Abstract
Background
Over the last few years multiple studies have been published showing a great diversity in size of chloroplast genomes (cpGenomes), and in the arrangement of gene clusters, in the Euglenales. However, while these genomes provided important insights into the evolution of cpGenomes across the Euglenales and within their genera, only two genomes were analyzed in regard to genomic variability between and within Euglenales and Eutreptiales. To better understand the dynamics of chloroplast genome evolution in early evolving Eutreptiales, this study focused on the cpGenome of Eutreptiella pomquetensis, and the spread and peculiarities of introns.
Methods
The Etl. pomquetensis cpGenome was sequenced, annotated and afterwards examined in structure, size, gene order and intron content. These features were compared with other euglenoid cpGenomes as well as those of prasinophyte green algae, including Pyramimonas parkeae.
Results and Discussion
With about 130,561 bp the chloroplast genome of Etl. pomquetensis, a basal taxon in the phototrophic euglenoids, was considerably larger than the two other Eutreptiales cpGenomes sequenced so far. Although the detected quadripartite structure resembled most green algae and plant chloroplast genomes, the gene content of the single copy regions in Etl. pomquetensis was completely different from those observed in green algae and plants. The gene composition of Etl. pomquetensis was extensively changed and turned out to be almost identical to other Eutreptiales and Euglenales, and not to P. parkeae. Furthermore, the cpGenome of Etl. pomquetensis was unexpectedly permeated by a high number of introns, which led to a substantially larger genome. The 51 identified introns of Etl. pomquetensis showed two major unique features: (i) more than half of the introns displayed a high level of pairwise identities; (ii) no group III introns could be identified in the protein coding genes. These findings support the hypothesis that group III introns are degenerated group II introns and evolved later.
Keywords: Eutreptiella pomquetensis, Introns, Twintrons, Genome structure, Chloroplast genome
Introduction
Recent analyses of chloroplast genomes (cpGenomes) have been largely used to retrace evolutionary steps of phototrophic euglenoids. Members of the genera Euglena, Monomorphina, Euglenaformis, Colacium, Strombomonas and recently Phacus (Bennett, Wiegert & Triemer, 2012; Bennett, Wiegert & Triemer, 2014; Bennett & Triemer, 2015; Bennett, Shiu & Triemer, 2017; Dabbagh & Preisfeld, 2016; Gockel & Hachtel, 2000; Hallick et al., 1993; Kasiborski, Bennett & Linton, 2016; Pombert et al., 2012; Wiegert, Bennett & Triemer, 2013) cpGenomes of the ‘crown group’ Euglenales have been studied intensely. Overall aims were to tackle questions of relatedness, gene arrangement, synteny and genome size as well as possession and dispersal of introns. However, the knowledge on cpGenomes of the basal lineage, Eutreptiales, is comparatively low. The two known genomes were reported to show a smaller genome size and display only seven and 27 introns in Eutreptiella gymnastica and Eutreptia viridis, respectively (Hrdá et al., 2012; Wiegert, Bennett & Triemer, 2012). Fitting into a scheme of increasing intron quantity and genome size, the invasion of introns in euglenoids was assumed to have started with very low intron numbers and as a consequence small cpGenomes in Eutreptiales, which both increased during diversification of photosynthetic euglenoids (Bennett & Triemer, 2015; Hrdá et al., 2012; Thompson et al., 1995; Wiegert, Bennett & Triemer, 2012). This hypothesis was initially corroborated by the fact that in Pyramimonas parkeae, as the closest living relative of the euglenoid plastid, only one intron was detected (Turmel et al., 2009). However, this was later refuted by analysis of different lineages in the Euglenales, all of which presented species with large cpGenomes and more than 110 introns (both E. gracilis strains, S. acuminata, C. vesiculosum) in addition to small cpGenomes with low intron numbers like M. aenigmatica (Bennett & Triemer, 2015; Hallick et al., 1993; Pombert et al., 2012; Wiegert, Bennett & Triemer, 2013). Although it could be assumed that introns spread independently within the lineages, it was unknown whether a small or a large cpGenome was present when phototrophic euglenoids emerged and how (un)evenly these early introns were distributed in the Eutreptiales.
In the present study Eutreptiella pomquetensis (McLachlan, Seguel & Fritz) Marin & Melkonian in Marin et al. (2003) was analyzed as a member of the scarcely investigated Eutreptiales. It was originally isolated from shallow, cold, marine habitats and is the only known phototrophic euglenoid with four flagella (McLachlan, Seguel & Fritz, 1994). It was classified as an obligate psychrophilic species, which is an unusual characteristic for euglenoids, and worthy of investigation.
The Eutreptiales only consist of two genera, Eutreptiella da Cunha, with ten described species, and Eutreptia Perty, with eight known species. They are regarded as basal phototrophic euglenoids in aspects of morphology (Leander, Witek & Farmer, 2001; Leedale, 1967) as well as in molecular analyses and molecular studies combined with morphological characters (Linton et al., 1999; Linton et al., 2000; Marin et al., 2003; Preisfeld et al., 2001; Yamaguchi, Yubuki & Leander, 2012) and hence of particular interest, where the evolution of euglenoid chloroplasts is reflected upon. The capacity for photosynthesis in euglenoids was found to have originated with the acquisition of chloroplasts by a phagotrophic euglenoid via secondary endocytobiosis of a green alga in a marine environment, which is still unknown (Gibbs, 1978, Gibbs, 1981). Presumably, the donor was a relative of the partly obligatory psychrophilic genus Pyramimonas (Marin, 2004; Turmel et al., 2009).
Thus, it was our interest to investigate the psychrophilic Eutreptiella pomquetensis for two reasons: First, to compare this cpGenome with that of P. parkeae (Turmel et al., 2009), and the other two Eutreptiales (Hrdá et al., 2012; Wiegert, Bennett & Triemer, 2012) with regard to genome structure and size, intron number and propagation, and gene content as well as arrangement; second, to diminish the bias in taxon sampling in euglenoid cpGenomic analyses.
Materials and Methods
Growth, isolation, sequencing and assembly
Eutreptiella pomquetensis (McLachlan, Seguel & Fritz) Marin & Melkonian in Marin et al. (2003) strain CCMP 1491 cells were grown in modified L1-Si Medium (Guillard & Hargraves, 1993) with artificial seawater Sea-Pure (CaribSea, Inc. Fort Pierce, USA) at 2–4 °C with changing 3:3 light:dark cycle using ExoTerra Natural Light PT2190 (Hagen, Holm Germany).
Three-hundred mL of cell culture were harvested by centrifugation and submitted to cell cleaning and chloroplast isolation protocol as described in Dabbagh & Preisfeld (2016) with a slight change during sonication of cells. Purified cells were subjected to sonication twice for three seconds with the amplitude set at 50% and a pulse rate of 0.1 s (Bandelin Sonopuls HD 60; Bandelin, Berlin, Germany). The DNA was sequenced with 454 sequencing according to the GS FLX ++ chemistry Rapid Shotgun Library Preparation Method technology (Eurofins Genomics Ebersberg, Germany). In total 60,225 reads were produced in segment of a full run with an average size of 608 bases. Automatic assembly of reads by Eurofins Genomics in Newbler (Roche, Basel, Switzerland) resulted in 668 contigs (N50 contig size was 1,157 bases).
Annotation of the plastid genome
Using a BLASTn homology search (Altschul et al., 1990) the four largest contigs were identified as major parts of the plastid genome, and were subsequently linked by fill-in PCR from the end of each contig using whole genomic DNA. Contig 1 consists of 10,450 number of reads with an average coverage depth of 131.70, contig 2 of 3,918 number of reads with an average coverage depth of 129.90, contig 3 of 3,398 number of reads with an average coverage depth of 112.60. Contig 4 was identified as major part of the chloroplast rRNA operon and showed an average coverage depth of 211.30. The average depth is the mean read coverage and helps to identify repetitive parts of the chloroplast genome. Based on coverage depth of the ribosomal operon components (5S, 16S, 23S) compared to single copy protein coding genes, it appears that at least two copies of the operon are present on the genome. Closing the circle failed in spite of many different approaches using PCR experiments from rpl32 to psaC and further from each rRNA gene to psaC with specifically designed primers. Experiments to close the circle were performed with both a Long Range PCR Kit (Qiagen GmbH, Hilden, Germany) and a Long Amp Taq DNA Polymerase (New England BioLabs GmbH, Frankfurt am Main, Germany).
The final annotation of the chloroplast sequence was performed with Geneious 9 Pro (version 9.1.3, Kearse et al., 2012) with the option to translate the nucleotide sequence in all frames selected, and the “Genetic Code” was identified as “Bacterial”.
Protein coding genes were manually aligned in MEGA 7 (Tamura et al., 2011) against the nucleotide coding DNA sequences (CDS) from other photosynthetic euglenoids and prasinophyte representatives to determine exon-intron boundaries as well as start and stop of each gene. In all cases, a traditional methionine (ATG) start codon was preferred. CDS was verified by BLASTx, “Genetic code” set at “Bacteria and Archaea (11)” and Emboss Sixpack Sequence translation (EMBL- EBI 2015) “Codon Table” set at “Bacterial” and added to the annotation. The introns within protein coding genes were analyzed for the presence of potential twintrons as described in Bennett & Triemer (2015). This analysis was modified such that the 3′ motifs were established using a Python script instead of a manual search. The script browsed the homologous external introns for the conserved 3′ motifs (abcdef (3–8 nucleotides) f′e′d′A∗c′b′a′ (four nucleotides)). Afterwards, all 51 introns were searched for the conserved 5′ insertion sequence GUGYG. RNA secondary structure for group II introns was created by RNA folding via Mfold web server using default settings (Zuker, 2003), manually optimized and illustrated with the PseudoViewer web application (Byun & Han, 2006). For roaA a pairwise sequence comparison of the amino acid sequence of E. gracilis with a putative amino acid sequence of Etl. pomquetensis was performed using Exonerate 2.4.0 by Slater & Birney (2005) to reveal intron boundaries and start/ stop of the searched gene.
tRNAscan-SE 1.21 (Schattner, Brooks & Lowe, 2005), with the default settings and the source given as mito/chloroplast, was used to identify tRNAs. Uncharacterized open reading frames (ORFs) were identified with ORF finder within Geneious, with the genetic code set to bacterial. Only ORFs which were at least 300 bp, did not overlap with the coding region of another gene, and lacked BLASTp evidence (default settings) for being a previously identified chloroplast protein-coding gene were included in the annotation. ORFs were named according to the number of amino acids in the coding region. To evaluate the proportion of short repeated sequences the variable number of tandem repeats was scanned with the online version of REPuter (Kurtz et al., 2001) under the same settings as described in Bennett & Triemer (2015) and with Tandem Repeats Finder, with the option “Basic”, using default parameters (Benson, 1999).
The start/stop areas of the 16S and 23S rRNA genes were identified using RNAmmer 1.2 (Lagesen et al., 2007), with “Bacteria” chosen as the sequence kingdom of origin. The 5S rRNA start/stop regions were identified using Rfam 12.1 Sequence Search (Burge et al., 2013). The number of rRNA operons flanked by the protein-coding genes rpoC2 and psbA were confirmed using PCR. One further rRNA operon was identified by PCR experiments next to the protein coding gene rpl32 by long range PCR. To verify the exact sequence a Long Range PCR was performed with primers (forward 5′ -AGAGTTTGATCCTGGCTCAG- 3′; reverse 5′-TGCTTCCATACACTTTTACGCATA- 3′) from the beginning of the 16S to the rpl32 gene. Primers were created manually by Primer3Plus (Untergasser et al., 2012) based on the nucleotide sequence. The PCR product (5,080 bp) was purified and used as DNA matrix for further PCRs to determine the sequence of the rRNA genes and the noncoding regions in between. The number of rRNA operons next to the rpl32 gene was performed using long-range PCR. The long-range PCR approach to measure the copies of the RNA operon yielded only one product.
Synteny between the cpGenomes of all three sequenced Eutreptiales was determined using Mauve (Darling et al., 2004), as a plugin for Geneious, with the alignment algorithm set as progressive Mauve. Each genome was displayed as a linear sequence with blocks representing a homologous gene cluster. In the Mauve alignment the repeat regions of rRNA were not included because Mauve will not align repeat regions which have multiple matches on both genomes. The circular genome map was created using GenomeVx (Conant & Wolfe, 2008).
Results and Discussion
General genome analyses
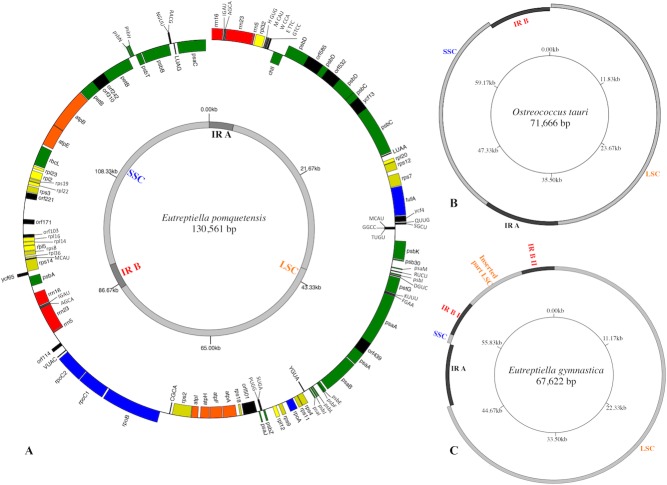
The cpGenome of Etl. pomquetensis is presented as an incomplete circle, because attempts to close the gap between the 16S rRNA gene and the protein coding gene psaC were unsuccessful, even with long range approaches. Thus, the cpGenome contained at least 130,561 bp, which is twice the size of Eutreptiella gymnastica with 67,622 bp (Hrdá et al., 2012) and Eutreptia viridis with 65,523 bp (Wiegert, Bennett & Triemer, 2012). The new cpGenome resembled the members of the Euglenales E. gracilis var. bacillaris (132,034 bp) and C. vesiculosum (128,892 bp, Table S1) in size. The content of genes was similar to those of other phototrophic euglenoids and reduced as compared to P. parkeae (Turmel et al., 2009) or Ostreococcus tauri (Robbens et al., 2007). The organization of the whole genome, however, resembled those of higher plants and algae (Cattolico et al., 2008; Lemieux, Otis & Turmel, 2007; Ravi et al., 2008; Robbens et al., 2007; Turmel et al., 2009) more than other euglenoids. The genome was composed of a large single copy region (LSC 80,941 bp), a small single copy region (SSC 39,856 bp) and two inverted repeats (IR) containing the rRNA genes in a way similar to O. tauri, but different in gene content (Figs. 1A & 1B). In the cpGenome of P. parkeae, the putative chloroplast donor for euglenoids, the organization is very much alike, but lacks the 5S rRNA in both inverted repeats. However, the possibility of non-recognition of the sequence as described by Turmel et al. (2009) still has to be considered. The fact that one operon was localized on the positive and one on the negative strand points at another similarity between the green algae P. parkeae, O.tauri and Etl. pomquetensi s. In the close relative Etl. gymnastica, the rRNA operon consisted of two incomplete copies, without a 5S rRNA, as in P. parkeae, but additionally one operon was divided into two parts separated by parts of the LSC (Hrdá et al., 2012, Fig. 1C). The G+C base composition of 35.1% again resembled that of Etl. gymnastica and P. parkeae and was higher than that of Et. viridis with 28.6% (Table S1).
Figure 1. Gene maps of chloroplast genomes.
(A) Map of the plastid genome of Eutreptiella pomquetensis. Boxes of different colors represent genes of similar functional groups: red, ribosomal rRNAs; green, photosystem/photosynthesis genes; yellow, ribosomal proteins (rpl, rps); orange, atp genes; blue: transcription/translation- related genes (rpo, tufA); black, conserved hypothetical proteins (ycf), open reading frames (ORF), tRNAs. Boxes are proportional to their sequence length. Outer ring: Genes on the outside of the circle are considered on the positive strand, genes inside the circle on the negative strand. Inner circle shows the large single copy region (LSC) and short single copy region (SSC) in light grey and inverted repeats (IR) in dark grey. (B–C) Simplified maps of the plastid genomes of Ostreococcus tauri (B) and Eutreptiella gymnastica (C) to demonstrate similarities and differences of genome structures. Copies of the IR sequences are represented in dark grey and LSC and SSC in light grey.
Analysis of gene content and arrangement
In total, 94 genes were identified and annotated in the cpGenome of Etl. pomquetensis, including 60 protein coding genes, two complete copies of the rRNA operon and 28 tRNAs (Fig. 1A). Alignments and analysis of protein coding genes indicated that the coding regions were more similar to those of P. parkeae than to those of the Euglena clade. For example, a pairwise comparison between psbD coding regions from P. parkeae and Etl. pomquetensis pointed out an 84.4% identity at the nucleotide level, whereas the same region from E. gracilis and Etl. pomquetensis showed only an 80.5% identity making the resemblance of Etl. pomquetensis to P. parkeae more apparent. Traditional methionine start codons (ATG) were found for each protein coding gene, except rpoA, where an alternative start codon (ATA) was accepted. Four protein coding genes were annotated with alternative start codons in Etl. gymnastica and Et. viridis (Table 1; Hrdá et al., 2012; Wiegert, Bennett & Triemer, 2012) and three in P. parkeae (Turmel et al., 2009). The number of the protein coding genes (60) was most similar to Etl. gymnastica (59), where psaI was missing. Et. viridis lacked psaM, ycf12 (psb30) and ycf65 and hence counted only 57 protein coding genes. No mat5 or mat2 genes have been identified in Etl. pomquetensis, but mat1 (ycf13) was detected, as was expected from results in other Eutreptiales (Hrdá et al., 2012; Wiegert, Bennett & Triemer, 2012). Just like Et. viridis (Wiegert, Bennett & Triemer, 2012), Etl. pomquetensis also lacked the common land plant chloroplast genes rpl33, infA, clpP, frxB, ndhA-K, petA, petD, psbM, rps15 and rps16.
Table 1. Alternative start codons usage in protein coding genes of cpGenomes of Eutreptiales and Pyramimonas parkeae (Chlorophyta).
| Alternative start codons: | ||||
|---|---|---|---|---|
| Etl.pomquetensis | Etl. gymnastica | Et. viridis | P. parkeae | |
| Total number | 1 | 4 | 4 | 3 |
| Gene/start | rpoA (ATA) | rpoA (TTG) | psaI (ATT) | rps11 (GTG) |
| psbC (TAT) | rps11 (ATT) | rpoA (GTG) | ||
| ycf13 (GTG) | atpE (ATT) | rps18 (GTG) | ||
| atpF (TTG) | petB (GTG) | |||
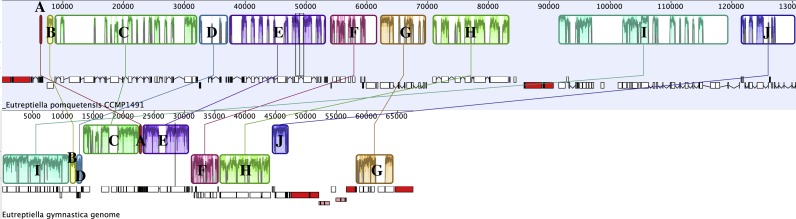
Progressive Mauve was used to analyze related chloroplast genomes (Darling et al., 2004). A comparison of Etl. pomquetensis and Etl. gymnastica gene content and arrangement identified 10 conserved gene clusters (Fig. 2, Table 2). Although gene content was similar in the two studied Eutreptiella species, the gene clusters showed significant rearrangements in position and strand orientation between Etl. gymnastica and Etl. pomquetensis. Block I was the largest in Etl. pomquetensis, included 18 genes, and was more than 19 kb long. The clusters themselves showed that extensive rearrangements occurred between Etl. gymnastica and Etl. pomquetensis. This lack of synteny was surprising, because high intrageneric variability between other taxa had not been noted so far. For example, a comparison between M. aenigmatica and M. parapyrum or E. gracilis and E. viridis cpGenomes revealed only one and two blocks, respectively. But, although Etl. gymnastica and Etl. pomquetensis are described as belonging to one genus, the evolutionary distance between euglenoid taxa is usually relatively high and makes differences probable. On the other hand, Etl. pomquetensis lives under psychrophilic conditions, whereas Etl. gymnastica lives under moderate marine conditions, which means that the environmental pressure is varying.
Figure 2. Progressive Mauve analysis comparing the cpGenomes of Etl. pomquetensis and Etl. gymnastica.
Each box represents a cluster of homologous genes with Eutreptiella pomquetensis as the reference genome. Like blocks are labelled by letters A–J. See Table 4 for a list of genes contained in each block. In the Mauve alignment the repeat regions of rRNA were not included, because Mauve will not align repeat regions, which have multiple matches on both genomes.
Table 2. Gene clusters resulting from Progressive Mauve cpGenome analysis of the two Eutreptiella species.
Gene clusters (blocks) are labelled with letters (A–J) and relevant genes listed. Bars in blocks C, H, I, J mark positions of a second Progressive Mauve analysis of the three Eutreptiales, where blocks are divided (see Fig. S1).
| Block | Gene Clusters |
|---|---|
| A | tRNA-His, tRNA-Met, tRNA-Trp, tRNA-Glu, tRNA-Gly |
| B | chlI |
| C | psbD psbC tRNA-Leu /rpl20 rps12 rps7 tufA ycf4 tRNA-Gln tRNA-Ser |
| D | tRNA-Arg psaM psb30 (syn. ycf12) psbK tRNA-Thr tRNA-Gly tRNA-Met |
| E | psb I tRNA-Asp petG tRNA-Lys tRNA-Phe psaA psaB psbE psbF psbL psbJ |
| F | rps18 psaJ tRNA-Pro tRNA-Ser psbZ rpl12 rps9 rpoA rps11 rps4 tRNA-Tyr |
| G | tRNA-Cys rps2 atpI atpH atpF atpA |
| H | tRNA-Val /rpoC2 rpoC1 rpoB |
| I | petB atpB atpE /rbcL rpl23 rpl2 rps19 rpl22 rps3 rpl16 rpl14 rpl5 rps8 rpl36 tRNA-Met rps14 ycf65 psbA |
| J | psbN psbH psbT psbB tRNA-Asn tRNA-Arg tRNA-Leu /psaC |
The noted difference in gene density between Etl. pomquetensis and Etl. gymnastica was not only due to an increase of introns from seven introns in Etl. gymnastica (total amount of intron space 6,893 bp) to 51 introns in Etl. pomquetensis (total amount of intron space 52,999 bp), but additionally to an increased intergenic space in Etl. pomquetensis. The intergenic space of Etl. pomquetensis comprised more than 23 kb, which was more than twice in that of Etl. gymnastica. While most of the blocks in Etl. gymnastica were quite compact with little intergenic or intron space in blocks C, E and G, all of the identified clusters showed heavily fragmented blocks in Etl. pomquetensis, except A and B (Fig. 2).
A second Mauve analysis of Etl. pomquetensis and the two other basal phototrophic Eutreptiales Et. viridis and Etl. gymnastica identified 14 conserved gene clusters (Fig. S1). The gene order within the clusters was mostly conserved and equal to the ten clusters found in the previous analysis. However, four gene clusters were further divided into two clusters each (Table 2, bar in blocks C, H, I, J).
Three additional Mauve analyses using Etl. pomquetensis identified 31 clusters with P. parkeae, 26 with P. provasolii, and 21 with O. tauri (Fig. S2). A comparison of the Mauve analyses found more homologous regions between Etl. pomquetensis and the other Eutreptiales than with the prasinophytes (the group containing the putative chloroplast donor). As the phototrophic euglenoids have a reduced amount of protein coding genes in contrast to the green algae, this high number of clusters was expected.
Open reading frames
Ten uncharacterized open reading frames (ORFs) were found in Etl. pomquetensis. A BLASTp analysis was performed against the NCBI nonredundant protein sequences (nr) database to determine whether any of the ORFs had functional similarity to previously sequenced genes (Table 3). The psbD gene of Etl. pomquetensis contained two ORFs (orf585 and orf532). The intron encoded orf585 of Etl. pomquetensis psbD I2 shared strong similarity with the orf583 of atpB I1 in the chloroplast genome of Pycnococcus provasolii (Turmel et al., 2009), with an e-value of 0.0. Turmel et al. (2009) determined that the Pycnococcus and Ostreococcus intron ORFs share strong similarity with each other, and for example, also with mat4 in Euglena myxocylindracea (Turmel et al., 2009). The open reading frames orf171 and orf103 next to the rpl16 gene showed weak similarity to the roaA gene annotated in some Euglenales chloroplast genomes. However, in either case the best match is reported for putative reverse transcriptase and intron maturase. Further, exonerate 2.4.0 (Slater & Birney, 2005) and a manual alignment were performed to evaluate if the two ORFs were part of the roaA gene. Neither of these methods yielded clear results, and no exact exon-intron boundaries or start/ stop regions could be identified. Additionally, RT-PCR experiments for detecting a putative intron between orf103 and orf171 failed, indicating that these ORFs may not have a true function in vivo.
Table 3. Open Reading Frames.
BLASTp analysis of ten uncharacterized ORFs in the Eutreptiella pomquetensis cpGenome against NCBI nonredundant protein sequences (nr) database. For each ORF the best match is reported.
| ORF | Accession number | Best BLASTp match | ||
|---|---|---|---|---|
| Organism | Product | E-value | ||
| 585 | YP_002600812.1 | Pycnococcus provasolii | putative reverse transcriptase and intron maturase | 0.0 |
| 532 | WP_041039849.1 | Tolypothrix campylonemoides | group II intron reverse transcriptase/maturase | 3e − 57 |
| 439 | YP_009306333.1 | Caulerpa cliftonii | hypothetical protein | 2e − 39 |
| 501 | WP_050045085.1 | Tolypothrix bouteillei | group II intron reverse transcriptase/maturase | 4e − 65 |
| 114 | − | − | no significant similarity found | − |
| 310 | BAM65725.1 | Helminthostachys zeylanica | maturase K | 9e − 14 |
| 242 | WP_061793822.1 | Bacillus firmus | hypothetical protein | 0.14 |
| 221 | AOC61650.1 | Gloeotilopsis planctonica | putative reverse transcriptase and intron maturase | 5e − 35 |
| 171a | AOC61481.1 | Gloeotilopsis sarcinoidea | putative reverse transcriptase and intron maturase | 3e − 12 |
| 103a | AOC61650.1 | Gloeotilopsis planctonica | putative reverse transcriptase and intron maturase | 7e − 09 |
Notes.
maybe roaA.
There is no evidence of a VNTR (variable number of tandem repeat) sequence, though this could be a result of our inability to circularize the genome.
Intron sequence similarity
Twenty-three out of the 60 protein-coding genes contained one or more introns, resulting in a total of 51 introns with likely twintrons measured as one insertion site. psaA contained the highest count with six introns (Table S1). The number of introns revealed, is twice as high as in Et. viridis (27), nearly eight times higher than found in Etl. gymnastica (7), and consequently constitutes the highest intron number known in the Eutreptiales (Hrdá et al., 2012; Pombert et al., 2012; Wiegert, Bennett & Triemer, 2012). Upon closer inspection of the intron sequences, we discovered 90% pairwise identities in introns of different genes in Etl. pomquetensis.
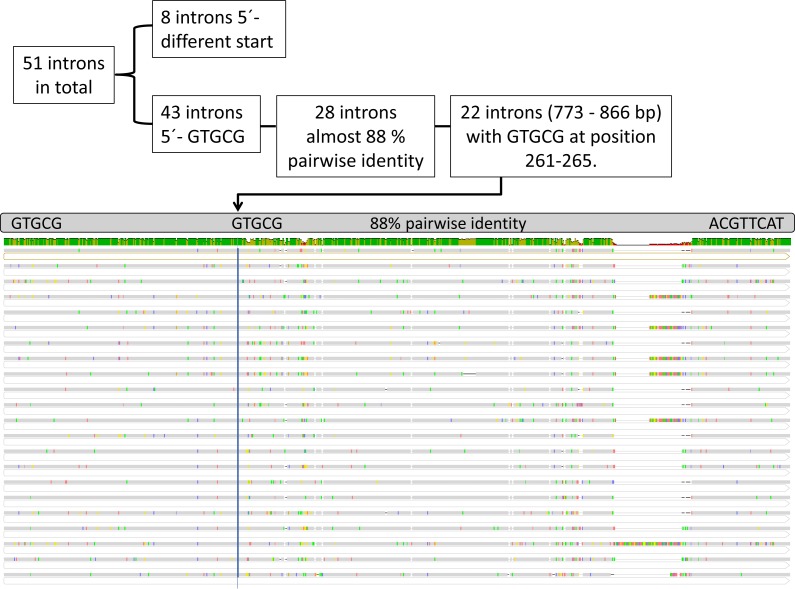
Therefore, and to gather information on the relatedness of the introns in basal euglenoids, we aligned all intron sequences and detected 28 introns (773–1,578 bp, Table S2 marked bold) in Etl. pomquetensis with pairwise identities of 87.4% and identical 5′-GTGCG boundaries typical for group II introns. Since group II introns in euglenoids are short for group II intron membership and usually do not show high sequence similarities, except in bounding regions, the strongly conserved GAAA terminal loop and portions of the domain V stem and, if present, in maturases (Michel & Ferat, 1995; Thompson et al., 1997) it was surprising to discover pairwise identities of about 90% in introns of different genes in Etl. pomquetensis. Moreover, 3′ boundaries always showed matching ACGTTCAT motifs (except for petG I1 and psaC I2) with the presumed “branch-point” *A for splicing at position eight in domain VI, where the first transesterification takes place (Lambowitz & Belfort, 2015). The last two nucleotides AY represent the typical conserved ending for group II-introns (Lambowitz & Belfort, 2015). As expected, domain V, known to play a catalytic role in intron excision, showed a highly conserved secondary structure (Kelchner, 2002; Michel & Ferat, 1995; Thompson et al., 1997; Toor, Hausner & Zimmerly, 2001). The 28 introns scrutinized, except for petG I1 and psaC I2 (Table S2 marked bold), showed a highly conserved domain V with 24 out of 34 nucleotides identical. Beside the fact that three base pairs (5′- …AGC …GUU…-3′) near the base of the stem were completely identical (Fig. S3), the secondary structure was unambiguously the same as the secondary structure of group IIB introns predicted by Kelchner (2002). Also of interest was that more than half out of the 51 nucleotides forming the stem and loop of domain VI were identical and resulted in the same secondary structure (Fig. S3).
Twenty- two of these introns (773–866 bp) in Etl. pomquetensis additionally showed the same GTGCG motif at positions nt 261 to 265 upstream from base one of the intron, with pairwise identities of 88% (Fig. 3, Table S2 highlighted in gray).
Figure 3. Alignment of group II introns.
Intron identity according to boundaries and position of additional GTGCG (blue line). Geneious nucleotide alignment with absolute pairwise identities (green in first line, grey in alignment, different nucleotides in colored bars) of 21 introns in Etl. pomquetensis in various genes. Introns top down: atpB I2, atpB I3, atpB I4, atpE I2, atpH I1, psaA I1, psaA I2, psaA I3, psaA I4, psaA I6, psaB I2, psaC I3, psbB I2, psbC I3, psbC I5, psbD I1, psbD I5, rbcL I1, rpl32 I1, rpoB I1, rps7 I1, rps12 I1.
We assume that all of these 28 introns of Etl. pomquetensis with high pairwise identity were closely related and arose from a single ancestor proliferating via retrotransposition and moved horizontally into DNA target sequences, which resembled the homing site. According to Lambowitz & Zimmerly (2011) and Lambowitz & Belfort (2015), retrotransposition to ectopic sites plays a major role in intron dissemination to novel locations, so that the many and very similar introns in Etl. pomquetensis could be explained.
Possible proliferation of group II introns
Still the question remained of how these introns could be spliced without an ORF including maturase activity in domain IV. One possibility was that they rely on trans-acting RNAs or proteins with two feasible splicing mechanisms: (1) The introns of Etl. pomquetensis used host encoded proteins to promote splicing, reverse splicing and mobility, which is typical for most mitochondrial and plant chloroplast group II introns (Lambowitz & Zimmerly, 2011). Chlamydomonas reinhardtii even utilized nuclear-encoded maturases for splicing of the trans-spliced group II introns (Merendino et al., 2006).
(2) All these introns could be spliced by a single IEP (Intron-Encoded Protein) that could either be free-standing or located in a functional intron. This would provide an accessible splicing apparatus and allow all but one intron to lose its own IEP (Dai & Zimmerly, 2003; Lambowitz & Belfort, 2015; Lambowitz & Zimmerly, 2011). Brouard et al. (2016) assumed that the freestanding orf1311 in Oedocladium (Chlorophyceae), with an intron encoded maturase, could function as promoter for splicing the ORF-less group II introns. Turmel, Otis & Lemieux (2016) detected introns in G. planctonica without ORFs, which may reflect an evolutionary pressure for a smaller and more compact intron structure enabling increased efficiency of splicing and mobility, when maturase activity is provided from elsewhere. Furthermore, it might be assumed that an early event in the Etl. pomquetensis cpGenome was the deletion of an intron encoded ORF, which appeared to have occurred prior to the spreading of introns across the genome and that other group II introns with encoded IEPs or freestanding ORFs acted in trans to promote splicing and mobility of ORF-free introns (Doetsch, Thompson & Hallick, 1998). To gain information about DNA target sites, which the introns of Etl. pomquetensis use for retrotransposition, we checked the insertion sites and the sequences of flanking exons. The exon nucleotides at the 5′-insertion site of the intron did not show any similarity, which might be due to a not strictly controlled transposition/ retrotransposition processes (Pombert et al., 2012), thus helping with random intron invasion all over the genome, and on both strands. The only conspicuous DNA target site the 28 homologous introns with high sequence similarity used for reverse splicing was a pyrimidine base, which represented the first nucleotide of the following exon (except for atpE exon 3, I2). The gene psaA contained the most of these introns, and five of six introns contained high similarity.
A search for related introns in Etl. gymnastica, Et. viridis and P. parkeae and all other euglenoid cpGenomes did not reveal any sequential or positional homology. Insertion sites found in Etl. pomquetensis were unique to that taxon.
The highest pairwise identity of introns was found in E. gracilis var. bacillaris with 56.7%, but only for three small 97 bp long group III introns (rps16 I1, rpoC1 I7 and rps19 I2). Also, outside of euglenoid chloroplast introns, very few species showed high pairwise similarity. For instance, Brouard et al. (2016) found six group IIA introns in the chlorophyte Oedocladium carolinianum with high levels of nucleotide identities, which displayed over 80% pairwise identity. As well Turmel, Otis & Lemieux (2016) found several group II introns with high nucleotide identities also at various insertion sites, but only in small numbers. The introns of ycf3 and psbH in Gloeotilopsis sarcinoides were 85.6% identical. To our knowledge, Etl. pomquetensis is the first organism with more than 50 introns within protein coding genes and half of those sharing a pairwise identity of 90%.
Lack of group III introns in the genome
The second peculiarity in Etl. pomquetensis is the absence of group III introns. Group III introns are believed to be the descendants of group II introns which only retained domains DI and DVI (Christopher & Hallick, 1989). The 5′ -boundaries are more variable than in group II introns, but most group III introns have a U at position 2 and a G at position 5. Most of them are of dyad symmetry near the 3′ -end similar to domain VI of group II introns. The motif driving the symmetry follows abcdef (3–8)f′e′d′A∗c′b′a′ (Drager & Hallick, 1993). The 3′ sequence of group II and III introns are variable, although the branch-point A∗ is usually at position eight, sometimes at seven, and occasionally at position nine. Interestingly, none of the 51 identified introns of Etl. pomquetensis complied with the typically confined group III intron size of 91–120 nucleotides (Christopher & Hallick, 1989; Copertino & Hallick, 1993; Doetsch, Thompson & Hallick, 1998; Drager & Hallick, 1993). Underpinning these findings, 43 of 51 introns started with a typical group II 5′-GTGCG (Table S2, start marked bold) and even the smallest intron was over 300 bp long (rpoC1 I1 356 bp). Furthermore, the intron size was even larger than group III twintrons (group III introns within group III introns), which were found in the chloroplast genome of E. gracilis (Copertino, Shigeoka & Hallick, 1992; Copertino et al., 1994). The smallest introns of Etl. gymnastica and Et. viridis were rpoB I1 with 179 bp (re-analyses of data from (Hrdá et al., 2012; Wiegert, Bennett & Triemer, 2012) and 156 bp, respectively, and these were larger than group III introns. Hence, we assumed that group III introns probably evolved after Etl. pomquetensis diverged. Secondary the structure of domain V and VI of rpoB I1 in Etl. gymnastica and Et. viridis was recognizable when some mismatches were allowed in the analyses (Fig. S4). Degeneration and mutation of group II introns in euglenoids have been described before and are known to impact secondary structure elements. Even domain V tolerates a surprising number of mismatches (Michel & Ferat, 1995). To our present knowledge, such introns best resemble mini-group II introns, which lack different domains (Doetsch, Thompson & Hallick, 1998). Under the presumption that rpoB I1 of Etl. gymnastica and Et. viridis are mini group II introns and not group III introns, we assume that group III introns evolved probably within the Euglenales after fresh-water and brackish environments became accessible together with warmer temperatures. The impact of the environmental medium could have been a driving force on degenerating group II introns. The change from group II intron to group III introns was observed in the psbC intron containing mat1 (ycf13). It is clearly a group II intron/ twintron in all Eutreptiales, but a group III twintron in E. gracilis with an open reading frame (ycf13, mat1) within the internal group III intron (Copertino et al., 1994; Table 4). Copertino et al. (1994) proposed that mat1 may be involved in group III intron metabolism and is required for group III intron excision and/or mobility in Euglena and Astasia. The ORF of Euglena gracilis psbC I4 has detectable similarity to the RT domain of group II intron ORFs, although it lacks characteristics of functional RT activity (Copertino et al., 1994; Doetsch, Thompson & Hallick, 1998; Mohr, Perlman & Lambowitz, 1993).
Table 4. Features of the presumed ancestral psbC twintron in all cpGenomes of phototrophic euglenoids.
| Intron containing mat1 | psbC total Intron length (bp) | length mat1 (bp) | psbC intron length without mat1 (bp) | |
|---|---|---|---|---|
| E. gracilis | I4 | 1,605 | 1,377 | 228 |
| E. gracilis var. bacillaris | I4 | 1,605 | 1,377 | 228 |
| E. viridis | I2 | 1,612 | 1,359 | 258 |
| E. viridis epitype | I2 | 1,617 | 1,359 | 258 |
| E. mutabilis | I2 | 3,406 | 3,149 | 257 |
| Era. anabaena | I2 | 1,945 | 1,683 | 262 |
| M. parapyrum | I2 | 1,613 | 1,338 | 275 |
| M. aenigmatica | I2 | 1,618 | 1,389 | 229 |
| Cr. skujae | I3 | 1,629 | 1,362 | 267 |
| S. acuminata | I2 | 1,686 | 1,371 | 315 |
| T. volvocina | I2 | 2,534 | 1,672 | 862 |
| C. vesiculosum | a | 2,742 | ||
| Efs. proxima | I3 | 3,349 | 2,669 | 680 |
| P. orbicularis | I1 | 1,716 | 1,533 | 183 |
| Et. viridis | I1 | 4,350 | 3,609 | 741 |
| Etl. gymnastica | I1 | 1,778 | 1,137 | 641 |
| Etl. pomquetensis | I1 | 2,580 | 1,389 | 1,191 |
Notes.
annotation mistake.
Based on the greater length of the psbC intron in Etl. pomquetensis, and a typical group II intron 5′ -boundary, it seems likely that the psbC intron is instead a group II intron/twintron. All three Eutreptiales have a psbC intron including mat1 (ycf13) that is at least three times larger than the group III twintron (I4) including mat1 of E. gracilis (Table 4). These findings, and the fact that E. gracilis contained a group II -type maturase in a group III twintron (Doetsch, Thompson & Hallick, 1998; Mohr, Perlman & Lambowitz, 1993), underpin the possibility that group II introns evolved first in basally branching euglenoid species. Subsequently, they degenerated by loss of different domains (in more derived species) to group III introns, containing only DI-like and DVI-like structures (Doetsch, Thompson & Hallick, 1998; Lambowitz & Belfort, 2015). This finding is also supported by identification of two maturase encoded introns and their predicted secondary structure models in Lepocinclis buetschlii by Doetsch, Thompson & Hallick (1998). The authors interpreted these introns as group II/group III intermediates just in the process of losing group II intron domains and they were designated as mini-group-II introns.
Summarizing, we presume that group II introns appeared first in an intron-less ancestral genome and gave rise to group III introns and from there on degeneration went on independently in different lineages. Further on, either the Etl. pomquetensis group II intron mat1 or another intron encoded protein (IEP) act in trans to promote splicing and mobility of ORF-less introns.
Intron trends in Euglenoids
In their characterization of Euglenaceae, Bennett & Triemer (2015) noted that all Euglenaceae, but no Eutreptiales, contained an intron or twintron in petB (I1) and that this intron/twintron may be a synapomorphy for at least the Euglenaceae. Kasiborski, Bennett & Linton (2016) identified a homologous intron/twintron within petB I1 of P. orbicularis and discussed this intron/ twintron as a putative synapomorphy for the order Euglenales. However, in the cpGenome of Etl. pomquetensis two introns were detected in petB. The first was found at the identical insertion site, but nearly two times larger than that of E. gracilis strain Z and five times larger than that of P. orbicularis. All petB I1 introns started with a typical group II 5′ -GUGYG (P. orbicularis re-analysis, Table S1). This means, a group II intron in petB could neither be a synapomorphy for the Euglenales, nor for the Euglenaceae, but evidently evolved at least in Eutreptiella.
Twintron analysis
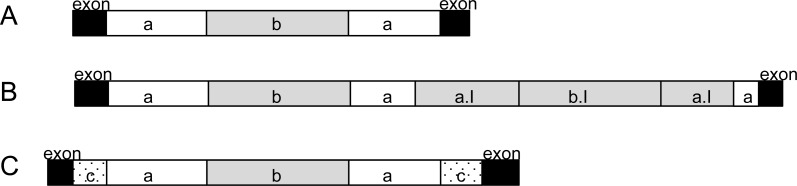
All 51 external introns were investigated for the presence of potential twintrons using a Python script, which searched for the conserved 3′ motif of group II and group III introns reported in Copertino & Hallick (1993). The search resulted in 28 external introns which contained at least one 3′ motif (see GenBank accession). Sixteen of the 28 introns contained four kinds of repeated 3′ motifs (Table S2, indicated by number of asterisks). Additionally, four potential group II twintrons were found (rpoB I1, rps2 I2, psbC I2, psbD I4, added to annotation) with only one 3′ motif and only one 5′ -GUGYG prior to the identified 3′ motif. Two of these potential group II twintrons (rpoB I1 and psbD I4) were those which share strong nucleotide identity with half of the introns detected in Etl. pomquetensis. We assume that all 28 introns (Table S2 marked bold except for petG I1 and psaC I2) with equal intron organization (5′ motif GTGCG, 3′ motif ACGTTCAT and further GTGCG at nt 261-265) are potential twintrons with an external and internal group II intron (Fig. 4A). Secondary structure analysis of domain V and VI of the potential internal introns of rpoB I1and psbD I4 in Etl. pomquetensis showed recognizable counterparts, when mismatches were allowed in the analyses (Fig. S5). For the potential internal introns in rpoB I1 and psbD I4 the conserved three base pairs (5′- …AGC …−3′) near the base of the stem of domain V were detectable, but the secondary structure showed a slightly altered terminal loop and no branch-point A∗ was detectable in domain VI (Michel & Ferat, 1995; Thompson et al., 1997). Since the Phyton script only detects an unaltered conserved 3′ motif, only two of the close related introns have been detected as potential twintrons. This underpins several statements, that group II introns of phototrophic euglenoids are highly degenerated and persistent to detailed analysis (Michel & Ferat, 1995; Mohr, Ghanem & Lambowitz, 2010). Two introns, psaC I2 and petG I1, out of the 28 potential twintrons with high sequence similarity were significantly larger and thus investigated for the presence of potential complex twintrons.
Figure 4. Analysis of potential twintrons with high sequence similarity.
(A) Highly conserved introns are shown. (B) Structure of the petG I1 complex twintron. (C) Structure of psaC I2. Black boxes represent exons. White boxes (a) are external introns of twintrons, white dotted boxes (c) are external introns of complex twintrons. Grey boxes (b, a.I, b.I) represent internal introns, whereby a.I showed high sequence similarity to external intron a and b.I to internal intron b.
psaC I2 analysis: The intron psaC I2 of Etl. pomquetensis was 1,294 bp long and by this more than 400 bp longer than the average. The nucleotide sequence alignment of all 28 introns (Table S2) was remarkably well conserved. It showed that psaC I2 is a complex twintron with an external intron interrupted by the same potential internal twintron as all the others (Figs. 4A and 4C).
The potential internal twintron (825 bp) shared 88% pairwise identity with the other 27 potential twintrons (Table S2). It is located 281 bp downstream of the external 5′ splice site. Comparing the secondary structure of domain V of the external intron a of the internal twintron (Fig. 4C) with the other highly conserved twintrons (Fig. 4A) resulted in identical stems and loops with only two out of 34 nucleotides differing (Fig. S3).
A BLASTn search for the external intron of psaC I2 (Fig. 4C dotted intron c) revealed weak similarity with psbC I2 (containing a still unspecified maturase) of Etl. gymnastica. Secondary structure analysis of domains V and VI of psbC I2 from Etl. gymnastica, realigned by Dabbagh & Preisfeld (2016), and the external intron of psaC I2 in Etl. pomquetensis revealed highly conserved structures of domains V (Fig. S6). They only differed in six nucleotides and contained the AGC motif near the base of the stem from the 5′-boundary (Thompson et al., 1997). We presume that the external intron of psaC I2 in Etl. pomquetensis (Fig. 4C dotted intron c) is closely related to and arose from the same ancestral intron as psbC I2 in Etl.gymnastica and that the intron degeneration and loss of the maturase in Etl. pomquetensis took place afterwards.
petG I1 analysis: We were also interested in closely investigating petG I1, because it was more than twice the size of all other highly conserved potential twintrons, but shared pairwise identities of 87.4%. This resulted in the identification of petG I1 as a complex twintron with high pairwise identities of internal and external twintrons (Fig. 4B). The two twintrons in petG I1 were the same and showed 90% pairwise identity. Both started with a 5′-GTGCG boundary, a 3′ -boundary ACGTTCAT motif and an additional GTGCG at insertion site 261. A comparison of the secondary structure of the introns (Fig. 4B intron a/ intron a.I) with the consensus domain V from the other highly conserved potential twintrons (Fig. S3) showed that 33 out of the 34 nucleotides were identical. The internal twintron comprised 799 bp and was located three nucleotides upstream from the 3′ splice site of the external twintron. It seems reasonable that the internal twintron proliferated into the external twintron and that both originated from the same twintron as the other ones (Figs. 4A and 4B).
Conclusion
Analysis of the genome of all euglenoids sequenced so far in regard to sequence and structural levels makes it apparent that the green algae origin is most visible in the cpGenome of Etl. pomquetensis. This can be seen by high pairwise identities in coding regions with the putative chloroplast ancestor P. parkeae and a typical green algae and land plant quadripartite genome structure. Still, independent evolution of the genomes since secondary endosymbiosis can also be observed in Etl. pomquetensis by decreased protein coding gene content and increased intron numbers compared to P. parkeae.
The cpGenome size of Etl. pomquetensis was substantially larger than those of other Eutreptiales published so far due to an increased number of introns and intergenic space, and was closest in size to the largest known euglenoid cpGenomes. This contradicts earlier assumptions that introns invaded cpGenomes massively in Euglenales. Interestingly, and unique within the phototrophic euglenoids, we detected a high similarity between more than half of the 51 introns. Another singularity was that no group III introns, or group III twintrons could be identified. This underlines the hypothesis that group II introns arrived first in basally branching euglenoid species and group III introns emerged from group II introns.
Finally, we speculate that future investigations could explore the possibility of a psychrophilic member of the Pyramimonas genus as a putative chloroplast donor to the euglenoid lineage and that Etl. pomquetensis may very well be the nearest relative up to date.
Supplemental Information
Each box represents a cluster of homologous genes with Eutreptiella pomquetensis as the reference genome. Like blocks are labelled by letters A-J. See Table 4 for a list of genes contained in each block. In the Mauve alignment the repeat regions of rRNA were not included, because Mauve will not align repeat regions, which have multiple matches on both genomes.
Each box represents a cluster of homologous genes between Eutreptiella pomquetensis as the reference genome and Pyramimonas parkeae (A), Pycnococcus provasolii (B) and Ostreococcus tauri (C). In the Mauve alignment the repeat regions of rRNA were not included, because Mauve will not align repeat regions, which have multiple matches on both genomes.
Consensus secondary structure model of domain V and VI of the highly conserved introns of Etl. pomquetensis based on the model proposed by Michel, Umesono & Ozeki, (1989) and on comparative analysis of other euglenoid group II introns (Thompson et al., 1997). The three base pairs (5’- ...AGC ... GUU…-3’) near the base of stem V were invariant (red box). Introns that form consensus sequence: atpB I1- I4; atp E I2; atp H I1; psa A I1- I4 & I6; psa B I1-I2; psa C I3; psb B I2; psb C I3 & I5; psb D I1& I4-I5; rbc L I1; rpl 32 I1; rpo B I1& I3; rps 7 I1; rps 12 I1.
Domain V and VI of rpo B I1 of Et. viridis (A) with branch-point A* at position 8 of domain VI and conserved three base pairs (5’- …AGC …-3’) near the base of the stem of domain V (red box). Domain V and VI of r po B I1 of Etl. gymnastica (B) with slightly altered base pairs (5’- …AGUC …-3’) of domain V (red box) but without branch-point A* at position 8 of domain VI.
Internal introns of rpo B I1 of Etl. pomquetensis (A) with conserved three base pairs (5’- …AGC …-3’) near the base of the stem of domain V (red box). Internal intron of psb D I4 of Etl. pomquetensis (B) with conserved three base pairs (5’- …AGC …-3’) near the base of the stem of domain V (red box).
Secondary structure model of putative domain V and VI of psa C I2 external intron of Etl. pomquetensis (A) with branch-point A* at position 7 of domain VI and conserved three base pairs (5’- …AGC …-3’) near the base of the stem of domain V (red box). Secondary structure model of putative domain V and VI of psb C I2 of Etl. gymnastica (B) with slightly altered branch-point AU* at positions 7 and 8 of domain VI and conserved three base pairs (5’- …AGC …-3’) near the base of the stem of domain V (red box).
* Chloroplast circle not closed. a Twintrons were counted as single insertion sites. b Including rRNA repeats and intermediate tRNAs. c Includes the identified 5S, for S. acuminata the two identified 5S. d Includes the two introns in rps18. e Includes the one intercistronic intron rps4-rps11. f First exon could not be identified, so gene length is a minimum. g Realigned, but with alternative start codon. h Start codon not determined, due to undetermined exon1 i: New intron start after re-analyses
Acknowledgments
The authors thank Onur Baltaci and Sabine Stratmann-Lettner for lab assistance. Additional thank is due to A. Donath (MITOS) for consultations regarding exonerate.
Funding Statement
ND received a doctoral scholarship from the Bergische University of Wuppertal, Germany. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Nadja Dabbagh conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables.
Matthew S. Bennett performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, reviewed drafts of the paper.
Richard E. Triemer contributed reagents/materials/analysis tools.
Angelika Preisfeld conceived and designed the experiments, contributed reagents/materials/analysis tools, prepared figures and/or tables, reviewed drafts of the paper.
DNA Deposition
The following information was supplied regarding the deposition of DNA sequences:
The sequences have been uploaded as Supplemental Files. The data is also available under GenBank accession number KY706202.
Data Availability
The following information was supplied regarding data availability:
The raw data has been supplied as a Supplementary File.
References
- Altschul et al. (1990).Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. Journal of Molecular Biology. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bennett, Shiu & Triemer (2017).Bennett MS, Shiu SH, Triemer RE. A rare case of plastid protein-coding gene duplication in the chloroplast genome of Euglena archaeoplastidiata (Euglenophyta) Journal of Phycology. 2017;53:493–502. doi: 10.1111/jpy.12531. [DOI] [PubMed] [Google Scholar]
- Bennett & Triemer (2015).Bennett MS, Triemer RE. Chloroplast genome evolution in the euglenaceae. Journal of Eukaryotic Microbiology. 2015;62:773–785. doi: 10.1111/jeu.12235. [DOI] [PubMed] [Google Scholar]
- Bennett, Wiegert & Triemer (2012).Bennett MS, Wiegert KE, Triemer RE. Comparative chloroplast genomics between Euglena viridis and Euglena gracilis (Euglenophyta) Phycologia. 2012;51:711–718. doi: 10.2216/12-017.1. [DOI] [Google Scholar]
- Bennett, Wiegert & Triemer (2014).Bennett MS, Wiegert KE, Triemer RE. Characterization of Euglenaformis gen. nov. and the chloroplast genome of Euglenaformis [Euglena] proxima (Euglenophyta) Phycologia. 2014;53:66–73. doi: 10.2216/13-198.1. [DOI] [Google Scholar]
- Benson (1999).Benson G. Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Research. 1999;27:573–580. doi: 10.1093/nar/27.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouard et al. (2016).Brouard J, Turmel M, Otis C, Lemieux C. Proliferation of group II introns in the chloroplast genome of the green alga Oedocladium carolinianum (Chlorophyceae) PeerJ. 2016;4:e2627. doi: 10.7717/peerj.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge et al. (2013).Burge SW, Daub J, Eberhardt R, Tate J, Barquist L, Nawrocki EP, Eddy SR, Gardner PP, Bateman A. Rfam 11.0: 10 years of RNA families. Nucleic Acids Research Database. 2013;41:D226–D232. doi: 10.1093/nar/gks1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun & Han (2006).Byun Y, Han K. PseudoViewer: web application and web service for visualizing RNA pseudoknots and secondary structures. Nucleic Acids Research. 2006;34:W416–W422. doi: 10.1093/nar/gkl210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattolico et al. (2008).Cattolico R, Jacobs MA, Zhou Y, Chang J, Duplessis M, Lybrand T, McKay J, Ong H, Sims E, Rocap G. Chloroplast genome sequencing analysis of Heterosigma akashiwo CCMP452 (West Atlantic) and NIES293 (West Pacific) strains. BMC Genomics. 2008;9:211. doi: 10.1186/1471-2164-9-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher & Hallick (1989).Christopher DA, Hallick RB. Euglena gracilis chloroplast ribosomal protein operon: a new chloroplast gene for ribosomal protein L5 and description of a novel organelle intron category designated group III. Nucleic Acids Research. 1989;17:7591–7608. doi: 10.1093/nar/17.19.7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conant & Wolfe (2008).Conant GC, Wolfe KH. GenomeVx: simple web-based creation of editable circular chromosome maps. Bioinformatics. 2008;24:861–862. doi: 10.1093/bioinformatics/btm598. [DOI] [PubMed] [Google Scholar]
- Copertino et al. (1994).Copertino DW, Hall ET, Van Hook FW, Jenkins KP, Hallick RB. A group III twintron encoding a maturase-like gene excises through lariat intermediates. Nucleic Acids Research. 1994;22:1029–1036. doi: 10.1093/nar/22.6.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copertino & Hallick (1993).Copertino DW, Hallick RB. Group II and group III introns of twintrons: potential relationships with nuclear pre-mRNA introns. Trends in Biochemical Sciences. 1993;18:467–471. doi: 10.1016/0968-0004(93)90008-B. [DOI] [PubMed] [Google Scholar]
- Copertino, Shigeoka & Hallick (1992).Copertino DW, Shigeoka S, Hallick RB. Chloroplast group III twintron excision utilizing multiple 5′- and 3′-splice sites. The EMBO Journal. 1992;11:5041–5050. doi: 10.1002/j.1460-2075.1992.tb05611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabbagh & Preisfeld (2016).Dabbagh N, Preisfeld A. The chloroplast genome of Euglena mutabilis —Cluster arrangement, intron analysis, and intrageneric trends. Journal of Eukaryotic Microbiology. 2016;64:31–44. doi: 10.1111/jeu.12334. [DOI] [PubMed] [Google Scholar]
- Dai & Zimmerly (2003).Dai L, Zimmerly S. ORF-less and reverse-transcriptase-encoding group II introns in archaebacteria, with a pattern of homing into related group II intron ORFs. RNA. 2003;9:14–19. doi: 10.1261/rna.2126203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling et al. (2004).Darling AC, Mau B, Blattner FR, Perna NT. Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Research. 2004;14:1394–1403. doi: 10.1101/gr.2289704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch, Thompson & Hallick (1998).Doetsch NA, Thompson MD, Hallick RB. A maturase-encoding group III twintron is conserved in deeply rooted euglenoid species: are group III introns the chicken or the egg? Molecular Biology and Evolution. 1998;15:76–86. doi: 10.1093/oxfordjournals.molbev.a025850. [DOI] [PubMed] [Google Scholar]
- Drager & Hallick (1993).Drager RG, Hallick RB. A complex twintron is excised as four individual introns. Nucleic Acids Research. 1993;21:2389–2394. doi: 10.1093/nar/21.10.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs (1978).Gibbs SP. The chloroplasts of Euglena may have evolved from symbiotic green algae. Canadian Journal of Botany. 1978;56:2883–2889. doi: 10.1139/b78-345. [DOI] [Google Scholar]
- Gibbs (1981).Gibbs SP. The chloroplasts of some algal groups may have evolved from endosymbiotic eukaryotic algae. Annals of the New York Academy of Sciences. 1981;361:193–208. doi: 10.1111/j.1749-6632.1981.tb54365.x. [DOI] [PubMed] [Google Scholar]
- Gockel & Hachtel (2000).Gockel G, Hachtel W. Complete gene map of the plastid genome of the nonphotosynthetic euglenoid flagellate Astasia longa. Protist. 2000;151:347–351. doi: 10.1078/S1434-4610(04)70033-4. [DOI] [PubMed] [Google Scholar]
- Guillard & Hargraves (1993).Guillard RRL, Hargraves PE. Stichochrysis immobilis is a diatom, not a chrysophyte. Phycologia. 1993;32:234–236. doi: 10.2216/i0031-8884-32-3-234.1. [DOI] [Google Scholar]
- Hallick et al. (1993).Hallick RB, Hong L, Drager RG, Favreau MR, Monfort A, Orsat B, Spielmann A, STUTZ E. Complete sequence of Euglena gracilis chloroplast DNA. Nucleic Acids Research. 1993;21:3537–3544. doi: 10.1093/nar/21.15.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrdá et al. (2012).Hrdá Š, Fousek J, Szabová J, Hampl V, Vlček Č. The plastid genome of Eutreptiella provides a window into the process of secondary endosymbiosis of plastid in euglenids. PLOS ONE. 2012;7:e33746. doi: 10.1371/journal.pone.0033746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasiborski, Bennett & Linton (2016).Kasiborski BA, Bennett MS, Linton EW. The chloroplast genome of Phacus orbicularis (Euglenophyceae): an initial datum point for the phacaceae. Journal of Phycology. 2016;52:404–411. doi: 10.1111/jpy.12403. [DOI] [PubMed] [Google Scholar]
- Kearse et al. (2012).Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelchner (2002).Kelchner SA. Group II introns as phylogenetic tools: structure, function, and evolutionary constraints. American Journal of Botany. 2002;89:1651–1669. doi: 10.3732/ajb.89.10.1651. [DOI] [PubMed] [Google Scholar]
- Kurtz et al. (2001).Kurtz S, Choudhuri JV, Ohlebusch E, Schleiermacher C, Stoye J, Giegerich R. REPuter: the manifold applications of repeat analysis on a genomic scale. Nucleic Acids Research. 2001;29:4633–4642. doi: 10.1093/nar/29.22.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagesen et al. (2007).Lagesen K, Hallin P, Rødland EA, Staerfeldt H, Rognes T, Ussery DW. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Research. 2007;35:3100–3108. doi: 10.1093/nar/gkm160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambowitz & Belfort (2015).Lambowitz AM, Belfort M. Mobile bacterial group II introns at the crux of eukaryotic evolution. Microbiology Spectrum. 2015;3:MDNA3-0050-2014. doi: 10.1128/microbiolspec.MDNA3-0050-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambowitz & Zimmerly (2011).Lambowitz AM, Zimmerly S. Group II introns: mobile ribozymes that invade DNA. Cold Spring Harbor Perspectives in Biology. 2011;3:a003616. doi: 10.1101/cshperspect.a003616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leander, Witek & Farmer (2001).Leander BS, Witek RP, Farmer MA. Trends in the evolution of the euglenid pellicle. Evolution; International Journal of Organic Evolution. 2001;55:2215–2235. doi: 10.1111/j.0014-3820.2001.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Leedale (1967).Leedale GF. Euglenoid flagellates. New Jersey: Prentice Hall; 1967. p. 242. [Google Scholar]
- Lemieux, Otis & Turmel (2007).Lemieux C, Otis C, Turmel M. A clade uniting the green algae Mesostigma viride and Chlorokybus atmophyticus represents the deepest branch of the Streptophyta in chloroplast genome-based phylogenies. BMC Biology. 2007;5:2–10. doi: 10.1186/1741-7007-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton et al. (1999).Linton EW, Hittner D, Lewandowski C, Auld T, Triemer RE. A molecular study of euglenoid phylogeny using small subunit rDNA. The Journal of Eukaryotic Microbiology. 1999;46:217–223. doi: 10.1111/j.1550-7408.1999.tb04606.x. [DOI] [PubMed] [Google Scholar]
- Linton et al. (2000).Linton EW, Nudelman MA, Conforti V, Triemer RE. A molecular analysis of the Euglenophytes using SSU rDNA. Journal of Phycology. 2000;36:740–746. doi: 10.1046/j.1529-8817.2000.99226.x. [DOI] [PubMed] [Google Scholar]
- Marin (2004).Marin B. Origin and fate of chloroplasts in the euglenoida. Protist. 2004;155:13–14. doi: 10.1078/1434461000159. [DOI] [PubMed] [Google Scholar]
- Marin et al. (2003).Marin B, Palm A, Klingberg M, Melkonian M. Phylogeny and taxonomic revision of plastid-containing euglenophytes based on SSU rDNA sequence comparisons and synapomorphic signatures in the SSU rRNA secondary structure. Protist. 2003;154:99–145. doi: 10.1078/143446103764928521. [DOI] [PubMed] [Google Scholar]
- McLachlan, Seguel & Fritz (1994).McLachlan JL, Seguel MR, Fritz L. Tetreutreptia pomquetensis gen. et sp. nov. (Euglenophyceae): a quadriflagellate, phototrophic marine euglenoid. Journal of Phycology. 1994;30:538–544. doi: 10.1111/j.0022-3646.1994.00538.x. [DOI] [Google Scholar]
- Merendino et al. (2006).Merendino L, Perron K, Rahire M, Howald I, Rochaix JD, Goldschmidt-Clermont M. A novel multifunctional factor involved in trans-splicing of chloroplast introns in Chlamydomonas. Nucleic Acids Research. 2006;34:262–274. doi: 10.1093/nar/gkj429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel & Ferat (1995).Michel F, Ferat JL. Structure and activities of group II introns. Annual Review of Biochemistry. 1995;64:435–461. doi: 10.1146/annurev.bi.64.070195.002251. [DOI] [PubMed] [Google Scholar]
- Mohr, Ghanem & Lambowitz (2010).Mohr G, Ghanem E, Lambowitz AM. Mechanisms used for genomic proliferation by thermophilic group II introns. PLOS Biology. 2010;8:e1000391. doi: 10.1371/journal.pbio.1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr, Perlman & Lambowitz (1993).Mohr G, Perlman PS, Lambowitz AM. Evolutionary relationships among group II intron-encoded proteins and identification of a conserved domain that may be related to maturase function. Nucleic Acids Research. 1993;21:4991–4997. doi: 10.1093/nar/21.22.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pombert et al. (2012).Pombert J, James ER, Janouškovec J, Keeling PJ, McCutcheon J. Evidence for transitional stages in the evolution of euglenid group II introns and twintrons in the Monomorphina aenigmatica plastid genome. PLOS ONE. 2012;12:e53433. doi: 10.1371/journal.pone.0053433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preisfeld et al. (2001).Preisfeld A, Busse I, Klingberg M, Talke S, Ruppel HG. Phylogenetic position and inter-relationships of the osmotrophic euglenids based on SSU rDNA data, with emphasis on the Rhabdomonadales (Euglenozoa) International Journal of Systematic and Evolutionary Microbiology. 2001;51:751–758. doi: 10.1099/00207713-51-3-751. [DOI] [PubMed] [Google Scholar]
- Ravi et al. (2008).Ravi V, Khurana JP, Tyagi AK, Khurana P. An update on chloroplast genomes. Plant Systematics and Evolution. 2008;271:101–122. doi: 10.1007/s00606-007-0608-0. [DOI] [Google Scholar]
- Robbens et al. (2007).Robbens S, Derelle E, Ferraz C, Wuyts J, Moreau H, Van de Peer Y. The complete chloroplast and mitochondrial DNA sequence of Ostreococcus tauri: organelle genomes of the smallest eukaryote are examples of compaction. Molecular Biology and Evolution. 2007;24:956–968. doi: 10.1093/molbev/msm012. [DOI] [PubMed] [Google Scholar]
- Schattner, Brooks & Lowe (2005).Schattner P, Brooks AN, Lowe TM. The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Research Web Server. 2005;33:W686–W689. doi: 10.1093/nar/gki366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater & Birney (2005).Slater GStC, Birney E. Automated generation of heuristics for biological sequence comparison. BMC Bioinformatics. 2005;1:31. doi: 10.1186/1471-2105-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura et al. (2011).Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson et al. (1995).Thompson MD, Copertino DW, Thompson E, Favreau MR, Hallick RB. Evidence for the late origin of introns in chloroplast genes from an evolutionary analysis of the genus Euglena. Nucleic Acids Research. 1995;23:4745–4752. doi: 10.1093/nar/23.23.4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson et al. (1997).Thompson MD, Zhang L, Hong L, Hallick RB. Extensive structural conservation exists among several homologs of two Euglena chloroplast group II introns. Molecular and General Genetics. 1997;257:45–54. doi: 10.1007/s004380050622. [DOI] [PubMed] [Google Scholar]
- Toor, Hausner & Zimmerly (2001).Toor N, Hausner G, Zimmerly S. Coevolution of group II intron RNA structures with their intron-encoded reverse transcriptases. RNA. 2001;7:1142–1152. doi: 10.1017/S1355838201010251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turmel et al. (2009).Turmel M, Gagnon M, O’Kelly CJ, Otis C, Lemieux C. The chloroplast genomes of the green algae Pyramimonas, Monomastix, and Pycnococcus shed new light on the evolutionary history of prasinophytes and the origin of the secondary chloroplasts of euglenids. Molecular Biology and Evolution. 2009;26:631–648. doi: 10.1093/molbev/msn285. [DOI] [PubMed] [Google Scholar]
- Turmel, Otis & Lemieux (2016).Turmel M, Otis C, Lemieux C. Mitochondrion-to-chloroplast DNA transfers and intragenomic proliferation of chloroplast group II introns in Gloeotilopsis green algae (Ulotrichales, Ulvophyceae) Genome Biology and Evolution. 2016;8:2789–2805. doi: 10.1093/gbe/evw190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Untergasser et al. (2012).Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG. Primer3—new capabilities and interfaces. Nucleic Acids Research. 2012;40:e115. doi: 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegert, Bennett & Triemer (2012).Wiegert KE, Bennett MS, Triemer RE. Evolution of the chloroplast genome in photosynthetic euglenoids: a comparison of Eutreptia viridis and Euglena gracilis (Euglenophyta) Protist. 2012;163:832–843. doi: 10.1016/j.protis.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Wiegert, Bennett & Triemer (2013).Wiegert KE, Bennett MS, Triemer RE. Tracing patterns of chloroplast evolution in euglenoids: contributions from Colacium vesiculosum and Strombomonas acuminata (Euglenophyta) Journal of Eukaryotic Microbiology. 2013;60:214–221. doi: 10.1111/jeu.12025. [DOI] [PubMed] [Google Scholar]
- Yamaguchi, Yubuki & Leander (2012).Yamaguchi A, Yubuki N, Leander BS. Morphostasis in a novel eukaryote illuminates the evolutionary transition from phagotrophy to phototrophy: description of Rapaza viridis n. gen. et sp. (Euglenozoa, Euglenida) BMC Evolutionary Biology. 2012;12:29. doi: 10.1186/1471-2148-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuker (2003).Zuker M. Mfold web server for nuclei acid folding and hybridization prediction. Nucleic Acids Research. 2003;31:3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Each box represents a cluster of homologous genes with Eutreptiella pomquetensis as the reference genome. Like blocks are labelled by letters A-J. See Table 4 for a list of genes contained in each block. In the Mauve alignment the repeat regions of rRNA were not included, because Mauve will not align repeat regions, which have multiple matches on both genomes.
Each box represents a cluster of homologous genes between Eutreptiella pomquetensis as the reference genome and Pyramimonas parkeae (A), Pycnococcus provasolii (B) and Ostreococcus tauri (C). In the Mauve alignment the repeat regions of rRNA were not included, because Mauve will not align repeat regions, which have multiple matches on both genomes.
Consensus secondary structure model of domain V and VI of the highly conserved introns of Etl. pomquetensis based on the model proposed by Michel, Umesono & Ozeki, (1989) and on comparative analysis of other euglenoid group II introns (Thompson et al., 1997). The three base pairs (5’- ...AGC ... GUU…-3’) near the base of stem V were invariant (red box). Introns that form consensus sequence: atpB I1- I4; atp E I2; atp H I1; psa A I1- I4 & I6; psa B I1-I2; psa C I3; psb B I2; psb C I3 & I5; psb D I1& I4-I5; rbc L I1; rpl 32 I1; rpo B I1& I3; rps 7 I1; rps 12 I1.
Domain V and VI of rpo B I1 of Et. viridis (A) with branch-point A* at position 8 of domain VI and conserved three base pairs (5’- …AGC …-3’) near the base of the stem of domain V (red box). Domain V and VI of r po B I1 of Etl. gymnastica (B) with slightly altered base pairs (5’- …AGUC …-3’) of domain V (red box) but without branch-point A* at position 8 of domain VI.
Internal introns of rpo B I1 of Etl. pomquetensis (A) with conserved three base pairs (5’- …AGC …-3’) near the base of the stem of domain V (red box). Internal intron of psb D I4 of Etl. pomquetensis (B) with conserved three base pairs (5’- …AGC …-3’) near the base of the stem of domain V (red box).
Secondary structure model of putative domain V and VI of psa C I2 external intron of Etl. pomquetensis (A) with branch-point A* at position 7 of domain VI and conserved three base pairs (5’- …AGC …-3’) near the base of the stem of domain V (red box). Secondary structure model of putative domain V and VI of psb C I2 of Etl. gymnastica (B) with slightly altered branch-point AU* at positions 7 and 8 of domain VI and conserved three base pairs (5’- …AGC …-3’) near the base of the stem of domain V (red box).
* Chloroplast circle not closed. a Twintrons were counted as single insertion sites. b Including rRNA repeats and intermediate tRNAs. c Includes the identified 5S, for S. acuminata the two identified 5S. d Includes the two introns in rps18. e Includes the one intercistronic intron rps4-rps11. f First exon could not be identified, so gene length is a minimum. g Realigned, but with alternative start codon. h Start codon not determined, due to undetermined exon1 i: New intron start after re-analyses
Data Availability Statement
The following information was supplied regarding data availability:
The raw data has been supplied as a Supplementary File.