), house dust mite, and cockroach allergen within the first 3 wk after birth caused mucus overproduction in wild-type (WT) mice (Fig. 1B–E and Supplemental Fig. 1A–E). In contrast, NT4−/− mice were resistant to mucus overproduction after similar exposures without any defects in allergic inflammation (Fig. 1B–E and Supplemental Fig. 1A–E) (17). In support of this finding, blockade of TrkB signaling in TrkBF616A/TrkBF616A mice by 1NMPP1 during OVA exposure reduced mucus overproduction (Supplemental Fig. 1F, G) (17). TrkBF616A/TrkBF616A mice harbor a point mutation rendering the receptor sensitive to 1NMPP1 inhibition (28). Given that TrkB is selectively expressed by nerves in airways (17), these findings collectively indicate that NT4-dependent airway innervation is required for allergen-induced mucus overproduction in early life.
Figure 1.
NT4 was required for mucus overproduction after early life allergen exposure. A) Experimental scheme of early life OVA exposure in mice. Control mice were challenged with saline (PBS). Lungs were collected at P21. B) Serum levels of IL-13 measured by ELISA. Each mark represents 1 mouse. C) Differential immune cell counts in BAL. Relative abundance of macrophages (Mac), eosinophils (Eos), neutrophils (Neut), and lymphocytes (Lymph) was quantified as percentage of cells in BAL; n = 9 per group. D) Representative images of PAS staining in airways of WT and NT4−/− mice challenged with PBS or OVA. Mucin+ cells are marked by arrows. Enlarged epithelium staining is shown in inserts. E) Muc5ac mRNA expression in WT and NT4−/− mice that were challenged with PBS or OVA; n = 14 mice for each group. F) Muc5ac mRNA levels in lung slices of P21 WT mice (n = 3) after 48 h in culture. Results were normalized to PBS-challenged controls. G) Muc5ac mRNA levels in lung slices of P21 NT4−/− mice (n = 6) after 48 h in culture with and without neurotransmitter treatment. Results were normalized to PBS-challenged slices without treatment. Neurotransmitters tested included GABA (10 mg/ml), CGRP (10 µM), substance P (SP, 10 µM), dopamine (dopa, 10 µM), and Mch (10 μM). Data in C–E represent means ± sem from 3 independent experiments. *P < 0.05, **P < 0.01. Scale bar, 100 µm.
To elucidate the role of NT4 in mucus overproduction, we tested several neurotransmitters in Muc5ac gene expression using NT4−/− lung slices as an ex vivo system. After 2 d in culture, lung slices from WT and NT4−/− mice at P21 with and without OVA exposure maintained differential Muc5ac mRNA expression as in vivo (Fig. 1E–G). Therefore, lung slice cultures maintained relevant cell types and pathways and thus were an enabling ex vivo tool for the rescue assay in NT4−/− mice. We tested the following neurotransmitters on the basis of their likely presence in PNEC innervating nerves and their previously characterized roles in mucus production: GABA, CGRP, substance P, dopamine, and Mch. Only GABA increased Muc5ac mRNA levels by 3-fold in NT4−/− lung slices, and the effect was evident only in the presence of prior OVA exposure (Fig. 1G).
PNECs provided a critical source of GABA for allergen-induced mucus overproduction in early life
To connect GABA with NT4-dependent nerves, we speculated that GABA may be released from GABAergic nerves or by cells innervated by nerves. To distinguish between these two possibilities, we characterized the source of GABA in control and OVA-exposed mice at P21. We utilized a Gad1-GFP reporter line that expressed green fluorescent protein (GFP) under the control of the GAD1 promoter (29). Notably, the GAD1 gene encodes the GABA biosynthetic enzyme GAD67. We found no GFP+ nerves in GAD1-GFP airways (Fig. 2A). Instead, GFP was detected in a subset of airway epithelial cells that also expressed CGRP and thus were PNECs (Fig. 2A). Double staining confirmed specific Gad67 expression in ∼85% of PNECs (Fig. 2A). These findings were further supported by PNEC-specific expression of the GABA vesicular transporter A1 (Slc32a1) (30). No Gad65 expression was detected in lungs by lineage tracing using Gad2CreERT2/+;Rosa(lacZ) mice (Supplemental Fig. 3A). Therefore, the only cellular source of GABA in the airway is from innervated PNECs. Our finding is at odds with ubiquitous epithelial Gad67 expression in a previous report (7). The discrepancy may be explained by technical issues of antibody staining in the previous study.
Figure 2.
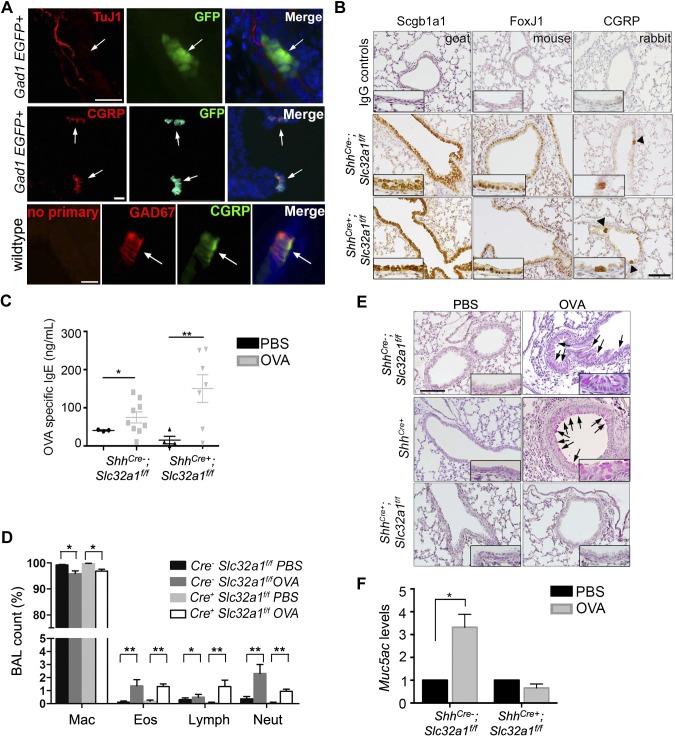
GABA from PNECs was required for mucus overproduction after early life OVA exposure. A) PNEC expression of GAD67 assayed at P21. In lungs of GAD1-GFP mice, PNECs were GFP+ while TuJ1+ nerves were not. Double staining for Gad67 and PNEC marker CGRP also showed colocalization. Arrows indicate PNECs. Scale bars, 25 µm. B) Characterization of airway epithelium in ShhCre−;Slc32a1f/f control littermates and ShhCre+;Slc32a1f/f mice at P21 by staining for specific markers of club cells (Scgb1a1), ciliated cells (Foxj1), and PNECs (CGRP). Negative controls were matched IgGs. Arrowheads indicate PNEC clusters. Insets show enlarged images of epithelium staining. Scale bars, 100 µm. C) Serum levels of OVA-specific IgE in saline and OVA-exposed littermates and ShhCre+;Slc32a1f/f mice at P21. Each mark indicates sample from 1 mouse. D) Differential immune cell count in BAL of PBS- and OVA-exposed control (Cre−) and ShhCre+;Slc32a1f/f (Cre+) mice at P21. Percentages of macrophages (Mac), eosinophils (Eos), lymphocytes (Lymph), and neutrophils (Neut) are shown. E) Representative images of PAS staining in airways of ShhCre−;Slc32a1f/f and ShhCre+ controls and ShhCre+;Slc32a1f/f mutant mice at P21. Arrows indicate mucin-positive cells. Scale bars, 100 µm. Insets show enlarged images of stained epithelium. F) Muc5ac gene expression in lungs of littermate controls (PBS; n = 4; OVA, n = 11) and ShhCre+;Slc32a1f/f mutant mice (PBS, n = 6; OVA, n = 5). Data in C, D, and F represent means ± sem from 3 independent experiments. *P < 0.05, **P < 0.01.
To establish an essential role of GABA in mucus overproduction and circumvent off-target effects often associated with pharmaceutical approaches in previous studies (7, 8), we generated tissue-specific GABA-deficient mice by crossing the ShhCre+ line into Gad1f/f and Slc32a1f/f background. The ShhCre+ line is a widely used genetic tool in pulmonary research because it acts in the entire epithelium, including PNECs (31). ShhCre+ mice had no defects in mucus overproduction after allergen exposure (Fig. 2E). We chose not to use neuroendocrine cell-specific Cre lines, including Ascl1CreER/+ and CGRPCreER/+ mice (32, 33), because of concerns of haploinsufficient phenotypes, as Ascl1 and CGRP are involved in PNEC development, inflammation, and sensory neuron function (24, 34, 35). Characterization of ShhCre+;Gad1f/f and ShhCre+;Slc32a1f/f mutant lines revealed normal airway epithelium and no change in baseline mucin expression (Fig. 2B and Supplemental Fig. 3B). Because ShhCre+;Gad1f/f mice exhibited hydrocephalus and died when they were about 3 to 4 wk old, we focused on ShhCre+;Slc32a1f/f mice that were healthy and fertile. Loss of Slc32a1 in airway epithelium had no effect on allergic inflammation assayed by serum levels of OVA-specific IgE and differential BAL counts (Fig. 2C, D), consistent with the previous finding that GABA signaling is dispensable for allergen-induced inflammation (7). However, after OVA exposure, compared to Cre− littermates that had excessive mucus in the airway, ShhCre+;Slc32a1f/f mice showed no change in baseline mucus levels (Fig. 2E, F). Consistently, 2 ShhCre+;Gad1f/f mice that survived were also resistant to OVA-induced mucus overproduction (Supplemental Fig. 3C). These findings demonstrate that PNEC-derived GABA is essential for allergen-induced mucus overproduction.
NT4 was required for PNEC innervation and PNEC hyperinnervation after early life allergen exposure
Neuroendocrine cells that reside in multiple organs are mediators of the communication between the nervous system and endocrine secretion to regulate fundamental body functions. We speculated that a similar paradigm may apply to NT4-dependent innervation and GABA secretion in allergic inflammation. To test this hypothesis, we characterized PNEC development in postnatal mouse lungs (36). We first assessed whether PNECs continued to form during the first 3 wk after birth. To accommodate for the rapid increase in lung size during postnatal development, we collected all the lung slices (150 µm thick) from the entire right cranial lobe before CGRP staining and confocal imaging to quantify solitary PNECs, NEBs, and the total number of PNECs. We found that the abundance of solitary PNECs and NEBs remained relatively unchanged (Fig. 3A). However, the total number of PNECs almost tripled at P21 from birth as a result of an increase in cell numbers within NEBs during the second week (Fig. 3A). The PNEC population stabilized around P14 (Fig. 3A).
Figure 3.
PNEC innervation during postnatal development was dependent on NT4. A) Quantification of solitary PNECs, clusters, and total number of PNECs at birth and at P7, 14, and 21 in WT mice. PNECs were quantified after CGRP staining of tissue sections collected from entire right cranial lobe. B) PNEC expression of NT4 by double staining for NT4 and CGRP. NT4−/− lung sections were negative control for NT4 antibody. Arrows indicate PNECs. Scale bars, 100 µm. C) Representative images of postnatal TrkB lineage tracing using TrkBCreERT2/+;Rosa(tmRed) mouse line. PNECs (arrows) were stained for CGRP indicated by arrows. Nerves (arrowheads) were labeled by tomato red (tmRed) fluorescence. Scale bar, 100 µm. D) Assessment of solitary PNECs and clusters in WT and NT4−/− lungs with and without OVA challenges at P21. PNECs were labeled by CGRP staining of medial lung slices from left lobe. Each mark indicates results from 1 lung slice; n = 3 for each group. E) Assessment of NEB innervation in WT and NT4−/− mice with and without OVA exposure. Lung slices were stained with CGRP and TuJ1 antibodies followed by confocal microscopy to assess spatial association between NEBs and nerves. NEBs with 5 or more PNECs were counted. NEBs without associated nerves (none), nerves at base (basal), and nerves penetrating into clusters (penetrating) were grouped and presented as percentages of all NEBs. F) Representative images of NEBs with penetrating nerves in WT and NT4−/− mice with and without OVA challenges at P21. Scale bar, 50 µm. G) Quantification of NEB innervation density. Density of nerves was calculated by normalizing TuJ1-immunoreactive area by number of cells within NEBs in WT and NT4−/− mice with and without OVA challenges. For data in E–G, total of 45–50 NEBs in lung slices from 3 mice were scored for each group. N.s., not significant. *P < 0.05.
As a second step, on the basis of our finding that postnatal airway innervation requires NT4 (17), we tested whether NT4 was expressed by PNECs as a neurotrophic factor for the innervating nerves during postnatal development. We assessed NT4 expression in PNECs by double staining for NT4 and CGRP. Compared to NT4−/− mouse as the negative control, NT4 was detected in all CGRP+ PNECs in WT mice (Fig. 3B). In addition, TrkB linage labeling during neonatal allergen exposure found no TrkB expression by PNECs using tamoxifen-inducible TrkBCreERT2/+;Rosa(tmRed) mice (Fig. 3C). Instead, the lineage labeling identified nerves in the airway (Fig. 3C) (17) and type II pneumocytes in the alveoli (Supplemental Fig. 2). The discreet expression of NT4 and TrkB in PNECs and the innervating nerves, respectively, support NT4 as a candidate target-derived neurotrophic factor for PNEC innervation.
As a third step, we assessed the role of NT4 in PNEC innervation. Lung slices of WT and NT4−/− mice were double labeled for CGRP and neuron-specific β-tubulin III before confocal microscopy and 3-D rendering of the images. Each lung slice had an average of 2 to 3 single PNECs and 10 clusters, half of which contained 5 or more cells in both WT and NT4−/− mice (Fig. 3D). These findings indicated that NT4 is dispensable for PNEC development, which is consistent with a lack of TrkB expression by PNECs (Fig. 3C). To assess PNEC innervation, we evaluated the innervation of NEBs containing 5 or more cells because a majority of nerves were associated with relatively large clusters. We noted 3 distinct spatial patterns of nerves in relationship to NEBs: no associated nerves (none), nerves only at the base (basal), and nerves sprouting into the NEB (penetrating). Each pattern was scored and presented as a percentage of all NEBs. Compared to WT mice, NT4−/− mice had more noninnervated NEBs (none) [59 vs. 22% (WT)] but fewer basal NEBs [26 compared to 54% (WT)] and penetrating NEBs [15 compared to 25%, (WT)] (Fig. 3E). Early life allergen exposure has been shown to elevate the levels of NT4 (17). While it had no effect on the total number of PNECs and their solitary and clustered forms (Fig. 3D), OVA exposure in WT mice increased the abundance of penetrating NEBs to 66% and elevated the density of nerves within penetrating NEBs by 4-fold compared to PBS controls (Fig. 3E–G). In contrast, OVA exposure marginally increased the overall level of PNEC innervation and had no effect on the density of penetrating nerves in NT4−/− mice (Fig. 3E–G). Together, early life allergen exposure causes aberrant increases in PNEC innervation by deregulating the essential neurotrophic factor NT4 during postnatal development.
Early life OVA exposure deregulated sensory neuron activity by elevating NT4 levels
In response to injury, airway epithelium releases ATP that activates purinergic receptors expressed by sensory nerves (37). The nodose ganglion provides a major source of sensory innervation for ASM and PNECs (23, 38, 39). A large majority of nodose ganglion neurons express a purinergic P2X3 receptor that is required for ATP-induced activities (40). Because the development of nodose ganglion neurons during embryogenesis is dose-dependent on brain-derived neurotrophic factor and NT4, both of which signal through the TrkB receptor (41, 42), we speculated that elevated levels of NT4 after early life OVA exposure might affect nodose sensory neuron activities. To test this hypothesis, we examined the neuronal cell number and P2X3 expression in WT and NT4−/− mice at P21. NT4 was required for the survival of ∼50% of nodose ganglion neurons (Fig. 4A, B) while having no effect on the percentages of P2X3+ neurons (Fig. 4C) (42). In addition, OVA exposure had no effect on the number of neurons and the percentage of P2X3+ neurons in the nodose ganglion (Fig. 4B, C). However, OVA exposure increased the P2X3 mRNA levels by 3-fold in WT mice while having no effect in NT4−/− mice (Fig. 4D). These findings suggest that allergen exposure may increase the level of P2X3 expression per neuron and thus cause neural hyperactivities in response to ATP stimulation.
Figure 4.
NT4 was required for elevated purinergic receptor gene expression and increased nodose ganglion neuronal activities after early life OVA exposure. A) Representative images of double staining of medial nodose ganglions in WT and NT4−/− mice at P21 for pan neuronal marker Uchl1 and P2X3 receptor. Nuclei were labeled by DAPI. Scale bars, 250 µm. B) Quantification of neuronal numbers in medial sections of nodose ganglions from WT and NT4−/− mice at P21. Eighteen sections from 6 ganglions of 3 mice were quantified. C) Quantification of percentages of P2X3+ neurons in nodose ganglions from WT and NT4−/− mice at P21. D) P2X3 mRNA levels in nodose ganglions from WT and NT4−/− mice with and without OVA exposure. Six ganglions from 3 mice were pooled as 1 sample. Three samples were analyzed for each condition. E) Representative images of P2X3 staining of primary nodose ganglion neurons after 72 h in culture treated with control BSA or NT4. Arrowheads indicate P2X3+ neurons. F) Ca2+ imaging of control BSA- and NT4-treated primary nodose ganglion neurons in response to ATP stimulation. Relative fluorescence (Ft/F0) of Fluo-3 AM before and after ATP stimulation (arrow) was measured. To block P2X3 activities, NT4-treated cultures were pretreated with A-317491 (1 µM) for 20 min before ATP stimulation and during entire course of Ca2+ imaging. Results represent 2 independent experiments. Data in B–D represent means ± sem of 3 independent experiments. **P < 0.01.
To test whether NT4 may affect sensory neuron activity, we isolated nodose ganglions from P18 mice and established primary nodose sensory neuron cultures in defined neurobasal medium. Because dissection severed the axons, neurons were allowed to regenerate neurites in culture for 3 d before Ca2+ imaging using a fluorescent dye Fluo-3 AM for the measurement of their activities in response to ATP. We found postnatal nodose sensory neurons no longer required NT4 for survival, as both BSA-treated controls and NT4-treated cultures (100 ng/ml) exhibited little cell death (Fig. 4E). In addition, a majority of nodose sensory neurons were P2X3+ (Fig. 4E). ATP stimulation (100 µM) triggered a spike of intracellular Ca2+ shown by a ∼15% increase above the baseline fluorescence that was reduced by 40 to 50% after 15 s of stimulation (Fig. 4F). Compared to control neurons, NT4-treated cultures exhibited a ∼45% increase in fluorescence above the baseline on ATP stimulation and maintained a higher level of intracellular Ca2+ over time (Fig. 4F). Therefore, NT4 treatment increases the activity of nodose sensory neurons to ATP. However, neurons treated with NT4 in the presence of A-317491, a specific P2X3 blocker, exhibited a faster return to baseline fluorescence while maintaining the initial Ca2+ spike (Fig. 4F). These findings provide evidence for functional involvement of P2X3 in NT4-induced nodose sensory neurons hyperactivity. In addition, NT4 may affect other ATP receptor expression to account for the elevated Ca2+ spike immediately after ATP stimulation.
NT4 was required for GABA hypersecretion to induce mucus overproduction after early life allergen exposure
To test whether aberrant increases in PNEC innervation may deregulate GABA secretion from PNECs after early life allergen exposure, we measured serum levels of GABA in WT and NT4−/− mice by ELISA, as neuroendocrine cells secrete laterally and basally into the circulation (21). NT4 had no effect on baseline serum levels of GABA (∼200 ng/ml) (Fig. 5A). However, OVA exposure elevated serum levels of GABA by ∼60% in WT mice while having no effect on NT4−/− mice (Fig. 5A). We found no change in Gad1 and GABA receptor mRNA expression in purified airway epithelium cells at P21 after early life allergen exposure (Fig. 5B and Supplemental Fig. 4A). These findings provide evidence that NT4-dependent PNEC hyperinnervation deregulates PNEC secretion of GABA.
Figure 5.
NT4 was required for GABA secretion and mucus overproduction after early life OVA exposure. A) Serum levels of GABA in WT and NT4−/− mice with and without OVA challenges measured by ELISA. Data represent means ± sem from 10 to 16 mice of each group. B) Gad1 mRNA expression in airway epithelium of WT and NT4−/− mice after PBS or OVA exposure at P21; n = 6. C, D) Serum levels of IgE (C) and IL-13 (D) in BAL of PBS-exposed NT4−/− mice and OVA-exposed NT4−/− mice with and without intratracheal GABA treatment. Each mark indicates sample from 1 mouse. E) Representative images of PAS staining of lungs sections of WT and NT4−/− mice with indicated treatment. Arrows indicate mucin+ cells. Insets show enlarged images of epithelium staining. F) Muc5ac gene expression in PBS- and OVA-exposed WT and NT4−/− mice with and without GABA treatment. Each group had minimum of 7 mice. Data represent means ± sem of at least 3 independent experiments. G) Foxa3 staining of lung sections of WT and NT4−/− mice with and without OVA exposure and GABA treatment. Arrows indicate Foxa3+ cells. H) Percentages of Foxa3+ cells in airway epithelium of each experimental group. Data represent means ± sem from 15 airways of 3 mice for each experimental condition. Scale bars, 100 μm. *P < 0.05, **P < 0.01, ***P < 0.001.
We tested whether GABA administration would restore mucus overproduction in NT4−/− mice. GABA (10 µg/g body weight) was installed intratracheally between P14 and 20. Because of the relatively long half-life of GABA in vivo (5 h) (43), GABA was provided once a day. As a control neurotransmitter, Mch (30 mg/ml) was nebulized daily. Mice were analyzed at P21. We found that GABA alone had no effect on baseline mucus levels (Fig. 5E). In addition, GABA administration in OVA-exposed WT mice had no effect on already high levels of mucin expression (Fig. 5E). In contrast, compared to saline in NT4−/− mice, GABA fully restored mucus overproduction in OVA-exposed NT4−/− mice with no effect on allergic inflammation assayed by serum levels of IgE and IL-13 (Fig. 5C–F). In contrast, Mch had no such rescuing activities (Fig. 5E). These findings indicate that NT4 is specifically required for GABA signaling during early life allergen-induced mucus overproduction. To provide further evidence, we evaluated the epithelial expression of Foxa3, a transcriptional factor associated with mucus overproduction in both animal models and people with asthma (5, 6, 44). GABA partially recovered Foxa3 expression in ∼13% airway epithelial cells in OVA-exposed NT4−/− mice compared to WT mice, which had ∼34% Foxa3+ cells in airway epithelium after OVA exposure (Fig. 5G, H). Furthermore, GABA had no rescue activities in PBS-exposed NT4−/− mice (Fig. 5F), similar to the results in mouse lung slice cultures (Fig. 1G).
DISCUSSION
Our study used a combination of lineage tracing and in vivo and ex vivo models to investigate the development and neural regulation of pulmonary neuroendocrine system. Our results revealed an orchestrating role for NT4 in PNEC innervation and neuroendocrine secretion of GABA. Building on these findings, we propose a model of NT4-dependent mucus overproduction after early life allergen exposure (Fig. 6). In the model, NT4 is expressed by PNECs and serves to attract the innervating nerves during postnatal development. Allergen exposure to developing lungs, which elevates the levels of NT4 (17), causes aberrant increases in PNEC innervation and sensory neuron activity that feeds on efferent nerves to induce GABA hypersecretion. Excessive GABA signaling and IL-13 in airway epithelium ultimately induces mucus overproduction (Fig. 6). Blockade of NT4 and TrkB receptor signaling during allergen exposure prevents neural deregulation GABA secretion from PNECs and therefore prevents mucus overproduction (Fig. 6). Our findings, in combination with our previous work concerning ASM innervation and hyperreactivity, highlight the profound impact of allergen exposure on the pattern and degree of innervation of both ASM and PNECs in developing postnatal lungs. Our previous study identified mast cells as a de novo source of NT4 for allergen-induced ASM hyperinnervation (18). Whether mast cells play a similar role in aberrant PNEC innervation in the disease model warrants further investigation.
Figure 6.
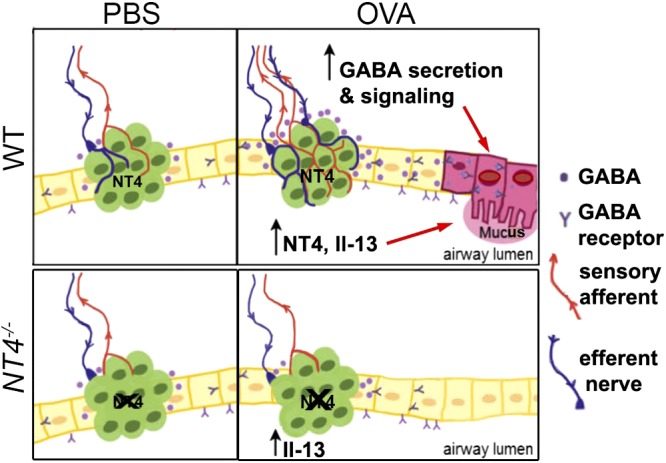
Model of NT4-PNEC axis in GABA secretion and mucus overproduction after early life allergen exposure. PNECs are innervated by both sensory afferents and efferent nerves. During development, NT4 expressed by PNECs serves as target-derived trophic factor for innervating nerves to establish innervation. Allergen exposure during postnatal PNEC development elevates levels of NT4, which in turn causes PNEC hyperinnervation and hyperactive nodose sensory neurons that feed on efferent nerves. Hyperactive neurocircuitry deregulates GABA secretion from PNECs. Aberrant levels of GABA act together with IL-13 to induce mucus overproduction. Loss of NT4 reduces PNEC innervation at baseline and prevents allergen-induced PNEC hyperinnervation. There is thus no induction of GABA secretion from PNECs in allergen-exposed NT4−/− mice. IL-13, in absence of GABA secretion, fails to induce mucus overproduction.
Our findings indicate that IL-13 provides a permissive signal for the essential GABA function in mucus overproduction after allergen exposure. This may be mediated through the multifaceted role of IL-13 in gene activation and in epithelial cell survival and differentiation to enable GABA signaling (7). In addition, allergic inflammation, which damages the barrier function of airway epithelium (45, 46), may also provide a permissive barrier mechanism for paracrine GABA signaling by facilitating the distribution of GABA after PNEC secretion in vivo. The apical–lateral distribution of a GABA receptor by club cells (Supplemental Fig. 4B) supports the possibility that GABA may interact with the receptors after basal–lateral secretion from PNECs. Furthermore, GABA receptor antagonists had no worsening effect on allergen-induced airway hyperreactivity (Supplemental Fig. 5). Therefore, targeting NT4 along the nerve–PNEC axis to block GABA secretion may be a valuable treatment strategy for mucus overproduction in asthma.
Allergen exposure to developing postnatal lungs elevates the neurogenic signal NT4 to disrupt the neurocircuitry that innervates PNECs. In addition to quantitative increases in PNEC innervation, we also provide evidence for NT4-induced sensory neuron hyperactivity in response to ATP. Whether PNEC-innervating efferent nerves are altered by early life allergen exposure warrants future work, as the efferent signal that induces GABA secretion from PNECs remains to be identified. However, independent of changes in efferent nerves, hyperactive sensory afferents are expected to feed on efferent nerves to deregulate the activity of the neurocircuitry, which in turn induces GABA hypersecretion. In contrast to neonatal mice, we showed that adult mice had no change in airway innervation after allergen exposure (17). Without the increase in innervation, we speculate that GABA secretion from PNECs may be triggered by the neurocircuitry activated in response to the stimuli present in the inflammatory milieu, such as ATP. Identification of the stimuli and investigation of their induction by injury, such as allergen exposure, cigarette smoke, and viral infection, may help explain why mucus overproduction occurs in a variety of airway diseases.
In summary, our study provides evidence that the nerve–PNEC axis may be involved in the pathogenesis of asthma after early episodes of exposures. Targeting NT4-dependent PNEC innervation may provide a novel strategy for the treatment of mucus overproduction.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Y. Bai and K. Trieu (both from Brigham and Women’s Hospital) for technical assistance, and K. C. Kim, (University of Arizona, Tucson, AZ, USA) for critical reading. This work is supported by an American Asthma Foundation award (12-0086), U.S. National Institutes of Health, National Heart, Lung, and Blood Institute (NIH/NHLBI) Grants 1R01HL116163 and 1R01HL132991 (to X.A.), and NIH/NHLBI F31 Training Grant HL007035 (to J.B.).
Glossary
- ASM
airway smooth muscle
- BAL
bronchoalveolar lavage
- BSA
bovine serum albumin
- CGRP
calcitonin gene-related peptide
- GFP
green fluorescent protein
- Mch
methacholine
- NEB
neuroendocrine body
- NT4
neurotrophin 4
- OVA
ovalbumin
- PAS
periodic acid–Schiff
- PNEC
pulmonary neuroendocrine cell
- TrkB
tropomyosin receptor kinase B
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
X. Ai conceived the project; J. Barrios, K. R. Patel, and L. Aven designed the experiments; X. Ai supervised the project; J. Barrios, L. Aven, and K. R. Patel performed most of the experiments; M. S. Minns, Y. Lee, and V. E. Trinkaus-Randall performed Ca2+ imaging for nodose ganglion neurons; J. Barrios and X. Ai compiled the data; J. Barrios and X. Ai prepared the figures and wrote the article; and all authors provided feedback on the article.
REFERENCES
- 1.Ordoñez C. L., Khashayar R., Wong H. H., Ferrando R., Wu R., Hyde D. M., Hotchkiss J. A., Zhang Y., Novikov A., Dolganov G., Fahy J. V. (2001) Mild and moderate asthma is associated with airway goblet cell hyperplasia and abnormalities in mucin gene expression. Am. J. Respir. Crit. Care Med. 163, 517–523 [DOI] [PubMed] [Google Scholar]
- 2.Evans C. M., Williams O. W., Tuvim M. J., Nigam R., Mixides G. P., Blackburn M. R., DeMayo F. J., Burns A. R., Smith C., Reynolds S. D., Stripp B. R., Dickey B. F. (2004) Mucin is produced by clara cells in the proximal airways of antigen-challenged mice. Am. J. Respir. Cell Mol. Biol. 31, 382–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pardo-Saganta A., Law B. M., Gonzalez-Celeiro M., Vinarsky V., Rajagopal J. (2013) Ciliated cells of pseudostratified airway epithelium do not become mucous cells after ovalbumin challenge. Am. J. Respir. Cell Mol. Biol. 48, 364–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wills-Karp M., Luyimbazi J., Xu X., Schofield B., Neben T. Y., Karp C. L., Donaldson D. D. (1998) Interleukin-13: central mediator of allergic asthma. Science 282, 2258–2261 [DOI] [PubMed] [Google Scholar]
- 5.Chen G., Korfhagen T. R., Xu Y., Kitzmiller J., Wert S. E., Maeda Y., Gregorieff A., Clevers H., Whitsett J. A. (2009) SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J. Clin. Invest. 119, 2914–2924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajavelu P., Chen G., Xu Y., Kitzmiller J. A., Korfhagen T. R., Whitsett J. A. (2015) Airway epithelial SPDEF integrates goblet cell differentiation and pulmonary Th2 inflammation. J. Clin. Invest. 125, 2021–2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiang Y. Y., Wang S., Liu M., Hirota J. A., Li J., Ju W., Fan Y., Kelly M. M., Ye B., Orser B., O’Byrne P. M., Inman M. D., Yang X., Lu W. Y. (2007) A GABAergic system in airway epithelium is essential for mucus overproduction in asthma. Nat. Med. 13, 862–867 [DOI] [PubMed] [Google Scholar]
- 8.Fu X. W., Wood K., Spindel E. R. (2011) Prenatal nicotine exposure increases GABA signaling and mucin expression in airway epithelium. Am. J. Respir. Cell Mol. Biol. 44, 222–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maddox L., Schwartz D. A. (2002) The pathophysiology of asthma. Annu. Rev. Med. 53, 477–498 [DOI] [PubMed] [Google Scholar]
- 10.Stern D. A., Morgan W. J., Halonen M., Wright A. L., Martinez F. D. (2008) Wheezing and bronchial hyper-responsiveness in early childhood as predictors of newly diagnosed asthma in early adulthood: a longitudinal birth-cohort study. Lancet 372, 1058–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guilbert T. W., Morgan W. J., Zeiger R. S., Mauger D. T., Boehmer S. J., Szefler S. J., Bacharier L. B., Lemanske R. F. Jr., Strunk R. C., Allen D. B., Bloomberg G. R., Heldt G., Krawiec M., Larsen G., Liu A. H., Chinchilli V. M., Sorkness C. A., Taussig L. M., Martinez F. D. (2006) Long-term inhaled corticosteroids in preschool children at high risk for asthma. N. Engl. J. Med. 354, 1985–1997 [DOI] [PubMed] [Google Scholar]
- 12.Dakhama A., Park J. W., Taube C., El Gazzar M., Kodama T., Miyahara N., Takeda K., Kanehiro A., Balhorn A., Joetham A., Loader J. E., Larsen G. L., Gelfand E. W. (2005) Alteration of airway neuropeptide expression and development of airway hyperresponsiveness following respiratory syncytial virus infection. Am. J. Physiol. Lung Cell. Mol. Physiol. 288, L761–L770 [DOI] [PubMed] [Google Scholar]
- 13.Larson S. D., Schelegle E. S., Walby W. F., Gershwin L. J., Fanuccihi M. V., Evans M. J., Joad J. P., Tarkington B. K., Hyde D. M., Plopper C. G. (2004) Postnatal remodeling of the neural components of the epithelial–mesenchymal trophic unit in the proximal airways of infant rhesus monkeys exposed to ozone and allergen. Toxicol. Appl. Pharmacol. 194, 211–220 [DOI] [PubMed] [Google Scholar]
- 14.Yu M., Zheng X., Peake J., Joad J. P., Pinkerton K. E. (2008) Perinatal environmental tobacco smoke exposure alters the immune response and airway innervation in infant primates. J. Allergy Clin. Immunol. 122, 640–647.e1 [DOI] [PubMed] [Google Scholar]
- 15.Wu Z. X., Hunter D. D., Kish V. L., Benders K. M., Batchelor T. P., Dey R. D. (2009) Prenatal and early, but not late, postnatal exposure of mice to sidestream tobacco smoke increases airway hyperresponsiveness later in life. Environ. Health Perspect. 117, 1434–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Z. X., Hunter D. D., Batchelor T. P., Dey R. D. (2016) Side-stream tobacco smoke–induced airway hyperresponsiveness in early postnatal period is involved nerve growth factor. Respir. Physiol. Neurobiol. 223, 1–8 [DOI] [PubMed] [Google Scholar]
- 17.Aven L., Paez-Cortez J., Achey R., Krishnan R., Ram-Mohan S., Cruikshank W. W., Fine A., Ai X. (2014) An NT4/TrkB-dependent increase in innervation links early-life allergen exposure to persistent airway hyperreactivity. FASEB J. 28, 897–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel K. R., Aven L., Shao F., Krishnamoorthy N., Duvall M. G., Levy B. D., Ai X. (2016) Mast cell–derived neurotrophin 4 mediates allergen-induced airway hyperinnervation in early life. Mucosal Immunol. 9, 1466–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tortorolo L., Langer A., Polidori G., Vento G., Stampachiacchere B., Aloe L., Piedimonte G. (2005) Neurotrophin overexpression in lower airways of infants with respiratory syncytial virus infection. Am. J. Respir. Crit. Care Med. 172, 233–237 [DOI] [PubMed] [Google Scholar]
- 20.Szczepankiewicz A., Rachel M., Sobkowiak P., Kycler Z., Wojsyk-Banaszak I., Schöneich N., Skibinska M., Bręborowicz A. (2012) Serum neurotrophin-3 and neurotrophin-4 levels are associated with asthma severity in children. Eur. Respir. J. 39, 1035–1037 [DOI] [PubMed] [Google Scholar]
- 21.Linnoila R. I. (2006) Functional facets of the pulmonary neuroendocrine system. Lab. Invest. 86, 425–444 [DOI] [PubMed] [Google Scholar]
- 22.Cutz E., Pan J., Yeger H., Domnik N. J., Fisher J. T. (2013) Recent advances and contraversies on the role of pulmonary neuroepithelial bodies as airway sensors. Semin. Cell Dev. Biol. 24, 40–50 [DOI] [PubMed] [Google Scholar]
- 23.Chang R. B., Strochlic D. E., Williams E. K., Umans B. D., Liberles S. D. (2015) Vagal sensory neuron subtypes that differentially control breathing. Cell 161, 622–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Branchfield K., Nantie L., Verheyden J. M., Sui P., Wienhold M. D., Sun X. (2016) Pulmonary neuroendocrine cells function as airway sensors to control lung immune response. Science 351, 707–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wojcik S. M., Katsurabayashi S., Guillemin I., Friauf E., Rosenmund C., Brose N., Rhee J. S. (2006) A shared vesicular carrier allows synaptic corelease of GABA and glycine. Neuron 50, 575–587 [DOI] [PubMed] [Google Scholar]
- 26.Oswald D. J., Lee A., Trinidad M., Chi C., Ren R., Rich C. B., Trinkaus-Randall V. (2012) Communication between corneal epithelial cells and trigeminal neurons is facilitated by purinergic (P2) and glutamatergic receptors. PLoS One 7, e44574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jarvis M. F., Burgard E. C., McGaraughty S., Honore P., Lynch K., Brennan T. J., Subieta A., Van Biesen T., Cartmell J., Bianchi B., Niforatos W., Kage K., Yu H., Mikusa J., Wismer C. T., Zhu C. Z., Chu K., Lee C. H., Stewart A. O., Polakowski J., Cox B. F., Kowaluk E., Williams M., Sullivan J., Faltynek C. (2002) A-317491, a novel potent and selective non-nucleotide antagonist of P2X3 and P2X2/3 receptors, reduces chronic inflammatory and neuropathic pain in the rat. Proc. Natl. Acad. Sci. USA 99, 17179–17184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen X., Ye H., Kuruvilla R., Ramanan N., Scangos K. W., Zhang C., Johnson N. M., England P. M., Shokat K. M., Ginty D. D. (2005) A chemical–genetic approach to studying neurotrophin signaling. Neuron 46, 13–21 [DOI] [PubMed] [Google Scholar]
- 29.Chattopadhyaya B., Di Cristo G., Higashiyama H., Knott G. W., Kuhlman S. J., Welker E., Huang Z. J. (2004) Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J. Neurosci. 24, 9598–9611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schnorbusch K., Lembrechts R., Pintelon I., Timmermans J. P., Brouns I., Adriaensen D. (2013) GABAergic signaling in the pulmonary neuroepithelial body microenvironment: functional imaging in GAD67-GFP mice. Histochem. Cell Biol. 140, 549–566 [DOI] [PubMed] [Google Scholar]
- 31.Harris K. S., Zhang Z., McManus M. T., Harfe B. D., Sun X. (2006) Dicer function is essential for lung epithelium morphogenesis. Proc. Natl. Acad. Sci. USA 103, 2208–2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim E. J., Ables J. L., Dickel L. K., Eisch A. J., Johnson J. E. (2011) Ascl1 (Mash1) defines cells with long-term neurogenic potential in subgranular and subventricular zones in adult mouse brain. PLoS One 6, e18472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song H., Yao E., Lin C., Gacayan R., Chen M. H., Chuang P. T. (2012) Functional characterization of pulmonary neuroendocrine cells in lung development, injury, and tumorigenesis. Proc. Natl. Acad. Sci. USA 109, 17531–17536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ito T., Udaka N., Yazawa T., Okudela K., Hayashi H., Sudo T., Guillemot F., Kageyama R., Kitamura H. (2000) Basic helix–loop–helix transcription factors regulate the neuroendocrine differentiation of fetal mouse pulmonary epithelium. Development 127, 3913–3921 [DOI] [PubMed] [Google Scholar]
- 35.Van Rossum D., Hanisch U. K., Quirion R. (1997) Neuroanatomical localization, pharmacological characterization and functions of CGRP, related peptides and their receptors. Neurosci. Biobehav. Rev. 21, 649–678 [DOI] [PubMed] [Google Scholar]
- 36.Hung K. S. (1982) Development of neuroepithelial bodies in pre- and postnatal mouse lungs: scanning electron microscopic study. Anat. Rec. 203, 285–291 [DOI] [PubMed] [Google Scholar]
- 37.Lazarowski E. R., Sesma J. I., Seminario L., Esther C. R. Jr., Kreda S. M. (2011) Nucleotide release by airway epithelia. Subcell. Biochem. 55, 1–15 [DOI] [PubMed] [Google Scholar]
- 38.Brouns I., Adriaensen D., Burnstock G., Timmermans J. P. (2000) Intraepithelial vagal sensory nerve terminals in rat pulmonary neuroepithelial bodies express P2X(3) receptors. Am. J. Respir. Cell Mol. Biol. 23, 52–61 [DOI] [PubMed] [Google Scholar]
- 39.Adriaensen D., Brouns I., Timmermans J. P. (2015) Sensory input to the central nervous system from the lungs and airways: a prominent role for purinergic signalling via P2X2/3 receptors. Auton. Neurosci. 191, 39–47 [DOI] [PubMed] [Google Scholar]
- 40.Cockayne D. A., Dunn P. M., Zhong Y., Rong W., Hamilton S. G., Knight G. E., Ruan H. Z., Ma B., Yip P., Nunn P., McMahon S. B., Burnstock G., Ford A. P. (2005) P2X2 knockout mice and P2X2/P2X3 double knockout mice reveal a role for the P2X2 receptor subunit in mediating multiple sensory effects of ATP. J. Physiol. 567, 621–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu X., Ernfors P., Wu H., Jaenisch R. (1995) Sensory but not motor neuron deficits in mice lacking NT4 and BDNF. Nature 375, 238–241 [DOI] [PubMed] [Google Scholar]
- 42.Erickson J. T., Conover J. C., Borday V., Champagnat J., Barbacid M., Yancopoulos G., Katz D. M. (1996) Mice lacking brain-derived neurotrophic factor exhibit visceral sensory neuron losses distinct from mice lacking NT4 and display a severe developmental deficit in control of breathing. J. Neurosci. 16, 5361–5371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li J., Zhang Z., Liu X., Wang Y., Mao F., Mao J., Lu X., Jiang D., Wan Y., Lv J. Y., Cao G., Zhang J., Zhao N., Atkinson M., Greiner D. L., Prud’homme G. J., Jiao Z., Li Y., Wang Q. (2015) Study of GABA in healthy volunteers: pharmacokinetics and pharmacodynamics. Front. Pharmacol. 6, 260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park S. W., Verhaeghe C., Nguyenvu L. T., Barbeau R., Eisley C. J., Nakagami Y., Huang X., Woodruff P. G., Fahy J. V., Erle D. J. (2009) Distinct roles of FOXA2 and FOXA3 in allergic airway disease and asthma. Am. J. Respir. Crit. Care Med. 180, 603–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stevens P. T., Kicic A., Sutanto E. N., Knight D. A., Stick S. M. (2008) Dysregulated repair in asthmatic paediatric airway epithelial cells: the role of plasminogen activator inhibitor-1. Clin. Exp. Allergy 38, 1901–1910 [DOI] [PubMed] [Google Scholar]
- 46.Saatian B., Rezaee F., Desando S., Emo J., Chapman T., Knowlden S., Georas S. N. (2013) Interleukin-4 and interleukin-13 cause barrier dysfunction in human airway epithelial cells. Tissue Barriers 1, e24333 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.