Abstract
Objective
This study investigated variants of TPH1, TPH2, and SLC6A4 in the moderation of the subjective effects of cocaine.
Methods
Non-treatment seeking cocaine-dependent individuals (N = 66) were intravenously administered saline and cocaine (40 mg) in randomized order. Participants self-reported subjective effects of cocaine using a visual analog scale starting before administration of saline or cocaine (−15 min) to up to 20 min post-infusion. Self-report ratings on the visual analog scale ranged from 0 (no effect) to 100 (greatest effect). Participants were genotyped for the TPH1 rs1799913, TPH2 rs4290270, and SLC6A4 5-HTTLPR variants. Repeated measures analysis of covariance (ANCOVA) was used to examine change in subjective effect scores over time while controlling for population structure.
Results
Participants carrying the TPH1 rs1799913 A allele reported greater subjective response to cocaine for ‘stimulated’ and ‘access’ relative to the CC genotype group. Those carrying the TPH2 rs4290270 A allele reported higher ‘good effect’ and lower ‘depressed’ effect relative to the TT genotype group. Those carrying the SLC6A4 5-HTTLPR S′ allele reported greater ‘desire’ and ‘access’ compared to the L′L′ genotype group.
Conclusions
These findings indicate that TPH1, TPH2, and SLC6A4 variants moderate the subjective effect of cocaine in non-treatment seeking cocaine-dependent participants.
Keywords: cocaine, tryptophan hydroxylase, genetics, serotonin, polymorphism, subjective, variant, substance use, TPH, transporter
Introduction
In the United States, over 38 million individuals greater than 11 years old reported cocaine use in their lifetime (SAMHSA, 2014). Several psychosocial and biological risk factors predict the trajectory from cocaine use to addiction including high impulsivity (Molander et al., 2011), changing method of drug administration (e.g., switching to smoking or intravenous injection; Gawin & Khalsa-Denison, 1996), and episodes of binging (Dackis & O’brien, 2001). Individuals with cocaine use disorder (CUD) are a heterogeneous population and up to 56% of the heritability and variance of their cocaine use may be due to genetic factors (Gelernter et al., 2014, Kendler et al., 2003, Tsuang et al., 1999). For example, a recent genome-wide association studies (GWAS) supports the role of genetic factors in vulnerability to develop cocaine dependence (Gelernter et al., 2014).
Our laboratory previously reported that the subjective effects of cocaine use are moderated, in part, by variants in the ankyrin repeat and kinase domain-containing 1 (ANKK1) (Spellicy et al., 2014), dopamine transporter (DAT1, SLC6A4), and serotonin transporter (SLC6A4) 5-HTTLPR (Liu et al., 2015) genes. Herein, we continue to investigate the relation between genetic variants and subjective effects of cocaine by exploring the role of the serotonergic system in the subjective effects of cocaine use in non-treatment seeking cocaine-dependent individuals.
Serotonin (5-hydroxytryptamine; 5-HT) is a neurotransmitter associated with psychobiological reward processes (Russo & Nestler, 2013). We previously have reviewed the role of the 5-HT system in substance use disorders, including tryptophan hydroxylase 1 (TPH1; rs1799913), tryptophan hydroxylase 2 (TPH2; rs4290270), and the serotonin transporter promoter (5-HTTLPR) (Bauer et al., 2015, Nielsen et al., 2012, Nielsen et al., 2008, Nielsen et al., 2014, Yuferov et al., 2010).
The biosynthesis of 5-HT relies on the conversion of tryptophan by TPH, the rate-limiting enzyme in the biosynthesis of serotonin (Cooper & Melcer, 1961). Specifically, after an individual consumes food containing tryptophan, tryptophan is absorbed by the intestine and transported by the blood to the brain, where it is transported across the blood-brain barrier and biotransformed into 5-HT. TPH is encoded by two separate genes, TPH1, located on chromosome 11p15.1, and TPH2, located on chromosome 12q21.1. Both TPH isozymes convert L-tryptophan via hydroxylation into 5-hydroxytryptophan (5-HTP), which is subsequently decarboxylated to 5-HT. TPH2 is expressed primarily in the raphe nuclei of the brain (Patel et al., 2004, Walther & Bader, 2003, Walther et al., 2003, Zill et al., 2004, Zill et al., 2007) whereas TPH1 is expressed mainly in the pineal gland, the raphe nuclei during late development, and the enterochromaffin cells of the gut (Nakamura et al., 2006, Zill et al., 2007).
The tightly linked variants (rs1799913 and rs1800532) in TPH1 (originally designated TPH prior to the discovery of TPH2) have been associated with behaviors including suicidality (González-Castro et al., 2014, Nielsen et al., 1994), alcoholism (Nielsen et al., 1998), impulsive behavior (Staner et al., 2002), and with cerebrospinal fluid (CSF) 5-hydroxyindoleacetic acid (5-HIAA) levels (Jönsson et al., 1997, Nielsen et al., 1994). Specifically, male TPH1 A allele carriers have been shown to have lower levels of serotonin production as measured by CSF 5-HIAA levels (Jönsson et al., 1997) and decreased risk for suicide attempt (González-Castro et al., 2014, Nielsen et al., 1994). TPH2 rs4290270 has been shown to have differential allelic expression in the cortex, hypothalamus, thalamus, hippocampus, amygdala, and cerebellum (Lim et al., 2007). The T allele is expressed at two times the level of the A allele in heterozygous subjects. The TT genotype may be related to the production of more serotonin compared to that in A allele carriers. TPH1 and TPH2 are expressed in several regions associated with reward processes (e.g., hippocampus, amygdala, frontal cortex; Russo & Nestler, 2013, Zill et al., 2004). Genetic variation in TPH1 and TPH2 may be related to altered serotonergic function and thus may impact reward processes (e.g., in the hippocampus, amygdala, frontal cortex) associated with the serotonergic system including effects of cocaine. Particularly, the low serotonin production associated with TPH1 A-allele and TPH2 A-allele carriers may increase an individual’s vulnerability to the subjective effects of cocaine.
A promoter variant in the promoter region of the serotonin transporter (5-HTT) gene (SLC6A4), 5-HTTLPR, alters the transcription and availability of 5-HT in humans (Lesch et al., 1996, Murphy et al., 2004). This polymorphism contains 16 or 14 repeats characterized as either the long (L) or short (S) form, respectively. The L allele typically has higher transcription activity than the S allele (Lesch et al., 1996), however, an A to G transition (rs25531) occurs in the L allele of 5-HTTLPR. The L allele containing the G transition codes for an allele (LG) with low transcriptional activity similar to that of the S allele (Hu et al., 2006, Praschak-Rieder et al., 2007). These two low transcription alleles (LG and S) are referred to as S′, while the LA allele, which has higher transcriptional activity, is referred to as L′. Recent findings suggest that individuals with the S′ allele may be more sensitive to the environment than those with the L′ L′ genotype (Fox et al., 2011, Graham et al., 2013). Specifically, S′ allele carriers appear more easily biased by environmental stimuli and more attuned to their perceived limitations.
We hypothesized that individuals who are carriers of alleles associated with lower serotonergic production (TPH1 A and TPH2 A alleles) or lower levels of serotonin transporter (5-HTTLPR S′ allele) would demonstrate more sensitivity to the positive and negative effects of cocaine (see Subjective Effects section below for description). Specifically: 1) participants who were carriers of the TPH1 rs1799913 A allele would have higher positive and lower negative subjective ratings to cocaine administration relative to participants with the CC genotype, 2) carriers of the TPH2 rs4290270 A allele would have higher positive and lower negative subjective ratings to cocaine administration compared to participants with the TT genotype, and 3) carriers of the S′ allele of 5-HTTLPR would have higher positive and lower negative subjective ratings to cocaine. Non-treatment seeking cocaine-dependent individuals enrolled in the present study were genotyped and completed self-report measures on the subjective effects of cocaine versus saline. TPH1, TPH2, and 5-HTTLPR genotype differences were examined on the self-reported subjective effects. Additionally, cardiovascular effects were investigated to examine differences between subjective and objective effects of cocaine.
Experimental Methods
Participants
Sixty-six participants between the ages of 18 and 55 were recruited from March 2010–July 2012 through an ongoing research trial at Baylor College of Medicine (see cohort details in prior publication; Brewer et al., 2015). Briefly, inclusion criteria for the study were that subjects: (1) gave informed consent; (2) had a negative pregnancy test for the women; (3) were administered the Mini-International Neuropsychiatric Interview (M.I.N.I.) (Sheehan et al., 1998) and met Diagnostic and Statistical Manual of Mental Disorders 4th Edition (DSM-IV-TR; Association, 2000) criteria for cocaine-dependence; and (4) were non-treatment seeking. Exclusion criteria included: (1) a history of head trauma, epilepsy, heart disease, acquired immunodeficiency syndrome (AIDS), asthma, or other serious medical conditions, (2) dependence on drugs other than cocaine or nicotine, (3) inability to sense the effects of cocaine, (4) presence of any other axis I psychiatric disorder, or (5) use of psychotropic medications or medications affecting blood pressure. All participants This study was approved by the Institutional Review Board of Baylor College of Medicine and the Research and Development committee of the Michael E. DeBakey Veteran Affairs Medical Center.
Subjective & Objective Effects
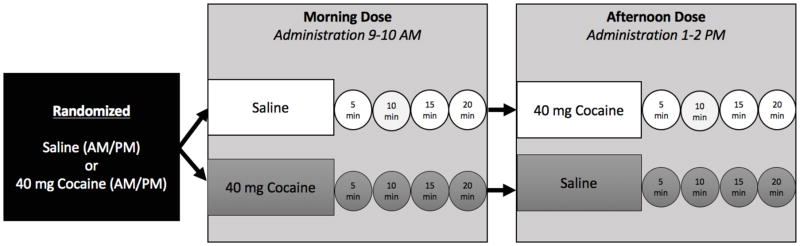
Congruent with previously published studies from our laboratory (Brewer et al., 2015, Spellicy et al., 2014), a double-blind, placebo-controlled, within-subjects experimental design was used. Participants were randomized and intravenously administered 0 mg (saline) or 40 mg of cocaine in the morning or afternoon. Each participant received either: 1) one morning dose (administered at approximately 9 AM or 10 AM) of saline and one afternoon dose of cocaine (administered at approximately 1 PM or 2 PM) or 2) one morning dose cocaine (at approximately 9 AM or 10 AM) and one afternoon dose of saline (at approximately 1 PM or 2 PM). Four hours separated the doses (i.e., patients received doses at 9 AM and 1 PM or 10 AM and 2 PM) to minimize any carry over effects. Participants rated baseline subjective effects fifteen minutes prior to receiving an infusion. Subjective effect ratings also were collected at 5, 10, 15, and 20 minutes post-infusion (see Figure 1). Participants rated subjective effects on a visual analog scale that ranged from 0 (“no effect”) to 100 (“most effect”). Positive subjective effects were: ‘high’ (“How high are you right now?”), ‘any drug effect’ (“Do you feel any drug effect right now?”), ‘stimulated’ (“How stimulated do you feel right now?”), ‘good effect’ (“Does the drug have any good effects right now?”), ‘desire’ (“How much do you desire the drug right now?”), ‘access’ (“If you had access to the drug right now how likely would you be to use it right now?”), and ‘like’ (“How much do you like the drug right now?”). Negative effects were: ‘bad effect’ (“Does the drug have any bad effects right now?”), ‘anxious’ (“How anxious do you feel right now?”), and ‘depressed’ (“How depressed do you feel right now?”). Heart rate and systolic and diastolic blood pressure were measured throughout using standard hospital equipment, GE Dash 3000 (GE Medical Systems, Milwaukee, WI). Cardiovascular effects were investigated to highlight differences between subjective and objective effects.
Figure 1.
Flow chart of challenge applied (squares) and timing of subjective effect ratings (circles).
Genotyping
DNA was extracted from participant’s blood using the Gentra Puregene blood kit (Qiagen, Germantown, MD) per manufacturer protocol. A researcher, who was blind to participant clinical status, determined the TPH1 rs1799913 and TPH2 rs4290270 genotypes in duplicate using 5′-fluorogenic exonuclease assays (TaqManÒ, Applied Biosystems, Foster City, CA). The TaqManÒ primer-probe sets ID C__2645661_10 was used to genotype TPH1 rs1799913 and the primer probe set C__26385365_10 for the TPH2 rs4290270 using the PlatinumÒ quantitative PCR SuperMix-UDG (Invitrogen, Carlsbad, CA) on a ViiA 7 (Applied Biosystems) in duplicate. Data analysis was conducted with ViiA 7 Software v1.1. The TPH1 rs1799913 variant is an A to C transversion with the A allele being the ancestral allele and the C allele the derived allele. The TPH2 rs4290270 variant is an A to T transition with the A allele being the ancestral allele and the T allele the derived allele. The 5-HTTLPR is a repeat of either 14 (short) or 16 (long) copies of a 22 base pair repeat in the promoter region of the SLC6A4 gene (Lesch et al., 1996). In the 14 copy repeat is rs25531, an T to C transition, when examined in the same orientation as the SLC6A4 gene. Both repeats are derived from a longer repeat region of 18 or 20 repeats found great apes, including chimpanzee, gorilla, and orangutan (Lesch et al., 1997). Negative controls were empty wells containing no DNA and positive controls were wells containing DNA from control samples that are standardized across our studies.
The serotonin transporter 5-HTTLPR was determined as described in Nielsen et al., 2012 (Nielsen et al., 2012). Briefly, the classic long “L” and short “S” alleles (rs4795541) (Lesch et al., 1996) were determined by PCR amplification. The L allele yields a 181 base pair (bp) fragment and the S allele a 138 bp fragment The internal variant rs25531 in the L allele was genotyped by digestion of the amplified DNA with HpaII (New England Biolabs, Ipswich, MA) (Hu et al., 2006). The “LG” (Stein et al., 2006) G containing allele is into 96 and 85 bp fragments, while the “LA” A containing allele remains undigested. The functionally similar LG and S alleles are designated as S′ and the higher transcriptional rate LA allele as L′.
Sex was confirmed by genotyping SRY (Kosten et al., 2013). Population structure was calculated by genotyping ten ancestry informative markers (AIMs). Data from the current participant sample were compared to the Centre d’Etude du Polymorphisme Humain–Human Genome Diversity Panel (CEPH-HGDP) samples (1,035 subjects of 51 populations) as described (Kosten et al., 2013). Previously, it has been demonstrated that 94.6% of the maximum informativity value is obtained using these ten AIMs (Lao et al., 2006).
Statistical analyses
R version 2.9.1 (R_Development_Core_Team, 2009) was used to conduct all statistical analyses. Participant’s subjective effect values were calculated by 1) subtracting baseline (−15 min) cocaine or saline values from all post-administration subjective effect values (normalization) and then 2) subtracting the normalized saline subjective effect values from the normalized cocaine subjective effect values. A dominant model was used for all statistical analysis. A repeated measures analysis of covariance (ANCOVA) was used to examine the change in subjective effects scores over time while controlling for population structure. Repeated measures ANCOVA was used to examine genotype differences between groups: TPH1 rs1799913 genotype (0 = CC genotype vs. 1 = AA/AC), TPH2 rs4290270 genotype (0 = TT genotype vs. 1 = AA/AT), 5-HTTLPR minor S′ allele (0 = L′L′ vs. 1 = L′S′/S′S′), and by genotype pattern (0 = AA/AC, AA/AT, L′S′/S′S′ vs. 1 = others). Between group (i.e., genotype) differences in demographic variables were analyzed using analysis of variance (ANOVA) and Fisher’s exact tests. Effect size was calculated as a partial eta-squared statistic using condition or polymorphism variance over residual variance and were compared to the established scale of small (η2 = .01), medium (η2 = .06), and large (η2 = .014) effects (Cohen, 1988). Note: although there were 52 statistical tests performed, the subjective and objective effect outcomes were highly correlated and dependent (r between .224–.924); therefore, correction for multiple hypothesis testing (e.g., Bonferroni) was not completed since these corrections are only valid when outcome variables are independent and uncorrelated (Blakesley et al., 2009).
Outside of the SRY assay and the ten AIMs, 27 variants have been examined for pharmacogenetic moderation of cocaine subjective effect with this cohort. As such, corrections for multiple testing were performed to evaluate experiment-wise significance (P <.05/27 = 0.0019) by applying a Bonferroni correction. Results presented below are the moderation analyses that demonstrated findings at P < 0.0019.
Results
Demographics
Between group (CC vs. AC/AA) differences were found for ethnicity (P = 0.029) and race (P = 0.006) for TPH1. An experiment-wise difference remained for race after using the Bonferroni correction. There was a point-wise difference with more Hispanic individuals having the TPH1 AA/AC, TPH2 AA/AT, 5-HTTLPR L′S′/S′S′ genotype pattern (P = 0.032) when compared to all other genotype groups, but this did not remain significant after Bonferroni correction. No point- or experiment-wise differences were found between TPH2 or 5-HTTLPR genotype groups for any of the demographic variables.
Cocaine use
Daily cocaine use was compared between those with the AA/AC, AA/AT, L′S′/S′S′ genotype pattern and those without this genotype pattern (Table 1). Greater cocaine daily use reported in the combined AA/AC, AA/AT, L′S′/ S′S′ genotype pattern group (P = 0.039; 3.3 grams ± 3.6 s.d.) compared to the other participants (1.9 grams ± 1.3 s.d.).
Table 1.
Demographic comparison between genotype groups.
|
TPH1
|
TPH2
|
5-HTTLPR
|
Genotype Pattern
|
|||||
|---|---|---|---|---|---|---|---|---|
| Genotype group | CC | AA/AC | TT | AA/AT | L′L′ | L′S′/S′S′ | AA/AC AA/AT L′S′/S′S′ |
Other |
| N | 45 | 21 | 15 | 51 | 23 | 43 | 14 | 52 |
| Male (%) | 78.8 | 81.0 | 80.0 | 80.4 | 69.6 | 86.0 | 85.71 | 78.85 |
| African American (%) | 84.4 | 47.6 | 53.3 | 78.4 | 73.9 | 72.1 | 64.29 | 75 |
| Caucasian (%) | 11.1 | 42.9 | 40.0 | 15.7 | 21.7 | 20.9 | 28.57 | 19.23 |
| Other (%) | 4.4 | 9.5a | 6.7 | 5.9 | 4.3 | 7.0 | 7.14 | 5.77 |
| Hispanic (%) | 4.4 | 23.8b | 20.0 | 7.8 | 4.3 | 14.0 | 28.57 | 5.77 c |
| Education, years (SD) | 12.7 (1.8) | 12.5 (1.9) | 12.3(1.8) | 12.7 (1.8) | 12.7 (1.9) | 12.6 (1.8) | 12.6 (2.0) | 12.6 (1.8) |
| Age, years (SD) | 43.8 (6.1) | 40.7 (8.2) | 43.9 (6.8) | 42.5 (7) | 43.0 (7.1) | 42.7 (6.9) | 40.3 (7.9) | 43.5 (6.6) |
| Weight, lbs. (SD) | 195.5 (35.4) | 180.2 (37.7) | 177.4 (30.7) | 192.8 (37.4) | 187.6 (34.6) | 190.2 (37.7) | 184.7 (42.4) | 190.5 (35.0) |
| Nicotine use, years (SD) | 21.7 (7.4) | 20.5 (9.6) | 20.8 (9.1) | 21.4 (8) | 19.8 (8.9) | 22.1 (7.7) | 20.4 (8.1) | 21.5 (8.2) |
| Daily cocaine use, grams (SD) d | 2.0 (1.4) | 2.8 (3.0) | 1.8 (1.4) | 2.3 (2.2) | 2.4 (1.5) | 2.2 (2.3) | 3.3 (3.6) | 1.9 (1.3) |
| Years of cocaine use (SD) | 17.8 (7.6) | 15.2 (7.5) | 16.4 (7.7) | 17.2 (7.7) | 17.0 (8.0) | 17.0 (7.5) | 15.4 (6.7) | 17.5 (7.9) |
| Past 30 days cocaine use, % (SD) | 19.1 (8.5) | 16.1 (6.5) | 19.5 (8.6) | 17.8 (7.9) | 20.2 (6.6) | 17.1 (8.5) | 17.6 (6.9) | 18.4 (8.3) |
Note.
P = 0.029
P = 0.006
P = 0.032
P = 0.039
Subjective Effects
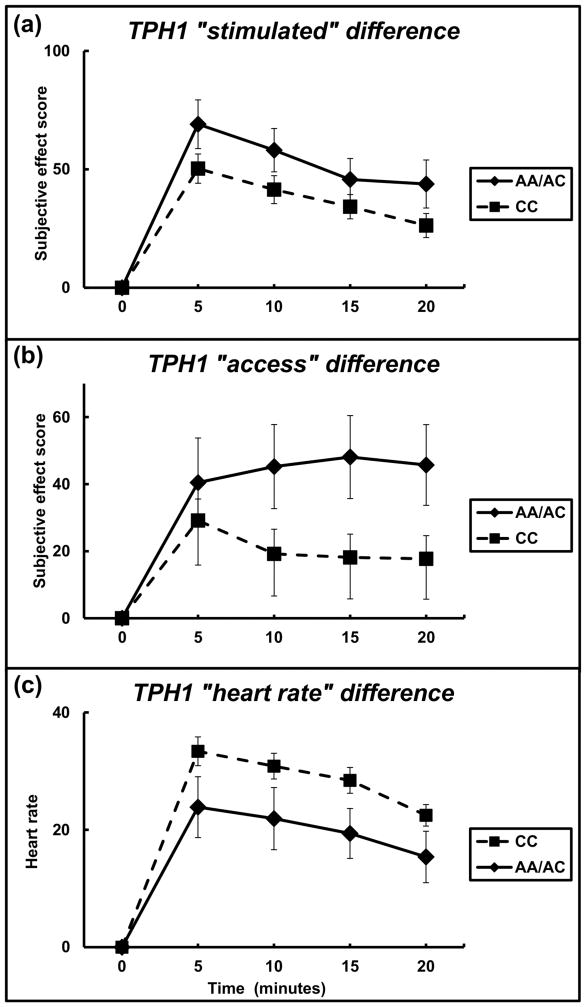
The role of TPH1 rs1799913 variant in moderating participants’ subjective effects to cocaine was evaluated. For the subjective score of ‘stimulated’ (for description of subjective effect measures please see Subjective Effects section in the methods above) there was a main effect of genotype group (F = 13.67; df = 1, 255; P < 0.001, with an effect size of 0.054) and time (F = 15.26; df = 1, 255; P < 0.001, with an effect size of 0.060) (Figure 2A). The AA/AC genotypes group (carriers of the A allele) demonstrated greater ‘stimulated’ across time. The largest difference between the genotype groups was at 5 minutes for ‘stimulated,’ where the AA/AC genotypes group had values of 69.1 ± 10.8 (s.e.m.) and the CC genotype group had values of 50.3 ± 6.2.
Figure 2.
Subjective effect scores by TPH1 genotype. (A) Change over time (in minutes) of participant-reported subjective effect of “stimulated” by AA/AC genotypes (n = 21) vs. CC genotype (n = 45) groups. (B) Change over time (in minutes) of participant-reported subjective effect of “access” by AA/AC genotypes vs. CC genotype groups (TPH1 rs1799913 minor T allele). Data reflect mean +/− S.E.M.
Regarding the subjective report of ‘access’, a main effect of genotype group (F = 17.57; df = 1, 255; P < 0.001 with an effect size of 0.069) was found (Figure 2B). The AA/AC genotypes group reported higher ‘access’ relative to the CC genotype group. The greatest difference between the genotype groups for ‘access’ was at 15 minutes where the AA/AC genotype group had values of 48.1 ± 12.4 and the CC genotype group had values of 18.3 ± 7.0.
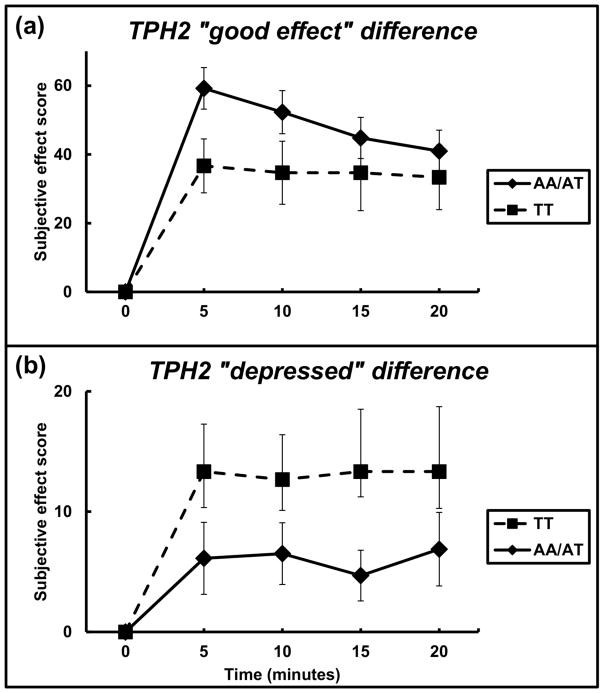
The TPH2 rs4290270 variant also was examined for its moderation of the subjective effects of cocaine. For the subjective score of ‘good effect’ there was a significant main effect of genotype group (F = 12.3; df = 1, 255; P = 0.001, with an effect size of 0.048) (Figure 3A). The AA/AT genotype group reported greater ‘good effect’ than the TT genotype. The largest difference between the genotype groups was at 5 minutes for ‘good effect’ where the AA/AT genotypes group had values of 59.2 ± 6.0 and the TT genotype group had values of 36.7 ± 7.8.
Figure 3.
Subjective effect scores by TPH2 genotype. (A) Change over time (in minutes) of participant-reported subjective effect of “good effect” by TT genotype (n = 15) versus AA/AT genotype (n = 51) groups. (B) Change over time (in minutes) of participant-reported subjective effect of “depressed” by TT genotype versus AA plus AT genotype groups (TPH2 rs4290270 minor A allele). Data reflect mean +/− S.E.M.
For the subjective scores of ‘depressed’ there was a main effect of genotype group (F = 10.40; df = 1, 255; P = 0.001, with an effect size of 0.041) (Figure 3B). Participants with the TT genotype reported a greater ‘depressed’ subjective effect than those carrying an A allele. The largest difference between the genotype groups for ‘depressed’ was at 15 minutes, where the AA/AT genotypes group had values of 4.7 ± 2.1 and the TT genotype group had values of 13.3 ± 5.2.
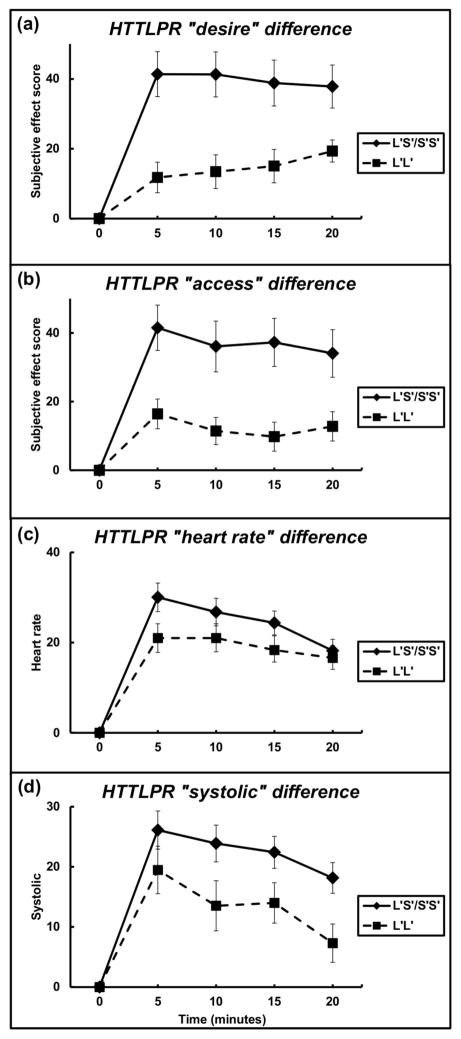
The subjective effects of cocaine were also examined for 5-HTTLPR. For the subjective scores of ‘desire’, there was a main effect of genotype (F = 17.98; df = 1, 255; P < 0.001, with an effect size of 0.070) (Figure 4A). The L′S′/S′S′ genotypes group reported higher ‘desire’ than the L′L′ genotype group. The difference between the genotype groups was the largest for ‘desire’ at 5 minutes into the trial where the L′S′/S′S′ genotypes group had values of 41.4 ± 6.4 and the L′L′ genotype group had values of 11.8 ± 4.4.
Figure 4.
Subjective effect scores by SLC6A4 genotype. (A) Change over time (in minutes) of participant-reported subjective effect of “desire” by the L′S′/S′S′ (n = 43) and the L′L′ genotype (n = 23) groups. (B) Change over time (in minutes) of participant-reported subjective effect of “access” by the L′S′/S′S′ and the L′L′ genotype groups (5-HTTLPR minor S′ allele). Data reflect mean +/− S.E.M.
Subjective effect scores of ‘access’ showed a main effect for genotype group (F = 14.62; df = 1, 255; P < 0.001, with an effect size of 0.060) (Figure 4B). The L′S′/S′S′ genotypes group reported higher ‘access’ than the L′L′ genotype group. Genotype groups reported the greatest difference for ‘access’ at 15 minutes where the L′S′/S′S′ genotypes group had values of 37.3 ± 7.0 and the L′L′ genotype group had values of 9.8 ± 4.2.
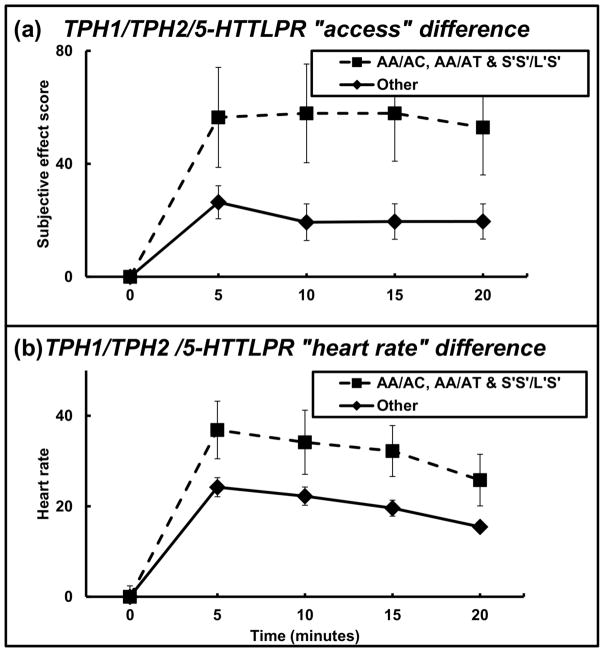
The subjective effects of cocaine were compared between the genotype pattern of those participants carrying a TPH1 A-, TPH2 A-, and 5-HTTLPR S′-alleles to those who were not TPH1 A, TPH2 A, and 5-HTTLPR S′ allele carriers (had either a TPH1 CC, TPH2 TT, or 5-HTTLPR L′L′ genotype). There was a main effect for the genotype pattern group for ‘access’ (F = 24.09; df = 1, 253; P < 0.001, with an effect size of 0.100) (Figure 5A), with the AA/AC, AA/AT, L′S′/S′S′ group self-reporting significantly higher ‘access.’ The largest difference between genotype groups was at 10 minutes where the genotype pattern AA/AC, AA/AT, L′S′/S′S′ group had values of 57.9 ± 17.5 compared to the other participants who had values of 19.3 ± 6.5.
Figure 5.
Subjective effect scores by genotype pattern. (A) Change over time (in minutes) of participant-reported subjective effect of “access” by the AA/AC (TPH1 rs1799913 minor T allele), AA/AT, and L′S′/S′S′ group (n = 14) and other participants (n = 52). (B) Change over time (in minutes) of participant-reported subjective effect of “heart rate” by the AA/AC, AA/AT, and L′S′/S′S′ group and “other” participants. Data reflect mean +/− S.E.M.
Objective Effects
A genotype group main effect was found (F = 13.17; df = 1, 255; P < 0.001 with an effect size of 0.051) for heart rate (Figure 2C). The CC genotypes group had higher heart rate relative to the AA/AC genotype group. Heart rate had the greatest group difference at 5 minutes where the AA/AC genotype group had values of 33.4 ± 5.2 and the CC genotype group had values of 23.9 ± 2.3.
A main effect of genotype (F = 4.47; df = 1, 255; P < 0.05, with an effect size of 0.020) was found for heart rate (Figure 4C). The L′S′/S′S′ genotype group had higher heart rate relative to the L′L′ group. Heart rate demonstrated the greatest difference between the genotype groups at 5 minutes where the L′S′/S′S′ genotypes group had values of 30.0 ± 3.2 and the L′L′ genotype group had values of 21.0 ± 3.2.
Systolic blood pressure also had a main effect of genotype (F = 15.45; df = 1, 255; P < 0.001, with an effect size of 0.060) (Figure 4D). The L′S′/S′S′ genotype group had higher systolic blood pressure relative to the L′L′ group. The largest difference between the genotype groups was systolic blood pressure at 20 minutes where the L′S′/S′S′ genotypes group had values of 18.1 ± 2.1 and the L′L′ genotype group had values of 7.3 ± 3.2.
There was a main effect for genotype group for heart rate (F = 16.39; df = 1, 253; P < 0.001, with an effect size of 0.060) (Figure 5B), with the AA/AC, AA/AT, L′S′/S′S′ genotype pattern group having higher heart rate. Heart rate differences were greatest between genotype groups at 5 minutes where the AA/AC, AA/AT, L′S′/ S′S′ group had values of 36.9 ± 6.4 and the other participants had values of 24.2 ± 2.4.
All other subjective and objective effect variables did not significantly differ by the aforementioned genotype groups and are not reported.
Discussion
The present study investigated the role of TPH1, TPH2, and SLC6A4 variants in the moderation of the subjective effect of cocaine in non-treatment seeking cocaine-dependent individuals. Our findings indicate that alleles associated with low serotonin production (TPH1 A, TPH2 A) or low serotonin transporter levels (5-HTTLPR S allele) are related to more positive self-reported subjective effects of cocaine post-cocaine administration.
The TPH1 AA/AC genotypes group reported greater subjective response to cocaine for ‘stimulated’ and ‘access’ relative to the CC genotype group, the TPH2 AA/AT genotypes group reported significantly more ‘good effect’ and less ‘depressed’ effect relative to the TT group, and the 5-HTTLPR L′S′/S′S′ genotypes group reported greater ‘desire’ and ‘access’ compared to the L′L′ genotype group. Further, when these alleles associated with low serotonin production or transporter levels were combined into one genotype pattern group (i.e., TPH1 AA/AC, TPH2 AA/AT, 5-HTTLPR L′S′/S′S′) and compared to those without these genotype patterns, the TPH1 A, TPH2 A, and 5-HTTLPR S′ allele carrier genotype pattern group demonstrated more positive subjective effects to cocaine regarding “access” than those without this genotype pattern. The positive subjective effects of the low serotonin genotype pattern group (AA/AC, AA/AT, L′S′/S′S′) was also associated with greater daily cocaine use. Physiological measurement of heart rate and blood pressure during the study indicated that the TPH1 AA/AC genotype groups had higher heart rate post-cocaine administration compared to the CC genotype group, the 5-HTTLPR L′S′/S′S′ genotype groups had higher heart rate and systolic blood pressure relative to the L′L′ genotype group, and the AA/AC, AA/AT, L′S′/S′S′ genotype pattern group had higher heart rate compared to the other participants.
Neurotransmitter and brain mechanisms related to reward processes help an individual make quick judgments regarding the aversive or rewarding aspects of a stimulus. The outcome of this cognitive processing (i.e., aversive or rewarding judgment) informs an individual’s future response to stimuli (for review see Schultz, 2011). Therefore it is not surprising that alterations of the serotonergic system have been associated with psychiatric diagnosis and symptomatology, including mood disorders, suicidality, impulsivity, and substance-related disorders (Mann, 1999). Cocaine has a particularly disruptive effect by inhibiting serotonin uptake (Han & Gu, 2006) and after long term exposure results in reduced 5-HT levels that increase cocaine-seeking and may maintain addictive behaviors (e.g., Kirby et al., 2011, Pelloux et al., 2012).
The lower serotonin levels associated with the TPH1 A and TPH2 A alleles may indicate an altered vulnerability to cocaine addiction due to their association with greater positive subjective effects of cocaine and increased daily cocaine use. In contrast, individuals without these alleles and thus higher basal serotonin levels may experience a “saturation” of serotonin. Further, serotonin levels are not able to increase upon using cocaine as much as in participants with lower baseline serotonin levels (i.e., carriers of the TPH1 A and TPH2 A); therefore, leading to less positive subjective effects of cocaine. Moreover, it is interesting that the participants with the AA/AC, AA/AT, L′S′/S′S′ genotype pattern group experienced more positive and less negative effects of cocaine, greater physiological response, and more daily cocaine use. These findings support prior literature that demonstrates that lower serotonin production associated with TPH1 A and TPH2 A is related to suicidality, alcoholism, and impulsive behavior (González-Castro et al., 2014, Nielsen et al., 1994, Nielsen et al., 1998, Slof-Op’t Landt et al., 2013, Staner et al., 2002). This indicates a potential genetic marker of vulnerability to cocaine addiction. It is possible that these genotypes, which are related to lower serotonin levels, and lower levels of the serotonin transporter drive increased cocaine-seeking and cocaine addiction (e.g., Kirby et al., 2011, Pelloux et al., 2012). Specifically, in the current study, these lower levels of serotonin and serotonin transporter are related to an increased likelihood that an individual would continue to use cocaine if they had access to it and to a greater physiological response (i.e., higher heart rate). Both this increased likelihood of continuing to use and being in the physiologically mobilized state (i.e., higher heart rate) may be mechanisms by which cocaine seeking and addiction are drive and contribute to this group’s higher daily use of cocaine. Participants with lower levels of serotonin and serotonin transporter may require less cocaine than individuals with higher levels of basal serotonin in order to feel similar subjective and objective (physiological) effects to cocaine use. Notably, the S′ allele of 5-HTTLPR also has been associated with response to treatment for cocaine dependence in prior studies (Nielsen et al., 2012). Additionally, it is possible that low serotonin and serotonin transporter levels reflect an increased sensitivity to impulsivity and to the positive subjective effects of cocaine (as well as other substances, like alcohol) and place an individual at greater risk for addiction.
In participants with low serotonin transporter levels, cocaine would saturate a higher proportion of transporter sites than in those participant’s with higher serotonin transporter levels. Hence, low amounts of cocaine would have a greater effect than in those individuals with low 5-HTT levels.
Limitations were present in this study. First, the combined AA/AC, AA/AT, L′S′/S′S′ genotype pattern group had a greater number of Hispanics then the group without the genotype pattern (although population structure was controlled for in all analyses). Secondly, we examined the subjective effects of only one dose of cocaine (40 mg). Thirdly, our sample size was small for a molecular genetic study; thus, replication of these findings will be needed to confirm these findings. Future studies could examine the subjective effects of several different doses a day (e.g., 0, 10, 20, and 40 mg; De La Garza et al., 2015).
In summary, we demonstrated that genetic variation in the 5-HT system accounts for differences in the subjective and physiological effects of cocaine in non-treatment-seeking cocaine-dependent individuals. We also demonstrated that specific serotonergic alleles were associated with higher daily cocaine use. These results suggest that the variants associated with low serotonin and transporter production could potentially be used as markers for individuals who may have a greater propensity for relapse – similar to when individuals are not as sensitive to the subjective effects of alcohol, then they are more likely to become addicted to alcohol (Schuckit, 1984, Schuckit et al., 2000). As such, these at-risk individuals may require intensive, personalized intervention in order to ensure maximal benefit.
Acknowledgments
Supported by: McNair Medical Institute (MP), NIH/NIDA DA023624 (RDLG), NIH/NIDA 5 P50 DA018197-05 (DN), through MD Anderson’s Cancer Center Support Grant NIH/NIDA DA026120 (DN), and the Toomim Family Fund (DN). This material is the result of work supported with resources and the use of facilities at the Michael E. DeBakey VA Medical Center, Houston, TX.
References
- American Psychiatric Association. Diagnostic and Statistic Manual of Mental Disorders (DSM-IV-TR) American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Bauer IE, Graham DP, Soares JC, Nielsen DA. Serotonergic gene variation in substance use pharmacotherapy: A systematic review. Pharmacogenomics. 2015;16:1305–1312. doi: 10.2217/pgs.15.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakesley RE, Mazumdar S, Dew MA, Houck PR, Tang G, Reynolds CF, III, Butters MA. Comparisons of methods for multiple hypothesis testing in neuropsychological research. Neuropsychology. 2009;23:255. doi: 10.1037/a0012850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer A, Nielsen DA, Spellicy CJ, Hamon SC, Gingrich J, Thompson-Lake DGY, Nielsen EM, Mahoney JJ, Kosten TR, Newton TF, De La Garza R. Genetic variation of the dopamine transporter (DAT1) influences the acute subjective responses to cocaine in volunteers with cocaine use disorders. Pharmacogenetics and genomics. 2015;25:296–304. doi: 10.1097/FPC.0000000000000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen . Statistical power analysis for the behavioral sciences. Erlbaum; Hillsdale, NJ: 1988. [Google Scholar]
- Cooper JR, Melcer I. The enzymic oxidation of tryptophan to 3-hydroxytryptophan in the biosynthesis of serotonin. Journal of Pharmacology and Experimental Therapeutics. 1961;132:265–268. [PubMed] [Google Scholar]
- Dackis CA, O’Brien CP. Cocaine dependence: A disease of the brain’s reward centers. Journal of Substance Abuse Treatment. 2001;21:111–117. doi: 10.1016/s0740-5472(01)00192-1. [DOI] [PubMed] [Google Scholar]
- De La Garza R, Bubar MJ, Carbone CL, Moeller FG, Newton TF, Anastasio NC, Harper TA, Ware DL, Fuller MA, Holstein GJ. Evaluation of the dopamine beta-hydroxylase (DβH) inhibitor nepicastat in participants who meet criteria for cocaine use disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2015;59:40–48. doi: 10.1016/j.pnpbp.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E, Zougkou K, Ridgewell A, Garner K. The serotonin transporter gene alters sensitivity to attention bias modification: evidence for a plasticity gene. Biological Psychiatry. 2011;70:1049–1054. doi: 10.1016/j.biopsych.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawin FH, Khalsa-Denison ME. Is craving mood-driven or self-propelled? Sensitization and “street” stimulant addiction. NIDA Research Monogrraph. 1996;163:224–250. [PubMed] [Google Scholar]
- Gelernter J, Sherva R, Koesterer R, Almasy L, Zhao H, Kranzler HR, Farrer L. Genome-wide association study of cocaine dependence and related traits: FAM53B identified as a risk gene. Molecular psychiatry. 2014;19:717–723. doi: 10.1038/mp.2013.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Castro TB, Juárez-Rojop I, López-Narváez ML, Tovilla-Zárate CA. Association of TPH-1 and TPH-2 gene polymorphisms with suicidal behavior: A systematic review and meta-analysis. BMC psychiatry. 2014;14:196. doi: 10.1186/1471-244X-14-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DP, Helmer DA, Harding MJ, Kosten TR, Petersen NJ, Nielsen DA. Serotonin transporter genotype and mild traumatic brain injury independently influence resilience and perception of limitations in veterans. Journal of Psychiatric Research. 2013;47:835–842. doi: 10.1016/j.jpsychires.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han DD, Gu HH. Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. BMC Pharmacology. 2006;6:6. doi: 10.1186/1471-2210-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, Xu K, Arnold PD, Richter MA, Kennedy JL, Murphy DL, Goldman D. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. American journal of human genetics. 2006;78:815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jönsson EG, Goldman D, Spurlock G, Gustavsson JP, Nielsen DA, Linnoila M, Owen MJ, Sedvall GC. Tryptophan hydroxylase and catechol-O-methyltransferase gene polymorphisms: relationships to monoamine metabolite concentrations in CSF of healthy volunteers. European Archives of Psychiatry and Clinical Neuroscience. 1997;247:297–302. doi: 10.1007/BF02922258. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Jacobson KC, Prescott CA, Neale MC. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. American Journal of Psychiatry. 2003;160:687–695. doi: 10.1176/appi.ajp.160.4.687. [DOI] [PubMed] [Google Scholar]
- Kirby L, Zeeb F, Winstanley C. Contributions of serotonin in addiction vulnerability. Neuropharmacology. 2011;61:421–432. doi: 10.1016/j.neuropharm.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, Domingo CB, Hamon SC, Nielsen DA. DBH gene as predictor of response in a cocaine vaccine clinical trial. Neurosci Lett. 2013;541:29–33. doi: 10.1016/j.neulet.2013.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao O, van Duijn K, Kersbergen P, de Knijff P, Kayser M. Proportioning whole-genome single-nucleotide-polymorphism diversity for the identification of geographic population structure and genetic ancestry. Am J Hum Genet. 2006;78:680–690. doi: 10.1086/501531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch K, Meyer J, Glatz K, Flügge G, Hinney A, Hebebrand J, Klauck S, Poustka A, Poustka F, Bengel D. The 5-HT transporter gene-linked polymorphic region (5-HTTLPR) in evolutionary perspective: Alternative biallelic variation in rhesus monkeys. Journal of Neural Transmission. 1997;104:1259–1266. doi: 10.1007/BF01294726. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lim JE, Pinsonneault J, Sadee W, Saffen D. Tryptophan hydroxylase 2 (TPH2) haplotypes predict levels of TPH2 mRNA expression in human pons. Molecular psychiatry. 2007;12:491–501. doi: 10.1038/sj.mp.4001923. [DOI] [PubMed] [Google Scholar]
- Liu S, Maili L, Lane SD, Schmitz JM, Spellicy CJ, Cunningham KA, Moeller FG, Nielsen DA. Serotonin transporter gene promoter polymorphism predicts relationship between years of cocaine use and impulsivity. Psychiatric Genetics. 2015;25:213–214. doi: 10.1097/YPG.0000000000000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann JJ. Role of the serotonergic system in the pathogenesis of major depression and suicidal behavior. Neuropsychopharmacology. 1999;21:99S–105S. doi: 10.1016/S0893-133X(99)00040-8. [DOI] [PubMed] [Google Scholar]
- Molander AC, Mar A, Norbury A, Steventon S, Moreno M, Caprioli D, Theobald DE, Belin D, Everitt BJ, Robbins TW. High impulsivity predicting vulnerability to cocaine addiction in rats: some relationship with novelty preference but not novelty reactivity, anxiety or stress. Psychopharmacology. 2011;215:721–731. doi: 10.1007/s00213-011-2167-x. [DOI] [PubMed] [Google Scholar]
- Murphy DL, Lerner A, Rudnick G, Lesch KP. Serotonin transporter: gene, genetic disorders, and pharmacogenetics. Molecular Interventions. 2004;4:109–123. doi: 10.1124/mi.4.2.8. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Sugawara Y, Sawabe K, Ohashi A, Tsurui H, Xiu Y, Ohtsuji M, Lin QS, Nishimura H, Hasegawa H. Late developmental stage-specific role of tryptophan hydroxylase 1 in brain serotonin levels. The Journal of Neuroscience. 2006;26:530–534. doi: 10.1523/JNEUROSCI.1835-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen D, Harding M, Hamon S, Huang W, Kosten T. Modifying the role of serotonergic 5-HTTLPR and TPH2 variants on disulfiram treatment of cocaine addiction: A preliminary study. Genes, Brain and Behavior. 2012;11:1001–1008. doi: 10.1111/j.1601-183X.2012.00839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen DA, Barral S, Proudnikov D, Kellogg S, Ho A, Ott J, Kreek MJ. TPH2 and TPH1: Association of variants and interactions with heroin addiction. Behavior Genetics. 2008;38:133–150. doi: 10.1007/s10519-007-9187-7. [DOI] [PubMed] [Google Scholar]
- Nielsen DA, Goldman D, Virkkunen M, Tokola R, Rawlings R, Linnoila M. Suicidality and 5-hydroxyindoleacetic acid concentration associated with a tryptophan hydroxylase polymorphism. Archives of General Psychiatry. 1994;51:34–38. doi: 10.1001/archpsyc.1994.03950010034005. [DOI] [PubMed] [Google Scholar]
- Nielsen DA, Nielsen EM, Dasari T, Spellicy CJ. Pharmacogenomics in Drug Discovery and Development. Springer; New York: 2014. Pharmacogenetics of addiction therapy; pp. 589–624. [DOI] [PubMed] [Google Scholar]
- Nielsen DA, Virkkunen M, Lappalainen J, Eggert M, Brown GL, Long JC, Goldman D, Linnoila M. A tryptophan hydroxylase gene marker for suicidality and alcoholism. Archives of General Psychiatry. 1998;55:593–602. doi: 10.1001/archpsyc.55.7.593. [DOI] [PubMed] [Google Scholar]
- Patel PD, Pontrello C, Burke S. Robust and tissue-specific expression of TPH2 versus TPH1 in rat raphe and pineal gland. Biological Psychiatry. 2004;55:428–433. doi: 10.1016/j.biopsych.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Pelloux Y, Dilleen R, Economidou D, Theobald D, Everitt BJ. Reduced forebrain serotonin transmission is causally involved in the development of compulsive cocaine seeking in rats. Neuropsychopharmacology. 2012;37:2505–2514. doi: 10.1038/npp.2012.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praschak-Rieder N, Kennedy J, Wilson AA, Hussey D, Boovariwala A, Willeit M, Ginovart N, Tharmalingam S, Masellis M, Houle S. Novel 5-HTTLPR allele associates with higher serotonin transporter binding in putamen: A [11 C] DASB positron emission tomography study. Biological Psychiatry. 2007;62:327–331. doi: 10.1016/j.biopsych.2006.09.022. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nature Reviews Neuroscience. 2013;14:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA. NSDUH Series H-41. Rockville, MD: 2014. Results from the 2013 National Survey on Drug Use and Health (NSDUH) [Google Scholar]
- Schuckit MA. Subjective responses to alcohol in sons of alcoholics and control subjects. Archives of General Psychiatry. 1984;41:879–884. doi: 10.1001/archpsyc.1984.01790200061008. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Kalmijn J, Tsuang J, Hesselbrock V, Bucholz K. Response to alcohol in daughters of alcoholics: a pilot study and a comparison with sons of alcoholics. Alcohol and Alcoholism. 2000;35:242–248. doi: 10.1093/alcalc/35.3.242. [DOI] [PubMed] [Google Scholar]
- Schultz W. Potential vulnerabilities of neuronal reward, risk, and decision mechanisms to addictive drugs. Neuron. 2011;69:603–617. doi: 10.1016/j.neuron.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Sheehan D, Lecrubier Y, Sheehan K, Amorim P, Janavs J, Weiller E, Herqueta T, Baker R, Dunbar G. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Slof-Op’t Landt MC, Bartels M, Middeldorp CM, van Beijsterveldt CE, Slagboom PE, Boomsma DI, van Furth EF, Meulenbelt I. Genetic variation at the TPH2 gene influences impulsivity in addition to eating disorders. Behavior Genetics. 2013;43:24–33. doi: 10.1007/s10519-012-9569-3. [DOI] [PubMed] [Google Scholar]
- Spellicy C, Harding M, Hamon S, Mahoney J, Reyes J, Kosten T, Newton T, De La Garza R, Nielsen D. A variant in ANKK1 modulates acute subjective effects of cocaine: A preliminary study. Genes, Brain and Behavior. 2014;13:559–564. doi: 10.1111/gbb.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staner L, Uyanik G, Correa H, Tremeau F, Monreal J, Crocq MA, Stefos G, Morris-Rosendahl DJ, Macher JP. A dimensional impulsive-aggressive phenotype is associated with the A218C polymorphism of the tryptophan hydroxylase gene: A pilot study in well-characterized impulsive inpatients. American Journal of Medical Genetics. 2002;114:553–557. doi: 10.1002/ajmg.10405. [DOI] [PubMed] [Google Scholar]
- Stein MB, Seedat S, Gelernter J. Serotonin transporter gene promoter polymorphism predicts SSRI response in generalized social anxiety disorder. Psychopharmacology (Berl) 2006;187:68–72. doi: 10.1007/s00213-006-0349-8. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Harley RM, Xian H, Eisen S, Goldberg J, True WR, Faraone SV. Genetic and environmental influences on transitions in drug use. Behavior Genetics. 1999;29:473–479. doi: 10.1023/a:1021635223370. [DOI] [PubMed] [Google Scholar]
- Walther DJ, Bader M. A unique central tryptophan hydroxylase isoform. Biochemical Pharmacology. 2003;66:1673–1680. doi: 10.1016/s0006-2952(03)00556-2. [DOI] [PubMed] [Google Scholar]
- Walther DJ, Peter JU, Bashammakh S, Hörtnagl H, Voits M, Fink H, Bader M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- Yuferov V, Levran O, Proudnikov D, Nielsen DA, Kreek MJ. Search for genetic markers and functional variants involved in the development of opiate and cocaine addiction and treatment. Annals of the New York Academy of Sciences. 2010;1187:184–207. doi: 10.1111/j.1749-6632.2009.05275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zill P, Büttner A, Eisenmenger W, Bondy B, Ackenheil M. Regional mRNA expression of a second tryptophan hydroxylase isoform in postmortem tissue samples of two human brains. European Neuropsychopharmacology. 2004;14:282–284. doi: 10.1016/j.euroneuro.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Zill P, Büttner A, Eisenmenger W, Möller HJ, Ackenheil M, Bondy B. Analysis of tryptophan hydroxylase I and II mRNA expression in the human brain: A post-mortem study. Journal of Psychiatric Research. 2007;41:168–173. doi: 10.1016/j.jpsychires.2005.05.004. [DOI] [PubMed] [Google Scholar]