Abstract
As a weak acid, methotrexate (MTX) is bound to serum albumin and has variable protein binding. The purpose of this study was to assess serum albumin’s relationship with MTX pharmacokinetics by comparing MTX clearance and toxicities between patients with normal serum albumin to those with hypoalbuminemia. This single-center retrospective study included adult patients with leukemia or lymphoma who received their first MTX at a dose ≥ 1 gram/m2. Hypoalbuminemia was defined as serum albumin ≤ 3.4 g/dL. MTX clearance was defined as the first documented time the MTX level ≤ 0.05 micromolar. Fisher’s Exact tests and Wilcoxon Rank Sum tests were used to examine differences in toxicities and Cox proportional hazards regression was used to assess relationship with time to clearance. Of 523 patients identified, 167 patients were evaluable. One hundred thirty five patients had normal serum albumin and 32 had hypoalbuminemia. Hypoalbuminemia was associated with a higher proportion of patients experiencing edema, ascites or pleural effusions (34% vs. 12% p=0.006) and the concomitant use of nephrotoxic agents (41% vs. 20% p=0.021). Hypoalbuminemia was associated with a significantly longer time to methotrexate clearance (median: 96 hours vs 72 hour p=0.004). In addition, patients with hypoalbuminemia had a higher proportion of hyperbilirubinemia and significantly longer hospitalization (median 14 days vs. 5 days p<0.001). In conclusion, hypoalbuminemia was associated with increased time to MTX clearance and increase length of hospitalization. High dose MTX is safe to administer in patients with low albumin levels, with appropriate leucovorin rescue and good supportive care.
Keywords: Methotrexate, hypoalbuminemia, pharmacokinetics, toxicity, clearance, albumin, leukemia, lymphoma, high dose methotrexate
Introduction
Methotrexate(MTX), 2,4-diamino-N10-methyl propylglutamic acid, is an antimetabolite used for over 50 years and is a key chemotherapeutic agent for many oncology indications such as leukemia, lymphoma and solid tumor malignancies[1]. The inclusion of high dose methotrexate with leucovorin rescue enhanced the efficacy of regimens treating lymphomas and acute leukemias [2–6]. Methotrexate is an inhibitor of dihydrofolate reductase (DHFR) and directly inhibits the folate-dependent enzymes of de-novo purine and thymidylate synthesis [1]. Further, it requires active transport to enter mammalian cells due to its polarity. Only free unbound drug can be transported via folate transporter inside tumor cells and the blood brain barrier [1]. Once transported intracellularly, folates and methotrexate are converted to polyglutamates, which have unique properties and are preferentially retained inside the cells in the absence of the extracellular drug. This can lead to increased cytotoxicity once the drug is converted intracellularly. Additionally, the volume of distribution into third space fluid collections, such as pleural effusions and ascitic fluid, can substantially alter methotrexate pharmacokinetics. The presence of fluid collections typically results in methotrexate retention, slower clearance and a longer half life, requiring longer leucovorin rescue. Methotrexate is rapidly removed from circulation through the kidneys and clearance is mediated by glomerular filtration and tubular secretion [7]. About 80 to 90% of an administered dose is eliminated unchanged in the urine in the first 48 hours in patients with normal renal function.
Methotrexate is a weak acid and binds to serum albumin [8,9]. Methotrexate protein binding varies but has been reported to be approximately 50%. Albumin is alkalotic and binds weak acids in the serum; it has a long half life of approximately 19 days and is the primary protein responsible for intravascular oncotic pressure [10]. Some experimental evidence suggest that albumin preferentially accumulates in tumors [8,9]. This hypothesis led to the formulation of human serum albumin as a drug carrier for methotrexate. Methotrexate conjugated to albumin showed enhanced activity within tumors in mice, having greater suppression of tumor growth compared to unbound methotrexate. While bound to albumin, methotrexate can be internalized into tumor cells by endocytosis, independent of folate carrier transport required with conventional methotrexate. In animal models using methotrexate conjugated to albumin, investigators found an increased delivery of methotrexate to the liver and a decreased accumulation in the kidney, but also increased accumulation to other organs such as lung, heart and spleen[11]. In summary, methotrexate bound to albumin may have an enhanced anti-tumor effect and a decreased clearance.
However, it has been postulated that low albumin levels are associated with delayed clearance and increased toxicity. Choi et al. performed a pharmacokinetic study using intravenous methotrexate in analbuminemic rats which showed delayed methotrexate clearance compared to the control rats[12]. Additionally, several case reports and retrospective reviews reported a higher frequency of methotrexate toxicity in patients with hypoalbuminemia. A multicenter case-control study evaluated risk factors for methotrexate induced lung injury in rheumatoid arthritis patients and found that those with low levels of serum albumin had a 10 fold higher odds for developing methotrexate toxicity [13].
Given the lack of robust data, the main objective of our study was to assess albumin’s effect on high dose methotrexate clearance.
Patients and Methods
Study Design
This study was approved by the Institutional Review Board and all procedures followed were in accordance with ethical standards and informed consent was waived for this retrospective review. This was a single center observational retrospective cohort study performed at Memorial Sloan Kettering Cancer Center (MSK) comparing time to methotrexate clearance in patients with hypoalbuminemia versus patients with normal albumin levels.
Patients and MSK’s High Dose Methotrexate Administration Practice
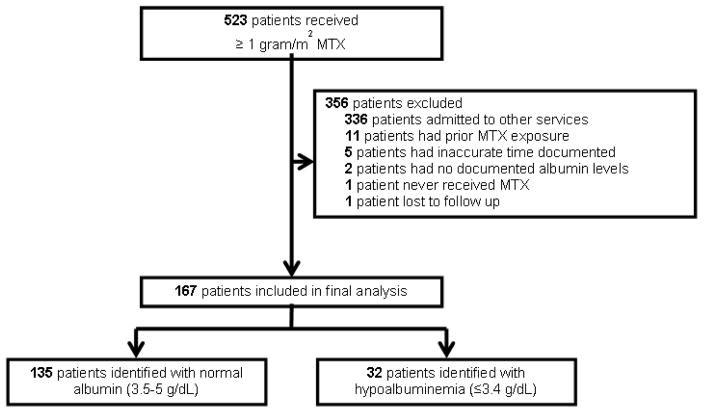
Patients treated between January 2007 and November 2014 with leukemia or lymphoma admitted to their respective inpatient services were identified through the pharmacy database and electronic medical records. Patients included were 18 years of age or older who had received methotrexate at a dose of 1 gram/m2 or higher. Patients with prior methotrexate, no reported albumin levels drawn prior to infusion, or inaccurate methotrexate levels drawn were excluded. Also, other services, such as neurology, followed different protocols for high dose methotrexate administration and monitoring and therefore were excluded from this study (Figure 1). Patients were evaluated during their first exposure to methotrexate only. Patients were categorized based on their baseline serum albumin defined as the level recorded the day of administration of methotrexate or the most recent serum albumin within two weeks prior to administration. Patients considered to have normal serum albumin had a serum albumin between 3.5–5 g/dL, while those with hypoalbuminemia had a serum albumin 3.4 g/dL or less.
Figure 1.
Prior to administering high dose methotrexate, all patients received sodium bicarbonate and hydration until urine output was greater than 150 mL/hour and urine pH was 7.5 or greater. After high dose methotrexate was administered, urinalysis was checked every 6 hours to ensure appropriate alkalinity. Additionally, input and output, patient weights, basic metabolic panel and complete blood counts were monitored daily. Twenty four hours after the start of methotrexate infusion, leucovorin was administered to all patients at a dose of 25 mg intravenously or orally every 6 hours. Methotrexate levels are scheduled to be drawn 24 hours after the start of the infusion for short infusions and 48 hours after the start of continuous infusions. Adjustment of the leucovorin dose was based on the serum methotrexate level. Patients remained on leucovorin until the methotrexate level was less than 0.05 micromolars.
End Points and Assessments
The primary objective of this study was to compare time to methotrexate clearance between patients with normal albumin levels vs patients with hypoalbuminemia. It was defined as the time between methotrexate administration and the first documented time that the serum methotrexate level was less than or equal to 0.05 micromolars. The second objective was to identify other predictors of methotrexate clearance, including renal impairment, drug interactions, urine pH <7, ascites, presence of fluid retention (defined has the patient either having edema, pleural effusions or edema), concomitant use of nephrotoxic agents, and methotrexate dosing level. Methotrexate dosing was defined as low-high dose, intermediate-high dose or high-high dose if the dose was between 1 to 2.9 grams/m2, 3–5.9 grams/m2 or 6 grams/m2 and higher, respectively. The third objective was to compare toxicity rates between patients with normal serum albumin versus those with hypoalbuminemia. All toxicities were graded according to the common terminology criteria for adverse events (CTCAE) version 4.03. Toxicities of particular interest included acute nephrotoxicity, hepatotoxicity, liver function test (LFT) abnormalities, myelosuppression, mucositis, and pneumonitis.
Statistical Analysis
Frequencies and percents were used to describe categorical variables, and medians and ranges were used to describe continuous variables. In addition to hypoalbuminemia, dosing level (low, intermediate, and high), urine pH less than 7, kidney impairment, fluid retention, nephrotoxic agents, and drug interactions were hypothesized to be associated with time to MTX clearance. The relationship between hypoalbuminemia and clinical variables was assessed with Fisher’s exact test for categorical variables and with the Wilcoxon Rank Sum test for continuous variables.
Kaplan Meier methods were used to visualize the relationship between hypoalbuminemia with time to methotrexate clearance. Time to methotrexate clearance was defined as the interval between the start of the methotrexate infusion and methotrexate level less than 0.05 micromolars. Patients who did not experience clearance were censored at the time of the last methotrexate level drawn. The univariate associations between predictors and clearance were assessed with Cox proportional hazards regression. Any variables significant at p<0.05 were considered for multivariate analysis. Interactions between predictors and hypoalbuminemia were examined and entered into the multivariate model if significant. Cox proportional hazards regression was also used to build the multivariate clearance model. As treatment dose does not remain constant over time, a sensitivity analysis was run treating dose as a time-dependent covariate in a bivariate Cox model with hypoalbuminemia.
Toxicity grade was grouped into 0 (no toxicity), grade 1–2, and grade 3–4. The relationship between toxicity grades and hypoalbuminemia was examined using Fisher’s exact test. P values less than 0.05 were considered statistically significant. All analyses were performed using SAS 9.4 (The SAS Institute, Cary, NC).
Results
Patient Population
A total of 523 patients received high dose methotrexate between and 356 were excluded (figure 1); the majority because they were treated by services other than the lymphoma and leukemia service. Table 1 outlines the baseline characteristics of the remaining 167 patients. 126 patients were managed by the lymphoma service (75.4%) and 41 patients by the leukemia service (24.6%). The median age of all patients was 51.3 years, 62.3% were male and 76.6% were Caucasian. The most common regimens used were R-CODOX/M-IVAC (21%), “pediatric-inspired” acute lymphoblastic leukemia regimens (15%), HyperCVAD (12%), SMILE (11.4%), and MR-CHOP (10%). Among the 167 patients, serum albumin levels were normal in 135 (80.8%) and hypoalbuminemia was present in 32 patients (19.2%). High dose methotrexate was given in first line regimens in 74.8% of normal serum albumin cohort and 56.3% in the hypoalbuminemia cohort. Baseline characteristics were similar between groups except a higher proportion of patients in the hypoalbuminemia cohort had fluid retention (34% vs. 12%, p=0.0055) and had concomitant use of nephrotoxic agents (41% vs. 20%, p=0.0205).No relationship was found between hypoalbuminemia and dosing level (p=0.40), urine pH > 7 (p=0.83), kidney impairment (p=1.00) or drug interactions (p=0.16).
Table 1.
| Characteristic | All patients (n=167) | Hypoalbuminemia (n=32) | Normal (n=135) | p-value |
|---|---|---|---|---|
| Age at Diagnosis-year (range) | 51.3 (18.5–83.7) | 55.8 (20.6–78.2) | 49.1 (18.5–83.7) | 0.07 |
| Gender-male-no. (%) | 104 (62.3) | 18 (56.3) | 86 (63.7) | 0.54 |
| Median Length of Stay-days (range) | 6 (2–164) | 14 (3–164) | 5 (2–97) | <0.001 |
| Race | ||||
| Caucasian-no. (%) | 128 (76.6) | 25 (78.1) | 103 (76.3) | 0.45 |
| Asian-no. (%) | 20 (12) | 2 (6.3) | 18 (13.3) | |
| Black-no. (%) | 15 (9) | 4 (12.5) | 11 (8.1) | |
| Latino/Hispanic-no. (%) | 2 (1.2) | 0 (0) | 2 (1.5) | |
| Other-no. (%) | 2 (1.2) | 1 (3.1) | 1 (0.7) | |
| Service | ||||
| Leukemia-no. (%) | 41 (24.6) | 7 (21.9) | 34 (25.2) | 0.82 |
| Lymphoma-no. (%) | 126 (75.4) | 25 (78.1) | 101 (74.8) | |
| Factors effecting methotrexate clearance | ||||
| Presence of Edema-no. (%) | 20 (12) | 8 (25) | 12 (8.9) | 0.029 |
| Presence of Ascites/Pleural Effusion-no. (%) | 13 (7.8) | 8 (25) | 5 (3.7) | 0.001 |
| Presence of Fluid Retention-no. (%) | 27 (16.2) | 11 (34.4) | 16 (11.9) | 0.006 |
| Use of Concomitant Nephrotoxic Agents-no. (%) | 40 (24) | 13 (40.6) | 27 (20) | 0.021 |
| Drug Interactions-no. (%) | 100 (59.9) | 23 (71.9) | 77 (57) | 0.16 |
| Urine pH < 7 | 47 (28.1) | 8 (25) | 39 (28.9) | 0.83 |
| Baseline Kidney Impairment | 57 (34.1) | 11 (34.4) | 46 (34.1) | 1.00 |
| Baseline median creatinine-g/dL | 0.8 (0.3–1.5) | 0.7 (0.4–1.3) | 0.8 (0.3–1.5) | 0.17 |
| Baseline median creatinine clearance-mL/min | 107.9 (46.2–279) | 114.3 (49.7–180.5) | 106.3 (46.2–279) | 0.69 |
| Dosing category | ||||
| Low-high dose methotrexate (1–2.9 g/m2)-no (%) | 46 (27.5) | 11 (34.4) | 35 (25.9) | 0.40 |
| Intermediate-high dose methotrexate (3–5.9 g/m2)-no (%) | 100 (59.9) | 19 (59.4) | 81 (60) | |
| High-high dose methotrexate (≥6 g/m2)-no (%) | 21 (12.6) | 2 (6.3) | 19 (14.1) | |
Time to Methotrexate Clearance
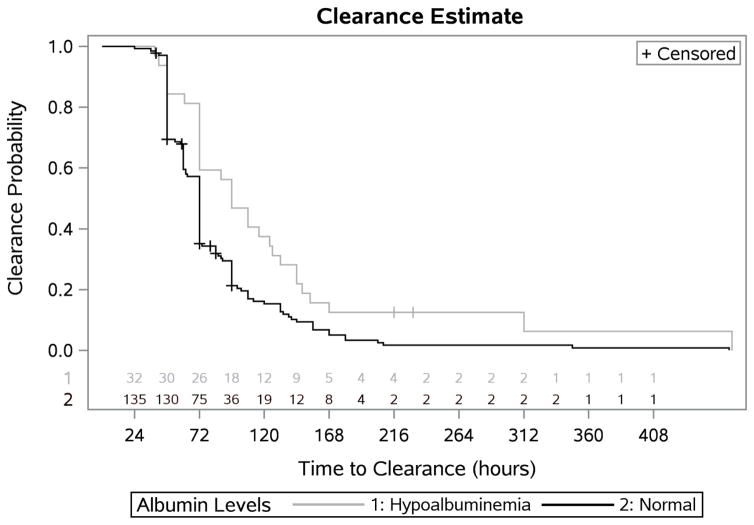
Hypoalbuminemia was associated with a significantly longer time to methotrexate clearance (median KM estimate: 96 hours (95% CI: 72–132 hours) vs. 72 hours (95% CI: 63–72 hours), p=0.004) (figure 2, table 2).
Figure 2.
Table 2.
| Group | N (Events) | Median Estimate (Hours) (95% CI) |
|---|---|---|
| Hypoalbuminemia | 32 (30) | 96 (72–132) |
| Normal | 132 (128) | 72 (63–72) |
| p=.004 |
In univariate Cox proportional hazards regression, the following factors were significantly associated with methotrexate clearance: albumin level (p=0.010), dosing level (p=0.009), and nephrotoxic agents (p=0.003) (Table 3). Patients with hypoalbuminemia had a decreased likelihood of clearance (HR 0.58, 95% CI: 0.39–0.88) compared to patients with normal serum albumin. Additionally, those with higher doses, ≥ 6 gram/m2, compared to lower doses, and those who received nephrotoxic agents compared to those who did not were also estimated to be at a decreased risk for clearance(HR 0.44, 95% CI:0.26–0.74, HR 0.56 95%CI:0.38–0.82, respectively) (table 4). The presence of fluid retention, defined ad presence of edema, ascites or pleural effusions, had a trend towards clearance risk (HR 0.66, 95% CI 0.43–1.02). In a multivariate Cox proportional hazard model, hypoalbuminemia (HR 0.57, p=0.009), dosing level (HR 0.38, p=0.002) and nephrotoxic agents (HR0.61, p=0.015) were significant independent predictors of methotrexate clearance (table 4). A sensitivity analysis was run to determine the effect of a time dependent dose level on the relationship between albumin and clearance. No significant change was found in the relationship between albumin and clearance when including dose level as a time-dependent covariate (p<0.05 for both albumin and dosing category).
Table 3.
| Variable | HR | [95% CI] | p-value |
|---|---|---|---|
|
| |||
| Albumin Level | |||
| Hypoalbuminemia | 0.58 | [0.39 – 0.88] | 0.01 |
| Normal | REF | ||
|
| |||
| Dosing Category | |||
| High (≥6 g/m2) | 0.44 | [0.26 – 0.74] | 0.002 |
| Intermediate (3–5.9 g/m2) | 0.75 | [0.53 – 1.08] | 0.12 |
| Low (1–2.9 g/m2) | REF | ||
|
| |||
| Urine pH < 7(Yes) | 0.79 | [0.55 – 1.13] | 0.20 |
|
| |||
| Baseline Kidney Impairment | 0.86 | [0.62 – 1.19] | 0.37 |
|
| |||
| Presence of Fluid Retention (presence of either edema, ascites or pleural effusion) | 0.66 | [0.43 – 1.02] | 0.06 |
|
| |||
| Use of Nephrotoxic Agents | 0.56 | [0.38 – 0.82] | 0.003 |
|
| |||
| Drug Interactions | 0.75 | [0.54 – 1.03] | 0.08 |
HR = Hazard Ratio CI = Confidence Interval
Table 4.
| Variable | HR | [95% CI] | p-value |
|---|---|---|---|
|
| |||
| Albumin Level | |||
| Hypoalbuminemia | 0.57 | [0.38 – 0.87] | 0.009 |
| Normal | REF | ||
|
| |||
| Dosing Category | |||
| High (≥6 g/m2) | 0.38 | [0.22 – 0.65] | 0.001 |
| Intermediate (3–5.9 g/m2) | 0.71 | [0.50 – 1.02] | 0.06 |
| Low (1–2.9 g/m2) | REF | ||
|
| |||
| Use of Nephrotoxic Agents | 0.61 | [0.41 – 0.91] | 0.015 |
HR = Hazard Ratio CI = Confidence Interval
Toxicity
The proportion of the toxicity rates in patients with hypoalbuminemia and patients with normal albumin graded are shown in Table 5. Patients with hypoalbuminemia had higher rates of bilirubin elevations compared to those with normal serum albumin (grade 3–4: 12.5% vs. 0.0%, grade 1–2: 15.6% vs. 12.6%, p=0.001, respectively). Anemia was also associated with hypoalbuminemia (p=0.013). Of the hypoalbuminemia patients 31% (10/32) had grade 1–2, and 25% (8/32) had grade 3–4; in contrast, of the patients with normal albumin levels, 53% (71/135) had grade 1–2, and 8% (11/135) had grade 3–4. Since anemia was also associated with hypoalbuminemia (p=0.013) we also performed a sensitivity subgroup analysis examining whether this difference persists based on baseline hemoglobin levels. No significant difference was found in treatment related anemia for hypoalbumenia patients in patients with anemia at baseline (p=0.13). Also, no significant difference was found in the proportion of patients who experienced ALT/AST elevations, nephrotoxicity, mucositis, leukopenia, neutropenia, or pneumonitis (p-value ranges 0.08–0.99). Patients in the low albumin cohort had significantly longer hospitalization (median 14 days vs. 5 days, p<0.0001).
Table 5.
| Toxicity | Grade | Hypoalbuminemia | Normal | p-value |
|---|---|---|---|---|
| ALT | 0 | 15 (46.9) | 49 (36.3) | 0.40 |
| 1–2 | 14 (43.8) | 76 (56.3) | ||
| 3–4 | 3 (9.4) | 10 (7.4) | ||
| AST | 0 | 19 (59.4) | 81 (60) | > 0.99 |
| 1–2 | 12 (37.5) | 49 (36.3) | ||
| 3–4 | 1 (3.1) | 5 (3.7) | ||
| GI | 0 | 32 (100) | 135 (100) | >0.99 |
| Leukopenia | 0 | 6 (18.8) | 52 (38.5) | 0.08 |
| 1–2 | 8 (25) | 29 (21.5) | ||
| 3–4 | 18 (56.3) | 54 (40) | ||
| Mucositis | 0 | 27 (84.4) | 108 (80) | >0.99 |
| 1–2 | 5 (15.6) | 23 (17) | ||
| 3–4 | 0 (0) | 4 (3) | ||
| Nephrotoxicity | 0 | 24 (75) | 106 (78.5) | 0.65 |
| 1–2 | 7 (21.9) | 21 (15.6) | ||
| 3–4 | 1 (3.1) | 8 (5.9) | ||
| Neutropenia | 0 | 12 (37.5) | 68 (50.4) | 0.16 |
| 1–2 | 8 (25) | 17 (12.6) | ||
| 3–4 | 12 (37.5) | 50 (37) | ||
| Pneumonitis | 0 | 32 (100) | 135 (100) | 0.16 |
| Bilirubin | 0 | 23 (71.9) | 118 (87.4) | 0.001 |
| 1–2 | 5 (15.6) | 17 (12.6) | ||
| 3–4 | 4 (12.5) | 0 (0) | ||
| Anemia | 0 | 14 (43.8) | 53 (39.3) | 0.012 |
| 1–2 | 10 (31.3) | 71 (52.6) | ||
| 3–4 | 8 (25) | 11 (8.1) |
Two patients in the retrospective review experienced grade 4 toxicities. One patient, with newly diagnosed diffuse large B cell lymphoma with CNS involvement and treated with MR-CHOP, had normal serum albumin. Shortly after high dose methotrexate administration he developed acute renal failure and serum creatinine increased from 0.8 g/dL to 9.6 g/d. After hemodialysis methotrexate was clear at 132 hours and the kidney function had recovered. The patient had no additional methotrexate toxicity. Patient number #2 had received SMILE for newly diagnosed NK/T Cell lymphoma received had hypoalbuminemia. Shortly after receiving methotrexate the patient had acute renal failure with minimal urine output, the patient received glucarpidase within 72 hours, but ultimately died due to sepsis before the methotrexate cleared.
Discussion
Our study demonstrated that patients with hypoalbuminemia had significantly longer time to methotrexate clearance in patients with leukemia or lymphoma. To our knowledge, this is the first study to show that hypoalbuminemia is an independent predictor for methotrexate clearance. Longer time to methotrexate clearance with delayed elimination of the drug may potentially increase the risk of drug-related toxicity; however, we saw few differences in toxicity rates that dissipated once baseline levels were taken into account. The only toxicity difference noted was that patients with hypoalbuminemia were more likely to have bilirubin elevations. Wiczer et al analyzed factors that can contribute to methotrexate induced renal toxicity and found that patients who experienced nephrotoxicity had a higher rate low serum albumin (albumin <3 g/dL) compared those without nephrotoxicity (31.9% vs 15.9% p=0.04) [14]. However, our study did not confirm this finding, which may be a result of their more stringent criterion for hypoalbuminemia (<3 g/dL vs. 3.4 g/dL).
We identified several characteristics that were significantly different in patients with hypoalbuminemia that could contribute to delayed clearance, such as concomitant use of nephrotoxic agents and the presence of fluid retention defined as ascites, pleural effusions or edema [15]. In our multivariate analysis, hypoalbuminemia remained a predictor of clearance.
Additionally, a higher proportion of patients in the hypoalbuminemia group had fluid retention compared to patients with normal albumin (34.4% vs 11.9%, p=0.006). Since albumin is responsible for approximately 80% of intravascular oncotic pressure, low serum albumin can decrease oncotic pressure leading to third spacing by shifting fluids into interstitial spaces [10]. By being a polar molecule, MTX can accumulate in third space fluid such as pleural effusions and ascites fluid [15]. MTX is slowly released from third space fluid resulting in prolonged clearance. However, the presence of fluid retention did not cross the threshold for statistical significance in the univariate cox proportional hazards regression for methotrexate clearance (HR0.66, p=0.06). This trend is possibly due to the relative small sample size.
One potential approach to reduce the risk for delayed clearance is to correct serum albumin levels by human serum albumin which is commercially available for plasma volume expansion and maintenance of cardiac output in patients with shock and hypovolemia [16–17]. However, most of the externally administered human serum albumin is redistributed within 48 hours into extravascular space and therefore unlikely to impact methotrexate pharmacokinetics[18]. Further, low serum albumin levels are known to be associated with increased risk of severe ifosfamide-induced neurotoxicity [19]. Yet, in one study, prophylactic administration of exogenous albumin did not prevent ifosfamide-induced encephalopathy [20]. Taken together, although our study showed low albumin levels is associated with methotrexate clearance, given the unlikelihood of redistribution and amelioration of encephalopathy, we do not recommend albumin repletion to prevent delayed clearance prior to methotrexate administration.
Our study had several limitations; firstly, it is a retrospective chart review with a small sample size, having only 32 patients with hypoalbuminemia. Second, most our patients received high dose methotrexate as part of multi-agent chemotherapeutic regimens which does not take into account the differences between of their overall toxicity rates. An appropriately powered study that matches baseline characteristics such a case-control study is necessary confirm our observations.
Conclusion
Hypoalbuminemia was associated with prolonged methotrexate clearance, and it remained significant after adjusting for the methotrexate dose and concomitant use of nephrotoxic agents. Additionally, patients with hypoalbuminemia had significantly longer lengths of hospitalization. Patients with hypoalbuminemia were more likely to have bilirubin elevation, but are both markers of synthetic liver function. Despite differences in bilirubin elevations, high dose methotrexate is safe to administer in patients with low albumin levels, with appropriate leucovorin rescue and good supportive care. While hypoalbuminemia can predict longer time to clearance, based on our safety data we cannot support prophylactic administration of human albumin in an attempt to decrease MTX clearance.
Footnotes
Conflict of interest disclosure
The authors declare that they have no conflict of interest.
Contributor Information
Samantha Reiss, Memorial Sloan Kettering Cancer Center, 1250 First Avenue, Schwartz Building S-710, New York, NY 10065.
Larry Buie, Memorial Sloan Kettering Cancer Center, 1250 First Avenue, Schwartz Building S-710, New York, NY 10065.
Nelly Adel, Memorial Sloan Kettering Cancer Center, 1250 First Avenue, Schwartz Building S-710, New York, NY 10065.
Debra A. Goldman, Memorial Sloan Kettering Cancer Center, 485 Lexington Avenue 2nd Floor, New York, NY 10017.
Sean M. Devlin, Memorial Sloan Kettering Cancer Center, 485 Lexington Avenue 2nd Floor, New York, NY 10017.
Dan Douer, Memorial Sloan Kettering Cancer Center, 1250 First Avenue, New York, NY 10065.
References
- 1.Jolivet J, Cowan KH, Curt GA, et al. The pharmacology and clinical use of methotrexate. N Engl J Med. 1983;309:1094–1104. doi: 10.1056/NEJM198311033091805. [DOI] [PubMed] [Google Scholar]
- 2.Kantarjian HM, O’Brien S, Smith TL, et al. Results of treatment with hyper-CVAD, a dose intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol. 2000;18(3):547–61. doi: 10.1200/JCO.2000.18.3.547. [DOI] [PubMed] [Google Scholar]
- 3.Magrath I, Adde M, Venzon D, et al. Adults and Children with small non-cleaved cell lymphoma have similar excellent outcome when treated with the same chemotherapy regimen. J Clin Oncol. 1996;14:925–34. doi: 10.1200/JCO.1996.14.3.925. [DOI] [PubMed] [Google Scholar]
- 4.Mead GM, Sydes MR, Walewski J, et al. An international evaluation of CODOX-M and CODOX-M alternating with IVAC in adult Burkitt’s lymphoma: results of United Kingdom Lymphoma Group LY06 study. Ann Oncol. 2002;13:1264–1274. doi: 10.1093/annonc/mdf253. [DOI] [PubMed] [Google Scholar]
- 5.Yamaguchi M, Kwong YL, Kim WS, et al. Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: the NK-Cell Tumor Study Group study. J Clin Oncol. 2011;29:4410–6. doi: 10.1200/JCO.2011.35.6287. [DOI] [PubMed] [Google Scholar]
- 6.Paci A, Veal G, Bardin C, et al. Review of therapeutic drug monitoring of anticancer drugs part 1-Cytotoxics. European Journal of Cancer. 2014:2010–2019. doi: 10.1016/j.ejca.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Bleyer WA. The clinical pharmacology of methotrexate. Cancer. 1978;41:36–51. doi: 10.1002/1097-0142(197801)41:1<36::aid-cncr2820410108>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 8.Stehle G, Sinn H, Wunder A, Schrenk HH, Schütt S, Heene DL, Maier-Borst W. The loading rate determines tumor targeting of methotrexate-albumin conjugates in rats. Anticancer Drugs. 1999;8:677–685. [PubMed] [Google Scholar]
- 9.Stehle G, Wunder A, Sinn H, et al. Pharmacokinetics of methotrexate-albumin conjugates in tumor bearing rats. Anticancer Drugs. 1997;8:835–844. doi: 10.1097/00001813-199710000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Boldt J. Use of albumin: an update. Br J Anaesth. 2010;104:276–84. doi: 10.1016/j.bja.2020.07.039. [DOI] [PubMed] [Google Scholar]
- 11.Hartung G, Stehle G, Sinn H, et al. Phase I Trial of Methotrexate-Albumin in a Weekly Intravenous Bolus Regimen in Cancer Patients. Clin Canc Res. 1999;5:753–759. [PubMed] [Google Scholar]
- 12.Choi YH, Bae SK, Oh JM, et al. Pharmacokinetics of Intravenous Methotrexate in Mutant Nagase Analbuminemic Rats. Bipharm Drug Dispos. 2007;28:385–392. doi: 10.1002/bdd.565. [DOI] [PubMed] [Google Scholar]
- 13.Alarcon GS, Kremer JM, Macaluso M, et al. Risk Factors for Methotrexate-Induced Lung Injury in Patients with Rheumatoid Arthritis. Ann Intern Med. 1997;127:356–364. doi: 10.7326/0003-4819-127-5-199709010-00003. [DOI] [PubMed] [Google Scholar]
- 14.Wiczer T, Dotson E, Tuten A, et al. Evaluation of incidence and risk factors for high-dose methotrexate-induced nephrotoxicity. J Oncol Pharm Pract. 2016;22:430–6. doi: 10.1177/1078155215594417. [DOI] [PubMed] [Google Scholar]
- 15.Evans WE, Pratt CB. Effect of pleural effusion on high-dose methotrexate kinetics. Clin Pharmacol Ther. 1978;23:68–72. doi: 10.1002/cpt197823168. [DOI] [PubMed] [Google Scholar]
- 16.Finfer S, Bellomo R, Boyce N, et al. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247–2256. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]
- 17.The Official Statement of the American Thoracic Society. Evidence-based Colloid Use in the Critically Ill: American Thoracic Society Consensus Statement. Am J Respir Crit Care Med. 2004;170:1247–1259. doi: 10.1164/rccm.200208-909ST. [DOI] [PubMed] [Google Scholar]
- 18.Guthrie RD, Jr, Hines C., Jr Use of intravenous albumin the critically ill patient. Am J Gastroenterol. 1991;86:255–63. [PubMed] [Google Scholar]
- 19.Curtin JP, Koonings PP, Gutierrez M, et al. Ifosfamide-induced neurotoxicity. Gynecol Oncol. 1991;42(3):193–6. doi: 10.1016/0090-8258(91)90344-5. [DOI] [PubMed] [Google Scholar]
- 20.Kettle JK, Grauer D, Folker TL, et al. Effectiveness of exogenous albumin administration for the prevention of ifosfamide-induced encephalopathy. Pharmacotherapy. 2010;30:812–7. doi: 10.1592/phco.30.8.812. [DOI] [PubMed] [Google Scholar]