Abstract
Purpose
Cryoablation is used in the treatment of atrial fibrillation and other cardiac arrhythmias. This study evaluated a novel 10 cm flexible nitrous oxide cryoprobe in an ovine model of atrial ablation.
Description
Six sheep were anesthetized, underwent left thoracotomy, and were placed on cardiopulmonary bypass. A left atriotomy was made, and the cryoprobe was applied endocardially for 120 seconds at less than −40°C to four sites on the left atrium. The atrium was closed and the animals were recovered. After 30 days, the animals were sacrificed. Transmurality was evaluated in 5 mm sections of each lesion using 2,3,5-triphenyltetrazolium chloride (TTC) and Masson’s trichrome staining.
Evaluation
All animals survived. 104/106 sections (98%) were transmural by TTC; 103/106 (97%) were transmural by trichrome staining. There was no late atrial perforation, intraluminal thrombus or thromboembolism.
Conclusions
The device reliably produced transmural lesions in a chronic ovine model. Its performance was equivalent to that of other nitrous oxide cryoablation systems.
Technology
Atrial fibrillation is the most common sustained cardiac arrhythmia. Available treatments for atrial fibrillation include antiarrhythmic drugs, catheter-directed ablation and surgical ablation. The Cox-Maze procedure, the first effective interventional treatment for atrial fibrillation, was introduced in 1987 by Dr. James Cox. His cut-and-sew procedure was highly efficacious but technically difficult and was not widely adopted. Alternative energy sources including radiofrequency ablation, cryoablation, microwave ablation, and laser ablation have been used for surgical ablation.(1) To be effective, these tools need to reliably create transmural lesions that cause conduction block while causing minimal damage to adjacent tissues. Of the available technologies, bipolar radiofrequency ablation and cryoablation have proven to be the most effective and are commonly used today.
Cryoablation in cardiac surgery was first described 1951.(2) Since that time, cryoablation has been used to treat Wolff-Parkinson-White syndrome, ventricular tachycardia, and atrial fibrillation. Cryoablation was a critical part of the primarily cut-and-sew Cox-Maze III procedure for ablation of the coronary sinus(3) and around the tricuspid and mitral valve annuli, areas which were difficult to divide and reconstruct.(4) Cryoablation has been used in modified Maze procedures, notably the Cox-Maze IV procedure described by Damiano and colleagues in 2004(5) and the CryoMaze procedure described by Gammie and colleagues in 2005.(6) Others have reported good outcomes using similar approaches.(7, 8)
Cryoablation injures tissue through several mechanisms, described by Gage and Baust.(9) At nonfreezing temperatures, hypothermia slows enzyme function and can directly cause cell death. Extracellular ice crystals form at 0°C to −10°C, drawing water out of cells by osmosis. Dehydration damages cell contents but is not reliably lethal. The cytosol is somewhat protected from freezing by its high concentration of solutes. Intracellular ice crystals form heterogeneously at −15°C and appear in all cells by −40°C. During thawing, ice can recrystallize into larger crystals, damaging cell membranes. These changes can be visualized acutely as shrunken cardiomyocytes with sarcoplasmic vacuolization, membrane damage, nuclear pyknosis and increased intercellular distance; after one month, viable myocytes cannot be found in ablated tissues.(10) Late effects include apoptosis,vascular thrombosis, edema, inflammation and fibrosis. Due to the heterogeneous and time-variant effects of cryoablation, its effectiveness is more reliably evaluated after sufficient time has been allowed for fibrosis to occur.
Taylor’s early report of cardiac cryoablation used a two-minute duration of freezing to −60°C, and this practice has persisted to the present time.(2) Modern cardiac cryoablation systems use either nitrous oxide or argon. Several types of cryoablation probe are in use, including rigid T-shaped and bell-shaped probes and rigid or malleable linear probes with smooth or corrugated walls.
Our laboratory has previously evaluated the performance of a disposable aluminum linear cryoprobe (Cryo1, AtriCure, Inc., West Chester, OH) in a chronic animal study and found it to be equivalent to that of a reusable probe (3011 Maze Linear Probe, AtriCure, Inc., West Chester, OH) used with nitrous oxide.(11) The Cryo1 probe created transmural lesions in 99% of sections compared with 98% for the 3011 probe.
The purpose of this study was to evaluate the performance of a novel disposable cryoprobe (cryoFORM, AtriCure, Inc., West Chester, OH) in a chronic ovine model.
Technique
Six sheep weighing 69–85 kg were used in this study. The protocol was in accordance with the Guide for the Care and Use of Laboratory Animals (National Academy Press, Washington, DC, 1996) and was approved by the Institutional Animal Care and Use Committee at the University of Minnesota, Twin Cities.
The device used was a 10 cm linear cryoprobe (cryoFORM, AtriCure, Inc., West Chester, OH; Figure 1). The probe was 4 mm in diameter. The cryoFORM is dramatically different from previous cryoprobes on the market using nitrous oxide in three major ways: it is manufactured from stainless steel rather than aluminum, it is corrugated, not smooth, and it is much more flexible. Because of these significant design changes, it is necessary to rigorously evaluate the efficacy of this new device. Nitrous oxide was delivered using a control console with a rapid defrost feature (cryoICE BOX V6, AtriCure, Inc., West Chester, OH).
Figure 1.

The cryoprobe used in this study. Courtesy of AtriCure, Inc., used with permission.
Each animal underwent left atrial ablation on cardiopulmonary bypass with asystolic arrest. Following overnight fasting, animals were premedicated with sustained release buprenorphine (0.12–0.27 mg/kg). General anesthesia was induced using ketamine (10 mg/kg) and propofol (2–6 mg/kg) and maintained with isoflurane (1–3%) and propofol. Ceftiofur (3 mg/kg) was administered preoperatively and continued for ten days. Endotracheal and orogastric tubes, ECG, end-tidal CO2, oxygen saturation, and temperature monitors were placed. Methylprednisolone (250 mg) was administered to blunt the inflammatory response to cardiopulmonary bypass. Animals were placed on the operating table in right lateral decubitus position, shaved, prepped and draped in sterile fashion. Succinylcholine (0.4–0.8 mg/kg) was administered. A thoracotomy was made in the fourth left intercostal space. A catheter was placed in the left internal mammary artery for pressure monitoring. Heparin (250 units/kg) was given to achieve an activated clotting time of >350 s. Cardiopulmonary bypass was instituted via cannulae placed in the descending thoracic aorta and the right atrial appendage. A flowrate of 40–70 ml/kg/min, sweep of 2 L/min, and FDO2 70% were used. The animal was cooled to 27°C. A left ventricular vent was placed in the apex. The ascending aorta was cannulated and cross-clamped. Cold cardioplegia (Custodiol HTK, Essential Pharmaceuticals, LCC, Ewing, NJ) was administered until cardiac arrest. An atriotomy was made from the apex of the left atrial appendage to the base of the left atrial free wall, and the posterior left atrium and mitral valve were exposed.
The cryoprobe was used to make four endocardial lesions; on the free wall superior to the atriotomy, on the free wall inferior to the atriotomy, along the roof of the atrium, and between the pulmonary veins toward the mitral valve annulus, as depicted in Figure 2. The locations were chosen to maximize lesion length without intersection and to avoid the atrioventricular node. Each lesion was marked with sutures. The tissue was frozen to a temperature of −40°C or colder, measured at the proximal end of the probe, for 120 seconds. The probe reached an average temperature nadir of −67°C.
Figure 2.
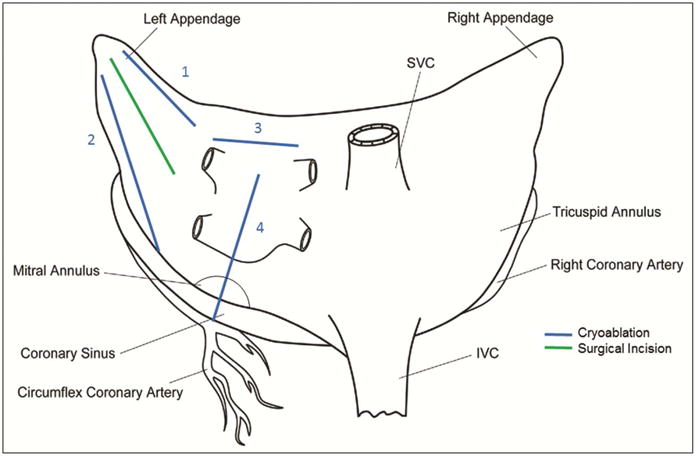
Lesions used for the study, drawn on a posteroanterior schematic of the atria. Blue lines denote cryoablation; the green line denotes the atriotomy.
After ablation, the left atrium was closed with a running polypropylene suture. The heart was de-aired, and the aortic cross-clamp was removed. The animals were given additional methylprednisolone and were weaned from cardiopulmonary bypass. Protamine was administered for heparin reversal if needed. The thoracotomy was closed, and the animals were recovered. Postoperatively the animals were given subcutaneous heparin twice daily for two days and furosemide as needed for diuresis.
The animals were survived for 30–31 days. Each animal was given 300 units/kg of heparin intravenously and euthanized humanely with phenytoin and phenobarbital. The heart and lungs were excised en bloc. The heart was perfused with 250 ml 5% 2,3,5-triphenyl tetrazolium chloride (TTC) via the aortic root. The brain, lungs, liver, spleen, kidneys and gastrointestinal tract were examined by a veterinary pathologist. The left atrium was opened, and each lesion was excised. Each lesion was sectioned every 5 mm along its length, as shown in Figure 3, and immersed in 1% TTC for a minimum of 20 minutes. Each section was photographed using a high-resolution digital camera with a ruler for calibration. The tissue was then fixed in 10% neutral buffered formalin and embedded. Five-micron sections were prepared and stained with Masson’s trichrome.
Figure 3.
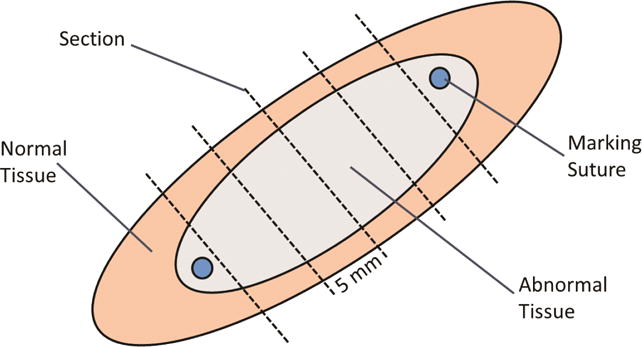
Schematic diagram of section preparation. Each lesion was excised with a rim of normal tissue surrounding it. Sections were taken transversely every 5 mm along the lesion.
The lesion depth and tissue thickness were measured on each photograph and section. Each histologic section was evaluated by a veterinary pathologist. A transmural lesion was defined as a continuous area of scar (unstained by TTC, uptake of blue and not red stain on trichrome) from endocardium to epicardium or adipose tissue. Descriptive statistics were performed using R version 3.2.4 (R Foundation, Vienna, Austria). Data are expressed as mean ± standard deviation unless otherwise noted.
Clinical Experience
All animals survived without strokes or other embolic complications. At necropsy, the pericardium was diffusely adherent to the left atrium. The left atrial diameter was 3.8 ± 0.5 cm. Two animals had thickening of the main pulmonary artery. One animal had an intrapericardial hematoma. There were no intracavitary or intraluminal thrombi.
Analysis of the TTC-stained gross photographs revealed a tissue thickness of 2.93 ± 1.78 mm and a lesion depth of 2.90 ± 1.73 mm. The lesion width was 13 ± 5 mm. 104/106 sections (98%) were transmural. Lesion depth and tissue thickness are shown in Figure 4.
Figure 4.
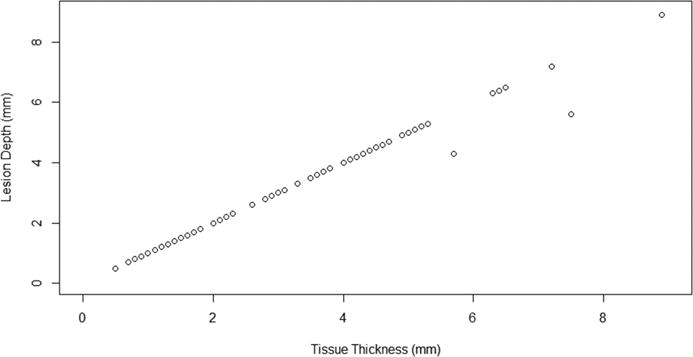
Lesion depth versus tissue thickness by TTC. Transmural lesions fall along a line of unity; non-transmural lesions lie below this line of unity.
Analysis of trichrome-stained sections revealed a tissue thickness of 2.93 ± 1.47 mm. The lesion depth was 2.89 ± 1.45 mm. 101/106 sections (95%) were transmural at the center of the lesion. Lesion depth and tissue thickness are shown in Figure 5. 103/106 sections (97%) were transmural at any area of the lesion. 21/24 lesions (88%) were transmural at every section along their length. One non-transmural section was located at the distal end of the probe. Another non-transmural section had a 2.8 mm lesion width, suggestive of poor contact between the probe and the tissue. The remaining non-transmural section was in an area of thick tissue (6.3 mm by trichrome). The non-transmural sections identified by TTC differed from those identified by trichrome, with only one non-transmural section identified by both techniques. One nontransmural section by trichrome was found at site 2, one at site 3, and one at site 4. Examples of transmural and non-transmural sections are shown in Figure 6.
Figure 5.
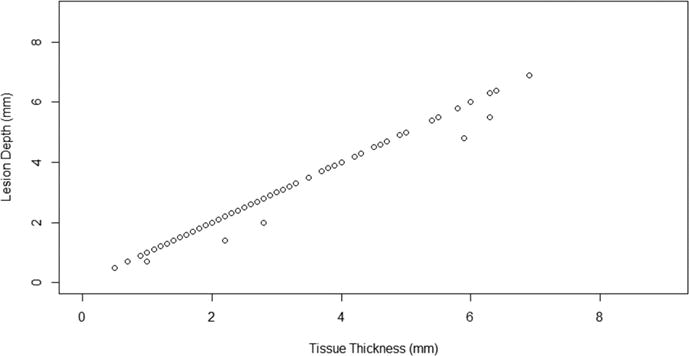
Lesion depth (measured at center of lesion) versus tissue thickness by Masson’s trichrome. Transmural lesions fall along a line of unity; non-transmural lesions lie below this line of unity.
Figure 6.
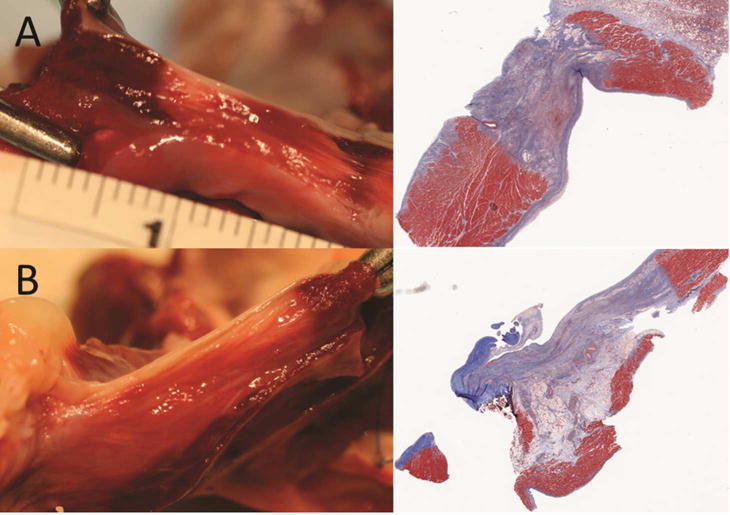
Representative transmural (A) and non-transmural (B) sections by TTC (left) and Masson’s trichrome (right). Viable tissue stains dark red with TTC; nonviable tissue appears yellow to white. Collagen in scar tissue stains blue with Masson’s trichrome; muscle tissue stains red.
Comment
This novel ablation device was effective in producing transmural lesions on the arrested heart as evaluated by TTC and trichrome stains. It performed similarly to previous devices that our laboratory has investigated.(11) The difference in transmurality as determined by TTC compared to trichrome implies that either both stains or testing for conduction block should be used in future studies of ablation device performance. The presence of rare non-transmural sections in areas of poor contact and at the distal end of the probe may allow the possibility of inadequate lesions if surgeons are not vigilant about probe contact with tissue. This is especially important given the fact that this device is significantly more flexible than previous cryoprobes.
This study has several limitations. First, a Cox-Maze lesion set was not used. No right atrial lesions were performed. No repeat ablation was performed when gaps in epicardial frost were visualized. Additionally, temperature was measured on the cryoablation probe and not in the ablated tissue. Only the distal portion of the cryoprobe was used due to the limited size of the healthy sheep left atrium, preventing assessment of the full length of the probe. The manufacturer has determined that the temperature variation along the length of the probe at the end of the freeze cycle is no more than 10 °C (per AtriCure, Inc., data on file), but we did not independently test this. Furthermore, an ablation time of 120 seconds was used. Due to the fact that less than 100% of nitrous oxide cryolesions were transmural in two studies we have performed,(1, 11) some surgeons including our center use longer cryoablation times. Additionally, the device was used in an arrested open heart model. This eliminated the heat sink effect of circulating blood. Cryoablation on the beating heart may not yield equivalent results.
In summary, the novel cryoablation probe produced transmural lesions in 97% of histologic sections examined in the arrested heart in a chronic ovine model. This probe is a viable alternative to previous devices and has similar efficacy. Surgeons should be vigilant about maintaining probe contact with tissue across the entire lesion, especially if the full length of the flexible probe is used. Overlapping lesions should be created clinically. Repeat ablation of areas of poor contact may improve device performance.
Acknowledgments
Disclosure and Freedom of Investigation
The authors acknowledge the technical assistance of Matthew Lahti, John Carney, Dr. Rebecca Rose and the staff of University of Minnesota Experimental Surgical Services.
This study was sponsored by AtriCure, Inc., the manufacturer of the device. The authors attest that they have examined all data and had full freedom of investigation and freedom to publish results regardless of the outcome of the study. Expenses for Washington University staff were paid through a research grant to Dr Ralph J. Damiano Jr from AtriCure, Inc. Dr Matthew R. Schill is supported by NIH grant T32-HL007776. Drs Ralph J. Damiano Jr and Richard B. Schuessler are supported by NIH grant R01-HL032257.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schuessler RB, Lee AM, Melby SJ, et al. Animal studies of epicardial atrial ablation. Heart Rhythm. 2009;6(12 Suppl):S41–45. doi: 10.1016/j.hrthm.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor CB, Davis CB, Jr, Vawter GF, Hass GM. Controlled myocardial injury produced by a hypothermal method. Circulation. 1951;3(2):239–253. doi: 10.1161/01.cir.3.2.239. [DOI] [PubMed] [Google Scholar]
- 3.Cox JL, Ad N. The importance of cryoablation of the coronary sinus during the maze procedure. Semin Thorac Cardiovasc Surg. 2000;12(1):20–24. doi: 10.1016/s1043-0679(00)70012-8. [DOI] [PubMed] [Google Scholar]
- 4.Cox JL, Jaquiss RD, Schuessler RB, Boineau JP. Modification of the maze procedure for atrial flutter and atrial fibrillation. Ii. Surgical technique of the maze iii procedure. J Thorac Cardiovasc Surg. 1995;110(2):485–495. doi: 10.1016/S0022-5223(95)70245-8. [DOI] [PubMed] [Google Scholar]
- 5.Gaynor SL, Diodato MD, Prasad SM, et al. A prospective, single-center clinical trial of a modified cox maze procedure with bipolar radiofrequency ablation. J Thorac Cardiovasc Surg. 2004;128(4):535–542. doi: 10.1016/j.jtcvs.2004.02.044. [DOI] [PubMed] [Google Scholar]
- 6.Gammie JS, Laschinger JC, Brown JM, et al. A multi-institutional experience with the cryomaze procedure. Ann Thorac Surg. 2005;80(3):876–880. doi: 10.1016/j.athoracsur.2005.03.075. discussion 880. [DOI] [PubMed] [Google Scholar]
- 7.Ad N, Henry L, Friehling T, Wish M, Holmes SD. Minimally invasive stand-alone cox-maze procedure for patients with nonparoxysmal atrial fibrillation. Ann Thorac Surg. 2013;96(3):792–798. doi: 10.1016/j.athoracsur.2013.05.007. discussion 798–799. [DOI] [PubMed] [Google Scholar]
- 8.Watkins AC, Young CA, Ghoreishi M, et al. Prospective assessment of the cryomaze procedure with continuous outpatient telemetry in 136 patients. Ann Thorac Surg. 2014;97(4):1191–1198. doi: 10.1016/j.athoracsur.2013.10.041. discussion 1198. [DOI] [PubMed] [Google Scholar]
- 9.Gage AA, Baust J. Mechanisms of tissue injury in cryosurgery. Cryobiology. 1998;37(3):171–186. doi: 10.1006/cryo.1998.2115. [DOI] [PubMed] [Google Scholar]
- 10.Manasse E, Colombo P, Roncalli M, Gallotti R. Myocardial acute and chronic histological modifications induced by cryoablation. Eur J Cardiothorac Surg. 2000;17(3):339–340. doi: 10.1016/s1010-7940(99)00361-9. [DOI] [PubMed] [Google Scholar]
- 11.Weimar T, Lee AM, Ray S, Schuessler RB, Damiano RJ., Jr Evaluation of a novel cryoablation system: In vivo testing in a chronic porcine model. Innovations (Phila) 2012;7(6):410–416. doi: 10.1097/IMI.0b013e31828534e5. [DOI] [PMC free article] [PubMed] [Google Scholar]


