Abstract
Airborne particulate matter (PM) is a global concern because exposure is associated with adverse cardiorespiratory effects. To better understand source-orientated PM toxicity, a comparative study of the biological effects of fine PM (diameter ≤ 2.5 µm, PM2.5) from two geographically different regions of the world known for high PM air pollution, Shanxi Province in China and the Central Valley in California in the United States was conducted on an equal mass basis. PM2.5 was collected in the capital cities of Shanxi Province (Taiyuan) and California (Sacramento) during the winter season. The overarching hypothesis for this study was to test whether the chemical composition of particulate matter on an equal mass basis from two urban areas, one in China and one in California, can lead to significantly different effects of acute toxicity and inflammation in the lungs of healthy young mice. Male, 8-week old BALB/C mice (n=10/group) were given a single 50 µg dose of vehicle, Taiyuan PM or Sacramento PM by oropharyngeal aspiration. Animals were sacrificed 24 hours later to capture peak inflammation following exposure. Bronchoalveolar lavage, ELISA and histopathology were performed to determine biological effects, along with chemical analysis of PM composition. Sacramento PM was found to have a greater proportion of oxidized organic material than Taiyuan PM. Additionally, Sacramento PM was associated with significantly increased neutrophil numbers and elevated CXCL-1 and TNF-α protein levels compared to the Taiyuan PM. The findings suggest, on an equal mass basis, Sacramento PM was associated with a greater inflammatory response compared to that of Taiyuan PM that could be driven by a higher oxidized state of organic carbon and possibly greater copper content.
Keywords: air pollution, lung, inflammation, cytokines, chemokines
1. INTRODUCTION
Particulate matter (PM) air pollution is a worldwide health problem associated with adverse effects on the cardiorespiratory system, such as asthma, COPD, and myocardial infarction. Worldwide air pollution related annual mortalities have been estimated at 7 million (WHO 2015). PM has a wide variety of physicochemical characteristics which depend on the source and atmospheric aging of these particles. Fine PM, also known as PM2.5 (Dp ≤ 2.5 µm), is especially harmful because it can readily deposit deep in the lung and be retained, irritating lung parenchyma or moving into the blood stream (Churg and Brauer 1997; Madl et al. 2014; Mannucci et al. 2015).
PM pollution has increased with industrialization and climate change. It is especially prevalent in areas of rapid economic growth fueled by fossil fuels, such as China, or arid regions with geographical/meteorological conditions that trap PM for long periods of time and concentrate it, such as in the large valleys of the Western United States. This paper describes a comparative study of the biological effect of PM2.5 from two parts of the world known for high PM air pollution, Shanxi Province in China and the Central Valley in California in the United States. The study was a joint effort to define the influence of the chemical composition of PM from diverse urban sources of these two countries on an equal mass basis in measured biological toxicity of the lungs following acute exposure.
PM was collected in the capital cities of Shanxi Province and the state of California, Taiyuan and Sacramento, respectively, based on the fact that both cities are heavily urbanized, have relatively dry, sunny winters, economies dominated by agriculture and industry, and a long history of unhealthy levels of PM2.5, especially during the winter season. Because the economy of Taiyuan is dominated by abundant coal production and combustion, while the economy of Sacramento is largely based on government, transportation, and agriculture, it was expected that the study would provide an opportunity to better understand how PM source influences pulmonary toxicity.
To compare the biological effects of the two geographic PM samples, young male BALB/C mice were exposed to the collected PM2.5 from Taiyuan or Sacramento by oropharyngeal aspiration (50 µg) on an equal mass basis. The PM was collected at both sites during winter since higher air pollution during this season has been associated with increased hospital admissions and the incidence of cardiovascular and respiratory disease (Rodopoulou et al. 2015). Animals were sacrificed 24 hours post-exposure to capture peak inflammation, as is well known to occur following gas and particle exposure. Patterns of pulmonary toxicity were assessed by bronchoalveolar lavage (BAL), enzyme-linked immunosorbent assays (ELISA) and histopathologic assessment. In addition, the chemical composition of each PM sample was analyzed to determine if chemical differences could help explain potential differences in pulmonary toxicity.
2. MATERIALS AND METHODS
2.1 Particle Collection
Sampling was done during the winter of 2012 in Taiyuan and 2013 in Sacramento to collect sufficient PM mass for toxicological and chemical characterization.
The sampling site in Taiyuan was located on the rooftop of the five story building of the College of Environmental Science and Resources on the Shanxi University campus (N37°47′, E112°34′) in downtown Taiyuan, surrounded by a mixture of residential, commercial and industrial buildings. The sampling site in Sacramento was located on the rooftop of a two story building at the northeast corner of T St. and 13th St. (N38°34', W121°29'), also surrounded by a mixture of residential, commercial and industrial buildings and within a quarter mile of a major freeway interchange.
In Taiyuan, PM2.5 was collected for one day on 3 µm pore size quartz microfiber filters (Whatman) using a high-volume particle collector with a flow rate of 40 cfm (Thermo Anderson, USA). The quartz filters were preheated at 450°C for 24 hours before sampling to eliminate endotoxin. Filters were pre- and post-weighed to calculate the PM mass and then subsequently cut into fragments, placed in a 250 mL conical flask with 30 mL Milli-Q water and sonicated for 10 minutes (repeated three times for a total of 30 minutes). The PM extract was filtered through six layers of sterile gauze. These extraction steps were repeated three times. The collected solution was lyophilized to powder and stored at −80 °C until use. Prior to use, the powder was weighed, and suspended in Milli-Q water to a concentration of 1mg/mL.
In Sacramento, PM2.5 was collected for seven days with a high-volume sampler system (Tisch Environmental Inc., TE-6070V-2.5-HVS) was equipped with a PM10 size-selective head (Tisch Environmental Inc., TE-6001), operating at a flow rate of 40 cfm and loaded with Teflon coated borosilicate glass microfiber filters (Pall Corporation, TX40H120WW-8×10) for collecting PM2.5. Glass filters were pre-cleaned via successive sonication in Milli-Q water, dichloromethane and hexane. Field blanks were included. The sample filters were weighed to calculate the PM concentration, placed in Milli-Q water and sonicated for 1 hour. The sonication extract was filtered using a 0.2 µm pore size syringe filter. The collected solution (approximately 100 mL) was lyophilized and then resuspended in Milli-Q water to a final PM concentration of 1 mg/mL. Detailed extraction methods can be found in Bein and Wexler (Bein and Wexler 2015).
The difference in collection times between China and California (1 day vs. 7 days, respectively) was due to China having much higher levels of air pollution. Because the China collection filters had significantly higher areal density (heavier loading) of PM than California filters, the sonification times for the China PM and California PM preparations differed accordingly (30 minutes vs 60 minutes, respectively). It is acknowledged these differences in extraction and sonication times might contribute to different efficiencies in complete particle extraction for each PM sample and could represent a possible limitation of the study.
2.2 PM Suspension Preparation
To achieve identical particle concentrations for both CA and CH PM samples prior to oropharyngeal aspiration, extracted PM samples from CA and CH were lyophilized to dryness in order to measure a precise PM mass that was suspended in nanopure water and sonicated for 20 minutes to derive an identical mass (1 µg/µL) for each PM sample. PM samples :were analyzed for endotoxin using a chromogenic Limulus Amebocyte Lysate (LAL) test with a PM sample concentration of 1 mg/mL.
2.3 Hydrodynamic Particle Size
Following 20 minute bath sonication of each PM suspension, dynamic light scattering (DLS) was used to determine hydrodynamic particle size distribution of each sample immediately prior to oropharyngeal aspiration.
2.4 Chemical Analysis of PM samples
All chemical analyses were performed at the University of California, Davis. Frozen 1 mg/mL stock solutions of Taiyuan and Sacramento PM2.5 were thawed to ~ 4°C and sonicated for 20 minutes to ensure thorough dispersion of the PM.
2.4.1 High-Resolution Aerosol Mass Spectrometry
PM2.5 samples were analyzed for organic species, as well as nitrate, sulfate, chloride, and ammonium, and particle size using a high-resolution time-of-flight aerosol mass spectrometer (HR-AMS), which has been used extensively to quantitatively characterize the bulk composition of ambient aerosols (Canagaratna et al. 2007). Suspended PM samples were diluted by a factor of 10, and 1 mL of each sample was atomized using a constant output aerosol generator with argon. The generated aerosol passed through a silica gel drier before being sampled by the HR-AMS. The elemental composition of the organic aerosols in each of the samples was determined by the method of Aiken and colleagues (Aiken et al. 2008) to indicate the average degree of oxidation of organic matter in particles. Greater detail on the analytical methods and data analysis for the chemical analyses of PM extracts are described elsewhere (e.g. Sun et al. 2010; Sun et al. 2011).
2.4.2 Inductively Coupled Plasma-Mass Spectrometry
Metals were measured using an Agilent 7500ce Inductively Coupled Plasma-Mass Spectrometer (ICP-MS) at the Interdisciplinary Center for Plasma Mass Spectrometry at the University of California, Davis (http://icpms.ucdavis.edu/). PM2.5 samples were analysed for a broad range of elements, including vanadium (V), chromium (Cr), iron (Fe), nickel (Ni), copper (Cu), and lead (Pb). To each of the liquid suspensions, 0.75 mL of concentrated nitric acid (15.968M, Fischer Scientific) was added, followed by the addition of Milli-Q water. The final volume of each sample was 2.5 mL and the resulting concentrations of the samples analyzed by ICP-MS were 0.65 mg/mL for CA and 0.42 mg/mL for China. Aerosolized particles were generated by nebulizing the sample solution that was then introduced by carrier gas into a high temperature argon plasma. Calibration standards were run as well.
2.5 Animals and Exposure
Thirty male, 8-week old BALB/C mice were purchased (Envigo, Hayward, CA). All animal procedures were approved by the UC Davis Institutional Animal Care and Use Committee in accordance with the US Animal Welfare Act. Animals were housed four per cage on sterile laboratory bedding with a 12 hour light / dark cycle and access to water and food ad libitum. The mice were maintained for one week prior to the beginning of the experiment to allow for acclimation and then randomly assigned to three exposure groups (n=10/ group) by random number generation: 1) vehicle control (Milli-Q water), 2) Taiyuan PM, and 3) Sacramento PM. Fifty microliters of Milli-Q water, Taiyuan PM (1 µg/µL) or Sacramento PM (1 µg/µL) was delivered to the lungs of mice via oropharyngeal aspiration after mice were anesthetized via inhalation of isoflurane with oxygen (3:1 ratio) (Gilmour et al. 2007; Plummer et al. 2015). For each exposure group, six animals were used to collect BALF and, while four animals were used for histological assessment only.
2.6 BALF and Lung Tissue
Cell and tissue collection occurred 24 hours after oropharyngeal aspiration. All mice were euthanized with Beuthanasia-D (120 mg/kg) and exsanguinated. A subset of six mice per exposure group underwent lung lavage with two aliquots of Dulbecco’s Phosphate Buffered Saline (PBS) (first 0.7 ml and then 0.6 ml). The collected bronchoalveolar lavage fluid (BALF) was centrifuged at 4°C at 2000 xg for 15 minutes to pellet the cells. The supernatant was collected and saved for total protein determination by Lowry Assay. The pelleted cells were resuspended in PBS to determine total cell number and viability via Trypan Blue exclusion assay. Cytospin slides were prepared and stained with DiffKwik Differential Stain kit (American MasterTech, Lodi, California) for cell differentials (500 cells/slide were counted) using a light microscope. The cranial, middle, caudal and accessory lobes of the right lung of these animals were placed in cryovials and stored at −80°C for further analysis. The right caudal lobes were used for all ELISA following homogenization. The left lung lobes were fixed with 4% paraformaldehyde for 48 hours and subsequently transferred into 70% ethanol. Left lung lobes (cut into four transverse slices) were embedded in paraffin and sectioned for histological staining.
2.7 ELISA
ELISAs (Biolegend, San Diego, CA) for tumor necrosis factor alpha (TNF-α); monocyte chemoattractant protein-1 (MCP-1); chemokine (C-X-C Motif) ligand 1 (CXCL-1); interleukins 1 beta, 5 and 6 (IL-1β, IL-5, IL-6); and heme oxygenase 1 (HO-1: Abcam, Cambridge, MA) were performed on lung homogenates from the caudal lobe of the right lung. Samples were analyzed in duplicate, and the plates were read with a SpectroMax plate reader (Molecular Devices, Sunnyvale, CA) at 450 nm. Protein concentrations were standardized to total lung protein and are expressed as pg/mg of lung tissue.
2.8 Histology
All lung lobes from each set of four animals from all groups not lavaged were embedded in paraffin and used for histological analysis. Sections (5 µm thick) were stained with hematoxylin and eosin stain (H&E, Harris Hematoxylin, and Eosin Y Stain: American MasterTech, Lodi, CA). Lung tissue sections were examined for the presence of inflammation, cellular infiltrates and epithelial abnormalities in alveolar ducts, blood vessels, and airways.
A semi-quantitative scoring numeric system was used to rank the degree of alveolar, bronchiolar, perivascular, particle-associated, and pleural inflammation in H&E-stained tissue sections as described previously (Silva et al., 2013, 2014). In brief, an initial blind, qualitative assessment was performed to evaluate the range of inflammatory responses. Subsequently, to minimize observer scoring subjectivity, a scoring rubric was made with categorical definitions extent (focal to diffuse on a scale of 0 to 3), as well as pictorial guidelines of severity, again with ordinal scores (0–3) corresponding to no, minimal, moderate, and marked inflammation, respectively. Blind semi-quantitative histological assessment of all samples was then performed with the product of extent and severity scores to achieve a final histopathological score.
2.9 Statistical Analysis
Data analysis was done by IBM SPSS Statistics 22 software (IBM Analytics, Armonk, NY). Shapiro-Wilk test and Leneve test was used to detect normality and homogeneity of variance. One-way analysis of variance (ANOVA) and post hoc Tukey’s tests were used to determine significant differences with a significance level of P ≤ 0.05. BALF data was log transformed to meet the requirements of ANOVA. Graphpad Prism 5 (GraphPad Software, Inc., La Jolla, CA) was used to present BALF and ELISA data (mean ± standard error of the mean).
3. RESULTS
3.1 BAL Fluid: Total Cell Number and Differentials
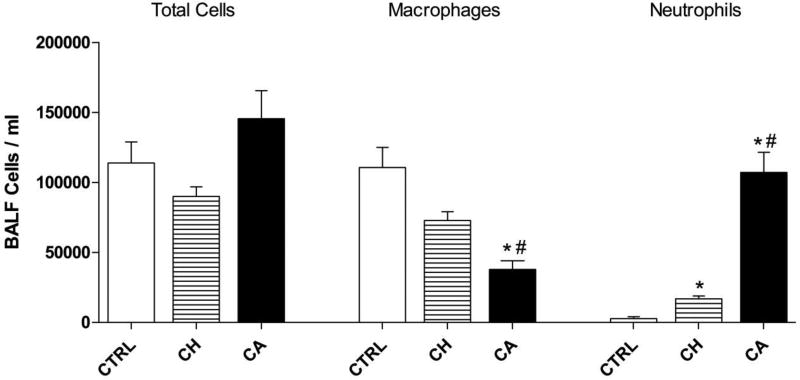
Total cell number and differential cell counts were determined from the collected BAL fluid (Figure 1). There was a trend for the number of total cells in the Sacramento PM group to be greater than either the control or Taiyuan PM groups, although no statistical significance was noted. Macrophages and neutrophils were the predominant cell types found in all three exposure groups. The Sacramento PM group had significantly fewer macrophages (26% of the total cell differential) than the control (97% of total cells) and Taiyuan PM (81% of total cells) groups. Neutrophil number was significantly increased with PM exposure compared to the control, regardless of country of origin. However, animals exposed to Sacramento PM had significantly more neutrophils (74% of cell total) than animals exposed to Taiyuan PM (19% of cell total).
Figure 1. Total and differential cell count of BAL fluid.
Total and differential cell count of BAL fluid. Neutrophil numbers following Taiyuan (CH) PM is significantly different from sham control (CTRL), while exposure to Sacramento (CA) PM is much higher than both Taiyuan and sham control numbers. Data is presented as mean (SEM) from 6 animals. * indicates significant difference to sham control. # indicates significant difference to Taiyuan PM (p<0.05 by one-way ANOVA).
3.2 ELISA: Chemokines and Cytokines Associated with Inflammation
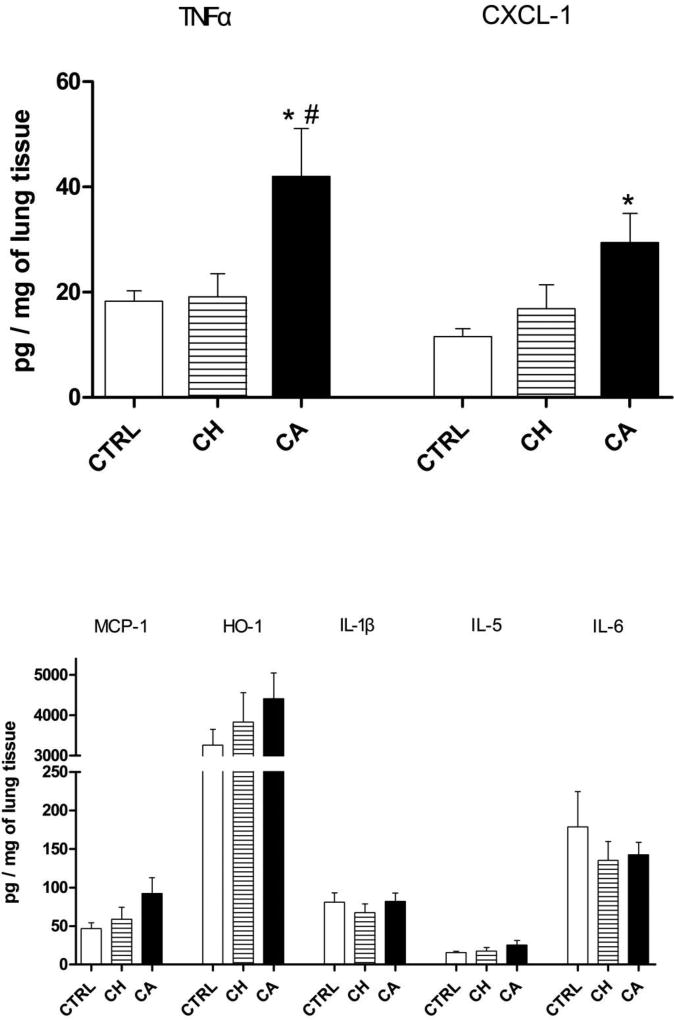
ELISA was used to quantitate select chemokines and cytokines associated with inflammation (Figure 3). TNF-α expression in mice exposed to CA PM was significantly higher than both the control and CH PM groups. CXCL-1 expression, which is associated with neutrophil chemoattraction, was significantly increased in the CA PM group compared to the control. No statistically significant differences were found for MCP-1, HO-1, IL-1β, IL-5 or IL-6 levels with either of the exposures, although there was a pattern of higher levels of MCP-1, HO-1, and IL-5 for CA PM, but not to a level of statistical significance.
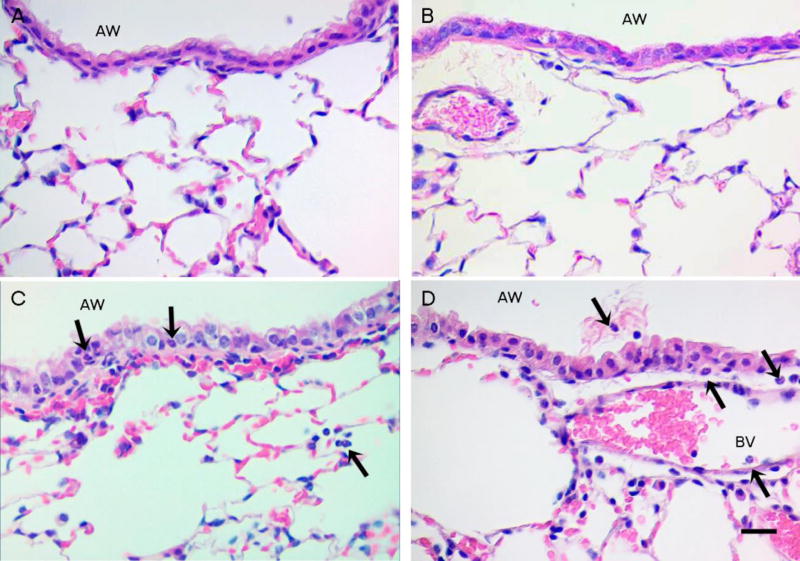
Figure 3. Peribronchiolar lung region.
The peribronchiolar region of the lung shows no neutrophils and limited macrophages in controls (A) and mice exposed to China PM (B), In contrast, neutrophils were observed in the peribronchiolar region of mice exposed to Sacramento PM (C, D). Please note a visible thickening and vacuolization of the airway epithelium following exposure to Sacramento PM (C,D) in contrast to the airway epithelium of the sham controls and mice exposed to China PM (A, B). Black arrows show the position of neutrophils. AW = airway, BV = blood vessel. Scale bar is 20µm.
3.3 Histological analysis
Sectioned lung tissue was stained with H&E to examine the airways, parenchyma and peribronchiolar regions of the lungs for each exposure group (Figure 3). Control mice and mice exposed to CH PM had no visible neutrophils and few macrophages in the airways, peribronchiolar regions or alveolar parenchyma (Figure 3A and 3B). In contrast, neutrophil influx into the lungs was clearly apparent in peribronchiolar regions following PM exposure (Figure 3C and 3D), in particular with CA PM. This exposure group had numerous neutrophils within the alveolar air spaces as well as the walls and epithelium of the lower conducting airways. Neutrophils within the bronchial epithelium appeared to be in the process of migrating to the airway lumen from the underlying vasculature (Figure 3D).
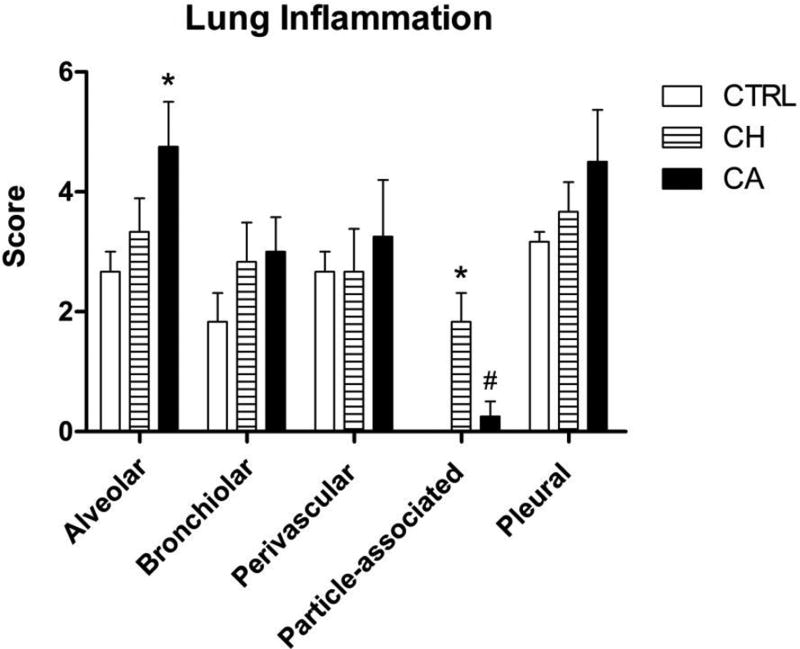
Histological scoring of the lung tissues for inflammation is shown in Figure 4. Lung structures evaluated included the alveoli, airways, blood vessels and pleura, as well as a general score for particle-associated inflammation in four completely different regions of the lungs. Scoring demonstrated significantly greater avleolitis in the lungs of mice exposed to CA PM compared to control, while particle-associated inflammation was significantly greater in the lungs of mice exposed to CH PM compared CA PM or sham control mice.
Figure 4. Histopathologic Scoring.
Semiquantitative scores of lung inflammation within alveolar, bronchiolar, perivascular and pleural regions, as well as particle-associated inflammation throughout the lungs. In general, inflammatory scores were higher following exposure to CA PM, compared to controls or CH PM exposure. However, only the inflammation of the alveoli was significantly different following exposure to CA PM compared to control (* p<0.05). In contrast, particle-associated inflammation was significantly greater in mice exposed to CH PM, compared to control (* p<0.05) or to CA PM (# p<0.05). This difference is likely to be due to the greater amounts of black soot found in CH PM.
3.4 Chemical analysis of PM
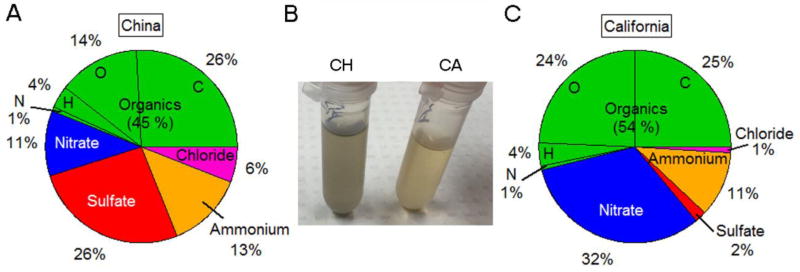
Both PM samples consisted primarily of organic compounds with 45% for Taiyuan PM (Figure 4A) and 54% for Sacramento PM (Figure 4C). However, while the organic matter of Taiyuan PM was composed of approximately 58% carbon, 31% oxygen, 9% hydrogen and 2% nitrogen, the Sacramento PM was more oxidized, consisting of approximately 46% carbon, 45% oxygen, 7% hydrogen and 2% nitrogen on mass basis. Another major difference was that Taiyuan PM had a much higher percent of sulfate (Taiyuan PM 26% versus Sacramento PM 2%), whereas Sacramento PM had a much greater percent of nitrate (Taiyuan PM 11% versus Sacramento PM 32%). A wide range of metals was detected in both samples (Table 1). Notable differences in metal concentration (greater than a two-fold difference in value) between the two PM samples included the following: calcium, copper, magnesium, barium, lead and vanadium. Particle size as determined by AMS demonstrated no differences between the two PM samples. Endotoxin assays of these PM samples demonstrated no signal (EU/mL) above detection limit using the Limulus Amebocyte Lysate (LAL) test.
Table 1.
Concentration and fold difference of various elements measured in the California and China samples by ICP-MS (ppm). Gray highlights greatest fold differences (CA compared to China).
| Sample concentration (ppm) |
|||
|---|---|---|---|
| Element | CA | China | Fold difference |
| K | 9.9 | 12 | 0.83 |
| Ca | 4 | 19 | 0.21 |
| B | 3.1 | 3.7 | 0.84 |
| Zn | 2.9 | 3.6 | 0.81 |
|
| |||
| Cu | 1.3 | 0.054 | 24 |
|
| |||
| Mg | 1.2 | 14 | 0.086 |
| Ba | 0.77 | 3.3 | 0.23 |
| Fe | 0.66 | 0.66 | 1 |
| Pb | 0.028 | 0.36 | 0.078 |
|
| |||
| Ni | 0.019 | 0.015 | 1.3 |
|
| |||
| Cr | 0.015 | 0.031 | 0.48 |
3.5 DLS
Hydrodynamic particle size was determined for each PM suspension immediately within 30 minutes of sonication for 20 minutes and shown in Figure 1. The average hydrodynamic particle size for CA PM was 312 nm, while the CH average hydrodynamic particle size was 728 nm.
4. DISCUSSION
The purpose of this study was to better understand PM2.5 toxicity on an equal mass basis from two different parts of the world experiencing periods of high air pollution: the city of Taiyuan in Shanxi Province, China and Sacramento, California, United States. To the best of our knowledge, this study is the first of its kind. In this study, healthy young mice received an acute dose (50 µg) of PM2.5 by oropharyngeal aspiration and were evaluated for pulmonary toxicity 24 hours later. Despite appearing visually less dark in suspension (Figure 4B), Sacramento PM2.5 was found to be more toxic than Taiyuan PM2.5, with a significant influx of neutrophils in the bronchiolar lavage fluid associated with elevations in CXCL-1 and TNF-α protein expression in lung tissue. Extensive chemical analysis revealed differences in organic matter, sulfate, nitrate, and metal composition between the Taiyuan and Sacramento PM, which most likely influence the toxicity of each PM sample.
Although conducted on different continents, PM sample collection and extraction protocols were desgined to work with similar PM2.5 samples. However, some differences between collection and extraction protocols included collection time, filter types for extraction and sonication time to orignially disaggregate the particulate matter collected. It is well-known PM composition and concentration in the atmosphere are influenced by emission sources, formation and removal processes, and meteorological conditions that can change on time scales ranging from seconds to days. So there would be differences between samples collected over different time periods, not just in China but everywhere. Nevertheless, since human activities (e.g., driving and cooking) and meteorological conditions (e.g., solar radiation and wind patterns) tend to vary diurnally, the average composition of PM collected over 1 day in general represent that collected over multiple days. Since PM concentration in China was almost an order of magnitude higher than in California, it was necessary that we sampled longer time in California to collect a comparable amount of PM sample as in China.
While we acknowledge some differences in extraction methods used (sterile gauze versus nucleopore filters) for the PM collected from these two sites, we have minimized all other parameters to allow for a direct comparison of each PM sample on an equal mass basis for acute toxicity measurements in mice following oropharyngeal aspiration. Both collection sites were selected so as to be in the middle of their respective cities to give an accurate reflection of the PM to which residents are exposed. Sample collection occurred during winter because PM concentrations tend to be higher in winter than summer due to fuel combustion heating of buildings as well as meteorological conditions that favor the accumulation of pollutants. Air pollution levels have been found to be approximately three times higher and aerosol optical depth five times higher during the heating (winter) season compared to the non-heating season in China (Cao et al. 2007; Xiao et al. 2015). Winter climate conditions, such as air stagnation, also play an important role in increasing PM concentration during this season. Wintertime inversions occur in many of the valleys of the Western United States, such as the California’s Central Valley in which Sacramento is located (Holmes et al. 2015), and these periods are associated with increased hospital admissions and incidence of cardiovascular and respiratory disease (Rodopoulou et al. 2015).
Twenty-four hours following exposure, mice exposed to either PM sample had significantly more neutrophils recovered in bronchiolar lavage fluid than mice exposed to vehicle control. However, the Sacramento PM group also had significantly more neutrophils than the Taiyuan PM group. Neutrophil influx is most frequently associated with acute lung injury (Abraham 2003; Grammes and Soehnlein 2011; Lee and Downey 2001), and the relative number of neutrophils entering the lungs typically reflects the severity of the biological response. Neutrophil recruitment under the conditions of this study was surmised to occur by egress from the capillaries of the airways through the epithelium into the airway lumen and alveoli (Grammes and Soehnlein 2011) based on the histological evidence of neutrophils in pulmonary capillaries, interstitium, epithelial cells, and airspace. Animals exposed to Sacramento PM may have had higher numbers of neutrophils in the lung because they had significantly increased levels of TNF-α, which is involved in acute lung inflammation and the acute phase reaction (Li et al. 2013), compared to animals exposed to the vehicle control and Taiyuan PM. CXCL-1, a neutrophil chemoattractant (De Filippo et al. 2008), was also significantly elevated following exposure to Sacramento PM compared to the vehicle control. MCP-1 is a chemoattractant responsible for monocyte and macrophage recruitment. However, growing evidence shows MCP-1 could also be involved in attracting neutrophils (Balamayooran et al. 2011; Reichel et al. 2009). Therefore, the increased level of MCP-1 expression in the Sacramento PM group could also be associated with the observed elevation in neutrophil number in the lungs.
Because of the growing evidence that particle chemical composition plays an important role in eliciting health effects (Dreher 2000; Plummer et al. 2015; Valavanidis et al. 2008), the chemical composition of both PM samples was determined. Atmospheric PM is very complex, comprising a large number of compounds including organics, inorganic species, elemental carbon, crustal components, and metals. Organic aerosols often represent a major component of the total fine PM mass (Kanakidou et al. 2005; Zhang et al. 2007), as was the case for both the Taiyuan and Sacramento PM. As organic aerosols can be composed of hundreds of carbon-containing compounds, they have the potential to differentially influence PM toxicity. Such is the case here where the Sacramento PM had higher levels of oxidized organic compounds compared with the Taiyuan PM. Previous studies indicate that oxidized organic compounds in fine PM are strongly linked with inflammatory responses (Happo et al. 2010; Plummer et al. 2015). Thus, it could be that the higher levels of oxidized organic compounds in the Sacramento PM are responsible for its ability to generate a greater acute inflammatory response in the lungs.
While the bulk of the PM was largely organic material, both samples also contained nitrates, sulfates, and ammonium. Similar sources of particulate emission are present in both CH (Taiyuan) and CA (Sacramento) during the winter season, such as fossil fuels used for heating and cooking, transportation (gas and diesel emissions), and agricultural production. However, the Sacramento area has greater wood burning, whereas Taiyuan has greater coal burning (Ge et al. 2012; Young et al. 2016). The higher emission of sulfate in the Taiyuan PM is most likely due to the burning of coal. In addition, mass spectral fingerprints for wood combustion were observed in Sacramento whereas coal combustion signatures were detected in Taiyuan samples.
Metals are associated with various inflammatory responses and oxidative stress (e.g. Miousse et al. 2015) and have been implicated in a range of pulmonary health effects in numerous studies (Costa and Dreher 1997). CH PM and CA PM contained a wide range of metals; however, they differed in concentration. In particular, the concentration of copper was much higher in the CA PM sample than the CH sample. It was recently reported that Cu produces hydrogen peroxide in surrogate lung fluid in a study of ambient PM components and their ability to produce reactive oxygenation species (Charrier et al. 2014).
Limitations of this study include only one post-exposure time point observed, differences in PM collection times, along with differences in filter extraction procedures and sonication time between the CA and CH two PM samples. Although inhalation studies are preferable to understand the biological effects of PM depositing in the lungs, the goal of this study was to compare the two PM samples on an equal mass basis. This requires precise dosing, which can be accomplished with oropharyngeal aspiration, but is much more difficult to achieve with inhalation. Oropharyngeal aspiration also allowed us to test the two samples under identical conditions. Future studies would benefit from either repeated exposure to PM and/or more time points post-exposure to determine inflammatory time-lag effects of PM from each country.
5. Conclusion
The results from this study indicate chemical composition is an important factor of PM toxicity, especially since the CA and CH PM samples were evaluated on an equal mass basis. The CA (Sacramento) PM produced a greater inflammatory response that is thought to be due to its higher oxidized state than the CH (Taiyuan PM). However, differences in copper and hydrodynamic particle size as measured by ICP-MS and DLS, respectively, for these samples of CA and CH PM may also be important drivers in the acute toxicity differences observed. It is unlikely that just one component is solely responsible for the toxicity and possible synergistic effects between the organics and metals may be occurring. International collaborations such as this study are needed to more fully understand how chemical composition of PM correlates with adverse health effects. These findings provide strong scientific evidence that highlight a need to develop source-specific regulations that support greater protection of human health.
Figure 2. Lung ELISA.
Lung ELISA: TNF-α in the lungs following exposure to Sacramento (CA) PM is significantly higher than either the controls (CTRL) or mice exposed to Taiyuan (CH) PM, while CXCL-1 expression in mice exposed to Sacramento (CA) PM is significantly higher only than control. A trend but no significance is noted for MCP-1, HO-1 and IL-5 levels for CA PM. Data is presented as mean (SEM) from 6 animals. * indicates significant difference to sham control. # indicates significant difference to Taiyuan (CH) PM (p<0.05 by one-way ANOVA).
Figure 5. Chemical composition.
Chemical composition of winter time Taiyuan, China (A) and Sacramento, California (C) PM at equal mass concentrations (1µg/µl) in suspension (B). Inorganics dominate Taiyuan (CH) PM (55%), with sulfate being particularly important. Organics (54%) and nitrate (32%) are the largest contributors to the mass in the case of Sacramento (CA) PM.
Acknowledgments
The authors thank Imelda Espiritu and Dale Uyeminami for technical assistance during the course of this study and the use of the Cellular and Molecular Imaging Core at the Center for Health and the Environment. We thank Rouzbeh Rahai, Jennifer Lien and Mengqi Su from the laboratory of Professor Ting Guo, University of California, Davis for their assistance in DLS measurements of the PM suspensions used in this study.
FUNDING SOURCES
This work was supported by the National Institute of Occupational Safety and Health (U54 0H07550 and P30 ES023513) and the National Institutes of Health (T32 HL007013 andT32 GM099608).
References
- Abraham E. Neutrophils and acute lung injury. Critical care medicine. 2003;31:S195. doi: 10.1097/01.CCM.0000057843.47705.E8. [DOI] [PubMed] [Google Scholar]
- Aiken AC, Decarlo PF, Kroll JH, Worsnop DR, Huffman JA, Docherty KS, et al. O/c and om/oc ratios of primary, secondary, and ambient organic aerosols with high-resolution time-of-flight aerosol mass spectrometry. Environmental Science & Technology. 2008;42:4478–4485. doi: 10.1021/es703009q. [DOI] [PubMed] [Google Scholar]
- Balamayooran G, Batra S, Balamayooran T, Cai S, Jeyaseelan S. Monocyte chemoattractant protein 1 regulates pulmonary host defense via neutrophil recruitment during escherichia coli infection. Infection and immunity. 2011;79:2567–2577. doi: 10.1128/IAI.00067-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bein K, Wexler AS. Compositional variance in extracted particulate matter using different filter extraction techniques. Atmospheric Environment. 2015;107:24–34. [Google Scholar]
- Canagaratna M, Jayne J, Jimenez J, Allan J, Alfarra M, Zhang Q, et al. Chemical and microphysical characterization of ambient aerosols with the aerodyne aerosol mass spectrometer. Mass Spectrometry Reviews. 2007;26:185–222. doi: 10.1002/mas.20115. [DOI] [PubMed] [Google Scholar]
- Cao J, Lee S, Chow JC, Watson JG, Ho K, Zhang R, et al. Spatial and seasonal distributions of carbonaceous aerosols over china. Journal of Geophysical Research: Atmospheres. 2007:112. [Google Scholar]
- Charrier JG, McFall AS, Richards-Henderson NK, Anastasio C. Hydrogen peroxide formation in a surrogate lung fluid by transition metals and quinones present in particulate matter. Environmental science & technology. 2014;48:7010–7017. doi: 10.1021/es501011w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churg A, Brauer M. Human lung parenchyma retains pm2. 5. American journal of respiratory and critical care medicine. 1997;155:2109–2111. doi: 10.1164/ajrccm.155.6.9196123. [DOI] [PubMed] [Google Scholar]
- Costa DL, Dreher KL. Bioavailable transition metals in particulate matter mediate cardiopulmonary injury in healthy and compromised animal models. Environmental health perspectives. 1997;105:1053. doi: 10.1289/ehp.97105s51053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippo K, Henderson RB, Laschinger M, Hogg N. Neutrophil chemokines kc and macrophage-inflammatory protein-2 are newly synthesized by tissue macrophages using distinct tlr signaling pathways. The Journal of Immunology. 2008;180:4308–4315. doi: 10.4049/jimmunol.180.6.4308. [DOI] [PubMed] [Google Scholar]
- Dreher KL. Particulate matter physicochemistry and toxicology: In search of causality—a critical perspective. Inhalation Toxicology. 2000;12:45–57. doi: 10.1080/08958378.2000.11463230. [DOI] [PubMed] [Google Scholar]
- Ge X, Setyan A, Sun Y, Zhang Q. Primary and secondary organic aerosols in fresno, california during wintertime: Results from high resolution aerosol mass spectrometry. Journal of Geophysical Research: Atmospheres. 2012:117. [Google Scholar]
- Gilmour MI, McGee J, Duvall RM, Dailey L, Daniels M, Boykin E, et al. Comparative toxicity of size-fractionated airborne particulate matter obtained from different cities in the united states. Inhalation toxicology. 2007;19:7–16. doi: 10.1080/08958370701490379. [DOI] [PubMed] [Google Scholar]
- Grammes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Molecular Medicine. 2011;17:293. doi: 10.2119/molmed.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happo M, Salonen R, Hälinen A, Jalava P, Pennanen A, Dormans J, et al. Inflammation and tissue damage in mouse lung by single and repeated dosing of urban air coarse and fine particles collected from six european cities. Inhalation toxicology. 2010;22:402–416. doi: 10.3109/08958370903527908. [DOI] [PubMed] [Google Scholar]
- Holmes HA, Sriramasamudram JK, Pardyjak ER, Whiteman CD. Turbulent fluxes and pollutant mixing during wintertime air pollution episodes in complex terrain. Environmental science & technology. 2015;49:13206–13214. doi: 10.1021/acs.est.5b02616. [DOI] [PubMed] [Google Scholar]
- Kanakidou M, Seinfeld J, Pandis S, Barnes I, Dentener F, Facchini M, et al. Organic aerosol and global climate modelling: A review. Atmospheric Chemistry and Physics. 2005;5:1053–1123. [Google Scholar]
- Lee WL, Downey GP. Neutrophil activation and acute lung injury. Current opinion in critical care. 2001;7:1–7. doi: 10.1097/00075198-200102000-00001. [DOI] [PubMed] [Google Scholar]
- Li T, Luo N, Du L, Zhou J, Zhang J, Gong L, et al. Tumor necrosis factor-α plays an initiating role in extracorporeal circulation-induced acute lung injury. Lung. 2013;191:207–214. doi: 10.1007/s00408-012-9449-x. [DOI] [PubMed] [Google Scholar]
- Madl A, Kadir T, Pinkerton KE. Proceedings of the Elsevier Inc. 2014. Particle toxicities. [Google Scholar]
- Mannucci PM, Harari S, Martinelli I, Franchini M. Effects on health of air pollution: A narrative review. Internal and emergency medicine. 2015;10:657–662. doi: 10.1007/s11739-015-1276-7. [DOI] [PubMed] [Google Scholar]
- Miousse IR, Chalbot M-CG, Lumen A, Ferguson A, Kavouras IG, Koturbash I. Response of transposable elements to environmental stressors. Mutation Research/Reviews in Mutation Research. 2015;765:19–39. doi: 10.1016/j.mrrev.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer LE, Carosino CM, Bein KJ, Zhao Y, Willits N, Smiley-Jewell S, et al. Pulmonary inflammatory effects of source-oriented particulate matter from california's san joaquin valley. Atmospheric Environment. 2015;119:174–181. doi: 10.1016/j.atmosenv.2015.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CA, Rehberg M, Lerchenberger M, Berberich N, Bihari P, Khandoga AG, et al. Ccl2 and ccl3 mediate neutrophil recruitment via induction of protein synthesis and generation of lipid mediators. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:1787–1793. doi: 10.1161/ATVBAHA.109.193268. [DOI] [PubMed] [Google Scholar]
- Rodopoulou S, Samoli E, Chalbot M-CG, Kavouras IG. Air pollution and cardiovascular and respiratory emergency visits in central arkansas: A time-series analysis. Science of The Total Environment. 2015;536:872–879. doi: 10.1016/j.scitotenv.2015.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva RM, Teesy C, Franzi L, Weir A, Westerhoff P, Evans JE, Pinkerton KE. Biological response to nano-scale titanium dioxide (TiO2): role of particle dose, shape, and retention. J Toxicol Environ Health A. 2013;76(16):953–72. doi: 10.1080/15287394.2013.826567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva RM, Doudrick K, Franzi LM, TeeSy C, Anderson DS, Wu Z, Mitra S, Vu V, Dutrow G, Evans JE, Westerhoff P, Van Winkle LS, Raabe OG, Pinkerton KE. Instillation versus inhalation of multiwalled carbon nanotubes: exposure-related health effects, clearance, and the role of particle characteristics. ACS Nano. 2014;8(9):8911–31. doi: 10.1021/nn503887r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Zhang Q, Anastasio C, Sun J. Insights into secondary organic aerosol formed via aqueous-phase reactions of phenolic compounds based on high resolution mass spectrometry. Atmospheric Chemistry and Physics. 2010;10:4809–4822. [Google Scholar]
- Sun Y, Zhang Q, Zheng M, Ding X, Edgerton ES, Wang X. Characterization and source apportionment of water-soluble organic matter in atmospheric fine particles (pm2. 5) with high-resolution aerosol mass spectrometry and gc-ms. Environmental science & technology. 2011;45:4854–4861. doi: 10.1021/es200162h. [DOI] [PubMed] [Google Scholar]
- Valavanidis A, Fiotakis K, Vlachogianni T. Airborne particulate matter and human health: Toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. Journal of Environmental Science and Health, Part C. 2008;26:339–362. doi: 10.1080/10590500802494538. [DOI] [PubMed] [Google Scholar]
- WHO. Climate and health country profile 2015 [Google Scholar]
- Xiao Q, Ma Z, Li S, Liu Y. The impact of winter heating on air pollution in china. PloS one. 2015;10:e0117311. doi: 10.1371/journal.pone.0117311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D, Kim H, Parworth C, Zhou S, Zhang X, Cappa C, et al. Influences of emission sources and meteorology on aerosol chemistry in a polluted urban environment: Results from discover-aq california. Atmospheric Chemistry and Physics Discussions. 2016;16:5427–5451. [Google Scholar]
- Zhang Q, Jimenez J, Canagaratna M, Allan J, Coe H, Ulbrich I, et al. Ubiquity and dominance of oxygenated species in organic aerosols in anthropogenically-influenced northern hemisphere midlatitudes. Geophysical Research Letters. 2007:34. [Google Scholar]