Abstract
Polyurethane foam (PUF) in upholstered furniture frequently is treated with flame retardant chemicals (FRs) to reduce its flammability and adhere to rigorous flammability standards. For decades, a commercial mixture of polybrominated diphenyl ethers (PBDEs) called PentaBDE was commonly applied to foam to fulfill these regulations; however, concerns over toxicity, bioaccumulation, and persistence led to a global phase-out in the mid-2000s. Although PentaBDE is still detected in older furniture, other FR compounds such as tris(1,3-dichloroisopropyl) phosphate (TDCIPP) and Firemaster® 550 (FM550) have been increasingly used as replacements. While biomonitoring studies suggest exposure is widespread, the primary sources of exposure are not clearly known. Here, we investigated the relationships between specific FR applications in furniture foam and human exposure. Paired samples of furniture foam, house dust and serum samples were collected from a cohort in North Carolina, USA and analyzed for FRs typically used in PUF. In general, the presence of a specific FR in the sofa of a home was associated with an increase in the concentration of that FR in house dust. For example, the presence of PentaBDE in sofas was associated with significantly higher levels of BDE-47, a major component of PentaBDE, in house dust (10β=6.4, p<0.001). A similar association was observed with a component of FM550, 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (EH-TBB), with levels that were approximately 3 times higher in house dust when FM550 was identified in the sofa foam (p<0.01). These relationships were modified by dust loading rates in the living room and the ratio of sofa size to room size. Interestingly, levels of TDCIPP and tris(1-chloro-2-isopropyl) phosphate (TCIPP) were also higher in dust with detections in sofa foam; however, these associations were not statistically significant and may suggest there are other prominent sources of these compounds in the home. In addition, the presence of PentaBDE in sofa foam was associated with significantly higher levels of BDE-47 in serum (p<0.01). These results suggest that FR applications in sofas are likely major sources of exposure to these compounds in the home.
Keywords: Brominated flame retardants, polyurethane foam, house dust, exposure, serum biomarkers
Introduction
Flame retardants (FRs) are applied to polyurethane foam (PUF) in upholstered furniture to reduce its flammability. Implemented in 1975, the State of California’s Technical Bulletin 117 (TB 117) mandated that all upholstered furniture pass a 12-second open flame test (State of California BEARHFTI, 2000). To pass these flammability tests, FR chemicals have been added to the PUF, and sometimes other components of furniture such as textile coverings. The polybrominated diphenyl ether (PBDE) commercial mixture known as PentaBDE was a common FR used in furniture foam; however, it was phased out in the mid-2000s. In response, organophosphate FRs such as tris(1,3-dichloroisopropyl)phosphate (TDCIPP), tris(1-chloro-2-propyl)phosphate (TCIPP), and the commercial FR mixture Firemaster® 550 (FM550), which contains triphenyl phosphate (TPHP), isopropylated triaryl phosphates (ITPs), 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (EH-TBB) and bis(2-ethylhexyl)-tetrabromophthalate (BEH-TEBP), were increasingly applied as replacements (Stapleton et al., 2012b). In a recent study analyzing over one thousand foam samples collected between 2014 and 2016, TDCIPP was found to be the most common FR detected in furniture foam (Cooper et al., 2016). Despite the phase-out of PentaBDE and the recent amendment to TB 117 in 2013, which replaced the open flame test with a smolder test, flame retardants, including PentaBDE, are still detected in home furnishings and other foam-containing consumer products. This is due, in part, to slow product turnover and recycling of products themselves as well as the use of PUF in applications (Cooper et al., 2016; State of California BEARHFTI, 2013).
PBDE exposure and toxicity have been studied and characterized over the last few decades. The PBDE congeners associated with the PentaBDE mixture have been measured extensively in the environment and in human tissues, and exposure has been associated with negative impacts on thyroid hormone regulation and neurodevelopment, both in human and animal studies (Costa et al., 2014; Hale et al., 2003; Herbstman et al., 2010; Hites, 2004; Rahman et al., 2001; Zhou et al., 2002). Although less is known about other FRs, research conducted in the late 1970s suggested that TDCIPP is mutagenic, and more recently, that it may also be neurotoxic (Babich, 2006; Behl et al., 2016; Dishaw et al., 2011; Freudenthal and Henrich, 2000; Gold et al., 1978). Additionally, TDCIPP was added to California’s Proposition 65 List of Possible Carcinogens in 2011. In contrast to PBDEs, TDCIPP is rapidly metabolized and excreted. The metabolite, bis(1,3-dichloroisopropyl)phosphate (BDCIPP), has been measured ubiquitously in urine samples, suggesting widespread human exposure to TDCIPP in North America, Australia, and Europe; however, TDCIPP exposure appears to be higher in the U.S. population compared to other countries (Cequier et al., 2015b; Cooper et al., 2011; Dodson et al., 2014; Hoffman et al., 2017a; Van den Eede et al., 2015). Comparatively less is known about the toxicity of TCIPP, although it has been associated with endocrine disruption and limited neurodevelopmental changes in a few animal studies (Dishaw et al., 2014; Farhat et al., 2013). Like TDCIPP, exposure to TCIPP is also widespread. The hydroxylated metabolite of TCIPP has been ubiquitously detected in humans in several recent studies (Hammel et al., 2016; Van den Eede et al., 2015, 2013). Components of FM550 have been associated with endocrine and metabolic disruption in exposed rodents, and they have been shown to bind and activate nuclear receptors that regulate adipogenic pathways in in vitro models (Belcher et al., 2014; Fang et al., 2014; Patisaul et al., 2013; Pillai et al., 2014). Metabolites of both the organophosphate and brominated components of FM550, identified through dosed rodent studies and in vitro studies, are now frequently detected in human urine (Butt et al., 2016a, 2014; Hoffman et al., 2014; Phillips et al., 2016; Roberts et al., 2012). Notably, all of the aforementioned FR compounds have been widely detected in indoor house dust, which may serve as an important exposure pathway via hand-to-mouth activity (Bergh et al., 2011; Hoffman et al., 2015b; Stapleton et al., 2008; Van den Eede et al., 2012). In fact, several studies have found significant positive associations between PBDE levels in house dust and serum or breast milk in the US, reinforcing house dust as a primary exposure pathway (Johnson et al., 2010; Stapleton et al., 2012a; Watkins et al., 2012a; Wu et al., 2007). More recent studies have suggested that diet may play a larger role than dust for exposure to PBDEs, especially in European populations where PentaBDE was not used as pervasively in furniture as in North America. One recent study found no association between PBDEs in dust and serum but did find significant associations with various dietary items (Cequier et al., 2015a).
Despite these studies, research linking specific FR applications in products to house dust or biomarker levels is limited. Previously, portable x-ray fluorescence (XRF) measurements of bromine in products found in the home were shown to be highly correlated with Penta-BDE levels in paired house dust and serum samples (Allen et al., 2008; Imm et al., 2009). However, these portable XRF instruments are only sensitive to specific elements, such as bromine, and are not capable of differentiating between PBDEs or FM550, for example. Furthermore, XRF appears to have limited utility for chlorinated FRs (Stapleton et al., 2011). The presence of foam-containing napping equipment in early childhood education facilities also was associated with higher levels of tris(2-chloroethyl) phosphate (TCEP) and TDCIPP in dust (Bradman et al., 2014). A significant reduction in dust PBDE levels were observed with the removal of older furniture and carpets between sampling in 2006 and 2011; however, furniture was not verified to contain any specific FR chemicals, and the source of the reduction (e.g. furniture, dust-loading, or FRs in carpet padding) was not evaluated (Dodson et al., 2012). FR levels found on product surface wipes have been associated with dust levels; however, only prominent electronic products found in the home were evaluated, and most of the relationships were observed among these products and plastic casings, such as televisions and computers (Abbasi et al., 2016). In one study, counts of baby products used in the home were correlated with urinary BDCIPP levels in infants, highlighting a possible link between product use and exposure (Hoffman et al., 2015a).
In the present study, we sought to further examine specific relationships between FR application in furniture foam and human exposure. Our goal was to identify and quantify specific FR applications in PUF from study participants’ sofas in the main living area and determine how the presence or absence of a specific FR related to levels measured in house dust and residents’ serum levels. Further, we sought to determine whether characteristics of the furniture or living space modified this relationship. To our knowledge, this is the first study to compare levels of specific FRs in a consumer product, particularly levels in upholstered furniture, to a known biomarker and to house dust.
Materials and Methods
Study Design
Participants were recruited as a part of a case-control thyroid cancer study between April 2014 and January 2016. Papillary thyroid cancer patients treated at the Duke Cancer Institute and Duke University Medical Center were invited to participate in the study by their physicians, and willing participants were contacted by our study team for enrollment (n=72). Control participants (n=81) were recruited via flyers in the Duke University Medical Center facilities or randomly selected as other Duke patients undergoing routine wellness care or care for unrelated illnesses. We assume that case status does not affect concentrations of flame retardants in furniture, dust or serum; hence, we included both cases and controls in the current study. The study population is described in greater detail in Hoffman et al. (2017b). Once enrolled, study personnel visited each participant’s home to conduct questionnaires and collect environmental samples and biospecimens. The participants in the study all lived within 50 miles of Duke University and had lived in their homes for at least two years, ensuring that their current homes reflected several years of past exposure. On average, participants in this study lived in their homes approximately 11 years. All study protocols and related materials were approved by the Duke Medicine Institutional Review Board for Clinical Investigations, and all participants gave informed consent before providing any information or samples.
Sample Collection
All participants were instructed not to vacuum their homes in the two days before the visit. During each visit, the entire floor surface of main living area in the participant’s home was vacuumed using a Eureka Mighty Mite vacuum fitted with a cellulose thimble in the hose attachment for dust collection (Stapleton et al., 2012a). The thimbles containing the dust were wrapped in aluminum foil and immediately frozen at −20°C. One small piece of foam (approximately 1–3 cm3) was removed from the sofa located in the main living area, wrapped in foil, and immediately frozen. Almost all homes contained one sofa, but if other upholstered furniture (e.g. loveseats, chairs, ottomans, etc.) were present in the room, they were noted in the study log. Only PUF from the sofa, and not textile or fabric samples, were analyzed as part of this study. All participants provided non-fasting blood samples in serum-separator tubes which were centrifuged and frozen for analysis of PBDEs. Urine was not collected as part of this protocol, and thus no biomarkers of organophosphate flame retardants were measured. Although all participants were asked to provide all samples, under some circumstances a particular sample could not be collected. For example, samples could not be collected from sofas that did not have accessible foam (e.g. leather sofas with no zippers to the cushion compartment). As such, there was not complete overlap in the number of participants with each sample type. Additional information on the sample sizes is included in the supplementary material (Figure S1).
Dust Extraction
Dust samples were extracted and analyzed similar to the methods developed by Van den Eede et al. (2012). In brief, dust samples (about 100 mg) were spiked with the following internal standards: d15-tris(1,3-dichloro-2-propyl)phosphate (d15-TDCIPP; 154.8 ng), 13C-triphenyl phosphate (13C-TPHP; 100.0 ng), 13C-2-ethylhexyl-2,3,4,5-tetrabromobenzoate (13C-EH-TBB; 100.0 ng), 13C-bis(2-ethylhexyl)-tetrabromophthalate (13C-BEH-TEBP; 100.0 ng), 4-fluoro-2,3,4,6-tetrabromodiphenylether (FBDE-69; 30.0 ng), and 13C-decabromodiphenyl ether (13C-BDE209; 67.0 ng). The dust was extracted with 1:1 dichloromethane/hexane (v/v) via sonication extraction then concentrated to 1.0 mL using a nitrogen evaporator system. These extracts were purified using Florisil® solid-phase extraction cartridges (Supelclean ENVI-Florisil, 6 mL, 500 mg bed weight; Supelco), eluting the F1 fraction with 6 mL hexane (brominated compounds) and the F2 fraction with 10 mL ethyl acetate (PFRs). Each fraction was concentrated to 1 mL and then transferred to an autosampler vial for analysis by GC/MS. Recovery of the internal standards was assessed using 13C labeled 2,2’,3,4,5,5’-hexachlorodiphenyl ether (13C-CDE141; 50 ng) for FBDE-69, d9-tris(2-chloroethyl) phosphate (d9-TCEP; 227 ng) for d15-TDCIPP, and d15-triphenyl phosphate (d15-TPHP; 128 ng) for 13C-TPHP. Recoveries of FBDE-69, 13C-BDE-209, d15-TDCIPP, and 13C-TPHP were on average 109 ± 4%, 109 ± 7%, 90.6 ± 2%, and 79.6 ± 2%, respectively. Laboratory blanks and house dust standard reference materials (SRM 2585; National Institute of Standards and Technology, Gaithersburg, MD) were analyzed in each batch for quality assurance and quality control. Dust samples were analyzed in three separate batches, and MDLs were consistent across matches. Detection frequencies were determined based on the corresponding batch’s MDL, and samples were blank-corrected by batch as well. Measurements of PBDEs in SRM 2585 were very similar to their certified levels, and ranged from 73 to 111% relative to the certified values. Levels of the FM550 components (TPHP, EH-TBB, BEH-TEBP) in SRM 2585 were 60–98% of the values reported in published literature (Ali et al., 2012; Van den Eede et al., 2012, 2011).
Foam Extraction
Foam samples were extracted and analyzed as in previously described methods (Stapleton et al., 2012b). Briefly, ~50 mg of foam was accurately weighed and extracted via sonication with dichloromethane (DCM) and then filtered with a 25 mm syringe filter with a 0.2 µm PTFE membrane. A 100 µL aliquot of each extract was removed and diluted to 1 mL for an initial screening of flame retardant additives by gas chromatography/mass spectrometry (GC/MS) in full scan using both electron ionization and electron capture negative chemical ionization. Three laboratory blanks (DCM with no foam) were analyzed with each batch of samples. Significant peaks in the total ion chromatogram were compared against a custom spectral library of known FRs (Cooper et al., 2016). Based on the FRs identified in the scans, d15-TDCIPP, 13C-TPHP, 13C-EH-TBB, 13C-BEH-TEBP, and FBDE-69 were spiked into sample extracts for quantification by GC/MS. Due to the lack of standards at the time of analysis, the isopropylated triaryl phosphate (ITP) compounds were not quantified, but retention times and m/z ions were verified based on similarity with the FM550 mixture (Klosterhaus et al., 2009). The TBPP mixture (containing TPHP and mono-, di- and tri-tert-butylated phenyl phosphate esters) and MPP mixture (containing methyl phenyl phosphate esters) were similarly matched to a custom spectral library of FRs from previous characterization (Cooper et al., 2016; Stapleton et al., 2012b). It should be noted that FM550 was classified in this study by detection of EH-TBB and BEH-TEBP. Although typically associated with the ITP compounds, EH-TBB and BEH-TEBP were also observed in combination with the TBPP mixture (Cooper et al., 2016). Similar to dust, foam samples were analyzed in three separate batches, and detection frequencies were determined by each batch’s MDL. MDLs were consistent across batches and therefore an average MDL was reported.
Serum Analysis
Serum was analyzed for PBDEs and extracted according to methods described in Butt et al. (2016). Briefly, the samples were spiked with 2.5 ng of FBDE-69 and 13C-BDE-209. The samples were sonicated with 2.0 mL 0.1 M formic acid and 6.0 mL water to denature the serum proteins. Following column conditioning with 5.0 mL DCM, methanol, and water, the samples were loaded on a Waters Oasis® HLB column (500 mg bed weight, 6 mL) and washed with 5.0 mL water. PBDE analytes were eluted with 10.0 mL of 1:1 DCM/ethyl acetate (v/v), then concentrated to near dryness using a nitrogen evaporator and reconstituted in 1.0 mL hexane. These samples were further cleaned using a silica column cartridge (1 g, Waters, Sep-Pak), eluting the F1 fraction with 10.0 mL hexane for the PBDEs. The F1 fraction was concentrated to approximately 100 µL and spiked with 5.0 ng 13C-CDE-141 to assess recovery of FBDE-69. This fraction was analyzed using GC/MS in electron capture negative chemical ionization mode for 27 PBDEs. Average recovery of FBDE-69 in the samples was 65 ± 2%. PBDE masses in serum were normalized to mass serum extracted as well as total lipid content. Serum triglycerides (TG) and total cholesterol (CHOL) were measured via enzymatic techniques (LabCorps, Burlington, NC). Total lipid content (TL) in serum was estimated using the equation reported by Covaci et al. (2006). Lab blanks and serum SRM 1957 (National Institute of Standards and Technology, Gaithersburg, MD) were extracted alongside the samples for quality assurance and quality control. The serum samples were analyzed in two separate batches, and detection frequencies as well as blank-correction was performed within each batch of samples. Measurements in SRM 1957 relative to the certified values were 129% for BDE-47 and 75% for BDE-153, and the range for all PBDEs measured in the SRM relative to certified values was 71 to 141%.
Product and Living Space Characteristics
To examine how product characteristics impacted analyzed relationships in this study, sofa footprint and dust-loading were defined based on measurements taken during home visits. The sofa footprint represents the sofa size relative to the size of a room, which allowed us to account for the amount of foam present in a main living space. This was calculated by dividing the two-dimensional surface area of the sofa (length × width) by the surface area of the vacuumed main living space. Dichotomized at the median footprint, a small footprint represented sofas that took up <17.5% of the room, whereas a large footprint was indicative of a couch that took up >17.5% of the room. Dust-loading was determined by dividing the total mass of dust collected while vacuuming the main living space by the surface area of that room. Again, dichotomized at the median of 1.66 µg dust/in2, low dust-loading is representative of lower dust masses collected from the surface area of the room, whereas high dust represented large masses of dust per area vacuumed.
Statistical Analyses
All analyses were conducted using SAS statistical software (version 9.4; SAS Institute Inc., Cary, NC). Analyses were performed for analytes detected in >70% of samples. Method detection limits (MDLs) were calculated by the standard deviation of the blanks multiplied by three and divided by the average mass of sample extracted. For all concentrations below the MDL, the concentration was replaced by MDL/2 (Antweiler and Taylor, 2008; Hornung and Reed, 1990). The FR concentrations in dust, foam, and serum were all log-normally distributed. For analyses, FR detections in foam were assessed as a dichotomized variable (FR present or absent in foam) due to the smaller sample sizes associated with each FR application. Positive identification of a FR in foam was determined as a concentration of at least 1 mg FR/g foam.
Linear regression models were performed to determine if FR presence in PUF and other variables were associated with dust or serum FR concentrations (log10-transformed analyte concentrations used as outcomes). Beta coefficients were exponentiated to assist with interpreting the results and are representative of multiplicative change in the outcome relative to the reference category. Through all analyses, statistical results were assessed at a level of α=0.05 for significance.
Results and Discussion
Study Population
Overall, 153 participants were recruited for this study, and 115 provided a foam sample. Most of the study participants were female, and the mean age was 48 years. Further details about this cohort can be found in Hoffman et al., 2017b. In brief, the cases and controls were similar in race and ethnicity, household income, and health history as well as average time reported living in the address at which the home visit was conducted (mean of 11 years).
FRs in Individual Matrices
Polyurethane Foam
Of the 115 foam samples collected, about two-thirds contained at least one flame retardant. The ITP mixture, which is associated with FM550 but also may be applied in other mixtures, was the most frequently detected flame retardant application (29% of total foam samples), followed by TDCIPP and then the brominated components (EH-TBB and BEH-TEBP) in FM550 (Table 1). Although it was the most frequently detected chemical in the foam samples (44%), TPHP is not typically applied on its own as a flame retardant. To our knowledge, TPHP is always co-applied with another flame retardant mixture, such as with PentaBDE, or found with a mixture of phosphates as in the ITP mixture and the TBPP mixture. Therefore, while it is commonly detected, it is not the most frequently detected flame retardant application. The average FR levels in this study are similar to previously reported measurements in foam (Stapleton et al., 2012b). Penta-BDEs were still detected in over 15% of foam samples collected despite their voluntary phase-out in upholstered furniture occurring over a decade ago. While most of these particular sofas were reported as purchased prior to 2005, others were purchased second-hand or handed down; therefore, the original manufacturer and purchase date is unknown. Concentrations of FRs in foam reported in Table 1 are for individual FRs; however, many of these FRs are applied as mixtures. Generally, the total concentration of all FRs applied to the foam were >1% of the product by weight.
Table 1.
Detections, geometric means, and ranges (mg/g foam) for flame retardants in polyurethane foam samples (n = 115).
| Compound | Number of Detects |
% Detect of Total Samples |
Average MDL |
Geometric Mean |
Minimum | Maximum |
|---|---|---|---|---|---|---|
| EH-TBB | 20 | 17.4 | 0.227 | 8.79 | 1.97 | 15.9 |
| BEH-TEBP | 20 | 17.4 | 0.341 | 3.91 | 0.514 | 7.54 |
| ΣPentaBDE* | 18 | 15.6 | 0.055 | 3.74 | 0.941 | 35.0 |
| TCIPP | 7 | 6.08 | 0.261 | 6.32 | 0.768 | 46.0 |
| TDCIPP | 22 | 19.1 | 0.236 | 19.9 | 0.534 | 88.1 |
| TPHP | 51 | 44.3 | 0.124 | 0.983 | 0.0013 | 15.0 |
| ITP Compounds# | 33 | 28.7 | n.a. | n.a. | n.a. | n.a. |
| TBPP Compounds% | 14 | 12.2 | n.a. | n.a. | n.a. | n.a. |
| MPP Mixture& | 4 | 3.47 | n.a. | n.a. | n.a. | n.a. |
| No FR | 37 | 32.2 | n.a. | n.a. | n.a. | n.a. |
ΣPenta-BDEs includes BDE-17, 28, 47, 49, 66, 85, 99, 100, 153, 154, and 155.
ITP compounds refer to a mixture of isopropylated triaryl phosphate esters.
TBPP compounds refer to a mixture of tert-butylated phenyl phosphate esters.
MPP Mixture refers to a mix of methyl phenyl phosphate esters.
Serum
Serum samples were analyzed for a suite of PBDE compounds but only BDE-47 and BDE-153 were detected in >70% of the samples and are the only congeners analyzed in this study for associations with FR applications in foam (other congeners were detected in under 50% of samples) (Table 2). More information on the serum PBDE measurements can be found in Hoffman et al. 2017b. In general, the levels of these two compounds were lower than previously reported among U.S. adults, which likely reflects a decline in overall exposure to Penta-BDEs following their phase-out (Buttke et al., 2013; Sjödin et al., 2008). While such a decrease is expected with a now decade-old global phase-out of Penta-BDEs, detection of these two BDEs in serum suggests continued exposure to these compounds.
Table 2.
Geometric means and selected percentiles for FRS in serum (n = 135) and house dust (n = 137).
| Percentile | |||||||
|---|---|---|---|---|---|---|---|
| Matrix and Compound | Percent Detect |
Average MDL |
Geometric Mean |
25th | 50th | 75th | Maximum |
| Serum (ng/g lipid) | |||||||
| BDE-47 | 77.8 | 1.321 | 7.855 | 3.852 | 8.891 | 16.01 | 144.2 |
| BDE-153 | 94.8 | 0.5490 | 4.541 | 2.386 | 4.371 | 8.576 | 143.2 |
| Dust (ng/g dust) | |||||||
| EH-TBB | 94.2 | 3.833 | 239.4 | 82.86 | 188.4 | 754.4 | 16,320 |
| BEH-TEBP | 81.8 | 15.60 | 117.1 | 44.67 | 160.4 | 420.6 | 11,190 |
| TCIPP | 94.2 | 90.90 | 2,141 | 823.5 | 2,118 | 6,350 | 66,210 |
| TDCIPP | 92.0 | 56.97 | 1,384 | 640.7 | 1,220 | 3,269 | 59,490 |
| TPHP | 95.6 | 5.000 | 1,409 | 475.5 | 1,345 | 4,049 | 111,300 |
| PBDEs | |||||||
| BDE-47 | 92.0 | 78.50 | 222.2 | 71.65 | 202.2 | 518.5 | 17,010 |
| BDE-99 | 96.4 | 74.30 | 346.1 | 100.3 | 310.4 | 789.7 | 32,820 |
| BDE-100 | 70.1 | 20.70 | 62.51 | 18.35 | 44.38 | 155.0 | 6,396 |
| BDE-153 | 87.6 | 10.37 | 43.99 | 11.90 | 34.49 | 124.5 | 4,488 |
| BDE-154 | 81.8 | 8.100 | 31.88 | 9.067 | 27.27 | 77.79 | 3,657 |
| BDE-209 | 93.4 | 20.13 | 524.1 | 184.2 | 536.9 | 1198 | 28,490 |
House Dust
Of the FRs quantified in foam, all were detected in 70% or more of the dust samples (Table 2). Overall, the geometric means of organophosphate FRs in dust were at least an order of magnitude greater than the brominated compounds, which suggests greater exposure potential for organophosphate compounds via dust ingestion. The most abundant organophosphate FR in dust was TCIPP, which follows a trend observed in the U.S. and parts of Europe and Asia (Araki et al., 2014; Brommer and Harrad, 2015; Cequier et al., 2014; Dodson et al., 2014; Stapleton et al., 2014). Although PBDEs have been largely phased out, detections and concentrations of the more widely used PBDEs (BDE-47 and BDE-99 in PentaBDE and BDE-209 of the DecaBDE mixture) in dust were similar to the levels of the other brominated compounds, EH-TBB and BEH-TEBP. This suggests that despite decreased use, house dust serves as a continued repository for PBDEs, and sources of PBDEs may still be present in homes.
Comparing PBDEs in Foam and Serum
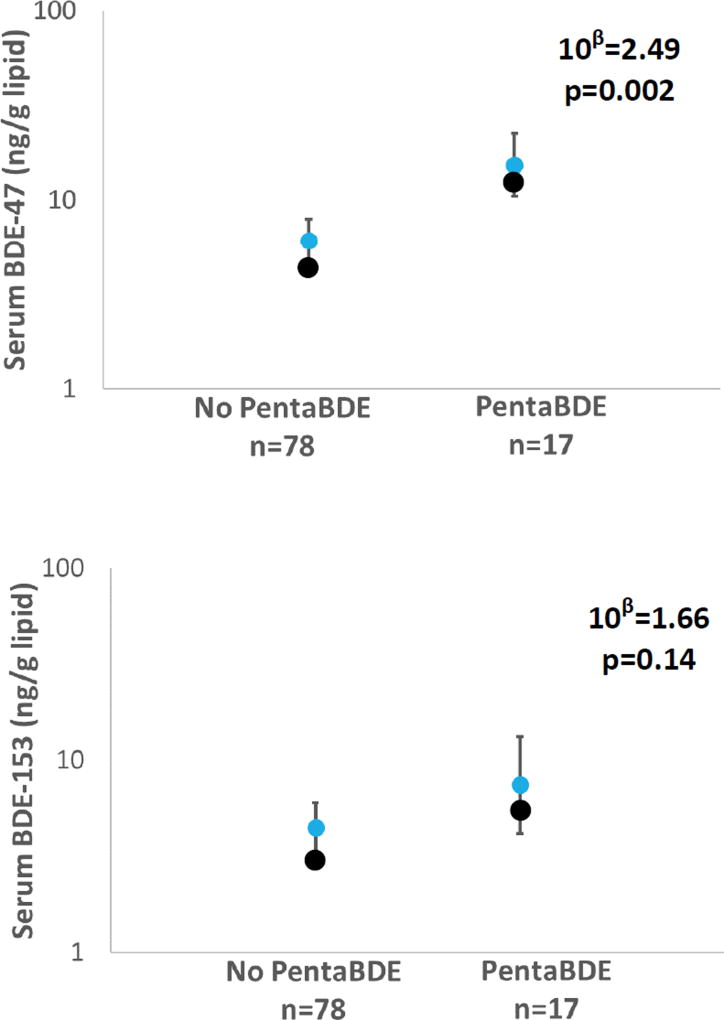
Levels of serum BDE-47 and BDE-153 were greater in participants who lived in a home with a sofa containing Penta-BDEs (Figure 1). Serum BDE-47 levels were 2.5 times as high in these participants compared to those whose sofa did not contain Penta-BDE (95% CI: 1.4, 4.5; p<0.01). Although not statistically significant, a similar pattern was observed for serum BDE-153 levels, with BDE-153 in serum being about 1.7 times as high if the participants had a Penta-BDE-containing sofa (95% CI: 0.84, 3.3; p=0.14). The lack of a significant association between BDE-153 in serum and Penta-BDE in foam may be due to a difference in the half-lives of BDE-153 compared to BDE-47, or differences in exposure. Additionally, diet may be a greater source of exposure for BDE-153 in the present day than it did prior to the global phase-out due to its persistence in the environment. BDE-47 has been estimated to have a half-life of 1.8 years, while BDE-153’s half-life has been estimated to be 6.5 years (Geyer et al., 2004). Therefore, BDE-153 measured in serum would likely reflect longer-term average exposures from either past furniture containing Penta-BDE or other sources present in the last several years of the participants’ exposure, which could include diet or other microenvironments (Watkins et al., 2012b). Additionally, the relationship between Penta-BDE in sofa foam and BDE-47 in serum was slightly stronger if individuals spent an average of 13 hours or more in their homes per day (10β=2.8, p=0.01) compared to people who did not have Penta-BDE in their sofas. Furthermore, BDE-47 in dust was significantly correlated with paired BDE-47 serum levels in this cohort (rs=0.35, p=0.004) but a similar relationship between dust and serum was not observed for BDE-153 (Hoffman et al., 2017b). Past studies have shown significant associations between serum and house dust for certain PBDEs (rs=0.8 for BDE-47 in adults, r=0.3 for a summed BDE-47, −99, and −100 in toddlers; p<0.01), but not for BDE-153 (Johnson et al., 2010; Stapleton et al., 2012a). To our knowledge, serum PBDE levels have not been previously linked to a specific application in a product. The significant association between foam Penta-BDE and serum BDE-47 suggests that these pieces of upholstered furniture serve as major sources of BDE-47 in humans. Because there are no other reliable serum biomarkers for the additional FRs investigated here, only relationships with PentaBDE were investigated with serum.
Figure 1.
Geometric means and 95% confidence intervals of serum PBDE levels based on presence or absence of PentaBDE in paired foam. Absence of PentaBDE in foam was the reference category for determining the magnitude of effect on serum BDE-47 and BDE-153, which was determined by linear regression.
FRs in Foam and Dust
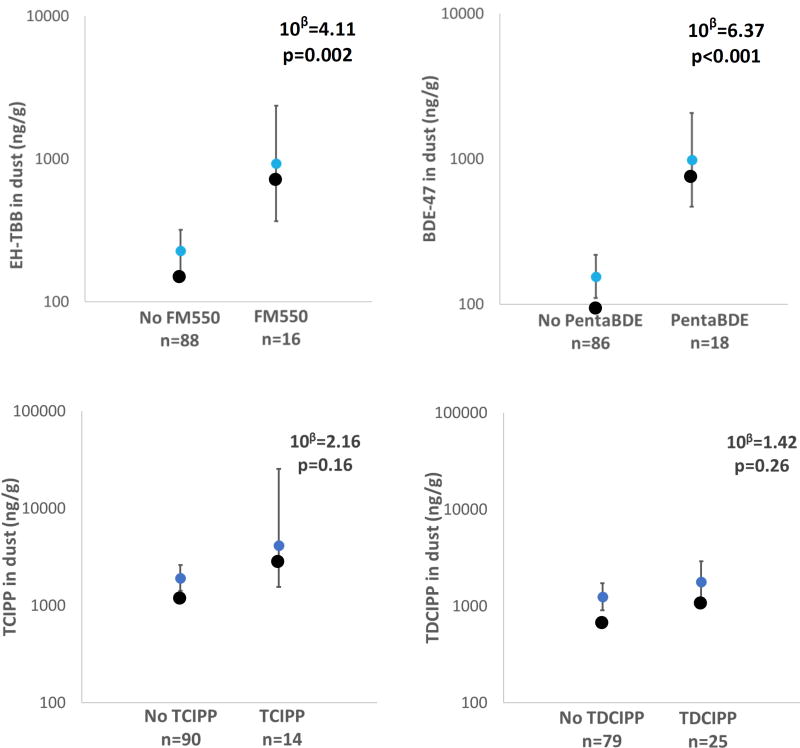
The presence of FM550 and Penta-BDEs in the sofa foam were significantly associated with higher levels of EH-TBB and BDE-47 in dust, respectively (Figure 2). The EH-TBB and BDE-47 dust levels were between 4 to 6.5 times as high when a sofa containing that specific FR was in the same living area (FM550 95% CI: 1.7, 9.8; PentaBDE 95% CI: 2.8, 14.3; p<0.01). While sofas may not be the sole source of FRs, associations indicate that foam-containing furniture contributes heavily to house dust and serves as a major source of brominated FRs in house dust. This implies that having a sofa that does not contain either of these brominated FR mixtures could potentially decrease a person’s exposure to these compounds via decreases in FR levels in dust. The magnitude of effect between Penta-BDE detection in foam and BDE-47 dust levels was much greater than the effect with BDE-47 in serum (6.5 times in dust compared to 2.5 in serum), which suggests that the relationship observed between foam and serum is attenuated, perhaps by the time spent in other micro-environments (e.g. home, work), handwashing, and/or dietary sources, even if the sofa may serve as a major source of exposure (Watkins et al., 2012b). Relationships between dust levels of other PBDEs (BDE-99, −100, −153, −154) were evaluated with Penta-BDE in foam and were found to be significantly associated (10β=3.2–3.9, p<0.01) with one exception being BDE-153 (10β=2.2; p=0.07). Dust levels of BEH-TEBP were also assessed with FM550 presence in foam and were found to be statistically significant (10β=3.8, 95% CI: 1.3, 11.2, p<0.01). Because BDE-47 and EH-TBB showed the strongest relationship with their respective applications in foam, and BEH-TEBP may be applied in other commercial mixtures in the home, the results with BDE-47 and EH-TBB are presented in the figures and further evaluated with other characteristics.
Figure 2.
Geometric means and 95% confidence intervals of dust concentrations based on the corresponding FR’s presence or absence in paired foam. The magnitude of increase in outcome and associated p-value with the FR foam presence compared to absence (reference category) are displayed in the top right of each panel, which was assessed by linear regression.
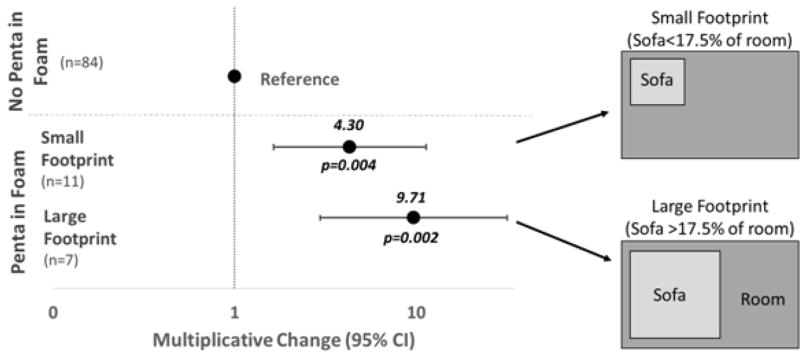
The relationships between foam and dust were investigated further based on various product characteristics and living space traits. When the Penta-BDE relationship between foam and dust was assessed based on the sofa footprint, levels of BDE-47 in dust were found to be 9.7 times as high when the sofa had a large footprint compared to only 4.3 times as high with a small footprint (large footprint 95% CI: 3.0, 31.8; small footprint 95% CI: 1.6, 11.3; p<0.01; Figure 3), relative to dust levels in homes without a Penta-BDE sofa. A similar trend was observed with FM550 and EH-TBB in dust (large footprint 10β=4.5, 95% CI: 1.5, 13.3; small footprint 10β=7.6, 95% CI: 1.5, 39.8; p<0.05), although the sample sizes for each category were small, resulting in overlapping confidence intervals in the small and large footprint categories. Overall, the trend suggests that sofas that take up more of the surface area of the room contribute to higher levels of these brominated FRs in dust.
Figure 3.
Multiplicative change and 95% confidence intervals of BDE-47 in dust depending on the presence of PentaBDE in paired foam and the size of the sofa relative to the vacuumed room, designated as a footprint. The sofa footprint was dichotomized at the median percent of the room covered by the sofa (17.5%).
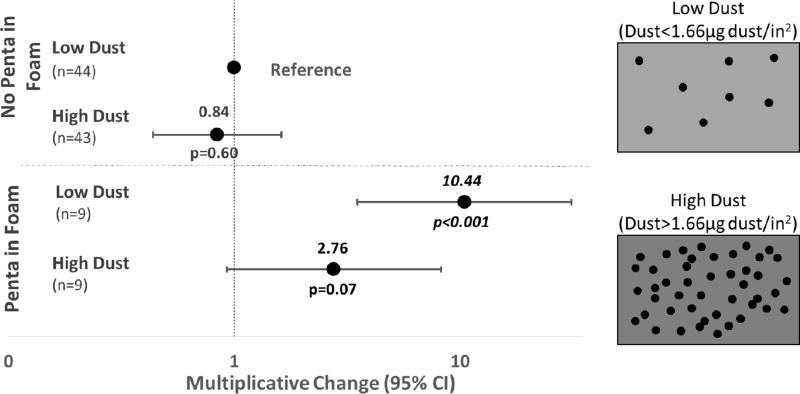
Dust-loading of the vacuumed room was also examined as a potential modifying factor in the foam-dust relationship. When compared to the reference category (low dust mass loadings and no Penta-BDE in foam), a significant relationship between Penta-BDE in foam and BDE-47 levels in dust was observed with low dust-loading (10β=10.4; 95% CI: 3.5, 31.1; p<0.0001; Figure 4). A significant relationship was not observed with high dust-loading levels, although the results were suggestive of a trend (10β=2.8; 95% CI: 0.9, 8.2; p=0.07). Again, the same trend was observed with FM550 in foam and EH-TBB in dust (low dust 10β=6.5; 95% CI: 1.8, 22.8; high dust 10β=4.3; 95% CI: 1.2, 15.1; p<0.05), but sample sizes were not sufficiently large, resulting in overlapping confidence intervals. This finding may suggest that a dilution effect is occurring, whereby the brominated compounds are diluted in a room that accumulates more soil or dust particles (leading to higher dust mass in a room). Therefore, dust-loading may be an important variable to consider when assessing relationships between product use and house dust levels, as it could modify the observed relationships and lead to incorrect estimates.
Figure 4.
Multiplicative change and 95% confidence intervals of BDE-47 in dust depending on the presence of PentaBDE in paired foam and dust-loading of the vacuumed room. Dust-loading was dichotomized at the median dust mass per area vacuumed, which was 1.66 µg/in2.
As for the organophosphate FRs, higher levels of TCIPP and TDCIPP in dust were observed when detected in the sofa foam; however, the relationships were not statistically significant (10β=1.6–2.5, p>0.1; Figure 2). Interestingly, when TCIPP and TDCIPP were not detected in the sofa foam, the GM dust levels of these two organophosphate compounds were an order of magnitude greater than EH-TBB and BDE-47. This suggests that product sources other than sofas in the main living area may be contributing to the dust concentrations of these two organophosphate FRs, or TCIPP and TDCIPP may have different migration behaviors than the brominated compounds from foam to dust due to physicochemical properties. However, the upholstered furniture seems to be a major source of FM550 and Penta-BDE in the home. TDCIPP and TCIPP relationships between foam and dust were assessed based on footprint and dust-loading but no associations were observed, further suggesting that sofa foam assessed in this study may not be the main source of these organophosphates in dust. For instance, TCIPP is frequently used in thermal insulation, and both of these organophosphates could be used in a variety of applications in textiles (Andresen et al., 2004; Ionas et al., 2015; van der Veen and de Boer, 2012).
Limitations
Although participants in this study were recruited for the purpose of a case-control study to examine thyroid cancer, this should not bear any effects on this particular study since case status should not impact foam, dust, or serum levels of FRs. As shown in Hoffman et al. 2017b, there was no significant difference in serum BDE-47 or BDE-153 levels by case status. Still, our results should be interpreted in the context of a few limitations. First, one foam sample was assessed per household for associations with house dust; this does not account for the possible contribution of other upholstered furniture in the room or adjacent rooms. Due to different applications of FRs in foam, the sample sizes of foam samples containing a specific FR were relatively small compared to those that did not. As a result, relationships between foam and dust were assessed based on detection (>1 mg/g foam) rather than on a continuous basis. Detailed behavioral information such as how much time an individual spent in the main living area or on the sampled sofa and frequency of handwashing could provide additional insights, as these factors could modify the relationship between foam and human exposure. Future studies including analyses with urine measurements from participants may also be helpful to determine how organophosphates assessed in foam relate to biomarkers for exposure.
Conclusion
Our results indicate that foam in sofas serves as an important source of flame retardants for exposure in humans, suggesting that removal of FRs from the foam or furniture pieces in the home could indeed reduce human exposure to these compounds. FR compounds were measured in foam samples and evaluated for their associations with paired serum and house dust samples. Significantly higher levels of serum BDE-47 were observed when the participant had a sofa containing Penta-BDE in the main living area with a similar but non-significant relationship observed with serum BDE-153. Detections of FM550 and Penta-BDE in foam were also indicative of significantly greater levels of EH-TBB and BDE-47 in dust, suggesting that foam in upholstered furniture containing these brominated mixtures serves as a major source of these compounds in the home environment. These relationships were strengthened with larger furniture pieces per area vacuumed (sofa footprint) and with lower dust-loading. Foam-dust relationships were not significant for TDCIPP or TCIPP, suggesting that other sources of these two compounds may be contributing to the levels measured in house dust or the compounds migrate from foam to dust in a manner different from the brominated compounds.
Supplementary Material
Highlights.
-
-
PentaBDE and FM550 in foam is associated with higher levels in dust.
-
-
Foam-dust associations were not observed for TDCIPP and TCIPP.
-
-
Sofa footprint in the living area and dust loading rates modified associations.
-
-
PentaBDE in foam was significantly associated with higher BDE-47 in serum.
Acknowledgments
Funding for this research was provided by Fred & Alice Stanback through a research grant to the Duke Cancer Institute. Additional support for the authors was provided by grants from the Environmental Protection Agency (Grant 83564201) and NIEHS (R01 ES016099). We thank Kristen Lynam and Marlo Evans for their efforts in recruiting participants and coordinating study-related activities. We also gratefully acknowledge the samples provided by our research participants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Notes
JAS is a member of the Data Monitoring Committee of the Medullary Thyroid Cancer Consortium Registry supported by Novo Nordisk, GlaxoSmithKline, Astra Zeneca, and Eli Lilly. SCH, KH, AML, AC, ALP, CMB, TFW, and HMS declare no competing financial interest.
References
- Abbasi G, Saini A, Goosey E, Diamond ML. Product screening for sources of halogenated flame retardants in Canadian house and office dust. Sci. Total Environ. 2016;545–546:299–307. doi: 10.1016/j.scitotenv.2015.12.028. [DOI] [PubMed] [Google Scholar]
- Ali N, Dirtu AC, Van den Eede N, Goosey E, Harrad S, Neels H, ‘t Mannetje A, Coakley J, Douwes J, Covaci A. Occurrence of alternative flame retardants in indoor dust from New Zealand: indoor sources and human exposure assessment. Chemosphere. 2012;88:1276–82. doi: 10.1016/j.chemosphere.2012.03.100. [DOI] [PubMed] [Google Scholar]
- Allen JG, McClean MD, Stapleton HM, Webster TF. Linking PBDEs in House Dust to Consumer Products using X-ray Fluorescence. Environ. Sci. Technol. 2008;42:4222–4228. doi: 10.1021/es702964a. [DOI] [PubMed] [Google Scholar]
- Andresen JA, Grundmann A, Bester K. Organophosphorus flame retardants and plasticisers in surface waters. Sci. Total Environ. 2004;332:155–166. doi: 10.1016/j.scitotenv.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Antweiler RC, Taylor HE. Evaluation of Statistical Treatments of Left-Censored Environmental Data using Coincident Uncensored Data Sets: I. Summary Statistics. Environ. Sci. Technol. 2008;42:3732–3738. doi: 10.1021/es071301c. [DOI] [PubMed] [Google Scholar]
- Araki A, Saito I, Kanazawa A, Morimoto K, Nakayama K, Shibata E, Tanaka M, Takigawa T, Yoshimura T, Chikara H, Saijo Y, Kishi R. Phosphorus flame retardants in indoor dust and their relation to asthma and allergies of inhabitants. Indoor Air. 2014;24:3–15. doi: 10.1111/ina.12054. [DOI] [PubMed] [Google Scholar]
- Babich MA. CPSC Staff Preliminary Risk Assessment of Flame Retardant FR Chemicals in Upholstered Furniture Foam. Bethesda, MD: 2006. [Google Scholar]
- Behl M, Rice JR, Smith MV, Co CA, Bridge MF, Hsieh J-H, Freedman JH, Boyd WA. Comparative Toxicity of Organophosphate Flame Retardants and Polybrominated Diphenyl Ethers to Caenorhabditis elegans. Toxicol. Sci. 2016;154:241–252. doi: 10.1093/toxsci/kfw162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcher SM, Cookman CJ, Patisaul HB, Stapleton HM. In vitro assessment of human nuclear hormone receptor activity and cytotoxicity of the flame retardant mixture FM 550 and its triarylphosphate and brominated components. Toxicol. Lett. 2014;228:93–102. doi: 10.1016/j.toxlet.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergh C, Torgrip R, Emenius G, Ostman C. Organophosphate and phthalate esters in air and settled dust - a multi-location indoor study. Indoor Air. 2011;21:67–76. doi: 10.1111/j.1600-0668.2010.00684.x. [DOI] [PubMed] [Google Scholar]
- Bradman A, Castorina R, Gaspar F, Nishioka M, Colón M, Weathers W, Egeghy PP, Maddalena R, Williams J, Jenkins PL, McKone TE. Flame retardant exposures in California early childhood education environments. Chemosphere. 2014;116:61–66. doi: 10.1016/j.chemosphere.2014.02.072. [DOI] [PubMed] [Google Scholar]
- Brommer S, Harrad S. Sources and human exposure implications of concentrations of organophosphate flame retardants in dust from UK cars, classrooms, living rooms, and offices. Environ. Int. 2015;83:202–207. doi: 10.1016/j.envint.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Butt CM, Congleton J, Hoffman K, Fang M, Stapleton HM. Metabolites of organophosphate flame retardants and 2-ethylhexyl tetrabromobenzoate in urine from paired mothers and toddlers. Environ. Sci. Technol. 2014;48:10432–8. doi: 10.1021/es5025299. [DOI] [PubMed] [Google Scholar]
- Butt CM, Hoffman K, Chen A, Lorenzo A, Congleton J, Stapleton HM. Regional comparison of organophosphate flame retardant (PFR) urinary metabolites and tetrabromobenzoic acid (TBBA) in mother-toddler pairs from California and New Jersey. Environ. Int. 2016a;94:627–634. doi: 10.1016/j.envint.2016.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt CM, Miranda ML, Stapleton HM. Development of an analytical method to quantify PBDEs, OH-BDEs, HBCDs, 2,4,6-TBP, EH-TBB, and BEH-TEBP in human serum. Anal. Bioanal. Chem. 2016b;408:2449–2459. doi: 10.1007/s00216-016-9340-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttke DE, Wolkin A, Stapleton HM, Miranda ML. Associations between serum levels of polybrominated diphenyl ether (PBDE) flame retardants and environmental and behavioral factors in pregnant women. J. Expo. Sci. Environ. Epidemiol. 2013;23:176–82. doi: 10.1038/jes.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cequier E, Ionas AC, Covaci A, Marcé RM, Becher G, Thomsen C. Occurrence of a Broad Range of Legacy and Emerging Flame Retardants in Indoor Environments in Norway. Environ. Sci. Technol. 2014;48:6827–6835. doi: 10.1021/es500516u. [DOI] [PubMed] [Google Scholar]
- Cequier E, Marce RM, Becher G, Thomsen C. Comparing human exposure to emerging and legacy flame retardants from the indoor environment and diet with concentrations measured in serum. Environ. Int. 2015a;74:54–59. doi: 10.1016/j.envint.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Cequier E, Sakhi AK, Marcé RM, Becher G, Thomsen C. Human exposure pathways to organophosphate triesters — A biomonitoring study of mother-child pairs. Environ. Int. 2015b;75:159–165. doi: 10.1016/j.envint.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Cooper EM, Covaci A, van Nuijs ALN, Webster TF, Stapleton HM. Analysis of the flame retardant metabolites bis(1,3-dichloro-2-propyl) phosphate (BDCPP) and diphenyl phosphate (DPP) in urine using liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2011;401:2123–32. doi: 10.1007/s00216-011-5294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper EM, Kroeger G, Davis K, Clark CR, Ferguson PL, Stapleton HM. Results from Screening Polyurethane Foam Based Consumer Products for Flame Retardant Chemicals: Assessing Impacts on the Change in the Furniture Flammability Standards. Environ. Sci. Technol. 2016;50:10653–10660. doi: 10.1021/acs.est.6b01602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, de Laat R, Tagliaferri S, Pellacani C. A mechanistic view of polybrominated diphenyl ether (PBDE) developmental neurotoxicity. Toxicol. Lett. 2014;230:282–294. doi: 10.1016/j.toxlet.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covaci A, Voorspoels S, Thomsen C, van Bavel B, Neels H. Evaluation of total lipids using enzymatic methods for the normalization of persistent organic pollutant levels in serum. Sci. Total Environ. 2006;366:361–366. doi: 10.1016/j.scitotenv.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Dishaw LV, Hunter DL, Padnos B, Padilla S, Stapleton HM. Developmental exposure to organophosphate flame retardants elicits overt toxicity and alters behavior in early life stage zebrafish (Danio rerio) Toxicol. Sci. 2014;142:445–54. doi: 10.1093/toxsci/kfu194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishaw LV, Powers CM, Ryde IT, Roberts SC, Seidler FJ, Slotkin Ta, Stapleton HM. Is the PentaBDE replacement, tris (1,3-dichloro-2-propyl) phosphate (TDCPP), a developmental neurotoxicant? Studies in PC12 cells. Toxicol. Appl. Pharmacol. 2011;256:281–9. doi: 10.1016/j.taap.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson RE, Perovich LJ, Covaci A, Van den Eede N, Ionas AC, Dirtu AC, Brody JG, Rudel RA. After the PBDE Phase-Out: A Broad Suite of Flame Retardants in Repeat House Dust Samples from California. Environ. Sci. Technol. 2012;46:13056–13066. doi: 10.1021/es303879n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson RE, Van den Eede N, Covaci A, Perovich LJ, Brody JG, Rudel RA. Urinary Biomonitoring of Phosphate Flame Retardants: Levels in California Adults and Recommendations for Future Studies. Environ. Sci. Technol. 2014;48:13625–13633. doi: 10.1021/es503445c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M, Webster TF, Ferguson PL, Stapleton HM. Characterizing the Peroxisome Proliferator–Activated Receptor (PPARγ) Ligand Binding Potential of Several Major Flame Retardants, Their Metabolites, and Chemical Mixtures in House Dust. Environ. Health Perspect. 2014 doi: 10.1289/ehp.1408522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhat A, Crump D, Chiu S, Williams KL, Letcher RJ, Gauthier LT, Kennedy SW. In Ovo Effects of Two Organophosphate Flame Retardants--TCPP and TDCPP--on Pipping Success, Development, mRNA Expression, and Thyroid Hormone Levels in Chicken Embryos. Toxicol. Sci. 2013;134:92–102. doi: 10.1093/toxsci/kft100. [DOI] [PubMed] [Google Scholar]
- Freudenthal RI, Henrich RT. Chronic Toxicity and Carcinogenic Potential of Tris-(1,3-Dichloro-2-propyl) Phosphate in Sprague-Dawley Rat. Int. J. Toxicol. 2000;19:119–125. [Google Scholar]
- Geyer HJ, Schramm K-W, Darnerud PO, Aune M, Feicht EA, Fried KW, Henkelmann B, Lenoir D, Schmid P, McDonald T. Terminal elimination half-lives of the brominated flame retardants TBBPA, HBCD, and lower brominated PBDEs in humans. Organohalogen Compd. 2004;66:3820–3825. [Google Scholar]
- Gold MD, Blum A, Ames BN. Another flame retardant, tris-(1,3-dichloro-2-propyl)-phosphate, and its expected metabolites are mutagens. Science (80−.) 1978;200:785–7. doi: 10.1126/science.347576. [DOI] [PubMed] [Google Scholar]
- Hale RC, Alaee M, Manchester-Neesvig JB, Stapleton HM, Ikonomou MG. Polybrominated diphenyl ether flame retardants in the North American environment. Environ. Int. 2003;29:771–9. doi: 10.1016/S0160-4120(03)00113-2. [DOI] [PubMed] [Google Scholar]
- Hammel SC, Hoffman K, Webster TF, Anderson KA, Stapleton HM. Measuring Personal Exposure to Organophosphate Flame Retardants Using Silicone Wristbands and Hand Wipes. Environ. Sci. Technol. 2016;50:4483–4491. doi: 10.1021/acs.est.6b00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbstman JB, Sjödin A, Kurzon M, Lederman SA, Jones RS, Rauh V, Needham LL, Tang D, Niedzwiecki M, Wang RY, Perera F. Prenatal Exposure to PBDEs and Neurodevelopment. Environ. Health Perspect. 2010;118:712–719. doi: 10.1289/ehp.0901340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hites RA. Polybrominated Diphenyl Ethers in the Environment and in People: A Meta-Analysis of Concentrations. Environ. Sci. Technol. 2004;38:945–956. doi: 10.1021/es035082g. [DOI] [PubMed] [Google Scholar]
- Hoffman K, Butt CM, Chen A, Limkakeng AT, Stapleton HM. High Exposure to Organophosphate Flame Retardants in Infants: Associations with Baby Products. Environ. Sci. Technol. 2015a;49:14554–14559. doi: 10.1021/acs.est.5b03577. [DOI] [PubMed] [Google Scholar]
- Hoffman K, Butt CM, Webster TF, Preston EV, Hammel SC, Makey C, Lorenzo AM, Cooper EM, Carignan C, Meeker JD, Hauser R, Soubry A, Murphy SK, Price TM, Hoyo C, Mendelsohn E, Congleton J, Daniels JL, Stapleton HM. Temporal Trends in Exposure to Organophosphate Flame Retardants in the United States. Environ. Sci. Technol. Lett. 2017a doi: 10.1021/acs.estlett.6b00475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, Fang M, Horman B, Patisaul HB, Garantziotis S, Birnbaum LS, Stapleton HM. Urinary tetrabromobenzoic acid (TBBA) as a biomarker of exposure to the flame retardant mixture Firemaster® 550. Environ. Health Perspect. 2014;122:963–9. doi: 10.1289/ehp.1308028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, Garantziotis S, Birnbaum LS, Stapleton HM. Monitoring indoor exposure to organophosphate flame retardants: hand wipes and house dust. Environ. Health Perspect. 2015b;123:160–5. doi: 10.1289/ehp.1408669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, Lorenzo A, Butt CM, Hammel SC, Henderson BB, Roman SA, Scheri RP, Stapleton HM, Sosa JA. Exposure to Flame Retardant Chemicals and the Occurrence and Severity of Papillary Thyroid Cancer: A Case-Control Study. Environ. Int. Accepted. 2017b doi: 10.1016/j.envint.2017.06.021. [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. Estimation of Average Concentration in the Presence of Nondetectable Values. Appl. Occup. Environ. Hyg. 1990;5:46–51. doi: 10.1080/1047322X.1990.10389587. [DOI] [Google Scholar]
- Imm P, Knobeloch L, Buelow C, Anderson HA. Household Exposures to Polybrominated Diphenyl Ethers (PBDEs) in a Wisconsin Cohort. Environ. Health Perspect. 2009;117:1890–1895. doi: 10.1289/ehp.0900839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionas AC, Ballesteros Gómez A, Uchida N, Suzuki G, Kajiwara N, Takata K, Takigami H, Leonards PE, Covaci A. Comprehensive characterisation of flame retardants in textile furnishings by ambient high resolution mass spectrometry, gas chromatography-mass spectrometry and environmental forensic microscopy. Environ. Res. 2015;142:712–719. doi: 10.1016/j.envres.2015.09.012. [DOI] [PubMed] [Google Scholar]
- Johnson PI, Stapleton HM, Sjodin A, Meeker JD. Relationships between Polybrominated Diphenyl Ether Concentrations in House Dust and Serum. Environ. Sci. Technol. 2010;44:5627–5632. doi: 10.1021/es100697q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klosterhaus S, Konstantinov A, Davis E, Klein J. Characterization of organophosphorus chemicals in a PentaBDE replacement mixture and their detection in biosolids 2009 [Google Scholar]
- Patisaul HB, Roberts SC, Mabrey N, McCaffrey KA, Gear RB, Braun J, Belcher SM, Stapleton HM. Accumulation and endocrine disrupting effects of the flame retardant mixture Firemaster® 550 in rats: an exploratory assessment. J. Biochem. Mol. Toxicol. 2013;27:124–36. doi: 10.1002/jbt.21439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AL, Chen A, Rock KD, Horman B, Patisaul HB, Stapleton HM. Transplacental and Lactational Transfer of Firemaster® 550 Components in Dosed Wistar Rats. Toxicol. Sci. 2016;153:246–257. doi: 10.1093/toxsci/kfw122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai HK, Fang M, Beglov D, Kozakov D, Vajda S, Stapleton HM, Webster TF, Schlezinger JJ. Ligand Binding and Activation of PPARγ by Firemaster® 550: Effects on Adipogenesis and Osteogenesis in Vitro. Environ. Health Perspect. 2014 doi: 10.1289/ehp.1408111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman F, Langford KH, Scrimshaw MD, Lester JN. Polybrominated diphenyl ether (PBDE) flame retardants. Sci. Total Environ. 2001;275:1–17. doi: 10.1016/s0048-9697(01)00852-x. [DOI] [PubMed] [Google Scholar]
- Roberts SC, Macaulay LJ, Stapleton HM. In Vitro Metabolism of the Brominated Flame Retardants 2-Ethylhexyl-2,3,4,5-Tetrabromobenzoate (TBB) and Bis(2-ethylhexyl) 2,3,4,5-Tetrabromophthalate (TBPH) in Human and Rat Tissues. Chem. Res. Toxicol. 2012;25:1435–1441. doi: 10.1021/tx300086x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjödin A, Wong L-Y, Jones RS, Park A, Zhang Y, Hodge C, Dipietro E, McClure C, Turner W, Needham LL, Patterson DG. Serum concentrations of polybrominated diphenyl ethers (PBDEs) and polybrominated biphenyl (PBB) in the United States population: 2003–2004. Environ. Sci. Technol. 2008;42:1377–84. doi: 10.1021/es702451p. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Allen JG, Kelly SM, Konstantinov A, Klosterhaus S, Watkins D, Mcclean MD, Webster TF. Alternate and new brominated flame retardants detected in U.S. house dust. Environ. Sci. Technol. 2008;42:6910–6916. doi: 10.1021/es801070p. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Eagle S, Sjödin A, Webster TF. Serum PBDEs in a North Carolina toddler cohort: Associations with handwipes, house dust, and socioeconomic variables. Environ. Health Perspect. 2012a;120:1049–1054. doi: 10.1289/ehp.1104802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Klosterhaus S, Keller A, Ferguson PL, van Bergen S, Cooper E, Webster TF, Blum A. Identification of flame retardants in polyurethane foam collected from baby products. Environ. Sci. Technol. 2011;45:5323–31. doi: 10.1021/es2007462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Misenheimer J, Hoffman K, Webster TF. Flame retardant associations between children’s handwipes and house dust. Chemosphere. 2014 doi: 10.1016/j.chemosphere.2013.12.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Sharma S, Getzinger G, Ferguson PL, Gabriel M, Webster TF, Blum A. Novel and high volume use flame retardants in US couches reflective of the 2005 PentaBDE phase out. Environ. Sci. Technol. 2012b;46:13432–9. doi: 10.1021/es303471d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- State of California BEARHFTI. Technical Bulletin 117–2013. State of California 2013 [Google Scholar]
- State of California BEARHFTI. Technical Bulletin 117: Requirements, Test Procedure and Apparatus for Testing the Flame Retardance of Resilient Filling Materials Used in Upholstered Furniture. State of California 2000 [Google Scholar]
- Van den Eede N, Dirtu AC, Ali N, Neels H, Covaci A. Multi-residue method for the determination of brominated and organophosphate flame retardants in indoor dust. Talanta. 2012;89:292–300. doi: 10.1016/j.talanta.2011.12.031. [DOI] [PubMed] [Google Scholar]
- Van den Eede N, Dirtu AC, Neels H, Covaci A. Analytical developments and preliminary assessment of human exposure to organophosphate flame retardants from indoor dust. Environ. Int. 2011;37:454–61. doi: 10.1016/j.envint.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Van den Eede N, Heffernan AL, Aylward LL, Hobson P, Neels H, Mueller JF, Covaci A. Age as a determinant of phosphate flame retardant exposure of the Australian population and identification of novel urinary PFR metabolites. Environ. Int. 2015;74:1–8. doi: 10.1016/j.envint.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Van den Eede N, Maho W, Erratico C, Neels H, Covaci A. First insights in the metabolism of phosphate flame retardants and plasticizers using human liver fractions. Toxicol. Lett. 2013;223:9–15. doi: 10.1016/j.toxlet.2013.08.012. [DOI] [PubMed] [Google Scholar]
- van der Veen I, de Boer J. Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis. Chemosphere. 2012;88:1119–53. doi: 10.1016/j.chemosphere.2012.03.067. [DOI] [PubMed] [Google Scholar]
- Watkins DJ, McClean MD, Fraser AJ, Weinberg J, Stapleton HM, Sjödin A, Webster TF. Impact of Dust from Multiple Microenvironments and Diet on PentaBDE Body Burden. Environ. Sci. Technol. 2012a;46:1192–1200. doi: 10.1021/es203314e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins DJ, McClean MD, Fraser AJ, Weinberg J, Stapleton HM, Sjödin A, Webster TF. Impact of Dust from Multiple Microenvironments and Diet on PentaBDE Body Burden. Environ. Sci. Technol. 2012b;46:1192–1200. doi: 10.1021/es203314e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N, Herrmann T, Paepke O, Tickner J, Hale R, Harvey E, La Guardia M, McClean MD, Webster TF. Human Exposure to PBDEs: Associations of PBDE Body Burdens with Food Consumption and House Dust Concentrations. Environ. Sci. Technol. 2007;41:1584–1589. doi: 10.1021/es0620282. [DOI] [PubMed] [Google Scholar]
- Zhou T, Taylor MM, DeVito MJ, Crofton KM. Developmental exposure to brominated diphenyl ethers results in thyroid hormone disruption. Toxicol. Sci. 2002;66:105–16. doi: 10.1093/toxsci/66.1.105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.