Abstract
Purpose
With aging, there tends to be an increase in retrograde and oscillatory shear in peripheral conduit arteries of humans. Whether the increase in shear rate is due to the aging process or an effect of a less active lifestyle that often accompanies aging is unknown. Therefore, we examined whether chronic endurance exercise training attenuates conduit artery retrograde and oscillatory shear in older adults.
Methods
Brachial and common femoral artery mean blood velocities and diameter were determined via Doppler ultrasound under resting conditions, and shear rate was calculated in 13 young (24 ± 2 years), 17 older untrained (66 ± 3 years), and 16 older endurance exercise-trained adults (66 ± 7 years).
Results
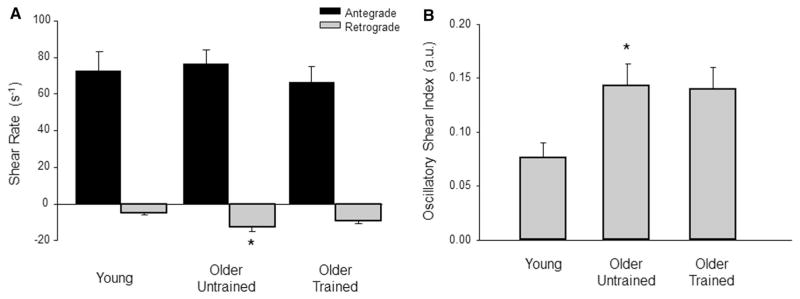
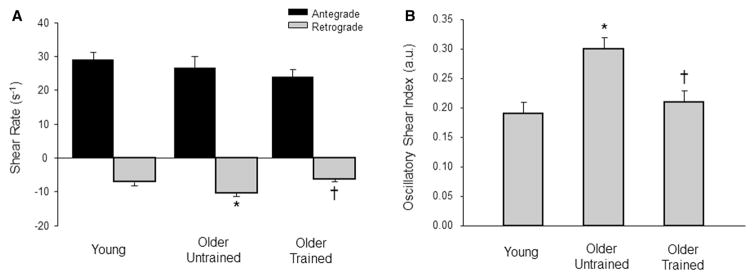
Brachial artery retrograde (−9.1 ± 6.4 vs. −12.6 ± 9.4 s−1; P = 0.35) and oscillatory (0.14 ± 0.08 vs. 0.14 ± 0.08 arbitrary units; P = 0.99) shear were similar between the older trained and untrained groups, whereas brachial artery retrograde and oscillatory shear were greater in older untrained compared to young adults (−5.0 ± 3.4, 0.08 ± 0.05 s−1 arbitrary units, P = 0.017 and 0.048, respectively). There was no difference between the young and older trained brachial retrograde (P = 0.29) and oscillatory (P = 0.07) shear. Common femoral artery retrograde (−6.3 ± 2.9 s−1) and oscillatory (0.21 ± 0.08 arbitrary units) shear were reduced in older trained compared to the older untrained group (−10.4 ± 4.1 and 0.30 ± 0.09 s−1 arbitrary units, both P = 0.005 and 0.006, respectively), yet similar to young adults (−7.1 ± 3.5 and 0.19 ± 0.06 s−1 arbitrary units, P = 0.81 and 0.87, respectively).
Conclusion
Our results suggest that chronic endurance exercise training in older adults ameliorates retrograde and oscillatory shear rate patterns, particularly in the common femoral artery.
Keywords: Aging, Shear rate, Conduit artery, Exercise training
Introduction
Peripheral conduit arteries often present with a bidirectional blood flow pattern across the cardiac cycle. That is, there is antegrade flow towards the periphery during systole followed by a brief episode of retrograde flow directed back toward the heart during diastole (Blackshear et al. 1979). This pattern of blood flow results in oscillations in the frictional forces (i.e., shear stress) exerted on the arterial wall, which consequently influences endothelial cell function. In this context, in vitro data from endothelial cells in culture, of isolated arteries, and of arteries in intact animals suggest that antegrade shear is thought to be beneficial and promote an antiatherogenic endothelial phenotype (Laughlin et al. 2008; Wang et al. 2013), whereas increased retrograde and oscillatory shear can induce proatherogenic effects on endothelial cells (Chien 2008; Conway et al. 2010; Hahn and Schwartz 2009; Lu and Kassab 2004). Evidence in humans further demonstrates that acute (30 min) and longer term (2 weeks) increases of retrograde and oscillatory shear impair endothelial function in young adults (Schreuder et al. 2014; Thijssen et al. 2009, 2015; Johnson et al. 2013; Totosy de Zepetnek et al. 2014). Of clinical importance, arterial regions characterized by an oscillatory shear stress environment are correlated with the localization of atherosclerotic plaque (Ku et al. 1985). Moreover, the peripheral conduit arteries of the lower limbs, vascular regions characterized by elevated levels of oscillatory shear (Wu et al. 2004; Newcomer et al. 2008), demonstrate a greater prevalence and severity of atherosclerosis compared to conduit arteries of the upper extremities (Kroger et al. 1999; Stary et al. 1995).
We and others have demonstrated that aging is associated with greater retrograde and oscillatory shear under resting conditions in both the brachial and femoral arteries of humans (Casey et al. 2012; Padilla et al. 2011b; Young et al. 2010). The age-related increases in retrograde and oscillatory shear in the brachial artery have been attributed to an enhanced α-adrenergic vasoconstriction and/or reduced nitric oxide bioavailability in the downstream resistance vessels (Casey et al. 2012; Padilla et al. 2011b). However, the previous studies reporting age-associated alterations in shear patterns have only been performed in older adults who were sedentary or moderately active with no formal exercise training. There is strong evidence from cross-sectional observations as well as exercise training studies that chronically endurance-trained older adults demonstrate profound improvements in measures of peripheral vascular and endothelial function (DeSouza et al. 2000; Pierce et al. 2011a, b; Seals et al. 2008; Taddei et al. 2000). Furthermore, regular exercise and physical activity have been shown to reduce the risk of cardiovascular disease across the lifespan, as well as extend longevity (Blair and Morris 2009). To our knowledge, there are no reports addressing whether exercise training has favorable effects on peripheral conduit artery shear profiles. In addition, it is unknown whether the potential favorable effects would be more apparent in conduit arteries supplying blood flow to muscle mainly involved in most endurance-type exercise and/or peripheral arteries more susceptible to atherosclerotic development (i.e., common femoral artery). Therefore, in a cross-sectional study, we tested the hypothesis that chronic endurance exercise training is associated with an attenuation of the expected age-related increase in peripheral conduit artery retrograde and oscillatory shear, and this exercise-induced benefit on shear rate patterns would be more evident in the common femoral artery of older adults.
Methods
Subjects
Thirteen young (24 ± 2 years) and 33 older (66 ± 6 years) adults volunteered to participate in this study, which was part of a larger protocol with some of the data previously published (Hughes et al. 2016). All subjects completed a general health history screening and written informed consent and were generally healthy, free of any diagnosed cardiovascular or metabolic complications, nonobese (body mass index: ≤30 kg/m2), nonsmokers, and not taking any vasoactive medications. The young subjects were self-reported as sedentary or recreationally active, with no regular physical training. The older adults consisted of two separate groups based on reported exercise history. One group (n = 17) was self-reported as sedentary or recreationally active, with no regular physical training and thus classified as the ‘older untrained’ group. The other group of older adults (n = 16) self-reported chronic endurance exercise training ≥4 days per week and ≥1 h per day for at least the past year and were classified as the ‘older exercise-trained’ group. On average, the older exercise-trained adults reported meeting these training requirements for the past 19 ± 13 years and several of them were still actively competing in races (both running and cycling). Eight subjects (four older trained, three older untrained, and one young) reported taking a daily multivitamin, five (three older trained and two older untrained) reported taking vitamin D, four (three older trained and one older untrained) reported taking calcium, and seven reported taking fish oil. Subjects taking vitamins and/or supplements were asked to withhold them for 3 days prior to their study visit. Blood flow studies were performed after an overnight fast, and subjects refrained from exercise, alcohol, and caffeine for 24 h before reporting to the laboratory. All measurements (aside from maximal exercise testing) were performed in the morning between the hours of 7:00–9:00 a.m. in a quiet, light-attenuated room with stable ambient temperature ~22 °C. Young female subjects were studied during the early follicular phase of their menstrual cycle or the placebo phase of oral contraceptives to control for the potential influence of sex hormones on primary outcome variables (Minson et al. 2000). All older female subjects were post-menopausal and were not taking any form of hormone replacement therapy. All study protocols were approved by the Institutional Review Board at the University of Iowa.
Brachial and femoral artery blood flow
Brachial artery and common femoral artery (~2–3 cm proximal to bifurcation) mean blood velocity and diameter were determined with a 12 MHz linear-array Doppler probe (model M12L; Vivid 7, General Electric, Milwaukee, WI). Blood velocity was measured with a probe insonation angle previously calibrated to 60°. Sample volume was adjusted to cover the width of the brachial and common femoral artery to encompass the entire lumen of the vessel, and the cursor was set at mid-vessel. Measured velocity waveforms were synchronized to a data acquisition system (WinDaq; DATAQ Instruments, Akron, OH) via a Doppler audio transformer (Herr et al. 2010). Artery diameter measurements were obtained at end diastole. Brachial measurements were performed with the subject in a supine position, and femoral measurements were acquired with the subjects in a semirecumbent (~140°)-seated position. The use of a semirecumbent-seated position for the common femoral measurements was a result of this data being derived as part of a larger protocol that involved single knee extensions on a custom-made leg ergometer that did not allow the seat to recline to a fully supine position (Hughes et al. 2016). All blood flow and shear data collected for the present study were performed under resting conditions and prior to any single knee extensions used for the aforementioned larger study (Hughes et al. 2016). Limb blood flow was calculated as the product of mean blood velocity (cm s−1) and artery cross-sectional area (cm2) and expressed as milliliters per minute (ml min−1). All blood flow measurements were performed and analyzed by a single investigator.
Blood pressure
Brachial artery pressure was measured in duplicate and averaged using an automated cuff (Cardiocap/5, Datex-Ohmeda, Louisville, CO, USA) at the beginning of each subject’s study visit following 15 min of supine rest. If the two consecutive blood pressure measurements (systolic or diastolic) differed by more than 5 mmHg, a third was taken. In addition, systemic blood pressure was assessed (beat-to-beat) via finger plethysmography (Nexfin; Edwards Lifesciences, Irvine, CA) throughout the blood flow measurements and used to estimate vascular resistance in the arm and leg.
Measurement of exercise capacity
On a separate day (preceding the actual blood flow study day), peak exercise oxygen consumption (VO2peak) was determined in all subjects using 12-lead ECG and respiratory gas analysis (Parvo Medics TrueOne 2400, Sandy, UT, USA) during incremental treadmill exercise using a Bruce protocol performed to exhaustion as previously described (Fielding et al. 1997). Graded maximal exercise testing was performed to evaluate exercise capacity differences between groups as well as to rule out clinical evidence of cardiopulmonary disease. All subjects that volunteered for this study and underwent the 12-lead ECG exercise stress test did not demonstrate any signs of coronary heart disease (i.e., angina and/or ECG changes).
Data analysis and statistics
Data were collected at 250 Hz, stored on a computer, and analyzed offline with the signal-processing software (WinDaq, DATAQ Instruments, Akron, OH). Mean arterial pressure (MAP) was determined from the Nexfin pressure waveform. Time-average mean blood velocity (i.e., velocity over the entire cardiac cycle), antegrade mean velocity (i.e., velocity during the systolic phase of the cardiac cycle), and retrograde mean velocity (i.e., velocity during the diastolic phase of the cardiac cycle) were calculated. Diameter and velocity measures were used to estimate brachial and common femoral artery shear rates. Mean shear rate (s−1) was defined as 4 Vm/D, where Vm is mean blood velocity (cm s−1) and D is arterial diameter (cm) (Casey et al. 2012; Padilla et al. 2009, 2011b). For calculations of antegrade and retrograde shear rate, antegrade and retrograde mean that blood velocities were used, respectively. Oscillatory shear index is a dimensionless parameter that can be used as an indicator of the magnitude of oscillation and can be defined as follows: |Retrograde shear|/(|Antegrade shear| + |Retrograde shear|) (Casey et al. 2012; Padilla et al. 2009, 2010, 2011b). Note that the values for oscillatory shear range from 0 to 0.5, where a value of 0 corresponds to a unidirectional shear rate throughout the cardiac cycle, and a value of 0.5 represents pure oscillation with a time-average shear equal to 0. Estimated vascular resistance for each limb was calculated as mean arterial pressure/blood flow and expressed as millimeters of mercury per milliliter of flow per minute (mmHg ml−1 min−1). All measurements were averaged over the last minute of data collection. The analysis of velocity profiles and calculation of shear rates were performed by a single investigator. Previous pilot data from our lab in young and older adults demonstrate excellent intraclass correlation coefficients for retrograde shear rate and oscillatory shear index in the brachial (0.93–0.98) and common femoral artery (0.94–0.99) over multiple measurements on a single day, respectively.
All values are expressed as mean ± SD unless stated otherwise. Descriptive statistics were used to characterize the young and older groups of subjects. One-way analysis of variance (ANOVA) tests was used to compare shear values in the brachial and common femoral artery between subject groups. When significance was detected, Tukey’s post hoc analysis was used to identify differences between groups. Pearson’s correlation coefficients were calculated to assess the relationship between shear and vascular resistance. All statistical analyses were completed using the SigmaPlot software version 11.0 (Systat Software Inc., San Jose, CA). Statistical difference was set a priori at P ≤ 0.05.
Results
Subject characteristics are shown in Table 1. All three groups (young, older untrained, and older trained) were of similar height, weight, and body mass index. In addition, the three groups exhibited similar brachial blood pressures. However, older untrained adults demonstrated lower peak exercise oxygen consumption (VO2peak) compared to the young (P < 0.001) and older exercise-trained (P = 0.02) groups.
Table 1.
Young and older untrained and older trained characteristics
| Variable | Young | Older untrained | Older trained |
|---|---|---|---|
| n | 13 | 17 | 16 |
| Sex (men/women) | 8/5 | 10/7 | 9/7 |
| Age (years) | 24 ± 2 | 66 ± 3 | 66 ± 7 |
| Height (m) | 175 ± 10 | 172 ± 9 | 170 ± 8 |
| Weight (kg) | 75 ± 12 | 77 ± 11 | 72 ± 11 |
| Body mass index (kg m−2) | 24.6 ± 2.4 | 26.1 ± 2.9 | 24.7 ± 3.0 |
| Systolic blood pressure (mmHg) | 117 ± 7 | 123 ± 11 | 123 ± 14 |
| Diastolic blood pressure (mmHg) | 72 ± 7 | 74 ± 8 | 77 ± 7 |
| MAP (mmHg) | 87 ± 6 | 91 ± 8 | 92 ± 8 |
| VO2 (ml/kg/min) | 43.3 ± 9.3 | 30.2 ± 5.9* | 37.5 ± 7.6† |
Data are mean ± SD
P ≤ 0.05 vs. young
P ≤ 0.05 vs. older untrained
Shear rate patterns
Resting brachial and common femoral artery diameters and velocity profiles are summarized in Table 2. Brachial artery retrograde and oscillatory shear were similar between the older untrained and exercise-trained groups (P = 0.35 and 0.99, respectively; Fig. 1a, b). However, the older untrained adults demonstrated greater brachial retrograde and oscillatory shear compared to young adults (P = 0.017 and 0.048, respectively; Fig. 1a, b). Common femoral artery retrograde and oscillatory shear was greater in older untrained adults compared to young adults (P = 0.041 and 0.002, respectively; Fig. 2a, b). Conversely, older exercise-trained adults demonstrated reduced retrograde and oscillatory shear in the common femoral artery compared to the older untrained adults (P = 0.005 and 0.006, respectively) and similar retrograde and oscillatory shear as the young group (P = 0.81 and 0.87, respectively, Fig. 2a, b).
Table 2.
Limb hemodynamics in young and older untrained and older trained subjects
| Variable | Young (n = 13) | Older untrained (n = 17) | Older trained (n = 16) |
|---|---|---|---|
| Brachial artery | |||
| Artery diameter (cm) | 0.37 ± 0.07 | 0.40 ± 0.07 | 0.41 ± 0.07 |
| Mean velocity (cm s−1) | 5.9 ± 3.0 | 6.0 ± 1.8 | 5.4 ± 2.6 |
| Mean shear rate (s−1) | 67.0 ± 38.5 | 63.7 ± 27.9 | 57.0 ± 33.7 |
| Antegrade mean velocity (cm s−1) | 6.4 ± 2.9 | 7.2 ± 1.8 | 6.4 ± 2.5 |
| Retrograde mean velocity (cm s−1) | −0.5 ± 0.3 | −1.2 ± 0.8 | −0.9 ± 0.6 |
| Common femoral artery | |||
| Artery diameter (cm) | 0.88 ± 0.10 | 0.94 ± 0.12 | 1.02 ± 0.14* |
| Mean velocity (cm s−1) | 4.7 ± 1.3 | 3.7 ± 2.5 | 4.4 ± 1.7 |
| Mean shear rate (s−1) | 21.9 ± 6.9 | 16.4 ± 12.4 | 17.6 ± 7.6 |
| Antegrade mean velocity (cm s−1) | 6.3 ± 1.6 | 6.0 ± 2.7 | 5.9 ± 1.8 |
| Retrograde mean velocity (cm s−1) | −1.5 ± 0.7 | −2.4 ± 0.8 | −1.6 ± 0.7 |
Data are mean ± SD
P ≤ 0.05 vs. young
Fig. 1.
Brachial artery antegrade and retrograde shear (a) and oscillatory shear index (b) at rest in young and older adults. Values are expressed in mean ± SE. *P ≤ 0.05 vs. young
Fig. 2.
Common femoral artery antegrade and retrograde shear (a) and oscillatory shear index (b) at rest in young and older adults. Values are expressed in mean ± SE. *P ≤ 0.05 vs. young; †P ≤ 0.05 vs. older untrained
Limb vascular resistance
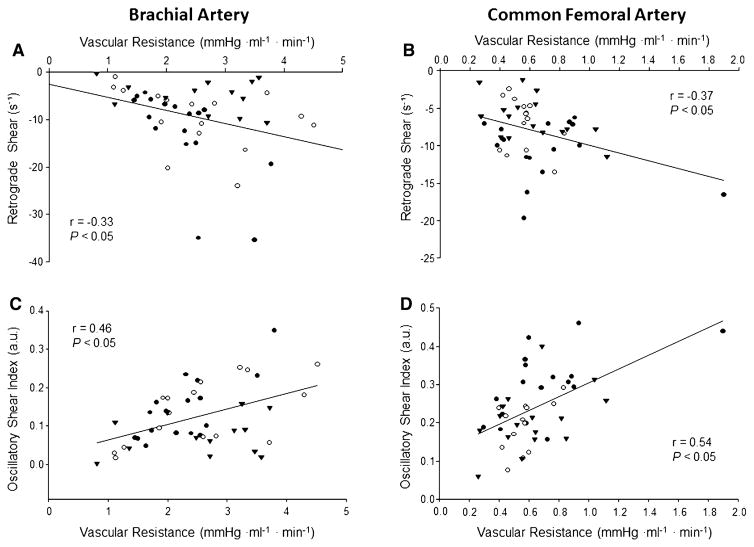
Regional estimates of vascular resistance in the arm (2.58 ± 0.98 vs. 2.26 ± 0.65 vs. 2.55 ± 1.05 mmHg ml−1 min−1; P = 0.56) and leg (0.56 ± 0.13 vs. 0.71 ± 0.36 vs. 0.61 ± 0.25 mmHg ml−1 min−1; P = 0.32) did not differ between the young, older untrained, and older trained groups, respectively. However, moderate correlations between vascular resistance and retrograde and oscillatory shear were observed in both the arm and leg of the entire group (Fig. 3). When separated by group, arm vascular resistance was inversely related to retrograde shear for the older untrained adults only (r = −0.68, P = 0.003) and positively related to oscillatory shear in both the older untrained (r = 0.75, P < 0.0001) and trained groups (r = 0.61, P = 0.01). In the leg, vascular resistance was only related to oscillatory shear in the older untrained adults (r = 0.57, P = 0.02).
Fig. 3.
Linear regression analysis of the relationship between vascular resistance and retrograde shear (a and b) and oscillatory shear index (c and d) in the brachial and common femoral arteries, respectively, of young (triangles), older untrained (black circles), and older trained (open circles) adults
Potential sex-related differences
Due to the similar male-to-female ratio in each group, we examined whether potential sex-related differences in shear profiles existed in the current study cohort. Comparing each sex as a whole (not separated by groups), women demonstrated a greater antegrade shear in the brachial (93.5 ± 33.5 vs. 56.7 ± 25.3, P < 0.001) and common femoral artery (31.5 ± 11.8 vs. 22.6 ± 8.5, P < 0.01) compared to men. No differences in retrograde shear were noted in the brachial (−10.8 ± 10.2 vs. −8.3 ± 4.8, P = 0.27) or common femoral artery (−8.4 ± 4.4 vs. −7.8 ± 3.6, P = 0.61) in all women vs. men. Consequently, there was a trend for a lower oscillatory shear in the brachial (0.10 ± 0.07 vs. 0.14 ± 0.08, P = 0.06) and common femoral artery (0.21 ± 0.07 vs. 0.26 ± 0.10, P = 0.09) in women. Examining the correlations between vascular resistance and shear rate patterns revealed stronger relationships in men compared to women for retrograde shear in the arm (r = −0.63 vs. −0.08) and leg (r = −0.48 vs. −0.20), as well as oscillatory shear in the arm (r = 0.74 vs. 0.32) and leg (r = 0.64 vs. 0.28).
Mean values for each sex within each group are presented in Table 3. When separated by group, brachial artery antegrade shear was higher in older women (both untrained and trained) compared to their male counterparts. No other within group sex differences in brachial shear patterns were noted. In the common femoral artery, the only sex-related differences noted were greater antegrade shear and lower oscillatory shear in older untrained women compared to men.
Table 3.
Shear rate patterns in men and women
| Variable | Young | Older untrained | Older trained |
|---|---|---|---|
| Brachial artery | |||
| Antegrade shear rate (s−1) | |||
| Males (n = 8) | 66.6 ± 39.1 | 57.7 ± 15.1 | 46.7 ± 16.6 |
| Females (n = 5) | 82.5 ± 37.4 | 102.9 ± 31.9* | 92.0 ± 34.9* |
| Retrograde shear rate (s−1) | |||
| Males (n = 10) | −5.5 ± 3.4 | −10.7 ± 4.9 | −8.14 ± 4.9 |
| Females (n = 7) | −4.1 ± 3.4 | −15.3 ± 10.6 | −11.23 ± 7.9 |
| Oscillatory shear index (a.u.) | |||
| Males (n = 9) | 0.09 ± 0.05 | 0.16 ± 0.09 | 0.16 ± 0.09 |
| Females (n = 7) | 0.05 ± 0.03 | 0.14 ± 0.07 | 0.12 ± 0.08 |
| Common femoral artery | |||
| Antegrade shear rate (s−1) | |||
| Males (n = 8) | 27.0 ± 7.9 | 20.4 ± 8.5 | 21.3 ± 8.6 |
| Females (n = 5) | 32.3 ± 8.4 | 35.2 ± 16.3* | 27.3 ± 8.2 |
| Retrograde shear rate (s−1) | |||
| Males (n = 10) | −7.6 ± 3.1 | −9.8 ± 3.5 | −5.7 ± 3.1† |
| Females (n = 7) | −6.3 ± 4.3 | −11.2 ± 4.9 | −7.0 ± 2.4 |
| Oscillatory shear index (a.u.) | |||
| Males (n = 9) | 0.21 ± 0.05† | 0.34 ± 0.09 | 0.21 ± 0.1† |
| Females (n = 7) | 0.16 ± 0.08 | 0.25 ± 0.07* | 0.21 ± 0.04 |
Data are mean ± SD
P ≤ 0.05 vs. males
P ≤ 0.05 vs. older untrained
Discussion
Previous studies have demonstrated that aging is associated with increased levels of retrograde and oscillatory shear in peripheral conduit arteries of humans (Casey et al. 2012; Padilla et al. 2011b; Young et al. 2010). In the present study, we aimed to examine whether the increase in conduit artery retrograde and oscillatory shear with aging is offset with the long-term endurance exercise training. Our primary novel findings indicate that the age-related changes in shear rate patterns can be improved with chronic endurance exercise training in apparently healthy older adults, and this benefit might be limb and/or artery specific. That is, endurance exercise-trained older adults demonstrate significantly lower retrograde and oscillatory shear in the common femoral but not brachial artery, compared to their less active (untrained) counterparts. Moreover, the conduit artery shear patterns in older adults that have engaged in the long-term endurance exercise resemble that of young adults.
Although aging is associated with arterial dysfunction at the conduit artery and microvascular level (Celermajer et al. 1994; Gerhard et al. 1996; Taddei et al. 1995; Seals et al. 2014), there is substantial evidence supporting the benefits of exercise training and physical activity on measures of endothelial function in older adults. Indeed, both habitual endurance exercise as well as shorter term (i.e., 8–12 weeks) exercise interventions have been shown to be effective in ameliorating the age-associated decrements in brachial artery flow-mediated dilation (Eskurza et al. 2005; Pierce et al. 2011a; Yoshizawa et al. 2010; Akazawa et al. 2012; Pierce et al. 2011b) and acetylcholine mediated vasodilator responsiveness (Taddei et al. 2000; DeSouza et al. 2000) in humans. In the current study, we report another potential vascular benefit of the long-term exercise training in older adults. That is, chronically endurance exercise-trained older adults demonstrate an improved resting shear profile (i.e., reduced retrograde and oscillatory shear) in the conduit arteries compared to their age matched sedentary counterparts. Considering the majority of the older exercise-trained adults in the present study performed exercises mainly involving muscles of the lower body (i.e., running or cycling), the more pronounced improvements in common femoral artery retrograde and oscillatory shear were not that surprising (Fig. 2).
In addition to being a potential benefit of exercise training, the improved shear patterns could also serve as a possible mechanism for the exercise-induced improvements in endothelial function commonly reported in older adults (Akazawa et al. 2012; DeSouza et al. 2000; Eskurza et al. 2005; Pierce et al. 2011a, b; Taddei et al. 2000; Yoshizawa et al. 2010). Along these lines, the transient removal of retrograde and oscillatory shear, in conjunction with increased antegrade shear, augments flow mediated dilation in young adults (Tinken et al. 2009). This possibility is further supported by observations that acute exercise abolishes the age-related increase in retrograde shear in the brachial artery (Padilla et al. 2011b). However, the majority of studies demonstrating the beneficial effects of exercise on endothelial function in older adults have primarily used measures involving the brachial artery or forearm vasculature (Akazawa et al. 2012; DeSouza et al. 2000; Eskurza et al. 2005; Pierce et al. 2011a, b; Taddei et al. 2000; Yoshizawa et al. 2010). In the current study, retrograde and oscillatory shear were not different in the brachial artery between the older untrained and trained groups. Conversely, while we observed marked improvements in the resting common femoral artery shear profile, the previous studies have suggested that exercise training does not improve endothelial function of the conduit arteries in the lower limb of healthy older adults (Wray et al. 2006). Therefore, it is unclear whether improvements in resting conduit artery shear patterns with the long-term exercise training actually contribute to improved endothelial function in older adults. In this context, the resting shear might not influence function, whereas the repeated episodes of antegrade and mean shear represent the primary physiological signal for endothelial adaptations to exercise training (Padilla et al. 2011a).
The idea that aging is associated with increases in retrograde and oscillatory shear in the femoral artery that was originally reported by Young and colleagues (Young et al. 2010) has recently been challenged. Evidence from Trinity et al. (Trinity et al. 2014) suggests that although aging is associated with reductions in femoral artery mean shear rate, it is primarily a result of reductions in antegrade shear and not an elevation in retrograde shear. The reason for the discrepancy between these two aforementioned studies is not entirely clear but has been attributed to differences in age (60 vs. 75 years) and femoral artery diameter (0.86 vs. 1.05 cm) between the two respective older groups. Other contributing factors that could possibly explain why Trinity et al. (Trinity et al. 2014) failed to detect age-related differences in retrograde shear may be explained by their small sample size (n = 8) or the inclusion of only men. Whereas the study by Young and colleagues (Young et al. 2010) included a significantly greater number of older adults (n = 18) and included both men and women. The inclusion of women likely explains the differences in femoral artery diameter between studies. Our current data in older untrained adults which included a similar number of subjects (n = 16) and included both sexes are in agreement with Young and colleagues (Young et al. 2010), in that retrograde shear appears to be increased in the common femoral artery. Despite the differences and similarities between our data and those previously published in untrained older adults, the novel finding of the present study is chronic exercise training can ameliorate the retrograde and oscillatory shear pattern in the common femoral artery and thus effectively eliminate the observed age-related differences (Fig. 2).
The mechanism(s) by which the long-term exercise training improves conduit artery shear patterns in older adults is presently unknown but could be explained by factors, such as enhanced nitric oxide (NO) mediated vasodilation and/or decreased sympathetic vasoconstrictor restraint in the downstream resistance vasculature. Along these lines, acute inhibition of NO synthase in the forearm circulation of young adults increases retrograde and oscillatory shear (Padilla et al. 2011b) as well as decreases antegrade and increases retrograde and oscillatory shear in the femoral artery of both young and older adults (Trinity et al. 2014). In addition, lifelong physical activity and exercise have been linked to preventing age-related reductions in arterial and skeletal muscle NO bioavailability (Nyberg et al. 2012; Taddei et al. 2000). Taken together, these studies suggest that increased NO bioavailability and/or enhanced NO-mediated vasodilation in the resistance vessels could contribute to the improved shear patterns in upstream conduit arteries in exercise-trained older adults. We have also previously demonstrated that α-adrenergic vasoconstriction of the downstream resistance vessels contributes to the age-related increases in brachial artery retrograde and oscillatory shear at rest (Casey et al. 2012). Moreover, exercise training is effective in attenuating α-adrenergic vasoconstriction in skeletal muscle arterioles from old rats (Donato et al. 2007) and, therefore, is a plausible mechanism for the improvements in retrograde and oscillatory shear in exercise-trained older adults. Regardless of the precise mechanism, alterations in downstream vascular resistance are thought to play a significant role in the increased retrograde and oscillatory shear in conduit arteries of older adults. However, in the current study, we did not find statistical differences in regional estimates of vascular resistance in the arm or leg between the three groups (young, older untrained, and older trained). When comparing only the older adults, there was a trend for a reduced estimated vascular resistance in the leg (P = 0.09) of the trained group. In addition, we found moderate correlations between vascular resistance and retrograde (inverse) and oscillatory shear (positive) in the brachial and femoral artery (Fig. 3). Direct measures of pressure in the leg, as opposed to using measures derived via finger plethysmography, would have provided a stronger estimate of regional vascular resistance and might have revealed more significant differences between older trained and untrained adults as well as stronger relationships between downstream resistance and shear rates.
Experimental considerations
In the current study, we utilized a cross-sectional approach to address whether the long-term exercise training improves shear patterns in conduit arteries of older adults. This approach resulted in a cohort of older adults that performed different modes of endurance exercise training (although the majority performed cycling, running, or a combination of the two) as well as varying lengths of exposure to training (i.e., number of years). Therefore, we are unable to ascertain whether there is a certain type of exercise or duration of training that is needed or most beneficial for improving conduit artery shear patterns. In addition, since several of the trained older adults in this study participated in formal exercise training for a large majority of their adult lives (i.e., past 30+ years), our current results do not allow us to determine if an exercise intervention would be beneficial in improving shear profiles in a group of previously sedentary older adults. The relatively small sample size for each group (13–17 subjects per group) used in this cross-sectional study could also be viewed as a study limitation. However, when examining differences in two of the main outcome measures (retrograde and oscillatory shear in the common femoral artery) between groups, the power of the performed tests was 0.81 and 0.93, respectively. Another experimental consideration related to the present study is that the common femoral artery blood flow measurements were performed, while subjects were in a semirecumbant (~140°)-seated position. Recent evidence suggests that sitting (as compared to lying in a supine position) evokes a proatherogenic shear pattern in the femoral artery of young healthy adults, whereas little or no difference in the shear profile was observed in older adults between seated and supine positions (Trinity et al. 2014). Therefore, it is possible that the age-related differences in common femoral artery retrograde and oscillatory shear previously reported might have been slightly underestimated in the current study. However, since all older adults were studied in the same position this experimental consideration should not diminish our novel finding that exercise training ameliorates the age-associated changes in common femoral artery retrograde and oscillatory shear.
Conclusion
While we have previously demonstrated that conduit artery retrograde and oscillatory shear of older subjects are reduced during acute rhythmic exercise (Padilla et al. 2011b), the present study is the first to report a beneficial effect of chronic endurance exercise training on resting peripheral conduit artery shear profiles in older adults. Specifically, our data suggest that the age-related increases in femoral artery retrograde and oscillatory shear are prevented in older adults that have engaged in long-term endurance exercise training as opposed to those that are sedentary. We believe that these findings illustrate another positive effect of chronic exercise training on vascular function in aging humans. Moving forward it will be important to establish whether shorter term exercise interventions are effective in improving conduit artery retrograde and oscillatory shear in individuals with preexisting proatherogenic shear profiles.
Acknowledgments
The authors are grateful to the study volunteers for their participation. We thank David Treichler, Charles Ganger IV, William Hughes, and Samuel Norton for their technical assistance.
Funding
This research was supported by National Heart, Lung, and Blood Institute Research Grant HL-105467 (to D.P. Casey).
Abbreviations
- ANOVA
Analysis of variation
- MAP
Mean arterial pressure
Footnotes
Conflict of interest
No conflicts of interest, financial, or otherwise.
References
- Akazawa N, Choi Y, Miyaki A, Tanabe Y, Sugawara J, Ajisaka R, Maeda S. Curcumin ingestion and exercise training improve vascular endothelial function in postmenopausal women. Nutr Res. 2012;32(10):795–799. doi: 10.1016/j.nutres.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Blackshear WM, Jr, Phillips DJ, Strandness DE., Jr Pulsed Doppler assessment of normal human femoral artery velocity patterns. J Surg Res. 1979;27(2):73–83. doi: 10.1016/0022-4804(79)90113-6. [DOI] [PubMed] [Google Scholar]
- Blair SN, Morris JN. Healthy hearts-and the universal benefits of being physically active: physical activity and health. Ann Epidemiol. 2009;19(4):253–256. doi: 10.1016/j.annepidem.2009.01.019. [DOI] [PubMed] [Google Scholar]
- Casey DP, Padilla J, Joyner MJ. Alpha-adrenergic vasoconstriction contributes to the age-related increase in conduit artery retrograde and oscillatory shear. Hypertension. 2012;60(4):1016–1022. doi: 10.1161/HYPERTENSIONAHA.112.200618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24(2):471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- Chien S. Effects of disturbed flow on endothelial cells. Ann Biomed Eng. 2008;36(4):554–562. doi: 10.1007/s10439-007-9426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway DE, Williams MR, Eskin SG, McIntire LV. Endothelial cell responses to atheroprone flow are driven by two separate flow components: low time-average shear stress and fluid flow reversal. Am J Physiol Heart Circ Physiol. 2010;298(2):H367–H374. doi: 10.1152/ajpheart.00565.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102(12):1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Lesniewski LA, Delp MD. Ageing and exercise training alter adrenergic vasomotor responses of rat skeletal muscle arterioles. J Physiol. 2007;579(Pt 1):115–125. doi: 10.1113/jphysiol.2006.120055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol. 2005;568(Pt 3):1057–1065. doi: 10.1113/jphysiol.2005.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding RA, Frontera WR, Hughes VA, Fisher EC, Evans WJ. The reproducibility of the Bruce protocol exercise test for the determination of aerobic capacity in older women. Med Sci Sports Exerc. 1997;29(8):1109–1113. doi: 10.1097/00005768-199708000-00018. [DOI] [PubMed] [Google Scholar]
- Gerhard M, Roddy MA, Creager SJ, Creager MA. Aging progressively impairs endothelium-dependent vasodilation in forearm resistance vessels of humans. Hypertension. 1996;27(4):849–853. doi: 10.1161/01.hyp.27.4.849. [DOI] [PubMed] [Google Scholar]
- Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol. 2009;10(1):53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr MD, Hogeman CS, Koch DW, Krishnan A, Momen A, Leuenberger UA. A real-time device for converting Doppler ultrasound audio signals into fluid flow velocity. Am J Physiol Heart Circ Physiol. 2010;298(5):H1626–H1632. doi: 10.1152/ajpheart.00713.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes WE, Ueda K, Casey DP. Chronic endurance exercise training offsets the age-related attenuation in contraction-induced rapid vasodilation. J Appl Physiol. 2016;120(11):1335–1142. doi: 10.1152/japplphysiol.00057.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BD, Mather KJ, Newcomer SC, Mickleborough TD, Wallace JP. Vitamin C prevents the acute decline of flow-mediated dilation after altered shear rate patterns. Physiologie appliquee nutrition et metabolisme. 2013;38(3):268–274. doi: 10.1139/apnm-2012-0169. [DOI] [PubMed] [Google Scholar]
- Kroger K, Kucharczik A, Hirche H, Rudofsky G. Atherosclerotic lesions are more frequent in femoral arteries than in carotid arteries independent of increasing number of risk factors. Angiology. 1999;50(8):649–654. doi: 10.1177/000331979905000805. [DOI] [PubMed] [Google Scholar]
- Ku DN, Giddens DP, Zarins CK, Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis. 1985;5(3):293–302. doi: 10.1161/01.atv.5.3.293. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Newcomer SC, Bender SB. Importance of hemodynamic forces as signals for exercise-induced changes in endothelial cell phenotype. J Appl Physiol. 2008;104(3):588–600. doi: 10.1152/japplphysiol.01096.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Kassab GS. Nitric oxide is significantly reduced in ex vivo porcine arteries during reverse flow because of increased superoxide production. J Physiol. 2004;561(Pt 2):575–582. doi: 10.1113/jphysiol.2004.075218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation. 2000;101(8):862–868. doi: 10.1161/01.cir.101.8.862. [DOI] [PubMed] [Google Scholar]
- Newcomer SC, Sauder CL, Kuipers NT, Laughlin MH, Ray CA. Effects of posture on shear rates in human brachial and superficial femoral arteries. Am J Physiol Heart Circ Physiol. 2008;294(4):H1833–H1839. doi: 10.1152/ajpheart.01108.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg M, Blackwell JR, Damsgaard R, Jones AM, Hellsten Y, Mortensen SP. Lifelong physical activity prevents an age-related reduction in arterial and skeletal muscle nitric oxide bioavailability in humans. J Physiol. 2012;590(Pt 21):5361–5370. doi: 10.1113/jphysiol.2012.239053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla J, Sheldon RD, Sitar DM, Newcomer SC. Impact of acute exposure to increased hydrostatic pressure and reduced shear rate on conduit artery endothelial function: a limb-specific response. Am J Physiol Heart Circ Physiol. 2009;297(3):H1103–H1108. doi: 10.1152/ajpheart.00167.2009. [DOI] [PubMed] [Google Scholar]
- Padilla J, Young CN, Simmons GH, Deo SH, Newcomer SC, Sullivan JP, Laughlin MH, Fadel PJ. Increased muscle sympathetic nerve activity acutely alters conduit artery shear rate patterns. Am J Physiol Heart Circ Physiol. 2010;298(4):H1128–H1135. doi: 10.1152/ajpheart.01133.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla J, Simmons GH, Bender SB, Arce-Esquivel AA, Whyte JJ, Laughlin MH. Vascular effects of exercise: endothelial adaptations beyond active muscle beds. Physiology. 2011a;26(3):132–145. doi: 10.1152/physiol.00052.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla J, Simmons GH, Fadel PJ, Laughlin MH, Joyner MJ, Casey DP. Impact of aging on conduit artery retrograde and oscillatory shear at rest and during exercise: role of nitric oxide. Hypertension. 2011b;57(3):484–489. doi: 10.1161/HYPERTENSIONAHA.110.165365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce GL, Donato AJ, LaRocca TJ, Eskurza I, Silver AE, Seals DR. Habitually exercising older men do not demonstrate age-associated vascular endothelial oxidative stress. Aging Cell. 2011a;10(6):1032–1037. doi: 10.1111/j.1474-9726.2011.00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce GL, Eskurza I, Walker AE, Fay TN, Seals DR. Sex-specific effects of habitual aerobic exercise on brachial artery flow-mediated dilation in middle-aged and older adults. Clin Sci. 2011b;120(1):13–23. doi: 10.1042/CS20100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreuder TH, Green DJ, Hopman MT, Thijssen DH. Acute impact of retrograde shear rate on brachial and superficial femoral artery flow-mediated dilation in humans. Physiol Rep. 2014;2(1):e00193. doi: 10.1002/phy2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Desouza CA, Donato AJ, Tanaka H. Habitual exercise and arterial aging. J Appl Physiol. 2008;105(4):1323–1332. doi: 10.1152/japplphysiol.90553.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Kaplon RE, Gioscia-Ryan RA, LaRocca TJ. You’re only as old as your arteries: translational strategies for preserving vascular endothelial function with aging. Physiology. 2014;29(4):250–264. doi: 10.1152/physiol.00059.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Jr, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1995;92(5):1355–1374. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation. 1995;91(7):1981–1987. doi: 10.1161/01.cir.91.7.1981. [DOI] [PubMed] [Google Scholar]
- Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation. 2000;101(25):2896–2901. doi: 10.1161/01.cir.101.25.2896. [DOI] [PubMed] [Google Scholar]
- Thijssen DH, Dawson EA, Tinken TM, Cable NT, Green DJ. Retrograde flow and shear rate acutely impair endothelial function in humans. Hypertension. 2009;53(6):986–992. doi: 10.1161/HYPERTENSIONAHA.109.131508. [DOI] [PubMed] [Google Scholar]
- Thijssen DH, Schreuder TH, Newcomer SW, Laughlin MH, Hopman MT, Green DJ. Impact of 2-weeks continuous increase in retrograde shear stress on brachial artery vasomotor function in young and older men. J Am Heart Assoc. 2015 doi: 10.1161/JAHA.115.001968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinken TM, Thijssen DH, Hopkins N, Black MA, Dawson EA, Minson CT, Newcomer SC, Laughlin MH, Cable NT, Green DJ. Impact of shear rate modulation on vascular function in humans. Hypertension. 2009;54(2):278–285. doi: 10.1161/HYPERTENSIONAHA.109.134361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totosy de Zepetnek JO, Jermey TL, MacDonald MJ. Superficial femoral artery endothelial responses to a short-term altered shear rate intervention in healthy men. PLoS One. 2014;9(11):e113407. doi: 10.1371/journal.pone.0113407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinity JD, Groot HJ, Layec G, Rossman MJ, Ives SJ, Richardson RS. Impact of age and body position on the contribution of nitric oxide to femoral artery shear rate: implications for atherosclerosis. Hypertension. 2014;63(5):1019–1025. doi: 10.1161/HYPERTENSIONAHA.113.02854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Baker BM, Chen CS, Schwartz MA. Endothelial cell sensing of flow direction. Arterioscler Thromb Vasc Biol. 2013;33(9):2130–2136. doi: 10.1161/ATVBAHA.113.301826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray DW, Uberoi A, Lawrenson L, Richardson RS. Evidence of preserved endothelial function and vascular plasticity with age. Am J Physiol Heart Circ Physiol. 2006;290(3):H1271–H1277. doi: 10.1152/ajpheart.00883.2005. [DOI] [PubMed] [Google Scholar]
- Wu SP, Ringgaard S, Oyre S, Hansen MS, Rasmus S, Pedersen EM. Wall shear rates differ between the normal carotid, femoral, and brachial arteries: an in vivo MRI study. J Magn Reson Imaging JMRI. 2004;19(2):188–193. doi: 10.1002/jmri.10441. [DOI] [PubMed] [Google Scholar]
- Yoshizawa M, Maeda S, Miyaki A, Misono M, Choi Y, Shimojo N, Ajisaka R, Tanaka H. Additive beneficial effects of lactotripeptides intake with regular exercise on endothelium-dependent dilatation in postmenopausal women. Am J Hypertens. 2010;23(4):368–372. doi: 10.1038/ajh.2009.270. [DOI] [PubMed] [Google Scholar]
- Young CN, Deo SH, Padilla J, Laughlin MH, Fadel PJ. Proatherogenic shear rate patterns in the femoral artery of healthy older adults. Atherosclerosis. 2010;211(2):390–392. doi: 10.1016/j.atherosclerosis.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]