Abstract
Aim
The aim of this study was to determine the population pharmacokinetics of darbepoetin alfa in hypothermic neonates with hypoxic-ischemic encephalopathy treated with hypothermia.
Methods
Neonates ≥36 weeks gestation and <12 h postpartum with moderate to severe hypoxic-ischemic encephalopathy who were undergoing hypothermia treatment were recruited in this randomized, multicenter, investigational, new drug pharmacokinetic study. Two intravenous darbepoetin alfa treatment groups were evaluated: 2 and 10 μg/kg. Serum erythropoietin concentrations were measured using an enzyme-linked immunosorbent assay. Monolix 4.3.1 was used to estimate darbepoetin alfa clearance and volume of distribution. Covariates tested included: birthweight, gestational age, postnatal age, postmenstrual age, sex, Sarnat score, and study site.
Results
Darbepoetin alfa pharmacokinetics were well described by a one-compartment model with exponential error. Clearance and the volume of distribution were scaled by birthweight (centered on the mean) a priori. Additionally, gestational age (also centered on the mean) significantly affected darbepoetin alfa clearance. Clearance and volume of distribution were estimated as 0.0465 L/h (95 % confidence interval 0.0392–0.0537) and 1.58 L (95 % confidence interval 1.29–1.87), respectively.
Conclusions
A one-compartment model successfully described the pharmacokinetics of darbepoetin alfa among hypothermic neonates treated for hypoxic-ischemic encephalopathy. Clearance decreased with increasing gestational age.
1 Introduction
Hypoxic-ischemic encephalopathy (HIE) affects 1.5 infants per 1000 live births in developed countries [1]. Moderate to severe HIE is associated with severe disabilities in one in four survivors and a mortality of 15–25 % [2, 3]. Common disabilities include significant motor deficits, cerebral palsy, and persistent developmental delays [2, 3]. The standard of care for HIE is moderate hypothermia, in which the neonate is cooled to 33.5°C within 6 h of birth and hypothermia is maintained for 72 h [4–6]. A meta-analysis published by Edwards et al. [3] evaluated the neurologic outcomes of infants who were randomized to receive moderate hypothermia or normothermia and compared their clinical outcomes after a minimum of 18 months of follow-up. The authors found that the case fatality rate decreased from 33 to 26 % and abnormal neurologic outcomes at 18–22 months decreased from 40 to 28 % comparing normothermia with moderate hypothermia, respectively. A similar analysis by Tagin et al. [7] with 1214 newborns found that with hypothermia treatment, the risk ratio of death or major neurodevelopmental disability was 0.76 (95 % confidence interval 0.69–0.84) and the rate of survival with normal neurologic function at 18 months of age was improved (1.64; 1.36–1.95). While these results are encouraging, moderate to severe HIE despite hypothermia treatment still causes substantial mortality and morbidity, which supports the need for adjunctive therapies to improve outcomes.
Erythropoietin-stimulating agents have been shown to mediate adaptive tissue responses following stressful insults and may be neuroprotective [8, 9]. This is consistent with studies that have shown that the erythropoietin receptor is expressed throughout the human brain [10–12]. Preclinical studies using mice demonstrated that erythropoietin efficiently crosses the blood-brain barrier [13]. Daily administration of erythropoietin to animals has conferred histologic and behavioral neuroprotection after experimental intracerebral hemorrhage [14]. In two clinical trials, erythropoietin was found to exert neuroprotective effects among neonates who did not undergo hypothermia treatment [15, 16]. More recently, the Neonatal Erythropoietin in Asphyxiated Term Neonates trial showed that erythropoietin could be administered safely to neonates undergoing therapeutic hypothermia [17].
Darbepoetin alfa is a synthetic molecule designed to mimic the effects of erythropoietin [18–20]. Site-directed mutagenesis at five amino acid locations (Ala30Asn, His32Thr, Pro87Val, Trp88Asn, and Pro90Thr) added two additional N-linked carbohydrate chains, which extend the half-life [18–20]. Preclinical studies with darbepoetin alfa have shown that it crosses the blood-brain barrier and displays comparable neuroprotective activity when compared with erythropoietin [13, 21]. In animal models, weekly administration of darbepoetin alfa conferred histologic and behavioral neuroprotection after experimental intracerebral hemorrhage [14]. Early clinical studies have demonstrated the safety of darbepoetin alfa among preterm and term neonates [22, 23]. The aim of this study was to determine the population pharmacokinetics of darbepoetin alfa among neonates undergoing therapeutic hypothermia for the treatment of HIE in support of larger studies to evaluate its efficacy for neuroprotection.
2 Methods
2.1 Subjects and Study Design
This was a multicenter, placebo-controlled, randomized, double-blind, pharmacokinetic and safety trial that was approved by each site’s institutional review board (NCT01471015). The population pharmacokinetic analysis of this trial is described herein. Centers included the Seattle Children’s Hospital, Primary Children’s Hospital, University of Utah Hospital, Intermountain Medical Center, McKay-Dee Hospital, Vanderbilt Children’s Hospital, and the University of New Mexico Children’s Hospital. Parental permission was obtained before conducting any study procedures. Inclusion criteria included: gestational age ≥36 weeks, <12 h of age; evidence of moderate to severe hypothermia based on a modified Sarnat score of 2–3 [4]; and severe fetal or early (< 1 h of age) neonatal acidosis. Additional criteria included evidence of an acute intrauterine event, and either a 10-min Apgar score of ≤5 or assisted ventilation initiated at birth that continued for ≥10 min was required if a blood gas was not available or a blood gas at <1 h of age had a pH between 7.01 and 7.15, or a base deficit between 10 and 15.9 mEg/L. Neonates were excluded from the trial if they had a major congenital and/or chromosomal abnormality, severe growth restriction (≤1800 g at birth), a prenatal diagnosis of brain abnormality or hydrocephalus, a hematocrit >65 %, a platelet count >600,000/dL, neutropenia (absolute neutrophil count <500 μL), were receiving extracorporeal membrane oxygenation, or had a maternal history of major vascular thrombosis or multiple fetal losses (three or more spontaneous abortions).
Following informed consent, patients were randomized to one of three study arms: placebo (saline), 2 μg/kg, or 10 μg/kg darbepoetin alfa (Aranesp®, Amgen, Thousand Oaks, CA, USA). All three groups received therapeutic hypothermia according to the standard of care within 6 h of birth, which was maintained for 72 h. Neonates received the darbepoetin alfa or placebo dose intravenously over 5 min within 12 h of life, followed by a 2-mL saline flush over 5 min.
2.2 Sample Collection
Plasma samples were collected according to a population design, in which patients were randomized to one of two sampling schedules in a 1:1 ratio. One group had samples collected before treatment and at 4, 12, 24, and 60 h post-dose. The second group had samples collected before treatment and at 4, 18, 36, and 72 h post-dose. All blood samples were collected by a heel stick or from an arterial or central venous catheter.
2.3 Analytical Assay
Plasma samples were analyzed using a Quantikine IVD™ human erythropoietin ELISA (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s protocol. This ELISA cross-reacts with endogenous erythropoietin, which makes it impossible to distinguish darbepoetin alfa from endogenous erythropoietin. Standard curves included 2.5 [lower limit of quantification (LLOQ)], 5.0, 20, 50, 100, and 200 mU/mL of erythropoietin. A weighted (1/x2) quadratic regression model was fitted to each standard curve. Each individual sample well was quantified by interpolation, after which, duplicates were averaged. Samples that were below the LLOQ were excluded from the analysis (eight concentrations, 14 %). If samples were evaluated on more than 1 day, all wells were averaged for the final sample quantitation. Duplicates in the standard curve that had an intraday coefficient of variation >10 % or samples that had intra- or inter-day coefficients of variation >10 % were excluded from the analysis. In addition, standards that did not meet the United States Food and Drug Administration guidelines (±20 % for LLOQ, ±15 % for all others) were not included in the construction of the standard curve.
2.4 Pharmacokinetic Analysis
All darbepoetin alfa-treated concentrations were normalized to the median placebo concentration at the corresponding sampling time to account for the effect of endogenous erythropoietin contributions. Darbepoetin alfa pharmacokinetic parameters were estimated using a nonlinear mixed-effects model that was implemented in Monolix 4.3.1 (Lixoft, Orasy, France) using the stochastic approximation expectation maximization algorithm [24] combined with a Markov Chain Monte Carlo procedure. The number of Markov Chain Monte Carlo chains was fixed to four. One- and two-compartment structural models were evaluated and selected based on their goodness of fit. Model stability was assessed by altering the initial estimates for darbepoetin alfa clearance and volume of distribution. Unstable models and those that produced erroneous results (e.g., negative parameter estimates) were disregarded. Diagnostic plots were used to assess the model’s fit. Individual weighted residuals were plotted vs. time and individual predictions. Models were compared by assessing the biological plausibility of the parameter estimates, the variability of the parameter estimates, and the −2 × log likelihood or the objective function value (OFV).
Model variability and random effects were classified as one of two types of error: between-subject variability (BSV) and residual unexplained variability (RUV). BSV was assumed to be log-normally distributed according to an exponential equation:
| (1) |
where Pi, is the pharmacokinetic parameter of the ith individual, θpop is the population mean for P, and η represents the normally distributed between-subject random effect with a mean of zero and a variance of ω2. Additive, proportional, combined additive and proportional, and exponential RUV error models were evaluated. The final model used an exponential model of the form:
| (2) |
where Yij is the observed concentration for the ith individual at time j, Ŷij is the individual predicted concentration, and ε represents the normally distributed error term with a mean of zero and variance of σ2 .
2.5 Covariate Analysis
Several patient characteristics were tested for their influence on darbepoetin alfa pharmacokinetic parameters. The covariates that were tested included birthweight, gestational age, postnatal age, postmenstrual age, sex, Sarnat score, and study site. Birthweight centered on the mean was included a priori in the model for both clearance and the volume of distribution on the basis of its biological plausibility [25]. In addition, exponents were fixed at 1 based on model estimates of 1.2 and 0.992. Power, exponential, and linear models were evaluated for the remaining covariates. In addition, each covariate was also evaluated after it was centered on the population mean. Forward addition was used to determine significant covariates. A decrease in the OFV ≥3.84 was considered significant for 1 df at p = 0.05 based on the χ2 distribution. Backward elimination was used to remove covariates from the model with an increase in the OFV ≥6.63 corresponding to 1 df at p = 0.01.
2.6 Model Evaluation
Base and final models were evaluated using goodness-of-fit plots. Observed drug concentrations were inspected for their correlation with predicted concentrations. The −2 and +2 region criterion was used to assess the individual weighted residual plots that were constructed. Uncertainty in pharmacokinetic parameter estimates was quantitatively assessed by calculating standard errors and 95 % confidence intervals for all pharmacokinetic parameter estimates. Additionally, normalized prediction distribution errors were plotted against time and population-predicted darbepoetin alfa concentrations to assess for model misspecification. A prediction-corrected visual predictive check was also performed by simulating 1000 darbepoetin alfa concentrations at each time point [26].
3 Results
There were 10 patients with 32 concentration measurements who were randomized to the placebo group. In addition, there were 16 patients with 63 concentration measurements who received darbepoetin alfa. Supplemental Fig. 1 depicts the time-concentration curve of all patients. The demographics for the study subjects at the time of the dose are included in Table 1. The data were well described by a one-compartment model with zero-order input and first-order elimination as assessed by visual inspection of the diagnostic plots and the significant reduction of the OFV. After a priori inclusion of birthweight on clearance and the volume of distribution, additional covariate analyses indicated that gestational age centered on the mean exerted a significant influence on darbepoetin alfa clearance (decreasing BSV from 0.328 to 0.205, Supplemental Fig. 2). After including these covariates, no other covariates were found to significantly affect darbepoetin alfa pharmacokinetics. The final model estimates are presented in Table 2 and were derived from the following final model:
| (3) |
| (4) |
where CLi is the individual clearance, CLpop is the estimated population clearance, BWTi is the individual birth-weight, BWTpop is the mean population birthweight, GAi is the individual gestational age, GApop is the mean population gestational age, and θ is the estimated exponent for the effect of gestational age on darbepoetin alfa clearance. Similarly, Vi is the individual volume of distribution, Vpop is the population estimate, BWTi is the individual weight, and BWTpop is the mean population birthweight. Diagnostic plots were visually inspected to confirm the selection of the final model (Fig. 1a, b). To further evaluate the model, individual weighted residual and normalized prediction distribution error plots were examined (Fig. 2a–d). The data were equally distributed around zero and were in the −2to +2 range. Additionally, data were simulated to produce a visual predictive check comparing the 10th, 50th, and 90th percentiles (Fig. 3). The majority of the simulations were within these ranges, demonstrating acceptable agreement between observed concentrations and simulated darbepoetin alfa concentrations.
Table 1.
Patient demographics
| Characteristic | Placebo (n = 10)a | Darbepoetin alfa (n = 16)a |
|---|---|---|
| Sex (male) | 4 | 10 |
| GA (weeks) | 38.1 ± 1.7 | 38.4 ± 1.2 |
| PNA (h) | 9.0 ± 1.4 | 9.7 ± 2.2 |
| PMA (weeks) | 38.1 ± 1.7 | 38.4 ± 1.1 |
| BWT (kg) | 3.09 ± 0.39 | 3.03 ± 0.43 |
| Sarnat scoreb | ||
| 2 | 8 | 10 |
| 3 | 2 | 6 |
BWT birthweight, GA gestational age, HIE hypoxic ischemic encephalopathy, PMA postmenstrual age, PNA postnatal age
n or mean ± standard deviation
The Sarnat Grading Scale of Hypoxic Ischemic Encephalopathy is a scoring system used to grade the severity of an HIE injury. Scores of 2 and 3 represent moderate and severe HIE injuries, respectively
Table 2.
Final model parameter estimates
| Parameters | Mean parameter estimate | 95 % confidence interval |
|---|---|---|
| V (L) | 1.58 | 1.29–1.87 |
| CL (L/h) | 0.0465 | 0.0392–0.0537 |
| θ | 10.8 | 5.50–15.7 |
| ω-BSV | ||
| V | 0.153 | −0.161 to 0.467 |
| CL | 0.205 | 0.054–0.356 |
| σ-RUV | 0.421 | 0.325–0.517 |
CL clearance, BSV between-subject variability, RUV residual unexplained variability, V volume of distribution, θ represents the model-estimated coefficient for the effect of gestational age (weeks) on darbepoetin alfa clearance
Fig. 1.
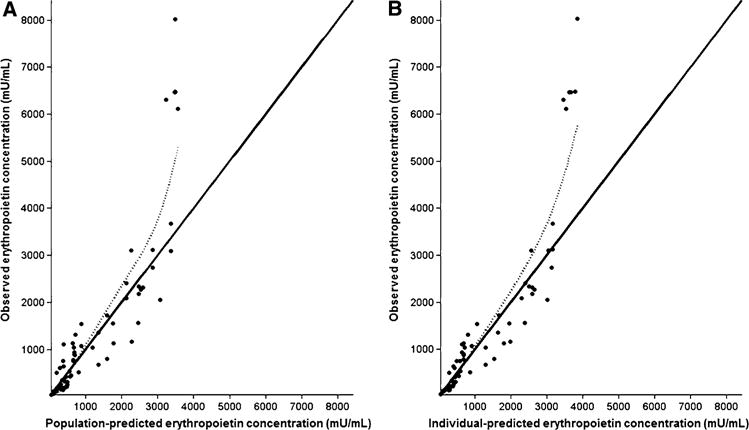
Observed erythropoietin concentrations vs. population-and individual-predicted erythropoietin concentrations for hypothermic darbepoetin alfa-treated neonates are presented in a and b, respectively, for the final model. The solid line represents the line of reference and the dotted line represents the spline of the model
Fig. 2.
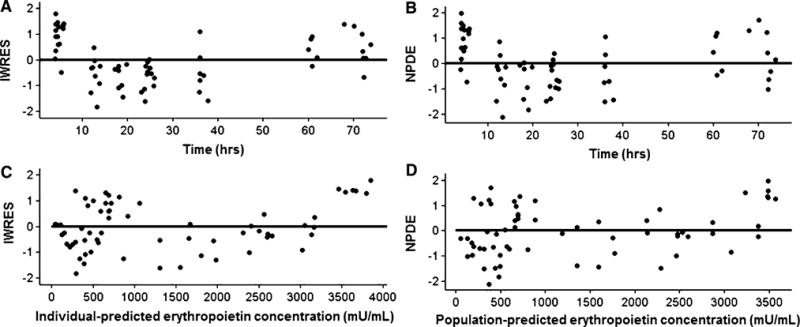
The individual weighted residuals (IWRES) and normalized prediction distribution errors (NPDE) vs. time- and population-predicted erythropoietin concentrations are presented in a–d. The solid line is the reference line at zero
Fig. 3.
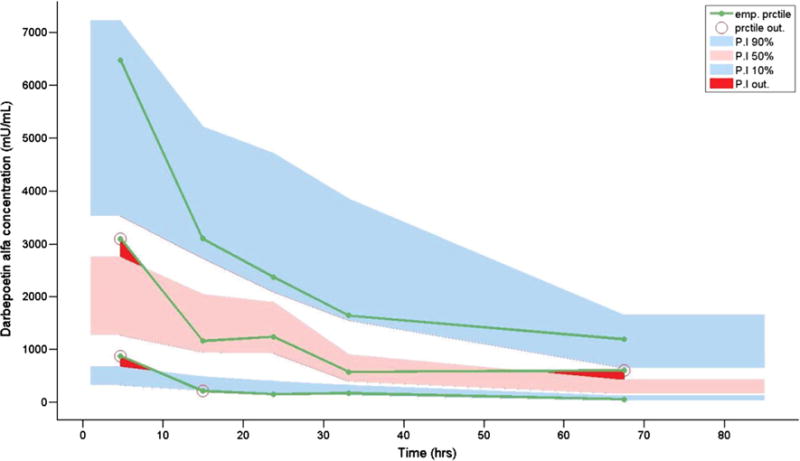
Visual predictive check of the final neonatal darbepoetin alfa model. The blue shading represents the 10th and 90th percentiles. Pink shading represents the 50th percentile. The open circles and red shading represent data that did not fall within the 10th, 50th, or 90th percentiles. emp empirical, prctile percentile
4 Discussion
This is the first study to evaluate the pharmacokinetics of darbepoetin alfa among neonates with HIE undergoing therapeutic hypothermia. This study also featured a placebo arm, such that endogenous erythropoietin concentrations could be used to normalize erythropoietin concentrations for the darbepoetin alfa-treated patients. This study demonstrated that darbepoetin alfa pharmacokinetics in neonates with HIE was well described by a one-compartment model, with a population clearance equal to 0.0465 L/h (0.015 L/h/kg) and volume of distribution equal to 1.58 L (0.511 L/kg). Covariate analyses indicated that after the inclusion of birthweight on volume of distribution and clearance, gestational age also significantly affected clearance, with increasing gestational age associated with decreased darbepoetin alfa clearance. Darbepoetin alfa is a synthetic derivative of erythropoietin and is primarily cleared by binding to erythropoietin receptors on progenitor cells [27–32]. It has been demonstrated in lambs that the clearance of erythropoietin occurs most rapidly in fetuses and gets progressively lower as sheep mature to adulthood [33], supporting the findings of this study.
A majority of the adult pharmacokinetic studies pooled data from patients who received darbepoetin alfa intravenously and via subcutaneous injection [34–36]. These studies used a two-compartment model to describe darbepoetin alfa pharmacokinetics [34–36]. It is unclear whether the one-compartment model used in the current study performed better than the two-compartment model because of sparse sampling in this vulnerable neonatal population or whether the pharmacokinetics of darbepoetin alfa are better represented by a one-compartment model in neonates rather than the two-compartment model seen in adults. The only other study to evaluate intravenous darbepoetin alfa pharmacokinetics in neonates was conducted in 10 anemic patients with a mean gestational age of 31.1 weeks at birth who did not have HIE [22]. The authors found that darbepoetin alfa pharmacokinetics were best described using a mono-exponential model; however, two patients were better fit with a dual-exponential pharmacokinetic model. The mean volume of distribution and clearance were 1.29 L and 0.0882 L/h, respectively, resulting in a half-life of about 10.1 h. The population estimates reported herein resulted in a half-life of 23.6 h. Patients in this population analysis were on average 7 weeks older at birth than those in the study conducted by Warwood et al. [22]. Unlike prior studies, the current analysis adopted a population pharmacokinetic approach, which allowed for the identification of influential covariates that were used to reduce the proportion of unexplained variability in darbepoetin alfa pharmacokinetic parameters. The population pharmacokinetic model revealed that after accounting for the influence of birthweight, darbepoetin alfa clearance was inversely correlated with gestational age. This finding suggests that the difference in the mean gestational age of the cohort studied by Warwood et al. and that of the cohort included in this analysis may explain, at least in part, the difference in darbepoetin alfa half-lives reported previously and those observed in this study. Additionally, patients in our study were also treated with hypothermia. It is therefore unclear whether the differences in half-lives between these studies can be attributed to differences in gestational age, the use of therapeutic hypothermia, other unmeasured factors, or a combination of the above.
Some limitations should be considered when interpreting this study’s findings. First, patients had differing lengths of hypoxic episodes, which were not quantifiable. Hypoxic episodes increase erythropoietin production and it is believed that longer hypoxic episodes result in more erythropoietin production [37]. Even with a representative placebo group, it is possible that early darbepoetin alfa concentrations may have been overestimated because of higher endogenous erythropoietin concentrations at birth among darbepoetin alfa-treated patients. Second, the assay used quantifies both darbepoetin alfa and erythropoietin, and as such, median concentrations from placebo patients had to be used as a baseline for the treated patients. Furthermore, a specific validation for this assay has not been completed in neonates, though this assay has been used previously for darbepoetin alfa quantification [38]. Additionally, the confidence interval for the BSV for the volume of distribution includes zero, which could be attributed to the sparse sampling that was needed to ethically and practically conduct this neonatal pharmacokinetic trial. With a larger number of subjects and/or additional concentration measurements, it may be possible to improve the precision of the BSV estimate for the volume of distribution. Furthermore, the relatively large RUV observed in this study (42 %) is in agreement with previous neonatal drug studies and emphasizes the difficulty in determining precise pharmacokinetic parameter estimates in this highly variable patient population, which undergoes profound developmental changes over the first few weeks of life [39]. Last, choosing to include birthweight a priori in the model could decrease the predictive performance of the model with the addition of other covariates. However, because of biological plausibility, we deemed it important to include birthweight in the model as a covariate before testing other covariates.
5 Conclusions
This study presents the first population pharmacokinetic analysis of darbepoetin alfa in hypothermic neonates with HIE. This study demonstrated that after accounting for the effect of birthweight on clearance and the volume of distribution, gestational age also significantly affected darbepoetin alfa clearance. Gestational age was inversely correlated with clearance, confirming results obtained in earlier studies involving fetal, neonatal, and adult sheep. Future analyses with larger numbers of subjects and long-term follow-up are warranted to further characterize the pharmacokinetics of darbepoetin alfa and their influence on clinical outcomes, including death and severe disability.
Supplementary Material
Key Points.
A population pharmacokinetic model for darbepoetin alfa in neonates with hypoxic-ischemic encephalopathy was developed.
After a priori inclusion of birthweight on the volume of distribution and clearance, gestational age was inversely correlated with darbepoetin alfa clearance.
Acknowledgments
JKR was supported by the Pharmacotherapy Subspecialty Award from the Primary Children’s Hospital Foundation; CS was supported by the American Foundation for Pharmaceutical Education’s Clinical Pharmaceutical Sciences Fellowship; MCB and this clinical study were supported by the Thrasher Foundation (10026771-F1); the REDCap database used to house the clinical data from the trial was supported by the Center for Clinical and Translational Sciences grant (8UL1TR000105 NCATS/NIH).
Footnotes
Beyond these disclosures, the authors have no financial relationships with any organizations that might have an interest in the submitted work, nor are there any other relationships or activities that could appear to have influenced the submitted work.
Electronic supplementary material The online version of this article (doi:10.1007/s40262-015-0286-y) contains supplementary material, which is available to authorized users.
Author Contributions JKR performed the pharmacokinetic modeling and analysis, and drafted the manuscript. CS provided advice regarding the pharmacokinetic modeling and critically reviewed the manuscript. RMW helped design the clinical study and critically reviewed the manuscript. JB performed analytical measurements, helped design the clinical study, and critically reviewed the manuscript. MCB was the principal investigator of the study, designed the clinical study, and critically reviewed the manuscript. MGS provided advice regarding the pharmacokinetic modeling, and critically reviewed the manuscript. CMTS provided advice regarding the pharmacokinetic modeling and critically reviewed the manuscript.
References
- 1.Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev. 2010;86(6):329–38. doi: 10.1016/j.earlhumdev.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Shankaran S. Hypoxic-ischemic encephalopathy and novel strategies for neuroprotection. Clin Perinatol. 2012;39(4):919–29. doi: 10.1016/j.clp.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Edwards AD, Brocklehurst P, Gunn AJ, Halliday H, Juszczak E, Levene M, et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ. 2010;340:c363. doi: 10.1136/bmj.c363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353(15):1574–84. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 5.Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365(9460):663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 6.Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361(14):1349–58. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- 7.Tagin MA, Woolcott CG, Vincer MJ, Whyte RK, Stinson DA. Hypothermia for neonatal hypoxic ischemic encephalopathy: an updated systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2012;166(6):558–66. doi: 10.1001/archpediatrics.2011.1772. [DOI] [PubMed] [Google Scholar]
- 8.Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, Cerami C, et al. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci USA. 2000;97(19):10526–31. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casals-Pascual C, Idro R, Gicheru N, Gwer S, Kitsao B, Gitau E, et al. High levels of erythropoietin are associated with protection against neurological sequelae in African children with cerebral malaria. Proc Natl Acad Sci USA. 2008;105(7):2634–9. doi: 10.1073/pnas.0709715105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasselblatt M, Ehrenreich H, Siren AL. The brain erythropoietin system and its potential for therapeutic exploitation in brain disease. J Neurosurg Anesthesiol. 2006;18(2):132–8. doi: 10.1097/00008506-200604000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Dame C, Bartmann P, Wolber E, Fahnenstich H, Hofmann D, Fandrey J. Erythropoietin gene expression in different areas of the developing human central nervous system. Brain Res Dev Brain Res. 2000;125(1–2):69–74. doi: 10.1016/s0165-3806(00)00118-8. [DOI] [PubMed] [Google Scholar]
- 12.Juul SE, Anderson DK, Li Y, Christensen RD. Erythropoietin and erythropoietin receptor in the developing human central nervous system. Pediatr Res. 1998;43(1):40–9. doi: 10.1203/00006450-199801000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Banks WA, Jumbe NL, Farrell CL, Niehoff ML, Heatherington AC. Passage of erythropoietic agents across the blood-brain barrier: a comparison of human and murine erythropoietin and the analog darbepoetin alfa. Eur J Pharmacol. 2004;505(1–3):93–101. doi: 10.1016/j.ejphar.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 14.Grasso G, Graziano F, Sfacteria A, Carletti F, Meli F, Maugeri R, et al. Neuroprotective effect of erythropoietin and darbepoetin alfa after experimental intracerebral hemorrhage. Neurosurgery. 2009;65(4):763–769. doi: 10.1227/01.NEU.0000347475.73347.5F. (discussion 769–70) [DOI] [PubMed] [Google Scholar]
- 15.Zhu C, Kang W, Xu F, Cheng X, Zhang Z, Jia L, et al. Erythropoietin improved neurologic outcomes in newborns with hypoxic-ischemic encephalopathy. Pediatrics. 2009;124(2):e218–26. doi: 10.1542/peds.2008-3553. [DOI] [PubMed] [Google Scholar]
- 16.Elmahdy H, El-Mashad AR, El-Bahrawy H, El-Gohary T, El-Barbary A, Aly H. Human recombinant erythropoietin in asphyxia neonatorum: pilot trial. Pediatrics. 2010;125(5):e1135–42. doi: 10.1542/peds.2009-2268. [DOI] [PubMed] [Google Scholar]
- 17.Wu YW, Bauer LA, Ballard RA, Ferriero DM, Glidden DV, Mayock DE, et al. Erythropoietin for neuroprotection in neonatal encephalopathy: safety and pharmacokinetics. Pediatrics. 2012;130(4):683–91. doi: 10.1542/peds.2012-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macdougall IC. Novel erythropoiesis stimulating protein. Semin Nephrol. 2000;20(4):375–81. [PubMed] [Google Scholar]
- 19.Ibbotson T, Goa KL. Darbepoetin alfa. Drugs. 2001;61(14):2097–2104. doi: 10.2165/00003495-200161140-00007. (discussion 105–106) [DOI] [PubMed] [Google Scholar]
- 20.Egrie JC, Browne JK. Development and characterization of novel erythropoiesis stimulating protein (NESP) Br J Cancer. 2001;84(Suppl 1):3–10. doi: 10.1054/bjoc.2001.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cherian L, Goodman JC, Robertson C. Improved cerebrovascular function and reduced histological damage with darbepoietin alfa administration after cortical impact injury in rats. J Pharmacol Exp Ther. 2011;337(2):451–6. doi: 10.1124/jpet.110.176602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warwood TL, Ohls RK, Lambert DK, Jones C, Scoffield SH, Gupta N, et al. Intravenous administration of darbepoetin to NICU patients. J Perinatol. 2006;26(5):296–300. doi: 10.1038/sj.jp.7211498. [DOI] [PubMed] [Google Scholar]
- 23.Warwood TL, Ohls RK, Wiedmeier SE, Lambert DK, Jones C, Scoffield SH, et al. Single-dose darbepoetin administration to anemic preterm neonates. J Perinatol. 2005;25(11):725–30. doi: 10.1038/sj.jp.7211387. [DOI] [PubMed] [Google Scholar]
- 24.Kuhn E, Lavielle M. Maximum likelihood estimation in nonlinear mixed effects models. Comput Stat Data Anal. 2005;49:1020–38. [Google Scholar]
- 25.Anderson BJ, Allegaert K, Holford NH. Population clinical pharmacology of children: modelling covariate effects. Eur J Pediatr. 2006;165(12):819–29. doi: 10.1007/s00431-006-0189-x. [DOI] [PubMed] [Google Scholar]
- 26.Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. Aaps J. 2011;13(2):143–51. doi: 10.1208/s12248-011-9255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chapel S, Veng-Pedersen P, Hohl RJ, Schmidt RL, McGuire EM, Widness JA. Changes in erythropoietin pharmacokinetics following busulfan-induced bone marrow ablation in sheep: evidence for bone marrow as a major erythropoietin elimination pathway. J Pharmacol Exp Ther. 2001;298(2):820–4. [PubMed] [Google Scholar]
- 28.Chapel SH, Veng-Pedersen P, Schmidt RL, Widness JA. Receptor-based model accounts for phlebotomy-induced changes in erythropoietin pharmacokinetics. Exp Hematol. 2001;29(4):425–31. doi: 10.1016/s0301-472x(01)00614-2. [DOI] [PubMed] [Google Scholar]
- 29.Freise KJ, Widness JA, Segar JL, Schmidt RL, Veng-Pedersen P. Increased erythropoietin elimination in fetal sheep following chronic phlebotomy. Pharm Res. 2007;24(9):1653–9. doi: 10.1007/s11095-007-9295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jelkmann W. The enigma of the metabolic fate of circulating erythropoietin (Epo) in view of the pharmacokinetics of the recombinant drugs rhEpo and NESP. Eur J Haematol. 2002;69(5–6):265–74. doi: 10.1034/j.1600-0609.2002.02813.x. [DOI] [PubMed] [Google Scholar]
- 31.Stohlman F, Jr, Brecher G. Humoral regulation of erythropoiesis: V. Relationship of plasma erythropoietine level to bone marrow activity. Proc Soc Exp Biol Med. 1959;100(1):40–3. doi: 10.3181/00379727-100-24516. [DOI] [PubMed] [Google Scholar]
- 32.Widness JA, Schmidt RL, Hohl RJ, Goldman FD, Al-Huniti NH, Freise KJ, et al. Change in erythropoietin pharmacokinetics following hematopoietic transplantation. Clin Pharmacol Ther. 2007;81(6):873–9. doi: 10.1038/sj.clpt.6100165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Widness JA, Veng-Pedersen P, Modi NB, Schmidt RL, Chestnut DH. Developmental differences in erythropoietin pharmacokinetics: increased clearance and distribution in fetal and neonatal sheep. J Pharmacol Exp Ther. 1992;261(3):977–84. [PubMed] [Google Scholar]
- 34.Takama H, Tanaka H, Nakashima D, Ogata H, Uchida E, Akizawa T, et al. Population pharmacokinetics of darbepoetin alfa in haemodialysis and peritoneal dialysis patients after intravenous administration. Br J Clin Pharmacol. 2007;63(3):300–9. doi: 10.1111/j.1365-2125.2006.02756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agoram B, Heatherington AC, Gastonguay MR. Development and evaluation of a population pharmacokinetic-pharmacodynamic model of darbepoetin alfa in patients with nonmyeloid malignancies undergoing multicycle chemotherapy. AAPS J. 2006;8(3):E552–63. doi: 10.1208/aapsj080364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agoram B, Sutjandra L, Sullivan JT. Population pharmacokinetics of darbepoetin alfa in healthy subjects. Br J Clin Pharmacol. 2007;63(1):41–52. doi: 10.1111/j.1365-2125.2006.02752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eckardt KU, Boutellier U, Kurtz A, Schopen M, Koller EA, Bauer C. Rate of erythropoietin formation in humans in response to acute hypobaric hypoxia. J Appl Physiol (1985) 1989;66(4):1785–8. doi: 10.1152/jappl.1989.66.4.1785. [DOI] [PubMed] [Google Scholar]
- 38.Lerner G, Kale AS, Warady BA, Jabs K, Bunchman TE, Heatherington A, et al. Pharmacokinetics of darbepoetin alfa in pediatric patients with chronic kidney disease. Pediatr Nephrol. 2002;17(11):933–7. doi: 10.1007/s00467-002-0932-0. [DOI] [PubMed] [Google Scholar]
- 39.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology: drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349(12):1157–67. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


