Abstract
The critical importance of thyroid hormones for fetal development is well established. The developing fetus is dependent on the mother for adequate thyroid hormone supply, and maternal thyroid dysfunction in pregnancy may result in suboptimal fetal development. Because exposure to childhood maltreatment (CM) has been associated with thyroid dysfunction in the non-pregnant state, we sought to test the hypothesis that exposure to CM may represent a risk factor for the development of maternal hypothyroidism in pregnancy. The study was conducted in a healthy cohort of 102 pregnant mothers who were followed across the entire course of pregnancy. At each trimester thyroid-stimulating hormone (TSH) and free thyroxine (fT4) were measured in maternal serum. Experience of CM was assessed using the Childhood Trauma Questionnaire. After adjusting for potentially confounding variables, CM exposure was associated with increased TSH concentrations across pregnancy (F1,94.6=11.52, p=.001) and with a 4- to 7-fold increased risk of TSH levels above the trimester-specific clinical cut-off values. Women with clinically elevated TSH concentrations did not differ in fT4 concentrations from women with normal TSH concentrations (p > .1), suggesting subclinical hypothyroidism. Our findings suggest that there is a substantial and clinically relevant increased risk for thyroid dysfunction during pregnancy among women exposed to abuse or neglect in their childhood. This could potentially have adverse consequences for fetal brain development. Thus, these findings highlight the critical importance of considering CM exposure as a potential risk factor for (subclinical) hypothyroidism in pregnancy.
Keywords: childhood maltreatment, pregnancy, thyroid dysfunction, subclinical hypothyroidism, TSH
1. Introduction
The thyroid hormones (THs), triiodothyronine (T3) and thyroxine (T4), are highly evolutionarily conserved tyrosine-based hormones produced by the thyroid gland that play a major role in the regulation of metabolism. During embryonic and fetal life THs also play an obligatory role in many fundamental processes underlying brain development, including neuronal proliferation, migration, neurite outgrowth and guidance, synaptogenesis, and myelination (Moog et al., 2017). The developing embryo/fetus is completely dependent on the mother for TH supply until around mid-gestation, at which time the fetal thyroid gland becomes functional (Williams, 2008). Even during the second half of gestation, a significant proportion of THs in the fetal compartment is obtained from the maternal compartment (Vulsma et al., 1989).
The hormonal and metabolic adaptations produced by the state of pregnancy induce major changes in maternal thyroid physiology. Free thyroxine (fT4) concentrations are typically highest in the first trimester and decrease slightly across gestation, whereas thyroid stimulating hormone (TSH) levels are typically suppressed in the first trimester and increase later in pregnancy. Overall, there is an increased demand on the maternal thyroid throughout gestation, which is reflected in an enlargement of the maternal thyroid in pregnancy by approximately 20% (Braunstein, 2011). These gestational changes, alone or in combination with pre-existing conditions such as the presence of thyroid autoantibodies (which are present in 5–20% of women of childbearing age), may lead to the onset of thyroid dysfunction or an exacerbation of milder pre-existing dysfunction (Glinoer et al., 1991). Thyroid dysfunction, i.e. hyper- and hypothyroidism, is estimated to affect about 4–6% of all pregnant women (Stagnaro-Green et al., 2011). For definitions and prevalence of dysfunctional thyroid states during pregnancy refer to Box 1.
Box 1. Definitions and prevalence of maternal thyroid dysfunction in pregnancy (De Groot et al., 2012; Stagnaro-Green et al., 2011).
Overt hypothyroidism (0.3–0.5%)
Serum TSH levels above the trimester-specific reference range (2.5–3.0 mU/l) in conjunction with a decreased fT4 concentration.
Subclinical hypothyroidism (2–2.5%)
Serum TSH levels above the trimester-specific reference range (2.5–3.0 mU/l) with normal fT4 concentrations.
Hyperthyroidism (1–3%)
Serum TSH levels below the trimester-specific reference range (0.1–0.3 mU/l) with (i.e. overt hyperthyroidism) or without (i.e. subclinical hyperthyroidism) elevated levels of fT4 and/or fT3, often transient in the first half of pregnancy.
Thyroid autoimmunity (5–20%)
Presence of TPO- and/or TG-antibodies in the serum, with or without changes in TSH and fT4 concentrations.
Severe forms of thyroid dysfunction, i.e. overt hypo- and hyperthyroidism, are often accompanied by marked symptomatology as well as fertility problems and increased embryonic morbidity, such that treatment is often necessary for a pregnancy to ensue or continue (Thangaratinam et al., 2011; van den Boogaard et al., 2011). On the other hand, more moderate forms of thyroid dysfunction, such as subclinical hypothyroidism, are less likely to be detected, and this may, therefore, paradoxically confer a higher burden of disease (risk to the developing fetus) from a public health perspective.
While a severe deficiency of maternal thyroid hormones during gestation produces grave cognitive, motor and sensory deficits in her child (Gilbert et al., 2012), even subtler forms of maternal hypothyroidism may produce long-lasting alterations in child brain structure and cognitive development, that confer increased risk for neurodevelopmental disorders (e.g., Ghassabian et al., 2011; Haddow et al., 1999; Pakkila et al., 2014; Williams et al., 2012; Willoughby et al., 2014). Moreover, these alterations appear to be largely irreversible by treatment after birth (de Escobar et al., 2004). Hence, the optimal regulation of maternal thyroid function during pregnancy is important not only for her own health, but likely also for the long-term health of her child.
Other than the presence of specific medical conditions (primarily thyroid autoimmunity) or iodine deficiency, relatively little is presently known about conditions and processes that confer increased risk of maternal hypothyroidism in pregnancy. We have previously advanced the hypothesis that maternal stress and stress-related biological processes during pregnancy may modulate maternal thyroid function (Moog et al., 2017). Here, we seek to test the hypothesis that exposure to stress that predates conception and extends back to the sensitive period of her own childhood may represent a novel and significant risk factor for the development of maternal hypothyroidism in pregnancy.
Substantial evidence in humans and animals demonstrates a tight coupling between the thyroid (hypothalamic-pituitary-thyroid (HPT)) and the stress (hypothalamic-pituitary-adrenal (HPA)) axes (for review see Moog et al., 2017). Based on the consideration that the onset and course of hypothyroidism is often insidious and may be precipitated by maternal states and conditions that precede pregnancy, we were particularly interested in considering the role of stress exposure from a maternal life course perspective. We selected exposure to childhood maltreatment (CM) as our primary variable of interest for the following reasons: a) CM represents among the most pervasive and pernicious stressors affecting around one third of the general population, with life-long biological, psychological and behavioral consequences (e.g., Heim et al., 2010); b) we and others have previously demonstrated that a woman’s exposure to CM can produce alterations in several features of gestational biology that relate to embryonic/fetal development (Cammack et al., 2011; Mason et al., 2016; Moog et al., 2016; Shea et al., 2007); c) CM exposure has been associated with reduced thyroid activity in the non-pregnant state (Haviland et al., 2006; Machado et al., 2015; Sinai et al., 2014), with thyroid dysfunction in the postpartum period (Plaza et al., 2010; Plaza et al., 2012), and, more generally, with a higher risk of autoimmune disorders (Dube et al., 2009; Goodwin and Stein, 2004); and d) children of women exposed to CM exhibit an increased risk of developing some of the same behavioral disorders that have been observed in children with moderate maternal thyroid dysfunction (Miranda et al., 2013; Rijlaarsdam et al., 2014; Thompson, 2007). In light of these observations, we hypothesized that women exposed to CM may exhibit an increased likelihood of thyroid dysfunction in pregnancy, a time period of particular importance for not only the mother but also for her developing fetus.
2. Materials and methods
2.1 Study design
The study was conducted at the University of California, Irvine, Development, Health and Disease Research Program in a clinical convenience cohort of 146 pregnant women. All participants had singleton, intrauterine pregnancies, with no known cord, placental, or uterine anomalies, fetal congenital malformations, or corticosteroid medication use. Participants were recruited in the first trimester of gestation and followed prospectively and serially through pregnancy. Study visits occurred in early (T1: 12.7 ± 1.8 weeks gestation, N=134), mid (T2: 20.5 ± 1.5 weeks, N=145), and late pregnancy (T3: 30.5 ± 1.4 weeks gestation, N=142). Study visit procedures included administration of structured socio-demographic and psychosocial interviews and questionnaires, collection of biological samples, and fetal ultrasonography. All study procedures were approved by the university’s institutional review board and all participants provided written informed consent.
2.2 Childhood maltreatment exposure
At the mid gestation study visit, exposure to CM was ascertained using the Childhood Trauma Questionnaire (CTQ, Bernstein and Fink, 1998a), one of the most widely-used, reliable, and validated instruments for determination of abuse and neglect experiences in childhood and adolescence. This 28-item measure assesses five dimensions of childhood maltreatment: emotional abuse, physical abuse, sexual abuse, emotional neglect, and physical neglect. Cut-off values for moderate or greater exposure were used to create dichotomous variables of exposure for each CTQ subscale (emotional abuse ≥13; physical abuse ≥10; sexual abuse ≥8; emotional neglect ≥15; and physical neglect ≥10). From these, a composite variable was computed indicating exposure to at least one category of moderate to severe abuse or neglect (CM+) vs. no or low exposure (CM−).
2.3 Thyroid function in pregnancy
Fasting maternal venous blood was collected in each trimester, processed and stored at −80°C, shipped to our research laboratory in Berlin, and re-stored in false bottom aliquot tubes (Sarstedt, Nümbrecht, Germany). Assays were performed in batches. Samples were thawed, centrifuged at 3,000 g for 15 min, and processed within 4 hours. TSH and fT4 concentrations were determined by electrochemiluminescent immunoassay (ECLIA) using the Cobas e 601 analyzer (Roche Diagnostics, Mannheim, Germany) in an accredited laboratory (Labor Berlin). The assay lower detection limits for TSH and fT4 were 0.005 mU/l and 0.23 ng/ml, respectively. Overall, 0.7–8.9% of thyroid parameters were missing, depending on time point during pregnancy. Outlier correction was performed by winsorizing TSH and fT4 values. Using The Endocrine Society’s guidelines (De Groot et al., 2012) and the guidelines of the American Thyroid Association (Stagnaro-Green et al., 2011), trimester-specific cut-off values for TSH (0.1–2.5 mU/l in the 1st trimester, 0.2–3.0 mU/l in the 2nd trimester, and 0.3–3.0 mU/l in the 3rd trimester) were used to create a binary variable at each trimester indexing hypothyroidism vs. euthyroidism. In addition, an fT4/TSH ratio was computed. Two of the study participants (2%) reported taking thyroid medication (levothyroxine) during pregnancy. Since CM exposure was evenly distributed between these two subjects and their TSH concentrations were still abnormal during at least one time point in pregnancy, they were included in the analyses to increase statistical power and ecological validity.
2.4 Covariates and potential confounders
Covariates and confounders were selected based on theoretical considerations and known associations with childhood maltreatment, thyroid function, or both. These included race/ethnicity, current socioeconomic status (SES), pre-pregnancy body-mass-index (BMI), maternal smoking during pregnancy, parity, and maternal depressive symptoms during pregnancy. In addition, we controlled for another important childhood condition, childhood socioeconomic status (cSES), because the prevalence of CM tends to be higher among low SES populations (Hussey et al., 2006).
cSES was quantified using a set of 19 questions that characterized distinct aspects of economic status (social privileges and disadvantages) during childhood. The questions focused on whether the family obtained regular medical check-ups, received public assistance, purchased new clothes on special occasions, could afford eating out in restaurants and taking music, dance or art lessons, and whether they owned a home, a car, television etc. Answers were given as 1 = “yes” or 0 = “no”. A total score was computed by summing up the number of social advantages (range 0–19). A higher score indicated higher cSES. A cSES score was missing in 20.5% of participants. Information regarding race/ethnicity, education, and parity was obtained from structured interviews. Educational level was originally assessed in categories from less than high school to advanced degree (master’s/doctorate) and then recoded into values from 1 through 5. Parity was coded into two dummy variables reflecting nulliparity and primiparity, which describes best the distribution in the study cohort (~ one third each for 0, 1, and ≥ 2 live births). Pre-pregnancy BMI was abstracted from the medical record. A dichotomous variable was created from BMI indicating normal weight (BMI between 18 and 25) vs. overweight/obesity (BMI ≥ 25). Maternal smoking during pregnancy was determined by maternal self-report and verified by measurement of urinary cotinine concentration. Urinary cotinine was assayed in maternal samples collected at each trimester using the Nicotine/COT(Cotinine)/Tobacco Drug Test Urine Cassette (http://www.meditests.com/nicuintescas.html), which involves transferring 4 drops of room temperature urine into the well of the cassette, and employs a cutoff for COT presence of 200ng/ml. Endorsement of smoking or detection of urinary COT in any trimester was coded as 1, and absence of evidence for smoking in any trimester coded as 0. Depressive symptoms were assessed at each trimester using the Center for Epidemiological Studies Depression scale (CES-D, Radloff, 1977). To account for singular missing items (i.e. ≤ 2 items) the mean responses were calculated for each time point and then multiplied by the number of items (i.e., 20). For participants with 3 or more missing items, no CES-D score was calculated for this time point. For all women an average CES-D score across pregnancy was calculated, which was based on at least 2 CES-D scores. Overall, 0–5.5% of CES-D scores were missing, depending on time point during pregnancy.
2.5 Statistical analysis
Pregnant women who exhibited transient hyperthyroidism in the first trimester (n=2), had a pre-pregnancy BMI <18.5 (n=3), or smoked during pregnancy (n=4) were excluded from the analyses because the limited variability of these measures in the study population precluded them from being entered as covariates (underweight, smoking) or being used as a separate outcome measure (hyperthyroidism). A linear mixed effects model was employed to determine the association between experience of CM and TSH concentrations across pregnancy. These models allow inclusion of all subjects with available measurements in the repeated measures variables (i.e. thyroid parameters, CES-D scores). The final sample that was considered in the mixed effects model consisted of N=102 pregnant women. Restricted maximum likelihood estimation was performed. The model included a random intercept term as well as a autoregressive (AR(1)) heterogeneous covariance structure in order to account for the expected within-subject correlation of TSH across time (study visits). The following covariates were included in the model: race/ethnicity, current SES, parity, pre-pregnancy BMI, cSES, and CES-D scores at each trimester. In our sample, cSES and current SES were moderately correlated (r=.36, p=.001) and could thus both be included in the model. As effect size estimates are not provided within linear mixed effects model analyses, these were calculated using the estimated marginal means and the standard errors were used to obtain the standard deviation in order to calculate Cohen’s d. To evaluate the risk of hypothyroidism at each trimester during pregnancy, logistic regression analysis was used to determine the association between CM and the risk for TSH concentrations above the trimester-specific clinical cut-off, controlling for the same covariates as in the models described above. Next, the associations between clinically elevated TSH (≥ two time points), CM and fT4 concentrations as well as between clinically elevated TSH, CM and the fT4/TSH ratio were determined with linear mixed effects models as described above. All analyses were performed with IBM SPSS Statistics 22 (IBM, Armonk, NY).
3. Results
3.1 Descriptives
As expected, and consistent with previously published estimates (17), exposure to CM was present in 28% (n=29) of the study population. One maltreatment category was reported by 17% (n=17), and multiple forms of maltreatment by 12% (n=12) of the study population. Table 1 depicts the key sociodemographic characteristics of the study population delineated by CM status (CM+ vs. CM−). The two groups differed with respect to SES, race/ethnicity and depressive symptoms. Women with CM were significantly less likely to be Non-Hispanic White compared to all other racial/ethnic categories, had a significantly lower educational level and a higher level of depressive symptoms across pregnancy. Women with CM also had a marginally lower cSES score. For further information on bivariate correlations between the main measures and covariates refer to Table 2.
Table 1.
Characteristics of the study population.
| Complete sample N = 102 | CM− group (no childhood maltreatment) N = 73 (72%) | CM+ group (≥ 1 type of childhood maltreatment) N = 29 (28%) | |
|---|---|---|---|
| Age in years, mean ± SD | 27.9 ± 5.5 | 28.4 ± 5.2 | 26.6 ± 6.1 |
| Race/ethnicity | |||
| Non-Hispanic White, N (%) | 41 (40.2) | 34 (46.6) | 7 (24.1)* |
| Hispanic White, N (%) | 36 (35.3) | 23 (31.5) | 13 (44.8) |
| Parity | |||
| Nulliparous, N (%) | 38 (37.7) | 26 (35.6) | 12 (41.4) |
| Primiparous, N (%) | 31 (30.4) | 24 (32.9) | 7 (24.1) |
| Pre-pregnancy body mass index ≥ 25, N (%) | 52 (51.0) | 38 (52.1) | 14 (48.3) |
| Childhood SES, mean ± SD | 13.0 ± 3.7 | 13.4 ± 3.4 | 12.0 ± 4.3† |
| Education, mean ± SD | 3.2 ± 1.0 | 3.4 ± 1.0 | 2.9 ± 0.9* |
| Depression in pregnancy (CES-D), mean ± SD | 13.0 ± 7.8 | 11.7 ± 7.5 | 16.3 ± 7.8** |
| Childhood Trauma Questionnaire | |||
| Total Score, mean ± SD | 34.8 ± 13.2 | 29.1 ± 4.1 | 49.0 ± 17.0** |
| Emotional Abuse, mean ± SD | 7.2 ± 3.6 | 5.8 ± 1.3 | 11.0 ± 4.9** |
| Physical Abuse, mean ± SD | 6.3 ± 3.0 | 5.5 ± 0.9 | 8.3 ± 5.0** |
| Sexual Abuse, mean ± SD | 6.1 ± 4.0 | 5.1 ± 0.4 | 8.8 ± 6.9** |
| Emotional Neglect, mean ± SD | 8.8 ± 3.8 | 7.3 ± 2.6 | 12.5 ± 3.7** |
| Physical Neglect, mean ± SD | 6.3 ± 2.3 | 5.5 ± 1.0 | 8.4 ± 3.2** |
Note. CM = childhood maltreatment; SES = Socio-economic status; CES-D = Center for Epidemiological Studies–Depression scale; CTQ = Childhood Trauma Questionnaire. Group differences were tested with independent sample t-tests or chi-square tests.
p < .1;
p < .05;
p < .01
Table 2.
Correlation table between the main measures and covariates.
| T1 TSH | T2 TSH | T3 TSH | CM | education | NHW | HW | ppBM I | nulliparity | primiparity | cSES | T1 CES-D | T2 CES-D | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T2 TSH | .808*** | ||||||||||||
| T3 TSH | .749*** | .845*** | |||||||||||
| CM | .252* | .299** | .264** | ||||||||||
| education | .064 | −.105 | −.017 | −.212* | |||||||||
| NHW | .007 | −.034 | −.066 | −.206* | .322** | ||||||||
| HW | −.015 | .020 | .031 | .126 | −.317** | −.605*** | |||||||
| ppBMI | .216* | .075 | .054 | −.034 | −.075 | −.076 | .191 | ||||||
| nulliparity | .293** | .231* | .193 | .054 | −.012 | −.094 | −.017 | .066 | |||||
| primiparity | −.076 | −.048 | −.054 | −.086 | .218* | .067 | −.042 | .008 | −.509*** | ||||
| cSES | −.018 | −.061 | −.082 | −.170 | .276** | .520*** | −.558*** | −.265** | −.009 | .089 | |||
| T1 CES-D | .225* | .276** | .091 | .268** | −.196 | −.090 | .044 | .128 | .201* | .008 | −.284** | ||
| T2 CES-D | .118 | .015 | −.095 | .214* | −.236* | −.088 | .047 | .205* | .259** | −.165 | −.223* | .607*** | |
| T3 CES-D | .194 | .110 | −.032 | .205* | −.201* | −.096 | .041 | .243* | .241* | −.125 | −.226* | .658*** | .722*** |
Note. Depicted are Pearson correlation coefficients. Significant correlations are indicated by asterisks. TSH = thyroid-stimulating hormone; CM = childhood maltreatment; NHW = Non-Hispanic White; HW = Hispanic White; ppBMI = pre-pregnancy body-mass-index; cSES = childhood socio-economic status; CES-D = Center for Epidemiological Studies–Depression scale
p < .05;
p < .01;
p < .001
Also as expected, TSH concentrations changed significantly across gestation (F2,117.9=18.47, p<.001). TSH levels in the first trimester were lower than those in the second and third trimesters (b = −0.43, 95% CI: −0.67, −0.18), likely due to peak secretion of hCG in early gestation.
3.2 Childhood Maltreatment and TSH concentrations across pregnancy
Exposure to CM was significantly associated with TSH concentrations across pregnancy (F1,94.6=11.52, p=.001). In each trimester, women with CM had higher concentrations of TSH compared to women without CM (b = 0.70, 95% CI: 0.3, 1.1; Cohen’s d = 0.76, corresponding to a “medium” effect size; see Fig. 1). The relationship between CM and TSH concentrations showed significant variance in intercepts across participants (Var = 0.50, Wald Z = 2.93, p = .003). The normal trajectory of TSH across gestation was preserved in women with CM (trimester*CM: F2,115.6=1.44, p=.240). Among the covariates only parity and depressive symptoms had a significant main effect on TSH across gestation; nulliparity was associated with higher TSH concentrations (F1,95.1=7.72, p=.007), and a higher level of depressive symptoms was associated with lower TSH concentrations (F1,226.2=7.80, p=.006). Excluding the two subjects with thyroid medication in pregnancy did not change these results.
Figure 1.
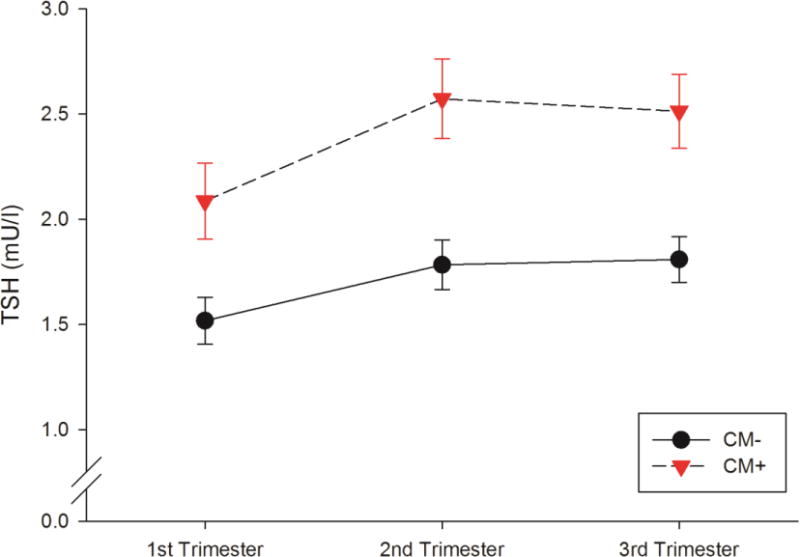
TSH concentrations across pregnancy in women with (CM+) and without a history of exposure to childhood maltreatment (CM−).
Note. Displayed are the mean TSH concentrations in each group adjusted for race/ethnicity, education, parity, pre-pregnancy BMI, childhood SES, and depressive symptoms in pregnancy. Error bars represent the standard error of the mean (SEM).
3.3 Childhood maltreatment and risk for hypothyroidism
In the next step, we examined whether the increase in TSH concentrations was clinically significant by using the trimester-specific cut-off ranges recommended by The Endocrine Society (De Groot et al., 2012). The numbers and percentages of women with TSH concentrations above the clinical cut-off at each trimester and across gestation are presented in Table 3. In our study population 8.9% of women had consistently clinically elevated TSH concentrations across gestation (i.e., they were hypothyroid); among CM+ women 20% were consistently hypothyroid, whereas this was only true for 4.6% of CM− women. The results of the logistic regression analyses indicated that there was a 4- to 7-fold increased risk for CM+ women of having TSH levels above the trimester-specific clinical cut-off values compared to women without CM (see Table 4).
Table 3.
Frequencies (N (%)) of pregnant women with TSH above the trimester specific cut-off in the total sample and delineated by CM status.
| Total sample | CM− | CM+ | |
|---|---|---|---|
| 1st trimester (TSH > 2.5 mU/l), N=94 | 18 (19.1) | 10 (15.2) | 8 (28.6) |
| 2nd trimester (TSH > 3.0 mU/l), N=101 | 12 (11.9) | 5 (6.8) | 7 (25.0) |
| 3rd trimester (TSH > 3.0 mU/l), N=99 | 12 (12.1) | 6 (8.3) | 6 (22.2) |
| Overall (TSH > trimester-specific cut-off in each trimester), N=90 | 8 (8.9) | 3 (4.6) | 5 (20.0) |
Note. CM = childhood maltreatment; TSH = thyroid-stimulating hormone. Trimester-specific reference ranges recommended by the American Thyroid Association and the Endocrine Society were applied.
Table 4.
Logistic regression analyses of the association between CM and risk of clinically elevated TSH concentrations at each trimester.
| 1st Trimester | 2nd Trimester | 3rd Trimester | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| B (SE) | OR [95% CI] | B (SE) | OR [95% CI] | B (SE) | OR [95% CI] | |
|
| ||||||
| CM | 1.35 (0.7) | 3.84 [1.0–14.5]* | 1.94 (0.8) | 6.98 [1.4–36.0]* | 1.80 (0.8) | 6.07 [1.3–27.5]* |
|
| ||||||
| Race/ethnicity | ||||||
| Non-Hispanic White | 0.70 (0.8) | 2.01 [0.4–9.7] | 0.36 (1.1) | 1.43 [0.2–12.8] | 1.33 (1.1) | 3.80 [0.4–32.6] |
| Hispanic White | 0.20 (0.9) | 1.22 [0.2–7.3] | −0.82 (1.1) | 0.44 [0.1–3.5] | 0.48 (1.1) | 1.61 [0.2–13.2] |
|
| ||||||
| Education | 0.22 (0.4) | 1.25 [0.6–2.6] | −1.31 (0.5) | 0.27 [0.1–0.7]* | −0.18 (0.4) | 0.84 [0.4–1.9] |
|
| ||||||
| Nulliparity | 2.66 (1.7) | 14.3 [1.5–139.9]* | 2.23 (1.2) | 9.29 [0.9–88.3] | 2.69 (1.2) | 14.7 [1.3–166.1]* |
|
| ||||||
| Primiparity | 1.77 (1.2) | 5.86 [0.5–62.6] | 1.82 (1.2) | 6.19 [0.6–64.8] | 2.10 (1.3) | 8.16 [0.7–97.8] |
|
| ||||||
| Pre-pregnancy BMI | 1.19 (0.7) | 3.30 [0.9–12.8] | 1.97 (0.9) | 7.14 [1.1–45.4]* | 1.36 (0.8) | 3.90 [0.8–19.8] |
|
| ||||||
| Childhood SES | 0.08 (0.1) | 1.08 [0.9–1.3] | −0.06 (0.1) | 0.94 [0.7–1.2] | −0.04 (0.1) | 0.96 [0.8–1.2] |
|
| ||||||
| Depression (CES-D) | 0.34 (0.8) | 1.40 [0.3–7.1] | −1.05 (1.1) | 0.35 [0.0–3.1] | −0.68 (1.0) | 0.51 [0.1–3.8] |
Note. BMI = body mass index; CM = childhood maltreatment; SES = Socio-economic status; CES-D = Center for Epidemiological Studies–Depression scale. BMI is dichotomized in normal weight (BMI < 25 and > 18.5) vs. overweight/obesity (BMI > 25). Logistic regressions were performed separately for each trimester. R2 = .27–.37 (Nagelkerke).
p < .05
3.4 Childhood maltreatment and TSH in association with fT4 concentrations and fT4/TSH ratio across pregnancy
fT4 concentrations decreased significantly across gestation (F2,92.4=80.94, p < .001). Women with clinically elevated TSH concentrations at two or more time points (with or without CM) did not differ in fT4 concentrations from women with TSH concentrations below the clinical cut-off at 2 or more time points (mean (SE): normal TSH: 11.03 (0.2) ng/l, high TSH: 10.84 (0.4) ng/l; F1,78.9=0.20, p = .658), indicating the presence of subclinical hypothyroidism. Women with clinically elevated TSH concentrations at two or more time points did, however, differ with respect to the fT4/TSH ratio (mean (SE): normal TSH: 8.62 (0.8) ng/l fT4 per unit TSH; high TSH: 5.03 (1.3) ng/l fT4 per unit TSH; F1,78.2=8.78, p=.004). The lower fT4/TSH ratio in women with clinically elevated TSH concentrations suggests that the thyroid capacity is impaired. CM was not independently associated with fT4 concentrations or the fT4/TSH ratio (p > .05). This pattern remains the same when contrasting women with consistently elevated TSH in all three trimesters against women who were consistently below the cut-off in pregnancy.
4. Discussion
The principal finding of our study is that women exposed to moderate to severe abuse or neglect during childhood were 4 to 7-fold more likely to exhibit subclinical hypothyroidism across the course of their pregnancy after accounting for the effects of key covariates. In our study population of relatively healthy pregnant women, approximately 9% had clinically elevated TSH across all three trimesters (see Table 2). This number is somewhat higher than the prevalence reported by previous studies, which may be due to differences in reference levels that were applied (Casey et al., 2005; Klein et al., 1991). Another reason for those discrepancies may lie in the current practice of targeted screening for subclinical hypothyroidism in only high-risk groups (e.g., family history, other autoimmune disorders, prior history of miscarriage/preterm birth) during pregnancy (Lazarus et al., 2014). However, such a strategy may miss 30% or more of women with thyroid dysfunction (Lazarus et al., 2014; Vaidya et al., 2007), suggesting that the risk factors that are presently known and utilized do not sufficiently characterize the group of women at high risk for developing thyroid dysfunction in pregnancy. Our findings support the implication that considering CM as an additional risk factor may improve the detection rate of hypothyroidism during pregnancy.
The observed CM-associated increases in TSH concentrations were not accompanied by reduced fT4 concentrations. But even when fT4 concentrations are within the normal range, maternal subclinical hypothyroidism in pregnancy may adversely affect fetal TH levels via two possible mechanisms. Firstly, subclinical hypothyroidism (in general, and as evidenced in our study population) is associated with a compromised capacity of the thyroid gland in terms of TH production, which, in the context of pregnancy may have downstream implications for the adequacy of supply of maternal THs to the developing embryo/fetus. Secondly, subclinical hypothyroidism is often accompanied by increased concentrations of anti-thyroid antibodies (e.g. thyroid peroxidase antibodies, TPOAb), which, during pregnancy, are known to pass freely across the placenta into the fetal compartment (Radetti et al., 1999; Seror et al., 2014), and then, consequently, may impair fetal thyroid function and the concentration of circulating THs in the fetal compartment. Most but not all studies indicate that moderate or subclinical hypothyroidism exerts an adverse effect on child brain and cognitive development and increased risk for neurodevelopmental disorders (for review see Moog et al., 2017). For instance, higher maternal levels of TSH and/or mild hypothyroidism during pregnancy have been associated with more externalizing problems and ADHD symptoms (Ghassabian et al., 2011; Pakkila et al., 2014), with lower IQ or cognitive performance (Haddow et al., 1999; Williams et al., 2012), and with lower hippocampal volume in their children (Willoughby et al., 2014).
Previous studies have also established that maternal exposure to childhood maltreatment is associated with a higher prevalence of social-emotional disorders in their children (Miranda et al., 2013; Thompson, 2007). Those findings, in conjunction with these of the present study, raise the possibility that maternal thyroid function in pregnancy may represent one pathway that mediates the impact of maternal CM exposure on child neurodevelopment outcomes. This, then, would also suggest that the process of intergenerational, mother-to-child transmission of the effects of maternal CM exposure may begin as early as during her child’s period of intrauterine development and involve aspects of gestational biology, in contrast to the prevailing notion that such intergenerational transmission occurs primarily during the child’s postnatal period of life (Rijlaarsdam et al., 2014; Thompson, 2007). Additionally, our finding supports the premise that not only events and conditions during gestation but also those that may have occurred prior to conception (in this case, experience of CM) may influence features of gestational biology (in this case, maternal thyroid function), which, in turn, may have important implications for fetal development and subsequent child and adult health outcomes.
The molecular basis underlying the long-term effects of CM exposure on maternal thyroid function in pregnancy is currently unknown. One possibility is a direct effect of CM on the integrity and function of the hypothalamic-pituitary-thyroid (HPT) axis via, for example, the production of stable epigenetic alterations. Exposure to abuse and neglect, specifically in interpersonal situations that may involve forced submission, is believed to induce a sense of helplessness and desperation. In this scenario, a strategy of energy conservation, withdrawal, and immobilization may be adaptive. Such a state may, in turn, be mediated by a reduction in thyroid function (Wang, 2006). In this manner, CM exposure may produce a permanent or long-lasting shift in the homeostatic set-point of the HPT axis, which may then increase susceptibility for developing thyroid dysfunction later in life.
A second possibility is that CM exposure may indirectly influence thyroid function via the long-term alterations it is known to produce on the integrity and function of the hypothalamic-pituitary-adrenal (HPA) axis (Heim et al., 2010). Since the HPA axis is strongly interconnected with the HPT axis, a dysregulation in one system likely has implications for the regulation of the other system.
A third possibility is that CM exposure may affect thyroid function via its long-term effects on immune function and inflammation. CM exposure is robustly associated with a higher pro-inflammatory milieu, e.g., higher levels of CRP, IL-6 and TNF-α; (Baumeister et al., 2016), which, in turn, is known to inhibit the synthesis and secretion of THs (Tominaga et al., 1991; Yamazaki et al., 1996). Increased concentrations of pro-inflammatory cytokines also are known to be associated with autoimmune thyroiditis (Figueroa-Vega et al., 2010; Heuer et al., 1996; Phenekos et al., 2004)–the most common cause of subclinical hypothyroidism in women of reproductive age–and also to stimulate the increased differentiation of TH17 lymphocytes in autoimmune thyroiditis (Figueroa-Vega et al., 2010), thereby accelerating its progression.
Our study has some limitations. The sample size was relatively small, which precluded us from examining the role of important CM-associated sequelae (i.e., depression) as potential moderators of the association between CM and thyroid function. In addition, only two unstimulated thyroid parameters during pregnancy were available in the present study. We suggest that future studies should include a more detailed characterization of thyroid function and examine interactions of the HPT with the HPA and immune systems, in order to further elucidate the mechanisms underlying our observed effects. Another limitation relates to the retrospective method of CM assessment, which may be subject to problems such as non-awareness (e.g. when CM occurred before the age of 3), non-disclosure, and reporting biases due to mood state. While we controlled for current depressive symptoms, which reduces the risk of reporting bias, and tried to limit the rate of false-positives by choosing a moderate cut-off score to classify exposure to CM (Bernstein and Fink, 1998b), this does not preclude the possibility of false negatives. Similarly, childhood SES was assessed retrospectively, which, again, may create vulnerability to bias particularly as a result of flawed or non-existent memory. The questions that were administered, however, probe for distinct, easy-to-remember aspects of the material and financial resources of the family (e.g. home ownership, ability to afford eating in restaurants or taking music lessons) rather than more abstract information such as the family income, which is unlikely to be remembered by a child.
5. Conclusions
In sum, our study suggests that among women, the long-term effects of stress-related events and conditions in early life may extend into pregnancy to impact her risk for thyroid dysfunction. Our findings specifically suggest that exposure to CM may represent a risk factor for the development of maternal subclinical hypothyroidism in pregnancy. More generally, our study supports the importance of adoption of a life course approach within the context of the fetal programming paradigm. Hypothyroidism is common in women of reproductive age, and maternal hypothyroidism has been associated during pregnancy with adverse neurodevelopmental outcomes in the child (Ghassabian et al., 2011; Haddow et al., 1999; Willoughby et al., 2014). Including CM as an additional factor in the risk profile that warrants screening for thyroid dysfunction may be useful to enhance the detection rate and treatment of hypothyroidism during pregnancy to improve clinical outcomes for pregnant women and their offspring.
Highlights.
Women exposed to childhood maltreatment (CM) have higher TSH levels in pregnancy.
TSH levels are also more often above the trimester-specific reference range.
fT4 levels are not reduced, indicating subclinical hypothyroidism in CM women.
Acknowledgments
This work was supported by the U.S. National Institutes of Health (R01 HD-060628 to PDW, R01 MH-105538 to PDW and CB, R01 MH-091351 to CB and PDW); NeuroCure Innovation Project to CMH; Elsa-Neumann-Stipendium des Landes Berlin to NKM; and Berlin School of Mind and Brain to NKM
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The authors report no conflicts of interest in this work.
References
- Baumeister D, Akhtar R, Ciufolini S, Pariante CM, Mondelli V. Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-alpha. Mol Psychiatr. 2016;21:642–649. doi: 10.1038/mp.2015.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DP, Fink L. Childhood Trauma Questionnaire: a retrospective self-report manual. The Psychological Corp; San Antonio, TX: 1998a. [Google Scholar]
- Bernstein DP, Fink L. Childhood Trauma Questionnaire: a retrospective self-report manual. The Psychological Corp; San Antonio, TX: 1998b. [Google Scholar]
- Braunstein GD. Endocrine Changes in Pregnancy. In: Melmed S, Polonsky KS, Larsen PR, Kronenberg HM, editors. Williams Textbook of Endocrinology. 12th. Saunders (Elsevier); Philadelphia, PA: 2011. [Google Scholar]
- Cammack AL, Buss C, Entringer S, Hogue CJ, Hobel CJ, Wadhwa PD. The association between early life adversity and bacterial vaginosis during pregnancy. Am J Obstet Gynecol. 2011;204:431 e431–438. doi: 10.1016/j.ajog.2011.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BM, Dashe JS, Wells CE, McIntire DD, Byrd W, Leveno KJ, Cunningham FG. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol. 2005;105:239–245. doi: 10.1097/01.AOG.0000152345.99421.22. [DOI] [PubMed] [Google Scholar]
- de Escobar GM, Obregon MJ, del Rey FE. Maternal thyroid hormones early in pregnancy and fetal brain development. Best Pract Res Clin Endocrinol Metab. 2004;18:225–248. doi: 10.1016/j.beem.2004.03.012. [DOI] [PubMed] [Google Scholar]
- De Groot L, Abalovich M, Alexander EK, Amino N, Barbour L, Cobin RH, Eastman CJ, Lazarus JH, Luton D, Mandel SJ, Mestman J, Rovet J, Sullivan S. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:2543–2565. doi: 10.1210/jc.2011-2803. [DOI] [PubMed] [Google Scholar]
- Dube SR, Fairweather D, Pearson WS, Felitti VJ, Anda RF, Croft JB. Cumulative childhood stress and autoimmune diseases in adults. Psychosom Med. 2009;71:243–250. doi: 10.1097/PSY.0b013e3181907888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa-Vega N, Alfonso-Perez M, Benedicto I, Sanchez-Madrid F, Gonzalez-Amaro R, Marazuela M. Increased circulating pro-inflammatory cytokines and Th17 lymphocytes in Hashimoto’s thyroiditis. J Clin Endocrinol Metab. 2010;95:953–962. doi: 10.1210/jc.2009-1719. [DOI] [PubMed] [Google Scholar]
- Ghassabian A, Bongers-Schokking JJ, Henrichs J, Jaddoe VWV, Visser TJ, Visser W, de Muinck Keizer-Schrama SMPF, Hooijkaas H, Steegers EAP, Hofman A, Verhulst FC, van der Ende J, De Rijke YB, Tiemeier H. Maternal Thyroid Function During Pregnancy and Behavioral Problems in the Offspring: The Generation R Study. Pediatr Res. 2011;69:454–459. doi: 10.1203/PDR.0b013e3182125b0c. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Rovet J, Chen Z, Koibuchi N. Developmental thyroid hormone disruption: prevalence, environmental contaminants and neurodevelopmental consequences. Neurotoxicology. 2012;33:842–852. doi: 10.1016/j.neuro.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Glinoer D, Soto MF, Bourdoux P, Lejeune B, Delange F, Lemone M, Kinthaert J, Robijn C, Grun JP, de Nayer P. Pregnancy in patients with mild thyroid abnormalities: maternal and neonatal repercussions. J Clin Endocrinol Metab. 1991;73:421–427. doi: 10.1210/jcem-73-2-421. [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Stein MB. Association between childhood trauma and physical disorders among adults in the United States. Psychol Med. 2004;34:509–520. doi: 10.1017/s003329170300134x. [DOI] [PubMed] [Google Scholar]
- Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, O’Heir CE, Mitchell ML, Hermos RJ, Waisbren SE, Faix JD, Klein RZ. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. New Engl J Med. 1999;341:549–555. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- Haviland MG, Sonne JL, Anderson DL, Nelson JC, Sheridan-Matney C, Nichols JG, Carlton EI, Murdoch WG. Thyroid hormone levels and psychological symptoms in sexually abused adolescent girls. Child Abuse Neglect. 2006;30:589–598. doi: 10.1016/j.chiabu.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Heim C, Shugart M, Craighead WE, Nemeroff CB. Neurobiological and psychiatric consequences of child abuse and neglect. Dev Psychobiol. 2010;52:671–690. doi: 10.1002/dev.20494. [DOI] [PubMed] [Google Scholar]
- Heuer M, Aust G, Ode-Hakim S, Scherbaum WA. Different Cytokine mRNA Profiles in Graves’ Disease, Hashimoto’s Thyroiditis, and Nonautoimmune Thyroid Disorders Determined by Quantitative Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) Thyroid. 1996;6:97–106. doi: 10.1089/thy.1996.6.97. [DOI] [PubMed] [Google Scholar]
- Hussey JM, Chang JJ, Kotch JB. Child maltreatment in the United States: prevalence, risk factors, and adolescent health consequences. Pediatrics. 2006;118:933–942. doi: 10.1542/peds.2005-2452. [DOI] [PubMed] [Google Scholar]
- Klein RZ, Haddow JE, Faix JD, Brown RS, Hermos RJ, Pulkkinen A, Mitchell ML. Prevalence of thyroid deficiency in pregnant women. Clin Endocrinol. 1991;35:41–46. doi: 10.1111/j.1365-2265.1991.tb03494.x. [DOI] [PubMed] [Google Scholar]
- Lazarus J, Brown RS, Daumerie C, Hubalewska-Dydejczyk A, Negro R, Vaidya B. 2014 European thyroid association guidelines for the management of subclinical hypothyroidism in pregnancy and in children. Eur Thyroid J. 2014;3:76–94. doi: 10.1159/000362597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado TD, Salum GA, Bosa VL, Goldani MZ, Meaney MJ, Agranonik M, Manfro GG, Silveira PP. Early life trauma is associated with decreased peripheral levels of thyroid-hormone T3 in adolescents. Int J Dev Neurosci. 2015;47:304–308. doi: 10.1016/j.ijdevneu.2015.10.005. Part B. [DOI] [PubMed] [Google Scholar]
- Mason SM, Tobias DK, Clark CJ, Zhang C, Hu FB, Rich-Edwards JW. Abuse in Childhood or Adolescence and Gestational Diabetes: A Retrospective Cohort Study. Am J Prev Med. 2016;50:436–444. doi: 10.1016/j.amepre.2015.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda JK, de la Osa N, Granero R, Ezpeleta L. Maternal childhood abuse, intimate partner violence, and child psychopathology: the mediator role of mothers’ mental health. Violence Against Wom. 2013;19:50–68. doi: 10.1177/1077801212475337. [DOI] [PubMed] [Google Scholar]
- Moog NK, Buss C, Entringer S, Shahbaba B, Gillen DL, Hobel CJ, Wadhwa PD. Maternal Exposure to Childhood Trauma Is Associated During Pregnancy With Placental-Fetal Stress Physiology. Biol Psychiatry. 2016;79:831–839. doi: 10.1016/j.biopsych.2015.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moog NK, Entringer S, Heim C, Wadhwa PD, Kathmann N, Buss C. Influence of maternal thyroid hormones during gestation on fetal brain development. Neuroscience. 2017;342:68–100. doi: 10.1016/j.neuroscience.2015.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakkila F, Mannisto T, Pouta A, Hartikainen AL, Ruokonen A, Surcel HM, Bloigu A, Vaarasmaki M, Jarvelin MR, Moilanen I, Suvanto E. The impact of gestational thyroid hormone concentrations on ADHD symptoms of the child. J Clin Endocrinol Metab. 2014;99:E1–8. doi: 10.1210/jc.2013-2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phenekos C, Vryonidou A, Gritzapis AD, Baxevanis CN, Goula M, Papamichail M. Th1 and Th2 serum cytokine profiles characterize patients with Hashimoto’s thyroiditis (Th1) and Graves’ disease (Th2) Neuroimmunomodulat. 2004;11:209–213. doi: 10.1159/000078438. [DOI] [PubMed] [Google Scholar]
- Plaza A, Garcia-Esteve L, Ascaso C, Navarro P, Gelabert E, Halperin I, Valdes M, Martin-Santos R. Childhood sexual abuse and hypothalamus-pituitary-thyroid axis in postpartum major depression. J Affect Disorders. 2010;122:159–163. doi: 10.1016/j.jad.2009.07.021. [DOI] [PubMed] [Google Scholar]
- Plaza A, Garcia-Esteve L, Torres A, Ascaso C, Gelabert E, Luisa Imaz M, Navarro P, Valdes M, Martin-Santos R. Childhood physical abuse as a common risk factor for depression and thyroid dysfunction in the earlier postpartum. Psychiat Res. 2012;200:329–335. doi: 10.1016/j.psychres.2012.06.032. [DOI] [PubMed] [Google Scholar]
- Radetti G, Persani L, Moroder W, Cortelazzi D, Gentili L, Beck-Peccoz P. Transplacental passage of anti-thyroid auto-antibodies in a pregnant woman with auto-immune thyroid disease. Prenatal Diag. 1999;19:468–471. [PubMed] [Google Scholar]
- Radloff The CESD scale: a self-report depression scale for research in the general population. Appl Psych Meas. 1977;1:385–401. [Google Scholar]
- Rijlaarsdam J, Stevens GW, Jansen PW, Ringoot AP, Jaddoe VW, Hofman A, Ayer L, Verhulst FC, Hudziak JJ, Tiemeier H. Maternal Childhood Maltreatment and Offspring Emotional and Behavioral Problems: Maternal and Paternal Mechanisms of Risk Transmission. Child Maltreat. 2014;19:67–78. doi: 10.1177/1077559514527639. [DOI] [PubMed] [Google Scholar]
- Seror J, Amand G, Guibourdenche J, Ceccaldi PF, Luton D. Anti-TPO antibodies diffusion through the placental barrier during pregnancy. PLoS ONE. 2014;9:e84647. doi: 10.1371/journal.pone.0084647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea AK, Streiner DL, Fleming A, Kamath MV, Broad K, Steiner M. The effect of depression, anxiety and early life trauma on the cortisol awakening response during pregnancy: preliminary results. Psychoneuroendocrinology. 2007;32:1013–1020. doi: 10.1016/j.psyneuen.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Sinai C, Hirvikoski T, Nordstrom AL, Nordstrom P, Nilsonne A, Wilczek A, Asberg M, Jokinen J. Hypothalamic pituitary thyroid axis and exposure to interpersonal violence in childhood among women with borderline personality disorder. Eur J Psychotraumatol. 2014;5 doi: 10.3402/ejpt.v5.23911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, Nixon A, Pearce EN, Soldin OP, Sullivan S, Wiersinga W, American Thyroid Association Taskforce on Thyroid Disease During P., Postpartum Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2011;21:1081–1125. doi: 10.1089/thy.2011.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangaratinam S, Tan A, Knox E, Kilby MD, Franklyn J, Coomarasamy A. Association between thyroid autoantibodies and miscarriage and preterm birth: meta-analysis of evidence. Brit Med J. 2011;342:d2616. doi: 10.1136/bmj.d2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. Mothers’ violence victimization and child behavior problems: examining the link. Am J Orthopsychiat. 2007;77:306–315. doi: 10.1037/0002-9432.77.2.306. [DOI] [PubMed] [Google Scholar]
- Tominaga T, Yamashita S, Nagayama Y, Morita S, Yokoyama N, Izumi M, Nagataki S. Interleukin 6 inhibits human thyroid peroxidase gene expression. Acta Endocrinol. 1991;124:290–294. doi: 10.1530/acta.0.1240290. [DOI] [PubMed] [Google Scholar]
- Vaidya B, Anthony S, Bilous M, Shields B, Drury J, Hutchison S, Bilous R. Detection of thyroid dysfunction in early pregnancy: universal screening or targeted high-risk case finding? J Clin Endocrinol Metab. 2007;92:203–207. doi: 10.1210/jc.2006-1748. [DOI] [PubMed] [Google Scholar]
- van den Boogaard E, Vissenberg R, Land JA, van Wely M, van der Post JAM, Goddijn M, Bisschop PH. Significance of (sub)clinical thyroid dysfunction and thyroid autoimmunity before conception and in early pregnancy: a systematic review. Hum Reprod Update. 2011;17:605–619. doi: 10.1093/humupd/dmr024. [DOI] [PubMed] [Google Scholar]
- Vulsma T, Gons MH, de Vijlder JJ. Maternal-fetal transfer of thyroxine in congenital hypothyroidism due to a total organification defect or thyroid agenesis. New Engl J Med. 1989;321:13–16. doi: 10.1056/NEJM198907063210103. [DOI] [PubMed] [Google Scholar]
- Wang S. Traumatic stress and thyroid function. Child Abuse Neglect. 2006;30:585–588. doi: 10.1016/j.chiabu.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Williams F, Watson J, Ogston S, Hume R, Willatts P, Visser T, Scottish Preterm Thyroid G Mild maternal thyroid dysfunction at delivery of infants born </=34 weeks and neurodevelopmental outcome at 5.5 years. J Clin Endocrinol Metab. 2012;97:1977–1985. doi: 10.1210/jc.2011-2451. [DOI] [PubMed] [Google Scholar]
- Williams GR. Neurodevelopmental and neurophysiological actions of thyroid hormone. J Neuroendocrinol. 2008;20:784–794. doi: 10.1111/j.1365-2826.2008.01733.x. [DOI] [PubMed] [Google Scholar]
- Willoughby KA, McAndrews MP, Rovet JF. Effects of maternal hypothyroidism on offspring hippocampus and memory. Thyroid. 2014;24:576–584. doi: 10.1089/thy.2013.0215. [DOI] [PubMed] [Google Scholar]
- Yamazaki K, Yamada E, Kanaji Y, Shizume K, Wang DS, Maruo N, Obara T, Sato K. Interleukin-6 (IL-6) inhibits thyroid function in the presence of soluble IL-6 receptor in cultured human thyroid follicles. Endocrinology. 1996;137:4857–4863. doi: 10.1210/endo.137.11.8895357. [DOI] [PubMed] [Google Scholar]


