Abstract
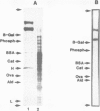
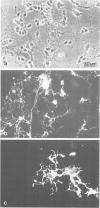
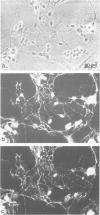
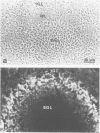
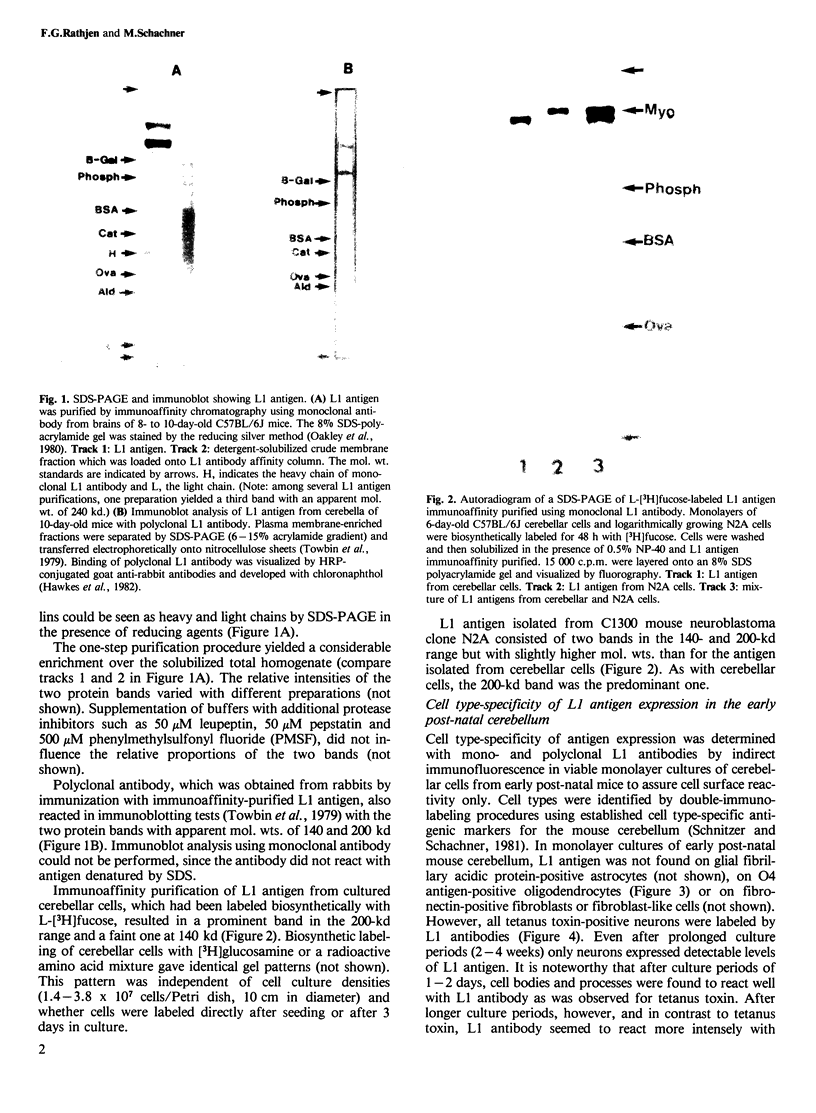
Monoclonal and polyclonal L1 antibodies react by indirect immunofluorescence with the cell surface of cultured tetanus toxin-positive neurons from post-natal cerebella of mice, but not with glial fibrillary acidic protein-positive astrocytes, O4 antigen-positive oligodendrocytes or fibronectin-positive fibroblasts or fibroblast-like cells. During cerebellar development L1 antigen is detectable on tetanus toxin-positive cells as early as embryonic day 13 after 3 days in culture. In sections of the early post-natal cerebellum, L1 antigen is found on pre-migratory neurons in the internal, but not in the external part of the external granular layer. In the adult cerebellum, L1 antigen is predominantly localized in the molecular layer and around Purkinje cells. Fibers in white matter and the granular layer are also L1 antigen-positive. Granule cell bodies and synaptic glomeruli are weakly antigen-positive. Several cell lines derived from neuroblastoma C1300 also express L1 antigen. The antigen is not detectable by enzyme-linked immunosorbent assay in tissue homogenates of liver, kidney, lung, heart, sperm or thymus. With polyclonal L1 antibodies, cross-reactive determinants are found in brains of rat, guinea pig, hamster, chicken, rabbit and man, but not in frog, while monoclonal antibody reacts detectably only with mouse brain. The molecular species recognized by both monoclonal and polyclonal antibodies display two prominent bands by SDS-PAGE under reducing and non-reducing conditions with apparent mol. wts. of 140 and 200 kd. L1 antigen isolated from cultured cerebellar cells consists mainly of a band in the 200-kd range and a faint one at 140 kd. L1 antigen from neuroblastoma N2A shows two bands with slightly higher apparent mol. wts. All molecular forms of L1 antigen can be labeled by [3H]fucose and [3H]glucosamine. Ca2+-independent re-aggregation of cerebellar cells from early post-natal C57BL/6J mice and of the continuous cell line N2A derived from the murine neuroblastoma C1300 is inhibited by Fab fragments of the polyclonal, but not of monoclonal antibody, both of which are known to react with the surface membrane of these cells.
Full text
PDF
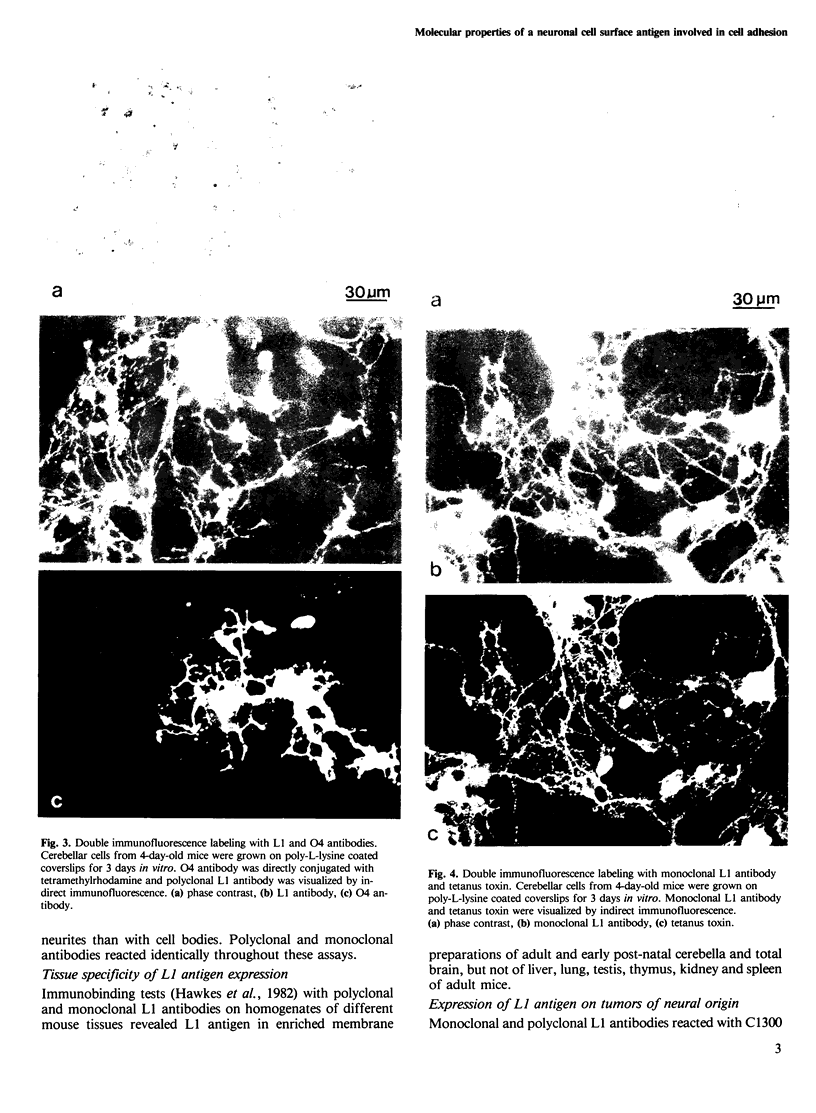




Images in this article
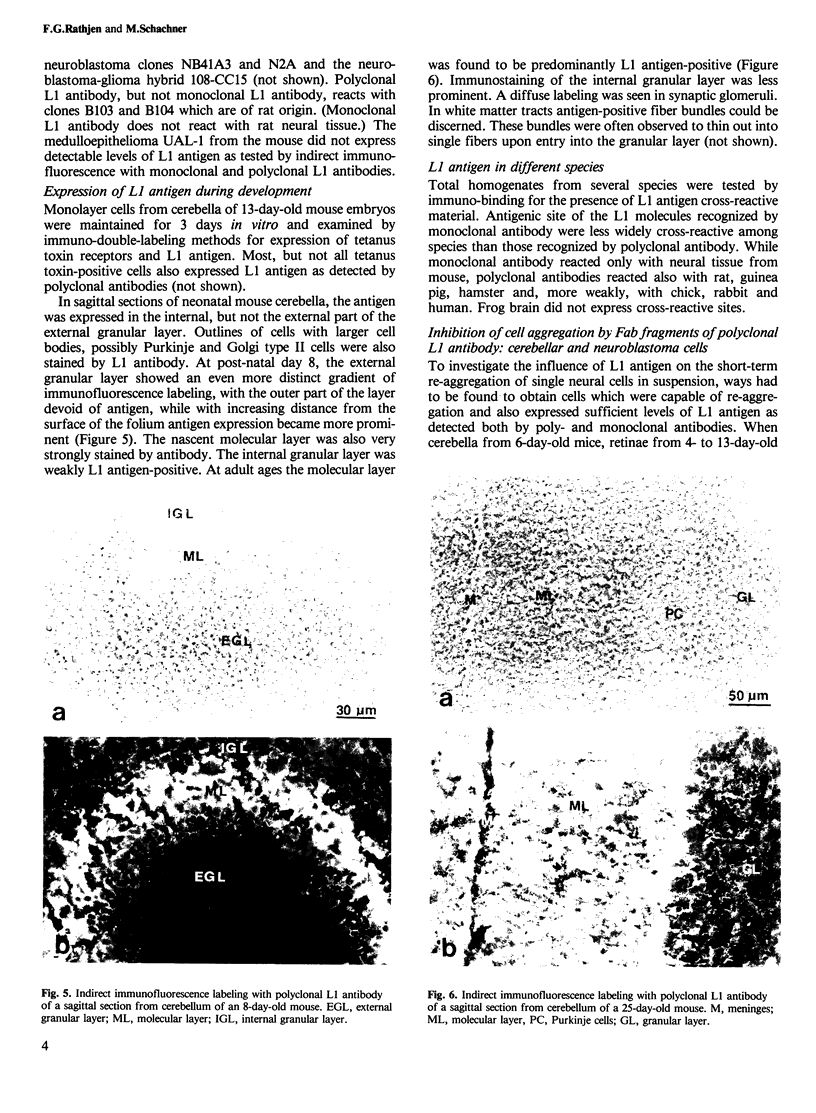
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
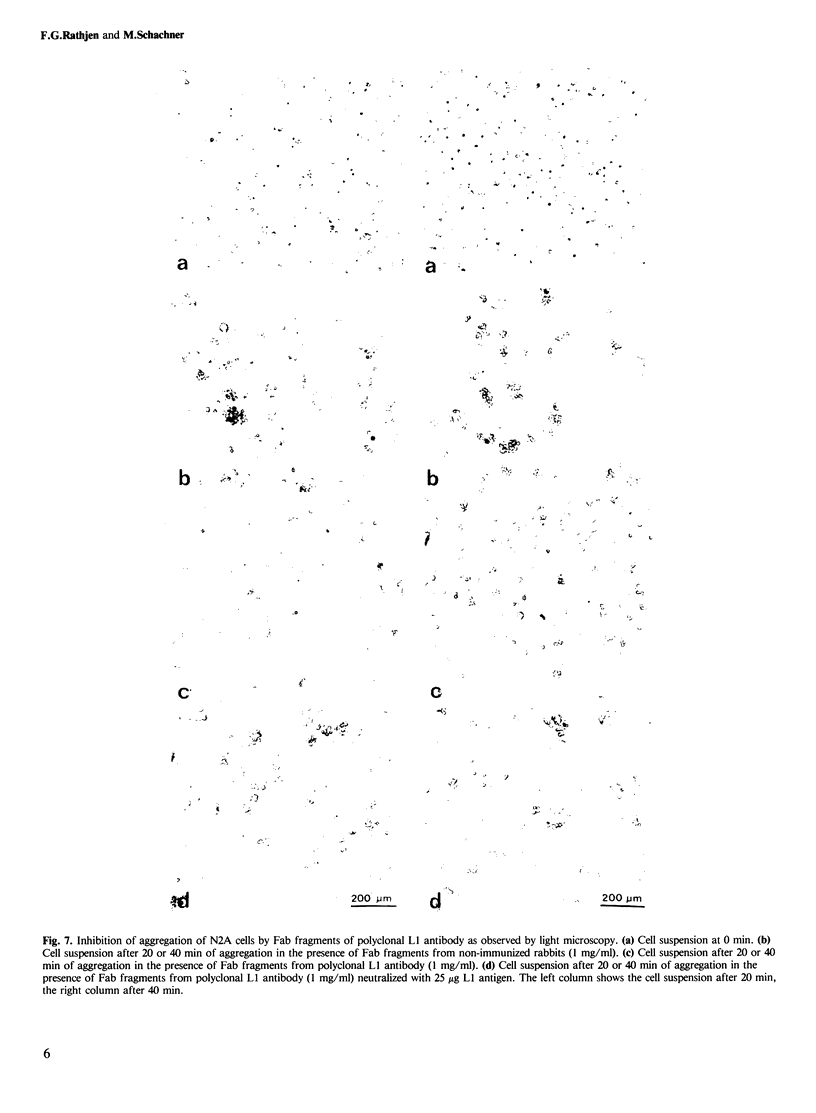
- Augusti-Tocco G., Sato G. Establishment of functional clonal lines of neurons from mouse neuroblastoma. Proc Natl Acad Sci U S A. 1969 Sep;64(1):311–315. doi: 10.1073/pnas.64.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balian G., Click E. M., Bornstein P. Location of a collagen-binding domain in fibronectin. J Biol Chem. 1980 Apr 25;255(8):3234–3236. [PubMed] [Google Scholar]
- Brackenbury R., Rutishauser U., Edelman G. M. Distinct calcium-independent and calcium-dependent adhesion systems of chicken embryo cells. Proc Natl Acad Sci U S A. 1981 Jan;78(1):387–391. doi: 10.1073/pnas.78.1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackenbury R., Thiery J. P., Rutishauser U., Edelman G. M. Adhesion among neural cells of the chick embryo. I. An immunological assay for molecules involved in cell-cell binding. J Biol Chem. 1977 Oct 10;252(19):6835–6840. [PubMed] [Google Scholar]
- Chuong C. M., McClain D. A., Streit P., Edelman G. M. Neural cell adhesion molecules in rodent brains isolated by monoclonal antibodies with cross-species reactivity. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4234–4238. doi: 10.1073/pnas.79.13.4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna G., Alexander S. S., Jr, Yamada K. M., Pastan I., Edelhoch H. The stability of cell surface protein to surfactants and denaturants. J Biol Chem. 1978 Nov 10;253(21):7787–7790. [PubMed] [Google Scholar]
- Fischer G., Schachner M. In vitro reaggregation of dissociated mouse cerebellar cells. I. Demonstration of different aggregation mechanisms. Exp Cell Res. 1982 Jun;139(2):285–296. doi: 10.1016/0014-4827(82)90253-1. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Hakomori S. Proteolytic and chemical fragmentation of galactoprotein a, a major transformation-sensitive glycoprotein released from hamster embryo fibroblasts. J Biol Chem. 1979 Jun 25;254(12):5442–5450. [PubMed] [Google Scholar]
- Galfrè G., Milstein C., Wright B. Rat x rat hybrid myelomas and a monoclonal anti-Fd portion of mouse IgG. Nature. 1979 Jan 11;277(5692):131–133. doi: 10.1038/277131a0. [DOI] [PubMed] [Google Scholar]
- Goridis C., Joher M. A., Hirsch M., Schachner M. Cell surface proteins of cultured brain cells and their recognition by anti-cerebellum (anti-NS-4) antiserum. J Neurochem. 1978 Aug;31(2):531–539. doi: 10.1111/j.1471-4159.1978.tb02669.x. [DOI] [PubMed] [Google Scholar]
- Goridis C., Martin J., Schachner M. Characterization of an antiserum to synaptic glomeruli from rat cerebellum. Brain Res Bull. 1978 Jan-Feb;3(1):45–52. doi: 10.1016/0361-9230(78)90060-6. [DOI] [PubMed] [Google Scholar]
- Hahn L. H., Yamada K. M. Isolation and biological characterization of active fragments of the adhesive glycoprotein fibronectin. Cell. 1979 Dec;18(4):1043–1051. doi: 10.1016/0092-8674(79)90217-4. [DOI] [PubMed] [Google Scholar]
- Hamprecht B. Structural, electrophysiological, biochemical, and pharmacological properties of neuroblastoma-glioma cell hybrids in cell culture. Int Rev Cytol. 1977;49:99–170. doi: 10.1016/s0074-7696(08)61948-8. [DOI] [PubMed] [Google Scholar]
- Hausman R. E., Moscona A. A. Immunologic detection of retina cognin on the surface of embryonic cells. Exp Cell Res. 1979 Mar 15;119(2):191–204. doi: 10.1016/0014-4827(79)90348-3. [DOI] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Hayman M. J., Crumpton M. J. Isolation of glycoproteins from pig lymphocyte plasma membrane using Lens culinaris phytohemagglutinin. Biochem Biophys Res Commun. 1972 May 26;47(4):923–930. doi: 10.1016/0006-291x(72)90581-5. [DOI] [PubMed] [Google Scholar]
- Hirn M., Ghandour M. S., Deagostini-Bazin H., Goridis C. Molecular heterogeneity and structural evolution during cerebellar ontogeny detected by monoclonal antibody of the mouse cell surface antigen BSP-2. Brain Res. 1983 Apr 11;265(1):87–100. doi: 10.1016/0006-8993(83)91337-9. [DOI] [PubMed] [Google Scholar]
- Hirn M., Pierres M., Deagostini-Bazin H., Hirsch M., Goridis C. Monoclonal antibody against cell surface glycoprotein of neurons. Brain Res. 1981 Jun 15;214(2):433–439. doi: 10.1016/0006-8993(81)91208-7. [DOI] [PubMed] [Google Scholar]
- Hoffman S., Sorkin B. C., White P. C., Brackenbury R., Mailhammer R., Rutishauser U., Cunningham B. A., Edelman G. M. Chemical characterization of a neural cell adhesion molecule purified from embryonic brain membranes. J Biol Chem. 1982 Jul 10;257(13):7720–7729. [PubMed] [Google Scholar]
- Jakoi E. R., Marchase R. B. Ligatin from embryonic chick neural retina. J Cell Biol. 1979 Mar;80(3):642–650. doi: 10.1083/jcb.80.3.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen O. S., Bock E. Brain specific synaptosomal membrane proteins demonstrated by crossed immunoelectrophoresis. J Neurochem. 1974 Oct;23(4):879–880. doi: 10.1111/j.1471-4159.1974.tb04419.x. [DOI] [PubMed] [Google Scholar]
- Jørgensen O. S., Delouvée A., Thiery J. P., Edelman G. M. The nervous system specific protein D2 is involved in adhesion among neurites from cultured rat ganglia. FEBS Lett. 1980 Feb 25;111(1):39–42. doi: 10.1016/0014-5793(80)80756-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lagenaur C., Sommer I., Schachner M. Subclass of astroglia in mouse cerebellum recognized by monoclonal antibody. Dev Biol. 1980 Oct;79(2):367–378. doi: 10.1016/0012-1606(80)90122-0. [DOI] [PubMed] [Google Scholar]
- Langley O. K., Ghandour M. S., Gombos G., Hirn M., Goridis C. Monoclonal antibodies as neural cell surface markers. Neurochem Res. 1982 Mar;7(3):349–362. doi: 10.1007/BF00965646. [DOI] [PubMed] [Google Scholar]
- Lemmon V., Staros E. B., Perry H. E., Gottlieb D. I. A monoclonal antibody which binds to the surface of chick brain cells and myotubes: cell selectivity and properties of the antigen. Brain Res. 1982 Mar;255(3):349–360. doi: 10.1016/0165-3806(82)90003-7. [DOI] [PubMed] [Google Scholar]
- Lindner J., Rathjen F. G., Schachner M. L1 mono- and polyclonal antibodies modify cell migration in early postnatal mouse cerebellum. 1983 Sep 29-Oct 5Nature. 305(5933):427–430. doi: 10.1038/305427a0. [DOI] [PubMed] [Google Scholar]
- Lotan R., Beattie G., Hubbell W., Nicolson G. L. Activities of lectins and their immobilized derivatives in detergent solutions. Implications on the use of lectin affinity chromatography for the purification of membrane glycoproteins. Biochemistry. 1977 May 3;16(9):1787–1794. doi: 10.1021/bi00628a004. [DOI] [PubMed] [Google Scholar]
- Magnani J. L., Thomas W. A., Steinberg M. S. Two distinct adhesion mechanisms in embryonic neural retina cells. I. A kinetic analysis. Dev Biol. 1981 Jan 15;81(1):96–105. doi: 10.1016/0012-1606(81)90351-1. [DOI] [PubMed] [Google Scholar]
- McClain D. A., Edelman G. M. A neural cell adhesion molecule from human brain. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6380–6384. doi: 10.1073/pnas.79.20.6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai N., Kobayashi S., Oguri M. Ultrastructural findings in medulloepitheliomatous neoplasms induced by human adenovirus 12 in rodents. Acta Neuropathol. 1974;28(4):293–304. doi: 10.1007/BF00685284. [DOI] [PubMed] [Google Scholar]
- Nelson P., Ruffner W., Nirenberg M. Neuronal tumor cells with excitable membranes grown in vitro. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1004–1010. doi: 10.1073/pnas.64.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhoff V., Philipp K., Zimmer H. G., Mesecke S. A simple, versatile, sensitive and volume-independent method for quantitative protein determination which is independent of other external influences. Hoppe Seylers Z Physiol Chem. 1979 Nov;360(11):1657–1670. doi: 10.1515/bchm2.1979.360.2.1657. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- PORTER R. R. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem J. 1959 Sep;73:119–126. doi: 10.1042/bj0730119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P., Sidman R. L. Weaver mutant mouse cerebellum: defective neuronal migration secondary to abnormality of Bergmann glia. Proc Natl Acad Sci U S A. 1973 Jan;70(1):240–244. doi: 10.1073/pnas.70.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer H., Schachner M. Surface proteins of cultured mouse cerebellar cells. J Neurochem. 1980 Oct;35(4):792–803. doi: 10.1111/j.1471-4159.1980.tb07075.x. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Hayman E. G., Kuusela P., Shively J. E., Engvall E. Isolation of a tryptic fragment containing the collagen-binding site of plasma fibronectin. J Biol Chem. 1979 Jul 10;254(13):6054–6059. [PubMed] [Google Scholar]
- Rutishauser U., Edelman G. M. Effects of fasciculation on the outgrowth of neurites from spinal ganglia in culture. J Cell Biol. 1980 Nov;87(2 Pt 1):370–378. doi: 10.1083/jcb.87.2.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser U., Gall W. E., Edelman G. M. Adhesion among neural cells of the chick embryo. IV. Role of the cell surface molecule CAM in the formation of neurite bundles in cultures of spinal ganglia. J Cell Biol. 1978 Nov;79(2 Pt 1):382–393. doi: 10.1083/jcb.79.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser U., Hoffman S., Edelman G. M. Binding properties of a cell adhesion molecule from neural tissue. Proc Natl Acad Sci U S A. 1982 Jan;79(2):685–689. doi: 10.1073/pnas.79.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoul R., Hirn M., Deagostini-Bazin H., Rougon G., Goridis C. Adult and embryonic mouse neural cell adhesion molecules have different binding properties. 1983 Jul 28-Aug 3Nature. 304(5924):347–349. doi: 10.1038/304347a0. [DOI] [PubMed] [Google Scholar]
- Schachner M., Wortham K. A., Carter L. D., Chaffee J. K. NS-4 (nervous system antigen-4), a cell surface antigen of developing and adult mouse brain and sperm. Dev Biol. 1975 Jun;44(2):313–325. doi: 10.1016/0012-1606(75)90402-9. [DOI] [PubMed] [Google Scholar]
- Schnitzer J., Schachner M. Expression of Thy-1, H-2, and NS-4 cell surface antigens and tetanus toxin receptors in early postnatal and adult mouse cerebellum. J Neuroimmunol. 1981 Dec;1(4):429–456. doi: 10.1016/0165-5728(81)90022-9. [DOI] [PubMed] [Google Scholar]
- Schubert D., Humphreys S., Baroni C., Cohn M. In vitro differentiation of a mouse neuroblastoma. Proc Natl Acad Sci U S A. 1969 Sep;64(1):316–323. doi: 10.1073/pnas.64.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi K., Hakomori S. Functional domain structure of fibronectin. Proc Natl Acad Sci U S A. 1980 May;77(5):2661–2665. doi: 10.1073/pnas.77.5.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer I., Schachner M. Cell that are O4 antigen-positive and O1 antigen-negative differentiate into O1 antigen-positive oligodendrocytes. Neurosci Lett. 1982 Apr 16;29(2):183–188. doi: 10.1016/0304-3940(82)90351-2. [DOI] [PubMed] [Google Scholar]
- Takeichi M., Ozaki H. S., Tokunaga K., Okada T. S. Experimental manipulation of cell surface to affect cellular recognition mechanisms. Dev Biol. 1979 May;70(1):195–205. doi: 10.1016/0012-1606(79)90016-2. [DOI] [PubMed] [Google Scholar]
- Thiery J. P., Brackenbury R., Rutishauser U., Edelman G. M. Adhesion among neural cells of the chick embryo. II. Purification and characterization of a cell adhesion molecule from neural retina. J Biol Chem. 1977 Oct 10;252(19):6841–6845. [PubMed] [Google Scholar]
- Thomas W. A., Steinberg M. S. Two distinct adhesion mechanisms in embryonic neural retina cells. II. An immunological analysis. Dev Biol. 1981 Jan 15;81(1):106–114. doi: 10.1016/0012-1606(81)90352-3. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D. D., Hynes R. O. Domain structure of fibronectin and its relation to function. Disulfides and sulfhydryl groups. J Biol Chem. 1979 Jul 25;254(14):6746–6754. [PubMed] [Google Scholar]
- Willinger M., Schachner M. GM1 ganglioside as a marker for neuronal differentiation in mouse cerebellum. Dev Biol. 1980 Jan;74(1):101–117. doi: 10.1016/0012-1606(80)90055-x. [DOI] [PubMed] [Google Scholar]
- de StGroth S. F., Scheidegger D. Production of monoclonal antibodies: strategy and tactics. J Immunol Methods. 1980;35(1-2):1–21. doi: 10.1016/0022-1759(80)90146-5. [DOI] [PubMed] [Google Scholar]