Distal sensory peripheral neuropathy (DSP) continues to be a prevalent complication associated with HIV (Ellis et al., 2010; Evans et al., 2011; Gonzalez-Duarte, Cikurel, & Simpson, 2007; Nicholas et al., 2010), despite medications effective in suppressing viral replication (Ances & Ellis, 2007; Evans et al., 2011; McArthur, Brew, & Nath, 2005). The pathogenesis of DSP in HIV is uncertain, but proposed mechanisms include cytokine dysregulation, viral protein-produced neurotoxicity, and mitochondrial dysfunction associated with antiretroviral medications (Cornblath & Hoke, 2006; Gonzalez-Duarte et al., 2007; Simpson, Estanislao, Brown, & Sampson, 2008). DSP occurs in an estimated 30% to 35% of persons with HIV, causing painful dysesthesias, numbness, and “pins and needles” sensations, predominantly in the feet (Centers for Disease Control and Prevention, 1993; Ferrari et al., 2006; Nicholas et al. 2002; Schifitto et al., 2002; Verma, Estanislao, & Simpson, 2005).
Currently, no U.S. Food and Drug Administration–approved therapy exists for HIV DSP. Studies of treatments to manage the neuropathic pain have largely focused on pharmacologic therapy such as nonsteroidal anti-inflammatory drugs (NSAIDs), tricyclic antidepressants, opioids, anticonvulsants, and topical analgesics (Dworkin et al., 2010; Gonzalez-Duarte et al., 2007; Keswani, Pardoa, Cherry, Hokea, & McArthur, 2002). Placebo-controlled trials have found these agents to be limited in efficacy and inconsistent in benefit for the symptoms of DSP (Silver, Blum, Grainger, Hammer, & Quessy, 2007; Simpson et al., 2010).
Many individuals with HIV report using complementary and alternative medicine (CAM) strategies to manage their symptoms (Fairfield, Eisenberg, Davis, Libman, & Phillips, 1998; Littlewood & Vanable, 2008). Acupuncture, a component of Chinese medicine, is notable as a treatment modality commonly used by individuals with HIV to manage symptoms of the disease and as an adjunctive therapy to manage pain (Fairfield et al., 1998; Gore-Felton et al., 2003; Nicholas et al., 2007). In Chinese medicine practice, acupuncture is traditionally paired with moxibustion, the burning of mugwort leaf (Artemisia vulgaris), to stimulate acupuncture points (see Methods). However, few Western studies of acupuncture have included moxibustion. Our exploratory study examined the use of a Traditional Chinese Medicine (TCM) approach, acupuncture and moxibustion (Acu/Moxa), to reduce pain severity and decrease associated symptoms of DSP (“pins and needles” sensation, numbness) in HIV. The objectives were: (a) to establish feasibility and methods of patient recruitment, retention, and protocol design, and (b) to estimate the effects of acupuncture and moxibustion for HIV DSP using a sham/placebo-controlled condition.
Materials and Methods
Design and Sample Study Participants
Fifty adults diagnosed with HIV and a moderate level of DSP pain were randomized to one of two groups in a sham/placebo-controlled, participant-and-evaluator-blinded clinical trial. Study procedures and specific details have been published previously (Anastasi, Capili, Chung, & Hammerschlag, 2010). In brief, participants who met eligibility criteria and average moderate pain or greater based on a 1-week symptom diary using the Gracely Pain Scale (GPS) were randomized in a 1:1 ratio to one of two groups using a plan of randomly permuted blocks with different block sizes (2, 4, or 6 assignments): true acupuncture/moxibustion (Acu/Moxa) or sham acupuncture/placebo moxibustion (Sham/Placebo) control. Treatment assignments were placed in sequentially numbered, sealed, opaque envelopes. The study coordinator released the assignments in numbered sequence. The principal investigator, study coordinator, neurology nurse practitioners, data manager, and statistician were blinded to group allocation until final data analysis.
Study participants attended 16 study sessions conducted over 15 weeks. Participants completed a screening/intake session, 12 treatment sessions administered twice a week for 6 weeks, and three nontreatment follow-up sessions at Week 9 (first follow-up), Week 11 (second follow-up), and Week 15 (third follow-up). Nontreatment follow-up sessions were included to assess longer-term effects of the Acu/Moxa treatment for DSP. Participants received $10 and a round-trip MetroCard valued at $4.50 for each completed study session. The study received institutional review board approval prior to the start of the study. Informed consent was obtained from all study participants.
The inclusion criteria were: men and women 18 years of age or older, diagnosed with HIV, lower extremity DSP, experiencing moderate pain recorded in a symptom diary, achieved a score of 24 or better on the Mini Mental State questionnaire, stable antiretroviral regimen (drug, dose, and frequency) for at least 8 weeks, stable analgesic regimen (drug, dose, and frequency) for at least 3 weeks, and committed to maintaining stable medications for the duration of the study. Any medication changes during the study were recorded. We obtained verification from the primary care provider of HIV status, DSP diagnosis, and medical history consistent with study criteria. Individuals were excluded if they had an acute medical condition, a diagnosis of diabetes mellitus, vitamin B-12 deficiency, coagulopathies, uncontrolled hypertension, pulmonary disease, or renal failure; were currently pregnant; were abusing alcohol or other substances; were using topically applied medications to the lower extremities, or using corticosteroids; had been treated with INH, dapsone, or metronidazole within 8 weeks prior to study enrollment; had received acupuncture within 6 months of study enrollment; or had a history of moxibustion use or other forms of CAM.
General Procedures
Licensed acupuncturists trained in TCM performed acupuncture and moxibustion procedures for both groups (Acu/Moxa and Sham/Placebo). Prior to the start of the study, the acupuncturists received training and passed both written and practical examinations of point locations and applications specific to our study protocol. The acupuncturists were also trained to perform the Sham/Placebo techniques. An unblinded study facilitator monitored all study treatment sessions and was tasked to observe the fidelity of the application of the study treatments including the sequence and timing requirements of the protocol. The study facilitator also ensured that no discussion occurred between the acupuncturist and subject during treatment sessions to avoid interaction effects. Both Acu/Moxa and Sham/Placebo participants were blindfolded during each treatment session to minimize the secondary effects of visual cues associated with the needling and moxibustion procedure. Nurse practitioners conducted neurologic assessments including cranial nerve, motor pathway, sensory pathway, gait, coordination, and reflexes at baseline, at the end of the twice-weekly treatment sessions, and at each follow-up session.
Condition 1: Acupuncture and Moxibustion (Acu/Moxa)
Acupuncture
Participants received acupuncture point stimulation de qi (termed “receiving Qi”) at traditional acupuncture point locations (Beijing College of Traditional Chinese Medicine, 1987). For Condition 1, the reinforcing/tonification needling method was used and administered by inserting the needle at the depth per classic text. Once inserted, the needle was rotated 9 times gently and slowly in a clockwise direction.
Moxibustion
Participants received the indirect moxibustion technique as described in the classic text, Chinese Acupuncture and Moxibustion (Liangyue, Yijun, & Shuhui, 1993). Moxa sticks made from the herb Artemisia vulgaris were lit and held 1 inch (2.54 cm) directly over the acupuncture points and rotated in a clockwise circular motion (Liangyue et al., 1993). Each acupuncture point was stimulated for 2 minutes with the moxa sticks.
Condition 2: Sham Acupuncture/Placebo Moxibustion (Sham/Placebo)
Sham acupuncture
Participants received sham acupuncture using established procedures (Birch, Hammerschlag, Trinh, & Zaslawski, 2002; Hammerschlag, 1997; Vincent & Lewith, 1995). Needles were inserted superficially, at a depth of 1–2 mm, sufficient for the needle to stand vertically, without eliciting a de qi response and 2–3 cm away from the true acupuncture point locations (not located on a channel/meridian). All sham point locations were anatomically specific and were diagrammed in the procedure manual.
Placebo moxibustion
A lit moxa stick was held approximately 8 inches (20.32 cm) above and 2–3 cm away from the true acupuncture point location for 2 minutes. During this procedure, the acupuncturists were trained to intermittently place a hand near the participant’s skin to assess for heat sensation. The placebo moxa technique allowed the moxa-naïve participant exposure to the smell of the burning moxa stick, but not the heat from the burning stick. It is important to note that the sequence and timing of Conditions 1 and 2 were the same.
Outcome Measures
Assessment of DSP pain
The assessment of lower-limb DSP pain was evaluated using a daily symptom diary (SD) that incorporated the GPS, the primary outcome, and the Subjective Peripheral Neuropathy Screen (SPNS). The GPS uses a 13-point, Likert-type measure of the sensory components of pain, which captures the average and the worst pain experienced during a 24-hour period. Each of the 13 word descriptors correspond to a log-scaled value of psychometrically validated “just-noticeable-difference” levels of pain intensity ranging from 0.00 to 1.77 (nothing, faint, very weak, weak, very mild, mild, moderate, barely strong, slightly intense, strong, intense, very intense, extremely intense; McArthur et al., 2000). The weekly average of the daily rated log scores was used for the primary analysis. The SPNS is a self-reporting screening tool used to evaluate the severity of DSP pain (McArthur, 1998). A description of the pain symptom, (e.g., aching/burning, pins and needles, or numbness), is first selected, and the severity of the pain symptom is rated on a 10-point scale. The Average Severity Score (SPNS Average) and Clinical Severity Grade (SPNS Grade) were computed for the analysis. The SPNS Average is the mean daily symptom severity score ranging from mild (1) to most severe (10), and the SPNS Grade is the highest symptom severity score of any symptom (aching/burning, pins and needles, or numbness).
Safety Measures
At every session, study staff collected and recorded adverse-event information by means of a scripted adverse-event elicitation form. A symptom checklist form was also included to monitor information about the progression of HIV disease and any side effects associated with the Acu/Moxa treatments (e.g., bruising, discomfort, skin irritation) if they occurred.
Credibility Assessment
The Credibility Assessment is a six-item, patientrated instrument adapted from an acupuncture credibility assessment scale (Borkovec & Nau, 1972). The instrument rates the confidence that the Acu/Moxa treatment received was true and not sham/placebo, the confidence in the logic of the treatment received, the success of the treatment in relieving DSP symptoms, the confidence that the improved symptoms were from the study treatment, the likelihood of the study treatment to decrease symptoms in other conditions, and whether the treatment would be recommended to a friend (Borkovec & Nau, 1972; Vincent & Lewith, 1995). Scores ranged from very confident (1) to not at all confident (6).
Statistical Analysis
Data collected on paper case report forms were transcribed into a Microsoft Excel database, which was exported to SAS v9.2 for data cleaning and statistical analysis. Univariate distributions of continuous variables were examined and assessed for approximation to normality with the Shapiro-Wilk test: none required transformation. Categorical variables were examined for distribution of counts and categories collapsed when necessary to form meaningful comparisons. Differences and percent differences from a subject’s baseline value were calculated for outcome variables. Between-treatment group differences in continuous measures at baseline were estimated with independent Student’s t tests and for categorical measures with chi-square or Fisher’s exact test. Baseline variables with group differences testing with p values less than .20 were evaluated as potential confounders in secondary analyses. Pearson correlations of baseline variable values with primary outcome level at the final follow-up visit were conducted to further identify potential time-independent effect modifiers, and those with p values less than .20 were entered in secondary analyses. The intent-to-treat analysis of the primary outcome, improvement in GPS score, used a linear mixed model for repeated measures with fixed effects for treatment group (Acu/Moxa vs. Sham/Placebo), time (baseline, treatment, first, second, and third follow-ups), and the group by time interaction; random effects for subject and error; the baseline value of the outcome variable entered as a continuous covariate; and a covariance structure for the autocorrelation of repeated measures within subject determined by empirical evaluation prior to hypothesis testing. The empirical evaluation of the covariance structure consisted of generating the Akaike and Bayesian Information Criteria for 15 potential covariance structures: the spatial Gaussian structure provided the best fit for all models tested. Following the primary intent-to-treat hypothesis tests, secondary tests incorporated potential confounders identified at baseline (high triglycerides) or potential effect modifiers (gender, ethnicity, duration of HIV, and the history of migraine headaches) as covariates in the linear mixed model. Secondary analyses limiting the cohort to those subjects who completed the 6-week course of treatment sessions and who attended follow-up visits were conducted to assess treatment group differences in those who adhered to the study protocol: a “per-protocol” analysis. In keeping with the goal of this pilot study to estimate treatment group differences for the purpose of designing a future study, unadjusted exact p values are reported.
Results
Fifty adults were randomized to Acu/Moxa (n = 25) treatment or Sham/Placebo (n = 25). See Figure 1 for the CONSORT diagram and Table 1 for baseline characteristics of study participants. Table 1 shows that the primary outcome measure of neuropathic pain (the GPS) was equally distributed in the Acu/Moxa and Sham/Placebo groups at baseline: 1.21 ± 0.04 versus 1.30 ± 0.04, p = .76, respectively. GPS improvement was greater in the Acu/Moxa group at first follow-up (p < .05) after the cessation of twice weekly active treatment (see Table 1).
Figure 1.
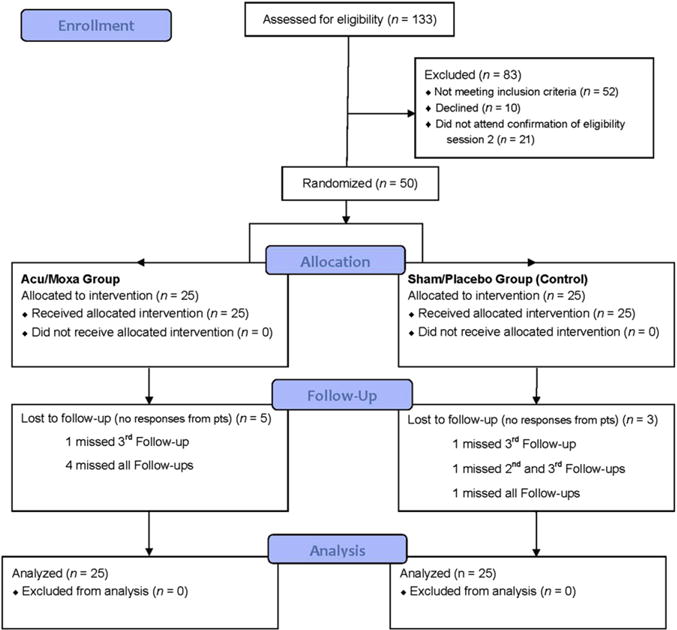
CONSORT Diagram (CONsolidated Standards of Reporting Trials). Note: Acu/Moxa = acupuncture and moxibustion; pts = patients.
Table 1.
Characteristics of Groups at Baseline
| Acu/Moxa N = 25 |
Control N = 25 |
p valuea | r- with outcomeb | ||
|---|---|---|---|---|---|
| Demographics | |||||
| Gender | N (%) male | 20 (80%) | 19 (76%) | 1.00 | 0.25/0.11 |
| Ethnicity | N (%) Hispanic | 4 (16%) | 4 (16%) | 1.00 | 0.26/0.10 |
| Race | N (%) Afr. Am. | 15 (60%) | 19 (76%) | .52 | −0.07/0.63 |
| Age (years) | Mean ± SD | 47.8 ± 7.2 | 47.6 ± 7.5 | .93 | −0.07/0.65 |
| Weight (pounds) | Mean ± SD | 180 ± 35 | 177 ± 36 | .80 | 0.02/0.88 |
| Duration HIV (years) | Mean ± SD | 16.4 ± 5.3 | 16.7 ± 6.3 | .83 | 0.22/0.18 |
| History | |||||
| Hepatitis B | N (%) positive | 2 (8%) | 2 (8%) | 1.00 | −0.04/0.77 |
| Hepatitis C | N (%) positive | 6 (24%) | 9 (36%) | .54 | 0.05/0.74 |
| CMV | N (%) positive | 1 (4%) | 0 (0%) | 1.00 | |
| Herpes | N (%) positive | 0 (0%) | 1 (4%) | 1.00 | |
| High BP | N (%) positive | 3 (12%) | 3 (12%) | 1.00 | −0.16/0.33 |
| Hypertension | N (%) positive | 0 (0%) | 2 (8%) | .48 | −0.14/0.41 |
| High cholesterol | N (%) positive | 2 (8%) | 2 (8%) | 1.00 | −0.03/0.87 |
| High triglycerides | N (%) positive | 0 (0%) | 4 (16%) | .12 | 0.19/0.24 |
| Osteoarthritis | N (%) positive | 1 (45%) | 1 (4%) | 1.00 | 0.20/0.21 |
| COPD | N (%) positive | 1 (4%) | 0 (0%) | 1.00 | |
| Depression | N (%) positive | 0 (0%) | 2 (8%) | .48 | 0.01/0.96 |
| Seizures | N (%) positive | 0 (0%) | 1 (4%) | 1.00 | |
| Back spasms | N (%) positive | 1 (4%) | 0 (0%) | 1.00 | |
| BPH | N (%) positive | 0 (0%) | 1 (4%) | 1.00 | |
| Anemia | N (%) positive | 0 (0%) | 1 (4%) | 1.00 | |
| Migraines | N (%) positive | 1 (4%) | 1 (4%) | 1.00 | 0.37/0.02 |
| Outcome Group Means at Baseline and Time Points
| ||||||
|---|---|---|---|---|---|---|
| Acu/Moxa | Baseline | Treatment | 1st Follow-up | 2nd Follow-up | 3rd Follow-up | p value |
| Study phase | ||||||
| GPS | 1.21 ± 0.04 | 0.96 ± 0.09 | 0.88 ± 0.09* | 0.88 ± 0.10+ | 0.85 ± 0.12+ | |
| SPNS Pain | 5.61 ± 0.56 | 4.35 ± 0.71 | 3.84 ± 0.56+ | 4.12 ± 0.62 | 3.56 ± 0.74 | |
| SPNS P&N | 5.91 ± 0.55 | 3.72 ± 0.88 | 4.02 ± 0.69 | 4.01 ± 0.73 | 3.46 ± 0.83 | |
| SPNS Numb | 5.84 ± 0.53 | 4.47 ± 0.78 | 4.21 ± 0.57 | 4.03 ± 0.73 | 3.85 ± 0.78 | |
| SPNS Avg. | 6.43 ± 0.32 | 5.11 ± 0.53 | 4.98 ± 0.45* | 5.10 ± 0.45 | 5.21 ± 0.52 | |
| SPNS Grade | 7.11 ± 0.32 | 5.73 ± 0.55 | 5.49 ± 0.46+ | 5.59 ± 0.46 | 5.70 ± 0.52 | |
| Control | ||||||
| GPS | 1.30 ± 0.04 | 1.12 ± 0.08 | 1.13 ± 0.08* | 1.11 ± 0.07+ | 1.10 ± 0.09+ | .05/.64 |
| SPNS Pain | 5.43 ± 0.50 | 4.40 ± 0.65 | 4.62 ± 0.55+ | 4.51 ± 0.59 | 4.68 ± 0.64 | .22/.42 |
| SPNS P&N | 5.67 ± 0.48 | 4.22 ± 0.73 | 4.07 ± 0.58 | 4.10 ± 0.60 | 3.85 ± 0.67 | .80/.81 |
| SPNS Numb | 5.84 ± 0.47 | 4.86 ± 0.67 | 4.09 ± 0.60 | 4.68 ± 0.58 | 4.87 ± 0.61 | .32/.45 |
| SPNS Avg. | 6.38 ± 0.22 | 5.70 ± 0.45 | 5.32 ± 0.34* | 5.54 ± 0.35 | 5.69 ± 0.40 | .17/.34 |
| SPNS Grade | 7.32 ± 0.20 | 6.31 ± 0.47 | 5.97 ± 0.32+ | 6.01 ± 0.34 | 6.15 ± 0.38 | .32/.46 |
Note: CMV = cytomegalovirus; High BP = high blood pressure; COPD = chronic obstructive pulmonary disease; BPH = benign prostatic hyperplasia; Afr. Am = African American; GPS = Gracely Pain Scale; SPNS = Subjective Peripheral Neuropathy Screen; SPSN Pain = Subjective Peripheral Neuropathy Screen Pain; SPNS P&N = Subjective Peripheral Neuropathy Screen Pins and Needles; SPNS Numb = Subjective Peripheral Neuropathy Screen Numbness; SPNS Avg = Subjective Peripheral Neuropathy Screen Average Severity Score; SPNS Grade = Subjective Peripheral Neuropathy Screen Clinical Severity Grade. Group means ± standard error shown. Bolded values are statistically different from baseline: p < .05, Group means at same time with plus (1) statistically differ with p < .10 and with asterisk (*) statistically differ with p < .05. p value for fixed effect of group/p value for fixed effect of group-by-time interaction.
Chi-square or Fisher’s Exact Test for categorical measures and Student’s independent t test for continuous variables.
r/p value for Pearson correlation between baseline and Gracely Pain Scale difference from baseline to the end of third follow-up.
The SPNS Average pain score showed improvement from baseline only in the Acu/Moxa group and showed superiority to Sham/Placebo at first follow-up. The SPNS Grade showed a trend toward the superiority of Acu/Moxa at first follow-up after treatment, but both groups showed parallel improvement relative to baseline. The results for the SPNS specific symptoms of pain, pins and needles, and numbness showed the Acu/Moxa group to be improved relative to baseline at first, second, and third follow-ups after treatment. A “per protocol” analysis of the outcomes, after restricting the sample to those 42 subjects completing treatment phase and providing follow-up data, showed between-group differences reported above increased only marginally in the GPS and SPNS Average and specific symptom scores.
Results from Credibility Assessment were as follows. At the second follow-up visit, 17 of 20 (85%) Acu/Moxa treatment subjects and 20 of 23 (86%) Sham/Placebo subjects reported being confident they received true Acu/Moxa treatment (p = 1.00). At the third follow-up, 16 of 20 (80%) Acu/Moxa and 18 of 22 (82%) Sham/Placebo reported confidence they received true treatment (p = 1.00). No adverse events of greater than mild severity (i.e., bruising at site of needle insertion) occurred during the conduct of this study.
Discussion
The participants in this study began with pain severity one to two levels more severe than moderate: barely strong to slightly intense. The reduction in the GPS score for the Acu/Moxa group was 0.25 points (reduced by two pain levels) immediately following 6 weeks of twice weekly treatment sessions and 0.36 points (reduced by three pain levels) at the third follow-up visit, after 9 weeks without further treatment: almost twice the reduction observed in the Sham/Placebo of 0.18 and 0.20 (reduced by one pain level), respectively. The benefit of Acu/Moxa was superior to Sham/Placebo at the first follow-up visit (p < .05), 3 weeks after the cessation of treatment, and retained a trend toward superiority at the second and third follow-up visits (p < .10). This pattern suggests that Acu/Moxa not only provided a reduction of pain during treatment but also provided relief for the duration of the nontreatment follow-up phase studied here.
Limitations
This study was conducted under the National Institute of Health R21 pilot study funding mechanism to provide data necessary for the design of a larger prospective clinical trial. As such, this study was limited by a small sample size. The level of DSP pain required by the eligibility criteria may be greater or lesser than the average level of pain experienced by persons living with HIV. The DSP pain level chosen was similar to that instituted in other studies. Our study utilized a standardized regimen of acupuncture/moxibustion points based on a conservative interpretation of the TCM diagnoses related to peripheral sensory abnormalities and was designed to be broadly applicable. In common TCM practice, diagnoses lead to patient-specific treatment prescriptions, and those prescriptions change over the course of treatment in response to the changing symptoms and diagnostic profile. Thus, while our chosen Acu/Moxa regimen facilitated manualization and training for practitioners interested in replicating these results, current practitioners may criticize the restrictive regimen as being unable to deliver the full benefit realizable by a more individualized approach.
Strengths
The masking procedures used for the Sham/Placebo condition were effective as nearly all participants in this group reported confidence they received true Acu/Moxa. All study participants were blindfolded for each session reducing the abilities of the participants to observe treatment procedures. Hence, study participants only had the sensory experience of needle insertion and moxibustion administration, minimizing other potential nonspecific effects. We also developed and implemented strict training and testing for our acupuncturists in administering both the Acu/Moxa and Sham/Placebo procedures. The Sham points used for the study were nonactive points, anatomically specific, well defined, and diagrammed in the procedure manual. A study facilitator monitored all sessions to ensure adherence and fidelity to the protocol.
Summary
Acu/Moxa shows promise in serving as a noninvasive therapy to manage the pain associated with DSP. Pharmacologic treatments commonly prescribed to manage DSP symptoms have not been shown to be superior to placebo in randomized controlled trials. Additionally, there are no U.S. Food and Drug Administration-approved agents to treat pain associated with DSP in HIV. A critical need exists to develop effective translational therapies to improve symptoms of DSP in HIV.
Acknowledgments
This study was funded by Grant No. 1R21AT003092 from the National Institutes of Health. A special thanks to Ann Chung, Londa Hackett, Gloria Rosenzweig, Lorena Gonzalez, Jessica Quinn, and Richard Hammerschlag for their assistance in this study.
Footnotes
Disclosures
The authors report no real or perceived vested interests that relate to this article that could be construed as a conflict of interest.
Contributor Information
Joyce K. Anastasi, Division of Special Studies in Symptom Management, New York University College of Nursing, New York, New York, USA.
Bernadette Capili, Division of Special Studies in Symptom Management, New York University College of Nursing, New York, New York, USA.
Donald J. McMahon, College of Physicians and Surgeons, Columbia University, Division of Endocrinology, New York, New York, USA.
Colin Scully, New York University College of Nursing, New York, New York, USA.
References
- Anastasi J, Capili B, Chung A, Hammerschlag R. Acupuncture/moxibustion RCT for distal sensory peripheral neuropathy in HIV/AIDS: Rationale, design, methods, procedures and logistics. European Journal of Oriental Medicine. 2010;6(4):40–52. [PMC free article] [PubMed] [Google Scholar]
- Ances B, Ellis R. Dementia and neurocognitive disorders due to HIV-1 infection. Seminars in Neurology. 2007;27(1):86–92. doi: 10.1055/s-2006-956759. [DOI] [PubMed] [Google Scholar]
- Beijing College of Traditional Chinese Medicine. Chinese acupuncture and moxibustion. Beijing, China: Foreign Languages Press; 1987. [Google Scholar]
- Birch S, Hammerschlag R, Trinh K, Zaslawski C. The non-specific effects of acupuncture treatment: When and how to control for them. Clinical Acupuncture and Oriental Medicine. 2002;3(1):20–25. [Google Scholar]
- Borkovec TD, Nau SD. Credibility of analogue therapy rationales. Journal of Behavioral Therapy & Experimental Pyschiatry. 1972;3(4):257–260. http://dx.doi.org/10.1016/0005-7916(72)90045-6. [Google Scholar]
- Centers for Disease Control and Prevention. Statement concerning clinical trial of acupuncture and amitriptyline for pain relief. 1993 Retrieved from http://ww1.aegis.org/pubs/Cdc_Fact_Sheets/1993/CDC93154.html.
- Cornblath D, Hoke A. Recent advances in HIV neuropathy. Current Opinion in Neurology. 2006;19(5):446–450. doi: 10.1097/01.wco.0000245366.59446.57. http://dx.doi.org/10.1097/01.wco.0000245366.59446.57. [DOI] [PubMed] [Google Scholar]
- Dworkin R, O’Connor A, Audette J, Baron R, Gourlay G, Haanpää M, Wells C. Recommendations for the pharmacological management of neuropathic pain: An overview and literature update. Mayo Clinic Proceedings. 2010;85(Suppl. 3):S3–S14. doi: 10.4065/mcp.2009.0649. http://dx.doi.org/10.4065/mcp.2009.0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R, Rosario D, Clifford D, McArthur J, Simpson D, Alexander T, Grant I. Continued high prevalence and adverse clinical impact of human immunodeficiency virus-associated sensory neuropathy in the era of combination antiretroviral therapy. Archives in Neurology. 2010;67(5):552–558. doi: 10.1001/archneurol.2010.76. http://dx.doi.org/10.1001/archneurol.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans S, Ellis R, Chen H, Yeh T, Lee A, Schifitto G, Clifford D. Peripheral neuropathy in HIV: Prevalence and risk factors. AIDS. 2011;25(7):919–928. doi: 10.1097/QAD.0b013e328345889d. http://dx.doi.org/10.1097/QAD.0b013e328345889d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairfield K, Eisenberg D, Davis R, Libman H, Phillips R. Patterns of use, expenditures, and perceived efficacy of complementary and alternative therapies in HIV-infected patients. Archives of Internal Medicine. 1998;158:2257–2264. doi: 10.1001/archinte.158.20.2257. [DOI] [PubMed] [Google Scholar]
- Ferrari S, Vento S, Monaco S, Cavallaro T, Cainelli F, Rizzuto N, Temesgen Z. Human immunodeficiency virus-associated peripheral neuropathies. Mayo Clinic Proceedings. 2006;81(2):213–219. doi: 10.4065/81.2.213. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Duarte A, Cikurel K, Simpson DM. Managing HIV peripheral neuropathy. Current HIV/AIDS Reports. 2007;4(3):114–118. doi: 10.1007/s11904-007-0017-6. http://dx.doi.org/10.1007/s11904-007-0017-6. [DOI] [PubMed] [Google Scholar]
- Gore-Felton C, Vosvick M, Power R, Koopman C, Ashton E, Bachmann MH, Spiegel D. Alternative therapies: A common practice among men and women living with HIV. Journal of the Association of Nurses in AIDS Care. 2003;14(3):17–27. doi: 10.1177/1055329003014003002. http://dx.doi.org/10.1177/1055329003014003002. [DOI] [PubMed] [Google Scholar]
- Hammerschlag R. Methodological and ethical issues in acupuncture research. NIH consensus development conference on acupuncture. 1997:45–49. Retrieved from http://consensus.nih.gov/1997/1997acupuncture107program.pdf.
- Keswani S, Pardoa CA, Cherry CL, Hokea A, McArthur JC. HIV-associated sensory neuropathies. AIDS. 2002;16(16):2105–2117. doi: 10.1097/00002030-200211080-00002. [DOI] [PubMed] [Google Scholar]
- Liangyue D, Yijun G, Shuhui J. Chinese acupuncture and moxibustion. Beijing, China: Foreign Languages Press; 1993. [Google Scholar]
- Littlewood RA, Vanable PA. Complementary and alternative medicine use among HIV+ people: Research synthesis and implications for HIV care. AIDS Care. 2008;20(8):1002–1018. doi: 10.1080/09540120701767216. http://dx.doi.org/10.1080/09540120701767216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur J. The reliability and validity of the subjective peripheral neuropathy screen. Journal of the Association of Nurses in AIDS Care. 1998;9(4):84–94. doi: 10.1016/S1055-3290(98)80048-4. http://dx.doi.org/10.1016/S1055-3290(98)80048-4. [DOI] [PubMed] [Google Scholar]
- McArthur J, Yiannoutsos C, Simpson DM, Adornato BT, Singer EJ, Hollander H, Clifford DB. A phase II trial of nerve growth factor for sensory neuropathy associated with HIV infection. Neurology. 2000;54(5):1080–1088. doi: 10.1212/wnl.54.5.1080. http://dx.doi.org/10.1212/WNL.54.5.1080. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Brew BJ, Nath A. Neurological complications of HIV infection. Lancet Neurology. 2005;4(9):543–555. doi: 10.1016/S1474-4422(05)70165-4. http://dx.doi.org/10.1016/S1474-4422(05)70165-4. [DOI] [PubMed] [Google Scholar]
- Nicholas P, Kemppainen J, Canaval G, Corless I, Sefcik E, Nokes K, Gallagher D. Symptom management and self-care for peripheral neuropathy in HIV/AIDS. AIDS Care. 2007;19(2):179–189. doi: 10.1080/09540120600971083. http://dx.doi.org/10.1080/09540120600971083. [DOI] [PubMed] [Google Scholar]
- Nicholas P, Kemppainen J, Holzmer W, Nokes K, Sanzero E, Corless I, Goodroad B. Self-care management for neuropathy in HIV disease. AIDS Care. 2002;14(6):763–771. doi: 10.1080/0954012021000031831. http://dx.doi.org/10.1080/0954012021000031831. [DOI] [PubMed] [Google Scholar]
- Nicholas P, Voss J, Wantland D, Lindgren T, Huang E, Holzemer W, Bain C. Prevalence, self-care behaviors, and self-care activities for peripheral neuropathy symptoms of HIV/AIDS. Nursing and Health Sciences. 2010;12(1):119–126. doi: 10.1111/j.1442-2018.2009.00505.x. http://dx.doi.org/10.1111/j.1442-2018.2009.00505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schifitto G, McDermott MP, McArthur JC, Marder K, Sacktor N, Epstein L, Kieburtz K. Incidence of and risk factors for HIV-associated distal sensory polyneuropathy. Neurology. 2002;58(12):1764–1768. doi: 10.1212/wnl.58.12.1764. http://dx.doi.org/10.1212/WNL.58.12.1764. [DOI] [PubMed] [Google Scholar]
- Silver M, Blum D, Grainger J, Hammer AE, Quessy S. Double-blind, placebo-controlled trial of lamotrigine in combination with other medications for neuropathic pain. Journal of Pain and Symptom Management. 2007;34(4):446–454. doi: 10.1016/j.jpainsymman.2006.12.015. http://dx.doi.org/10.1016/j.jpainsymman.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Simpson D, Estanislao L, Brown SL, Sampson J. An open-label pilot study of high concentration capsaicin patch in painful HIV neuropathy. Journal of Pain Symptom Mangement. 2008;35(3):299–306. doi: 10.1016/j.jpainsymman.2007.04.015. http://dx.doi.org/10.1016/j.jpainsymman.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Simpson D, Schifitto G, Clifford D, Murphy T, Durso-De Cruz E, Glue P, Freeman R. Pregabalin for painful HIV neuropathy. Neurology. 2010;74(5):413–420. doi: 10.1212/WNL.0b013e3181ccc6ef. http://dx.doi.org/10.1212/WNL.0b013e318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S, Estanislao L, Simpson DM. HIV-associated neuropathic pain: Epidemiology, pathophysiology and management. CNS Drugs. 2005;19(4):325–334. doi: 10.2165/00023210-200519040-00005. [DOI] [PubMed] [Google Scholar]
- Vincent C, Lewith G. Placebo controls for acupuncture studies. Journal of the Royal Society of Medicine. 1995;88(4):199–202. [PMC free article] [PubMed] [Google Scholar]


