Publisher's Note: There is an Inside Blood Commentary on this article in this issue.
Key Points
Enasidenib, a selective inhibitor of mutant IDH2 enzymes, was safe and well tolerated in patients with IDH2-mutated myeloid malignancies.
Enasidenib induced hematologic responses in patients with relapsed/refractory AML in this dose-escalation and expansion study.
Abstract
Recurrent mutations in isocitrate dehydrogenase 2 (IDH2) occur in ∼12% of patients with acute myeloid leukemia (AML). Mutated IDH2 proteins neomorphically synthesize 2-hydroxyglutarate resulting in DNA and histone hypermethylation, which leads to blocked cellular differentiation. Enasidenib (AG-221/CC-90007) is a first-in-class, oral, selective inhibitor of mutant-IDH2 enzymes. This first-in-human phase 1/2 study assessed the maximum tolerated dose (MTD), pharmacokinetic and pharmacodynamic profiles, safety, and clinical activity of enasidenib in patients with mutant-IDH2 advanced myeloid malignancies. We assessed safety outcomes for all patients and clinical efficacy in the largest patient subgroup, those with relapsed or refractory AML, from the phase 1 dose-escalation and expansion phases of the study. In the dose-escalation phase, an MTD was not reached at doses ranging from 50 to 650 mg per day. Enasidenib 100 mg once daily was selected for the expansion phase on the basis of pharmacokinetic and pharmacodynamic profiles and demonstrated efficacy. Grade 3 to 4 enasidenib-related adverse events included indirect hyperbilirubinemia (12%) and IDH-inhibitor–associated differentiation syndrome (7%). Among patients with relapsed or refractory AML, overall response rate was 40.3%, with a median response duration of 5.8 months. Responses were associated with cellular differentiation and maturation, typically without evidence of aplasia. Median overall survival among relapsed/refractory patients was 9.3 months, and for the 34 patients (19.3%) who attained complete remission, overall survival was 19.7 months. Continuous daily enasidenib treatment was generally well tolerated and induced hematologic responses in patients for whom prior AML therapy had failed. Inducing differentiation of myeloblasts, not cytotoxicity, seems to drive the clinical efficacy of enasidenib. This trial was registered at www.clinicaltrials.gov as #NCT01915498.
Introduction
Advances in the treatment of acute myeloid leukemia (AML) over the past 4 decades have been limited. In younger adults with de novo AML, overall survival at 5 years is 40% to 50%.1 Prognosis is significantly worse for older patients, those with secondary AML evolved from antecedent myeloid neoplasms, and those with relapsed or refractory disease, who have a 5-year survival rate of only 5% to 10%.1-3 However, an increasing understanding of molecular aberrations that trigger the development of AML, and growing use of next-generation sequencing, are advancing the development of investigational drugs against potential driver mutations in AML.
Recurrent mutations in the isocitrate dehydrogenase 1 and 2 genes (IDH1, IDH2) are found in myeloid malignancies.4,5 Enasidenib (AG-221/CC-90007) and ivosidenib (AG-120) are first-in-class, oral, selective, small-molecule inhibitors of IDH2- and IDH1-mutant enzymes, respectively. IDH2 mutations are relatively common in hematologic malignancies, occurring in ∼12% of patients with AML,6,7 are enriched in patients with normal karyotype, and increase in frequency with age.8-10
IDH2 enzymes catalyze conversion of isocitrate to α-ketoglutarate (αKG) in the mitochondria. Mutations within the conserved enzymatic active sites, R140 and R172, produce neomorphic activity, reducing αKG to R-2-hydroxyglutarate (R-2-HG).7 In preclinical models, increased levels of R-2-HG competitively inhibit αKG-dependent enzymes, leading to histone and DNA hypermethylation, chromatin modifications, and altered hypoxia responses.11-13 High R-2-HG concentrations are associated with differentiation arrest of hematopoietic cells in vivo,14 and R-2-HG levels are substantially increased in the sera of patients with mutant-IDH2 malignancies.15-17
In preclinical studies, enasidenib-induced inhibition of aberrant IDH2 protein decreased total serum 2-HG by more than 90%, reduced abnormal histone hypermethylation, and restored myeloid differentiation.18-20 Enasidenib was also associated with a dose-dependent survival advantage in a primary AML xenotransplant model.20 Enasidenib seems to act as a differentiation agent and is not cytotoxic. Bone marrow blasts from patients with mutant-IDH2 AML exposed to enasidenib ex vivo produce mature, fully functioning neutrophils with conserved mutant IDH2 allele frequency, indicating that the mature cells evolved from the mutant IDH2 blasts.20 Moreover, no apoptosis was observed in mutant-IDH2-R140 erythroleukemia (TF-1) cells treated with enasidenib for 7 days in vitro.20
This first-in-human, phase 1/2 study assessed the maximum tolerated dose (MTD), pharmacokinetic and pharmacodynamic profiles, safety, and clinical activity of enasidenib in patients with mutant-IDH2 advanced myeloid malignancies (NCT01915498; Phase 1/2 Study of AG-221 in Subjects With Advanced Hematologic Malignancies With an IDH2 Mutation). We report an overview of the pharmacokinetic and pharmacodynamic profiles of enasidenib, safety outcomes for all patients in the dose-escalation and phase 1 expansion portions of this study, and clinical efficacy in patients with relapsed/refractory AML, the largest subgroup of patients in this study.
Methods
This study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines. The trial was designed and monitored by the sponsors, Agios Pharmaceuticals, Inc. (Cambridge, MA) and Celgene Corporation (Summit, NJ), along with the study investigators. The protocol was approved by local human investigations committees at participating sites. Written informed consent was obtained from all patients before screening.
Patients
Patients age 18 years or older with mutant-IDH2 advanced myeloid malignancies and Eastern Cooperative Oncology Group performance status of 0 to 2 were eligible. Patients had a confirmed diagnosis (per World Health Organization 2008 classification21) of AML or of myelodysplastic syndromes with refractory anemia with excess blasts. IDH2 mutational status was evaluated locally at screening. Retrospective central review with investigational use of the Abbott RealTime IDH2 polymerase chain reaction–based assay and Abbott m2000rt technology (Abbott Molecular, Des Plaines, IL) showed high concordance with local assessments.
Study design
The primary objective was to determine the safety and MTD of enasidenib. Secondary objectives included characterizing the pharmacokinetic and pharmacodynamic profiles and clinical activity of enasidenib.
Dose-limiting toxicities (DLTs) were evaluated during treatment cycle 1. A single enasidenib dose was administered on day −3, followed by a 2-day pharmacokinetic evaluation period. Enasidenib was then administered orally once or twice per day in continuous 28-day cycles.
Dose-escalation was followed by an expansion phase comprising 4 cohorts of patients with IDH2 mutations, including those age 60 years or older with relapsed/refractory AML, or any age if they relapsed after hematopoietic cell transplantation; age younger than 60 years with relapsed/refractory AML and no prior transplantation; age 60 years or older with untreated AML and ineligible for induction chemotherapy; or ineligible for other expansion arms.
Study assessments
For the first 3 patients enrolled in a cohort during the dose-escalation phase of the study and the first 15 patients enrolled in each arm of the expansion phase, a single dose of enasidenib was administered on day −3 (ie, 3 days before the scheduled cycle 1 day 1 dose). Blood samples were drawn before single-dose administration of enasidenib (within 30 minutes) and at the following time points after administration for determining concentration-time profiles of enasidenib: 30 (±10) minutes and 1, 2, 3, 4, 6, 8, and 10 hours (±10 minutes), and 24, 48, and 72 hours (±1 hour) postdose. After 72 hours of blood sample collection, patients began continuous oral daily dosing of enasidenib on cycle 1 day 1. Pharmacokinetic parameters were determined by using noncompartmental analysis (NCA) methods.
In the dose-escalation phase, 5 enasidenib dose levels were administered twice per day (30, 50, 75, 100, and 150 mg), and 8 dose levels were administered once per day (50, 75, 100, 150, 200, 300, 450, and 650 mg). Serial blood sampling for assessment of 2-HG concentrations was performed predose in cycle 1 on days 1, 8, and 15; in cycles 2 and 3 on days 1 and 15; and on day 1 of cycle 4 and every cycle thereafter. The potential relationship between plasma levels of enasidenib and 2-HG was assessed by using WinNonLin software (Pharsight, Mountain View, CA).
Treatment-emergent adverse events (TEAEs), defined as events that began or worsened between the first enasidenib dose and 28 days after the last dose, were graded by using Common Terminology Criteria for Adverse Events, version 4.0. Serious TEAEs were those that were life-threatening, resulted in death, required hospitalization, or caused significant incapacity.
Efficacy outcomes are reported for the subgroup of patients with relapsed/refractory AML. Hematologic response was determined by investigator review of peripheral blood and bone marrow samples in accordance with modified 2003 International Working Group criteria for AML.22 Overall response rate (ORR) included complete remission, complete remission with incomplete hematologic or platelet recovery, partial remission, and morphologic leukemia-free state. Overall survival was defined as the time from first enasidenib dose to death by any cause. Event-free survival comprised the interval between first enasidenib dose and relapse (≥5% bone marrow blasts, reappearance of blasts in blood, or development of extramedullary disease), disease progression, or death.
Statistical analysis
Dose-escalation was performed by using a standard 3+3 design. If 2 or more patients within a 6-person cohort experienced a DLT, the previous dose was considered the MTD. Planned enrollment in the expansion phase was 25 patients in each of the 4 arms to provide a 93% probability of detecting TEAEs with an underlying rate of 10%, and a 72% probability of detecting TEAEs with an underlying rate of 5%. The 95% confidence intervals (CIs) for hematologic response are from a 2-sided exact binomial test. Overall survival and 1-year survival rates were estimated by using Kaplan-Meier methods.
Results
Between September 20, 2013, and April 15, 2016, 239 patients (113 in the dose-escalation phase and 126 in the 4-arm expansion phase) received enasidenib and comprised the intention-to-treat population. At data cutoff, 31 patients (13%) continued to receive enasidenib on-study, with median follow-up of 9.7 months (range, 3.7-20.8 months) (supplemental Figure 1, available on the Blood Web site). Baseline characteristics are provided in Table 1. Three-fourths of all patients had IDH2-R140 mutations and one-fourth had IDH2-R172 mutations. Of 175 patients with available cytogenetic data, 67% had National Comprehensive Cancer Network–defined23 intermediate-risk cytogenetics, including 43% with normal karyotype. The relapsed/refractory AML efficacy cohort comprised 176 patients (74% of all patients). At study entry, 94 patients (53%) had received 2 or more prior AML-directed regimens.
Table 1.
Baseline demographic and disease characteristics for patients with relapsed or refractory AML and all patients in the dose-escalation and expansion study phases
| Characteristic | Relapsed or refractory AML | All patients (N = 239) | ||||
|---|---|---|---|---|---|---|
| Enasidenib 100 mg per day (n = 109) | All doses (n = 176) | |||||
| No. | % | No. | % | No. | % | |
| Age, median (range), y | 67 (19-100) | 67 (19-100) | 70 (19-100) | |||
| Sex | ||||||
| Male | 46 | 42 | 90 | 51 | 137 | 57 |
| Female | 63 | 58 | 86 | 49 | 102 | 43 |
| AML classification* | 213 | 89 | ||||
| Myelodysplasia-related changes | 27 | 25 | 45 | 26 | 57 | 27 |
| Recurrent genetic abnormalities | 7 | 6 | 15 | 9 | 17 | 8 |
| Therapy-related myeloid neoplasms | 1 | <1 | 2 | 1 | 4 | 2 |
| Not otherwise specified | 62 | 57 | 91 | 52 | 111 | 52 |
| Missing† | 12 | 11 | 23 | 13 | 24 | 11 |
| Outcomes of prior AML therapyठ| ||||||
| Refractory to initial induction or re-induction treatment | 35 | 32 | 57 | 32 | — | |
| Relapsed/refractory to ≥2 cycles of first-line lower-intensity therapy§ | 25 | 23 | 43 | 24 | — | |
| Relapsed within 1 y of initial treatment | 27 | 25 | 41 | 23 | — | |
| Relapsed posttransplant | 12 | 11 | 24 | 14 | — | |
| In second or later relapse | 13 | 12 | 22 | 13 | — | |
| Relapsed >1 y after initial treatment | 8 | 7 | 15 | 9 | — | |
| Cytogenetic risk status | 80 | 73 | 128 | 73 | 175 | 73 |
| Intermediate | 51 | 64 | 85 | 66 | 117 | 67 |
| Poor | 29 | 36 | 43 | 34 | 58 | 33 |
| Co-occurring mutations | 47 | 43 | 100 | 57 | 134 | 56 |
| NPM1 | 8 | 17 | 16 | 16 | 17 | 13 |
| CEBPA | 1 | 2 | 6 | 6 | 10 | 8 |
| FLT3-ITD | 1 | 2 | 4 | 4 | 4 | 3 |
| Antecedent history of myelodysplastic syndromes | 17 | 16 | 30 | 17 | 30 | 13 |
| Prior stem cell transplantation | 12 | 11 | 24 | 14 | 28 | 12 |
| ECOG performance status|| | ||||||
| 0 | 25 | 23 | 39 | 22 | 55 | 23 |
| 1 | 68 | 62 | 106 | 60 | 139 | 58 |
| 2 | 16 | 15 | 31 | 18 | 45 | 19 |
| IDH2 mutation location | ||||||
| R140 | 83 | 76 | 130 | 74 | 179 | 75 |
| R172 | 25 | 23 | 45 | 26 | 57 | 24 |
| Other¶ | 1 | <1 | 1 | <1 | 3 | 1 |
| Bone marrow blasts, median (range), %# | 49 (0-96) | 49 (0-98) | 41 (0**-98) | |||
| Hematology, median (range) | ||||||
| WBC, ×109/L | 3.0 (0.2-88) | 2.7 (0.2-88) | 2.6 (0.2-88) | |||
| Hemoglobin, g/dL | 9.3 (6.9-13.8) | 9.2 (6.9-13.8) | 9.1 (6.9-15.2) | |||
| Platelets, ×109/L | 39 (1-372) | 44 (1-507) | 45 (1-644) | |||
ECOG, Eastern Cooperative Oncology Group; WBC, white blood cell count.
Per World Health Organization (WHO) 2008 AML classifications of myeloid neoplasms.21
During the study, the protocol was amended to determine AML subtypes according to the WHO classification scheme (AML classification was previously determined by French-American-British criteria).
Prior (initial) AML treatment of relapsed/refractory patients.
Individual patients may be counted in more than 1 category.
ECOG performance status scores of 0, 1, or 2 (on a scale from 0 to 5, with 0 indicating that the patient is fully active and higher numbers indicating greater disability).
One patient had an IDH2 G145fs mutation, and mutation site was not reported for 2 patients.
Local assessment.
Nine patients had extramedullary disease only at relapse.
Pharmacokinetics and pharmacodynamics
Enasidenib demonstrated high dose–proportional plasma exposure and an extended half-life (∼137 hours) after multiple doses, reaching steady state by cycle 2 day 1. A 100 mg once-per-day dose was chosen for the study expansion on the basis of robust steady-state drug concentrations, plasma 2-HG inhibition, and clinical activity. By cycle 2 day 1, enasidenib 100 mg per day reduced plasma 2-HG levels from baseline by a median of 93% and a maximum of 99% in patients with IDH2-R140Q mutations and by 28% and 94% in patients with IDH2-R172K mutations (supplemental Figure 2). No dose-dependent 2-HG inhibition was observed among doses below, at, or above 100 mg. Median 2-HG suppression from baseline was 92.4%, 90.4%, and 93.1% for those receiving enasidenib doses of less than 100 mg, 100 mg, and greater than 100 mg, respectively.
Safety
The safety-evaluable population comprised all 239 patients. Enasidenib doses of 50 mg to 650 mg per day were evaluated. The median number of enasidenib treatment cycles received was 5.0 (range, 1-25 cycles). Enasidenib was generally well tolerated; the MTD was not reached at doses of up to 650 mg once per day. Prolonged dosing with enasidenib 650 mg once per day was not well tolerated; 5 of 7 patients in this dose group had a dose reduction or modification as a result of treatment-related TEAEs that did not qualify as DLTs (supplemental Table 1).
Nearly all patients (n = 238) experienced a TEAE, and 195 patients (82%) experienced a treatment-related TEAE. The most common treatment-related TEAEs were indirect hyperbilirubinemia (38%) and nausea (23%) (supplemental Table 2); these were also the most common TEAEs regardless of cause (supplemental Table 3). Enasidenib-related grade 3 to 4 TEAEs occurred in 99 patients (41%), the most common being indirect hyperbilirubinemia (12%) and IDH-inhibitor–associated differentiation syndrome (IDH-DS) (6%) (the preferred term was “retinoic acid syndrome” because IDH-DS was not an established Medical Dictionary for Regulatory Activities preferred term when this study was conducted) (Table 2). Grade 3 to 4 treatment-related hematologic TEAEs and infections occurred in 10% and 1% of patients, respectively. Treatment-related serious TEAEs occurred in 58 patients (24%), the most common being IDH-DS (8%), leukocytosis (4%), tumor lysis syndrome (3%), nausea (2%), and hyperbilirubinemia (2%). Enasidenib-related TEAEs led to dose modification, interruption, or discontinuation for 7%, 22%, and 5% of patients, respectively. Supplemental Table 4 shows treatment-related TEAEs by daily enasidenib dose in the dose-escalation phase of this study.
Table 2.
Treatment-related TEAEs of grades 3 or 4 occurring in ≥2% of all patients
| TEAE | Enasidenib 100 mg per day (n = 153) | All patients (N = 239) | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| Hyperbilirubinemia* | 13 | 8 | 29 | 12 |
| IDH differentiation syndrome† | 11 | 7 | 15 | 6 |
| Anemia | 10 | 7 | 12 | 5 |
| Thrombocytopenia‡ | 8 | 5 | 15 | 6 |
| Tumor lysis syndrome | 5 | 3 | 8 | 3 |
| Decreased appetite | 3 | 2 | 6 | 3 |
| Leukocytosis | 2 | 1 | 6 | 3 |
| Fatigue | 2 | 1 | 6 | 3 |
| Nausea | 2 | 1 | 5 | 2 |
| Lipase increased | 2 | 1 | 5 | 2 |
A treatment-related TEAE was defined as any event that began or worsened on or after the start of enasidenib use until 28 days after the last dose and was considered by the treating physician to be possibly or probably related to enasidenib. TEAEs were coded by using the Medical Dictionary for Regulatory Activities, version 16.0.
Includes the preferred terms “hyperbilirubinemia” and “blood bilirubin increased.”
Preferred term is “retinoic acid syndrome.”
Includes the preferred terms “thrombocytopenia” and “platelet count decreased.”
IDH-DS occurred in 23 patients, 15 of whom had grade 3 to 4 IDH-DS. Median time to onset was 48 days (range, 10-340 days). IDH-DS was managed with systemic corticosteroids for 19 of 23 patients. Enasidenib dosing was interrupted for 10 patients with IDH-DS, but permanent drug discontinuation was not required (supplemental Table 5 further describes IDH-DS signs, symptoms, and management). Serious IDH-DS was reported for 18 patients; episodes resolved without sequelae for 16 of the 18 patients. A 62-year-old male died of staphylococcal sepsis, contracted while recovering from grade 3 IDH-DS, and an 83-year-old female developed pericardial effusion with increasing white blood cell and peripheral blast counts, and she died as a result of cardiac tamponade; retrospective review suggested death may have been triggered by IDH-DS.
Enasidenib may be associated with rapid myeloid proliferation, presenting as leukocytosis.24 Non–dose-dependent, non-infectious leukocytosis events (any cause) were reported for 41 patients (17%), primarily within the first 2 cycles. Leukocytosis was not necessarily accompanied by IDH-DS. Treatment-related leukocytosis was reported for 15 patients (6%), leading to study discontinuation by 1 patient and dose interruption for 6 patients. Grade 3 to 4 enasidenib-related leukocytosis occurred within the first 2 treatment cycles for 5 of 6 patients, 4 of whom showed hematologic evidence of myeloblast differentiation.
Enasidenib-induced indirect hyperbilirubinemia occurred in 35% of patients. Bilirubin increases did not seem to signal intrinsic liver toxicity, because there were no clinically meaningful alanine aminotransferase or aspartate aminotransferase increases over time in any patient. Increases may be the result of off-target inhibition of the UGT1A1 enzyme responsible for bilirubin metabolism (data on file; Agios Pharmaceuticals, Inc.), an effect similar to that of congenital UGT1A1 deficiency (eg, Gilbert syndrome).
IDH-DS, leukocytosis, and hyperbilirubinemia generally decreased in frequency as enasidenib treatment continued (supplemental Table 6).
Efficacy in relapsed/refractory AML
Response
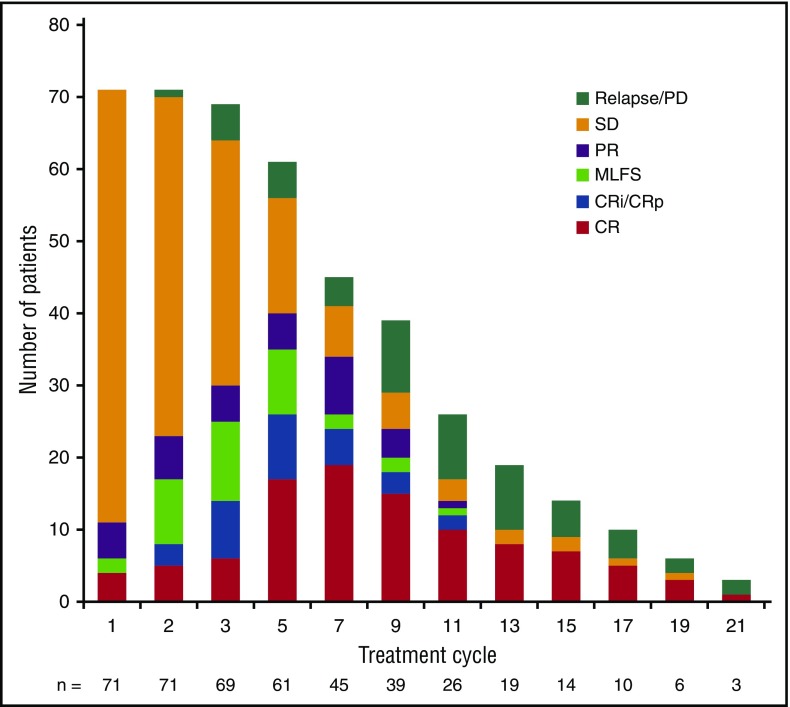
Thirty-four patients (19.3%) with relapsed/refractory AML attained complete remission. The ORR for all relapsed/refractory AML patients was 40.3% (95% CI, 33.0%-48.0%) (Table 3). Median time to first response was 1.9 months (range, 0.5-9.4 months); 87.3% of responding patients attained a first response by cycle 5. Of 34 patients who achieved complete remission, 7 (20.6%) did so by cycle 3, 23 (67.6%) by cycle 5, and 28 (82.4%) by cycle 7. Figure 1 shows the evolution of response at each treatment cycle among the 71 patients with relapsed/refractory AML who responded on-study. The ORR for patients treated with enasidenib 100 mg per day (n = 109) was 38.5%. Seventeen patients (10%) discontinued enasidenib treatment to proceed to stem cell transplant.
Table 3.
Investigator-reported hematologic response, time to response, and response duration among patients with relapsed/refractory AML treated with enasidenib
| Response | Relapsed or refractory AML | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Enasidenib 100 mg per day (n = 109) | All doses (N = 176) | |||||||||
| No. | % | 95% CI | Median | Range | No. | % | 95% CI | Median | Range | |
| ORR*† | 42 | 38.5 | 29.4-48.3 | 71 | 40.3 | 33.0-48.0 | ||||
| Best response | ||||||||||
| CR | 22 | 20.2 | 13.1-28.9 | 34 | 19.3 | 13.8-25.9 | ||||
| CR with incomplete hematologic recovery/CR with incomplete platelet recovery | 7 | 6.4 | 12 | 6.8 | ||||||
| Partial remission | 3 | 2.8 | 11 | 6.3 | ||||||
| Morphologic leukemia-free state | 10 | 9.2 | 14 | 8.0 | ||||||
| Stable disease‡ | 58 | 53.2 | 85 | 48.3 | ||||||
| Progressive disease§ | 5 | 4.6 | 9 | 5.1 | ||||||
| Not evaluable | 2 | 1.8 | 3 | 1. 7 | ||||||
| Time to first response, mo | 1.9 | 0.5-9.4 | 1.9 | 0.5-9.4 | ||||||
| Duration of response, mo | 3.8-9.7 | 5.6 | 3.9-7.4 | 5.8 | ||||||
| Time to CR, mo | 3.7 | 0.7-11.2 | 3.8 | 0.5-11.2 | ||||||
| Duration of response in patients who attained CR, mo || | 5.3-NR | 8.8 | 6.4-NR | 8.8 | ||||||
Responses were evaluated by study investigators and classified according to the 2003 revised International Working Group criteria for AML22
CR, complete remission; NR, not reached.
ORR includes patients with CR, CR with incomplete hematologic recovery, CR with incomplete platelet recovery, partial remission, or morphologic leukemia-free state.
Of 9 patients with extramedullary disease at study entry, 1 patient achieved CR with incomplete platelet recovery, 5 patients maintained stable disease, 1 patient experienced disease progression, and 2 patients were not evaluable for response.
Stable disease was defined as failure to achieve a response but not meeting criteria for disease progression for a period of more than 8 weeks.
Patients must have had previous partial remission or stable disease. For patients with 5% to 66% bone marrow blasts at nadir, progressive disease was defined as a >50% increase in bone marrow blast count percentage from the nadir and a blast percentage of ≥20%; for patients with ≥67% bone marrow blasts at nadir, progressive disease was defined as a doubling of the nadir absolute peripheral blood blast count with a final absolute peripheral blood blast count of >10 × 109/L.
Date of first documented response to date of relapse, disease progression, or death.
Figure 1.
Evolution of response during treatment of responding patients (n = 71). Bars reflect responses at each cycle. CR, complete response; CRi, CR with incomplete hematologic recovery; CRp, CR with incomplete platelet recovery; MLFS, morphologic leukemia-free state; PD, progressive disease; PR, partial response; SD, stable disease.
The ORR for patients with IDH2-R140 mutations was 35.4% (95% CI, 27.2%-44.2%) and for those with IDH2-R172 mutations, ORR was 53.3% (95% CI, 37.9%-68.3%). Rates of complete remission were 17.7% (95% CI, 11.6%-25.4%) in patients with IDH2-R140 mutations and 24.4% (95% CI, 12.9%-39.5%) for patients with IDH2-R172 mutations. The extent of 2-HG suppression from baseline at cycle 2 day 1 was not correlated with clinical response among all patients or within either site-mutation subgroup. Clinical activity was observed at all enasidenib doses (50-650 mg per day) (supplemental Table 7). Eighty-five patients (48.3%) maintained stable disease as best outcome on-study for a median of 4 treatment cycles (range, 1-23 cycles).
Nine patients had extramedullary disease at study entry. Among these patients, 1 patient achieved complete remission with incomplete platelet recovery, 5 patients maintained stable disease, 1 patient had only disease progression, and 2 patients were not evaluable for response (for 1 patient, blast counts remained between 1% and 2% at baseline and at all efficacy assessments; the second patient died on study day 11 and thus was never evaluated for response; however, the patient was included in survival analyses).
Bone marrow effects.
Bone marrow aspirates in clinical responders showed reduced myeloblasts and the morphologic appearance of mature myeloid forms with a normal immunophenotype (Figure 2A). Myeloid differentiation and trilineage hematopoietic recovery was observed, typically without intervening marrow aplasia or hypoplasia. Hematologic changes for patients with relapsed/refractory AML are shown in Figure 3. Mature granulocytes from patients in remission (Figure 2B) retained the IDH2 mutation and cytogenetic abnormalities, indicating that maturing cells evolved from the abnormal myeloblast population rather than from nonmalignant cells.
Figure 2.
Morphologic evidence of myeloid differentiation during enasidenib treatment. (A) Bone marrow (BM) blasts at screening (left). By cycle 3 day 1 (right), maturing forms including promyelocytes and myelocytes have largely replaced the immature myeloblasts, without initial marrow aplasia or hypoplasia at cycle 1 day 15 (middle). (B) Fluorescence in situ hybridization evidence of myeloid differentiation during enasidenib treatment. At screening, this patient with an IDH2-R140Q mutation had trisomy 8 in the majority of myeloblasts. By cycle 2 day 1, mature forms appeared with persistence of trisomy 8 in promyelocytes and mature granulocytes. In contrast, cells in the lymphoid compartment have a normal complement of chromosome 8.
Figure 3.
Mean (± standard error) platelet count, absolute neutrophil count, hemoglobin, and bone marrow blasts over time in patients with relapsed/refractory AML treated with enasidenib.
Survival.
At a median follow-up of 7.7 months (range, 0.4-26.7 months), median overall survival among patients with relapsed/refractory AML was 9.3 months (95% CI, 8.2-10.9 months) and estimated 1-year survival was 39%. Patients who attained complete or partial remission had a median survival of 19.7 months (95% CI, 11.6 months to not reached) or 14.4 months (95% CI, 7.5-26.7 months), respectively (Figure 4). For patients who had received 2 or more prior AML regimens, median survival was 8.0 months (95% CI, 5.9-9.0 months). Median event-free survival duration was 6.4 months (95% CI, 5.4-7.5 months). Thirty- and 60-day mortality rates were 5.1% and 13.1%, respectively.
Figure 4.
Overall survival. (A) Overall survival among all patients with relapsed/refractory (R/R) AML. (B) Overall survival among patients with relapsed/refractory AML in complete remission (CR), patients with a non-CR hematologic response, or no response. NE, not evaluated.
Discussion
AML is characterized by rapid proliferation of myeloid progenitors that fail to achieve multilineage differentiation.25 Despite decades of clinical investigation, the primary therapeutic approach is still intensive cytotoxic induction and consolidation chemotherapy.23 Patients with relapsed/refractory AML and those ineligible for intensive therapy have few therapeutic options. Our study suggests that continuous daily oral therapy with enasidenib can induce terminal differentiation of leukemic myeloblasts and lead to normal trilineage hematopoiesis in responding patients with mutant-IDH2 relapsed/refractory AML, most of whom had received multiple prior AML-directed treatments.
Enasidenib was well tolerated by these primarily older patients with mutant-IDH2 advanced myeloid malignancies. Although the pharmacokinetic profile, 2-HG reductions, and clinical efficacy support further investigation of a 100 mg once-per-day enasidenib dose, the MTD was not reached at doses of up to 650 mg per day. Because enasidenib is not myeloablative, patients with mutant-IDH2 hematologic malignancies may be spared the degree of hematologic toxicity associated with intensive treatment. In this study, only 5% of patients discontinued therapy as a result of a treatment-related TEAE. Enasidenib is not associated with bone marrow aplasia and susceptibility to severe infections seen with standard cytotoxic agents. Rates of enasidenib-related grade 3 to 4 hematologic TEAEs (10%) and infections (1%) were low compared with other AML treatments, for which rates can range from 20% to 90%.26-29
IDH-DS was reported for 10% of patients, which is less frequent than rates of differentiation syndrome reported for patients with acute promyelocytic leukemia treated with all-trans retinoic acid (∼25%30).31,32 IDH-DS has also been reported in patients receiving ivosidenib.33 Treatment of IDH-DS with intravenous corticosteroids until improvement may be appropriate for all-trans retinoic acid–related differentiation syndromes.30,33,34 Enasidenib can induce rapid myeloid proliferation, typically with a range of maturing cells in peripheral blood, resulting in rapid increases in white blood cell count, often without co-occurring infection or clinical signs of IDH-DS. Leukocytosis can be treated by initiating hydroxyurea or increasing hydroxyurea dose.33
Overall, 40.3% of patients with relapsed/refractory AML had a hematologic response during enasidenib treatment, including 34 patients with complete remissions and 11 with partial remissions. The median duration of response (5.8 months) with enasidenib may be clinically meaningful for this population, which included older patients in second or later relapse. Ten percent of patients proceeded to transplant, suggesting that enasidenib may provide a bridge to potentially curative treatment. Hematologic responses were attained regardless of IDH2 mutation site. Although suppression of 2-HG is likely required for clinical response, nonresponders also showed reductions in 2-HG. The lack of association between extent of 2-HG suppression and response suggests that additional mechanisms may contribute to the clinical activity of enasidenib that warrant further investigation.
Unlike cytarabine-based regimens, failure to obtain an early response with enasidenib did not presage treatment failure, as first responses were reported several months after beginning enasidenib treatment. Enasidenib, like hypomethylating agents, may produce a response pattern different from that of cytotoxic treatments.35 As with hypomethylating agent–based therapy, in the absence of disease progression, it may be prudent for patients to receive multiple enasidenib treatment cycles to induce or improve response. Notably, cellular differentiation with mutant-IDH inhibitors can cause transient increases in blast percentages that do not necessarily indicate progressive disease.36 The prognostic impact of the persistent mutant IDH2 clone remains unknown, but sustained enasidenib treatment may be necessary to suppress future clonal proliferation.
Median overall survival with enasidenib is especially promising at 9.3 months among all patients with relapsed/refractory AML, and 8.0 months in patients who had received 2 or more previous anti-cancer regimens. In a randomized phase 3 study in which patients with relapsed/refractory AML were treated with 1 or more of 7 available salvage treatments according to the investigator’s choice, median overall survival was only 3.3 months (95% CI, 2.9-4.4 months).37
In conclusion, data from this study showed that single-agent enasidenib was well tolerated, induced hematologic responses, and was associated with a median survival of more than 9 months in patients with relapsed or refractory mutant-IDH2 AML. A multicenter, randomized phase 3 study (NCT02577406; An Efficacy and Safety Study of AG-221 [CC-90007] Versus Conventional Care Regimens in Older Subjects With Late Stage Acute Myeloid Leukemia Harboring an Isocitrate Dehydrogenase 2 Mutation [IDHENTIFY]) is ongoing.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
The authors acknowledge Sheila Truten and Kelly Dittmore of MC2 (Wynnewood, PA), who provided editorial and administrative assistance during manuscript development.
This work was supported by Celgene Corporation.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: The sponsors (Agios Pharmaceuticals, Inc. and Celgene Corporation) collected and analyzed data in conjunction with the authors; E.M.S. prepared the first draft of the manuscript; and all authors helped revise the draft manuscript and reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: E.M.S. received grants and personal fees from Celgene Corporation and Agios Pharmaceuticals, Inc. C.D.D. received personal fees and clinical research support from Agios Pharmaceuticals, Inc. and clinical research support from Celgene Corporation. D.A.P. served on the advisory board for Pfizer, Karyopharm, Celgene Corporation, and Agios Pharmaceuticals, Inc. and received grants from Agios Pharmaceuticals, Inc. A.T.F. consulted for and received clinical trial support from Celgene Corporation and Seattle Genetics; served on advisory boards for Merck, Juno, Tolero, and Bexalata; and received clinical trial support from Takeda and Exelixis. G.J.R. consulted for Agios Pharmaceuticals, Inc., Celgene Corporation, Amgen, Amphivena, Astex, AstraZeneca, Celator, Genoptix, Janssen, Juno, MEI Pharma, MedImmune, Novartis, Onconova, Pfizer, Roche/Genentech, and Sunesis; and received research support from Cellectis. J.K.A. received personal fees from Syros, Janssen Pharmaceuticals, Novartis, Seattle Genetics, Spectrum, Ariad, Bristol-Myers Squibb, and Celgene Corporation; and funds to institution for trial participation from MethylGene Inc., Boehringer Ingelheim, Astellas, Agios Pharmaceuticals, Bristol-Myers Squibb, CSL Limited, Cyclacel, Epizyme, Genentech, Pfizer, BioLineRX, and Talon Therapeutics. R.M.S. served on an advisory board for Agios Pharmaceuticals, Inc., Novartis, Celgene Corporation, AbbVie, Karyopharm, Arog, Jansen, Celator/Jazz, Seattle Genetics, and Roche/Genetech; and served on data and safety monitoring boards for Celgene Corporation and Sunesis. D.J.D. served on the advisory board for Celgene Corporation. R.L.L. received research support from Celgene Corporation and serves on the supervisory board for Qiagen. I.W.F. received grants from Agios Pharmaceuticals, Inc., Acerta, Beigene, Celgene Corporation, Constellation, Curls, FortySeven, Genentech, Gilead, ImmunoGen, Incyte, Infinity, Janssen, Kite, Merck, Novartis, OncoMed, Pfizer, Portola, Seattle Genetics, Takeda, TG Therapeutics, and Trillium. R.C. received clinical research funding from Agios Pharmaceuticals, Inc. and Celgene Corporation. A.S. consulted for Amgen and Stemline. M.A.S. served on the advisory board for Celgene Corporation. R.T.S. received grants from Takeda and personal fees from Novartis, Agios Pharmaceuticals, Inc., and Celgene Corporation. B.C.M. served on advisory boards for Agios Pharmaceuticals, Inc. and Celgene Corporation. C.W. received grants from Agios Pharmaceuticals, Inc. A.T., Q.X., and R.D.K. are employees and stockholders of Celgene Corporation. K.E.Y. and S.A. are employees of Agios Pharmaceuticals, Inc. S.d.B. received personal fees from Agios Pharmaceuticals, Inc., Celgene Corporation, Novartis, Pfizer and Servier. The remaining authors declare no competing financial interests.
Correspondence: Eytan M. Stein, Leukemia Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: steine@mskcc.org.
References
- 1.Walter RB, Estey EH. Management of older or unfit patients with acute myeloid leukemia. Leukemia. 2015;29(4):770-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenblat TL, McDevitt MR, Mulford DA, et al. Sequential cytarabine and alpha-particle immunotherapy with bismuth-213-lintuzumab (HuM195) for acute myeloid leukemia. Clin Cancer Res. 2010;16(21):5303-5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oran B, Weisdorf DJ. Survival for older patients with acute myeloid leukemia: a population-based study. Haematologica. 2012;97(12):1916-1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DiNardo CD, Jabbour E, Ravandi F, et al. IDH1 and IDH2 mutations in myelodysplastic syndromes and role in disease progression. Leukemia. 2016;30(4):980-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medeiros BC, Fathi AT, DiNardo CD, Pollyea DA, Chan SM, Swords R. Isocitrate dehydrogenase mutations in myeloid malignancies. Leukemia. 2017;31(2):272-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiNardo CD, Ravandi F, Agresta S, et al. Characteristics, clinical outcome, and prognostic significance of IDH mutations in AML. Am J Hematol. 2015;90(8):732-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ward PS, Patel J, Wise DR, et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 2010;17(3):225-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marcucci G, Maharry K, Wu YZ, et al. IDH1 and IDH2 gene mutations identify novel molecular subsets within de novo cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. J Clin Oncol. 2010;28(14):2348-2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paschka P, Schlenk RF, Gaidzik VI, et al. IDH1 and IDH2 mutations are frequent genetic alterations in acute myeloid leukemia and confer adverse prognosis in cytogenetically normal acute myeloid leukemia with NPM1 mutation without FLT3 internal tandem duplication. J Clin Oncol. 2010;28(22):3636-3643. [DOI] [PubMed] [Google Scholar]
- 10.Abbas S, Lugthart S, Kavelaars FG, et al. Acquired mutations in the genes encoding IDH1 and IDH2 both are recurrent aberrations in acute myeloid leukemia: prevalence and prognostic value. Blood. 2010;116(12):2122-2126. [DOI] [PubMed] [Google Scholar]
- 11.Figueroa ME, Abdel-Wahab O, Lu C, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18(6):553-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu C, Ward PS, Kapoor GS, et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature. 2012;483(7390):474-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu W, Yang H, Liu Y, et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell. 2011;19(1):17-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kats LM, Reschke M, Taulli R, et al. Proto-oncogenic role of mutant IDH2 in leukemia initiation and maintenance. Cell Stem Cell. 2014;14(3):329-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fathi AT, Sadrzadeh H, Borger DR, et al. Prospective serial evaluation of 2-hydroxyglutarate, during treatment of newly diagnosed acute myeloid leukemia, to assess disease activity and therapeutic response. Blood. 2012;120(23):4649-4652. [DOI] [PubMed] [Google Scholar]
- 16.DiNardo CD, Propert KJ, Loren AW, et al. Serum 2-hydroxyglutarate levels predict isocitrate dehydrogenase mutations and clinical outcome in acute myeloid leukemia. Blood. 2013;121(24):4917-4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janin M, Mylonas E, Saada V, et al. Serum 2-hydroxyglutarate production in IDH1- and IDH2-mutated de novo acute myeloid leukemia: a study by the Acute Leukemia French Association group. J Clin Oncol. 2014;32(4):297-305. [DOI] [PubMed] [Google Scholar]
- 18.Fan B, Chen Y, Wang F, et al. Pharmacokinetic/pharmacodynamic (PK/PD) evaluation of AG-221, a potent mutant IDH2 inhibitor, from a phase 1 trial of patients with IDH2 mutation-positive hematologic malignancies [abstract]. Haematologica. 2015;100 Abstract 379.25261096 [Google Scholar]
- 19.Shih AH, Shank KR, Meydan C, et al. AG-221, a small molecule mutant IDH2 inhibitor, remodels the epigenetic state of IDH2-mutant cells and induces alterations in self-renewal/differentiation in IDH2-mutant AML model in vivo [abstract]. Blood. 2014;124(21). Abstract 437.25359993 [Google Scholar]
- 20.Yen K, Travins J, Wang F, et al. AG-221, a first-in-class therapy targeting acute myeloid leukemia harboring oncogenic IDH2 mutations. Cancer Discov. 2017;7(5):478-493. [DOI] [PubMed] [Google Scholar]
- 21.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937-951. [DOI] [PubMed] [Google Scholar]
- 22.Cheson BD, Bennett JM, Kopecky KJ, et al. ; International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642-4649. [DOI] [PubMed] [Google Scholar]
- 23.Clinical Practice Guidelines in Oncology. Acute Myeloid Leukemia, version 1.2017. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed 7 March 2017.
- 24.Wang F, Travins J, DeLaBarre B, et al. Targeted inhibition of mutant IDH2 in leukemia cells induces cellular differentiation. Science. 2013;340(6132):622-626. [DOI] [PubMed] [Google Scholar]
- 25.Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136-1152. [DOI] [PubMed] [Google Scholar]
- 26.Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30(21):2670-2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hämäläinen S, Kuittinen T, Matinlauri I, Nousiainen T, Koivula I, Jantunen E. Neutropenic fever and severe sepsis in adult acute myeloid leukemia (AML) patients receiving intensive chemotherapy: Causes and consequences. Leuk Lymphoma. 2008;49(3):495-501. [DOI] [PubMed] [Google Scholar]
- 28.Walter RB, Taylor LR, Gardner KM, Dorcy KS, Vaughn JE, Estey EH. Outpatient management following intensive induction or salvage chemotherapy for acute myeloid leukemia. Clin Adv Hematol Oncol. 2013;11(9):571-577. [PMC free article] [PubMed] [Google Scholar]
- 29.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. ; International Vidaza High-Risk MDS Survival Study Group. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10(3):223-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zuckerman T, Ganzel C, Tallman MS, Rowe JM. How I treat hematologic emergencies in adults with acute leukemia. Blood. 2012;120(10):1993-2002. [DOI] [PubMed] [Google Scholar]
- 31.Tallman MS, Andersen JW, Schiffer CA, et al. All-trans-retinoic acid in acute promyelocytic leukemia. N Engl J Med. 1997;337(15):1021-1028. [DOI] [PubMed] [Google Scholar]
- 32.Baljevic M, Park JH, Stein E, Douer D, Altman JK, Tallman MS. Curing all patients with acute promyelocytic leukemia: are we there yet? Hematol Oncol Clin North Am. 2011;25(6):1215-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Birendra KC, DiNardo CD. Evidence for clinical differentiation and differentiation syndrome in patients with acute myeloid leukemia and IDH1 mutations treated with the targeted mutant IDH1 inhibitor, AG-120. Clin Lymphoma Myeloma Leuk. 2016;16(8):460-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanz MA, Montesinos P. How we prevent and treat differentiation syndrome in patients with acute promyelocytic leukemia. Blood. 2014;123(18):2777-2782. [DOI] [PubMed] [Google Scholar]
- 35.Nazha A, Sekeres MA, Garcia-Manero G, et al. ; MDS Clinical Research Consortium. Outcomes of patients with myelodysplastic syndromes who achieve stable disease after treatment with hypomethylating agents. Leuk Res. 2016;41:43-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roboz GJ, Rosenblat T, Arellano M, et al. International randomized phase III study of elacytarabine versus investigator choice in patients with relapsed/refractory acute myeloid leukemia. J Clin Oncol. 2014;32(18):1919-1926. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.