Abstract
Young adults with psychiatric illnesses are more likely to use cannabis and experience problems from use. It is not known whether those with a lifetime psychiatric illness experience a prolonged cannabis withdrawal syndrome with abstinence. Participants were fifty young adults, aged 18–25, recruited from the Boston-area in 2015–2016, who used cannabis at least weekly, completed the Structured Clinical Interview for DSM-IV to identify Axis I psychiatric diagnoses (PD+ vs PD−), and attained cannabis abstinence with a four-week contingency management protocol. Withdrawal symptom severity was assessed at baseline and at four weekly abstinent visits using the Cannabis Withdrawal Scale. Cannabis dependence, age of initiation, and rate of abstinence were similar in PD+ and PD− groups. There was a diagnostic group by abstinent week interaction, suggesting a difference in time course for resolution of withdrawal symptoms by group, F(4,46)=3.8, p=0.009, controlling for sex, baseline depressive and anxiety symptoms, and frequency of cannabis use in the prior 90 days. In post hoc analyses, there was a difference in time-course of cannabis withdrawal. PD− had significantly reduced withdrawal symptom severity in abstinent week one [t(46)=−2.2, p=0.03], while PD+ did not report improved withdrawal symptoms until the second abstinent week [t(46) =−4.1, p=0.0002]. Cannabis withdrawal symptoms improved over four weeks in young people with and without a lifetime psychiatric diagnosis. However, those with a psychiatric illness reported one week delayed improvement in withdrawal symptom severity. Longer duration of cannabis withdrawal may be a risk factor for cannabis dependence and difficulty quitting.
Keywords: Marijuana, Cannabis, Mental Health, Cannabis Withdrawal Syndrome, Abstinence, Depression, Anxiety
Introduction
Cannabis use is common among young adults1, particularly among those with a current or lifetime psychiatric illness2–6. For example, national epidemiologic data indicate up to double the prevalence of cannabis use among those with a past year depressive episode versus those without7. This high prevalence of comorbidity may be clinically relevant as co-occurring mood and cannabis use disorders may complicate both substance use and mood disorder treatment.
The relationship between cannabis use and psychiatric illness is postulated to be bidirectional8. Cannabis use may precipitate or hasten the onset of psychotic9,10, depressive11,12, and anxiety disorders13. Cannabis use during adolescence and young adulthood has been associated with earlier onset14–17 and greater severity of mood symptoms18–20. Conversely, comorbid psychiatric illness may increase risk for problem cannabis use. Those with more severe depressive symptoms have been shown in longitudinal studies to have higher rates of subsequent cannabis use4,6, predicting an increase in frequency of weekly cannabis use by up to two-days per week from adolescence into young adulthood8.
Little is known about the association between lifetime psychiatric illness and the course of cannabis withdrawal symptom severity during the first weeks of cannabis abstinence. Cannabis withdrawal has been identified as a key criterion of cannabis use disorder21,22, impacting between 35% to 75% of adolescents and young adults with a use disorder attempting to reduce or discontinue use23. According to the DSM-V, the cannabis withdrawal syndrome involves the manifestation of three or more of the following six symptoms: irritability or anger; nervousness or anxiety; sleep difficulty (i.e., insomnia, disturbing dreams); decreased appetite or weight loss; restlessness; depressed mood; and at least one physical symptom causing discomfort (i.e., abdominal pain, shakiness/tremors, sweating, fever, chills, headache)24–26. Due to the long half-life of Δ9-tetrahydrocannabinol (THC) and its metabolites, the cannabis withdrawal syndrome may last for several days to weeks following last use. Importantly, the presence of withdrawal symptoms with abstinence has been found to be a marker for problematic cannabis use, predicting severity of use, rapid reinstatement of use during a quit attempt, and problems from use21,26,27 In a sample of adolescent cannabis users followed over one year, greater withdrawal symptom severity was associated with fewer days abstinent23
In a separate sample of 110 treatment-seeking emerging adults who were heavy cannabis users, those with significant cannabis withdrawal had a 53% greater risk of earlier resumption of cannabis use than those who did not report significant withdrawal symptoms28.
While associations have been reported between lifetime psychiatric diagnosis and cannabis use, and between cannabis withdrawal symptom severity and worse cannabis use outcomes, there are no reports to our knowledge of cannabis withdrawal trajectories by lifetime psychiatric diagnosis. It is plausible that those with a current or past psychiatric illness will have a more severe and prolonged cannabis withdrawal syndrome, as it is well known that psychiatric populations experience more intense withdrawal from other substances, such as nicotine29–33.
We investigated the time course and severity of cannabis withdrawal symptoms during four weeks of incentivized abstinence, and hypothesized that a lifetime psychiatric diagnosis would be associated with a slower rate of withdrawal symptom improvement among young adults who used cannabis at least weekly.
Methods
Participants
Eligible participants were otherwise healthy young adults, aged 18–25, who reported using cannabis at least weekly, recruited via peer referral and advertisements in the community that sought potential participants ‘who use marijuana and are between age 18 and 25.’ Inclusion criteria, determined via phone screen, included cannabis use in the week prior to the baseline visit, English language fluency, and willingness to stop using cannabis for 30 days. There was no requirement that potential participants wish to permanently discontinue cannabis use.
Assessments
At baseline, current and lifetime diagnoses of Axis I disorders were assessed with the Structured Clinical Interview for DSM-IV (SCID-IV)34. For this study, the lifetime psychiatric diagnosis group (PD+) was comprised of participants meeting diagnostic criteria for any current or lifetime Axis I disorder, with the exception that current or prior substance use disorder was not adequate for inclusion. Additional baseline assessments included the Wechsler Test of Adult Reading (WTAR)35 for predicted full-scale IQ, Mood and Anxiety Symptoms Questionnaire (MASQ)36, Cannabis Use Disorder Identification Test – Revised (CUDIT-R)37, and Alcohol Use Disorders Identification Test (AUDIT)38. Detailed interviews using a modified Timeline Followback method39 were conducted at baseline to approximate quantity and frequency of past 90-day cannabis and alcohol use.
At each study visit, cannabis use was assessed with a quantitative urine toxicology assessment and self-report40. Cannabis withdrawal symptom severity was assessed using the Cannabis Withdrawal Scale (CWS)26, a self-report assessment of severity of 19 cannabis withdrawal symptoms (e.g., headache, decreased appetite, irritability) over the past 24 hours using a 10-point Likert scale (0: Not at all to 10: Extremely). The total withdrawal intensity score was created by summing each item score to a maximum withdrawal intensity score of 190.
Intervention
This study was conducted between July and November 2016. A detailed description of procedures has been described previously40. All study procedures were approved by the Partners Healthcare Human Subjects Committee. Eligibility was confirmed and written informed consent was performed during this first in-person visit.
Participants were asked to arrive for the baseline assessment after overnight cannabis abstinence. Participants were also asked to refrain from using illicit drugs and alcohol on the day of all study visits. Following baseline assessments, participants began a contingency management (CM) program of financial incentives for four weeks of continuous abstinence40. Briefly, participants were enrolled in a four-week abstinence-based incentive program. Participants earned incentives based on a two-track system for attendance and abstinence, with escalating denominations for both attendance and abstinence to encourage study retention and achievement of longer periods of continuous abstinence. The first 35 participants earned $585 for 30 days of abstinence with full attendance ($405 for continuous abstinence and $180 for full attendance). Due to the overwhelming success of the CM paradigm at eliciting 30 days of cannabis abstinence, the payment schedule was reduced by approximately 30% for the final 15 participants ($315 for 30 days of continuous abstinence and $105 for full attendance). Incentives were distributed via reloadable credit cards through Clinical Trials Payer (CT Payer) on the day of the study visit for attendance and upon receipt of the quantitative urinalysis results confirming abstinence (described below).
Urine samples were shipped by overnight courier to Dominion Diagnostics (Kingstown, RI, USA) for quantitative assessment of concentration the 11-nor-9-carboxy-Δ9-tetrahydrocannabinol (THCCOOH) metabolite of THC, using liquid chromatography/tandem mass spectrometry (LC-MS/MS). Quantitative indices were tracked to determine if creatinine-adjusted THCCOOH levels decreased during the four-week monitoring period consistent with abstinence41, validated using a statistical model developed by Schwilke and colleagues42. Cannabis withdrawal rating data from participants who did not attain four weeks of abstinence were included in the analysis from visits with biochemically confirmed abstinence, and were excluded from the analysis for all visits subsequent to self-report or biochemical evidence for non-abstinence.
Analytic Approach
Data were analyzed from baseline and the four consecutive weekly post-baseline ‘abstinence’ assessments using Stata 13.1 and SAS 9.4. We inspected data for non-normal distribution and outliers, and performed rank-based nonparametric procedures (Mann-Whitney U tests, Spearman’s rank order correlations) when assumptions of normality were violated. A marginal model was conducted to detect longitudinal change in cannabis withdrawal intensity across four weeks of monitored abstinence. In the preplanned analysis, the following predictors were entered into the model: history of psychiatric diagnosis (dichotomous: PD+ versus PD−; between-subject effect), study week (range: 0–4; within-subject effect), the interaction of PD group and study week, and any baseline demographic, psychiatric or drug use factors that were different between PD groups. A significant interaction was followed by comparing adjusted means of withdrawal severity after controlling for baseline covariates, examining the effect of group at each study week as well as the pattern of change in withdrawal severity within each group. As exploratory analyses, correlations were conducted between levels of withdrawal intensity at the time point when PD groups were most different and parameters of cannabis use severity. Finally, sensitivity analyses using marginal models were conducted to evaluate the independent effect of covariates that differed between PD groups on level of cannabis withdrawal symptoms over time. Alpha was set at 0.05 for all statistical tests.
Results
Participant Characteristics
Forty-four of 50 (88%) participants maintained biochemically-confirmed continuous abstinence for the four-week CM protocol, 22 in the PD+ group and 22 in the PD− group. Data were included for all participants at all time points with verified abstinence (see Methods). Those in the PD+ group were more likely to be female and report greater baseline symptom severity on the MASQ Anxious Arousal and Anhedonic Depression subscales. Groups were otherwise comparable across assessed demographic, mood, and alcohol use indices, including frequency and amount of alcohol consumed in the 90 days prior to and during the intervention. The most frequent Axis I psychiatric diagnoses in the PD+ group were major depressive disorder (92%), generalized anxiety disorder (16%), posttraumatic stress disorder (12%), panic disorder (8%), and bulimia (8%). Thirty-two percent of the PD+ sample had more than one psychiatric diagnosis by SCID-IV criteria. Baseline characteristics of the samples are presented in the table.
Table 1.
Characteristics of Young Adult Cannabis Users Recruited in Boston from 2015 to 2016
| No Psychiatric Diagnosis (PD−; n=25) | Psychiatric Diagnosis (PD+; n=25) | p-value | |
|---|---|---|---|
|
Demographics
| |||
| Gender (n, (%) Female) | 7 (28) | 15 (60) | 0.02 |
| Age (years) | 20.9 (1.3) | 21.0 (1.8) | 0.79 |
| Education (years) | 14.5 (1.0) | 14.5 (1.6) | 0.45 |
| Race (%) | |||
| White | 68 | 64 | |
| Black | 20 | 4 | 0.22 |
| Asian | 4 | 4 | |
| More than One Race | 8 | 24 | |
| Other | 0 | 4 | |
| Ethnicity (n, (%) Hispanic) | 2 (8) | 3 (12) | 0.64 |
| IQ (WTAR) | 108.4 (8.4) | 107.2 (8.9) | 0.60 |
|
Psychiatric History | |||
| General Anxiety Symptom Subscale Score (MASQ) | 20 (6.1) | 21.8 (5.6) | 0.28 |
| Anxious Arousal Subscale Score (MASQ) | 22.8 (3.9) | 25.6 (5.2) | 0.03 |
| General Depressive Symptom Subscale Score (MASQ) | 22.4 (8.3) | 25.9 (7.8) | 0.13 |
| Anhedonic Depression Subscale Score (MASQ) | 52.3 (12.6) | 60.7 (12.8) | 0.02 |
| SCID-IV Psychiatric Diagnoses (Current/Lifetime; n) | |||
| Major Depressive Disorder | N/A | 1/23 | – |
| Bipolar I | N/A | 1/1 | – |
| Panic Disorder | N/A | 2/2 | – |
| Agoraphobia | N/A | 1/1 | – |
| Social Phobia | N/A | 0/0 | – |
| Specific Phobia | N/A | 0/1 | – |
| Obsessive Compulsive Disorder | N/A | 0/1 | – |
| Post Traumatic Stress Disorder | N/A | 1/3 | – |
| Generalized Anxiety Disorder (current only) | N/A | 4 | – |
| Anorexia | N/A | 0/0 | – |
| Bulimia | N/A | 1/2 | – |
| Psychotic Disorders | N/A | 0/0 | – |
| Co-Morbidity (n, (%) with 2+ SCID-IV Diagnoses) | N/A | 8 (32%) | – |
|
Alcohol Use | |||
| Age of Initiation (years) | 15.1 (1.9) | 14.9 (2.2) | 0.73 |
| Past 90 Day Alcohol Use | |||
| Days Alcohol Consumed | 27 (14.1) | 21.8 (11.5) | 0.15 |
| Drinks Consumed (Mdn, IQR) | 87.0 [70, 127] | 71.0 [48, 154] | 0.21 |
| Dependence Symptoms (AUDIT) | 9.2 (6.8) | 8.2 (4.7) | 0.53 |
| Alcohol Use During Intervention | |||
| Drinking Days in 1st Week/Overall | 3.0 (1.9)/10.1 (5.5) | 3.5 (2.6)/11.0 (5.8) | 0.46/0.57 |
|
Cannabis Use | |||
| Age of Initiation (First Use) | 15.9 (2.1) | 15.9 (1.7) | 1.00 |
| Past 90 Day Cannabis Use | |||
| Days Cannabis Consumed | 44.1 (22.5) | 63.5 (22.5) | 0.004 |
| Total Times Cannabis Consumed | 101.0 (77.9) | 159.7 (176.7) | 0.14 |
| Grams Consumed (Mdn, IQR) | 20.7 [7.8, 55.5] | 36.5 [11.6, 81.0] | 0.18 |
| Dependence Symptoms (CUDIT) | 14.0 (6.3) | 14.3 (4.5) | 0.82 |
| Days Since Last Use (Mdn, IQR) | 1 [1, 2] | 1 [1, 1] | 0.07 |
| 30-Day Abstinence (n, (%) Abstinent) | 22 (88%) | 22 (88%) | – |
Note: All values are means, standard deviations, unless otherwise noted; AUDIT, Alcohol Use Disorders Identification Test; CUDIT, Cannabis Use Disorder Identification Test; CN-THCCOOH, creatinine-adjusted THCCOOH levels; FSIQ, Wechsler Test of Adult Reading Full Scale IQ; IQR, Interquartile range; MASQ, Mood and Anxiety Symptom Questionnaire; Mdn, Median; MJ, cannabis; SCID-IV, Structured Clinical Interview for DSM-IV; TLFB, Timeline Followback (past 30 and 90 days).
Cannabis Use Severity
There was no group difference in reported age of cannabis initiation [15.96 (1.67) years for PD+, 15.96 (2.09) years for PD−; t=0, p=1.00) or number of symptoms of cannabis dependence [CUDIT scores: 14.32 (4.49) for +PD, 13.96 (6.29) for −PD; t=−0.23, p=0.82]. Those in the PD+ group reported using cannabis more frequently in the three months prior to enrollment [63.52 (22.53) days for PD+, 44.1 (22.5) days for PD−; t=−3.04, p=0.004].
Trajectories of Withdrawal during Four Weeks of Cannabis Abstinence
The figure illustrates the mean raw withdrawal scores by diagnostic group at each time point.
Figure 1.
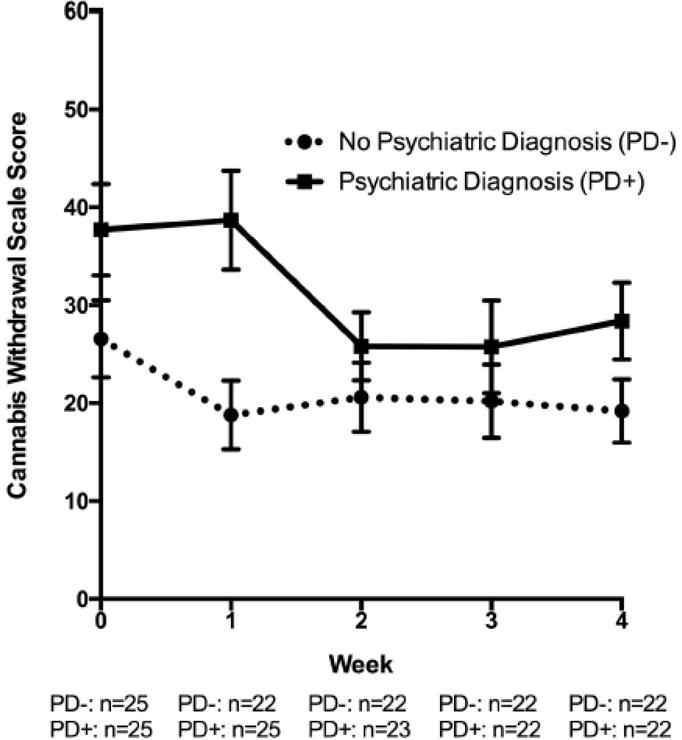
Weekly Ratings of Cannabis Withdrawal Intensity (Raw Scores) during Four Weeks of Verified Abstinence in Those with and without a Lifetime Psychiatric Illness from the Boston-Area in 2015/16
Note. All values represent raw means and standard errors.
After controlling for sex, self rating of anxious arousal and anhedonic depression, and past 90 day cannabis use frequency, there was a main effect of time (study week) [F(4,46) = 3.41, p = .02], but not PD group [F(1,46) = 0.09, p = 0.77] on cannabis withdrawal symptom severity. There was a significant PD group by time (study week) interaction [F(4,46) = 3.84, p = 0.009], suggesting that the group effect was modified by study week. Groups were not different at baseline and weeks 2, 3, and 4 [p-values >0.32], and there was a trend for the PD+ group to have higher withdrawal scores at week 1 [t(46)=1.87 p=0.07]. Additionally, there was significant reduction in withdrawal severity by 8.25 units from baseline to week 1 in the PD− group [t(46) = −2.24, p = 0.03], but no significant change from baseline to abstinent week 1 the PD+ group [t(46) = 0.27, p = 0.79]. From abstinent week 1 to 2, there was no significant reduction in withdrawal severity in the PD− group [t(46) = 0.83, p = 0.41], but there was significant reduction in withdrawal symptom severity score from week 1 to week 2 in the PD+ group [12.89 units; t(46) = −4.13, p = 0.0002]. Pairwise comparisons for withdrawal symptom severity in weeks 2 to 3 and 3 to 4 in each group were not significant [p-values > 0.48].
Exploratory correlation analyses suggested that withdrawal symptom intensity at one week of abstinence, when PD groups were most different, was positively associated with number of symptoms of cannabis dependence [ρ=0.38, p=0.009], frequency of baseline cannabis use [ρ=0.34, p=0.02], and there was a trend for a negative association with age of first cannabis use [ρ=−0.27, p=0.06]. There was no association between withdrawal symptom intensity at week 1 and amount of cannabis used in the 90 days prior to baseline [ρ=0.23, p=0.12].
Sensitivity Analyses
More frequent cannabis use and more severe self-rated symptoms of anhedonic depression prior to abstinence were associated with more severe withdrawal in the full sample [frequency of cannabis use F(1,48) = 4.41, p = 0.04; anhedonic depression symptoms: F(1,48) = 12.24, p = 0.001]. However, neither the effect of frequency of cannabis use nor depressive symptoms was modified by time [cannabis use frequency by time interaction: F(4,48) = 1.06, p = 0.39; anhedonic depression by time interaction: F(4,48) = 0.53, p = 0.71], suggesting that neither explained why withdrawal symptoms were changing differently over time among ±PD groups. Sex was neither associated with withdrawal severity [F(1,48) = 0.49, p = 0.49], nor time-course [F(4,48) = 0.46, p = 0.76]. In contrast, more severe baseline anxious arousal was associated with more symptoms of cannabis withdrawal [F(1,48) = 11.48, p = 0.001], and this effect varied with time [F(4,48) = 3.72, p = 0.01]. At baseline, each unit increase in anxious arousal severity predicted a 2.25 unit increase in cannabis withdrawal symptom severity at baseline [t(48)=3.92, p=0.0003], and this effect remained significant at weeks 1 and 2 [week 1 effect size=2.34, t(48)=3.67, p=0.0007; week 2 effect size=1.91, t(48)=4.50, p=<0.0001]. The effects of anxious arousal on cannabis withdrawal were not significant at weeks 3 and 4 [effect size= 0.54, t(48) = 0.83, p = 0.41 and effect size= 0.56, t(48) = 1.02, p = 0.31, respectively].
Discussion
Though the prevalence of psychiatric illness among young adult regular cannabis users is high, little is known about the impact of psychiatric illness on the time-course of the cannabis withdrawal syndrome. In this sample of 50 young adults who use cannabis regularly, lifetime psychiatric illness was associated with more persistent cannabis withdrawal symptoms during the first weeks of cannabis abstinence. We observed a protracted course of resolution of withdrawal symptoms during cannabis abstinence by one week in those with a current or lifetime psychiatric diagnosis. A protracted withdrawal syndrome by one week may be one factor accounting for differences in cannabis use prevalence, severity, and difficulty quitting among those with a lifetime psychiatric diagnosis.
Findings from this study replicate prior work suggesting that psychiatric diagnosis is associated with several indicators of greater severity of cannabis use4,6,8. In our sample, psychiatric history was associated with more frequent and heavier cannabis use in the prior 90 days, but unlike other studies27,43,44, not with earlier onset of use or more symptoms of a cannabis use disorder.
Consistent with our hypothesis, we found a shift in time to resolution of cannabis withdrawal symptoms, such that those with a psychiatric history required an additional week of abstinence before withdrawal symptoms significantly abated. Importantly, this effect remained after controlling for several potential confounds. Although frequency of cannabis use and anhedonic depression predicted level of withdrawal, their impact was constant over time. Therefore it is unlikely that these factors accounted for the differential rate of resolution of withdrawal symptoms by diagnostic group. Interestingly, severity of cannabis withdrawal after one week of abstinence was associated with more cannabis dependence symptoms, more frequent cannabis use, and earlier age of cannabis use initiation.
There are several potential explanations for the finding of more persistent cannabis withdrawal among young adult cannabis users with psychiatric illness. Young adults with psychiatric illness may be more likely to experience, or are more sensitive to, the symptoms of cannabis withdrawal, which overlap with symptoms of many psychiatric illnesses. This may be due to shared influences on the endocannabinoid system45–47, and may be particularly apparent in adolescence and young adulthood when the endocannabinoid system is maturing48. Despite the fact that only 16% of those in the PD+ group met criteria for a current Axis I diagnosis, it is also possible that those young adults with a lifetime psychiatric illness endorse greater withdrawal symptoms due to sub-syndromal current illness. This is supported by the finding that baseline anxiety independently predicted withdrawal in the first two weeks of abstinence. However, purely non-specific symptoms would not be expected to resolve after two weeks of cannabis abstinence.
The present results underscore the need to take into account co-morbidities when assessing cannabis withdrawal and calls for further work investigating the extent of symptomatic overlap between cannabis withdrawal and various psychiatric diagnoses. Yet these study results should be interpreted in light of the study limitations. The sample size was modest, hampering our ability to detect small effects, to detect the impact of specific withdrawal symptoms on the time course of resolution of total withdrawal scores (e.g., negative affect and sleep disruption49), and to evaluate potential moderators such as differential effects on withdrawal by sex50. The study also involved young adults, and generalizability to older or younger cannabis using populations is not known. Additionally, many prior studies on cannabis withdrawal investigated treatment-seeking cannabis users with more frequent, heavier and earlier initiation of use than the average participant enrolled in the current trial. It is therefore possible that patterns of withdrawal by psychiatric history may differ for more severe patient populations23, 50–51. Another noteworthy limitation is that, although the time since last cannabis use was within one week of the baseline visit, it was not standardized beyond that parameter. However, there was not a group difference in duration of abstinence at baseline, and therefore it is unlikely that time since last cannabis use accounted for the differential time-course of cannabis withdrawal observed. Finally, the average score on the CWS after four weeks of abstinence was not zero, and therefore it is not known whether these remaining low-level symptoms represent a full return-to-baseline. The scale items are non-specific and may reflect normal variability in daily functioning, sub-threshold symptoms common among people with a lifetime psychiatric diagnosis, or persistent withdrawal. This would be clarified by future studies that prospectively examine psychiatric history and cannabis withdrawal during regular use, acute deprivation, short-term sustained abstinence, and longer-term abstinence.
In summary, notwithstanding the above-mentioned limitations, this study supports the potential clinical significance of the presence of lifetime Axis I illness on the duration of the cannabis withdrawal syndrome among young adults, and has implications for prevention programming.52 As it is plausible that prolonged cannabis withdrawal may reduce success rates of abstinence attempts and increase relapse, and this may differentially impact those with a lifetime psychiatric illness, tertiary prevention interventions may help individuals identify strategies to cope with withdrawal in order to promote sustained cannabis abstinence. Indicated prevention approaches targeting youth with a psychiatric history prior to cannabis debut may be effective in reducing the prevalence of cannabis use and its associated consequences. Finally, universal prevention including behavioral health programs promoting emotional resiliency in all youth may have a downstream impact on cannabis use outcomes. These programs may preferentially target emotional risk factors such as anxious arousal, which was found to be uniquely associated with cannabis withdrawal in this sample and has been linked with severity of withdrawal from other substances including nicotine53–55. Particularly as more states are moving toward legalizing commercial markets for cannabis, it is both critical and timely to delineate potential risk factors for problematic cannabis use among the young adults most likely to use the drug and use the drug heavily, as well as develop interventions for those with or at risk for adverse cannabis use outcomes.
Highlights.
History of psychiatric illness was associated with more frequent cannabis use.
Cannabis users with psychiatric illness experienced more protracted withdrawal.
Early withdrawal intensity was associated with indicators of cannabis use severity.
Baseline anxiety independently also predicted withdrawal in early abstinence.
Acknowledgments
This publication was made possible by support from 1K23DA042946 (Schuster); 1K01DA034093 (Jodi Gilman); K24 DA030443 (Evins), the Norman E. Zinberg Fellowship in Addiction Psychiatry and Livingston Fellowship from Harvard Medical School (Schuster), and by the Louis V. Gerstner III Research Scholar Award (Schuster).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Substance Abuse and Mental Health Services Administration (SAMHSA) Results from the 2013 National Survey on Drug Use and Health: Summary of national findings. Rockville, MD: SAMHSA; 2014. [Google Scholar]
- 2.Buckner JD, Schmidt NB, Lang AR, Small JW, Schlauch RC, Lewinsohn PM. Specificity of social anxiety disorder as a risk factor for alcohol and cannabis dependence. J Psychiatr Res. 2008;42(3):230–239. doi: 10.1016/j.jpsychires.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feingold D, Weiser M, Rehm J, Lev-Ran S. The association between cannabis use and mood disorders: A longitudinal study. J Affect Disord. 2015;172:211–218. doi: 10.1016/j.jad.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Hooshmand S, Willoughby T, Good M. Does the direction of effects in the association between depressive symptoms and health-risk behaviors differ by behavior? A longitudinal study across the high school years. J Adolesc Health. 2012;50(2):140–147. doi: 10.1016/j.jadohealth.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 5.Marmorstein NR, White HR, Loeber R, Stouthamer-Loeber M. Anxiety as a predictor of age at first use of substances and progression to substance use problems among boys. J Abnorm Child Psychol. 2010;38(2):211–224. doi: 10.1007/s10802-009-9360-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wittchen HU, Frohlich C, Behrendt S, et al. Cannabis use and cannabis use disorders and their relationship to mental disorders: A 10-year prospective-longitudinal community study in adolescents. Drug Alcohol Depend. 2007;88(Suppl 1):S60–70. doi: 10.1016/j.drugalcdep.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 7.SAMHSA. Results from the 2015 National Survey on Drug Use and Health: Detailed tables. Rockville, MD: SAMHSA; 2016. [Google Scholar]
- 8.Wilkinson AL, Halpern CT, Herring AH, et al. Testing longitudinal relationships between binge drinking, marijuana use, and depressive symptoms and moderation by sex. J Adolesc Health. 2016;59(6):681–687. doi: 10.1016/j.jadohealth.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radhakrishnan R, Wilkinson ST, D’Souza DC. Gone to pot: A review of the association between cannabis and psychosis. Front Psychiatry. 2014;5:54. doi: 10.3389/fpsyt.2014.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sevy S, Robinson DG, Napolitano B, et al. Are cannabis use disorders associated with an earlier age at onset of psychosis? A study in first episode schizophrenia. Schizophr Res. 2010;120(1–3):101–107. doi: 10.1016/j.schres.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gage SH, Hickman M, Heron J, et al. Associations of cannabis and cigarette use with depression and anxiety at age 18: Findings from the Avon Longitudinal Study of Parents and Children. PLoS One. 2015;10(4):e0122896. doi: 10.1371/journal.pone.0122896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marmorstein NR, Iacono WG. Explaining associations between cannabis use disorders in adolescence and later major depression: A test of the psychosocial failure model. Addict Behav. 2011;36(7):773–776. doi: 10.1016/j.addbeh.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kedzior KK, Laeber LT. A positive association between anxiety disorders and cannabis use or cannabis use disorders in the general population: A meta-analysis of 31 studies. BMC Psychiatry. 2014;14:136. doi: 10.1186/1471-244X-14-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henquet C, Krabbendam L, de Graaf R, ten Have M, van Os J. Cannabis use and expression of mania in the general population. J Affect Disord. 2006;95(1– 3):103–110. doi: 10.1016/j.jad.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Lagerberg TV, Sundet K, Aminoff SR, et al. Excessive cannabis use is associated with earlier age at onset in bipolar disorder. Eur Arch Psychiatry Clin Neurosci. 2011;261(6):397–405. doi: 10.1007/s00406-011-0188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patton GC, Coffey C, Carlin JB, Degenhardt L, Lynskey M, Hall W. Cannabis use and mental health in young people: Cohort study. BMJ. 2002;325(7374):1195–1198. doi: 10.1136/bmj.325.7374.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Laar M, van Dorsselaer S, Monshouwer K, de Graaf R. Does cannabis use predict the first incidence of mood and anxiety disorders in the adult population? Addiction. 2007;102(8):1251–1260. doi: 10.1111/j.1360-0443.2007.01875.x. [DOI] [PubMed] [Google Scholar]
- 18.Wright NE, Scerpella D, Lisdahl KM. Marijuana use is associated with behavioral approach and depressive symptoms in adolescents and emerging adults. PLoS One. 2016;11(11):e0166005. doi: 10.1371/journal.pone.0166005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medina KL, Shear PK. Anxiety, depression, and behavioral symptoms of executive dysfunction in ecstasy users: Contributions of polydrug use. Drug Alcohol Depend. 2007;87(2–3):303–311. doi: 10.1016/j.drugalcdep.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubino T, Zamberletti E, Parolaro D. Adolescent exposure to cannabis as a risk factor for psychiatric disorders. J Psychopharmacol. 2012;26(1):177–188. doi: 10.1177/0269881111405362. [DOI] [PubMed] [Google Scholar]
- 21.Cornelius JR, Chung T, Martin C, Wood DS, Clark DB. Cannabis withdrawal is common among treatment-seeking adolescents with cannabis dependence and major depression, and is associated with rapid relapse to dependence. Addict Behav. 2008;33(11):1500–1505. doi: 10.1016/j.addbeh.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nocon A, Wittchen HU, Pfister H, Zimmermann P, Lieb R. Dependence symptoms in young cannabis users? A prospective epidemiological study. J Psychiatr Res. 2006;40(5):394–403. doi: 10.1016/j.jpsychires.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 23.Greene MC, Kelly JF. The prevalence of cannabis withdrawal and its influence on adolescents’ treatment response and outcomes: A 12-month prospective investigation. J Addict Med. 2014;8(5):359–367. doi: 10.1097/ADM.0000000000000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milin R, Manion I, Dare G, Walker S. Prospective assessment of cannabis withdrawal in adolescents with cannabis dependence: A pilot study. J Am Acad Child Adolesc Psychiatry. 2008;47(2):174–178. doi: 10.1097/chi.0b013e31815cdd73. [DOI] [PubMed] [Google Scholar]
- 25.Vandrey RG, Budney AJ, Hughes JR, Liguori A. A within-subject comparison of withdrawal symptoms during abstinence from cannabis, tobacco, and both substances. Drug Alcohol Depend. 2008;92(1–3):48–54. doi: 10.1016/j.drugalcdep.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allsop DJ, Norberg MM, Copeland J, Fu S, Budney AJ. The Cannabis Withdrawal Scale development: patterns and predictors of cannabis withdrawal and distress. Drug Alcohol Depend. 2011;119(1–2):123–129. doi: 10.1016/j.drugalcdep.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Budney AJ, Vandrey RG, Hughes JR, Thostenson JD, Bursac Z. Comparison of cannabis and tobacco withdrawal: Severity and contribution to relapse. J Subst Abuse Treat. 2008;35(4):362–368. doi: 10.1016/j.jsat.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis JP, Smith DC, Morphew JW, Lei X, Zhang S. Cannabis withdrawal, posttreatment abstinence, and days to first cannabis use among emerging adults in substance use treatment: A prospective study. J Drug Issues. 2016;46(1):64–83. doi: 10.1177/0022042615616431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gray KM, Baker NL, Carpenter MJ, Lewis AL, Upadhyaya HP. Attention-deficit/hyperactivity disorder confounds nicotine withdrawal self-report in adolescent smokers. Am J Addict. 2010;19(4):325–331. doi: 10.1111/j.1521-0391.2010.00048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pomerleau CS, Marks JL, Pomerleau OF. Who gets what symptom? Effects of psychiatric cofactors and nicotine dependence on patterns of smoking withdrawal symptomatology. Nicotine Tob Res. 2000;2(3):275–280. doi: 10.1080/14622200050147547. [DOI] [PubMed] [Google Scholar]
- 31.Reid HH, Ledgerwood DM. Depressive symptoms affect changes in nicotine withdrawal and smoking urges throughout smoking cessation treatment: Preliminary results. Addict Res Theory. 2016;24(1):48–53. doi: 10.3109/16066359.2015.1060967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soyster P, Anzai NE, Fromont SC, Prochaska JJ. Correlates of nicotine withdrawal severity in smokers during a smoke-free psychiatric hospitalization. Prev Med. 2016;92:176–182. doi: 10.1016/j.ypmed.2016.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xian H, Scherrer JF, Madden PA, et al. Latent class typology of nicotine withdrawal: genetic contributions and association with failed smoking cessation and psychiatric disorders. Psychol Med. 2005;35(3):409–419. doi: 10.1017/s0033291704003289. [DOI] [PubMed] [Google Scholar]
- 34.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-Patient Edition. New York: New York Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 35.Wechsler D. Wechsler Test of Adult Reading. San Antonio, TX: Pearson; 2001. [Google Scholar]
- 36.Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. J Abnorm Psychol. 1995;104(1):3–14. doi: 10.1037//0021-843x.104.1.3. [DOI] [PubMed] [Google Scholar]
- 37.Adamson SJ, Sellman JD. A prototype screening instrument for cannabis use disorder: the Cannabis Use Disorders Identification Test (CUDIT) in an alcohol-dependent clinical sample. Drug Alcohol Rev. 2003;22(3):309–315. doi: 10.1080/0959523031000154454. [DOI] [PubMed] [Google Scholar]
- 38.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on early detection of persons with harmful alcohol consumption–II. Addiction. 1993;88(6):791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 39.Robinson SM, Sobell LC, Sobell MB, Leo GI. Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use. Psychol Addict Behav. 2014;28(1):154–162. doi: 10.1037/a0030992. [DOI] [PubMed] [Google Scholar]
- 40.Schuster RM, Hanly A, Gilman J, Budney A, Vandrey R, Evins AE. A contingency management method for 30-days abstinence in non-treatment seeking young adult cannabis users. Drug Alcohol Depend. 2016;167:199–206. doi: 10.1016/j.drugalcdep.2016.08.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lafolie P, Beck O, Blennow G, et al. Importance of creatinine analyses of urine when screening for abused drugs. Clin Chem. 1991;37(11):1927–1931. [PubMed] [Google Scholar]
- 42.Schwilke EW, Gullberg RG, Darwin WD, et al. Differentiating new cannabis use from residual urinary cannabinoid excretion in chronic, daily cannabis users. Addiction. 2011;106(3):499–506. doi: 10.1111/j.1360-0443.2010.03228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Degenhardt L, Coffey C, Romaniuk H, et al. The persistence of the association between adolescent cannabis use and common mental disorders into young adulthood. Addiction. 2013;108(1):124–133. doi: 10.1111/j.1360-0443.2012.04015.x. [DOI] [PubMed] [Google Scholar]
- 44.Foster DW, Garey L, Buckner JD, Zvolensky MJ. Social anxiety and cannabis-related impairment: The synergistic influences of peer and parent descriptive and injunctive normative perceptions. Subst Use Misuse. 2016;51(7):912–921. doi: 10.3109/10826084.2016.1156701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hill MN, Miller GE, Carrier EJ, Gorzalka BB, Hillard CJ. Circulating endocannabinoids and N-acyl ethanolamines are differentially regulated in major depression and following exposure to social stress. Psychoneuroendocrino. 2009;34(8):1257–1262. doi: 10.1016/j.psyneuen.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zanettini C, Panlilio LV, Alicki M, Goldberg SR, Haller J, Yasar S. Effects of endocannabinoid system modulation on cognitive and emotional behavior. Front Behav Neurosci. 2011;5:57. doi: 10.3389/fnbeh.2011.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ashton CH, Moore PB. Endocannabinoid system dysfunction in mood and related disorders. Acta Psychiatr Scand. 2011;124(4):250–261. doi: 10.1111/j.1600-0447.2011.01687.x. [DOI] [PubMed] [Google Scholar]
- 48.Heng L, Beverley JA, Steiner H, Tseng KY. Differential developmental trajectories for CB1 cannabinoid receptor expression in limbic/associative and sensorimotor cortical areas. Synapse. 2011;65(4):278–286. doi: 10.1002/syn.20844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haney M, Bedi G, Cooper ZD, et al. Predictors of marijuana relapse in the human laboratory: robust impact of tobacco cigarette smoking status. Biol Psychit. 2013;73(3):242–248. doi: 10.1016/j.biopsych.2012.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herrmann ES, Weerts EM, Vandrey R. Sex differences in cannabis withdrawal symptoms among treatment-seeking cannabis users. Exp Clin Psychopharm. 2015;23(6):415–421. doi: 10.1037/pha0000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chung T, Martin CS, Cornelius JR, Clark DB. Cannabis withdrawal predicts severity of cannabis involvement at 1-year follow-up among treated adolescents. Addiction. 2008;103:787–799. doi: 10.1111/j.1360-0443.2008.02158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.National Research Council and Institute of Medicine. Preventing Mental, Emotional, and Behavioral Disorders Among Young People: Progress and Possibilities. Washington, DC: The National Academies Press; 2009. [PubMed] [Google Scholar]
- 53.Johnson KA, Stewart S, Rosenfield D, Steeves D, Zvolensky MJ. Prospective evaluation of the effects of anxiety sensitivity and state anxiety in predicting acute nicotine withdrawal symptoms during smoking cessation. Psychol Addict Behav. 2012;26(2):289–297. doi: 10.1037/a0024133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weinberger AH, Desai RA, McKee SA. Nicotine withdrawal in U.S. smokers with current mood, anxiety, alcohol use, and substance use disorders. Drug Alcohol Depend. 2010;108(1–2):7–12. doi: 10.1016/j.drugalcdep.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zvolensky MJ, Farris SG, Guillot CR, Levental AM. Anxiety sensitivity as an amplifier of subjective and behavioral tobacco abstinence effects. Drug Alcohol Depend. 2014;142:224–230. doi: 10.1016/j.drugalcdep.2014.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]


