Abstract
Whether malaria parasites can manipulate mosquito host choice in ways that enhance parasite transmission toward suitable hosts and/or reduce mosquito attraction to unsuitable hosts (i.e. specific manipulation) is unknown. To address this question, we experimentally infected three species of mosquito vectors with wild isolates of the human malaria parasite Plasmodium falciparum, and examined the effects of immature and mature infections on mosquito behavioural responses to combinations of calf odour, human odour and outdoor air using a dual-port olfactometer. Regardless of parasite developmental stage and mosquito species, P. falciparum infection did not alter mosquito activation rate or their choice for human odours. The overall expression pattern of host choice of all three mosquito species was consistent with a high degree of anthropophily, with infected and uninfected individuals showing higher attraction toward human odour over calf odour, human odour over outdoor air, and outdoor air over calf odour. Our results suggest that, in this system, the parasite may not be able to manipulate the early long-range behavioural steps involved in the mosquito host-feeding process. Future studies are required to test whether malaria parasites can modify their mosquito host choice at a shorter range to enhance transmission.
Introduction
Host behavioural manipulation by parasites is a widespread transmission strategy1–4. Trophically-transmitted parasites, for example, can alter the behaviour of their intermediate hosts in ways that increase predation rate by definitive hosts, hence favouring transmission5–7. However, altering the behaviour of intermediate hosts can also increase predation rates by unsuitable hosts8–10. This higher probability of being killed by dead-end predators can incur significant costs to manipulative parasites, especially when initial predation risk is high11. In response, some parasites have evolved specific manipulation, i.e. the ability to enhance transmission toward appropriate hosts and/or reduce predation by unsuitable hosts6, 7, 12–15.
In addition to its ecological and evolutionary relevance, host manipulation may also have profound implications for human health. Many manipulative parasites are responsible for devastating vector-borne diseases such as dengue fever, malaria, leishmaniasis, or sleeping sickness. Vector-borne parasites can indeed manipulate phenotypic traits of their vectors and hosts in ways that increase contacts between them, hence favouring parasite transmission16–22. A frequently reported change induced by vector-borne parasites is alteration of vector motivation and avidity to feed. For example in malaria mosquitoes, individuals infected with Plasmodium sporozoites (the mosquito to human transmission stage) can display increased response to host odours23, 24, increased landing and biting activity23, 25–29, increased number of feeds30 and increased blood volume intake28, 30, 31. In contrast, mosquitoes infected with oocysts (the immature non-transmissible stage of the parasite), are less persistent and less likely to attempt to feed24, 27, 28. Since biting is risky (e.g., host defensive behaviours can kill the vector and its parasite), reduced feeding attempts seems beneficial to the parasite32.
In natural conditions, these “stage-dependent” behavioural changes presumably increase the rate at which a mosquito will feed on a vertebrate blood-source, not all of which are suitable hosts for the parasite. The very few epidemiological models that have considered mosquito behavioural manipulation by malaria parasites have assumed that suitable hosts for parasite development were the only source of blood33, 34. These models have ignored the possibility that malaria parasites may increase biting rate on vertebrates that do not act as suitable hosts. Similarly, no study has, to our knowledge, investigated whether malaria parasites can manipulate mosquito vertebrate choice in ways that enhance parasite transmission toward suitable hosts and/or reduce mosquito attraction to unsuitable hosts (i.e. specific manipulation).
Mosquito choice for vertebrate blood-source is an important key predictor for the transmission intensity of vector-borne diseases. This choice may be influenced by genetic and environmental factors such as the innate preference of the mosquito and the availability of the vertebrate species35. While some malaria vectors can display propensity to feed on different vertebrate species (i.e. generalist or opportunistic feeding behaviour)36, the parasites they transmit are often highly host-specific, infecting only one or a few vertebrate species37. Because of this strong host specificity, it is possible that vector-borne parasites acquired, during the course of evolution, the ability to target appropriate host and/or avoid unsuitable ones38. Accordingly, generalist mosquitoes, once infected, should develop a feeding preference for vertebrates that are suitable for parasite development. Studies exploring this possibility may yield important information about the diversity of transmission strategies used by malaria parasites and show that mosquitoes infected with transmissible parasite stages not only bite “more” but perhaps also “better”.
Theoretically, there are several ways through which malaria parasites could maximise transmission towards suitable vertebrate hosts. First, the parasite may induce in the mosquito vector a sensory bias for host traits (e.g. specific odours) that are correlated with optimal suitability for the parasite. Second, the parasite may induce alteration of mosquito microhabitat choice, in a way that spatially matches the microhabitat of the suitable host species. Finally, the parasite may induce changes in time activity in a way that temporally matches the resting time of the suitable host.
Here, we explored the first possibility using the natural association between Plasmodium falciparum, which causes the most severe form of human malaria, the mosquito species Anopheles coluzzii, Anopheles gambiae and Anopheles arabiensis, three major vectors of P. falciparum in Africa, and calves and human, two common mosquito vertebrate hosts. An. coluzzii and An. gambiae are considered anthropophilic (they are attracted to human stimuli) throughout its distribution, whereas An. arabiensis can display a weak host tropism (plastic/opportunistic) and can display either anthropophilic or zoophilic preference depending on the geographic area and the relative abundance of cattle and human36, 39. P. falciparum displays an extreme form of specificity and can develop and reproduce in hominids only (predominantly in human and to a lesser extent in chimpanzee, bonobo, and gorilla)40–42, such that any mosquito bite on another vertebrate species, such as cattle, would be a dead-end for the parasite. We experimentally challenged local colonies of three mosquito species with sympatric field isolates of P. falciparum using direct membrane feeding assays in Burkina Faso, and examined the effects of immature (oocyst) and mature (sporozoite) infections on mosquito choice between human and calf odours using a dual-port olfactometer.
Methods
Mosquitoes
Anopheles gambiae and An. coluzzii mosquitoes originated from outbred colonies established in 2008 and repeatedly replenished with F1 from wild-caught mosquito females collected in Soumousso (An. gambiae) (11°01′00′N, 4°02′59′W) and Kou Valley (An. coluzzii) (11°23′14′N, 4°24′42′W), south-western Burkina Faso (West Africa) and identified by PCR-RFLP43, 44. Mosquitoes were maintained under standard insectary conditions (27 ± 2 °C, 70 ± 10% relative humidity, 12:12 LD). Mosquito colonies are maintained in separate rooms to avoid cross-contaminations and every 6 months 100 females from each colony are sampled to assess colony integrity by PCR-RFLP43, 44. Larvae were bred in the laboratory with ad libitum Tetramin® and adult mosquitoes were provided with a solution of 5% glucose.
Anopheles arabiensis mosquitoes originated from wild caught larvae in Dioulassoba (11°10′42′N, 4°18′26′W), a district of Bobo Dioulasso, where previous surveys ensured that An. arabiensis population was dominant45, 46. Collection of field larvae was conducted three times (twice in October 2014 and once in November 2014). Larvae were reared under the same standard insectary conditions as the mosquito colonies and F0 females were used for the experiments. Species identification of 35 individuals from each wild caught batch (a total of 105 mosquitoes) was performed to confirm that An. arabiensis was the dominant species43. Samples from the 1st and 2nd batches in October contained 60% and 90.91% of An. arabiensis respectively; sample from the 3rd batch in November contained 100% An. arabiensis.
Experimental infections
Experimental infections of mosquitoes were performed by membrane feeding of infectious blood (DMFA for Direct Membrane feeding Assay) as described previously47–51. Briefly, three- to five-days old females were fed through membranes on P. falciparum gametocyte (the human to mosquito transmission stage) -infected blood from malaria patients in Burkina Faso. Mosquitoes were starved of glucose solution for 12 h (An. gambiae and An. arabiensis) or 24 h (An. coluzzii) prior to the infection. Gametocyte carriers were selected by examining thick blood smears from children aged between 5 and 11 from two villages in southwestern Burkina Faso (Dande and Soumousso, located 60 km north and 40 km southeast of Bobo Dioulasso, respectively) and blood drawing was carried out at laboratory. For An. gambiae and An. arabiensis mosquitoes, when gametocytemia was below 160 gametocytes/µl, blood serum was replaced with European naive AB serum to limit potential effect of human transmission blocking immunity and hence maximize the number of successfully infected mosquitoes52. Serum was not changed in An. coluzzii experiments. As a negative control (uninfected mosquitoes), females were fed on the same blood in which gametocytes were heat-inactivated. Parasite inactivation was performed by placing the infectious blood in a thermo-mixer and heating at 43 °C for 15 min and 900 rpm. This heat-inactivation prevents from infectiousness of gametocytes and does not affect the blood nutritive quality53. Mosquitoes exposed/unexposed to infection were therefore fed with blood from the same individual, avoiding the potential confounding effects of different blood origins on mosquito behaviours. Mosquito blood feeding was performed by distributing three hundred µl of blood in membrane feeders maintained at 37 °C by water jackets; cups containing 60–80 mosquitoes were placed under the feeders to allow blood engorgement through Parafilm® membranes for 2 hours. Fully blood-fed females were sorted out and placed in new cages (30 × 30 × 30 cm) where they had constant access to 5% glucose solution on cotton wool pads until the behavioural assays. A total of eight experimental replicates using 9 different gametocyte carriers were performed (see Supplementary Table S1 for details).
Behavioural assays
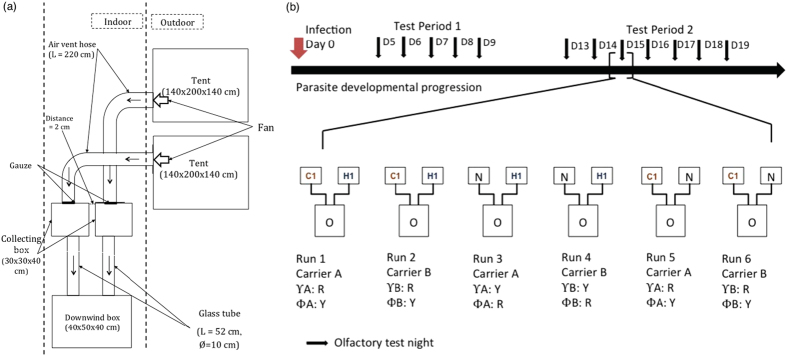
A dual-choice olfactometer was used to study odour-mediated host choice by infected and uninfected mosquitoes49, 54, 55. An. coluzzii behavioural assays were carried out as in ref. 49. There was one slight modification in the set-up when used for An. gambiae and An. arabiensis assays for which the two tents were placed next to each other (Fig. 1a). The olfactometer consisted of a source of two odours connected to two collecting boxes (30 × 30 × 40 cm) linked by two glass tubes (L = 52 cm, ϕ = 10 cm) to a downwind box (L × l × h = 60 × 40 × 40 cm) (Fig. 1a). Odour stimuli (from human, calf or outdoor air) came from two tents connected to the two collecting boxes of the olfactometer by air vent hoses (Scanpart®, D × L = 10 × 300 cm). Gauze was placed at the junction between the air vent hose and the collecting boxes to prevent mosquitoes entering the tent. A fan was set up at the junction of the air vent hose and the tent to draw air from the tent to the collecting box and the downwind box. The wind speed in the two downwind arms was regulated at 15 cm/s (±2 cm/s) using a 435–4 Testo multi-functional meter (Testor, Forbach, France) equipped with a hot wire probe (range: 0 to 20 m/s, accuracy: ±(0.03 m/s + 5% of mv)). The two tents were left outdoor while the collecting boxes and the downwind box were located inside an experimental room (Fig. 1a).
Figure 1.
Schematic representations of (a) the dual-choice olfactometer and (b) the behavioural assays. C1 represents the tent containing calf 1, H1 represents the tent containing human volunteer 1, N represents the tent with outdoor air (control), O represents the Olfactometer, Υ corresponds to mosquito infected status, Φ corresponds to mosquito uninfected status, R and Y represent the colours of the mosquitoes which are red and yellow respectively. The position of the tents was switched among replicates to account for side effect. Test period 1 and 2 correspond to the oocyst and sporozoite developmental stages in infected mosquitoes, respectively.
Mosquitoes were coloured with either red or yellow powders (Luminous Powder Kit, BioQuip) corresponding to their exposure status (received either an infectious blood-meal or a heat-inactivated uninfectious blood-meal)49, 56. The matching between exposure status and colours was switched between each run within a test day (Fig. 1b). To increase mosquito response to host odours in the olfactometer set-up, the three mosquito species were deprived from glucose solution for 10 hours prior to the behavioural tests. During this period, mosquitoes had access to water only. For An. gambiae and An. arabiensis assays, in a single test day, a maximum of 6 runs each lasting 30 minutes were conducted using one mosquito species, and a total of 3 odour combinations: human vs. calf odour (H-C), human vs. outdoor air (H-O) and calf vs. outdoor air (C-O) (Fig. 1b). For An. coluzzii experiments, only the human vs. calf odour (H-C) and human vs. outdoor air (H-O) combinations were carried out. In a single test day, 4 runs each lasting 30 minutes were carried out.
For each run, 20 uninfected controls and 20 gametocyte-exposed mosquitoes of similar age from the same experimental infection were simultaneously released in the downwind box of the apparatus. At the end of a run, the mosquitoes inside each of the two collecting boxes and the downwind box were removed with an aspirator, counted and kept in paper cups for subsequent analyses (dissection of mosquito midgut and head/thorax, see below). Each batch of mosquitoes was tested once, so that a fresh batch of naive mosquitoes was used for each run. After each run, the olfactometer was washed with 70% alcohol to remove odour contaminants left from previous tests. Latex gloves were worn by the experimenter to avoid contamination of the equipment. The chronological order of the odour combinations was changed to eliminate possible confounding effect of odour combination and test time. The side of the tent connected to the collecting boxes was also switched to avoid positional effect. Different combinations of calves and humans were used as odour sources on each testing day to obviate any individual effect (a total of 21 volunteers and 18 calves). All volunteers who served as the human odour source were male Burkinabe around 20–30 years old who lived in Bobo Dioulasso. Calves of about similar size and weight as human volunteers were used to equalize quantity of emitted odours.
Mosquito host choice was tested at different time points corresponding to two distinct phases of the parasite development: (i) Test period 1 (5–8 days post-infection (dpi)) corresponding to the period of immature parasite development in the midgut (i.e. oocyst stage), (ii) Test period 2 (13–19 dpi) corresponding to the period of parasite transmission potential (when the sporozoites have invaded the mosquito salivary glands). The total number of run performed for each mosquito species, test period and odour combination is indicated in Supplementary Table S1.
The day following behavioural testing, oocyst prevalence (proportion of P. falciparum-infected females) and intensity (number of oocysts in the midgut of infected females) were assessed by dissecting the midguts of mosquitoes that received an infectious blood-meal. Midguts were stained in a 1% mercurochrome solution and examined under a microscope49. Heads and thoraces were used to determine sporozoite prevalence (proportion of infected females) by PCR assays for An. coluzzii females57 and by qPCR assays for the two other species58. Three groups of mosquitoes were thus obtained: (i) females that received a gametocyte-positive blood and became successfully infected; (ii) females that received a gametocyte-positive blood and remained uninfected; and (iii) females that received a heat-treated gametocytic blood (uninfected control).
Statistical analysis
We performed two sets of analyses. In the first set, all data, including exposed-uninfected mosquitoes, were analysed. In the second set, we excluded exposed-uninfected individuals to focus on the difference between infected and uninfected control mosquitoes. The two sets of analyses yielded similar results, and, for the sake of clarity, only the second is reported in the main text (see Supplementary Tables S12–S15 for the detailed output of the first set of analyses).
All analyses were performed in R59. Binomial generalized linear mixed models (GLMMs) were fitted to investigate mosquito activation rate (proportion of mosquitoes caught in both collecting boxes out of the total number released) and odour choice (calculated separately for each odour combination, for example, human odour choice in the H-C combination was given as the proportion of mosquitoes entering human trap over the total mosquitoes entering both human and calf traps). In these models, infection treatment (two levels: infected and uninfected control), test period (two levels: test period 1 (oocyst stage) and test period 2 (sporozoite stage)), odour combination (three levels: H-C, H-O, and C-O for An. gambiae and An. arabiensis, and two levels: H-C, H-O for An. coluzzii) and relevant interactions were coded as fixed factors. Human volunteer and calf individual, and replicate were coded as random factors. Details of the fixed-effect and random-effect variables are shown in Supplementary Tables S2–S11. Because the An. arabiensis data were unbalanced (i.e. no data for carriers D and E during test period 1, see Supplementary Table S1), activation rate and odour choice we analysed separately for each test period. As there was complete separation of the data for one pair of human volunteer-calf individual in the H-C combination of test period 1 in the An. arabiensis data, volunteer was coded as a fixed factor in this host choice model only. We also verified for each combination, infection status and test period whether odour choice significantly differed from a random distribution between the two collecting boxes or whether mosquitoes displayed a statistically significant attraction to one odour.
For model selection, we used the stepwise removal of terms, followed by likelihood ratio tests (LRT). Term removals that significantly reduced explanatory power (P < 0.05) were retained in the minimal adequate model60. Post-hoc tests were carried out using the testFactor function in phia R package61.
Ethical statement
Ethical approval was obtained from the Centre Muraz Institutional Ethics Committee (A003–2012/CE-CM) and National Ethics Committee of Burkina Faso (2014-0040). The protocol conforms to the declaration of Helsinki on ethical principles for medical research involving human subjects (version 2002) and informed written consent were obtained from all volunteers. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by both the Office of Laboratory Animal Welfare of US Public Health Service (Assurance Number: A5928-01) and national committee of Burkina Faso (IRB registration #00004738 and FWA 00007038). Animals were cared for by trained personnel and veterinarians.
Results
Infection
Of the 1448 An. coluzzii mosquitoes exposed to an infectious blood meal, 622 became infected (percentage ± 95% confidence interval: 42.96 ± 2.55%, Supplementary Fig. S1), with a mean (±se) parasite intensity of 14.67 ± 1.41 (information for each carrier is in Supplementary Fig. S2, Supplementary Table S1). A total of 2161 An. coluzzii females (622 infected + 1539 uninfected control mosquitoes) were used for the analyses. Of the 744 exposed An. gambiae, 570 were infected (76.61 ± 3.07%, Supplementary Fig. S1), with a mean parasite intensity of 18.45 ± 1.71 (information for each carrier is in Supplementary Fig. S2, Supplementary Table S1). A total of 1260 An. gambiae females (570 infected + 670 control) were used for the analyses. Finally, of the 447 exposed An. arabiensis, 243 were infected (54.36 ± 4.62%, Supplementary Fig. S1), with a mean parasite intensity of 8.77 ± 0.61 (information for each carrier is in Supplementary Fig. S2, Supplementary Table S1. A total of 694 An. arabiensis females (243 infected + 451 uninfected controls) were used for the analyses. The differences in parasite prevalence and intensity observed across the different mosquito species can be explained by the use of different wild parasite isolates containing varying densities of gametocytes.
Activation rate
Anopheles coluzzii
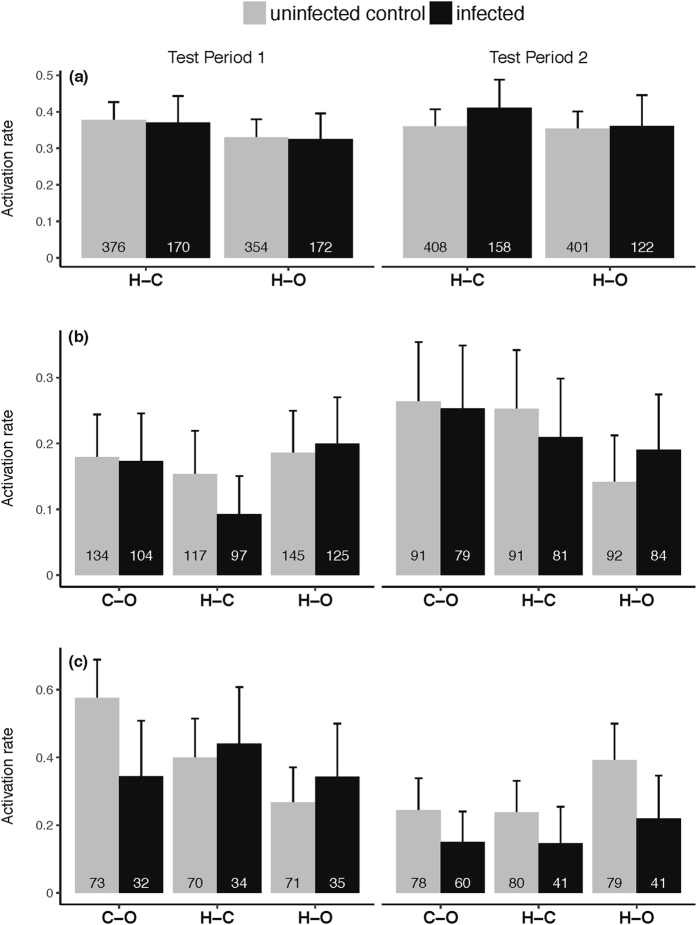
Overall, 228 of 622 infected mosquitoes and 548 of 1539 control mosquitoes left the downwind box of the olfactometer to fly upwind into one of the two collection boxes (activation rate of 36.66 ± 3.79% and 35.61 ± 2.39%, respectively). P. falciparum infection did not affect An. coluzzii activation rate (χ2 1 = 0.5, P = 0.48; Fig. 2a). An. coluzzii displayed similar activation rate between the two test periods (χ2 1 = 0.08, P = 0.78, Fig. 2a) and the three odour combinations (χ2 1 = 3.21, P = 0.07, Fig. 2a). Finally, there was no statistically significant interactions (test period × odour combination: χ2 1 = 1.06, P = 0.3; infection × odour combination: χ2 1 = 0.22, P = 0.64; infection × test period: χ2 1 = 1.13, P = 0.29; Supplementary Table S2, Fig. 2a).
Figure 2.
Mosquito activation rate, expressed as the proportion of mosquitoes caught in both collecting boxes out of the total number released in the downwind box for each treatment combination. (a) Anopheles coluzzii, (b) Anopheles gambiae, (c) Anopheles arabiensis. Numbers inside the bars indicate the total number of mosquitoes released across all runs. Error bars show the 95% confidence interval. C-O: for calf odour vs outdoor air combination, H-C: human odour vs calf odour combination, H-O: human odour vs outdoor air combination. Test Period 1 and Test Period 2 correspond to the oocyst and sporozoite stages in infected mosquitoes, respectively.
Anopheles gambiae
The overall activation rates of infected and uninfected control mosquitoes were 18.42 ± 3.18% (105 out of 570 mosquitoes) and 19.25 ± 2.99% (129 out of 670 mosquitoes) respectively. Infection did not significantly affect An. gambiae activation rate (χ2 1 = 0.22, P = 0.64, Fig. 2b). There was a marginally significant effect of test period (χ2 1 = 3.94, P = 0.047; Fig. 2b). Although there was no significant main effect of odour combination on An. gambiae activation rate (χ2 2 = 3.26, P = 0.2), there was a significant test period by odour combination interaction (χ2 2 = 8.44, P = 0.01, Supplementary Table S3) such that the H-O odour combination induced the highest mosquito activation rate during test period 1 and the lowest during test period 2 (Fig. 2b; Supplementary Table S4). Finally, there was no infection by test period interaction (χ2 1 = 0.23, P = 0.63), and no infection by combination interaction (χ2 2 = 2.21, P = 0.33; Supplementary Table S3).
Anopheles arabiensis
The activation rates of infected and uninfected control mosquitoes during test period 1 were 37.62 ± 9.45% (38 out of 101 mosquitoes) and 41.59 ± 6.6% (89 out of 214 mosquitoes) respectively. There was no influence of infection on An. arabiensis activation rate (χ2 1 = 0.58, P = 0.45; Fig. 2c; Supplementary Table S5). There was a significant effect of odour combination on activation rate (χ2 2 = 10.34, P = 0.005). In particular, mosquitoes were more activated in the C-O combination than in the H-O combination (post hoc tests: C-O vs. H-O: χ2 1 = 9.96, P = 0.002; H-C vs. C-O: χ2 1 = 1.79, P = 0. 18; H-O vs. H-C: χ2 1 = 3.43, P = 0.06; Supplementary Table S5). There was no significant interaction between infection and odour combination (χ2 2 = 4.95, P = 0.08; Supplementary Table S5). The activation rate of infected An. arabiensis during test period 2 (16.90 ± 6.16%, n = 142) was lower than that of uninfected individuals (29.11 ± 5.78%, n = 237) (χ2 1 = 6.69, P = 0.01; Fig. 2c). We also found a significant effect of odour combination (χ2 2 = 6.61, P = 0.04), such that mosquitoes were more activated in the H-O combination compared to the two other combinations (post hoc tests: H-O vs. H-C: χ2 1 = 4.97, P = 0.03; H-O vs. C-O: χ2 1 = 4.68, P = 0. 03; H-C vs. C-O: χ2 1 = 0.01, P = 0.9; Supplementary Table S6). We found no significant effect of infection by odour combination interaction (χ2 2 = 0.18, P = 0.92 Fig. 2c; Supplementary Table S5).
Odour choice
Anopheles coluzzii
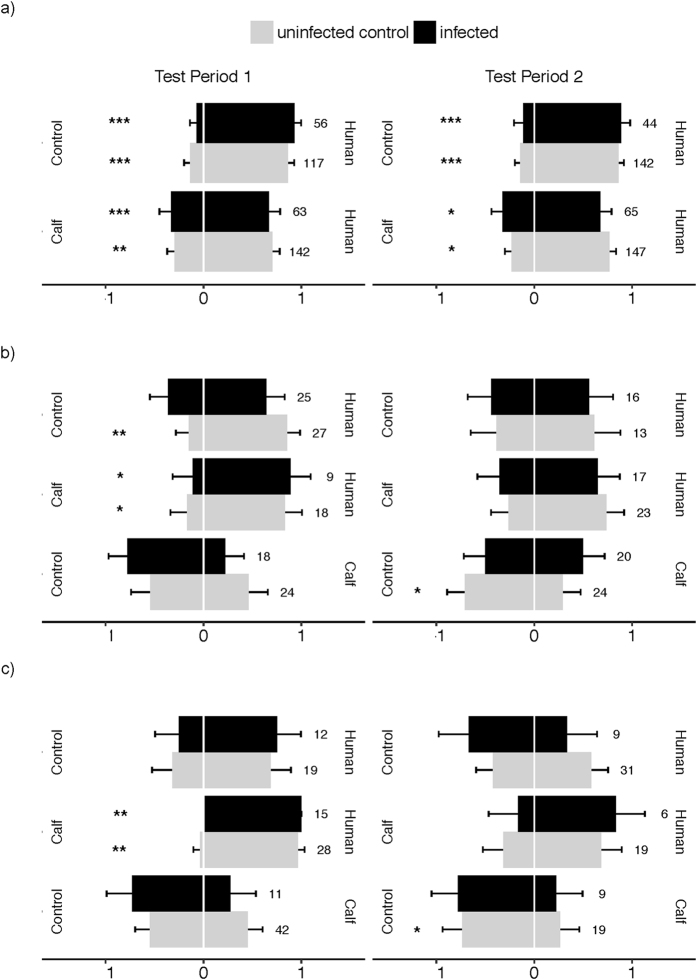
Infected and uninfected mosquitoes showed similar odour choice (H-C: χ2 1 = 0.73, P = 0.4; H-O: χ2 1 = 1.24, P = 0.43) with an overall attraction toward human odours of 71.70 ± 4.32% in the H-C and of 87.47 ± 3.42% in the H-O combinations (Fig. 3a). There was no significant difference in odour choice between test period 1 and 2 in both the H-C (χ2 1 = 0.03, P = 0.87) and the H-O odour combinations (χ2 1 = 0.02, P = 0.89). Finally, there was no interaction between test period and infection in both the H-C (χ2 1 = 0.31, P = 0.58) and H-O combinations (χ2 1 = 0.62, P = 0.43; Fig. 3a, Supplementary Table S8).
Figure 3.
Mosquito odour-mediated choice - expressed as the proportion of mosquitoes caught in one collecting box out of the total number retrieved from both collecting boxes. (a) An. coluzzii, (b) An. gambiae (c) An. arabiensis. Data show proportion ± 95% confidence interval across all runs. Numbers indicate the total numbers of mosquitoes in both traps across all runs. The annotation human, calf, control corresponds to source of odour the mosquitoes chose Test Period 1 and Test Period 2 correspond to the oocyst and sporozoite stages in infected mosquitoes, respectively. (* indicates significant bias toward an odour source; *P < 0.05, **P < 0.01, ***P < 0.001).
Anopheles gambiae
In all three odour combinations, infected and uninfected mosquitoes displayed similar odour choice (H-C: χ2 1 = 0.12, P = 0.73; H-O: χ2 1 = 2.92, P = 0.09; C-O: χ2 1 = 0.75, P = 0.75) with an overall attraction to human odours of 76.12 ± 10.21% in the H-C and 69.14 ± 10.06% in the H-O combinations, and a repulsion by calf odours of 62.79 ± 10.22% in the C-O combination (Fig. 3b). There was no significant effect of test period (H-C combination: χ2 1 = 1.96, P = 0.16; H-O combination: χ2 1 = 1.66, P = 0.2; C-O combination: χ2 1 = 0.4, P = 0.53) on An. gambiae odour choice. We found a significant infection by test period interaction for the C-O combination (χ2 3 = 4.67, P = 0.03) with calf odours inducing a stronger repellence to infected mosquitoes than to uninfected mosquitoes during test period 1 and inversely during test period 2 (Fig. 3b, Supplementary Table S9). Finally, there was no significant interaction between infection and test period in the H-C and H-O combinations (χ2 1 = 0.47, P = 0.49 and χ2 1 = 0.82, P = 0.37, respectively).
Anopheles arabiensis
For both test periods and all odour combinations, infected and uninfected mosquitoes displayed similar host choice (test period 1, H-C: χ2 1 = 0.87, P = 0.35; H-O: χ2 1 = 0.16, P = 0.69; C-O: χ2 1 = 1.2, P = 0.27; test period 2, H-C: χ2 1 = 0.36, P = 0.55, H-O: χ2 1 = 1.39, P = 0.24, C-O: χ2 1 = 0.06, P = 0.81; Fig. 3c, Supplementary Tables S10 and S11) with an attraction to human odours of 88.23 ± 7.66% in the H-C and 60.56 ± 11.37% in the H-O combinations and a repulsion by calf odour of 64.19 ± 10.44% in the C-O odour combination. There was no influence of volunteer/calf individual on odour choice (test period 1, H-C: χ2 1 = 0.69, P = 0.41; Supplementary Table S10).
Discussion
Several studies have demonstrated that malaria parasites can alter important behavioural features of their mosquito vectors in a stage dependant manner: oocyst-infected mosquitoes show reduced attraction to vertebrate odours and reduced avidity to feed, and, in contrast, sporozoite-infected individuals show enhanced attraction to vertebrate odours and enhanced avidity to feed16–19. These behavioural alterations likely increase parasite transmission, provided that mosquito feeds are taken on a suitable vertebrate host species for the parasite. Our results indicate that, regardless of parasite developmental stage, P. falciparum infection did not alter mosquito activation rate, a surrogate of mosquito motivation to feed. While this finding contrasts with earlier studies23, 24, 29, it supports two recent other studies, including one on An. coluzzii experimentally infected with sympatric wild isolates of P. falciparum 49, 62, and suggests that manipulation of mosquito activity and response to host odours may not be a universal phenomenon. The expression of parasite-induced behavioural alterations, like any other phenotypic traits, may depend on local coevolutionary processes63. Hence, natural selection might not favour the evolution of manipulation in the studied populations if, for example, mosquito behaviour already ensures high parasite transmission or if the mosquito vector has evolved resistance64. Further investigations using sympatric and allopatric host-parasite combinations will be essential to integrate these local co-adaptation phenomena.
Our results also showed similar attractiveness of host odours to infected and uninfected mosquitoes. The overall expression pattern of mosquito host choice was consistent with a high degree of anthropophily in both infected and uninfected An. coluzzii, An. gambiae and An. arabiensis. The only significant effect of infection on mosquito odour choice was seen in sporozoite-infected An. gambiae which were less repelled by calf odours compared to oocyst-infected counterparts (Fig. 3b). This result contrasts with the hypothesis of a parasite manipulation of mosquito odour preference and the expectation that sporozoite-infected individuals should be steered away from the odours of inappropriate vertebrate hosts. Although the precise reason behind this effect is currently unclear, it might be a mosquito response, and interactions among mosquito resources, infection and requirement of a blood-meal can be suspected. Because Plasmodium parasites utilize mosquito resources to develop and mature, sporozoite-infected An. gambiae females might be less choosy regarding the nature of the blood source, hence explaining this increased attraction to calf odour. At this stage, this remains speculative and further experiments are required to investigate the mechanisms underlying this effect and to explain why this pattern was not observed in An. coluzzii and An. arabiensis.
Our prediction was that sporozoites of P. falciparum may have evolved the ability to influence mosquito preference in a way that increases contact with humans - the appropriate vertebrate host of P. falciparum - by inducing in the mosquito vector a sensory bias for host odours that are correlated with suitability for the parasite. When simultaneously exposed to odours from calf and human, 71% and 76% of both sporozoite-infected and uninfected An. coluzzii and An. gambiae were retrieved from the human trap, hence confirming the anthropophilic behaviour of these species36, 39, 65, 66. Such anthropophily already ensures relatively high probability of transmission toward the appropriate host, perhaps making parasite manipulation of mosquito host choice useless in this system (i.e. weak selective pressures for its evolution). In other words, it is possible that, in this system, the balance between the transmission benefits of an increased preference for humans (e.g. from an anthropophily of 70 to 90%) and the costs associated with manipulation67 explain why this change has not evolved. Further experiments using vector populations with weaker human tropism will be required to test this hypothesis. Alternatively, it is possible that the parasite’s ability to modify its mosquito host choice did evolve but was not expressed here because of our experimental design.
First, while our olfactometer allows the study of long-range odour-mediated attractiveness, the full sequence of mosquito host-seeking process also includes short-range stimuli. Odour-mediated preference is critical at the initial step of host location; however final host decision might be influenced by cues other than odours, thereby determining alternative patterns of host preference. Indeed, olfactometer obviates stimuli such as visual cues, heat, moist, convective currents, and host movements. Under this scenario, sporozoite-infected An. gambiae would present similar host preference as uninfected counterparts in the early stages of the host-seeking process, when it mostly responds to host odours (i.e. long-range host preference), and then display increased attraction to humans at a shorter range, when other cues become more important (i.e. short-range host preference). In addition, we did not control the quantity of emitted host odour which could also affect mosquito behavioural responses65.
Second, our experiments were conducted between 6.30.pm and 11.30.pm while mosquito activation spans from 6.00.pm until early morning the following day with a peak of activity occurring around midnight. This activity peak is correlated with human resting behaviour to presumably maximize mosquito fitness68. During this time frame, human are less defensive, possibly facilitating feeding and reduce mosquito mortality. Malaria parasites may have evolved the ability to finetune manipulation to the temporal behaviour of both the vectors and the vertebrate host. Under this scenario, manipulation of host choice might occur later in the night (e.g. during the peak of mosquito activity), and we hence may have missed it.
Third, An. gambiae and An. coluzzii mosquitoes from colonies continually replenished with F1 from wild-caught females were used here and it will also be important to use F0 from field mosquitoes since rearing insects in the laboratory for many generations is unlikely to represent the genetic diversity observed in nature.
Fourth, the uninfected control mosquitoes were fed on the same blood as infected mosquitoes but in which gametocytes were heat-inactivated. This procedure allows avoiding confounding effects of different blood origins on mosquito fitness and behavioural responses. However, these heat-killed gametocytes might trigger a mosquito immune response, which in turn, might affect mosquito behaviour. Although no study has, to our knowledge, directly explored whether heat-killed gametocytes can stimulate a mosquito immune response, there is evidence that alive gametocytes blood triggers a mosquito immune response that does not occur with heat-killed gametocytes69, 70. In addition, the differences in immune gene expression between mosquitoes challenged with alive and dead P. falciparum gametocytes is similar to that observed between mosquitoes challenged with alive P. berghei gametocytes and mosquitoes that received a parasite-free blood-meal69, 70. These results suggest that heat-killed P. falciparum gametocytes and parasite-free blood-meal may induce similar mosquito immune response. However, a study showed that a challenge with heat-killed Escherichia coli can generate mosquito behavioural changes similar to that observed in mosquitoes infected with rodent malaria parasites24. Overall, future studies should ideally include two control groups: heat-inactivated gametocytes from the same carrier (to avoid effects of different blood-meal sources on mosquito behaviour) and (ii) uninfected blood from a parasite-free donor (to avoid possible effects of heat-killed gametocytes on mosquito behaviour).
Finally, it is possible that the expression of parasite manipulation of host preference is more pronounced in some mosquito-parasite combinations than in others. In particular, we predicted that the expression of parasite manipulation of vector host choice might be more obvious in An. arabiensis, a presumably more zoophilic/opportunistic vector species36. When simultaneously exposed to calf and human odours, we found that 90% of An. arabiensis were retrieved from the human trap. This result contrasts with most existing studies on An. arabiensis host preference, which report an overall high degree of zoophily36. Using odour-baited entry traps in Tanzania71, for example, showed a 90% zoophily in An. arabiensis. In contrast, of an estimated 1,800 field An. arabiensis collected using the same technique in Central Burkina Faso, slightly more than 8% were collected in the calf-baited trap, suggesting a high degree of anthropophily72. Our findings support the idea that West African populations of An. arabiensis may be generally more anthropophilic than East African ones39 and emphasizes that the anthropophilic/zoophilic label given to malaria mosquito species must be carefully interpreted and refer to populations rather than whole taxonomic unit.
We observed no parasite manipulation of mosquito odour-mediated host choice in the natural associations between P. falciparum and three of its major vector species, An. coluzzii, An. gambiae, and An. arabiensis. All three species were rather anthropophilic regardless of their infectious status. Further work is required to explore whether P. falciparum is able to modify its mosquito vertebrate choice in a way that increase transmission toward suitable host species. While our study examined the odour-mediated long-range mosquito host choice, determining the origin of blood-meals retrieved from uninfected, oocyst-infected and sporozoite-infected mosquitoes in the field may reveal the existence of specific manipulation. Future studies on specific manipulation in other vector systems would provide important information on the ecology and epidemiology of vector-borne diseases. A recent study suggested that the rodent- or bird-specialized Borrelia genospecies were unable to alter attraction of the generalist tick Ixodes ricinus to mouse odour73. However, this study used ticks collected from the field and was not able to establish a causal relationship between Borrelia infection and attraction to mouse odour73. Other possibly good model systems to study specific manipulation in vector-borne diseases are tsetse fly-transmitted trypanosomes. For example, Glossina palpalis gambiensis has a broad range of hosts in central Africa (humans, reptiles, bushbuck, and ox) and is the main vector of Trypanosoma brucei gambiense responsible for the medically important Human African trypanosomiasis. We would predict that once infected, flies are more attracted by human cues than by those of other vertebrates. Finally, parasite manipulation of mosquito host choice could theoretically occur at the intraspecific level (among different human individuals), with infected vectors biting more than expected less-immune hosts.
Electronic supplementary material
Acknowledgements
This work was supported by the Agence Nationale de Recherche (grant number: 11-PDOC-006-01). We would like to thank all children and their parents for participating in this study, the local authorities for their support, as well as all the volunteers for the dual-port olfactometer assays. We are very grateful to the IRSS staff in Burkina Faso for technical assistance. We also thanks the editor and two anonymous referees for helpful and constructive comments.
Author Contributions
A.V., A.C., F.T., L.-C.G., C.C., K.R.D. and T.L. conceived and designed the study. P.L.N., A.V., D.F.H., B.Y. and T.L. carried out the study. A.V., T.L. and P.L.N. performed the statistical analyses and drafted the manuscript. All authors contributed to the interpretation of the data and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Phuong L. Nguyen and Amélie Vantaux contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-09821-x
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Moore, J. Parasites and the behavior of animals. (Oxford University Press, 2002).
- 2.Lefèvre T, et al. Invasion of the body snatchers: the diversity and evolution of manipulative strategies in host-parasite interactions. Adv. Parasitol. 2009;68:45–83. doi: 10.1016/S0065-308X(08)00603-9. [DOI] [PubMed] [Google Scholar]
- 3.Hughes, D. P., Brodeur, J. & Thomas, F. Host Manipulation by Parasites. (Oxford University Press, 2012).
- 4.Poulin, R. In Advances in the study of behavior (eds. Brockmann, H. J. et al.) 151–186, doi:10.1016/S0065-3454(10)41005-0 (Elsevier Inc., 2010).
- 5.Lafferty KD, Morris AK. Altered behvavior of parasitized killifish increases susceptibility to predation by bird final hosts. Ecology. 1996;77:1390–1397. doi: 10.2307/2265536. [DOI] [Google Scholar]
- 6.Lagrue C, Kaldonski N, Perrot-Minnot M-J, Motreuil S, Bollache L. Modification of hosts’ behavior by a parasite: Field evidence for adaptive manipulation. Ecology. 2007;88:2839–2847. doi: 10.1890/06-2105.1. [DOI] [PubMed] [Google Scholar]
- 7.Lagrue C, Güvenatam A, Bollache L. Manipulative parasites may not alter intermediate host distribution but still enhance their transmission: field evidence for increased vulnerability to definitive hosts and non-host predator avoidance. Parasitology. 2013;140:258–265. doi: 10.1017/S0031182012001552. [DOI] [PubMed] [Google Scholar]
- 8.Mouritsen KN, Poulin R. Parasite-induced trophic facilitation exploited by a non-host predator: A manipulator’s nightmare. Int. J. Parasitol. 2003;33:1043–1050. doi: 10.1016/S0020-7519(03)00178-4. [DOI] [PubMed] [Google Scholar]
- 9.Kaldonski N, Perrot-Minnot MJ, Motreuil S, Cézilly F. Infection with acanthocephalans increases the vulnerability of Gammarus pulex (Crustacea, Amphipoda) to non-host invertebrate predators. Parasitology. 2008;135:627–32. doi: 10.1017/S003118200800423X. [DOI] [PubMed] [Google Scholar]
- 10.Seppälä O, Valtonen ET, Benesh DP. Host manipulation by parasites in the world of dead-end predators: adaptation to enhance transmission? Proc. R. Soc. London. Ser. B Biol. Sci. 2008;275:1611–1615. doi: 10.1098/rspb.2008.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seppälä O, Jokela J. Host manipulation as a parasite transmission strategy when manipulation is exploited by non-host predators. Biol. Lett. 2008;4:663–666. doi: 10.1098/rsbl.2008.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacquin L, Mori Q, Pause M, Steffen M, Médoc V. Non-specific manipulation of gammarid behaviour by P. minutus parasite enhances their predation by definitive bird hosts. PLoS One. 2014;9:1–12. doi: 10.1371/journal.pone.0101684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seppälä O, Karvonen A, Valtonen ET. Behavioural mechanisms underlying ‘specific’ host manipulation by a trophically transmitted parasite. Evol. Ecol. Res. 2012;14:73–81. [Google Scholar]
- 14.Médoc V, Beisel J. Field evidence for non-host predator avoidance in a manipulated amphipod. Naturwissenschaften. 2009;96:513–523. doi: 10.1007/s00114-008-0503-8. [DOI] [PubMed] [Google Scholar]
- 15.Médoc V, Rigaud T, Bollache L, Beisel J. A manipulative parasite increasing an antipredator response decreases its vulnerability to a nonhost predator. Anim. Behav. 2009;77:1235–1241. doi: 10.1016/j.anbehav.2009.01.029. [DOI] [Google Scholar]
- 16.Hurd H. Manipulation of medically important insect vectors by their parasites. Annu. Rev. Entomol. 2003;48:141–161. doi: 10.1146/annurev.ento.48.091801.112722. [DOI] [PubMed] [Google Scholar]
- 17.Lefèvre T, Thomas F. Behind the scene, something else is pulling the strings: Emphasizing parasitic manipulation in vector-borne diseases. Infect. Genet. Evol. 2008;8:504–519. doi: 10.1016/j.meegid.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Cator LJ, Lynch PA, Read AF, Thomas MB. Do malaria parasites manipulate mosquitoes? Trends Parasitol. 2012;28:467–470. doi: 10.1016/j.pt.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caljon G, et al. Alice in microbes’ land: adaptations and counter-adaptations of vector-borne parasitic protozoa and their hosts. FEMS Microbiol. Rev. 2016;40:664–685. doi: 10.1093/femsre/fuw018. [DOI] [PubMed] [Google Scholar]
- 20.De Moraes CM, et al. Malaria-induced changes in host odors enhance mosquito attraction. Proc. Natl. Acad. Sci. USA. 2014;111:11079–84. doi: 10.1073/pnas.1405617111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cornet S, Nicot A, Rivero A, Gandon S. Malaria infection increases bird attractiveness to uninfected mosquitoes. Ecol. Lett. 2013;16:323–329. doi: 10.1111/ele.12041. [DOI] [PubMed] [Google Scholar]
- 22.Lacroix R, Mukabana WR, Gouagna LC, Koella JC. Malaria infection increases attractiveness of humans to mosquitoes. PLoS Biol. 2005;3:1590–1593. doi: 10.1371/journal.pbio.0030298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossignol PA, Ribeiro JM, Spielman A. Increased biting rate and reduced fertility in sporozoite-infected mosquitoes. Am. J. Trop. Med. Hyg. 1986;35:277–279. doi: 10.4269/ajtmh.1986.35.277. [DOI] [PubMed] [Google Scholar]
- 24.Cator LJ, et al. ‘Manipulation’ without the parasite: altered feeding behaviour of mosquitoes is not dependent on infection with malaria parasites. Proc. R. Soc. London. Ser. B Biol. Sci. 2013;280 doi: 10.1098/rspb.2013.0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rossignol PA, Ribeiro JMC, Spielman A. Increased intradermal probing time in sporozoite-infected mosquitoes. Am. J. Trop. Med. Hyg. 1984;33:17–20. doi: 10.4269/ajtmh.1984.33.17. [DOI] [PubMed] [Google Scholar]
- 26.Wekesa JW, Copeland RS, Mwangi RW. Effect of Plasmodium falciparum on blood feeding behavior of naturally infected Anopheles mosquitoes in western Kenya. Am. J. Trop. Med. Hyg. 1992;47:484–488. doi: 10.4269/ajtmh.1992.47.484. [DOI] [PubMed] [Google Scholar]
- 27.Anderson RA, Koella JC, Hurd H. The effect of Plasmodium yoelii nigeriensis infection on the feeding persistence of Anopheles stephensi Liston throughout the sporogonic cycle. Proc. R. Soc. London. Ser. B Biol. Sci. 1999;266:1729–1733. doi: 10.1098/rspb.1999.0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koella JC, Rieu L, Paul REL. Stage-specific manipulation of a mosquito’s host-seeking behavior by the malaria parasite Plasmodium gallinaceum. Behav. Ecol. 2002;13:816–820. doi: 10.1093/beheco/13.6.816. [DOI] [Google Scholar]
- 29.Smallegange RC, et al. Malaria Infected Mosquitoes Express Enhanced Attraction to Human Odor. PLoS One. 2013;8:8–10. doi: 10.1371/journal.pone.0063602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koella JC, Sørensen FL, Anderson RA. The malaria parasite, Plasmodium falciparum, increases the frequency of multiple feeding of its mosquito vector, Anopheles gambiae. Proc. R. Soc. London. Ser. B Biol. Sci. 1998;265:763–768. doi: 10.1098/rspb.1998.0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koella JC, Packer MJ. Malaria parasites enhance blood-feeding of their naturally infected vector Anopheles punctulatus. Parasitology. 1996;113:105–109. doi: 10.1017/S0031182000066348. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz A, Koella JC. Trade-offs, conflicts of interest and manipulation in Plasmodium-mosquito interactions. Trends Parasitol. 2001;17:189–194. doi: 10.1016/S1471-4922(00)01945-0. [DOI] [PubMed] [Google Scholar]
- 33.Cator LJ, Lynch PA, Thomas MB, Read AF. Alterations in mosquito behaviour by malaria parasites: potential impact on force of infection. Malar. J. 2014;13 doi: 10.1186/1475-2875-13-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dobson AP. The population biology of parasite-induced changes in host behavior. Q. Rev. Biol. 1988;63:139–165. doi: 10.1086/415837. [DOI] [PubMed] [Google Scholar]
- 35.Lyimo IN, Ferguson HM. Ecological and evolutionary determinants of host species choice in mosquito vectors. Trends Parasitol. 2009;25:189–196. doi: 10.1016/j.pt.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 36.Takken, W. & Verhulst, N. O. Host Preferences of Blood-Feeding Mosquitoes. Annu. Rev. Entomol. 433–453 (2013). [DOI] [PubMed]
- 37.Perkins SL. Malaria’s many mates: past, present, and future of the systematics of the order Haemosporida. J. Parasitol. 2014;100:11–25. doi: 10.1645/13-362.1. [DOI] [PubMed] [Google Scholar]
- 38.Lefèvre T, et al. New prospects for research on manipulation of insect vectors by pathogens. PLoS Pathog. 2006;2:0633–0635. doi: 10.1371/journal.ppat.0020072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Costantini C, Sagnon N, della Torre A, Coluzzi M. Mosquito behavioural aspects of vector-human interactions in the Anopheles gambiae complex. Prassitologia. 1999;41:209–217. [PubMed] [Google Scholar]
- 40.Rayner, J. C., Liu, W., Peeters, M., Sharp, P. M. & Hahn, B. H. A plethora of Plasmodium species in wild apes: A source of human infection? Trends Parasitol.27, 222–229 (2011). [DOI] [PMC free article] [PubMed]
- 41.Prugnolle F, et al. A fresh look at the origin of Plasmodium falciparum, the most malignant malaria agent. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1001283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ngoubangoye B, et al. The host specificity of ape malaria parasites can be broken in confined environments. Int. J. Parasitol. 2016;46:737–744. doi: 10.1016/j.ijpara.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 43.Fanello C, Santolamazza F, Torre AD. Simultaneous identification of species and molecular forms of the Anopheles gambiae complex by PCR-RFLP. Med. Vet. Entomol. 2002;16:461–464. doi: 10.1046/j.1365-2915.2002.00393.x. [DOI] [PubMed] [Google Scholar]
- 44.Santolamazza F, et al. Insertion polymorphisms of SINE200 retrotransposons within speciation islands of Anopheles gambiae molecular forms. Malar. J. 2008;7 doi: 10.1186/1475-2875-7-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dabiré RK, et al. Occurrence of natural Anopheles arabiensis swarms in an urban area of Bobo-Dioulasso city, Burkina Faso, West Africa. Acta Trop. 2014;130:44–50. doi: 10.1016/j.actatropica.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 46.Dabiré RK, et al. Population dynamics of Anopheles gambiae s.l. in Bobo-Dioulasso city: bionomics, infection rate and susceptibility to insecticides. Parasit. Vectors. 2012;5:127–135. doi: 10.1186/1756-3305-5-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ouédraogo AL, et al. A protocol for membrane feeding assays to determine the infectiousness of P. falciparum naturally infected individuals to Anopheles gambiae. Malar. World J. 2013;4:17–20. [Google Scholar]
- 48.Vantaux A, Dabiré K, Cohuet A, Lefèvre T. A heavy legacy: offspring of malaria-infected mosquitoes show reduced disease resistance. Malar. J. 2014;13 doi: 10.1186/1475-2875-13-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vantaux A, et al. Host-seeking behaviors of mosquitoes experimentally infected with sympatric field isolates of the human malaria parasite Plasmodium falciparum: no evidence for host manipulation. Front. Ecol. Evol. 2015;3:1–12. doi: 10.3389/fevo.2015.00086. [DOI] [Google Scholar]
- 50.Roux O, et al. Evidence for carry-over effects of predator exposure on pathogen transmission potential. Proc. R. Soc. London. Ser. B Biol. Sci. 2015;282 doi: 10.1098/rspb.2015.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hien, D. F. S. et al. Plant-mediated effects on mosquito capacity to transmit human malaria. PLoS Pathog. 1–17 (2016). [DOI] [PMC free article] [PubMed]
- 52.Gouagna LC, et al. Stage-specific effects of host plasma factors on the early sporogony of autologous Plasmodium falciparum isolates within Anopheles gambiae. Trop. Med. Int. Heal. 2004;9:937–948. doi: 10.1111/j.1365-3156.2004.01300.x. [DOI] [PubMed] [Google Scholar]
- 53.Sangare I, et al. Studying fitness cost of Plasmodium falciparum infection in malaria vectors: validation of an appropriate negative control. Malar. J. 2013;12 doi: 10.1186/1475-2875-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lefèvre T, et al. Evolutionary lability of odour-mediated host preference by the malaria vector Anopheles gambiae. Trop. Med. Int. Heal. 2009;14:228–236. doi: 10.1111/j.1365-3156.2009.02206.x. [DOI] [PubMed] [Google Scholar]
- 55.Lefèvre T, et al. Beer consumption increases human attractiveness to malaria mosquitoes. PLoS One. 2010;5:1–8. doi: 10.1371/journal.pone.0009546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Verhulst NO, Loonen JACM, Takken W. Advances in methods for colour marking of mosquitoes. Parasit. Vectors. 2013;6 doi: 10.1186/1756-3305-6-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morassin B, Fabre R, Berry A, Magnaval JF. One year’s experience with the polymerase chain reaction as a routine method for the diagnosis of imported malaria. Am. J. Trop. Med. Hyg. 2002;66:503–508. doi: 10.4269/ajtmh.2002.66.503. [DOI] [PubMed] [Google Scholar]
- 58.Boissière A, et al. Application of a qPCR assay in the investigation of susceptibility to malaria infection of the M and S molecular forms of An. gambiae s.s. in Cameroon. PLoS One. 2013;8 doi: 10.1371/journal.pone.0054820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.R Development Core Team. R: A Language and Environment for Statistical Computing. At https://www.r-project.org/ (2008).
- 60.Crawley, M. J. The R book, doi:10.1017/CBO9781107415324.004 (John Wiley & Sons Ltd, England, 2007).
- 61.Rosario-Martinez, H. D., Fox, J. & Team, R. C. phia: Post-Hoc Interaction Analysis. (2015).
- 62.Cornet S, Nicot A, Rivero A, Gandon S. Both infected and uninfected mosquitoes are attracted toward malaria infected birds. Malar. J. 2013;12 doi: 10.1186/1475-2875-12-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thompson, J. N. The geographic mosaic theory of coevolution. University of Chicago Press (University of Chicago Press, 2005).
- 64.Daoust SP, et al. Making the best of a bad situation: Host partial resistance and bypass of behavioral manipulation by parasites? Trends Parasitol. 2015;31:413–418. doi: 10.1016/j.pt.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 65.Costantini C, et al. Mosquito responses to carbon dioxide in a west African Sudan savanna village. Med. Vet. Entomol. 1996;10:220–227. doi: 10.1111/j.1365-2915.1996.tb00734.x. [DOI] [PubMed] [Google Scholar]
- 66.Dekker T, Takken W, Braks AH. Innate preference for host-odour blends modulates degree of anthropophagy of Anopheles gambiae s.l. (Diptera: Culicidae) J. Med. Entomol. 2001;38:868–871. doi: 10.1603/0022-2585-38.6.868. [DOI] [PubMed] [Google Scholar]
- 67.Poulin R. The evolution of parasite manipulation of host behaviour: a theoretical analysis. Parasitology. 1994;109:S109–S118. doi: 10.1017/S0031182000085127. [DOI] [PubMed] [Google Scholar]
- 68.Lehane, M. J. The Biology of Blood-Sucking in Insects. (Cambridge University Press, 2005).
- 69.Mendes, A. M. et al. Conserved mosquito/parasite interactions affect development of Plasmodium falciparum in Africa. PLoS Pathog. 4 (2008). [DOI] [PMC free article] [PubMed]
- 70.Mendes AM, et al. Infection intensity-dependent responses of Anopheles gambiae to the African malaria parasite Plasmodium falciparum. Infect. Immun. 2011;79:4708–4715. doi: 10.1128/IAI.05647-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mahande A, Mosha F, Mahande J, Kweka E. Feeding and resting behaviour of malaria vector, Anopheles arabiensis with reference to zooprophylaxis. Malar. J. 2007;6 doi: 10.1186/1475-2875-6-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Costantini C, et al. Odor-mediated host preferences of West African mosquitoes, with particular reference to malaria vectors. Am. J. Trop. Med. Hyg. 1998;58:56–63. doi: 10.4269/ajtmh.1998.58.56. [DOI] [PubMed] [Google Scholar]
- 73.Berret J, Voordouw MJ. Lyme disease bacterium does not affect attraction to rodent odour in the tick vector. Parasit. Vectors. 2015;8 doi: 10.1186/s13071-015-0856-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.