Abstract
Purpose
There remains a paucity of direct visualization techniques for beating-heart intracardiac procedures. To address this need, we evaluated a novel cardioscope in the context of aortic paravalvular leaks (PVLs) localization and closure.
Description
A porcine aortic PVL model was created using a custom-made bioprosthetic valve and PVL presence was verified by epicardial echocardiography. Transapical delivery of occlusion devices guided solely by cardioscopy was attempted 13 times in a total of 3 pigs. Device retrieval after release was attempted 6 times. Echocardiography, morphological evaluation and delivery time were used to assess results.
Evaluation
Cardioscopic imaging enabled localization of PVLs via visualization of regurgitant jet flow in a paravalvular channel at the base of the prosthetic aortic valve. Occluders were successfully placed in 11 of 13 attempts (84.6%) taking on average 3:03±1:34min. Devices were cardioscopically removed successfully in 3 out of 6 attempts (50%) taking 3:41±1:46min. No damage to the ventricle or annulus were observed at necropsy.
Conclusions
Cardioscopy can facilitate intracardiac interventions by providing direct visualization of anatomical structures inside the blood-filled, beating heart model.
Keywords: Imaging, Cardiac catheterization/intervention, Endoscopy/endoscopic procedures, Heart valve repair
While intracardiac imaging has advanced in recent years, the positioning and deployment of devices remain challenging and success hinges on the experience level of the clinical team. Closure of PVLs in a beating heart is a specific example of such a challenge. PVL is a complication that can occur after both surgical and transcatheter prosthetic valve implantation. Their repair usually involves delivering one or more occluders into the leak to reduce or eliminate regurgitant flow [1]. Occluder deployment inside PVLs is currently performed using combinations of CT angiography, fluoroscopy and transesophageal echocardiography and requires an experienced interdisciplinary team of cardiovascular interventionalists and imaging experts [1,2]. Direct imaging of the valve annulus could substantially improve procedural success and reduce interventional time if it enables leak localization, visualization during deployment and evaluation of potential interference with valve function. Cardioscopy, i.e., endoscopy within the blood-filled heart, can provide these capabilities. In this paper, we describe the design of a hand-held cardioscope, that incorporates a working channel for deployment of various tools, and we demonstrate localization and repair of PVLs using a transapical approach in a swine model.
Technology
Using endoscopy inside the blood-filled heart involves creating an optically-clear path between imaging system and tissue. Early cardioscopes employed toroidal balloons pressed against tissue and cleared of blood by a continuous saline flow [3]. Alternative approaches have used clear plastic optical windows that are pressed against the tissue to exclude the blood. These devices have provided intracardiac images of unprecedented resolution [4,5].
In the design presented here, a chip-based camera and an LED (light emitting diode) for illumination are embedded in an optical window composed of soft, optically-clear silicone that incorporates a working channel for device delivery (Fig. 1). Since the image and illumination are transmitted electronically through very small diameter wires, the cardioscope can be easily integrated into both catheters and handheld surgical tools. Saline flow is also not necessary for tissue visualization through the clear silicone. Irrigation is provided through the working channel, however, to prevent blood backflow and to enable tool visualization inside the channel.
Fig. 1.
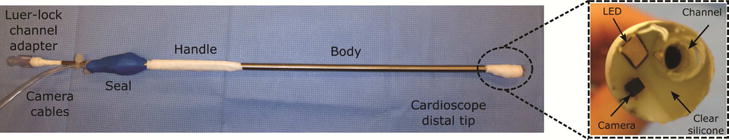
Cardioscope mounted on handheld catheter for transapical access.
The cardioscope design of Fig. 1 has a diameter of 10mm, but is scalable to achieve a specified field-of-view diameter and to accommodate various delivery routes, e.g., transfemoral or transapical. A 1mm3 CMOS camera and 1.6×1.6×0.5mm LED are molded inside the optical window, which also contains a 2.3mm internal diameter working channel. The working channel is positioned off center to facilitate device delivery around the outer edge of the annulus and its walls are transparent inside the optical window to enable device visualization inside the channel during delivery. The cardioscope is mounted on a straight 5mm diameter 35cm long handheld catheter. For PVL closure, the occlusion device is loaded inside a transparent delivery cannula that is advanced through the working channel. Both the handheld cardioscope and delivery cannula have integrated luer-lock male fittings for saline infusion to eliminate air bubbles and to clear blood from the channel.
Technique
A porcine model of PVLs was created using custom designed bioprosthetic valves incorporating features for producing three PVLs spaced ~120 degrees apart (Fig. 2). The bioprosthetic valves were comprised of a 3D printed titanium frame covered with a non-woven polyester fabric, a suturing ring made of polypropylene felt and leaflets made from glutaraldehyde-fixed porcine pericardium [6]. Three reinforced gaps in the suturing ring were included to allow paravalvular blood backflow. Valves were fabricated in several sizes to accommodate to individual porcine anatomies.
Fig. 2.
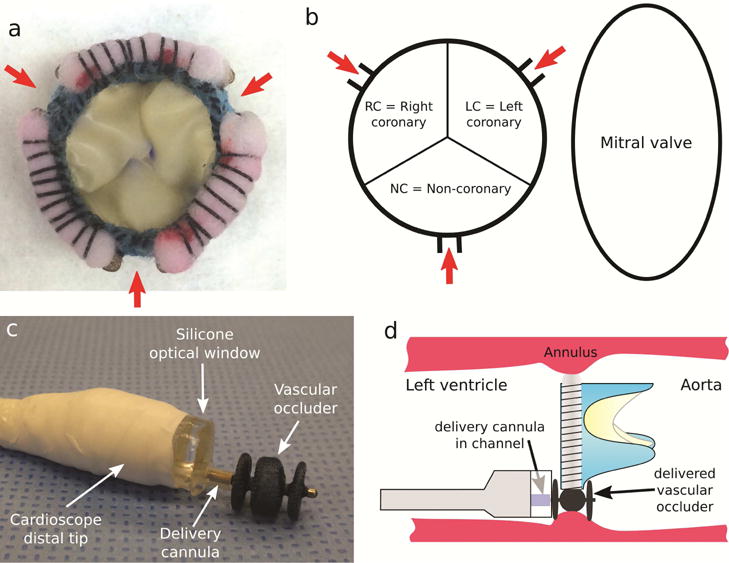
Paravalvular leak model and device deployment. (a) Bioprosthetic valve incorporating 3 PVLs. (b) Schematic showing PVL locations as viewed from ventricle. (c) Three-lobe occluder (Amplatzer AVP II) shown extended from delivery cannula as deployed from cardioscope. (d) Delivery process.
After having pre-medicated and sedated the swine, the thoracic cavity was accessed via a median sternotomy. Epicardial echocardiographic images of the native aortic valve were acquired for prosthetic valve implant size selection. After initiating cardiopulmonary bypass, performing aortic crossclamping and starting cardioplegia, a transverse arteriotomy was made in the aortic root to remove the native leaflets and implant the artificial valve.
With the valve placed, the animal was rewarmed and the aortotomy repaired followed by crossclamp release. Partial cardiopulmonary bypass was maintained to ensure hemodynamic and cardiac rhythm stability. Bioprosthetic valve function was then evaluated by epicardial short- and long-axis 2-dimensional and color Doppler echo to assess PVL location and size. Two pledgeted purse-string sutures were then placed on the cardiac apex, to stabilize it for introduction of the cardioscope, which was pre-loaded (Fig. 2c) with the delivery cannula containing an occlusion device (AMPLATZER™ Vascular Plug II, AGA Medical Corporation).
The handheld cardioscope was inserted through the apex and navigated to the valve annulus. Once the valve was reached, the catheter was navigated around the valve annulus to identify and inspect each leak. The catheter was then withdrawn from the annulus to just inside the apex. From this position, sequential closure of each PVL was attempted followed by device reloading.
The closure process consisted of the following steps. First, the cardioscope was navigated to the valve annulus and the targeted PVL (Fig. 2b) was localized. The working channel was positioned directly over the center of the PVL. The delivery sheath was then extended by ~3mm into the PVL to ensure that the device would expand inside the PVL and not proximal to it. The occluder was then advanced out of the delivery sheath with the operator able to feel a pop as each of the three device lobes were released. Finally, the sheath, the device and, if needed, the cardioscope, were slightly retracted until the proximal lobe of the occluder was positioned just inside the ventricle and flush with the annulus (Fig. 2d). If device position was satisfactory, it was released. Otherwise, the device was retracted back into its cannula and the process was repeated. The time needed to perform each closure was recorded.
Subsequent to device delivery, cardioscopy was used to inspect device placement. Echocardiography was also used to assess device placement and to evaluate reduction in PVL jet size. We note that blood flow through a properly delivered device can occur prior to endothelialization.
To investigate the potential for removal of a device after its release, we inserted 1.7mm diameter endoscopic forceps through the working channel in the last two experiments. After positioning the working channel over the screw connector of a deployed device, the connector was grasped with the forceps and the device was pulled out of the PVL. Since the diameter of the forceps precluded use of the occluder delivery sheath and to avoid damage to the silicone optical window, the occluder was not withdrawn inside the window. Instead, to remove the device from the heart, the cardioscope was withdrawn through the apical purse string sutures. At the conclusion of each experiment, a euthanasia solution was administered to the animal, and the heart was recovered for post-operative evaluation.
Pre-Clinical Experience
After a series of preliminary experiments to refine the PVL model and delivery protocol [7], the technique described above was performed in a series of animal experiments (n=3). In each experiment, three PVLs were successfully created at the aortic annulus (Fig. 2b) as validated using epicardial echocardiography (Fig. 3a,b,c).
Fig. 3.
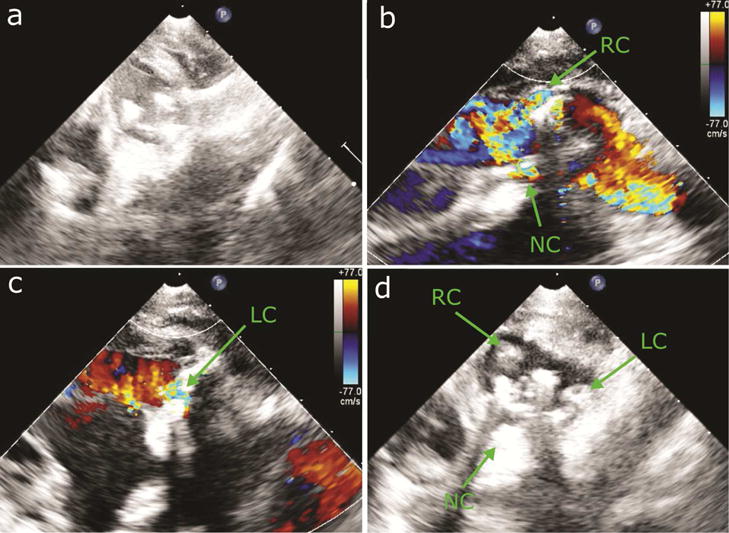
Epicardial echocardiography of PVLs. (a) Short-axis view of implanted valve. (b) Long-axis echo color Doppler showing regurgitant jet in RC and NC sinuses. (c) Long-axis echo color Doppler showing regurgitant jet in LC sinus. (d) Short-axis view after delivery of closure devices in 3 PVLs.
Navigation of the cardioscope tip from the apex to the aortic annulus using only cardioscopic guidance was performed on initial entry into the heart and, subsequently, for each PVL closure (Fig. 4, Video). Navigation around the entire annulus while maintaining cardioscopic visualization for inspection of the three PVLs was intuitive to perform (Video) requiring 54±22sec (n=3). PVLs were localized cardioscopically by identifying diastolic regurgitant blood flow external to the rim of the valve (Fig. 4b).
Fig. 4.
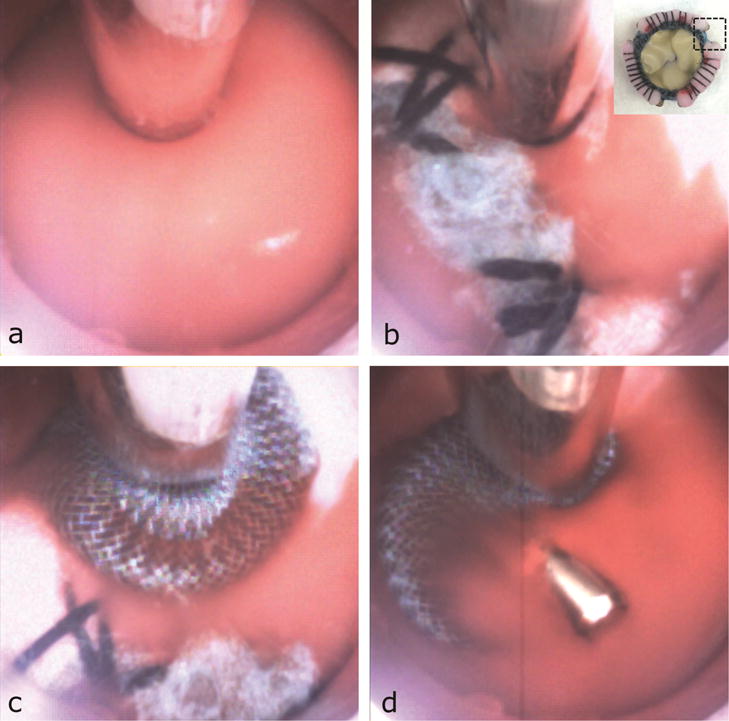
Cardioscopic images during PVL Closure. (a) View in blood. (b) Localizing PVL by visualization of regurgitation. Inset: Imaged region of valve. Blue fabric appears white in cardioscopic image due to lighting. (c) Device deployment. (d) Device inspection.
In total, 11 out of 13 occluders (84.6%) were successfully deployed and released in PVLs under cardioscopic guidance (Fig. 4, Video), (2/4 in non-coronary (NC), 4 in right coronary (RC), 5 in left coronary (LC) sinuses). The average time to navigate from the apex to the targeted PVL and deliver an occluder was 3:03±1:34min (n=13). In one unsuccessful case, the device was positioned in the PVL, but not released because it was cardioscopically observed to be undersized and shifting in position over the cardiac cycle. In the other case, remaining native leaflet tissue was covering the PVL channel and acting as a one-way valve. While regurgitant blood flow could be observed to be emerging from the edge of the leaflet tissue, it was not possible to safely insert the delivery sheath through the leaflet tissue. Of the 11 successfully deployed occluders, in 2 cases (18%), the devices were undersized and were found to have dislodged and been lost later in the procedure during cardioscopic and echocardiographic assessment.
Recovery of released occluders from PVLs was successfully performed in 3 out of 6 attempts (50%) (Fig. 5, Video), (2/4 from LC, 1/2 from RC sinus). The time to navigate from the apex to the device and to retrieve it from the heart was 3:41±1:46min. In the unsuccessful cases, one device dislodged into the aorta and a second device slipped from the forceps as it was pulled through the apical purse-string sutures. The third device was pushed deeper into the channel during attempted grasping such that it could not be grasped. Finally, during postmortem assessment, no significant damage to either the annulus or the septum was observed (Fig. 6).
Fig. 5.
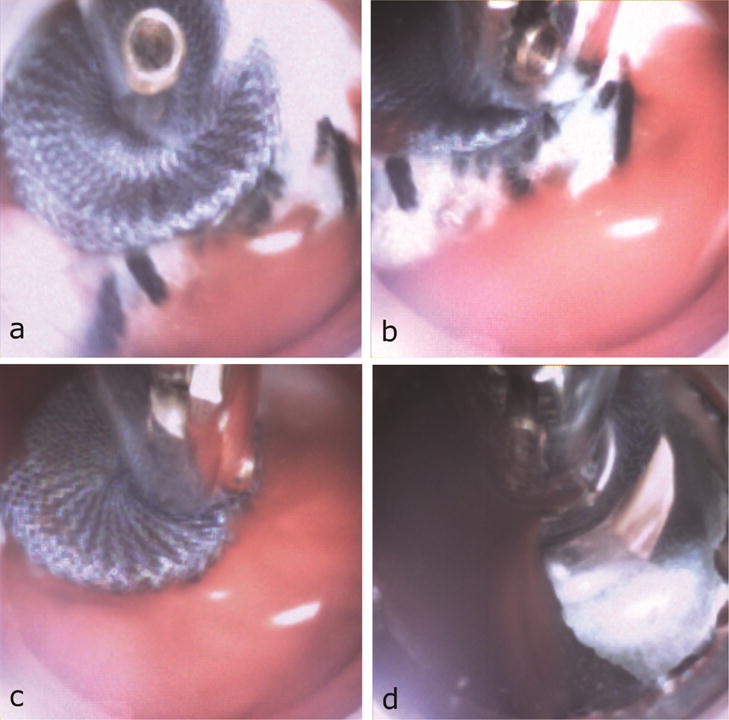
Cardioscopic images during device recovery. (a) Positioning device screw connector inside working channel. (b) Forceps grasping screw connector. (c) Device pulled from PVL. (d) Device removed through apex.
Fig. 6.
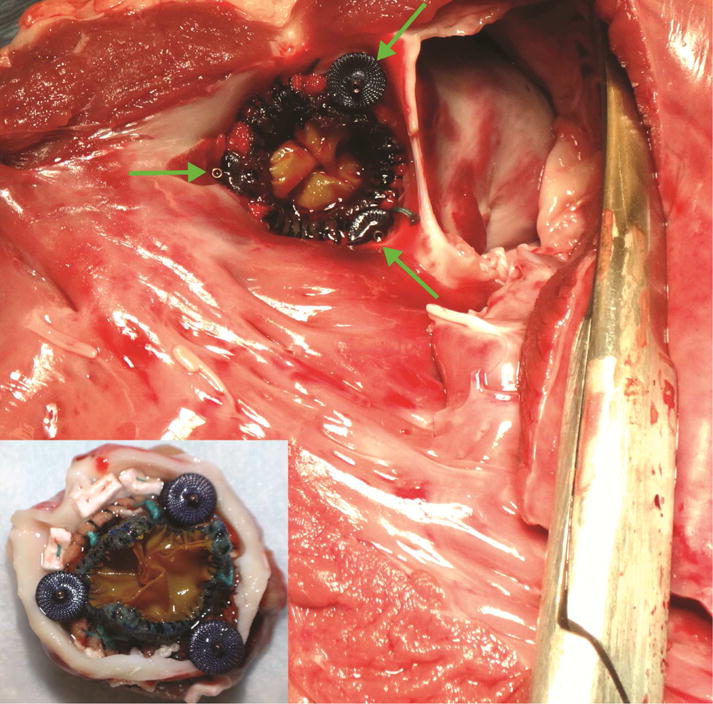
Postoperative assessment of device placement viewed from ventricle (anterior mitral leaflet resected). Inset: aortic view.
Comment
Cardioscopy is a very promising imaging modality for beating-heart interventions. In the context of PVL closure, it provided excellent tissue and bioprosthetic valve visualization. PVLs were easily localized by direct imaging of the regurgitant flow and the integrated working channel enabled device delivery under continuous direct visualization. With PVL closure times just over 3 minutes, the approach compares favorably with published reports for which fluoroscopy times alone are 29.9±24.5min [8]. While cardioscopy was used exclusively here for device deployment, we anticipate that clinically, additional imaging modalities would be needed, but that their need would be reduced.
The demonstration of cardioscopic technology on a straight transapically-introduced instrument was designed to simplify testing. The scalable design can be adapted for transfemoral access, which is often preferred for PVL closure [1,2]. Furthermore, the direct visualization during occluder delivery provided by cardioscopy may be equally applicable to the deployment of many valve replacement and repair devices.
Study limitations included some variability in our PVL model such that a few PVLs were larger than the devices we had on hand. This led to 2 instances of device dislodgement, which could be prevented through placement of larger devices. In addition, the technique of forceps-based device retrieval was intended to illustrate the potential for cardioscopically-guided retrieval, but would require modification for clinical applicability.
Supplementary Material
Acknowledgments
This work was supported by the NIH under grant R01HL124020.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures and Freedom of Investigation
The authors had freedom of investigation and full control of study design, methods used, outcome parameters and results, data analysis and production of the written report. Animal experiments were conducted under protocol approved by the IACUC of Boston Children’s Hospital.
References
- 1.Nietlispach F, Maisano F, Sorajja P, Leon MB, Rihal C, Feldman T. Percutaneous paravalvular leak closure: chasing the chameleon. Eur Heart J. 2016 doi: 10.1093/eurheartj/ehw165. [DOI] [PubMed] [Google Scholar]
- 2.Sharma S, Kliger C, Jelnin V, Ruiz C. Paravalvular leak closure: the apical approach. Cardiac Int Today. 2014:48–52. [Google Scholar]
- 3.Uchida Y. Recent advances in percutaneous cardioscopy. Current Cardiovascular Imaging Reports. 2011;4(4):317–327. doi: 10.1007/s12410-011-9092-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vasilyev NV, Martinez JF, Freudenthal FP, Suematsu Y, Marx G, del Nido PJ. Three-dimensional echo and videocardioscopy-guided atrial septal defect closure. Annals of Thoracic Surgery. 2006;4:1322–1326. doi: 10.1016/j.athoracsur.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Padala M, Jimenez JH, Yoganathan AP, Chin A, Thourani VH. Transapical beating heart cardioscopy technique for off- pump visualization of heart valves. Journal of Thoracic and Cardiovascular Surgery. 2012;144(1):231–234. doi: 10.1016/j.jtcvs.2011.11.038. [DOI] [PubMed] [Google Scholar]
- 6.Rosa B, Machaidze Z, Shin B, Manjila S, Brown D, Baird C, Mayer J, Dupont PE. A low cost bioprosthetic semilunar valve for research, disease modeling and surgical training applications. Interactive CardioVascular and Thoracic Surgery. doi: 10.1093/icvts/ivx189. In review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Machaidze Z, Rosa B, Mencattelli M, et al. TCT-246 Cardioscopy-Guided Repair of Aortic Paravalvular Leak in Porcine Beating Heart Model. Journal of the American College of Cardiology. 2016;68(18_S):B100. [Google Scholar]
- 8.Ruiz C, Jelnin V, Kronzon I, et al. Clinical outcomes in patients undergoing percutaneous closure of periprosthetic paravalvular leaks. Journal American College of Cardiology. 2011;58(21):2210–2217. doi: 10.1016/j.jacc.2011.03.074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


