Abstract
Hutchinson-Gilford progeria syndrome (HGPS) is a rare genetic disorder that is caused by a point mutation in the LMNA gene, resulting in production of a truncated farnesylated-prelamin A protein (progerin). We previously reported that XPA mislocalized to the progerin-induced DNA double-strand break (DSB) sites, blocking DSB repair, which led to DSB accumulation, DNA damage responses, and early replication arrest in HGPS. In this study, the XPA mislocalization to DSBs occurred at stalled or collapsed replication forks, concurrent with a significant loss of PCNA at the forks, whereas PCNA efficiently bound to progerin. This PCNA sequestration likely exposed ds-ssDNA junctions at replication forks for XPA binding. Depletion of XPA or progerin each significantly restored PCNA at replication forks. Our results suggest that although PCNA is much more competitive than XPA in binding replication forks, PCNA sequestration by progerin may shift the equilibrium to favor XPA binding. Furthermore, we demonstrated that progerin-induced apoptosis could be rescued by XPA, suggesting that XPA-replication fork binding may prevent apoptosis in HGPS cells. Our results propose a mechanism for progerin-induced genome instability and accelerated replicative senescence in HGPS.—Hilton, B. A., Liu, J., Cartwright, B. M., Liu, Y., Breitman, M., Wang, Y., Jones, R., Tang, H., Rusinol, A., Musich, P. R., Zou, Y. Progerin sequestration of PCNA promotes replication fork collapse and mislocalization of XPA in laminopathy-related progeroid syndromes.
Keywords: HGPS, lamin A, DNA DSBs, DNA repair, genome instability
Hutchinson-Gilford progeria syndrome (HGPS) is a laminopathy-based premature aging disease. It is caused by a de novo point mutation (1824C→T) in the LMNA gene (1, 2). The mutation results in sporadic activation of a cryptic donor splice site in exon 11 of the prelamin A premRNA, leading to sporadic production of a truncated prelamin A mRNA, resulting in a 150 base (coding for 50 aa residues) deletion near the 3′-end of the mRNA (1, 2). A direct consequence of this deletion is the loss of the Zmpste24 (also called FACE-1) endoproteolytic cleavage site (RSYLLG), which is required for the proteolytic maturation of prelamin A to lamin A (3). Formation of this aberrant mRNA results in production of a farnesylated–carboxymethylated truncated lamin A (progerin or LAΔ50). Lamin A, the proteolytically mature form of prelamin A, is an intermediate filament protein that is part of the nuclear lamina, which structurally supports the nucleus and organizes chromatin (4). Other genetic diseases arising from mutations in the lamin A gene or required processing proteases, as in restrictive dermopathy (RD), are collectively termed laminopathies (5, 6).
The replication rate of HGPS cells in culture has been shown to reach a level near senescence much more quickly than normal fibroblasts (7, 8). In addition, double-strand breaks (DSBs) accumulate in HGPS cells and, as a result, the cells exhibit genome instability that may contribute to accelerated replicative arrest and premature aging (7, 9–12). It has been suggested that cellular accumulation of DSBs could be due to a deficiency in DNA repair in progeria or senescing cells (13, 14). Our studies found that the DSB repair proteins Rad51 and -50 were absent at the progerin-induced DNA damage sites in progeria cells (14). These progerin-induced DSBs were resistant to repair in the progeria cells; however, repair of camptothecin (CPT)-induced DNA damage was still effective, although lower than normal human fibroblasts (BJ cells) (14). Unexpectedly, the nucleotide excision repair (NER) protein xeroderma pigmentosum group A (XPA) was found to form nuclear foci that colocalize with γ-variant of the H2A protein family (γ-H2AX), a marker for DSBs. Although the role of XPA in NER has been extensively studied (15–21), XPA has not been found to play any role in DSB repair. The mislocalization of XPA to or near the laminopathy-induced DSB sites blocked the accessibility of the damage sites to DSB-repair factors, thus inhibiting DNA repair (14).
In addition to its hallmark role in NER, we observed that XPA also can bind to double-strand/single-strand DNA (ds-ssDNA) junctions with 3′- and/or 5′-ssDNA overhangs. The binding affinity of XPA for these sites is 1–2 orders of magnitude higher than its ability to bind to bulky DNA adducts, and this binding is through an extended DNA-binding domain (22–24). The ds-ssDNA junction structures are the structural forms commonly found as intermediates during many DNA metabolic pathways, including DNA replication and repair. However, how these functions of XPA relate to its effects and observed phenotypes in HGPS is unclear.
Nuclear lamins directly interact with histones such as H2A; however, nuclear lamins also interact with DNA synthesis proteins such as proliferating cell nuclear antigen (PCNA) (25, 26). PCNA is a member of a family of sliding clamp proteins and is part of the replisome. It is essential for the progression of DNA synthesis/replication at the elongation phase (27). In addition, PCNA at the replication fork recruits DNA polymerases and enhances their processivity for DNA synthesis. The replication protein C (RFC) complex is essential for loading of PCNA onto replication forks. Our work also has demonstrated that RFC1, the large subunit of the complex, is increasingly degraded during HGPS cell growth (28). PCNA has also been shown to play a role in regulation of the cell cycle during replication through direct binding to the nuclear envelope proteins, specifically the lamins (25).
In the present study, we determined the mechanisms by which DSBs are produced and XPA is mislocalized to DSBs in progeroid cells. We found that γ-H2AX and XPA both colocalize with a subset of the DNA replication proteins in HGPS patient fibroblasts, suggesting that the DSBs may result from replication forks that have stalled or collapsed in HGPS cells and, thus, XPA is mislocalized to replication forks. This XPA mislocalization likely is caused by enhanced progerin binding to PCNA as compared to lamin A. The results suggest that the mislocalization of XPA to DSBs because of sequestration of PCNA by progerin may prevent repair of DSBs in HGPS cells. In addition, we found that this XPA-DNA interaction is regulated by the kinase Ataxia telangiectasia and Rad3-related (ATR), and the regulation may affect the expression and turnover of ribonucleotide reductase (RNR) in aging HGPS cells, likely through disruption of cell cycle transit. We also found that expression of progerin in XPA-deficient cells induced apoptotic cell death but that coexpression of progerin and XPA rescued these cells from apoptotic cell death, suggesting that binding of XPA to protein-depleted DNA junctions may play a role in reducing apoptosis in HGPS cells.
MATERIALS AND METHODS
Cell culture and drug treatment
Fibroblasts from 2 HGPS-bearing patients (AG11513A and AG11498B, designated HGPS-1 and -2, respectively) with the gene mutation at codon 608 (G608G) within exon 11 of the LMNA gene, were obtained from Coriell Cell Repository (Camden, NJ, USA). HGPS cells also were obtained from the Progeria Research Foundation (HGADFN178 designated as HGPS-4). Human RD fibroblasts were a gift from Dr. J. H. Miner (Washington University School of Medicine, St. Louis, MO, USA). The normal human BJ fibroblasts were purchased from American Type Culture Collection (ATCC; Rockville, MD, USA; CRL-2522). Normal fibroblasts harvested from the father of HGADFN178 (designated as 090) also were obtained from the Progeria Research Foundation. HeLa cells were purchased from ATCC (CCL-2). All cultures were maintained in DMEM supplemented with 10 or 15% fetal bovine serum for secondary and primary cells, respectively, and antibiotics (50 units/ml penicillin and 50 μg/ml streptomycin) at 37°C in an atmosphere containing 5% CO2. To induce DNA damage, cells were grown to 70% confluence, followed by incubation with 10 μM CPT for 6 h before analysis. Alternatively, cells were treated with 20 J/m2 UV-C irradiation using a 254-nm lamp at a fluorescence of 0.83 J·m−2·s followed by a 6-h recovery period before harvesting. The ATR kinase inhibitor NU6027 was purchased from EMD Millipore (189299) and the ataxia telangiectasia mutated (ATM) kinase inhibitor KU-55933 was purchased from Abcam (ab120637); each inhibitor was used at a final concentration of 10 μM in cell culture medium containing serum.
Transfection of small interfering RNA and plasmids
To knock down XPA or PCNA by RNAi, cells were transfected with a pool of small interfering RNAs (siRNAs) (sc-36853 and sc-29441, respectively; Santa Cruz Biotechnology, Dallas, TX, USA) or a scrambled sequence as a control using InteferIn transfection reagent (Polyplus-transfection SA, Illkirch-Graffenstaden, France) according to the manufacturer’s instructions. Further analyses were performed 72 h after transfection. Plasmid transfection was performed with JetPEI transfection reagent (Polyplus-transfection SA) according to the manufacturer’s instructions. Further analyses were performed 48 h after transfection.
Chromatin association assay
Cellular localization of XPA in HGPS cells was investigated by a chromatin fractionation technique based on the method of Mendez and Stillman (29). In brief, HGPS cells were plated in 100-mm dishes. At 80% confluence, cells were washed twice with ice-cold PBS, harvested, and collected by centrifugation at 500 g for 5 min. The cell pellet was resuspended in 100 μl solution A [10 mM HEPES–KOH (pH 7.9), 10 mM KCl, 1.5 mM MgCl2, 0.34 M sucrose, 1× protease inhibitor cocktail (78430; Thermo Fisher Scientific, Waltham MA, USA), 1 mM PMSF, and 0.1% Triton X-100). After a 5-min incubation on ice, samples were centrifuged at 1300 g for 4 min. The pellet was washed once in solution A and designated as intact nuclei. Nuclei were resuspended in 100 μl of solution B (3 mM EDTA, 0.2 mM EGTA, and 1× protease inhibitor cocktail) and incubated on ice for 10 min. The sample then was centrifuged at 13,000 g for 10 min. The pellet was washed once with solution B, and designated as the chromatin fraction.
Chromatin immunoprecipitation assay
For the chromatin immunoprecipitation (ChIP) assay (14), HGPS cells were grown in three 150 mm dishes per sample until reaching passage 12. At this passage, they were washed once with ice-cold PBS and then fixed in a 4% formaldehyde/PBS solution for 10 min at room temperature. The solution was then removed, and the remaining formaldehyde quenched by addition of 250 mM glycine/PBS. Cells then were washed twice with ice-cold PBS before harvest by scraping and centrifugation at 2500 rpm for 10 min at 4°C. The cell pellet was resuspended in 1 ml swelling buffer [25 mM HEPES-KOH (pH 7.8), 1.5 mM MgCl2, 10 mM KCl, 0.01% NP-40, 1 mM DTT, 1× protease/phosphatase inhibitor cocktail (78442; Thermo Fisher Scientific)]. After 10 min incubation on ice, samples were centrifuged at 4000 rpm for 10 min. The pellet was resuspended in 500 µl of NP-40 buffer [50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1% NP-40, and 1× protease/phosphatase inhibitor cocktail]. After a 10 min incubation on ice, lysates were sonicated at power level 4 for 10 s with a 1 s interval pulse and then at level 2 for 2 min with 1 s interval pulse while being suspended on an ethanol/water/ice slurry. Samples were centrifuged at 12,000 rpm for 10 min at 4°C to clear the samples. Supernatants then were incubated overnight with minichromosome maintenance complex (MCM)-7 antibody (sc-9966; Santa Cruz Biotechnology), followed by a 2 h incubation at 4°C with protein G magnetic beads (S1430S; New England Biolabs, Ipswich, MA, USA), and then 2 washes with NP-40 buffer. Samples were analyzed by 12% SDS-polyacrylamide gel and Western blot analysis.
Progerin antisense oligonucleotide transfection
Antisense or scrambled oligonucleotides to LMNA exon 11 (30) were transfected into HGPS cells (P12) using MegaTran 1.0 transfection reagent (TT200002; Origen, Rockville, MD, USA). Oligo transfections were performed at 6 μM for a total of 96 h. An initial transfection was performed for 48 h and then followed by a refresher transfection with a change of medium plus oligos for another 48 h before cell harvest or fixation for further experimentation. Efficiency of antisense oligos was assessed by Western blot analysis with antibody to LMNA (2032S; Cell Signaling Technology, Danvers, MA, USA).
Western blot analysis
Cells cultured in 100-mm dishes or 25-cm2 flasks were grown to 70% confluence and then trypsinized. The number of cells was counted with a hemocytometer, centrifuged at 500 g for 5 min, and washed twice with PBS in the presence of protease inhibitor cocktail. The cell pellet was lysed in 2× SDS gel loading buffer, and volumes corresponding to 5 × 106 cells were subjected to SDS-PAGE. Electrophoresis was carried out in 8 or 4–12% gels (Bio-Rad, Hercules, CA, USA). Proteins were transferred to PVDF membranes (Santa Cruz Biotechnology) and immunoblot analysis was performed with primary antibodies directed against XPA (MA5-13835; Thermo Fisher Scientific), lamin A/C (2032; Cell Signaling Technology), GAPDH (MA5-15738; Thermo Fisher Scientific), β-actin (A5316; Sigma-Aldrich, St. Louis, MO, USA), PCNA (sc-9857; Santa Cruz Biotechnology; and ab29; Abcam), MCM7 (sc-9966; Santa Cruz Biotechnology), Rad50 (sc-18291; Santa Cruz Biotechnology), poly ADP ribose polymerase (PARP)-1 (9542; Cell Signaling), p53 (sc-47698; Santa Cruz Biotechnology), phospho-p53 (Ser15) (9286; Cell Signaling), ribonucleotide reductase catalytic subunit (RRM)-1 (sc-11733; Santa Cruz Biotechnology), RRM2 (sc-10846; Santa Cruz Biotechnology), p53RRM2 (sc-10840; Santa Cruz Biotechnology), and polymerase δ (Polδ, sc-17776; Santa Cruz Biotechnology), followed by HRP-conjugated secondary antibodies (Santa Cruz Biotechnology). The complexes were revealed using SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific).
Immunofluorescence microscopy
Cells grown on coverslips were fixed with cold methanol (−20°C) for 10 min, or the cytoplasm was removed by extraction with 0.5% IGEPAL CA-630, followed by fixation with methanol. The fixed cells were incubated with primary antibodies against γ-H2AX (rabbit, Bethyl or mouse; Stressgen, San Diego, CA, USA), XPA [mouse (Thermo Fisher Scientific) or rabbit (Santa Cruz Biotechnology)], MCM7 (mouse; Santa Cruz Biotechnology) or PCNA (rabbit; Santa Cruz Biotechnology). Secondary antibodies included Alexa Fluor 488-conjugated donkey anti-rabbit IgG and Alexa Fluor 568-conjugated goat anti-mouse IgG (Molecular Probes, Eugene, OR, USA). Cells were counterstained with DAPI to visualize nuclear DNA. Two observers who randomly chose 100 cells for each experiment in a blinded procedure performed the counting of foci.
Click-iT 5-ethynyl-2′-deoxyuridine DNA synthesis assay
DNA in culture-aged HGPS and BJ cells was labeled with 5-ethynyl-2′-deoxyuridine (EdU; Thermo Fisher Scientific). The DNA was labeled for 1 h with 20 μM EdU. Cells were isolated, rinsed with PBS, and processed as described by the manufacturer (Click-iT EdU Manual; Thermo Fisher Scientific). EdU was detected after conjugation with Alexa Fluor 647 azide using Click-iT chemistry, and the DNA was stained with propidium iodide (Thermo Fisher Scientific) before analysis by flow cytometry. Cell cycle and cellular DNA synthesis were analyzed with FlowJo software (Tree Star, Ashland, OR, USA).
Duolink in situ proximity ligation assay
For Duolink microscopic detection of protein interactions, cells were grown on coverslips before 4% paraformaldehyde fixation and permeabilization with 0.2% Triton X-100 in PBS and blocked with 3% bovine serum albumin in PBS for 1 h at room temperature. Proteins of interest then were detected with primary antibodies, and the Duolink assay was performed according to the manufacturer’s protocol (DUO92101; Sigma-Aldrich). Images were taken using Evos FL Auto microscope (Thermo Fisher Scientific) or a confocal microscope (Olympus America, Melville, NY, USA). Statistical analysis was performed and standard deviations were determined based on a Student’s t test.
γ-H2AX association assay and coimmunoprecipitation
The γ-H2AX association assay was modified from the histone association assay described by Ricke and Bielinsky (31). In brief, cells were treated with 4% formaldehyde to cross-link interacting protein-DNA as well as protein–protein complexes. Nuclei were prepared by subcellular fractionation. The chromatin was sheared into 200- to 1500-bp fragments by sonication. The sheared chromatin was incubated with γ-H2AX antibody, followed by precipitation with protein G-Sepharose beads. The immunoprecipitates were boiled for at least 30 min to reverse the crosslinks. Proteins that coprecipitate with γ-H2AX-bound chromatin were detected by Western blot analysis. The Co-IP was performed with a Nuclear Complex Co-IP kit (Active Motif, Carlsbad, CA, USA), according to the manufacturer’s instructions.
Apoptosis assays
Tetramethylrhodamine ethyl ester (TMRE) staining (ab113852) was performed according to the manufacturer (Thermo Fisher Scientific). Samples were analyzed in an Accuri C6 flow cytometer (BD Biosciences, San Diego, CA, USA). The acetyl Asp-Glu-Val-Asp 7-amido-4-methylcoumarin (Ac-DEVD-AFC) Caspase-3 Fluorogenic Substrate Assay also was used to measure apoptosis. To detect active caspase-3, cells were lysed and incubated with the fluorescent substrate Ac-DEVD-AFC for 1 h at 37°C, and fluorescence was measured by spectrofluorometer. At least 3 independent experiments were conducted. Statistical analysis of samples for standard deviations was performed with Student’s t test, and a value of P < 0.05 was considered significant.
RESULTS
XPA replaces PCNA at stalled replication forks in HGPS cells
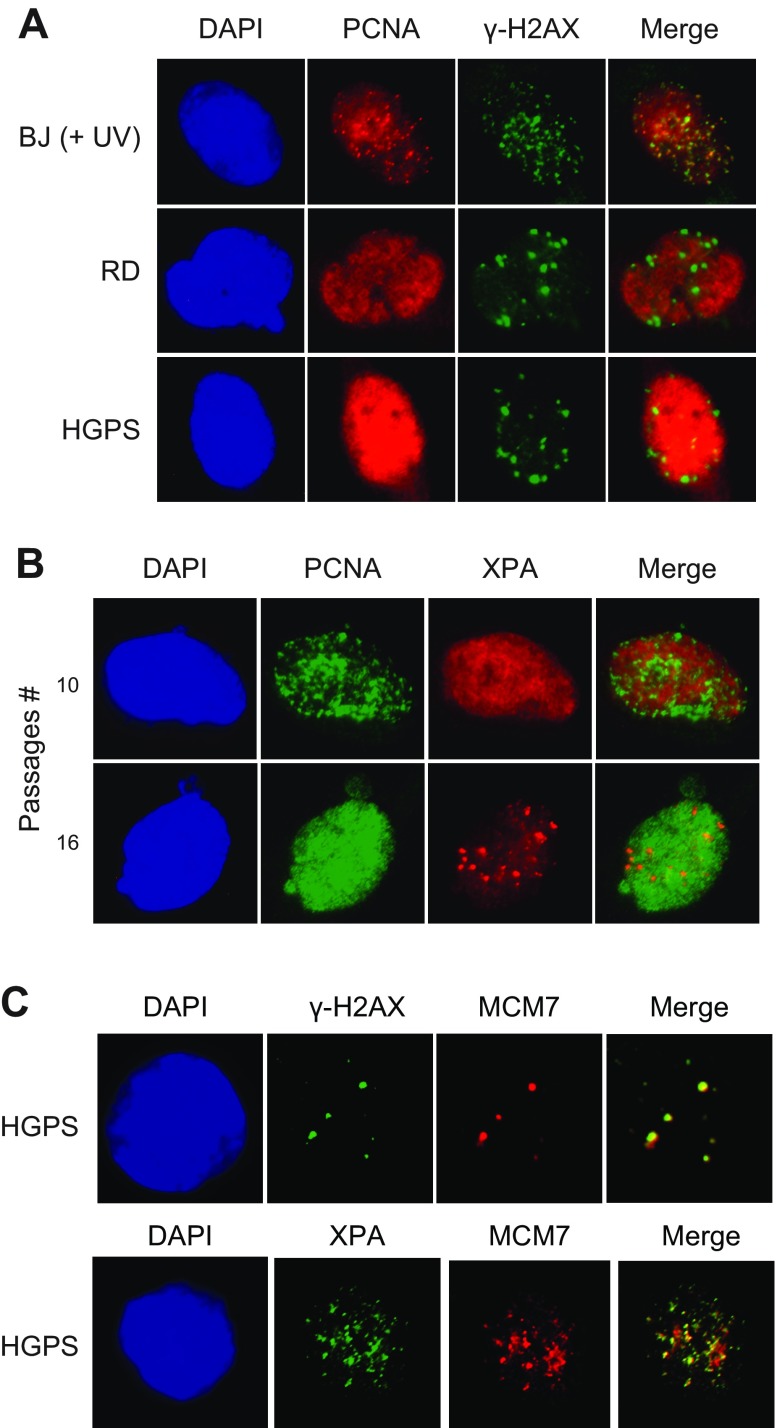
We first examined the status of PCNA-DSB colocalization in BJ, RD, and HGPS fibroblasts by probing PCNA and γ-H2AX focus formation using immunofluorescence microscopy. BJ cells were used in this study as the control. The BJ cells were treated with UV-C at 20 J/m2 to induce DNA damage for comparison. UV was used because the damage from UV irradiation causes replication-dependent strand breaks. γ-H2AX is a marker used to track DSBs. In UV-treated BJ cells there is an accumulation of γ-H2AX foci that overlap the PCNA foci (Fig. 1A) suggesting that PCNA and γ-H2AX occur at the same sites of DNA damage in BJ cells. In these cells, PCNA focus formation is consistent with active DNA synthesis, which in the treated cells would also include the sites of DNA repair synthesis. In both the RD and HGPS cells, which have been demonstrated to accumulate prelamin A and progerin with increased passage number, respectively (14), γ-H2AX is observed to form distinct foci without DNA damage treatment, consistent with previous reports of progerin-induced DSB formation (7, 14). PCNA, however, did not form discrete foci in these cells, suggesting that PCNA is not located at DSB sites, and that little or no repair synthesis occurs in the progeroid cells.
Figure 1.
XPA replaces PCNA at stalled replication forks in HGPS cells. A) Colocalization of γ-H2AX foci and the foci of PCNA in BJ (UV-treated) cells but not in HGPS and RD fibroblasts. B) PCNA formed nuclear foci in young HGPS cells, whereas XPA formed foci in late-passage HGPS cells. C) Colocalization of MCM7 foci and the foci of γ-H2AX and XPA in late-passage HGPS cells. Antigens were detected with corresponding antibodies by immunofluorescence microscopy. Uniform staining of the given protein throughout the nucleus indicates homogenous distribution of the protein without focus formation.
Because we had shown that XPA binds at DSB sites in HGPS cells (14), we next examined the nuclear localization of PCNA and XPA. As expected, in the young HGPS cells (P10), PCNA formed discrete foci indicating that replication was active, whereas nuclear XPA was diffusely distributed (Fig. 1B). Although XPA is known to accumulate at DSB sites in HGPS, it is unlikely that these rapidly growing, young-age cells had accumulated substantial DSBs. However, in the old-aged HGPS cells (P16), where PCNA was no longer found in the foci, but rather was diffuse throughout the nucleus, much like XPA in the young HGPS cells, discrete foci were remarkably observed for XPA in old HGPS cells. The formation of XPA foci in nuclei in old-age HGPS cells is consistent with our previous study, which is probably because of the loss of PCNA from chromatin (28). There is no colocalization of PCNA and XPA in either young or old cells, suggesting that these proteins interact differently with the DSBs in prematurely aging HGPS cells than in the normal BJ cells.
Next, we asked whether the XPA mislocalization at DSBs in older HGPS cells results from stalled replication forks. In old-aged HGPS cells, both γ-H2AX and XPA foci were colocalized with MCM7 foci (Fig. 1C). MCM7 is a subunit of the DNA replicative helicase in the replication complex and is involved in the initiation and elongation of replication (29, 32, 33). MCM7 helicase remains at the replication forks, even though the forks are stalled and strand breaks are induced (34, 35). γ-H2AX colocalization with MCM7 suggests that the DSBs formed in HGPS cells are the result of the collapse of stalled replication forks. These data further demonstrate that XPA can function outside of its known role as an NER factor.
XPA-associated DNA strand breaks form at replication forks in HGPS
To examine whether PCNA binds differentially to chromatin sites containing DNA DSBs induced by progerin vs. CPT (induces DSB by inhibiting DNA topoisomerase I), we used a modified chromatin immunoprecipitation (ChIP) assay. CPT-treated and untreated HGPS cells were subjected to the ChIP assay using γ-H2AX antibody for the immunoprecipitation followed by Western blot analysis to detect the proteins bound at the sites of DSBs. In the untreated HGPS cells little PCNA protein was bound in the DSB chromatin pulled down with γ-H2AX antibody, whereas significant MCM7 was bound (Fig. 2A), further demonstrating that PCNA does not bind γ-H2AX-tagged endogenous DSB chromatin sites in HGPS cells. In contrast, significantly more PCNA was pulled down with γ-H2AX-tagged DSB chromatin in CPT-treated HGPS cells. This result suggests that PCNA associates with the chromatin sites containing CPT-induced DSBs, likely because of the CPT-induced collapse of normally functioning replication forks (not affected by progerin) in the genomes or in the cells with less progerin accumulation in a heterogeneous cell population (11). In addition, the CPT-induced PCNA-DSBs association also may occur during repair of the damage (14, 36). However, PCNA did not bind well to endogenous DNA damage generated by progerin. When XPA was knocked down in the HGPS cells using XPA-specific siRNA, significantly more PCNA bound the endogenous DNA damaged chromatin (Fig. 2B). Consistently, significantly more Rad50, a DSB repair protein, was recruited to the damage sites in HGPS cells with XPA silencing than in control cells, as well as more replication Polδ for DNA damage repair at the stalled replication forks after XPA silencing in HGPS cells, as compared with the control (14, 37, 38). These data further confirm that the binding of XPA may prevent the binding of available PCNA at the DSB sites in aged HGPS cells.
Figure 2.
XPA-associated DSBs form at replication forks in HGPS. A) HGPS cells treated with or without CPT were subjected to the modified ChIP assay with pulldown by the anti-γ-H2AX antibody. The immunoprecipitated chromatin was analyzed for PCNA and MCM7 Co-IP by Western blot analysis. PCNA was largely absent at the replication forks with endogenous progerin-induced strand breaks, but was present in chromatin at CPT-induced strand breaks. MCM7 is a replication fork marker. B) HGPS cells were transfected with XPA siRNA, then mock or CPT treated, followed by analysis using modified ChIP assay with anti-γ-H2AX antibody. The successful knockdown of XPA by siRNA significantly restores the binding of PCNA and Polδ to the replication forks in the progeroid cells.
PCNA is sequestered by progerin in HGPS cells
As shown above, XPA binding to the chromatin sites containing DSBs in HGPS cells coincides with an absence of PCNA, likely at stalled or collapsed replication forks. Therefore, we speculated that XPA would bind to DSB sites generated in normal cells where replication forks are stalled by depletion of PCNA protein by siRNA knockdown. Thus, silencing of PCNA was performed in BJ cells by PCNA-specific siRNA. When PCNA was knocked down, XPA formed discrete foci in BJ cells, confirming that XPA locates to the PCNA-vacated chromatin sites, even in normal cells as a novel function for XPA (Fig. 3A). Because a low level of progerin also has been found in normal cells and increases with normal physiologic aging (39–43), further examination of BJ cells cultured in long-term to near senescence was conducted to determine whether an accumulation of XPA foci occurs with increasing passage number. As expected, this indeed was the case (Fig. 3B) and these XPA foci colocalized well with γ-H2AX foci in old-age BJ cells (P44). Western analysis demonstrated an accumulation of progerin in late-passage BJ cells. In contrast, for the relatively young BJ cells (P16), XPA was diffuse throughout the nucleus, and there were few or no distinguishable γ-H2AX foci present. A chromatin association assay further confirmed the passage-dependent accumulation of XPA at the chromatin in aging BJ cells.
Figure 3.
PCNA is sequestered by progerin in HGPS cells. A) XPA nuclear focus formation in normal BJ cells after PCNA knockdown by PCNA-specific siRNA. Western blot analysis demonstrated the efficiency of PCNA knockdown. B) Formation of XPA foci and colocalization with γ-H2AX in BJ cells with increasing passage number. One hundred cells were randomly chosen and counted. Cells that had at least 3 XPA foci were regarded as focus-positive cells. Statistical analysis of the data indicated that the changes were statistically significant between passage numbers 16 and 33 and between 16 and 44 (χ2 = 79; P < 0.01). Nuclear XPA is significantly associated with the chromatin in later passage BJ cells. Progerin accumulation with increasing passage number in BJ cells was analyzed by Western blot analysis of the whole-cell extracts. C) PLA using PCNA-specific and either lamin A- or progerin-specific antibodies in old-passage HGPS cells. A significant majority of PCNA was associated with progerin, not lamin A. After primary antibody incubation, secondary antibodies conjugated to complimentary oligonucleotides that were bound, ligated, and amplified to generate a red signal indicating interaction of the respective protein pairs. Interaction-positive foci were quantified and plotted. D) HGPS cells were subjected to a Co-IP assay with pulldown by the anti-progerin antibody. The immunoprecipitated progerin was analyzed for PCNA association by Western blot analysis. More PCNA pulldowns with progerin in untreated old HGPS cells (P18) than in younger HGPS cells (P9). The bead control contains HGPS cell lysate without antibody.
Next, to determine the molecular basis for PCNA vacancy at the replication forks in HGPS cells, we used the proximity ligation assay (PLA; Duolink) to test for the interaction between PCNA and the lamin A or progerin proteins. In this assay, 2 proteins must be within 30–40 nm in proximity to generate a signal. The proximity is likely related to protein–protein interaction. In old-age HGPS cells there was little PCNA-lamin A interaction; however, dramatically more PCNA was found to interact with progerin (Fig. 3C). Quantification of the data from analysis of 100 cells shows the significance of the results. To further confirm the interaction of PCNA and progerin a Co-IP assay was performed (Fig. 3D). In old-age HGPS cells (P18) progerin pulled down with significantly more PCNA than in younger HGPS cells. The PCNA-progerin interaction in old-age HGPS cells suggests that XPA binding to DSBs is a consequence of PCNA sequestration by progerin. Taken together, these observations suggest that XPA binds to DSB-containing chromatin sites in older HGPS cells where the DNA replication machinery is deficient because of the PCNA vacancy facilitated by its sequestration by progerin.
Progerin depletion restores PCNA presence at replication forks
To confirm that the sequestration of PCNA by progerin is a cause of the disruption of DNA replication activity and DSB accumulation in HGPS cells because of the loss of PCNA at the forks, HGPS cells were transfected with progerin antisense oligonucleotides to knock down progerin in the cells (30). The data presented in Fig. 4A demonstrate the efficient knockdown of progerin. The progerin-depleted HGPS cells then were subjected to a PLA (Duolink) for determination of the interaction between essential replication factor MCM7 and PCNA, revealed as PLA foci. As shown, the progerin depletion dramatically increased the interaction, demonstrating the restoration of PCNA at replication forks (Fig. 4B). In contrast, few MCM7-PCNA PLA foci were observed in the cells transfected with scrambled oligonucleotides. To further confirm the results, a ChIP assay was conducted. The same treated HGPS cells were fixed, followed by chromatin fragmentation by sonication. The fragmented DNA with associated proteins was immunoprecipitated by MCM7 antibody. Western blot analysis of the immunoprecipitates showed the restoration of PCNA association with replication forks in HGPS cells (Fig. 4C), which was fully consistent with the PLA data. These results confirm the role of progerin sequestration of PCNA from replication forks, thus disrupting replication activity and leading to DSB accumulation at stalled replication forks in HGPS cells.
Figure 4.
Depletion of progerin rescues PCNA association at replication forks in HGPS cells. A) Transfection of progerin antisense oligonucleotides resulted in dramatic reduction of progerin level in HGPS cells. B) PLA for detecting interaction of MCM7, an essential replication helicase factor, with PCNA were conducted in HGPS cells transfected with scrambled oligonucleotides or progerin antisense oligonucleotides. C) A modified ChIP assay (14) was performed with the same HGPS cells and treatments as in B. Chromatin fragments were immunoprecipitated with anti-MCM7 antibody for Western blot analysis.
XPA chromatin accumulation in HGPS cells is age dependent and regulated by ATR
XPA accumulation at chromatin is passage dependent in HGPS cells, as demonstrated using a chromatin association assay (Fig. 5A). In control BJ cells, XPA is found predominantly in the cytoplasm and nucleoplasm, whereas there is little, if any, XPA bound to the chromatin. In contrast, a significant amount of XPA became chromatin bound in the middle-age HGPS cells and increased as the cells aged further in culture (Fig. 5B). XPA was quantified and is shown as a ratio of chromatin-bound to not chromatin bound (free) XPA in the cells. XPA binding to the chromatin is likely a combined result of PCNA sequestration by the accumulated progerin and the moderate intrinsic affinity of XPA for ds-ssDNA junctions at replication forks (22). Because the checkpoint kinase ATR was shown previously to regulate XPA in response to UV-induced DNA damage (17, 44, 45), and ATM is a regulator for cellular damage responses to DSBs, we wondered whether ATR and ATM would have an effect on the XPA-related phenotypes observed in HGPS cells. By using inhibitors specific to ATM (KU-55933) and ATR (NU6027) we observed that inhibition of ATR but not ATM reduced the amount of chromatin-bound XPA in HGPS cells (Fig. 5C). p53 is a downstream substrate of both the ATM and ATR kinases. To demonstrate the efficiency of the kinase inhibition, the level of p53 (Ser15) phosphorylation in response to UV-induced DNA damage was monitored in whole-cell lysates.
Figure 5.
XPA chromatin accumulation in HGPS cells is age-dependent and regulated by ATR. A) Chromatin association assay of XPA binding in BJ and young (P13) and old (P18) HGPS fibroblasts. PARP was used as a control for the nuclear fraction, and histone 3 was used as a control for the chromatin-bound protein. B) The amount of chromatin-bound XPA was quantified in HGPS cells of 4 different ages (top) and normalized to the free XPA in the cell. C) ATR but not ATM kinase dependence of XPA chromatin association in old-age HGPS fibroblasts. The cells were treated with 10 μM ATR or ATM inhibitor for 1 h before chromatin isolation. Alternatively, the ATR/ATM inhibitor treatment was followed by 20 J/m2 UV-C treatment, followed by a 2-h recovery, to demonstrate the effectiveness of the inhibitors. Whole-cell lysates were generated and analyzed.
Altered expression of the RNR subunits results in abnormal replication in HGPS and a switch to repair DNA synthesis
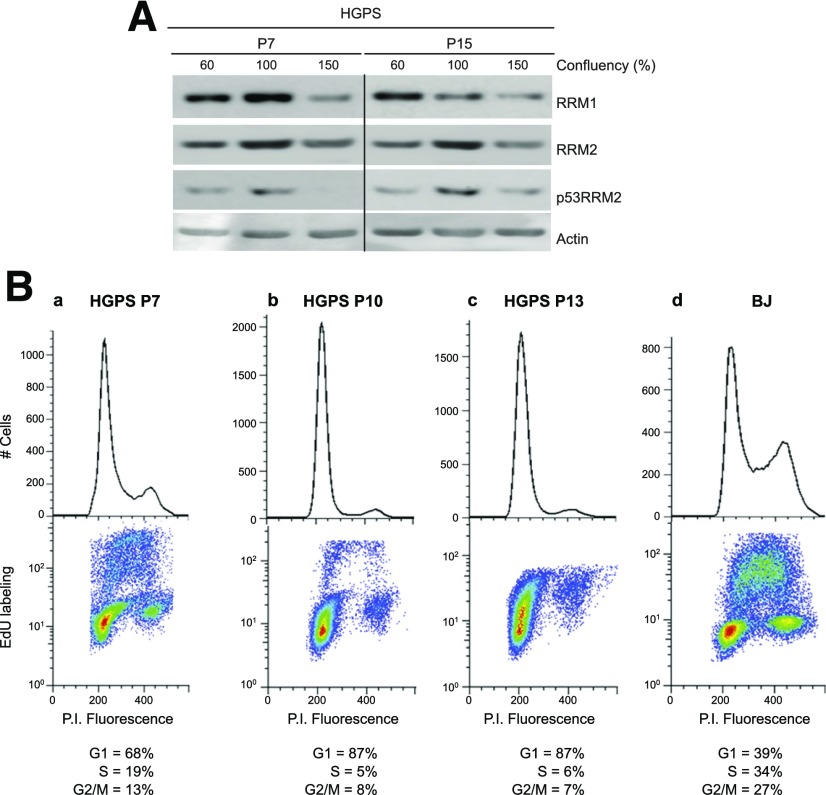
As mentioned previously, HGPS cells reach near senescence level much quicker than normal cells. Because the enzyme RNR is responsible for conversion in the nucleus of ribonucleotides to deoxyribonucleotides, essential substrates for DNA synthesis and repair, we hypothesized that the slow replication rate of HGPS cells would affect RNR subunit turnover. The RNR enzyme contains 2 R1 and 2 R2 subunits (RRM1 and -M2, respectively), forming a heterodimeric tetramer. RRM2 synthesis and turnover are dependent on cell-cycle progression from the G1/S phase transition through metaphase (46), whereas the RRM1 subunit, though highest in S phase, is maintained throughout the cell cycle. Recent work has demonstrated that RRM2 accumulation after DNA damage is regulated by checkpoint kinase ATR (47). As expected, the levels of RRM1 decreased as a nonsynchronous population of HGPS cell transition from rapid growth (60% confluence), to a contact-inhibited monolayer (100% confluence), to a quiescent state (150% confluence) (Fig. 6A). This pattern applied to younger (P7) and, especially, to older (P15) HGPS cells, reflecting the decreased need for ribonucleotides as cell replication rates decline. In contrast, the level of RRM2 increased in both younger and older cells as the cells neared confluence. This increase can be attributed to slowed proliferation and arrest of cell cycle progression as these cells become more confluent (Fig. 6B). Conversely, as the cells transitioned from confluence into quiescence (150%) the levels of RRM2 reverted to low confluence (60%) levels. The level of p53-inducible RRM2 subunit (p53RRM2) increased in both younger and older cells as the cells neared confluence, much like RRM2. However, after the young cells settled into confluence (150%) p53RRM2 was almost entirely depleted, whereas in the older cells, the p53RRM2 decreased only to preconfluence (60%) levels. The p53RRM2 expression being undetectable in the young HGPS is not surprising, as these cells have not been through as many passages and, therefore, have not accumulated the large amount of DSBs seen in the older cells. In the older HGPS cells, even after settling into quiescence, a priority is to constantly attempt to repair the strand breaks that the accumulated progerin continues to induce. These data suggest that the endogenous DNA damage brought about by the absence of PCNA and the mislocalization of XPA observed in old HGPS cells arrest progression through the cell cycle. This effect would upset the normal cell cycle turnover of the RRM2 subunit which normally degrades as the cells pass through the M phase (48). In addition, the increase in p53RRM2 in the older HGPS cells suggests an aging-related transition from replication to repair DNA synthesis (46) and is consistent with our previous finding that p53 is activated by the DNA damage response in older age HGPS cells (7).
Figure 6.
HGPS cells exhibit abnormalities in ribonucleotide reductase and in replication and repair DNA synthesis compared to normal fibroblasts. A) Expression levels of RNR subunits were observed by Western blot analysis. Cells were grown to the indicated confluence, where cells at 60% confluence were actively growing, cells at 100% confluence were entering a contact-arrested continuous monolayer, and cells at 150% confluence were in a quiescent state. B) Newly synthesized DNA in culture-aged HGPS cells (Ba–c) or in control BJ cells (Bd) was labeled for 1 h with 20 μM EdU. The cells were isolated, rinsed with PBS, and processed for flow cytometry analysis. Ba–c) The age of the HGPS cells, as measured by passage number is indicated.
To test this hypothesis, we examined the DNA synthesis parameters of HGPS cells and the ability of the cells to transit the cell cycle. DNA synthesis was monitored by flow cytometric analysis of incorporation of the thymidine analog EdU and Click-iT chemistry with the fluorescent Alexa Fluor 647 azide; nuclear DNA was monitored by propidium iodide staining (Fig. 6B). HGPS cells (P7 and older) grew more slowly than the BJ cells, as indicated by the greater number of cells in G1 and fewer in the S and G2/M phases. This finding was reflected also in the decreased number of EdU-label HGPS cells relative to the BJ cells. As the HGPS cells aged in culture (P7→P10→P13) the number of cycling cells decreased and all the cells traversed the cell cycle at a slower rate as indicated by the increased cell number in G1 and decreased number in S and G2/M, and a passage-dependent decrease in the number of cells with high EdU labeling (>50) in all cycle phases, especially the S phase. The latter was particularly noticeable relative to the intense S-phase labeling in BJ cells.
Note that there was significant but low (<50) EdU labeling in the cells in late G1 or at the G1/S phase transition, perhaps reflecting the initiation of the S phase in BJ and young HGPS cells. This result may also represent a stalling of initiated replication in older HGPS cells and partial repair because of accumulated DNA damage. The observed low level of labeling in G2/M phase cells may reflect a short end-of-S-phase DNA synthesis occurring early in the 1 h EdU labeling of cells transitioning to the G2 phase, DNA damage repair synthesis, and the reported low-level late DNA synthesis in the G2 phase (49). In younger cells (P7) DNA synthesis occurs as genomic replication in the S phase of the cell cycle. As the cells age, there is a shift of DNA synthesis from S phase to the G1/S phase transition (P10→P13), since replication is inhibited by activated DNA damage checkpoints. These data are in agreement with cell growth assays conducted with HGPS where the cells enter a senescence-like state in late passages (7).
XPA stalls progerin induction of apoptosis in old progeroid cells
Finally, we investigated the possible effects of XPA on HGPS cell death, a question that is clinically significant. We knocked down XPA in young and old HGPS cells. Apoptosis then was measured by a TMRE assay; TMRE preferentially stains active mitochondria with a polarized membrane. As shown in Fig. 7A, XPA depletion in old HGPS cells decreased the cell survival percentage, resulting in an increase in cells that initiated apoptosis when compared to mock knockdown with a control scrambled siRNA. Western blot analysis confirmed an increased apoptotic marker, the cleaving of caspase-3, in the siXPA-treated cells. These data suggest that XPA plays a role in preventing apoptosis in progeroid cells. Furthermore, XPA−/− cells (from a patient with xeroderma pigmentosum) expressing LAΔ50 (progerin) were observed to accumulate both degraded lamins (Fig. 7B) and an increase in PARP cleavage products relative to XPAWT cells, indicative of cells undergoing apoptosis. In addition, expression of LAΔ50 in XPA−/− cells more than doubled the number of apoptotic cells when compared to XPA−/− cells expressing control GFP (Fig. 7C, 1 vs. 2). In this experiment, apoptosis was measured in a caspase-3 activity assay with a fluorogenic substrate (Ac-DEVD-AFC). The relative fluorescence units were normalized to the protein concentration of the sample. Included in this study were LAΔ50- and GFP-expressing XPA-deficient cells complimented by transfection of a WT-XPA expression construct. Apoptosis was dramatically reduced in the LAΔ50-expressing cells by XPAWT protein and returned to near that of the GFP-expressing cells (Fig. 7C, 1 vs. 3, 4). CPT also was used as a control for comparison with DNA damage–induced apoptosis. CPT treatment greatly increased the number of cells that entered apoptosis, and the presence or absence of XPA had no effect on the CPT-induced apoptosis (Fig. 7C, 5 vs. 6), further supporting the unique role of XPA in suppressing HGPS cell death from endogenous DNA damage. The apoptosis assays and Western blot analyses demonstrate that XPA may play a role in suppressing apoptosis in progeroid cells. This function of XPA is independent of its role in NER. The data suggest that XPA-DSB mislocalization may function to suppress induction of apoptosis in aged HGPS cells.
Figure 7.
XPA stalls induction of apoptosis in old progeroid cells. A) HGPS cells at P8 (young) and P20 (old) passages were transfected with scrambled sequence (siCtrl) or an XPA-specific siRNA (siXPA). Five days after transfection, the cells were harvested. Cell survival was measured by flow cytometry using TMRE, which is an indicator of cells with functional, polarized mitochondria (left). Western blot analysis demonstrated caspase-3 cleavage and confirmed XPA-knockdown efficiency (right). B) HeLa, XPAWT and XPA−/− cells from a patient with xeroderma pigmentosum were transfected with plasmid expressing SSIM (a mutant lamin A that cannot be farnesylated), progerin (LAΔ50, GFP-LAΔ50), or an empty parent vector (GFP). At 24 h after transfection, the cells were harvested for analysis by Western blot analysis. The top blot was probed with an antibody against lamin A/C. The bottom blot was probed with an antibody against PARP. C) XPAWT and XPA−/− cells were transfected with the plasmid expressing progerin (LAΔ50) or empty parent vector (GFP). Cells were harvested 24 h after transfection. These cells also were separately treated with CPT for 6 h before assay. The caspase-3 activity was measured using a fluorogenic substrate (Ac-DEVD-AFC). The relative fluorescence units were normalized to the protein concentration of the sample.
DISCUSSION
A hallmark of premature aging syndromes is the accumulation of DSBs caused by the increased progerin levels, which lead to early replicative senescence; however, the mechanism behind this phenotype has not been fully described. The mechanisms underlying premature aging syndromes are thought to share similarities with normal aging in mammalian cells, in that these cells also accumulate progerin over time (41–43, 50). Our work uncovered an unexpected finding that the nucleotide excision repair protein XPA was localized at DSB sites in HGPS cells instead of the DSB repair proteins, inhibiting DSB repair (14). In an attempt to investigate the mechanisms of the unexpected XPA-DSB colocalization, we found that XPA and PCNA may compete for binding at the DSBs at stalled or collapsed replication forks in HGPS cells and that XPA binding is more favored because of PCNA sequestration by progerin. This novel non-NER binding of XPA to stalled or collapsed replication forks with DSBs is strongly supported by our finding that XPA recognizes ds-ssDNA junctions with 3′- and/or 5′-ssDNA overhangs containing no NER adducts (22, 23), a characteristic structure of replication forks. Remarkably, this binding affinity of XPA for these junctions is between 1 and 2 orders of magnitude higher than its ability to bind to bulky DNA damage (22, 23).
We showed that absence of PCNA at the stalled replication forks in HGPS cells is most likely related to the PCNA sequestration by progerin, which leaves the ds-ssDNA junctions at stalled replication forks unprotected for XPA binding. Indeed, through immunofluorescence microscopy we observed that, in HGPS cells, PCNA transitions from distinct focus formation to being diffuse throughout the nucleus in an age-dependent manner. Through the PLA, we found that, in old age, HGPS cell lamin A no longer interacts with PCNA, but there is significant interaction between PCNA and progerin. Furthermore, XPA forms nuclear foci in normal fibroblasts in which PCNA is depleted by siRNA knockdown. The dramatically enhanced binding of PCNA to progerin as compared to native lamin A in HGPS cells is loosely consistent with previous reports in which microinjection of a lamin A mutant protein (nonprogerin) into mammalian cells resulted in redistribution of PCNA and RFC, thus blocking the elongation phase of DNA replication. Those reports proposed that the replication blockage was probably caused by the loss of replicative PCNA because of its entrapment in lamin A aggregates (51, 52).
In a recent study, Cobb and colleagues (53) demonstrated that prelamin A competes for binding to PCNA with lamin A/C. Consequently, they found that the prelamin A-PCNA binding could reduce PCNA interaction with mature lamins, much like the progerin-PCNA interaction observed in the current study. They speculated that because prelamin A has a different nuclear localization and distribution than mature lamin A/C, the interaction of prelamin A and PCNA affects DNA replication. This finding is especially interesting in the context of RD, in which prelamin A accumulates because of a lack of Zmpste24, as well as the current therapeutic approach for treating HGPS patients with farnesyl-transferase inhibitors (54). Their work along with our current study suggests that prelamin A and progerin can interact with replication factors and ultimately have an effect on replication progression. The DSBs that are generated as a result of the stalled replication forks can then lead to chromosome aberrations that ultimately result in accelerated senescence or increased cell death.
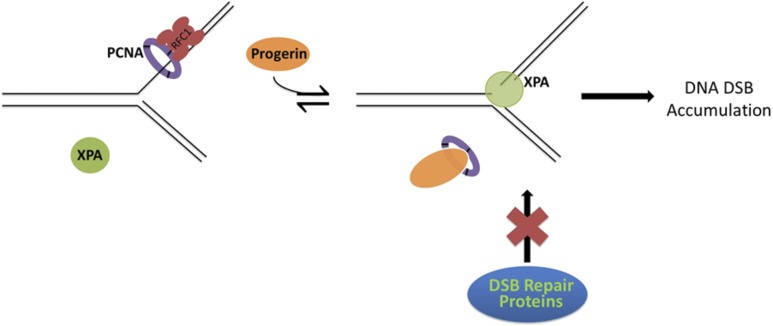
As proposed in Fig. 8, under normal conditions, PCNA is recruited by the RFC complex to replication forks and binds ds/ss-junctions with high affinity, which may prevent XPA-junction binding even though XPA also has a relatively high affinity for ds/ss-junctions. In this scenario the equilibrium between PCNA- and XPA-junction binding favors PCNA over XPA. However, when progerin accumulates in HGPS cells, the progerin-PCNA interaction sequesters PCNA away from the junction, shifting the equilibrium to favor XPA-junction binding over PCNA-junction binding. Thus, XPA may become more competitive in binding the junctions at stalled or collapsed replication forks in replacing the sequestered PCNA. A recent work has shown that PCNA and XPA may interact in response to UV-induced DNA damage via the APIM motif. It is worth noting that the APIM motif is located within the XPA minimum DNA-binding domain. Therefore, it is likely that in HGPS, XPA binding to DNA prevents XPA from binding to PCNA. In addition, the XPA-PCNA interaction at the replication fork described by Gilljam et al. (55) is related to the NER induced by UV damage; however, in the current study, XPA binds to ds/ss-junctions at replication forks lacking base adducts which are the normal NER substrates. When XPA is bound at ds/ss-junctions PCNA cannot bind XPA, potentially because of either a conformational change or steric hindrance.
Figure 8.
Proposed model for XPA binding at stalled replication forks as a result of PCNA sequestration by progerin. Under normal conditions in the cell the RFC complex loads the replication clamp, PCNA, onto the DNA to progress replication/synthesis. Normally, nuclear XPA is spread throughout the nucleus for activation in the event of a DNA damage. However, as HGPS cells age in culture, progerin accumulates and sequesters PCNA from the replication fork, allowing for XPA binding and inhibiting DNA replication and DNA repair. Ultimately, this process results in genomic instability in these cells.
Based on our model and the work of others, it appears that the defective lamina is at the root of what we are observing in the HGPS cells. Our current work may explain a piece of this puzzle. Because lamins interact with not only the DNA itself but also with proteins, including replication factors such as PCNA, disruptions to this interaction may lead to aberrant protein binding (XPA) which ultimately results in the accumulation of DNA damage. Targeting this mechanism can lead to novel treatments in not only premature aging syndromes, but aging in general. Looking more closely at how the lamins and PCNA interact in future studies may allow us to shed more light on this mechanism and how it fits into the normal aging process.
It is known that HGPS cells are susceptible to cell death and DSB accumulation generated from stalled or collapsed replication forks, if not properly repaired, could lead to cell death (10, 56, 57). This raises an interesting question about the possible role of the NER protein XPA in progerin-induced apoptosis. We demonstrated in the current study that, in old HGPS cells depleted of XPA, there is an increase in the number of apoptotic cells compared to mock-treated cells. This observation was further confirmed with XPA-deficient cells derived from a patient with xeroderma pigmentosum and XPAWT cells with overexpression of progerin. These data suggest that XPA plays a role in stabilizing the stalled or collapsed replication forks, though irreparable, and thus may serve to suppress onset of apoptotic death induced by progerin in HGPS cells.
ATR and ATM are DNA damage response kinases that can phosphorylate many downstream targets to initiate the checkpoint, halting replication and cell cycle transit, and appear to be involved in promoting structural stability of stalled forks by preventing dissociation of replisome components (58). ATR also can phosphorylate XPA in response to UV-induced DNA damage (19, 44). We speculated that ATR/ATM might play a role in regulating XPA binding at stalled replication forks. Indeed, inhibiting ATR kinase activity resulted in a decrease in XPA binding to chromatin in older HGPS cells and the release of XPA from the stalled replication forks. Also, ATR recently has been shown to suppress DNA damage by coordinating RRM2 accumulation at replication origins (47). The relatively high expression of RRM2 and p53RRM2 in old-age HGPS, even though the replication rate has decreased significantly in these cells, is potentially a consequence of ATR activation and increased levels of phosphorylated p53 (7). Increasing RNR activity in progeroid mice alleviates the severity of the premature aging phenotype (59). RNR also has been observed to promote fork restart after replicative stress (60). Thus, the accumulation of progerin in these aging HGPS cells has multiple effects in regulating DNA metabolism and altering progression through the cell cycle.
The findings presented in this study represent another step in uncovering the underlying mechanism of the DSB accumulation seen in the premature aging disease HGPS. Our results suggest a role for XPA in suppressing DSB repair while stalling induction of apoptosis in old HGPS cells by binding accessible replication forks deficient in PCNA because of progerin sequestration. Although great efforts have been made to fight this devastating disease (61), new strategies toward a more efficient treatment of HGPS remain badly needed. An interesting future study would be to test inhibitors that are capable of disrupting the PCNA-progerin interaction. DNA damage accumulation has been linked to normal aging as well as progerin accumulation (43, 62, 63). Further investigating the mechanisms behind the premature aging diseases also may lead to development of novel treatments to alleviate some age-related phenotypes.
ACKNOWLEDGMENTS
This study was supported by U.S. National Institutes of Health, National Institute on Aging Grant AG031503 (to Y.Z.), a Progeria Research Foundation grant (to Y.Z.), and East Tennessee State University Research Development Committee Grants E82083 and E31231 (to P.R.M.).
Glossary
- Ac-DEVD-AFC
acetyl Asp-Glu-Val-Asp 7-amido-4-methylcoumarin
- ATM
ataxia telangiectasia mutated
- ATR
ataxia telangiectasia and Rad3-related
- ChIP
chromatin immunoprecipitation
- Co-IP
coimmunoprecipitation
- CPT
camptothecin
- DSB
double-strand break
- ds-ssDNA
double-strand/single-strand DNA
- EdU
5-ethynyl-2′-deoxyuridine
- GFP
green fluorescent protein
- H2AX
variant of the H2A protein family
- HGPS
Hutchinson-Gilford progeria syndrome
- LMNA
lamin A/C
- MCM
minichromosome maintenance complex
- NER
nucleotide excision repair
- PARP
poly ADP ribose polymerase
- PCNA
proliferating cell nuclear antigen
- PLA
proximity ligation assay
- Polδ
polymerase δ
- RD
restrictive dermopathy
- RFC
replication protein C
- RNR
ribonucleotide reductase
- RRM1/M2
ribonucleotide reductase catalytic subunit M1/M2
- siRNA
small interfering RNA
- TMRE
tetramethylrhodamine ethyl ester
- XPA
xeroderma pigmentosum group A
AUTHOR CONTRIBUTIONS
B. A. Hilton, J. Liu, B. M. Cartwright, and Y. Liu performed most of the experiments; M. Breitman, Y. Wang, R. Jones, and H. Tang also made contributions to the work by conducting some experiments; B. A. Hilton, with the assistance of B. M. Cartwright, wrote the manuscript draft; A. Rusinol made contributions by helping with the project and provided some materials; P. R. Musich was involved in experimental design, experiment trouble-shooting, and manuscript editing; and Y. Zou is the senior author who oversaw and directed this study from initiation, experimental design, and performance through manuscript writing and revision.
REFERENCES
- 1.De Sandre-Giovannoli A., Bernard R., Cau P., Navarro C., Amiel J., Boccaccio I., Lyonnet S., Stewart C. L., Munnich A., Le Merrer M., Lévy N. (2003) Lamin a truncation in Hutchinson-Gilford progeria. Science 300, 2055 [DOI] [PubMed] [Google Scholar]
- 2.Eriksson M., Brown W. T., Gordon L. B., Glynn M. W., Singer J., Scott L., Erdos M. R., Robbins C. M., Moses T. Y., Berglund P., Dutra A., Pak E., Durkin S., Csoka A. B., Boehnke M., Glover T. W., Collins F. S. (2003) Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature 423, 293–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corrigan D. P., Kuszczak D., Rusinol A. E., Thewke D. P., Hrycyna C. A., Michaelis S., Sinensky M. S. (2005) Prelamin A endoproteolytic processing in vitro by recombinant Zmpste24. Biochem. J. 387, 129–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldman R. D., Gruenbaum Y., Moir R. D., Shumaker D. K., Spann T. P. (2002) Nuclear lamins: building blocks of nuclear architecture. Genes Dev. 16, 533–547 [DOI] [PubMed] [Google Scholar]
- 5.Hutchison C. J. (2011) The role of DNA damage in laminopathy progeroid syndromes. Biochem. Soc. Trans. 39, 1715–1718 [DOI] [PubMed] [Google Scholar]
- 6.Gonzalo S., Kreienkamp R. (2015) DNA repair defects and genome instability in Hutchinson-Gilford Progeria Syndrome. Curr. Opin. Cell Biol. 34, 75–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y., Rusinol A., Sinensky M., Wang Y., Zou Y. (2006) DNA damage responses in progeroid syndromes arise from defective maturation of prelamin A. J. Cell Sci. 119, 4644–4649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Musich P. R., Zou Y. (2011) DNA-damage accumulation and replicative arrest in Hutchinson-Gilford progeria syndrome. Biochem. Soc. Trans. 39, 1764–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu B., Wang J., Chan K. M., Tjia W. M., Deng W., Guan X., Huang J.-D., Li K. M., Chau P. Y., Chen D. J., Pei D., Pendas A. M., Cadiñanos J., López-Otín C., Tse H.-F., Hutchison C., Chen J., Cao Y., Cheah K. S. E., Tryggvason K., Zhou Z. (2005) Genomic instability in laminopathy-based premature aging. Nat. Med. 11, 780–785 [DOI] [PubMed] [Google Scholar]
- 10.Musich P. R., Zou Y. (2009) Genomic instability and DNA damage responses in progeria arising from defective maturation of prelamin A. Aging (Albany NY) 1, 28–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Constantinescu D., Csoka A. B., Navara C. S., Schatten G. P. (2010) Defective DSB repair correlates with abnormal nuclear morphology and is improved with FTI treatment in Hutchinson-Gilford progeria syndrome fibroblasts. Exp. Cell Res. 316, 2747–2759 [DOI] [PubMed] [Google Scholar]
- 12.Manju K., Muralikrishna B., Parnaik V. K. (2006) Expression of disease-causing lamin A mutants impairs the formation of DNA repair foci. J. Cell Sci. 119, 2704–2714 [DOI] [PubMed] [Google Scholar]
- 13.Sedelnikova O. A., Horikawa I., Zimonjic D. B., Popescu N. C., Bonner W. M. Barrett J. C. (2004). Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nat. Cell. Biol. 6, 168–170 [DOI] [PubMed] [Google Scholar]
- 14.Liu Y., Wang Y., Rusinol A. E., Sinensky M. S., Liu J., Shell S. M., Zou Y. (2008) Involvement of xeroderma pigmentosum group A (XPA) in progeria arising from defective maturation of prelamin A. FASEB J. 22, 603–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riedl T., Hanaoka F., Egly J. M. (2003) The comings and goings of nucleotide excision repair factors on damaged DNA. EMBO J. 22, 5293–5303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volker M., Moné M. J., Karmakar P., van Hoffen A., Schul W., Vermeulen W., Hoeijmakers J. H., van Driel R., van Zeeland A. A., Mullenders L. H. (2001) Sequential assembly of the nucleotide excision repair factors in vivo. Mol. Cell 8, 213–224 [DOI] [PubMed] [Google Scholar]
- 17.Wu X., Shell S. M., Liu Y., Zou Y. (2007) ATR-dependent checkpoint modulates XPA nuclear import in response to UV irradiation. Oncogene 26, 757–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Z., Musich P. R., Serrano M. A., Dong Z., Zou Y. (2011) XPA-mediated regulation of global nucleotide excision repair by ATR Is p53-dependent and occurs primarily in S-phase. PLoS One 6, e28326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu X., Shell S. M., Yang Z., Zou Y. (2006) Phosphorylation of nucleotide excision repair factor xeroderma pigmentosum group A by ataxia telangiectasia mutated and Rad3-related-dependent checkpoint pathway promotes cell survival in response to UV irradiation. Cancer Res. 66, 2997–3005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nouspikel T. (2009) DNA repair in mammalian cells: So DNA repair really is that important? Cell. Mol. Life Sci. 66, 965–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugasawa K., Okamoto T., Shimizu Y., Masutani C., Iwai S., Hanaoka F. (2001) A multistep damage recognition mechanism for global genomic nucleotide excision repair. Genes Dev. 15, 507–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Z., Roginskaya M., Colis L. C., Basu A. K., Shell S. M., Liu Y., Musich P. R., Harris C. M., Harris T. M., Zou Y. (2006) Specific and efficient binding of xeroderma pigmentosum complementation group A to double-strand/single-strand DNA junctions with 3′- and/or 5′-ssDNA branches. Biochemistry 45, 15921–15930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hilton B., Shkriabai N., Musich P. R., Kvaratskhelia M., Shell S., Zou Y. (2014) A new structural insight into XPA-DNA interactions. Biosci. Rep. 34, e00162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sugitani N., Shell S. M., Soss S. E., Chazin W. J. (2014) Redefining the DNA-binding domain of human XPA. J. Am. Chem. Soc. 136, 10830–10833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shumaker D. K., Solimando L., Sengupta K., Shimi T., Adam S. A., Grunwald A., Strelkov S. V., Aebi U., Cardoso M. C., Goldman R. D. (2008) The highly conserved nuclear lamin Ig-fold binds to PCNA: its role in DNA replication. J. Cell Biol. 181, 269–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mattout A., Goldberg M., Tzur Y., Margalit A., Gruenbaum Y. (2007) Specific and conserved sequences in D. melanogaster and C. elegans lamins and histone H2A mediate the attachment of lamins to chromosomes. J. Cell Sci. 120, 77–85 [DOI] [PubMed] [Google Scholar]
- 27.Moldovan G.-L., Pfander B., Jentsch S. (2007) PCNA, the maestro of the replication fork. Cell 129, 665–679 [DOI] [PubMed] [Google Scholar]
- 28.Tang H., Hilton B., Musich P. R., Fang D. Z., Zou Y. (2012) Replication factor C1, the large subunit of replication factor C, is proteolytically truncated in Hutchinson-Gilford progeria syndrome. Aging Cell 11, 363–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Méndez J., Stillman B. (2000) Chromatin association of human origin recognition complex, cdc6, and minichromosome maintenance proteins during the cell cycle: assembly of prereplication complexes in late mitosis. Mol. Cell. Biol. 20, 8602–8612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Misteli T., Scaffidi P. (2005) Genome instability in progeria: when repair gets old. Nat. Med. 11, 718–719 [DOI] [PubMed] [Google Scholar]
- 31.Ricke R. M., Bielinsky A.-K. (2005) Easy detection of chromatin binding proteins by the Histone Association Assay. Biol. Proced. Online 7, 60–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chibazakura T., Kamachi K., Ohara M., Tane S., Yoshikawa H., Roberts J. M. (2011) Cyclin A promotes S-phase entry via interaction with the replication licensing factor Mcm7. Mol. Cell. Biol. 31, 248–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Byun T. S., Pacek M., Yee M.-C., Walter J. C., Cimprich K. A. (2005) Functional uncoupling of MCM helicase and DNA polymerase activities activates the ATR-dependent checkpoint. Genes Dev. 19, 1040–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bailey R., Priego Moreno S., Gambus A. (2015) Termination of DNA replication forks: “breaking up is hard to do”. Nucleus 6, 187–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hicks W. M., Yamaguchi M., Haber J. E. (2011) Real-time analysis of double-strand DNA break repair by homologous recombination. Proc. Natl. Acad. Sci. USA 108, 3108–3115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Essers J., Theil A. F., Baldeyron C., van Cappellen W. A., Houtsmuller A. B., Kanaar R., Vermeulen W. (2005) Nuclear dynamics of PCNA in DNA replication and repair. Mol. Cell. Biol. 25, 9350–9359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maloisel L., Fabre F., Gangloff S. (2008) DNA polymerase delta is preferentially recruited during homologous recombination to promote heteroduplex DNA extension. Mol. Cell. Biol. 28, 1373–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith C. E., Lam A. F., Symington L. S. (2009) Aberrant double-strand break repair resulting in half crossovers in mutants defective for Rad51 or the DNA polymerase delta complex. Mol. Cell. Biol. 29, 1432–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao K., Blair C. D., Faddah D. A., Kieckhaefer J. E., Olive M., Erdos M. R., Nabel E. G., Collins F. S. (2011) Progerin and telomere dysfunction collaborate to trigger cellular senescence in normal human fibroblasts. J. Clin. Invest. 121, 2833–2844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao K., Capell B. C., Erdos M. R., Djabali K., Collins F. S. (2007) A lamin A protein isoform overexpressed in Hutchinson-Gilford progeria syndrome interferes with mitosis in progeria and normal cells. Proc. Natl. Acad. Sci. USA 104, 4949–4954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aliper A. M., Csoka A. B., Buzdin A., Jetka T., Roumiantsev S., Moskalev A., Zhavoronkov A. (2015) Signaling pathway activation drift during aging: Hutchinson-Gilford Progeria Syndrome fibroblasts are comparable to normal middle-age and old-age cells. Aging (Albany NY) 7, 26–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olive M., Harten I., Mitchell R., Beers J. K., Djabali K., Cao K., Erdos M. R., Blair C., Funke B., Smoot L., Gerhard-Herman M., Machan J. T., Kutys R., Virmani R., Collins F. S., Wight T. N., Nabel E. G., Gordon L. B. (2010) Cardiovascular pathology in Hutchinson-Gilford progeria: correlation with the vascular pathology of aging. Arterioscler. Thromb. Vasc. Biol. 30, 2301–2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scaffidi P., Misteli T. (2006) Lamin A-dependent nuclear defects in human aging. Science 312, 1059–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shell S. M., Li Z., Shkriabai N., Kvaratskhelia M., Brosey C., Serrano M. A., Chazin W. J., Musich P. R., Zou Y. (2009) Checkpoint kinase ATR promotes nucleotide excision repair of UV-induced DNA damage via physical interaction with xeroderma pigmentosum group A. J. Biol. Chem. 284, 24213–24222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Z., Musich P. R., Cartwright B. M., Wang H., Zou Y. (2013) UV-induced nuclear import of XPA is mediated by importin-α4 in an ATR-dependent manner. PLoS One 8, e68297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Håkansson P., Hofer A., Thelander L. (2006) Regulation of mammalian ribonucleotide reduction and dNTP pools after DNA damage and in resting cells. J. Biol. Chem. 281, 7834–7841 [DOI] [PubMed] [Google Scholar]
- 47.Buisson R., Boisvert J. L., Benes C. H., Zou L. (2015) Distinct but concerted roles of ATR, DNA-PK, and Chk1 in countering replication stress during Sphase. Mol. Cell 59, 1011–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chabes A., Thelander L. (2000) Controlled protein degradation regulates ribonucleotide reductase activity in proliferating mammalian cells during the normal cell cycle and in response to DNA damage and replication blocks. J. Biol. Chem. 275, 17747–17753 [DOI] [PubMed] [Google Scholar]
- 49.Widrow R. J., Hansen R. S., Kawame H., Gartler S. M., Laird C. D. (1998) Very late DNA replication in the human cell cycle. Proc. Natl. Acad. Sci. USA 95, 11246–11250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ding S.-L., Shen C.-Y. (2008) Model of human aging: recent findings on Werner’s and Hutchinson-Gilford progeria syndromes. Clin. Interv. Aging 3, 431–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spann T. P., Moir R. D., Goldman A. E., Stick R., Goldman R. D. (1997) Disruption of nuclear lamin organization alters the distribution of replication factors and inhibits DNA synthesis. J. Cell Biol. 136, 1201–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moir R. D., Spann T. P., Herrmann H., Goldman R. D. (2000) Disruption of nuclear lamin organization blocks the elongation phase of DNA replication. J. Cell Biol. 149, 1179–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cobb A. M., Murray T. V., Warren D. T., Liu Y., Shanahan C. M. (2016) Disruption of PCNA-lamins A/C interactions by prelamin A induces DNA replication fork stalling. Nucleus 7, 498–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Toth J. I., Yang S. H., Qiao X., Beigneux A. P., Gelb M. H., Moulson C. L., Miner J. H., Young S. G., Fong L. G. (2005) Blocking protein farnesyltransferase improves nuclear shape in fibroblasts from humans with progeroid syndromes. Proc. Natl. Acad. Sci. USA 102, 12873–12878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gilljam K. M., Müller R., Liabakk N. B., Otterlei M. (2012) Nucleotide excision repair is associated with the replisome and its efficiency depends on a direct interaction between XPA and PCNA. PLoS One 7, e49199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cortez D. (2015) Preventing replication fork collapse to maintain genome integrity. DNA Repair (Amst.) 32, 149–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ewald B., Sampath D., Plunkett W. (2008) Nucleoside analogs: molecular mechanisms signaling cell death. Oncogene 27, 6522–6537 [DOI] [PubMed] [Google Scholar]
- 58.Errico A., Costanzo V. (2012) Mechanisms of replication fork protection: a safeguard for genome stability. Crit. Rev. Biochem. Mol. Biol. 47, 222–235 [DOI] [PubMed] [Google Scholar]
- 59.Lopez-Contreras A. J., Specks J., Barlow J. H., Ambrogio C., Desler C., Vikingsson S., Rodrigo-Perez S., Green H., Rasmussen L. J., Murga M., Nussenzweig A., Fernandez-Capetillo O. (2015) Increased Rrm2 gene dosage reduces fragile site breakage and prolongs survival of ATR mutant mice. Genes Dev. 29, 690–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morafraile E. C., Diffley J. F. X., Tercero J. A., Segurado M. (2015) Checkpoint-dependent RNR induction promotes fork restart after replicative stress. Sci. Rep. 5, 7886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gordon L. B., Rothman F. G., López-Otín C., Misteli T. (2014) Progeria: a paradigm for translational medicine. Cell 156, 400–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pacheco L. M., Gomez L. A., Dias J., Ziebarth N. M., Howard G. A., Schiller P. C. (2014) Progerin expression disrupts critical adult stem cell functions involved in tissue repair. Aging (Albany NY) 6, 1049–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schumacher B., Garinis G. A., Hoeijmakers J. H. J. (2008) Age to survive: DNA damage and aging. Trends Genet. 24, 77–85 [DOI] [PubMed] [Google Scholar]