Abstract
Histone acetyltransferases and histone deacetylases (HDACs) are important epigenetic coregulators. It has been thought that HDACs associate with corepressor complexes and repress gene transcription; however, in this study, we have found that PU.1—a key master regulator for hematopoietic self-renewal and lineage specification—requires HDAC activity for gene activation. Deregulated PU.1 gene expression is linked to dysregulated hematopoiesis and the development of leukemia. In this study, we used erythroid differentiation as a model to analyze how the PU.1 gene is regulated. We found that active HDAC1 is directly recruited to active PU.1 promoter in progenitor cells, whereas acetylated HDAC1, which is inactive, is on the silenced PU.1 promoter in differentiated erythroid cells. We then studied the mechanism of HDAC1-mediated activation. We discovered that HDAC1 activates PU.1 gene transcription via deacetylation of TATA-binding protein–associated factor 9 (TAF9), a component in the transcription factor IID (TFIID) complex. Treatment with HDAC inhibitor results in an increase in TAF9 acetylation. Acetylated TAF9 does not bind to the PU.1 gene promoter and subsequently leads to the disassociation of the TFIID complex and transcription repression. Thus, these results demonstrate a key role for HDAC1 in PU.1 gene transcription and, more importantly, uncover a novel mechanism of TFIID recruitment and gene activation.—Jian, W., Yan, B., Huang, S., Qiu, Y. Histone deacetylase 1 activates PU.1 gene transcription through regulating TAF9 deacetylation and transcription factor IID assembly.
Keywords: TFIID, downstream promoter element, HDAC1, HDACi, acetylation
PU.1, an Ets family member, is a transcription factor that plays a key role in hematopoietic stem cell self-renewal (1, 2) and in directing multipotent hematopoietic stem cells toward lineage commitment by regulating lineage-specific gene expression, cell proliferation and differentiation (3–5). Expression of PU.1 fluctuates in various hematopoietic differentiation pathways (5, 6). PU.1 level is high in human hematopoietic stem cell CD34+ cells (6–9). PU.1 expression remains high in common myeloid progenitors and common lymphoid progenitors, and turns off as myeloid/erythroid precursor cells commit to the erythroid lineage (10–13). Inappropriate expression of PU.1 in specific hematopoietic cells can result in leukemic transformation, such as T-cell lymphomas, acute myeloid leukemia, and erythroleukemias (14–16). Several reports suggest that the proximal promoter regulates PU.1 expression in specific lineages (17, 18). Epigenetic regulation may also be a key in PU.1 gene expression (19).
Histone deacetylase 1 (HDAC1) deacetylates histones and nonhistone proteins and plays key roles in regulating a variety of cellular functions (20). A previous study has shown that HDAC1 can be dynamically acetylated and deacetylated (21, 22). Acetylation of HDAC1 by p300 attenuates its deacetylase activity (21). Acetylated HDAC1 can also trans-repress the activity of HDAC2, a deacetylase that often coexists with HDAC1 in corepressor complexes (23). Of interest, during erythroid differentiation, HDAC1 acetylation gradually increases, whereas HDAC1-associated deacetylase activity decreases (24). The reduction of HDAC activity that associates with GATA-1 transcription factor positively activates GATA-1–dependent gene transcription (24); therefore, the dynamic acetylation of HDAC1 regulates the activity of GATA-1 and erythropoiesis. PU.1 is a counteracting master regulator of GATA-1 (10, 11). It remains to be determined whether the down-regulation of HDAC1-associated deacetylase activity during erythropoiesis also directly affects the down-regulation of PU.1 gene expression.
Transcription factor IID (TFIID) is the first complex to be recruited to the core promoter element for preinitiation complex assembly. The TFIID complex consists of the TATA-binding protein (TBP) and a variety of TBP-associated factors (TAFs), which also serve to mediate signals between various activators and basal transcription machinery (25–27). TAF9 is a small TAF with an MW of approximately 32 kDa. The TAF9–TAF6 pair binds to a consensus DNA sequence, the downstream core promoter element (DPE) (28, 29). DPE is found in both TATA-containing and TATA-less promoters, although more commonly in TATA-less promoters (28). DPE is located at +28 to +32 relative to the transcription start site. DPE motifs are normally defined as sequences with at least a 5-of-6 match with the DPE consensus (30). TAF9 affects a large set of gene transcription during embryonic development in a variety of organisms (31–34). Deletion of TAF9 in insect cells causes codepletion of other TAFs and disruption of TFIID, which indicates that TAF9 is important for TFIID complex stability (35, 36).
In this study, we found that HDAC1 is required for PU.1 gene expression. During erythroid differentiation, the increase of HDAC1 acetylation reduced HDAC1-associated deacetylase activity and subsequently down-regulated PU.1 gene transcription. Furthermore, we identified that TAF9 is a target protein for HDAC1-mediated deacetylation and is required for PU.1 gene activation. Nonacetylated TAF9 associates with the PU.1 promoter and promotes gene activation. Upon induction of erythropoiesis, HDAC1 becomes acetylated at the PU.1 promoter and prevents the deacetylation of TAF9. Acetylated TAF9 not only loses its binding to the promoter, but also results in the disassembly of the entire TFIID complex from the promoter, which leads to transcriptional repression. Thus, our study mechanistically demonstrates that HDAC1 is a coactivator for PU.1 gene expression and unveils a novel mechanism by which genes are dynamically activated and repressed.
MATERIALS AND METHODS
Cell culture and plasmid constructs
K562 cells were cultured in RPMI 1640 medium that was supplemented with 10% fetal bovine serum (FBS). MEL cells were grown in DMEM with 10% FBS and induced by 1.5% DMSO for 0 or 3 d. RAW 264.7 cells were cultured in RPMI 1640 medium that was supplemented with 10% FBS and 1% minimum essential medium. CD34+ cells were cultured and expanded in StemSpan serum-free expansion medium (StemCell Technologies, Cambridge, MA, USA) that was supplemented with 75 ng/ml stem cell factor, 75 ng/ml Flt-3, 20 ng/ml IL-3, and 20 ng/ml IL-6 plus 1% penicillin/streptomycin. TAF9 expression plasmids were constructed by subcloning human TAF9 cDNA into pcDNA 3.1+ expression vector. GST-TAF9 was constructed by subcloning TAF9 cDNA into pGEX 2TK (Clontech Laboratories, Mountain View, CA, USA). GST-TAF9 K5Q or K108Q was constructed by converting the lysine at aa 5 or 108 to glutamine by using site-directed mutagenesis according to manufacturer protocol (Agilent Technologies, Santa Clara, CA, USA). Short hairpin RNA (shRNA)–resistant TAF9 was also constructed via site-directed mutagenesis by converting the shRNA target sequence from 5′-GAATATGAGCCAAGAGTTA-3′ to 5′-GAATACGAGCCTAGGGTCA-3′ without affecting protein sequences. pGL3-PU.1pro luciferase reporter plasmid was generated by PCR-mediated amplification of the mouse PU.1 promoter sequence from −334 to +147 and cloning into pGL3 basic plasmid. DPE mutation in pGL3-PU.1 was constructed via site-directed mutagenesis by converting the DPE sequence from 5′-GGCCCT-3′ to 5′-CTCATG-3′. All constructs were confirmed by DNA sequencing.
Transient transfection and reporter assays
Transient transfection was carried out in K562 or RAW 264.7 cells with Lipofectamine 2000 according to manufacturer protocol (Thermo Fisher Scientific, Waltham, MA, USA). The Renilla luciferase plasmid (PRL-CMV; Promega, Madison, WI, USA) was cotransfected as an internal control. After 40 h, cells were treated with trichostatin A (TSA), harvested 8 h after TSA treatment, and luciferase activities were measured by using the dual-luciferase reporter assay system (Promega).
Gene knockdown using inducible shRNA
TAF9 shRNA was cloned into the inducible pTRIPZ vector (Thermo Fisher Scientific) and transfected into K562 cells with Lipofectamine 2000 according to the manufacturer’s protocol. Two days after transfection, cells were selected in DMEM medium that contained 1 μg/ml puromycin for 10 d, then 5 μg/ml doxycycline was added to induce shRNA expression. The target sequences for shRNA are listed in Supplemental Table 1.
Gene expression analysis
Total cellular RNA was isolated from 1 × 106 cells and reverse transcribed into cDNA by using SuperScript reverse transcriptase and oligo(dT) primers (Thermo Fisher Scientific). Real-time PCR was performed by using Power SYBR Green PCR Master Mix (Bio-Rad, Hercules, CA, USA). Primers used are listed in Supplemental Table 1.
Each reaction was run in triplicate and data were normalized to glyceraldehyde 3-phosphate dehydrogenase expression. For statistical analysis, unpaired Student’s t tests were performed to determine the significance of differences between expression values. Significant differences in expression with a value of P < 0.05 are indicated with an asterisk. Error bars in all figures represent means ± sd (2 biologic replicates). Gaussian error propagation was applied for normalized data.
Immunoprecipitation and Western blot analysis
Immunoprecipitation and Western blot assays were performed as previously described (21) with the following Abs: anti–acetyl-lysine (EMD Millipore, Billerica, MA, USA), anti-HDAC1 (Thermo Fisher Scientific), anti-TAF9 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and anti–β-actin (Sigma-Aldrich, St. Louis, MO, USA).
Chromatin immunoprecipitation assay and data analysis
Chromatin immunoprecipitation (ChIP) assay was performed as previously described (24). In brief, 5 × 106 K562 or MEL cells were subjected to formaldehyde crosslink. Cells were sonicated to obtain chromatin fragments that ranged from approximately 300 to 500 bp. The crosslinked chromatin was subsequently immunoprecipitated with indicated Abs or normal lgG as control. Abs used for the ChIP assay are as follows: anti–acetyl-H3 (K9K14) and anti–acetyl-H4 (K5K8K12K16; EMD Millipore); and anti-TAF9, -TAF6, -TAF1, -TAF5, and -TBP (Santa Cruz Biotechnology). Abs against HDAC1 or acetylated HDAC1 were reported previously (24). Purified DNA from precipitated chromatin was subjected to real-time PCR amplification. Sequences of PCR primers are listed in Supplemental Table 1.
At least 2 biologic repeats were performed for each ChIP experiment. Each reaction was run in triplicate, and relative enrichment levels were normalized to input. Error bars in all figures represent means ± sd. Gaussian error propagation was applied for normalized data.
Reconstitution of mononucleosome in vitro
Mononucleosome particles were assembled by salt dilution as described in Chen and Manley (37), with core histone octamers purified from HeLa cell nuclei (38). In brief, 3.75 μg of a biotin-labeled PU.1 promoter sequence (−34 to +175) was incubated with 4.5 μg of core histones in a high-salt buffer (10 mM Tris, pH 7.5, 2 M NaCl, 0.1 mg/ml bovine serum albumin, 0.5 mM benzamidine). Samples were dialyzed successively against the same buffer, but contained 1.25, 1.0, and 0.75 M NaCl, each for 1 h at 4°C. Thereafter, samples were dialyzed against Tris EDTA for an additional 1 h at 4°C before being stored at 4°C.
Affinity pull-down assay
Biotin-tagged DNA or reconstituted mononucleosomes were immobilized to streptavidin-coupled Dynabeads (Thermo Fisher Scientific) by incubation in a reconstituted binding buffer (20 mM Tris HCl, pH 7.5, 150 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 5 mM DTT, 10% glycerol, 0.05% NP-40 and 1× protease inhibitor cocktail; Roche, Basel, Switzerland) at 4°C overnight. Immobilized nucleosomes or DNA were blocked by 1 mg/ml bovine serum albumin in the binding buffer for 30 min at room temperature, and then incubated with MEL cell nuclear extracts or GST-TAF9 proteins for 30 min at room temperature. Incubation was followed by 3 washes with binding buffer without NP-40. Bound proteins were then separated by SDS-PAGE and detected by Western blot with indicated Abs.
Acetylation and deacetylation in vitro
Expression and purification of FLAG-tagged p300 and HDAC1 from baculovirus, as well as the in vitro acetylation and deacetylation assays, have been previously described (21).
In vitro GST pull-down assay and protein purification
Bacteria-expressed, GST-tagged wild-type TAF9 and K5Q mutants were immobilized on glutathione-sepharose 4B beads (Pfizer, New York. NY, USA) as previously described (23). The quality and concentration of proteins were determined by SDS-PAGE. The GST pull-down assay was performed as previously described (23).
RESULTS
HDAC1 is required for PU.1 gene expression during erythroid lineage commitment
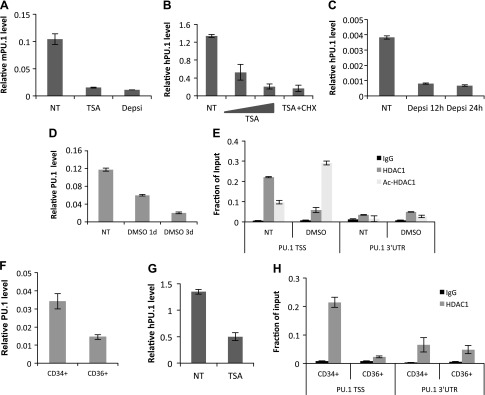
MEL cells are mouse erythroleukemia cells that are blocked in the erythroblast stage as a result of the deregulated overexpression of PU.1 (39). It is suggested that the overexpression of the PU.1 gene is the immediate cause for the leukemic phenotype of MEL cells by retaining the self-renewal capacity of transformed erythroblastic cells and by blocking the terminal differentiation program toward erythrocytes (40, 41). Human erythroleukemia K562 cells also express PU.1 (9, 42). Overexpression of PU.1 blocks the hemin-induced erythroid differentiation in K562 cells (42). It has been shown that an HDAC inhibitor (HDACi), such as TSA or sodium butyrate, can partially induce erythroid differentiation in MEL or K562 cells (24, 43, 44); however, the connection between the HDACi and PU.1 expression is poorly understood. It is reported that an HDACi represses PU.1 gene expression in macrophage cells (45); therefore, we tested whether an HDACi also represses PU.1 gene expression in erythroid progenitor cells. After TSA treatment, PU.1 mRNA was decreased in MEL and K562 cells (Fig. 1A, B). An HDAC1/2-specific inhibitor, depsipeptide, also reduced PU.1 mRNA at similar levels (Fig. 1A, C). Treatment with cycloheximide (CHX) did not affect TSA repression, which suggests that the repression of PU.1 gene expression by the HDACi does not require new protein synthesis, supporting the notion that HDAC1/2 may be needed for PU.1 gene transcription. If this is the case, then HDAC1 will be recruited to PU.1 promoter when the transcription is activated and will be absent when transcription is repressed. It has been shown that the chemical agent, DMSO, reduces PU.1 expression and induces terminal differentiation in MEL cells (46) (Fig. 1D). We tested HDAC1 recruitment at the PU.1 promoter in MEL cells that were treated with or without DMSO. HDAC1 was high at the promoter before DMSO treatment and significantly decreased after induction of differentiation (Fig. 1E). Of interest, acetylated HDAC1, which has no deacetylase activity, became enriched at the PU.1 promoter after induction of differentiation. This result suggests that active HDAC1 deacetylase activity may be required for PU.1 gene activation. Therefore, the effect of an HDACi in promoting erythroid differentiation, at least in part, may be a result of the inhibition of PU.1 expression.
Figure 1.
HDAC activity is required for PU.1 gene expression. A) MEL cells were treated with HDACi [100 nM TSA or 10 nM depsipeptide (Depsi)] for 8 h. PU.1 mRNA level was measured by using real-time PCR. B) K562 cells were treated with an increasing amount of TSA with or without 20 μm cycloheximide (CHX). PU.1 mRNA level was measured by using real-time PCR. C) K562 cells were treated with 10 nM Depsi for different time points. PU.1 mRNA level was measured by using real-time PCR. D) MEL cells were induced with 1.5% DMSO at different time points, and PU.1 mRNA level was measured by using real-time PCR. E) Recruitment of HDAC1 and acetyl-HDAC1 on mouse PU.1 promoter. ChIP assays with HDAC1 and acetylated HDAC1 Ab was performed in MEL cells induced with DMSO for 0 and 3 d. The resulting precipitated DNA was analyzed by using real-time PCR. F) Human CD34+ cells were induced for differentiation with Epo for 5 d, and CD36+ cells were collected via flow cytometry after differentiation. PU.1 mRNA level was measured by using real-time PCR. G) Human CD34+ cells were treated with TSA for 8 h. PU.1 mRNA level was measured by using real-time PCR. H) HDAC1 recruitment on human PU.1 promoter. ChIP assays with HDAC1 Ab were performed in CD34+ or CD36+ cells. The resulting precipitated DNA was analyzed by using real-time NT, no treatment; TSS, transcription start site; UTR, untranslated region. All data are represented as means ± sem.
We also tested whether an HDACi represses PU.1 gene expression during erythroid lineage commitment. PU.1 expression was high in human CD34+ cells and diminished in differentiated CD36+ cells (47) (Fig. 1F). Consistent with the results for MEL and K562 cells, PU.1 gene expression was repressed when human CD34+ cells were treated with TSA (Fig. 1G). We also tested whether the dynamic recruitment of HDAC1 is correlated with PU.1 gene activation during erythroid cell differentiation. ChIP results show that HDAC1 is present at the PU.1 promoter in CD34+ cells, but not in differentiated CD36+ cells (Fig. 1H). These results indicate that HDAC1 levels at the PU.1 promoter are positively correlated with PU.1 gene activation; therefore, HDAC1 may be a coactivator for PU.1 gene expression.
HDAC1 interacts with and deacetylates TAF9 on PU.1 core promoter
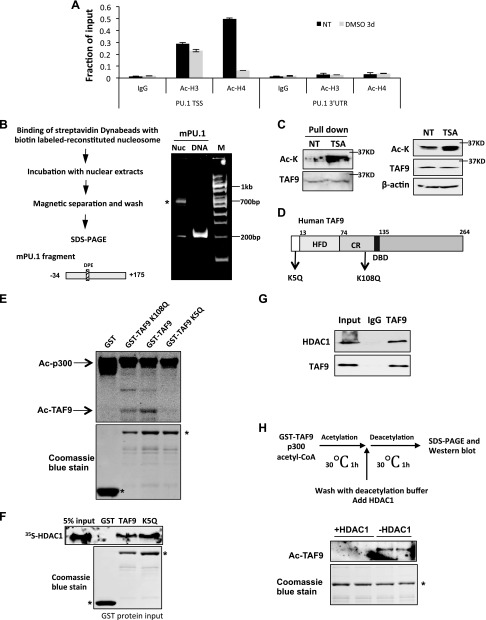
Because our results show that HDAC1 is mainly recruited to the core promoter region, we reason that the HDAC1 target is mainly located at the core promoter region. In addition, the target protein is deacetylated at the gene activation stage when active HDAC1 is recruited to the promoter, and is acetylated at the gene repression stage when HDAC1 is acetylated and subsequently loses deacetylase activity. To exclude the possibility that acetylated core histones at the core promoter may be the target for HDAC1, histone H3 and H4 acetylation among the promoter region were examined by ChIP assay. Histone H3 acetylation levels did not change significantly during the transcription cycle, and histone H4 is highly acetylated at the activation stage, but acetylation is reduced when PU.1 expression is repressed after DMSO induction (Fig. 2A). HDAC1 activities do not correlate with histone H3 and H4 deacetylation at the promoter; therefore, these results suggest that the HDAC1 target protein is a nonhistone protein that associates with the PU.1 core promoter region.
Figure 2.
TAF9 is acetylated on PU.1 core promoter, interacts with HDAC1, and is deacetylated by HDAC1. A) The recruitment of acetyl-histone H3 and -4 on mouse PU.1 promoter. ChIP assay with acetyl-H3 (Ac-H3) and acetyl-H4 (Ac-H4) Abs was performed in MEL cells induced with DMSO for 0 and 3 d. The resulting precipitated DNA was analyzed by using real-time PCR. Data are represented as means ± sem. B) Schematic representation of biotin-labeled nucleosome pull-down assay (left). Reconstituted nucleosome and control DNA were resolved in 5% native gel (right). Asterisk indicates reconstituted nucleosome. C) Proteins that bound to in vitro reconstituted PU.1 nucleosome from MEL nuclear extract treated with or without TSA were Western blotted with anti–acetyl-lysine (Ac-K) and anti-TAF9 Abs. The acetylated band colocalized with the TAF9 band. D) Schematic representation of TAF9 protein. K5 and K108 are reported acetylation sites. E) GST, GST-TAF9, K5Q, and K108Q mutants were purified from Escheria coli and incubated with p300 and 3H-labeled acetyl-CoA. The reaction mix was then subjected to SDS-PAGE and autoradiography. F) GST pull-down assay was performed by incubating immobilized GST, GST-TAF9, or K5Q mutant with in vitro translated [35S]-labeled HDAC1. The bond protein fraction was eluted and subjected to SDS-PAGE and autoradiography. G) K562 cell nuclear extract was immunoprecipitated with TAF9 Ab. The precipitated proteins were Western blotted with indicated Abs. H) Illustration of procedures of acetylation coupled deacetylation assay (top). In vitro acetylated TAF9 was incubated with or without HDAC1 (bottom). The reaction mix was Western blotted with acetyl-lysine Ab. CR, conserved region; DBD, DNA binding domain; HFD, histone fold domain; NT, no treatment; TSS, transcription start site; UTR, untranslated region.
To identify the HDAC1 target protein at the PU.1 core promoter, a 200-bp biotin-labeled PU.1 core promoter sequence was reconstituted to a single nucleosome and immobilized on magnetic beads (Fig. 2B). The nuclear extract from K562 cells that were treated with or without TSA was incubated with immobilized nucleosomes. PU.1 core promoter bound proteins were analyzed with anti–acetyl-lysine Ab. One acetylated band at the approximate size of 32 kDa was identified (Fig. 2C and Supplemental Fig. 1). By performing a survey of possible acetylated proteins that bind to the core promoter region, we found that TAF9—a component of the TFIID complex—has an approximate size of 32 kDa. Of more importance, TAF9 can be acetylated (48). Western blot with Abs against TAF9 confirmed that the acetylated protein overlaps with the TAF9 band (Fig. 2C).
It has been shown that TAF9 can be acetylated at lysine 5 and lysine 108 via a genome-wide acetylome survey (48) (Fig. 2D); however, the function of TAF9 acetylation was unknown. To confirm the acetylation sites of TAF9, lysine 5 or 108 was mutated to glutamine. Purified GST-TAF9 or TAF9 K108Q proteins, but not K5Q, were acetylated by p300 (Fig. 2E), which indicates that lysine 5 is the major acetylation site. We next tested whether TAF9 can interact with HDAC1. GST-TAF9 as well as GST-TAF9 K5Q mutant can directly interact with in vitro translated [35S]-labeled HDAC1 (Fig. 2F). In vivo, TAF9 also associates with HDAC1 from K562 cells (Fig. 2G). We then tested whether TAF9 is a substrate for HDAC1. GST-TAF9 was in vitro acetylated by recombinant p300 and subsequently incubated with purified active HDAC1 (Fig. 2H). HDAC1 efficiently deacetylated TAF9 in vitro (Fig. 2H and Supplemental Fig. 2). These results reveal that TAF9 is a target for HDAC1-mediated deacetylation, and that dynamic TAF9 acetylation patterns correlate with the inhibition of HDAC1 activity in cell extract and on the PU.1 promoter.
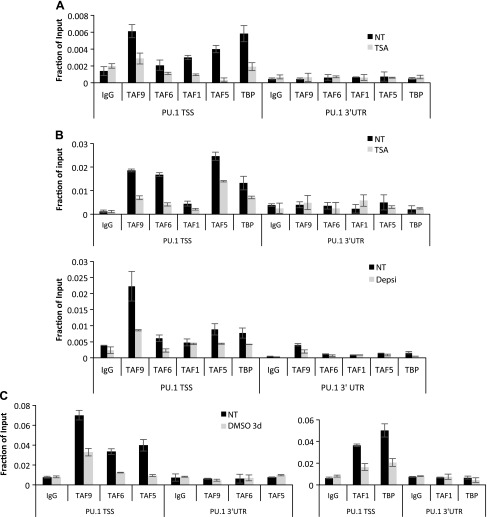
TAF9 and TFIID recruitment correlate with HDAC1 deacetylase activity at the PU.1 promoter
Because HDAC1 can deacetylate TAF9, we reasoned that an HDACi inhibits PU.1 transcription via up-regulation of acetylated TAF9. As TAF9 is a component of the TFIID complex, we next investigated whether TSA affects TFIID recruitment on the PU.1 chromatin. TFIID components, including TAFs and TBP, are recruited to the PU.1 promoter before TSA treatment. Treatment with TSA abolishes TBP recruitment as well as that of other TFIID components, which is consistent with the notion that TSA represses PU.1 transcription (Fig. 3A). Similarly, treatment with TSA in MEL cells also abolished the recruitment of the TFIID complex to the PU.1 promoter (Fig. 3B). Loss of TFIID recruitment was also observed in MEL cells when induced to differentiate with DMSO (Fig. 3C), which correlated with increased acetylation of HDAC1 at the promoter (Fig. 1E); therefore, our results indicate that the TFIID complex and HDAC1 are recruited to the PU.1 promoter when the promoter is active. Inactivation of HDAC1 via TSA treatment or induction of HDAC1 acetylation results in TAF9 acetylation, displacement of the TFIID complex, and down-regulation of promoter activity.
Figure 3.
TAF9 recruitment correlates with HDAC1 deacetylase activity on human and mouse PU.1 promoter. A) K562 cells were treated with or without TSA for 8 h, and ChIP assay was performed with the indicated Abs. The resulting precipitated DNA was analyzed by using real-time PCR. B) ChIP assays with indicated Abs were performed in MEL cells that were treated with TSA or depsipeptide for 0 and 8 h. The resulting precipitated DNA was analyzed by using real-time PCR. C) MEL cells were induced with DMSO for 0 and 3 d. The recruitment of the TFIID complex on mouse PU.1 promoter was measured by ChIP assays with indicated Abs. The resulting precipitated DNA was analyzed by using real-time PCR. Depsi, depsipeptide; NT, no treatment; TSS, transcription start site; UTR, untranslated region. Data are represented as means ± sem.
TAF9 is required for TFIID complex recruitment on the PU.1 promoter
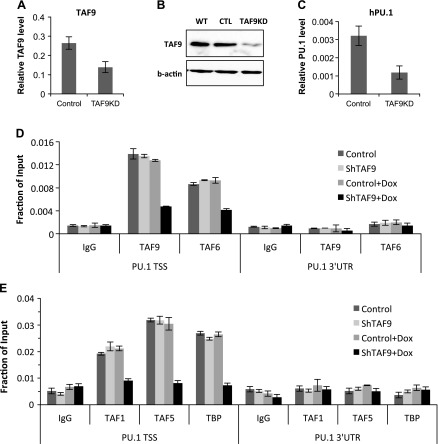
Because the inhibition of HDAC1 activity resulted in TAF9 acetylation and TFIID disassociation from the promoter and as acetylated TAF9 cannot bind to the promoter, we suspect that TAF9 is a key factor for TFIID complex recruitment to the PU.1 promoter. To demonstrate this, TAF9 was stably knocked down in K562 cells by shRNA using inducible lentiviral vector. When induced with doxycycline, cells showed decreased TAF9 mRNA and protein (Fig. 4A, B). Of importance, knockdown of TAF9 reduced PU.1 gene expression (Fig. 4C).
Figure 4.
TAF9 is required for PU.1 gene transcription and TFIID recruitment. A, B) TAF9 was stably knocked down in K562 cells by using inducible lentiviral shRNA. TAF9 gene expression was measured by using real-time RT-PCR (A), and cell extracts were subjected to Western blot against TAF9 and β-actin Ab (B). C) PU.1 gene expression was measured by using real-time RT-PCR from cells with or without induction of TAF9 knockdown for 3 d with doxycycline (Dox). D) ChIP assay was performed in K562 cells with induced TAF9 knockdown with Ab as indicated. The resulting precipitated DNA was analyzed by using real-time PCR. CTL, control; TSS, transcription start site; UTR, untranslated region; WT, wild type. Data are represented as means ± sem.
The recruitment of TFIID in control and TAF9-knockdown cells was evaluated by using ChIP assays. TAF9 knockdown reduced TAF9 recruitment at the PU.1 promoter (Fig. 4D). TAF9 knockdown also prevented the binding of TAF6, which supports the notion that TAF9 and TAF6 are corecruited. The knockdown also prevented the recruitment of other TAFs (Fig. 4D). These results indicate that the loss of TAF9 inhibits PU.1 transcription by preventing the recruitment of the entire TFIID complex at the PU.1 promoter.
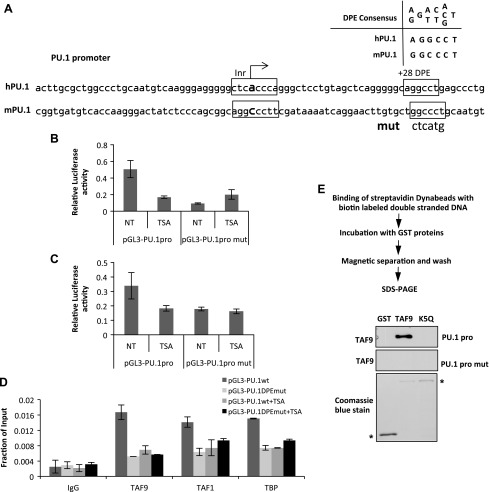
TAF9 binding to the DPE site is required for PU.1 gene activation
PU.1 contains a TATA-less promoter with a consensus DPE site at which TAF9/6 binds (28, 49) (Fig. 5A). If TAF9 DNA binding is required for PU.1 transcription, then mutation of the DPE site should reduce PU.1 transcription. To test this, we constructed a PU.1 reporter that comprised the PU.1 promoter region with or without a DPE mutation in the pGL3 mammalian reporter vector. DNA constructs were transfected into K562 cells. Similar to the endogenous PU.1 gene (Fig. 1B, C), pGL3-PU.1 reporter activity was repressed by TSA (Fig. 5B). The mutation of the DPE site repressed the reporter, and treatment with TSA did not further affect reporter activity (Fig. 5B). Similar results were observed in the macrophage cell line RAW 264.7, in which PU.1 gene expression is highly activated (Fig. 5C). These results suggest that TSA repression of PU.1 transcription is mainly mediated by the DPE site. To further confirm that the DPE site is bound by TAF9 in vivo, we performed ChIP assays using cells that were transfected with pGL3-PU.1 with or without the mutated DPE site. Mutation of the DPE site abolished the recruitment of TAF9 (Fig. 5D). Of importance, recruitment of other TAFs and TBP was also abolished (Fig. 5D). Results indicate that the DPE site is essential for TAF9 binding and TFIID complex recruitment. As TSA treatment resulted in TAF9 acetylation (Fig. 2C) and loss of TAF9 recruitment to the PU.1 promoter, we speculate that TAF9 acetylation abolishes TAF9 binding to the PU.1 promoter. To test this, the biotin-labeled PU.1 core promoter region (Fig. 1B) was immobilized on magnetic beads. Beads were then incubated with purified GST, GST-TAF9, or GST-TAF9 K5Q, the mutation that mimics acetylated TAF9 protein. TAF9 bound strongly to PU.1 core promoter DNA fragments; however, DNA elements with a DPE mutation abolished TAF9 binding (Fig. 5E). Acetylation significantly reduced DNA-binding affinity of TAF9 (Fig. 5E); therefore, our results indicate that TSA induces TAF9 acetylation and results in loss of TAF9 binding to DPE.
Figure 5.
TAF9 acetylation results in the loss of DNA binding and transcription repression. A) Schematic representation of PU.1 core promoter. It consists of a conserved DPE site in both mice and humans. B, C) DPE sequence on pGL3-PU.1pro plasmid was mutated and transfected to K562 cells (B) or RAW 264.7 cells (C). The luciferase activity of cells was determined with or without TSA treatment. D) pGL3-PU.1pro or pGL3-PU.1proDPEmut plasmid was transiently transfected into K562 cells, and ChIP assay was performed with the indicated antibodies. The resulting precipitated DNA was analyzed by using real-time PCR with primers specific for mouse PU.1 promoter and the pGL3 plasmid. E) Schematic representation of biotin-labeled DNA pull-down assay. GST, GST-TAF9, and GST-TAF9K5Q were incubated with biotin-labeled PU.1pro or PU.1pro mut DNA fragment, and the bound proteins were Western blotted with anti-TAF9 Ab. NT, no treatment. Asterisk indicates input GST and GST TAF9 proteins.
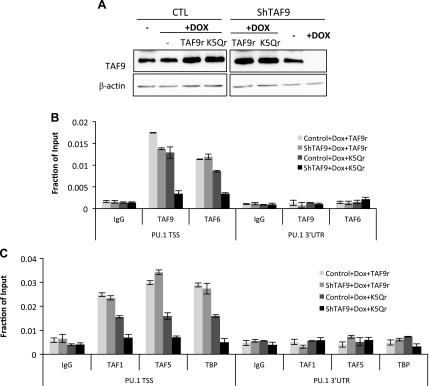
TAF9 acetylation prevents TFIID recruitment at the PU.1 promoter
Although we showed that TAF9 is required for the TFIID complex assembly at the PU.1 promoter and that acetylated TAF9 did not bind to the promoter, which suggests that TAF9 acetylation results in the disassociation of the TFIID complex, there is no evidence to directly link TAF9 acetylation to TFIID complex displacement. To test this, shRNA that was resistant TAF9 or TAF9 K5Q was re-expressed in knockdown K562 cells at levels that were similar to endogenous TAF9 cells compared with control cells (Fig. 6A). Re-expression of wild-type TAF9 re-established the binding of TAF9 (Fig. 6B) as well as that of the TFIID complex to the PU.1 promoter (Fig. 6C); however, TAF9 K5Q was not recruited to the PU.1 promoter in cells that overexpressed TAF9 K5Q (Fig. 6B). TAF9 K5Q also prevented the recruitment of TAF6 (Fig. 6B) and other TFIID subunits (Fig. 6C) to the promoter. These results reveal that TAF9 is essential for TFIID recruitment and that the acetylation status of TAF9 regulates TFIID recruitment at the core promoter.
Figure 6.
Acetylated TAF9 does not bind to chromatin and results in the disassociation of the entire TFIID complex from chromatin. A) Control and knockdown cells (shTAF9) were induced with or without doxycycline (Dox) for 3 d and transiently transfected with shTAF9-resistant TAF9 or TAF9 K5Q mutant (TAF9r or K5Qr). Cell extracts of treated cells were subjected to Western blot with indicated Abs. B, C) Knockdown cells (shTAF9) were induced with or without Dox for 3 d and transiently transfected with TAF9 or TAF9 K5Q mutant. Cells were subjected to ChIP assay with TAF9 and TAF6 (B); and TAF1, TAF5, and TBP (C). The resulting precipitated DNA was analyzed by using real-time PCR. CTL, control; TSS, transcription start site; UTR, untranslated region. Data are represented as means ± sem.
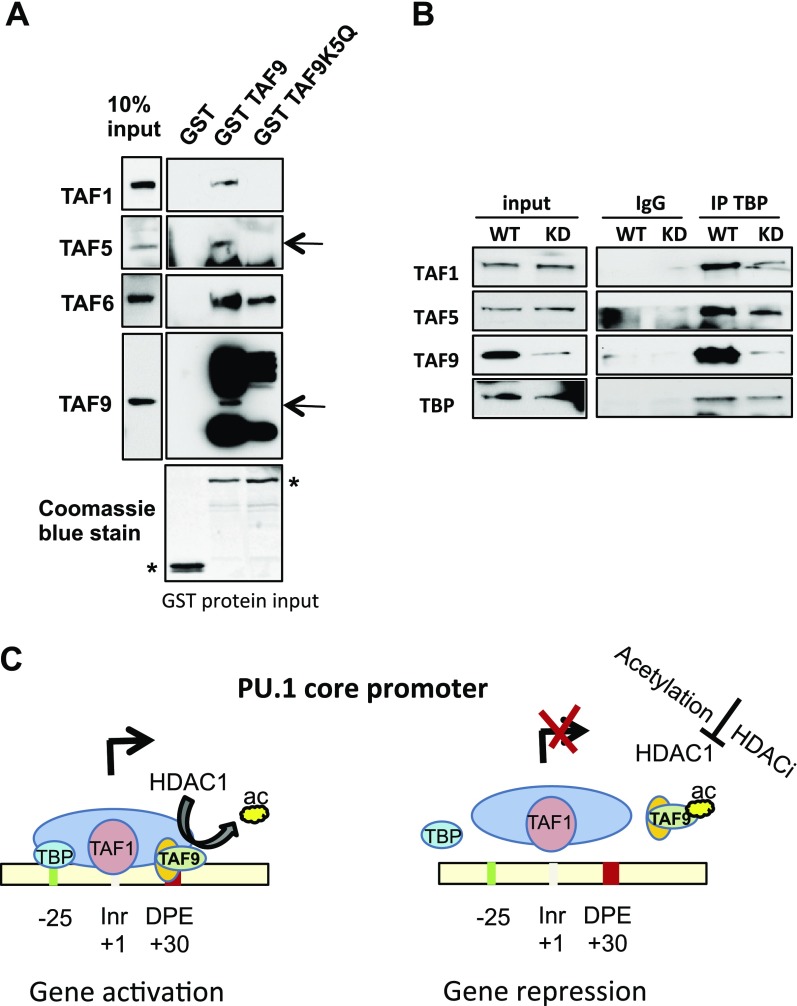
Acetylation of TAF9 results in disassembly of the TFIID complex
We also investigated whether acetylated TAF9 remains associate with the TFIID complex. Purified GST-TAF9 or TAF9 K5Q was incubated with nuclear extracts from 3134 cells, a murine mammary adenocarcinoma cell line (50). As expected, wild-type TAF9 could pull down components of TFIID; however, TAF9 K5Q did not interact with TFIID components except at reduced levels of TAF6 (Fig. 7A). Therefore, acetyl-mimic TAF9 does not associate with the TFIID complex. We next explored whether the absence of TAF9 affects TFIID complex formation. TAF9 knockdown did not affect the stability of TBP and other TAF proteins (Fig. 7B). Cell extracts from wild-type and TAF9 knockdown cells were immunoprecipitated with TBP and Western blotted with TFIID components. TBP associated with TAF1, TAF5, and TAF9 in extracts from wild-type cells; however, the association was significantly reduced in TAF9 knockdown cells (Fig. 7B). These results indicate that the absence of TAF9 results in the instability of the TFIID complex; thus, acetylation of TAF9 not only prevents the recruitment of the TFIID complex to the core promoter but also destabilizes the TFIID complex itself (Fig. 7C).
Figure 7.
Acetylation of TAF9 results in the disassembly of the TFIID complex. A) GST, GST-TAF9, or GST-TAF9 K5Q protein was immobilized on glutathione agarose beads and incubated with 3134 cell extracts. The associated protein was subjected to SDS-PAGE and was Western blotted with indicated Abs. B) Cell extracts from wild-type (WT) and TAF9-knockdown (KD) 3134 cells were immunoprecipitated (IP) with TBP Ab. The resulting immunoprecipitates were subjected to Western blot with indicated Abs. C) Model for HDAC1-dependent gene activation and acetylated HDAC1 mediated gene repression. TAF9 is deacetylated in the presence of HDAC1, and the TFIID complex is recruited to PU.1 promoter, which results in gene activation (left). HDAC1 is acetylated or inactivated by HDACi, which causes an inability to deacetylate TAF9 (right). Acetylated TAF9 is displaced from DNA. Acetylated TAF9 also causes the disassembly of the TFIID complex from the promoter, which results in gene repression. Inr, initiator. Asterisk indicates GST proteins.
DISCUSSION
HDAC1 often associates with corepressor complexes and represses gene transcription; however, in this study, we show that an HDACi directly represses PU.1 gene transcription. HDAC1 is recruited to the active PU.1 promoter, whereas acetylated HDAC1, which has no deacetylase activity, is recruited to the repressed PU.1 promoter. These data provide direct evidence that HDAC1 is a coactivator for PU.1 gene transcription. Similar results were also reported for the mouse mammary tumor virus promoter, in which unacetylated active HDAC1 was mainly recruited to the core promoter region during the gene activation stage (21, 51); however, the HDAC1 target for activation has not been identified. We reasoned that there is a nonhistone protein that needs to be deacetylated to mediate PU.1 gene activation, and we identified the target protein, TAF9, a component of TFIID complex, via affinity purification. TAF9 can be acetylated by p300 and deacetylated by HDAC1. The TAF9 acetylation pattern correlates with HDAC1 activity at the PU.1 promoter. TAF9 seems to be important for PU.1 transcription as TAF9 knockdown represses PU.1 gene expression. Of more importance, TAF9 recruitment is essential for TFIID complex recruitment. Acetylated TAF9 does not bind to the DPE region and precludes the recruitment of the TFIID complexes to the PU.1 promoter, which results in gene repression; therefore, our study provides a novel regulatory mechanism of TFIID complex recruitment and assembly.
Hematopoietic lineage specification is mediated by the activation of lineage-specific transcription regulators, such as PU.1 and GATA-1 (5, 52–54). Perturbation of these factors leads to various forms of anemia and myeloid malignancy (15, 55–59); however, little is known about the factors that regulate these master regulators to enhance differentiation toward the erythroid lineage. One of the most extensively studied epigenetic regulators in erythropoiesis is HDAC1, which is known to associate with many erythroid-specific transcription factors, such as GATA-1, TAL1, and EKLF, and to repress their gene expression (60–63). PU.1 is a critical regulator of erythropoiesis by preventing differentiation toward the erythroid lineage (1). We show here that HDAC1 directly activates PU.1 transcription; therefore, increased HDAC1 acetylation during erythroid differentiation plays dual roles in promoting the differentiation of erythroid cells, activation of GATA-1, and silencing of PU.1. An increased understanding of the mechanisms that regulate these factors, such as GATA-1 and PU.1—which are involved in lineage commitment and terminal differentiation—will advance our understanding of the etiology of such diseases as anemia, cancer, and other blood-related disorders.
The aberrant recruitment of HDAC1 has been linked to various types of leukemia, and an HDACi has shown clinical and preclinical promise as a result of its ability to induce leukemic cell differentiation and death (64–68). Conversely, blood-related adverse effects have been reported in clinical trials with various HDACis, including anemia, thrombocytopenia, neutropenia, and lymphopenia (69), which suggests that the HDACi affects hematopoietic lineage differentiation. In addition, reduction of the PU.1 level has been shown to be linked to the development of leukemia (16, 70); therefore, it is extremely important to understand how HDAC impacts normal hematopoiesis before an HDACi is administered to patients as a long-term therapeutic drug. Future studies should emphasize the fundamental understanding of cancer drug or therapy-related toxicity.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health (NIH) National Heart, Lung, and Blood Institute Grant R01-HL095674 (to Y.Q.), NIH National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK110108 (to S.H.), American Heart Association Grant AHA16GRNT31020032 (to S.H.), and the Florida Bankhead Coley Research Foundation (to Y.Q.). The authors thank Dr. Jim Bieker (Icahn School of Medicine at Mount Sinai, New York, NY, USA) for TAF9 plasmids and Dr. Robert Roeder (The Rockefeller University, New York, NY, USA) for discussion and constructive suggestions. The authors declare no conflicts of interest.
Glossary
- ChIP
chromatin immunoprecipitation
- DPE
downstream core promoter element
- FBS
fetal bovine serum
- HDAC
histone deacetylase
- HDACi
histone deacetylase inhibitor
- shRNA
short hairpin RNA
- TAF
TATA-binding protein–associated factor
- TBP
TATA-binding protein
- TFIID
transcription factor IID
- TSA
trichostatin A
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
W. Jian designed the research, performed the experiments, and analyzed the data; B. Yan performed experiments; S. Huang designed the research and interpreted the data; and Y. Qiu supervised the project, designed the research, and wrote the manuscript.
REFERENCES
- 1.Back J., Dierich A., Bronn C., Kastner P., Chan S. (2004) PU.1 determines the self-renewal capacity of erythroid progenitor cells. Blood 103, 3615–3623 [DOI] [PubMed] [Google Scholar]
- 2.Staber P. B., Zhang P., Ye M., Welner R. S., Nombela-Arrieta C., Bach C., Kerenyi M., Bartholdy B. A., Zhang H., Alberich-Jordà M., Lee S., Yang H., Ng F., Zhang J., Leddin M., Silberstein L. E., Hoefler G., Orkin S. H., Göttgens B., Rosenbauer F., Huang G., Tenen D. G. (2013) Sustained PU.1 levels balance cell-cycle regulators to prevent exhaustion of adult hematopoietic stem cells. Mol. Cell 49, 934–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolff L., Humeniuk R. (2013) Concise review: erythroid versus myeloid lineage commitment: regulating the master regulators. Stem Cells 31, 1237–1244 [DOI] [PubMed] [Google Scholar]
- 4.Yamada T., Abe M., Higashi T., Yamamoto H., Kihara-Negishi F., Sakurai T., Shirai T., Oikawa T. (2001) Lineage switch induced by overexpression of Ets family transcription factor PU.1 in murine erythroleukemia cells. Blood 97, 2300–2307 [DOI] [PubMed] [Google Scholar]
- 5.Mak K. S., Funnell A. P. W., Pearson R. C. M., Crossley M. (2011) PU.1 and haematopoietic cell fate: dosage matters. Int. J. Cell Biol. 2011, 808524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arinobu Y., Mizuno S., Chong Y., Shigematsu H., Iino T., Iwasaki H., Graf T., Mayfield R., Chan S., Kastner P., Akashi K. (2007) Reciprocal activation of GATA-1 and PU.1 marks initial specification of hematopoietic stem cells into myeloerythroid and myelolymphoid lineages. Cell Stem Cell 1, 416–427 [DOI] [PubMed] [Google Scholar]
- 7.Iwasaki H., Somoza C., Shigematsu H., Duprez E. A., Iwasaki-Arai J., Mizuno S., Arinobu Y., Geary K., Zhang P., Dayaram T., Fenyus M. L., Elf S., Chan S., Kastner P., Huettner C. S., Murray R., Tenen D. G., Akashi K. (2005) Distinctive and indispensable roles of PU.1 in maintenance of hematopoietic stem cells and their differentiation. Blood 106, 1590–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richardson C. L., Delehanty L. L., Bullock G. C., Rival C. M., Tung K. S., Kimpel D. L., Gardenghi S., Rivella S., Goldfarb A. N. (2013) Isocitrate ameliorates anemia by suppressing the erythroid iron restriction response. J. Clin. Invest. 123, 3614–3623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Q., Zhang M., Wang X., Yuan W., Chen D., Royer-Pokora B., Zhu T. (2007) A novel transcript of the LMO2 gene, LMO2-c, is regulated by GATA-1 and PU.1 and encodes an antagonist of LMO2. Leukemia 21, 1015–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rekhtman N., Radparvar F., Evans T., Skoultchi A. I. (1999) Direct interaction of hematopoietic transcription factors PU.1 and GATA-1: functional antagonism in erythroid cells. Genes Dev. 13, 1398–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang P., Behre G., Pan J., Iwama A., Wara-Aswapati N., Radomska H. S., Auron P. E., Tenen D. G., Sun Z. (1999) Negative cross-talk between hematopoietic regulators: GATA proteins repress PU.1. Proc. Natl. Acad. Sci. USA 96, 8705–8710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Back J., Allman D., Chan S., Kastner P. (2005) Visualizing PU.1 activity during hematopoiesis. Exp. Hematol. 33, 395–402 [DOI] [PubMed] [Google Scholar]
- 13.Nutt S. L., Metcalf D., D’Amico A., Polli M., Wu L. (2005) Dynamic regulation of PU.1 expression in multipotent hematopoietic progenitors. J. Exp. Med. 201, 221–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenbauer F., Owens B. M., Yu L., Tumang J. R., Steidl U., Kutok J. L., Clayton L. K., Wagner K., Scheller M., Iwasaki H., Liu C., Hackanson B., Akashi K., Leutz A., Rothstein T. L., Plass C., Tenen D. G. (2006) Lymphoid cell growth and transformation are suppressed by a key regulatory element of the gene encoding PU.1. Nat. Genet. 38, 27–37 [DOI] [PubMed] [Google Scholar]
- 15.Moreau-Gachelin F., Wendling F., Molina T., Denis N., Titeux M., Grimber G., Briand P., Vainchenker W., Tavitian A. (1996) Spi-1/PU.1 transgenic mice develop multistep erythroleukemias. Mol. Cell. Biol. 16, 2453–2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Will B., Vogler T. O., Narayanagari S., Bartholdy B., Todorova T. I., da Silva Ferreira M., Chen J., Yu Y., Mayer J., Barreyro L., Carvajal L., Neriah D. B., Roth M., van Oers J., Schaetzlein S., McMahon C., Edelmann W., Verma A., Steidl U. (2015) Minimal PU.1 reduction induces a preleukemic state and promotes development of acute myeloid leukemia. Nat. Med. 21, 1172–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kistler B., Pfisterer P., Wirth T. (1995) Lymphoid- and myeloid-specific activity of the PU.1 promoter is determined by the combinatorial action of octamer and ets transcription factors. Oncogene 11, 1095–1106 [PubMed] [Google Scholar]
- 18.Chen H., Zhang P., Radomska H. S., Hetherington C. J., Zhang D. E., Tenen D. G. (1996) Octamer binding factors and their coactivator can activate the murine PU.1 (spi-1) promoter. J. Biol. Chem. 271, 15743–15752 [DOI] [PubMed] [Google Scholar]
- 19.Amaravadi L., Klemsz M. J. (1999) DNA methylation and chromatin structure regulate PU.1 expression. DNA Cell Biol. 18, 875–884 [DOI] [PubMed] [Google Scholar]
- 20.Yang X. J., Seto E. (2007) HATs and HDACs: from structure, function and regulation to novel strategies for therapy and prevention. Oncogene 26, 5310–5318 [DOI] [PubMed] [Google Scholar]
- 21.Qiu Y., Zhao Y., Becker M., John S., Parekh B. S., Huang S., Hendarwanto A., Martinez E. D., Chen Y., Lu H., Adkins N. L., Stavreva D. A., Wiench M., Georgel P. T., Schiltz R. L., Hager G. L. (2006) HDAC1 acetylation is linked to progressive modulation of steroid receptor-induced gene transcription. Mol. Cell 22, 669–679 [DOI] [PubMed] [Google Scholar]
- 22.Dobbin M. M., Madabhushi R., Pan L., Chen Y., Kim D., Gao J., Ahanonu B., Pao P. C., Qiu Y., Zhao Y., Tsai L. H. (2013) SIRT1 collaborates with ATM and HDAC1 to maintain genomic stability in neurons. Nat. Neurosci. 16, 1008–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo Y., Jian W., Stavreva D., Fu X., Hager G., Bungert J., Huang S., Qiu Y. (2009) Trans-regulation of histone deacetylase activities through acetylation. J. Biol. Chem. 284, 34901–34910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang T., Jian W., Luo Y., Fu X., Noguchi C., Bungert J., Huang S., Qiu Y. (2012) Acetylation of histone deacetylase 1 regulates NuRD corepressor complex activity. J. Biol. Chem. 287, 40279–40291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bieniossek C., Papai G., Schaffitzel C., Garzoni F., Chaillet M., Scheer E., Papadopoulos P., Tora L., Schultz P., Berger I. (2013) The architecture of human general transcription factor TFIID core complex. Nature 493, 699–702 [DOI] [PubMed] [Google Scholar]
- 26.Cler E., Papai G., Schultz P., Davidson I. (2009) Recent advances in understanding the structure and function of general transcription factor TFIID. Cell. Mol. Life Sci. 66, 2123–2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Juven-Gershon T., Kadonaga J. T. (2010) Regulation of gene expression via the core promoter and the basal transcriptional machinery. Dev. Biol. 339, 225–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burke T. W., Kadonaga J. T. (1996) Drosophila TFIID binds to a conserved downstream basal promoter element that is present in many TATA-box-deficient promoters. Genes Dev. 10, 711–724 [DOI] [PubMed] [Google Scholar]
- 29.Burke T. W., Kadonaga J. T. (1997) The downstream core promoter element, DPE, is conserved from Drosophila to humans and is recognized by TAFII60 of Drosophila. Genes Dev. 11, 3020–3031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kutach A. K., Kadonaga J. T. (2000) The downstream promoter element DPE appears to be as widely used as the TATA box in Drosophila core promoters. Mol. Cell. Biol. 20, 4754–4764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen W. C., Bhaumik S. R., Causton H. C., Simon I., Zhu X., Jennings E. G., Wang T. H., Young R. A., Green M. R. (2003) Systematic analysis of essential yeast TAFs in genome-wide transcription and preinitiation complex assembly. EMBO J. 22, 3395–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walker A. K., Rothman J. H., Shi Y., Blackwell T. K. (2001) Distinct requirements for C. elegans TAF(II)s in early embryonic transcription. EMBO J. 20, 5269–5279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frontini M., Soutoglou E., Argentini M., Bole-Feysot C., Jost B., Scheer E., Tora L. (2005) TAF9b (formerly TAF9L) is a bona fide TAF that has unique and overlapping roles with TAF9. Mol. Cell. Biol. 25, 4638–4649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Z., Manley J. L. (2003) Core promoter elements and TAFs contribute to the diversity of transcriptional activation in vertebrates. Mol. Cell. Biol. 23, 7350–7362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright K. J., Marr M. T. II, Tjian R. (2006) TAF4 nucleates a core subcomplex of TFIID and mediates activated transcription from a TATA-less promoter. Proc. Natl. Acad. Sci. USA 103, 12347–12352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Z., Manley J. L. (2003) In vivo functional analysis of the histone 3-like TAF9 and a TAF9-related factor, TAF9L. J. Biol. Chem. 278, 35172–35183 [DOI] [PubMed] [Google Scholar]
- 37.Imbalzano A. N., Kwon H., Green M. R., Kingston R. E. (1994) Facilitated binding of TATA-binding protein to nucleosomal DNA. Nature 370, 481–485 [DOI] [PubMed] [Google Scholar]
- 38.Workman J. L., Taylor I. C., Kingston R. E., Roeder R. G. (1991) Control of class II gene transcription during in vitro nucleosome assembly. Methods Cell Biol. 35, 419–447 [DOI] [PubMed] [Google Scholar]
- 39.Yamada T., Kondoh N., Matsumoto M., Yoshida M., Maekawa A., Oikawa T. (1997) Overexpression of PU.1 induces growth and differentiation inhibition and apoptotic cell death in murine erythroleukemia cells. Blood 89, 1383–1393 [PubMed] [Google Scholar]
- 40.Atar O., Levi B. Z. (2005) PU.1 silencing leads to terminal differentiation of erythroleukemia cells. Biochem. Biophys. Res. Commun. 329, 1288–1292 [DOI] [PubMed] [Google Scholar]
- 41.Rao G., Rekhtman N., Cheng G., Krasikov T., Skoultchi A. I. (1997) Deregulated expression of the PU.1 transcription factor blocks murine erythroleukemia cell terminal differentiation. Oncogene 14, 123–131 [DOI] [PubMed] [Google Scholar]
- 42.Zhang P., Zhang X., Iwama A., Yu C., Smith K. A., Mueller B. U., Narravula S., Torbett B. E., Orkin S. H., Tenen D. G. (2000) PU.1 inhibits GATA-1 function and erythroid differentiation by blocking GATA-1 DNA binding. Blood 96, 2641–2648 [PubMed] [Google Scholar]
- 43.Pace B. S., Chen Y. R., Thompson A., Goodman S. R. (2000) Butyrate-inducible elements in the human gamma-globin promoter. Exp. Hematol. 28, 283–293 [DOI] [PubMed] [Google Scholar]
- 44.Lozzio C. B., Lozzio B. B., Machado E. A., Fuhr J. E., Lair S. V., Bamberger E. G. (1979) Effects of sodium butyrate on human chronic myelogenous leukaemia cell line K562. Nature 281, 709–710 [DOI] [PubMed] [Google Scholar]
- 45.Laribee R. N., Klemsz M. J. (2001) Loss of PU.1 expression following inhibition of histone deacetylases. J. Immunol. 167, 5160–5166 [DOI] [PubMed] [Google Scholar]
- 46.Oikawa T., Yamada T., Kihara-Negishi F., Yamamoto H., Kondoh N., Hitomi Y., Hashimoto Y. (1999) The role of Ets family transcription factor PU.1 in hematopoietic cell differentiation, proliferation and apoptosis. Cell Death Differ. 6, 599–608 [DOI] [PubMed] [Google Scholar]
- 47.Yu Y., Mo Y., Ebenezer D., Bhattacharyya S., Liu H., Sundaravel S., Giricz O., Wontakal S., Cartier J., Caces B., Artz A., Nischal S., Bhagat T., Bathon K., Maqbool S., Gligich O., Suzuki M., Steidl U., Godley L., Skoultchi A., Greally J., Wickrema A., Verma A. (2013) High resolution methylome analysis reveals widespread functional hypomethylation during adult human erythropoiesis. J. Biol. Chem. 288, 8805–8814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choudhary C., Kumar C., Gnad F., Nielsen M. L., Rehman M., Walther T. C., Olsen J. V., Mann M. (2009) Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 325, 834–840 [DOI] [PubMed] [Google Scholar]
- 49.Shao H., Revach M., Moshonov S., Tzuman Y., Gazit K., Albeck S., Unger T., Dikstein R. (2005) Core promoter binding by histone-like TAF complexes. Mol. Cell. Biol. 25, 206–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walker D., Htun H., Hager G. L. (1999) Using inducible vectors to study intracellular trafficking of GFP-tagged steroid/nuclear receptors in living cells. Methods 19, 386–393 [DOI] [PubMed] [Google Scholar]
- 51.Qiu Y., Stavreva D. A., Luo Y., Indrawan A., Chang M., Hager G. L. (2011) Dynamic interaction of HDAC1 with a glucocorticoid receptor-regulated gene is modulated by the activity state of the promoter. J. Biol. Chem. 286, 7641–7647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hattangadi S. M., Wong P., Zhang L., Flygare J., Lodish H. F. (2011) From stem cell to red cell: regulation of erythropoiesis at multiple levels by multiple proteins, RNAs, and chromatin modifications. Blood 118, 6258–6268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palis J., Segel G. B. (1998) Developmental biology of erythropoiesis. Blood Rev. 12, 106–114 [DOI] [PubMed] [Google Scholar]
- 54.Kueh H. Y., Champhekar A., Nutt S. L., Elowitz M. B., Rothenberg E. V. (2013) Positive feedback between PU.1 and the cell cycle controls myeloid differentiation. Science 341, 670–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kerenyi M. A., Orkin S. H. (2010) Networking erythropoiesis. J. Exp. Med. 207, 2537–2541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wong P., Hattangadi S. M., Cheng A. W., Frampton G. M., Young R. A., Lodish H. F. (2011) Gene induction and repression during terminal erythropoiesis are mediated by distinct epigenetic changes. Blood 118, e128–e138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kastner P., Chan S. (2008) PU.1: a crucial and versatile player in hematopoiesis and leukemia. Int. J. Biochem. Cell Biol. 40, 22–27 [DOI] [PubMed] [Google Scholar]
- 58.Koschmieder S., Rosenbauer F., Steidl U., Owens B. M., Tenen D. G. (2005) Role of transcription factors C/EBPalpha and PU.1 in normal hematopoiesis and leukemia. Int. J. Hematol. 81, 368–377 [DOI] [PubMed] [Google Scholar]
- 59.Steidl U., Steidl C., Ebralidze A., Chapuy B., Han H. J., Will B., Rosenbauer F., Becker A., Wagner K., Koschmieder S., Kobayashi S., Costa D. B., Schulz T., O’Brien K. B., Verhaak R. G., Delwel R., Haase D., Trümper L., Krauter J., Kohwi-Shigematsu T., Griesinger F., Tenen D. G. (2007) A distal single nucleotide polymorphism alters long-range regulation of the PU.1 gene in acute myeloid leukemia. J. Clin. Invest. 117, 2611–2620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rodriguez P., Bonte E., Krijgsveld J., Kolodziej K. E., Guyot B., Heck A. J., Vyas P., de Boer E., Grosveld F., Strouboulis J. (2005) GATA-1 forms distinct activating and repressive complexes in erythroid cells. EMBO J. 24, 2354–2366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hong W., Nakazawa M., Chen Y. Y., Kori R., Vakoc C. R., Rakowski C., Blobel G. A. (2005) FOG-1 recruits the NuRD repressor complex to mediate transcriptional repression by GATA-1. EMBO J. 24, 2367–2378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang S., Brandt S. J. (2000) mSin3A regulates murine erythroleukemia cell differentiation through association with the TAL1 (or SCL) transcription factor. Mol. Cell. Biol. 20, 2248–2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen X., Bieker J. J. (2004) Stage-specific repression by the EKLF transcriptional activator. Mol. Cell. Biol. 24, 10416–10424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Drummond D. C., Noble C. O., Kirpotin D. B., Guo Z., Scott G. K., Benz C. C. (2005) Clinical development of histone deacetylase inhibitors as anticancer agents. Annu. Rev. Pharmacol. Toxicol. 45, 495–528 [DOI] [PubMed] [Google Scholar]
- 65.Minucci S., Nervi C., Lo Coco F., Pelicci P. G. (2001) Histone deacetylases: a common molecular target for differentiation treatment of acute myeloid leukemias? Oncogene 20, 3110–3115 [DOI] [PubMed] [Google Scholar]
- 66.Ferrara F. F., Fazi F., Bianchini A., Padula F., Gelmetti V., Minucci S., Mancini M., Pelicci P. G., Lo Coco F., Nervi C. (2001) Histone deacetylase-targeted treatment restores retinoic acid signaling and differentiation in acute myeloid leukemia. Cancer Res. 61, 2–7 [PubMed] [Google Scholar]
- 67.McCaffrey P. G., Newsome D. A., Fibach E., Yoshida M., Su M. S. (1997) Induction of gamma-globin by histone deacetylase inhibitors. Blood 90, 2075–2083 [PubMed] [Google Scholar]
- 68.Fathallah H., Weinberg R. S., Galperin Y., Sutton M., Atweh G. F. (2007) Role of epigenetic modifications in normal globin gene regulation and butyrate-mediated induction of fetal hemoglobin. Blood 110, 3391–3397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wagner J. M., Hackanson B., Lübbert M., Jung M. (2010) Histone deacetylase (HDAC) inhibitors in recent clinical trials for cancer therapy. Clin. Epigenetics 1, 117–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Verbiest T., Bouffler S., Nutt S. L., Badie C. (2015) PU.1 downregulation in murine radiation-induced acute myeloid leukaemia (AML): from molecular mechanism to human AML. Carcinogenesis 36, 413–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.