Abstract
Intracellular compartmentalization and trafficking of molecules plays a critical role in complex and essential cellular processes. In lung cancer and other malignancies, aberrant nucleocytoplasmic transport of tumor suppressor proteins and cell cycle regulators results in tumorigenesis and inactivation of apoptosis. Pharmacologic targeting of this process, termed selective inhibition of nuclear export (SINE), has demonstrated anti-tumor efficacy in preclinical models and human clinical trials. Exportin-1 (XPO1)—which serves as the sole exporter of several tumor suppressor proteins and cell cycle regulators, including retinoblastoma (Rb), adenomatous polyposis coli (APC), p53, p73, p21, p27, FOXO, STAT3, IKB, topoisomerase II and PAR-4—is the principal focus of SINE drug development. The most extensively studied SINE to date, the XPO1 inhibitor selinexor (KPT-330; Karyopharm Therapeutics, Inc., Newton, MA), has demonstrated single-agent anticancer activity and synergistic effects in combination regimens against multiple cancer types, with principal toxicities of low-grade cytopenias and gastrointestinal effects. SINE may have particular relevance in KRAS-driven tumors, for which this treatment strategy demonstrates significant synthetic lethality. A multi-center phase 1/2 clinical trial of selinexor in previously treated advanced KRAS mutant non-small cell lung cancer is underway.
Keywords: Adenocarcinoma, Exportin-1, KRAS, Pathway, Selinexor, Targeted therapy, XP01
Intracellular compartmentalization and trafficking of molecules plays a critical role in complex and essential cellular processes. Aberrant nucleocytoplasmic transport of tumor suppressor proteins and cell cycle regulators—mediated by importins and exportins—can result in tumorigenesis and inactivation of apoptosis. Several malignancies, including lung cancer, feature over-expression of these nuclear transport receptors. Pharmacologic targeting of this process has demonstrated anti-tumor efficacy. In this review, we describe the mechanism, function, and therapeutic targeting of nuclear transport, with particular focus on application in lung cancer.
Nuclear export machinery
The nuclear envelope, comprising an inner and outer membrane, prevents the unrestricted diffusion of molecules larger than 40 kilodalton between the nucleus and the cytoplasm. This regulated nuclear-cytolasmic transport of proteins and other molecules plays a key role in cell functioning.1 Within the nuclear envelope, nuclear pore complexes provide an aqueous channel for the active transport of molecules. The karyopherin-B protein family, which includes both importins and exportins, facilitates transport across these nuclear pores.2 Cargo proteins destined for nuclear export have specific leucine-rich amino acid sequences known as nuclear export signals (NES), which are recognized by exportin proteins.3 Nuclear-cytoplasmic transport is an active process, requiring energy provided by RanGTP. A complex between the cargo protein, the exportin molecule, and RanGTP is formed and transported across the nuclear pore complex to the cytoplasm.4 In the cytoplasm, RanGTPase causes hydrolysis of the RanGTP, releasing and the cargo (which remains in the cytoplasm) and exportin protein (which is recycled back to the nucleus) (Figure 1A).5 At least 7 eukaryotic exportins have been identified (Table 1). While most of these are responsible for transport,6 Exportin-1 (XPO1, also known as chromosomal region maintenance 1, or CRM1) is a more ubiquitous receptor protein responsible for transporting approximately 220 proteins.7,8
Figure 1. Export through the nuclear pore complex.
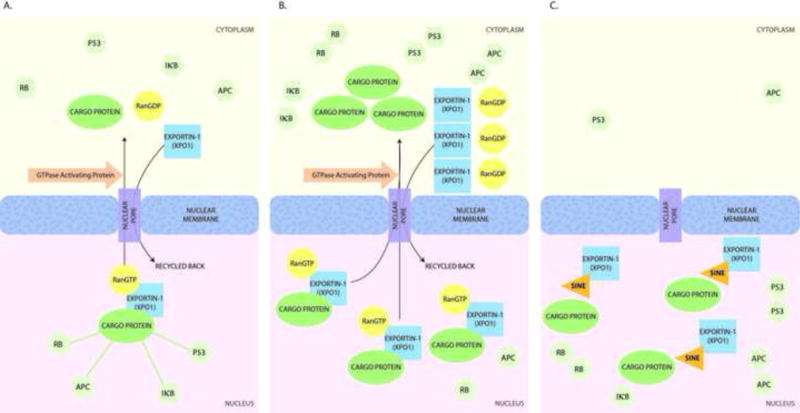
(A) Physiologic state. Export complexes containing Exportin-1, a cargo protein, and RanGTP are transported across the nuclear pore due to the RanGTP: RanGDP gradient. RanGTPase hydrolyzes RanGTP in the cytoplasm, leading to dissociation of the complex in the cytoplasm. (B) Cancer. Up-regulation of Exportin-1 results in dysregulated cytoplasmic transport of cell-cycle regulators, leading to their accumulation in the cytoplasm and inability to exert their effects. In turn, this state leads to aberrant growth signaling, inactivation of apoptosis, and tumor initiation and growth. (C) Pharmacologic inhibition. Selective Inhibitors of Nuclear Export (SINE) bind to Exportin-1 (XPO1) and prevent its interaction with cargo proteins, thereby inhibiting nuclear export. Cell-cycle regulators are retained in the nucleus, leading to growth inhibition.
Table 1.
Characteristics of exportin molecules
| Exportin protein | Alternative name | Chromosome | Cargo | Role in cancer | Reference |
|---|---|---|---|---|---|
| Exportin-1 (XPO1) | Chromosome region maintenance 1 (CRM1) protein | 2p15 | >200 macromolecules, including protein and RNA (including several tumor suppressor genes and cell cycle regulators, such as p53, p21, Rb, APC, FOXO) | Upregulated in multiple cancer types; associated with tumorigenesis and drug resistance | 8 |
| Exportin-2 | Cellular apoptosis susceptibility (CAS) protein, Chromosome segregation 1-like protein | 20q13.13 | Importin-alpha | Overexpressed in thyroid cancer | 23 |
| Exportin 3, (Exportin-T) | Karyopherin-beta, tRNA exportin | 12q14.2 | tRNA | – | 24 |
| Exportin-4 | KIAA1721, FLJ13046, | 13q12.11 | Broad substrate specificity including SMAD3, eIF5A | – | 25 |
| Exportin-5 | Ran-binding protein 21, KIAA1291 | 6p21.1 | Proteins bearing a double-stranded RNA binding domain and double-stranded RNAs, micro-RNA precursors, tRNA, eIF1A | Inactivating mutations associated with microsatellite instability | 26 |
| Exportin-6 | Ran-binding protein 20, KIAA0370, FLJ22519 | 16p11.2 | Actin | – | 27 |
| Exportin-7 | Ran-binding protein 16, KIAA0745 | 8p21.3 | eIF4A1, ARHGAP1, VPS26A, VPS29, VPS35 and SFN, p50RhoGAP | – | 28 |
APC, Adenomatous polyposis coli; ARHGAP1, Rho GTPase activating protein 1; eIF, eukaryotic translation initiation factor; FOXO, Forkhead box O; Rb, retinoblastoma; RNA, ribonucleic acid; SFN, Stratifin; SMAD3, Mothers against decapentaplegic homolog 3; tRNA, transfer RNA; VPS, Vacuolar protein sorting-associated protein
Role of nuclear export functions in normal cell physiology and cancer
XPO1 is the sole exporter of several tumor suppressor proteins and cell cycle regulators, including retinoblastoma (Rb), adenomatous polyposis coli (APC), p53, p73, p21, p27, FOXO, STAT3, IKB, topoisomerase II and PAR-4.9 Under physiological conditions, the regulated export of these molecules prevents their over-activity in the nucleus in the absence of oncogenic stimuli or DNA damage. In multiple cancer types, XPO1 overexpression leads to dysregulated export of these tumor suppressor proteins into the cytoplasm where they are unable to exercise their effects, thereby resulting in aberrant growth signaling, inactivation of apoptosis, and tumor initiation and growth (Figure 1B). XPO1 overexpression is also associated with drug resistance due to export of drug targets such as topoisomerase II and galectin-3.10,11
Nuclear export targeting
Given the critical role of nuclear export in cell cycle regulation and tumorigenesis, efforts to inhibit XPO1 pharmacologically have been undertaken. First generation XPO1 inhibitors include natural products such as leptomycin B (Table 1). Leptomycin B irreversibly alkylates an XPO1 cysteine residue (cysteine 528), preventing XPO1 binding to cargo protein nuclear export signals. This in turn leads to inhibition of export complex formation, as well as nuclear retention of tumor suppressor proteins (Figure 1C).12 Despite promising preclinical studies, strong dose-limiting toxicities (anorexia, nausea) and minimal clinical benefit in early studies limited development of leptomycin B.13 Newer pharmacological agents, termed selective inhibitors of nuclear export (SINE), reversibly bind the XPO1 cysteine 528 residue. To date, the most extensively studied SINE is selinexor (KPT-330; Karyopharm Therapeutics, Inc., Newton, MA). In multiple in vitro and in vivo models, selinexor has demonstrated single-agent anticancer activity and synergistic effects in combination regimens (Table 2). Globally, selinexor has been administered to more than 2,100 patients. Common adverse events include low-grade nausea (62%), fatigue (60%), anorexia (51%), thrombocytopenia (42%), and vomiting (37%), which have generally been readily managed with standard supportive care measures.
Table 2.
Inhibitors of nuclear export tested in humans
| Drug | Parent company | Molecular Target | Trial phase | Indication for human use | Clinical trials.gov Identifier(s) |
|---|---|---|---|---|---|
| First generation nuclear export inhibitor | |||||
| Leptomycin (also called elactocin) | Warner-Lambert | Exportin-1 | 1 | Advanced refractory solid cancers | |
| Second generation nuclear export inhibitors | |||||
| SL-801 | Stemline Therapeutics, Inc. | Exportin-1 | 1 | Advanced Solid Tumors | NCT02667873 |
| Selinexor (KPT-330) | Karyopharm Therapeutics, Inc | Exportin-1 | 2/3 | Acute myelogenous leukemia, myelodysplastic syndrome, T-cell lymphoma, B-cell lymphoma, chronic lymphocytic leukemia, multiple myeloma, glioblastoma, gynecological cancers, lung cancer, head and neck cancer, sarcoma, melanoma, breast cancer, prostate cancer, colon cancer, pancreatic cancer, gastric cancer, esophageal cancer, salivary gland tumors | (>50 trials total). Lung cancer trials: NCT02536495, NCT02351505, NCT03095612, NCT02250885, NCT02213133 |
| Verdinexor (KPT-335) | Karyopharm Therapeutics, Inc | Exportin-1 | 1 | Antiviral agent (approved for canine lymphoma) | NCT02431364 |
| KPT-8602 | Karyopharm Therapeutics, Inc | Exportin-1 | 1/2 | Relapsed refractory Multiple myeloma | NCT02649790 |
As monotherapy, selinexor has induced responses in hematologic malignancies and yielded disease control in solid tumors.14–16 In one study, 31% of evaluable patients had an objective response with use of selinexor across a spectrum of non-Hodgkin’s lymphoma subtypes, with a median duration of response exceeding 10 months.16 In another study, among 157 evaluable patients with advanced or metastatic solid tumors, single-agent selinexor resulted in an objective response rate of 4%, and a stable disease rate of 43%.17
Preclinical studies and clinical trials in lung cancer
XPO1 is overexpressed in lung cancer cells, particularly those arising in the setting of Nicotine-derived nitrosamine ketone (NNK, a tobacco carcinogen) exposure.18 Preclinical studies have demonstrated antitumor activity of SINEs in non-small cell lung cancer (NSCLC) cell lines and xenografts.19,20 SINEs have shown efficacy against epidermal growth factor receptor (EGFR) inhibitor-resistant NSCLC cell lines in a time- and dose- dependent manner.20 Synergism with chemotherapy and radiation therapy has been demonstrated in the presence of diverse molecular alterations, including EGFR, p53, RAS, and PIK3CA mutations.19,21
Efficacy against KRAS mutant lung adenocarcinoma, a disease setting lacking specific targeted therapies to date, appears particularly promising. In a multi-genomic screen of 4,700 biological processes in more than 100 human NSCLC cell lines, nuclear transport machinery emerged as the sole process exhibiting synthetic-lethal interactions in KRAS-driven cancers.22 In this study, the primary mechanism of cell kill was intolerance to nuclear accumulation of IкB with consequent inhibition of NFкB transcription activity. Rare cases (<20%) of intrinsic resistance were associated with FSTL5 mutations and attributed to YAP1 activation. With few exceptions, nuclear export inhibition had limited efficacy against KRAS wild type cell lines.
In summary, the broad genomic landscape in lung cancer makes it an attractive clinical setting for SINEs. To date, selinexor trials in advanced squamous cell lung cancer (NCT02536495) and relapsed small cell lung cancer (NCT02351505) have been initiated.17 A phase 1/2 trial in previously treated advanced KRAS mutant NSCLC is underway (NCT03095612).
Acknowledgments
None
Funding source: Funded in part by a National Cancer Institute (NCI) Midcareer Investigator Award in Patient-Oriented Research (K24CA201543-01; to DEG)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest/Disclosures: None
References
- 1.Schmidt HB, Gorlich D. Transport Selectivity of Nuclear Pores, Phase Separation, and Membraneless Organelles. Trends Biochem Sci. 2016;41(1):46–61. doi: 10.1016/j.tibs.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Ullman KS, Powers MA, Forbes DJ. Nuclear export receptors: from importin to exportin. Cell. 1997;90(6):967–970. doi: 10.1016/s0092-8674(00)80361-x. [DOI] [PubMed] [Google Scholar]
- 3.Dong X, Biswas A, Suel KE, et al. Structural basis for leucine-rich nuclear export signal recognition by CRM1. Nature. 2009;458(7242):1136–1141. doi: 10.1038/nature07975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monecke T, Guttler T, Neumann P, Dickmanns A, Gorlich D, Ficner R. Crystal structure of the nuclear export receptor CRM1 in complex with Snurportin1 and RanGTP. Science. 2009;324(5930):1087–1091. doi: 10.1126/science.1173388. [DOI] [PubMed] [Google Scholar]
- 5.Lui K, Huang Y. RanGTPase: A Key Regulator of Nucleocytoplasmic Trafficking. Mol Cell Pharmacol. 2009;1(3):148–156. doi: 10.4255/mcpharmacol.09.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guttler T, Gorlich D. Ran-dependent nuclear export mediators: a structural perspective. EMBO J. 2011;30(17):3457–3474. doi: 10.1038/emboj.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turner JG, Sullivan DM. CRM1-mediated nuclear export of proteins and drug resistance in cancer. Curr Med Chem. 2008;15(26):2648–2655. doi: 10.2174/092986708786242859. [DOI] [PubMed] [Google Scholar]
- 8.Stade K, Ford CS, Guthrie C, Weis K. Exportin 1 (Crm1p) is an essential nuclear export factor. Cell. 1997;90(6):1041–1050. doi: 10.1016/s0092-8674(00)80370-0. [DOI] [PubMed] [Google Scholar]
- 9.Fukuda M, Asano S, Nakamura T, et al. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature. 1997;390(6657):308–311. doi: 10.1038/36894. [DOI] [PubMed] [Google Scholar]
- 10.Takenaka Y, Fukumori T, Yoshii T, et al. Nuclear export of phosphorylated galectin-3 regulates its antiapoptotic activity in response to chemotherapeutic drugs. Mol Cell Biol. 2004;24(10):4395–4406. doi: 10.1128/MCB.24.10.4395-4406.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turner JG, Dawson J, Sullivan DM. Nuclear export of proteins and drug resistance in cancer. Biochem Pharmacol. 2012;83(8):1021–1032. doi: 10.1016/j.bcp.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meissner T, Krause E, Vinkemeier U. Ratjadone and leptomycin B block CRM1-dependent nuclear export by identical mechanisms. FEBS Lett. 2004;576(1–2):27–30. doi: 10.1016/j.febslet.2004.08.056. [DOI] [PubMed] [Google Scholar]
- 13.Newlands ES, Rustin GJ, Brampton MH. Phase I trial of elactocin. Br J Cancer. 1996;74(4):648–649. doi: 10.1038/bjc.1996.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garzon R, Savona M, Baz R, et al. A phase I clinical trial of single-agent selinexor in acute myeloid leukemia. Blood. 2017 doi: 10.1182/blood-2016-11-750158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gounder MM, Zer A, Tap WD, et al. Phase IB Study of Selinexor, a First-in-Class Inhibitor of Nuclear Export, in Patients With Advanced Refractory Bone or Soft Tissue Sarcoma. J Clin Oncol. 2016;34(26):3166–3174. doi: 10.1200/JCO.2016.67.6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuruvilla J, Savona M, Baz R, et al. Selective inhibition of nuclear export with selinexor in patients with non-Hodgkin’s lymphoma. Blood. 2017 doi: 10.1182/blood-2016-11-750174. [DOI] [PubMed] [Google Scholar]
- 17.Abdul Razak AR, Mau-Soerensen M, Gabrail NY, et al. First-in-Class, First-in-Human Phase I Study of Selinexor, a Selective Inhibitor of Nuclear Export, in Patients With Advanced Solid Tumors. J Clin Oncol. 2016;34(34):4142–4150. doi: 10.1200/JCO.2015.65.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao W, Lu C, Chen L, Keohavong P. Overexpression of CRM1: A Characteristic Feature in a Transformed Phenotype of Lung Carcinogenesis and a Molecular Target for Lung Cancer Adjuvant Therapy. J Thorac Oncol. 2015;10(5):815–825. doi: 10.1097/JTO.0000000000000485. [DOI] [PubMed] [Google Scholar]
- 19.Sun H, Hattori N, Chien W, et al. KPT-330 has antitumour activity against non-small cell lung cancer. Br J Cancer. 2014;111(2):281–291. doi: 10.1038/bjc.2014.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang S, Han X, Wang J, Yao J, Shi Y. Antitumor effects of a novel chromosome region maintenance 1 (CRM1) inhibitor on non-small cell lung cancer cells in vitro and in mouse tumor xenografts. PLoS One. 2014;9(3):e89848. doi: 10.1371/journal.pone.0089848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cromie MM, Gao W. Epigallocatechin-3-gallate enhances the therapeutic effects of leptomycin B on human lung cancer a549 cells. Oxid Med Cell Longev. 2015;2015:217304. doi: 10.1155/2015/217304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J, McMillan E, Kim HS, et al. XPO1-dependent nuclear export is a druggable vulnerability in KRAS-mutant lung cancer. Nature. 2016;538(7623):114–117. doi: 10.1038/nature19771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holzer K, Drucker E, Oliver S, et al. Cellular apoptosis susceptibility (CAS) is overexpressed in thyroid carcinoma and maintains tumor cell growth: A potential link to the BRAFV600E mutation. Int J Oncol. 2016;48(4):1679–1687. doi: 10.3892/ijo.2016.3388. [DOI] [PubMed] [Google Scholar]
- 24.Leisegang MS, Martin R, Ramirez AS, Bohnsack MT. Exportin t and Exportin 5: tRNA and miRNA biogenesis — and beyond. Biol Chem. 2012;393(7):599–604. doi: 10.1515/hsz-2012-0146. [DOI] [PubMed] [Google Scholar]
- 25.Kurisaki A, Kurisaki K, Kowanetz M, et al. The mechanism of nuclear export of Smad3 involves exportin 4 and Ran. Mol Cell Biol. 2006;26(4):1318–1332. doi: 10.1128/MCB.26.4.1318-1332.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melo SA, Moutinho C, Ropero S, et al. A genetic defect in exportin-5 traps precursor microRNAs in the nucleus of cancer cells. Cancer Cell. 2010;18(4):303–315. doi: 10.1016/j.ccr.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Stuven T, Hartmann E, Gorlich D. Exportin 6: a novel nuclear export receptor that is specific for profilin.actin complexes. EMBO J. 2003;22(21):5928–5940. doi: 10.1093/emboj/cdg565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mingot JM, Bohnsack MT, Jakle U, Gorlich D. Exportin 7 defines a novel general nuclear export pathway. EMBO J. 2004;23(16):3227–3236. doi: 10.1038/sj.emboj.7600338. [DOI] [PMC free article] [PubMed] [Google Scholar]


