Abstract
Objective
This report evaluates clinical experience with the Rezūm system after US Food and Drug Administration clearance in consecutive cases accrued by multiple community urologists for the treatment of lower urinary tract symptoms (LUTS) associated with benign prostatic hyperplasia (BPH). Treatment techniques for transurethral convective radiofrequency water-vapor thermal therapy and outcomes with up to 12 months’ follow-up are presented.
Materials and methods
A total of 131 patients with moderate–severe LUTS were included in a retrospective analysis of BPH procedures with the Rezūm system. Pre- and postprocedure assessments included International Prostate Symptom Score (IPSS), quality of life, peak urinary flow rate, voided volume, and postvoid residual urine volume. Urologists used their own discretion for patient selection, with variable prostate sizes, LUTS severity, urinary retention, or presence of an obstructing median lobe. Safety signals and surgical retreatment rates were monitored prospectively.
Results
Men aged 47–96 years with prostates 13–183 cm3 showed significant improvement in IPSS, quality of life, and postvoid residual volume durable through 12 months after thermal therapy. Patients with either moderate (IPSS 8–19) or severe (IPSS 20–35) symptoms achieved significantly improved scores. Postprocedure adverse events normally anticipated and related to endoscopic instrumentation were transient and mild–moderate in nature. No de novo erectile or ejaculatory dysfunction was reported.
Conclusion
This study corroborates prior published pilot and randomized controlled trial results indicating significant relief of urinary symptoms and reproducibility of responses to thermal therapy. Convective radiofrequency thermal therapy with the Rezūm system warrants consideration as a first-line treatment for LUTS/BPH as an alternative to the use of pharmaceutical agents.
Keywords: prostate, prostatic hyperplasia, lower urinary tract symptoms, convective RF thermal therapy, minimally invasive procedure
Introduction
How does one best approach and select the most efficient therapeutic management of benign prostatic hyperplasia (BPH) with lower urinary tract symptoms (LUTS)? BPH is a common chronic condition often associated with progressive development of voiding and obstructive LUTS. The ten most prominent and costly disease conditions in men over 50 years of age in the US include BPH with LUTS,1 and it is the commonest diagnosis made by urologists for men 45–74 years of age.2 A review of several large administrative and community-based cohorts through the year 2000 indicated a high prevalence of BPH, with almost 8 million visits made to physicians’ offices for a primary or secondary diagnosis of this condition.3 Over a broader time span, data from the National Ambulatory Medical Care Survey (1993–2010) identified over 101 million outpatient visits for men with a diagnosis of BPH/LUTS.4,5
Treatment options include medical, surgical, and a new wave of minimally invasive therapies. The treatment-strategy challenge relates to when to initiate treatment, substitute, or advance to more aggressive therapy. The objective should always focus on achievement of the best specific outcome (eg, improving LUTS and preserving erectile and ejaculatory function) for each patient, but with consideration of costs to both patients and payers. It is estimated that 20% of the population will reach 65 years of age or older by 2030 and those 85 years and older will represent the fastest-growing segment of our population.6 Therefore, the prevalence of symptomatic BPH will increase proportionally with this aging population and foretells significant health-care system financial pressures related to BPH care.7 The expenditure for this chronic condition exceeds US$1 billion in the Medicare program alone, and is estimated to be as high as $6 billion.3,8
The availability of minimally invasive surgical treatment (MIST) for LUTS/BPH now allows the urologist to tailor therapies in a continuum of care from medical management to more invasive surgical procedure approaches. A recent new MIST (Rezūm) employs a platform technology to convectively deliver to prostate tissue stored thermal energy in the form of water vapor (steam) created with radiofrequency (RF) current to produce instantaneous cell death in targeted tissue. The procedure is transurethral needle ablation, but with considerably more efficient delivery of thermal energy to heat and ablate the tissue. Convective RF thermal therapy with the Rezūm system results in rapid, significant, and durable improvements in LUTS, while at the same time preserving erectile and ejaculatory function.9–13 There are minimal transient perioperative side effects but resultant enhanced quality of life (QOL) in patients with LUTS due to BPH. The safety and effectiveness of convective RF thermal therapy with the Rezūm system is supported by selective evidence-based clinical studies.13 Although randomized controlled trials (RCTs) have provided guidelines and expectations within designated criteria for use of a device, acquisition of data after US Food and Drug Administration (FDA) clearance of a device provides great value in developing patient-management strategies and reassuring the community of practicing urologists concerning safety and efficacy in a broader patient population.
The purpose of this study was to evaluate the safety and efficacy of BPH treatments with the Rezūm system from community urology-practice groups following FDA clearance of the Rezūm system in 2015. The objective was to include detailed patient selection, evaluation, and surgical techniques of this next-generation transurethral thermal therapy. This is the first report of the clinical and practical assessments of a postmarket device to provide a more realistic and broader-spectrum view of real-world patients compared to patients in a registration-directed RCT with restrictive inclusion and exclusion criteria. The retrospective analysis includes outcomes to 12 months of postprocedure follow-up.
Materials and methods
Plan for patient accrual
Men with bothersome LUTS due to BPH were treated with Rezūm convective RF thermal therapy by multiple urologists in two large group practices from November 2015. Data accrual up to 12 months of follow-up continued until May 2017. These centers were early users of this thermal therapy, each with its own standard of practice for evaluation of LUTS, selection of patients appropriate for ablative thermal therapy, and assessment of response to the therapy. Compared to a formal clinical trial, clinicians had flexibility in patient selection with respect to variable prostate sizes, symptom severity, flow rate, and no morphological limitations, including patients with an obstructing median lobe and/or enlarged central zone. Patient selection and treatment did not follow a standardized protocol. Although the intent was to follow as many patients as possible, there was no obligation for patients to comply with follow-up evaluations. Each center provided prospectively accrued data on consecutively treated men for analysis; total accrual for evaluation was planned for at least 100 patients. Patient chart reviews were performed with the approval of the Western Institutional Review Board. Patient consent was not required by the board, as deidentified patient numbers were assigned for data accrual on Excel spreadsheets to maintain patient-data confidentiality.
Rezūm thermal therapy device
All convective RF thermal therapy procedures were conducted with the Rezūm system (NxThera, Maple Grove, MN, USA), which includes an RF power-supply generator and single-use transurethral delivery device, and utilizes a standard reusable 4 mm, 30° rigid lens allowing treatment needle placement under direct cystoscopic visualization. The stored thermal energy of water vapor is created by applying RF current against an inductive coil in the handle of the delivery device (Figure 1). The handheld control delivers thermal energy in the form of water vapor at approximately 103°C, providing a consistent energy dose of approximately 208 calories per treatment into the prostate tissue through a retractable 18-gauge polyetheretherketone (insulated plastic) needle. The Rezūm system and the principles of RF-generated water-vapor thermal energy based on the thermodynamic properties of convective versus conductive heat transfer to ablate tissue have been previously described.10,13 The device received Conformité Européene (European Conformity, CE) marking in 2013 and 510(k) clearance from the FDA in 2015. The commercially marketed device and therapeutic algorithm are the same used in the previous RCT.10,12 All treating physicians received training prior to use of the Rezūm system.
Figure 1.
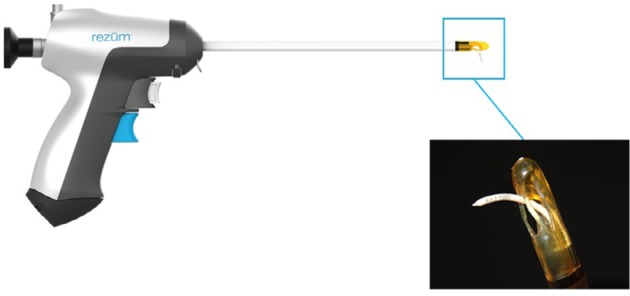
Rezūm delivery device and vapor needle.
Note: The vapor needle resides within the insulated lumen of the delivery device until it is deployed into the prostate tissue.
Patient selection and evaluation
Patients with moderate–severe LUTS were offered treatment with the Rezūm system to ameliorate the anatomical tissue interference that causes BPH and outlet obstruction as an alternative to medications for symptomatic relief, or after inadequate relief or intolerance of drug use. Similarly, the progression of prostate-tissue impediment may be halted as the result of treatment at an earlier stage. While early clinical experience suggests excellent outcomes in larger glands, a urologist’s early experience for treating BPH with the Rezūm system should be reserved for smaller glands until more experience has been gained. The anatomy of larger glands may be challenging, include the propensity for some bleeding. Patients may have anticoagulation withheld for the procedure and for a short time afterward in consultation with their cardiologist.
Routine assessments included a complete medical history and physical examination, prostate-specific antigen, cystoscopy, transrectal ultrasonography (TRUS), urinalysis, maximum urinary flow rate (Qmax) and postvoid residual (PVR) urine-volume measurements. Patients completed the International Prostate Symptom Score (IPSS) questionnaire, unless they were in urinary retention, and the IPSS QOL question. Urodynamic studies are helpful in assessing appropriate patients for treatment, but this practice is not uniformly recommended prior to proceeding with surgical therapy, based on American Urological Association Guidelines for management for BPH.14 One of the pitfalls of proceeding with treatment without urodynamics may involve ignorance of bladder function, including either impaired bladder contractility or overactivity that may contribute to the total LUTS complex. When obtained, urodynamics are felt to be invaluable to provide a basis for perioperative issues. The management of pain and anxiety is based on clinicians’ discretion. This can be assessed with preprocedure cystoscopy and/or TRUS. Options used in the two centers included either intravenous sedation or prostate block followed by posttreatment analgesics. Oral sedation only had been used predominantly for anesthesia in previous clinical trials.9,10
BPH procedures with the Rezūm system
Convective RF thermal therapy with the Rezūm system is performed with the patient in the dorsal lithotomy position; the treatment device is inserted into the urethra. Confirmation of the contours of the prostate and planned disbursement of thermal lesions as derived from baseline cystoscopy is an appropriate first pass. Examination of the bladder and specifically the ureteral orifices is important, particularly in the event that median-lobe tissue has elevated the orifice. Treatment begins with the needle tip visually positioned and inserted from approximately 1 cm distally to the bladder neck. It is suggested to complete all treatments on one side of the gland, in order to take advantage of the latent heat from prior treatments on that side, and then proceed to treat tissue on the contralateral side of the gland. Multiple thermal treatments are delivered with the retractable vapor needle, which penetrates a fixed 10.25 mm into the prostatic tissue. Each treatment delivers approximately 208 calories of thermal energy by converting 0.42 mL of sterile water into vapor thermal energy with the application of RF current to the inductive coil within the device. The treatment needle has a total of 12 small emitter holes spaced around its tip at 120° intervals to allow circumferential dispersion of thermal energy to create an approximate 1.5–2 cm lesion that remains confined within the anatomical zones of the prostate. A continuous saline (room temperature)-flush irrigation through the device lumen enhances visualization and cools the urethral surface to preserve the urethral lining.
Prostate adenomas in the transition and central zones can be precisely targeted. Any intravesical prostatic protrusions of either lateral or median lobes are injected from 1 cm from the proximal edge of the protrusion. This is an important point, as intravesical protrusions can be accurately and effectively targeted, which makes the Rezūm system unique as a minimally invasive procedure. Median-lobe treatments entail angulation of the treatment needle at 45° medially into the tissue. The treatment needle is retracted after each treatment and repositioned in 1 cm increments distally from the previous site to the end of the prostatic tissue just proximal to the verumontanum. The objective of the treatment is to create contiguous, overlapping lesions running parallel to the natural slope of the urethra, hence prior “mapping” of the prostate is important. Both preprocedure cystoscopy and TRUS help to “map” or plan the ideal treatment for the best outcomes. The total number of treatments in each lobe of the prostate is determined by the length of the hypertrophied prostatic tissue, and can be customized to the configuration of the gland including the median lobe.
Data analysis
Data analysis was performed by a biostatistician at an independent medical research organization. Descriptive statistics were used to describe baseline and postoperative follow-up values for all study variables, and data are presented as means, SD, mean changes, 95% CIs. For each outcome measure, the paired change from baseline to follow-up visits at 1, 3–6, and 12 months after Rezūm thermal therapy was analyzed with descriptive statistics and also with a longitudinal general estimation-equation model using an exchangeable working correlation structure. This model was used to assess the statistical significance of changes from baseline, as this method takes into account the correlation within a subject over time and uses that information to adjust the standard error of the estimates.15 Significance was determined by a P-value less than 0.05. Analysis also included the proportion of patients with a change in IPSS that met a clinically meaningful threshold, defined by the American Urological Association as ≥3-point increase in IPSS relative to baseline.14 Patients meeting or exceeding this level of improvement are considered responders.
Results
A total of 131 consecutive patients were treated with Rezūm convective RF water-vapor thermal therapy by seven urologists in large group-community practices. Patient demographics were similar to those in prior published reports for pilot studies and RCTs.9,10,12 Some notable differences were apparent compared to previous studies, including the older mean age of 70.9 (47–96) years, broader range of prostate volumes (mean 45.1 [12.9–183] cm3), and higher PVR volumes (mean 216.6 [0–2,000] mL), with 26% of patients having a PVR volume >250 mL and three in retention (Table 1). Several patients (12%) had prior surgery or MIST procedure, including transurethral resection of the prostate (three), transurethral conductive RF thermal therapy (nine), transurethral microwave thermal therapy (one), transurethral microwave thermal therapy and prostatic urethral lift (one), and Rezūm convective RF thermal therapy (two).
Table 1.
Baseline characteristics
| Mean (SD) Median (range) |
n | |
|---|---|---|
| Age, years | 70.9 (9.4) 71.3 (47.4–96.4) |
131 |
| Prostate volume (cm3) | 45.1 (23.4) 38 (12.9–183) |
130 |
| PSA (ng/mL) | 3.5 (5.6) 2.2 (0.1–52.2) |
109 |
| Qmax (mL/second) | 8.6 (4.9) 8.1 (1.5–23.1) |
94 |
| PVR volume (mL): all patients | 216.8 (286.6) 150 (0–2,000) |
115 |
| PVR >250 mL | 30 (26.1%) | 115 |
| IPSS* – all patients | 19.5 (6.6) 19 (9–35) |
128 |
| LUTS severity | ||
| Moderate (IPSS 8–19) | 68 (53.1%) | |
| Severe (IPSS 20–35) | 60 (46.9%) |
Note:
Range 0 (no symptoms) to 35 (maximal symptoms).
Abbreviations: PSA, prostate-specific antigen; Qmax, peak urinary flow; PVR, postvoid residual; IPSS, International Prostate Symptom Score; LUTS, lower urinary tract symptoms.
All treatments were successfully completed without perioperative device- or procedure-related adverse events. Procedures were performed with intravenous sedation (86%), general anesthesia (15%), or prostate block (6%). The total number of treatments in lateral lobes averaged 4.4 (range 2–12). The median lobe and/or enlarged central zone was identified and treated in 54 patients (41%) of 131, with an average of 1.6 (range 1–6) treatments.
Nonserious, procedure-related adverse events and anticipated events included postoperative acute urinary retention (10.7% [14 of 131]), and urinary frequency, urgency, frequency and urgency, hematuria, and nocturia in ≤3.8% of 131 patients. Adverse events were mild–moderate in severity, and most resolved within a short time after routine treatment or without treatment. Although this study did not collect sexual-function data via validated questionnaires, all patients were asked about sexual function, and there were no reports of de novo erectile or ejaculatory dysfunction. Three patients (2%) with obstructing residual tissue or insufficient improvement underwent a transurethral resection of the prostate procedure 7–12 months later; one patient had a second Rezūm procedure 12 months later.
Observed outcomes for all patients at 1, 3–6, and 12 months after thermal therapy are presented in Table 2. Three patients in retention did not have a baseline IPSS. Clinically significant relief of LUTS was observed with baseline IPSS reduced by 16%, 47%, and 45% at the three time-point evaluations, respectively (P<0.0001). Mean baseline IPSS of 19.5 decreased an average of 10.1 and 9.4 points at 3–6 months and 12 months. Among these were 54 patients that had a median lobe/enlarged central zone treated. They were more likely to have ≥3-point improvement in IPSS (91.7% versus 75.4%, P=0.03) after treatment of this prostate tissue. Furthermore, the 23 patients with prostates ≥60 cm3 had a similar likelihood of a meaningful clinical outcome of at least ≥3-point IPSS improvement compared with patients with prostates <60 cm3 (94.7% versus 78.9%, P=0.11). Significant improvements in QOL and PVR volume were commensurate with IPSS improvements. IPSS improvements are also presented in Figure 2, comparing outcomes of this postmarket study and replication of the profile of IPSS changes in a previous pilot study and RCT.
Table 2.
Changes in outcome measures after convective RF thermal therapy: all patients and patient subsets, based on severity of LUTS at baseline
| All LUTS categories: baseline IPSS 8–35
| ||||
|---|---|---|---|---|
| Outcome measure | Baseline | 1 month | 3–6 months | 12 months |
| IPSS | ||||
| n (paired values) | 128 | 93 | 115 | 87 |
| Baseline | 19.5 (6.6) | 19.9 (6.5) | 19.9 (6.7) | 19.4 (6.7) |
| Follow-up | 16 (8) | 9.8 (6.9) | 10.1 (7.2) | |
| Change | −3.9 (8.2) | −10.1 (8.8) | −9.4 (8.7) | |
| % change | −15.9 | −47.2 | −45.2 | |
| P-value (GEE) | <0.0001 | <0.0001 | <0.0001 | |
| QOL (IPSS question 8) | ||||
| n (paired values) | 112 | 81 | 104 | 74 |
| Baseline | 4.3 (1.2) | 4.4 (1.2) | 4.3 (1.2) | 4.4 (1.3) |
| Follow-up | 3.7 (1.8) | 2.3 (1.5) | 2.5 (1.4) | |
| Change | −0.6 (1.9) | −2 (1.7) | −1.9 (1.8) | |
| % change | −7.2 | −42.7 | −37.8 | |
| P-value (GEE) | 0.0007 | <0.0001 | <.00001 | |
| Qmax (mL/second) | ||||
| n (paired values) | 94 | 23 | 38 | 7 |
| Baseline | 8.6 (4.9) | 8.3 (3.8) | 8.7 (4.7) | 8.5 (3.5) |
| Follow-up | 9.6 (5.9) | 11.6 (7.7) | 10 (5) | |
| Change | 1.3 (5.1) | 3 (9) | 1.5 (5.9) | |
| % change | 20.7 | 75.3 | 51.4 | |
| P-value (GEE) | 0.2047 | 0.0388 | 0.4257 | |
| PVR volume (mL) | ||||
| n (paired values) | 115 | 83 | 89 | 35 |
| Baseline | 216.8 (286.6) | 209.9 (273.5) | 243.8 (316.7) | 236.6 (341.3) |
| Follow-up | 82.5 (144.2) | 85.8 (167.3) | 77.3 (122.1) | |
| Change | −127 (257.1) | −158 (221.8) | −159 (254.7) | |
| % change | 44.6 | −30.2 | −34.9 | |
| P-value (GEE) | <0.0001 | <0.0001 | <0.0001 | |
| Voided volume (mL) | ||||
| n (paired values) | 94 | 25 | 38 | 7 |
| Baseline | 163.2 (108.4) | 165.4 (122.8) | 192.3 (119.4) | 182.7 (119.4) |
| Follow-up | 114.6 (77.3) | 146.7 (100.6) | 138.4 (103.1) | |
| Change | −50.8 (133.9) | −45.5 (149.8) | −44.2 (146.6) | |
| % change | 12.9 | 30.9 | 0.5 | |
| P-value (GEE) | 0.0377 | 0.1948 | 0.5129 | |
|
| ||||
|
Moderate LUTS: baseline IPSS 8–19
| ||||
| Outcome measure | Baseline | 1 month | 3–6 months | 12 months |
|
| ||||
| IPSS | ||||
| n (paired values) | 68 | 47 | 59 | 46 |
| Baseline | 14.3 (2.9) | 14.5 (2.9) | 14.4 (2.9) | 14.1 (3) |
| Follow-up | 13.5 (6.3) | 8.3 (6) | 8.3 (6.6) | |
| Change | −1 (6.1) | −6.1 (6.5) | −5.8 (6.5) | |
| % change | −6 | −40.1 | −40.6 | |
| P-value (GEE) | 0.2077 | <0.0001 | <0.0001 | |
| QOL (IPSS question 8) | ||||
| n (paired values) | 58 | 41 | 54 | 39 |
| Baseline | 3.8 (1.2) | 3.9 (1.3) | 3.9 (1.2) | 3.8 (1.4) |
| Mean | 3.6 (1.7) | 2.1 (1.4) | 2.3 (1.5) | |
| Change | −0.3 (1.7) | −1.8 (1.5) | −1.5 (1.9) | |
| % change | 2.8 | −41 | −32.7 | |
| P-value (GEE) | 0.1029 | <0.0001 | <0.0001 | |
| Qmax (mL/second) | ||||
| n (paired values) | 50 | 14 | 15 | 3 |
| Baseline | 9.3 (5.1) | 8 (3.5) | 8.4 (5) | 8.1 (4.6) |
| Follow-up | 10.1 (5.5) | 14.5 (10.1) | 9.3 (3.2) | |
| Change | 2.1 (5.1) | 6.2 (10.8) | 1.2 (7.6) | |
| % change | 34.5 | 124.8 | 87.6 | |
| P-value(GEE) | 0.0960 | 0.0157 | 0.5722 | |
| PVR volume (mL) | ||||
| n (paired values) | 60 | 47 | 46 | 17 |
| Baseline | 188.8 (321.5) | 170.3 (292.4) | 213.5 (362.2) | 281.6 (461.6) |
| Follow-up | 72 (145.7) | 97.8 (213.6) | 96.6 (166.4) | |
| Change | −98.3 (271.8) | −116 (210.4) | −185 (340.5) | |
| % change | 32.3 | −16.9 | −8.4 | |
| P-value (GEE) | 0.0089 | <0.0001 | <0.0001 | |
| Voided volume (mL) | ||||
| n (paired values) | 50 | 15 | 15 | 3 |
| Baseline | 161.5 (100.5) | 174.9 (94.2) | 183.9 (113.9) | 223.7 (124.1) |
| Follow-up | 119.7 (89.2) | 142.9 (95.9) | 120 (129.2) | |
| Change | −55.1 (139.4) | −41 (122.3) | −104 (230.2) | |
| % change | −9.1 | 55.8 | −13 | |
| P-value | 0.1041 | 0.6527 | 0.3579 | |
|
| ||||
|
Severe LUTS: baseline IPSS 20–35
| ||||
| Outcome measure | Baseline | 1 month | 3−6 months | 12 months |
|
| ||||
| IPSS | ||||
| n (paired values) | 60 | 46 | 56 | 41 |
| Baseline | 25.5 (4.2) | 25.3 (4.4) | 25.7 (4.3) | 25.4 (4.1) |
| Follow-up | 18.5 (8.9) | 11.3 (7.5) | 12 (7.3) | |
| Change | −6.8 (9) | −14.4 (8.9) | −13.4 (9.2) | |
| % change | −26.1 | −54.7 | −50.4 | |
| P-value (GEE) | <0.0001 | <0.0001 | <0.0001 | |
| QOL (IPSS question 8) | ||||
| n (paired values) | 52 | 39 | 49 | 34 |
| Baseline | 4.9 (1) | 4.8 (1) | 4.9 (1) | 4.9 (0.9) |
| Follow-up | 3.9 (1.8) | 2.5 (1.5) | 2.6 (1.3) | |
| Change | −1 (2) | −2.3 (1.8) | −2.3 (1.6) | |
| % change | −17.8 | −45.5 | −43.4 | |
| P-value (GEE) | 0.0017 | <0.0001 | <0.0001 | |
| Qmax (mL/second) | ||||
| n (paired values) | 42 | 9 | 23 | 4 |
| Baseline | 8.1 (4.6) | 8.8 (4.4) | 8.9 (4.6) | 8.8 (3.2) |
| Follow-up | 8.9 (6.8) | 9.7 (5.1) | 10.5 (6.5) | |
| Change | 0.1 (5.2) | −0.9 (7.1) | −1.7 (5.5) | |
| % change | −0.9 | 43 | 24.2 | |
| P-value (GEE) | 0.7114 | 0.5352 | 0.8234 | |
| PVR volume (mL) | ||||
| n (paired values) | 52 | 35 | 40 | 18 |
| Baseline | 227 (221) | 240.5 (207.7) | 251.9 (238) | 194.1 (168.8) |
| Follow-up | 88.4 (137.1) | 58.4 (62) | 59 (55.2) | |
| Change | −152 (224.5) | −193 (220.3) | −135 (138.9) | |
| % change | 63.6 | −43.1 | −59.9 | |
| P-value (GEE) | <0.0001 | <0.0001 | <0.0001 | |
| Voided volume (mL) | ||||
| n (paired values) | 42 | 10 | 23 | 4 |
| Baseline | 169.2 (118.4) | 151.1 (161.2) | 197.7 (125.1) | 151.9 (123.7) |
| Follow-up | 106.9 (58.5) | 149.2 (105.6) | 152.3 (97.7) | |
| Change | −44.2 (132.5) | −48.5 (168) | 0.3 (38.4) | |
| % change | 45.9 | 14.6 | 10.6 | |
| P-value (GEE) | 0.2013 | 0.2384 | 0.7915 | |
Notes: Values presented as means (SD) and compared with baseline using paired Student t-test; P-values from Wald test for whether GEE estimate of change from baseline is different from 0.
Abbreviations: LUTS, lower urinary tract symptoms; GEE, general estimating equation; IPSS, International Prostate Symptom Score; QOL, quality of life; Qmax, peak urinary flow; PVR, postvoid residual: RF, radiofrequency.
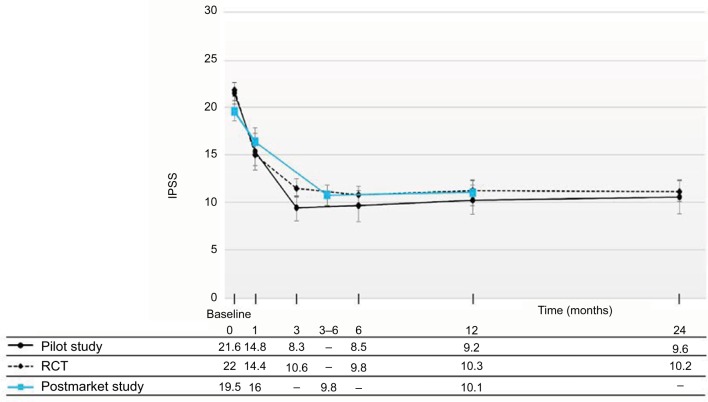
Figure 2.
IPSS improvements shown for all treated patients throughout 12 months after convective RF thermal therapy in this postmarket study.
Notes: These outcomes are compared with IPSS changes from the previously published pilot study9 and RCT10 showing similarity and durability of improvements in the three Rezūm system studies. Values are means, and error bars represent 95% CIs. IPSS improvements relative to baseline significant at all time points (P<0.001/P<0.0001).
Abbreviations: IPSS, International Prostate Symptom Score; RF, radiofrequency; RCT, randomized controlled trial.
Because of the broad spectrum of LUTS severity, outcomes were further evaluated separately in patients with moderate (IPSS 8–19) or severe (IPSS 20–35) LUTS, as shown in Table 2. Both severity groups were similar in size. In both LUTS-severity cohorts, convective RF water-vapor thermal therapy showed clinically and statistically significant improvements in IPSS, QOL, and PVR volume from 3 to 12 months (P<0.0001), and as early as 1 month for some variables. No earlier follow-up visits were conducted to determine the earliest time to response, as not all patients returned for all visits. Urinary flow rate was infrequently repeated. A significant reduction of 6.1 points in IPSS (40.1%) was achieved in the moderate LUTS cohort at 3–6 months, continuing with a 40.6% reduction through 12 months from a mean IPSS baseline of 14.3 (P<0.0001). In the severe LUTS cohort, a reduction of 14.4 points in IPSS (54.7%) from baseline was evident at 3–6 months, continuing to 12 months with a 13.4-point IPSS reduction (50.4%) from a mean IPSS baseline of 25.5 (P<0.0001).
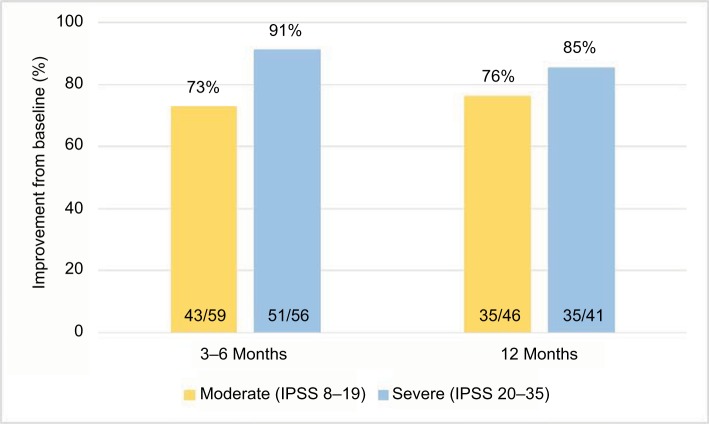
The number of individual patients achieving a meaningful clinical improvement is presented in Figure 3. The criterion for improvement was defined as ≥3-point decrease in IPSS.13 At 3–6 months after convective RF water-vapor thermal therapy, 73% of patients with moderate and 91% with severe LUTS had meaningful symptom relief. At 12 months, the relief continued in 76% and 85% of moderate and severe LUTS patients, respectively. The comparison of percentage of patients with moderate LUTS and those with severe LUTS was not significantly different at the 3–6-month and 12-month evaluations in either moderate LUTS (P=0.48) or severe LUTS (P=0.32) patients.
Figure 3.
Patients with meaningful clinical improvements in IPSS ≥3-point decrease relative to pretreatment.
Abbreviation: IPSS, International Prostate Symptom Score.
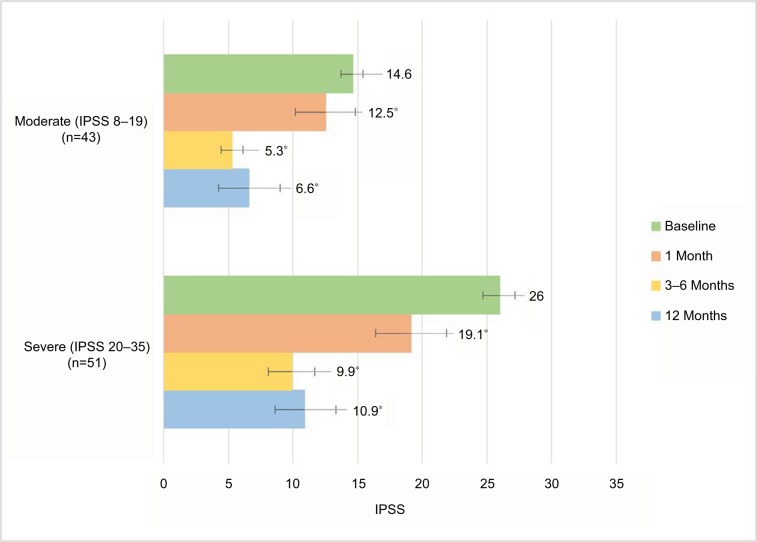
Observed IPSS values of ≥3 points at each clinic visit for all evaluable patients are shown in Figure 4. After thermal therapy, significant symptomatic relief was achieved in both LUTS-severity groups. The IPSS outcomes achieved at 3–6 months were sustained throughout 12 months. No significant differences were noted between these evaluation time points for patients with moderate LUTS (P=0.37) or severe LUTS (P=0.21). Logistic regression analysis examined the probability of patients achieving meaningful symptom relief after convective RF thermal therapy with the Rezūm system when the procedure was performed at the two urology centers or among the participating surgeons. The outcomes were not significantly different, indicative of the reproducibility of the therapeutic approach among patients with a wide spectrum of clinical conditions.
Figure 4.
Observed IPSS changes for subset of patients with meaningful clinical improvement in urinary symptoms, defined as ≥3-point decrease in IPSS.
Notes: Values are means, and error bars represent 95% CIs. *Improvements relative to baseline significant at all time points (P<0.02/P<0.0001).
Abbreviation: IPSS, International Prostate Symptom Score.
Discussion
The current data represent the first report of pooled accrued, consecutive cases of BPH treatments with the Rezūm system performed in two large community-group urology practices following commercial availability of convective RF water-vapor thermal therapy. Before FDA clearance, RCTs provided guidelines and expectations within designated and restrictive enrollment and exclusion criteria.9,10,12 However, the real-world application of the Rezūm system for treatment of LUTS/BPH adds another level of important clinical scrutiny. Compared to patients enrolled in previous trials, the cohort of patients in this retrospective analysis included a cross-section of patients with greater variability, many who were older with large prostates and some with previous invasive and MIST treatments or long-term use of BPH medications, yet continued bothersome urinary symptoms. This report also presents the first postmarket application for patients with prostates larger than 80 (80–183) cm3, all done without adverse events and with successful outcomes. Procedural details based on the experiences of the seven urologists offer valuable insights for patient selection, application to prostate zones, including the median lobe or elevated bladder neck in central-zone hyperplasia, and intraoperative techniques helpful to clinicians new to this efficient and versatile procedure.
The results of this study showed sustained improvements in urinary symptoms, QOL, and PVR volume through 1 year of follow-up. No adverse events related to sexual function were reported. The significant relief and profile of IPSS improvements reproduced and mirrored the responses to convective RF thermal therapy when compared with the pilot trial and RCT.13 The outcomes were also reproducible when comparing patients treated at two large urology groups. These observations further support the use of convective RF thermal therapy in traditional community clinical practices that encompass patients with considerable variability in LUTS/BPH. Differences in IPSS after treatment are known to be powerfully influenced by patient baseline scores.16 Accordingly, the evaluations based on LUTS severity showed that patients with either moderate (IPSS 8–19) or severe (IPSS 20–35) both achieved significantly improved scores.
All procedures were performed in an office or outpatient treatment setting. The majority of patients at these two sites were managed with intravenous conscious sedation. Management of pain and anxiety was at the discretion of the clinician and his or her standard of practice; no specific anesthesia has been suggested or required for this procedure. In the RCT, anesthesia was variable: 69% received oral sedation only, 10% intravenous sedation, and 21% a prostate block.
An inherent limitation of this study, unlike an RCT, is that follow-up times were not tightly controlled, nor inclusion criteria standardized. While considered a limitation, the patients treated and patterns of evaluation reflected those in a typical urologic practice. Unlike an RCT, patients did not present for treatment with a commitment to a protocol or a sense of altruism.
In conclusion, we believe this study highlights the merits of using convective RF water-vapor thermal therapy for rapid and durable relief of LUTS due to BPH. The thermal energy is contained within the zonal boundaries of the prostate glandular anatomy without compromising the integrity of the urinary sphincter, bladder, or rectum.17 Convective RF thermal therapy using the Rezūm system provides versatility for application to a variety of prostate-gland morphologies. The reproducibility of outcomes among the participating urologists in their practices and corroboration of results with prior clinical trials are important in meeting our expectations to provide the best care for our patients. For many symptomatic men, convective RF water-vapor thermal therapy will be an appropriate and low-risk treatment in the continuum between medical management and more invasive surgical approaches. The unique therapeutic modality warrants consideration for a first-line treatment for LUTS/BPH.
Acknowledgments
The authors appreciate the assistance of Kathleen Nunez for medical chart reviews and Tyson Rogers from NAMSA for statistical analyses.
Footnotes
Disclosure
MFD, EEA, and RRG have consulted for NxThera. The authors report no other conflicts of interest in this work.
References
- 1.Fenter TC, Naslund MJ, Shah MB, Eaddy MT, Black L. The cost of treating the 10 most prevalent diseases in men 50 years of age or older. Am J Manag Care. 2006;12:S90–S98. [PubMed] [Google Scholar]
- 2.Urology Care Foundation [website on the Internet] [Accessed June 9, 2017]. Available from: www.urologyhealth.org.
- 3.Wei JT, Calhoun E, Jacobsen SJ. Urologic Diseases in America project: benign prostatic hyperplasia. J Urol. 2005;173:1256–1261. doi: 10.1097/01.ju.0000155709.37840.fe. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention Ambulatory health care data. 2017. [Accessed June 4, 2017]. Available from: http://www.cdc.gov/nchs/ahcd.htm.
- 5.Filson CP, Wei JY, Hollingsworth Trends in medical management of men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Urology. 2013;82:1386–1392. doi: 10.1016/j.urology.2013.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention Public health and aging – trends in aging: United States and worldwide. JAMA. 2003;289:1371–1373. [PubMed] [Google Scholar]
- 7.Rensing AJ, Kuxhausen A, Vetter J, Strope SA. Differences in the treatment of benign prostatic hyperplasia: comparing the primary care physician and the urologist. Urol Pract. 2017;4:193–199. doi: 10.1016/j.urpr.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mundy AR. The future of urology. BJU Int. 2003;92:337–339. doi: 10.1046/j.1464-410x.2003.04381.x. [DOI] [PubMed] [Google Scholar]
- 9.Dixon CM, Cedano ER, Pacik D, et al. Two-year results after convective water vapor energy treatment of symptomatic benign prostatic hyperplasia. Res Rep Urol. 2016;8:207–216. doi: 10.2147/RRU.S119596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McVary KT, Gange SN, Gittelman MC, et al. Minimally invasive prostate convective water vapor energy ablation: a multicenter, randomized, controlled study for treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2016;195:1529–1538. doi: 10.1016/j.juro.2015.10.181. [DOI] [PubMed] [Google Scholar]
- 11.McVary KT, Gange SN, Gittelman MC, et al. Erectile and ejaculatory function preserved with convective water vapor energy treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia: randomized controlled study. J Sex Med. 2016;13:924–933. doi: 10.1016/j.jsxm.2016.03.372. [DOI] [PubMed] [Google Scholar]
- 12.Roehrborn CG, Gange SN, Gittelman MC, et al. Convective thermal therapy: durable 2-year results of randomized controlled and prospective crossover studies for treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. J Urol. 2017;197:1507–1516. doi: 10.1016/j.juro.2016.12.045. [DOI] [PubMed] [Google Scholar]
- 13.Woo HH, Gonzalez RR. Perspective on the Rezūm system: a minimally invasive treatment strategy for benign prostatic hyperplasia using convective radiofrequency water vapor thermal therapy. Med Devices (Auckl) 2017;10:71–80. doi: 10.2147/MDER.S135378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Urological Association Management of benign prostatic hyperplasia (BPH) 2010. [Accessed August 3, 2017]. Available from: http://www.auanet.org/guidelines/benign-prostatic-hyperplasia-(2010-reviewed-and-validity-confirmed-2014)
- 15.Cui L, Hung HM, Wang SJ. Modification of sample size in group sequential clinical trials. Biometrics. 1999;55:853–885. doi: 10.1111/j.0006-341x.1999.00853.x. [DOI] [PubMed] [Google Scholar]
- 16.Barry MJ, Williford WO, Chang Y, et al. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? J Urol. 1995;154:1770–1774. doi: 10.1016/s0022-5347(01)66780-6. [DOI] [PubMed] [Google Scholar]
- 17.Dixon CM, Cedano ER, Mynderse LA, Larson TR. Transurethral convective water vapor as a treatment for lower urinary tract symptomatology due to benign prostatic hyperplasia using the Rezūm system: evaluation of acute ablative capabilities in the human prostate. Res Rep Urol. 2015;7:13–18. doi: 10.2147/RRU.S74040. [DOI] [PMC free article] [PubMed] [Google Scholar]